SUMMARY
Melanocyte stem cells (McSCs) intimately interact with epithelial stem cells (EpSCs) in the hair follicle bulge and secondary hair germ (sHG). Together, they undergo activation and differentiation to regenerate pigmented hair. However, the mechanisms behind this coordinated stem cell behavior have not been elucidated. Here, we identified Wnt signaling as a key pathway that couples the behavior of the two stem cells. EpSCs and McSCs coordinately activate Wnt signaling at the onset of hair follicle regeneration within the sHG. Using genetic mouse models that specifically target either EpSCs or McSCs, we show that Wnt activation in McSCs drives their differentiation into pigment-producing melanocytes, while EpSC Wnt signaling not only dictates hair follicle formation but also regulates McSC proliferation during hair regeneration. Our data define a role for Wnt signaling in the regulation of McSCs and also illustrate a mechanism for regeneration of complex organs through collaboration between heterotypic stem cell populations.
INTRODUCTION
Successful regeneration of a functional organ relies on the organized and timely orchestration of molecular events among distinct stem/progenitor cell populations. The mammalian hair follicle (HF), containing several stem cell populations, serves as an advantageous model for the dissection of such collaboration among distinct cell types. The HF undergoes cyclical periods of growth (anagen) and rest (telogen), driven by the proliferation and differentiation of epithelial stem cells (EpSCs) residing in the bulge area as well as the secondary hair germ (sHG) of the HF (Cotsarelis et al., 1990, Greco et al., 2009, Zhang et al., 2009). The HF bulge and sHG areas maintain not only EpSCs that express Keratin 15 (K15) (Liu et al., 2003), but also hold melanocyte stem cells (McSCs) that are responsible for hair pigmentation (Nishimura et al., 2002). McSCs are undifferentiated and unpigmented melanocytes that reside in the bulge-sHG area. Developmentally, melanocytes originate from the neural crest (Rawles, 1947) and migrate through the dermis and epidermis to eventually reside in the HF. In adult mouse skin, melanocytes are located exclusively in HFs, while in human skin, melanocytes are maintained in the interfollicular epidermis as well.
During anagen, differentiated McSC progeny that are located in the hair bulb produce and transfer pigment to adjacent epithelial cells that differentiate into hair (Nishimura et al., 2002). Upon entry into telogen, differentiated melanocytes are no longer present as they undergo apoptosis in sync with degeneration of the lower part of the HF (Sharov et al., 2005). When EpSCs regenerate the lower follicle at the initiation of a new anagen phase, undifferentiated McSCs coordinately repopulate the hair bulb with differentiated pigment-producing progeny. These two distinct stem cell populations of developmentally distinct origins act in concert to regenerate pigmented hair with each hair cycle. However, the mechanisms behind this coordinated stem cell behavior have not been elucidated.
In this study, we ask how two adult stem cells of different lineages become activated to proliferate and differentiate in a synchronized manner at the onset of HF regeneration. Addressing this question is not only critical to understanding the molecular mechanisms regulating McSCs, but may also provide important insight into how a complex organ can form by cooperation between distinct stem/progenitor cells in adult mammals. Numerous studies have focused on the reciprocal interactions between tissue-producing EpSCs and inductive dermal cells during the induction of HF regeneration (Greco et al., 2009; Rendl et al., 2008). Little is known, however, about the molecular mechanisms of how different types of stem/progenitor cells, which form the complete HF, coordinate their behavior.
Large strides have been made to understand the molecular signals regulating EpSCs (Blanpain and Fuchs, 2009). Principal among these is the Wnt signaling pathway. Upon Wnt stimulation, GSK3b, which phosphorylates and targets β-catenin for degradation, is inhibited. β-catenin accumulates in the cytoplasm and then translocates to the nucleus, where it binds to TCF/LEF transcription factors to regulate transcription of Wnt target genes (Barker, 2008). The resulting changes in gene expression are the basis for the diverse roles of Wnt signaling in development, regeneration and tumorigenesis (Nusse, 2008). Wnt signaling is critical for HF development, as inhibition of embryonic Wnt/β-catenin signaling results in a lack of HFs (Andl et al., 2002; Huelsken et al., 2001). Activation of β-catenin promotes HF morphogenesis and differentiation (Gat et al., 1998; Zhang et al., 2008). Postnatally, Wnt/β-catenin signaling is activated in EpSCs and is essential for their proliferation and differentiation to regenerate the HF during anagen (Lowry et al., 2005; Van Mater et al., 2003). These studies established the role of Wnt signaling in governing HF development and the adult hair cycle. Interestingly, β-catenin activation in embryonic epidermis results in hyperpigmentation (Zhang et al., 2008), and ectopic hair follicles induced by forced activation of β-catenin contain melanocytes (Silva-Vargas et al., 2005), suggesting that epithelial β-catenin may influence behavior of other cell types, including melanocytes in the skin. However, the precise cellular behaviors of melanocytes or molecular mechanisms that resulted in the observed phenotypes have not been defined.
In contrast to EpSCs, relatively modest strides have been made toward understanding the signals that regulate McSCs (Nishikawa and Osawa, 2007). Previous elegant studies revealed that Notch (Moriyama et al., 2006; Schouwey et al., 2007) and TGF-β signaling (Nishimura et al., 2010) are essential for McSC establishment and maintenance, while molecular signals regulating the proliferation and differentiation of McSCs during HF regeneration remain largely unknown.
The importance of Wnt signaling in mouse melanocyte development is exemplified by studies showing that mice deficient in Wnt1 or Wnt3a expression, or the downstream effector, β-catenin, exhibit a loss of melanocyte formation from neural crest (Ikeya et al., 1997; Hari et al., 2002). Wnt signaling promotes the generation of melanocytes from neural crest cells (Dorsky et al., 1998; Dunn et al., 2000). β-catenin stabilization in mouse melanoblasts results in their reduced numbers in embryonic skin (Delmas et al., 2007). In vitro studies have shown that Wnts induce several melanogenic genes associated with melanocyte differentiation (Takeda et al., 2000). Conversely, Dickkopf 1, an inhibitor of canonical Wnt activity, suppresses melanocyte differentiation in human melanocyte cultures (Yamaguchi et al., 2004). However, despite these cumulative data on the role of Wnt signaling in in vitro melanocyte/melanoma studies (Larue and Delmas, 2006), the role of Wnt signaling in McSCs in the skin remains undetermined.
In this study, we examine the regulation of McSCs in adult skin by the Wnt signaling pathway through both intrinsic and extrinsic mechanisms. Using loss- and gain-of-function studies of β-catenin specifically in melanocytes, we find that Wnt/β-catenin signaling is a critical intrinsic pathway governing McSCs and hair pigmentation. Furthermore, we find two specific ways that EpSCs regulate McSCs, parallel and subsequent to the activation of Wnt signaling in EpSCs. First, although McSCs do not produce Wnt ligands themselves, we find that neighboring K15+ EpSCs produce canonical Wnt ligands capable of activating Wnt signaling in melanocytes. Second, by using a promoter that specifically targets K15+ EpSCs, we demonstrate that modulation of Wnt/β-catenin signaling in EpSCs extrinsically regulates McSC behavior. Specifically, β-catenin activation in K15+ EpSCs, which initiates HF regeneration during anagen, can simultaneously promote McSC proliferation partly through downstream secretion of Endothelins (Edns) that act as potent mitogens for melanocytes. From these results, we propose a model that explains the coordinated behavior of these two stem cell populations during HF regeneration. Collectively, our results indicate that Wnt signaling is a key intrinsic and extrinsic regulatory pathway that couples the activation of McSCs to that of EpSCs within the niche and may serve as a provocative target to manipulate McSCs in the treatment of pigmentation disorders.
RESULTS
EpSCs and McSCs Coactivate Wnt Signaling at Anagen Onset in the sHG
To investigate the potential role of canonical Wnt signaling in McSCs, we examined the immunoreactivity of β-catenin, a key downstream mediator of Wnt signaling, during the adult hair cycle. We analyzed melanocytes that stain positively for Trp2 (tyrosinase-related protein 2), a marker that is expressed in both differentiated and undifferentiated melanocytes (Nishimura et al., 2002). During telogen, Trp2+ McSCs were found in the lower permanent portion of the HF, corresponding to the bulge-sHG area, consistent with previous reports (Nishimura et al., 2002). We previously described that the sHG is a biochemically distinct but functionally closely related extension of the bulge (Ito et al., 2004). Recent studies showed that EpSCs in the sHG (CD34–) initiate hair growth in cooperation with bulge EpSCs (CD34+) in anagen (Greco et al., 2009, Zhang et al., 2009).
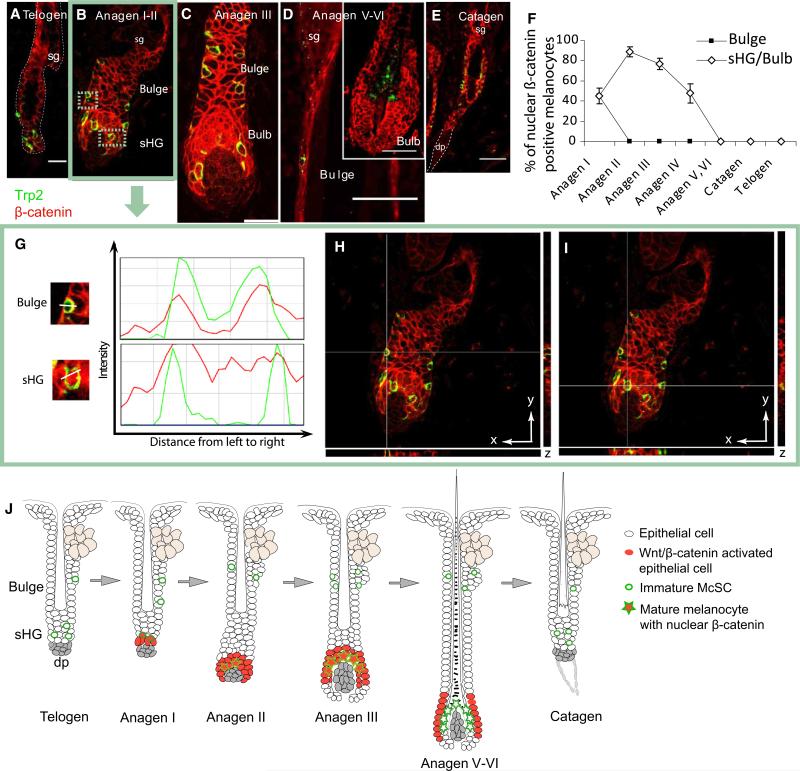
During telogen, β-catenin is confined to the cytoplasm of Trp2+ McSCs in the bulge-sHG area (Figure 1A). At anagen onset, both EpSCs and McSCs that colocalize in the sHG (CD34–) display nuclear β-catenin, an indicator of active canonical Wnt signaling (Figures 1B and 1G–1I and Figure S1 available online). As the HF grows, the sHG becomes unapparent with establishment of the hair bulb (Figure 1C). The melanocytes that show nuclear β-catenin positivity remains closely associated with the nuclear β-catenin-positive epithelial cells in the hair bulb (Figure 1C). The nuclear signal in differentiated bulb melanocytes then becomes downregulated by late anagen (Figure 1D), prior to the transition into catagen (Figure 1E). This pattern of nuclear β-catenin reactivity (Figure 1J) is also seen in human HFs, suggesting that the regulation of Wnt signaling is conserved between mouse and human follicular melanocytes (Figure S2).
Figure 1. Wnt/β-Catenin Signaling Is Suppressed in Bulge McSCs during the Adult Hair Cycle.
(A–E) Immunofluorescence of Trp2 and β-catenin on dorsal skin of 8-week-old mice.
(F) Percentage ± standard deviation (SD) of Trp2+ melanocytes with nuclear β-catenin in the bulge and sHG/bulb.
(G) Intensity histograms of bulge and sHG melanocytes. A minimum of three melanocytes per compartment were analyzed in 20 follicles.
(H–I) Orthogonal views of anagen onset HFs at indicated axes intersections.
(J) Schematic representation. HFs were staged as published (Müller-Röver et al., 2001).
Scale bars represent 20 μm (A) and 50 μm (C–E). sg, sebaceous gland; dp, dermal papilla; sHG, secondary hair germ. See also Figures S1 and S2.
Forced Activation of Wnt Signaling in McSCs Induces McSC Differentiation and Premature Hair Graying
To investigate the functional significance of Wnt activation in McSCs, we first analyzed the effects of forced activation of Wnt signaling in McSCs. We used an inducible transgenic mouse model (Tyr-CreERT2; β-cateninfl(ex3)/+) to conditionally express a dominant mutant form of β-catenin specifically in melanocytes in vivo(Bosenberg et al., 2006; Harada et al., 1999). The mutant β-catenin gene lacks exon 3, which is essential for its phosphorylation by GSK3b, resulting in constitutive activation of canonical Wnt signaling.
After tamoxifen (TAM) induction of the mutant β-catenin gene during early anagen, we found that bulge McSCs, which typically do not undergo β-catenin stabilization, exhibit nuclear localization of β-catenin during anagen (Figures 2F and 2H). Most noticeably, those McSCs that show aberrant β-catenin activation also display ectopic pigmentation within the bulge, a region that never has pigment in control mice (Figures 2G and 2K). Immunohistochemistry reveals that the pigmented McSCs in the bulge of the Tyr-CreERT2; β-cateninfl(ex3)/+ mice also ectopically upregu-late melanocyte differentiation markers (Figures 2J and 2M), in marked contrast to their usual undifferentiated state seen in control mice (Figures 2I and 2L). Further comprehensive analyses by quantitative PCR (qPCR) and immunohistochemistry (Figure S3) confirm upregulation of melanocyte differentiation markers in mutant McSCs. Differentiated bulb melanocytes from control and mutant mice show similar expression of melanocyte differentiation markers (Figures 2L and 2M). These results demonstrate that β-catenin stabilization in McSCs is sufficient to drive melanocyte differentiation.
Figure 2. Stabilization of β-Catenin in McSCs Drives the Melanocyte Differentiation Program.
(A) PCR genotyping of dorsal skin shows the floxed exon 3 (left) and its deletion (right) before and after TAM administration, respectively.
(B) Mice were administered TAM for 7 days during their second anagen and after depilation-induced third anagen. Gross appearance of control and mutant (Tyr-CreERT2;β-cateninfl(ex3)/+) mice at third anagen.
(C) Hair was considered gray when at least 20% of randomly picked hair shafts displayed lack of pigmentation. Ten mice from more than four separate litters were analyzed.
(D–G, I, J, L, and M) Immunohistochemistry for indicated melanocyte differentiation markers during third anagen, 5 days after depilation.
(H, K, and N) Quantification of histological and histochemical analysis at third anagen.
Data are reported as average ± SD. Arrowheads indicate pigmented bulge melanocytes. See also Figures S3 and S4.
We noticed that some of the TAM-induced mice grew unpigmented hair in the third anagen and that all mutant mice exhibited this phenotype by the fourth hair cycle (Figures 2B and 2C), in contrast to control mice that maintain pigmented hair. Previous studies showed that precocious differentiation of McSCs in the bulge occurs at the expense of stem cell self-renewal, leading to their eventual loss and causing hair graying (Nishimura et al., 2005). We analyzed whether pigmented McSCs in our model were similarly lost during successive hair cycles. After the initial TAM treatment during the second anagen, McSCs persisted throughout the immediate HF cycle (Figure S4A). However, by the end of the third hair cycle, when hair graying begins to be observed in the mutant mice, the number of Trp2+ McSCs decreased significantly (Figures S4B and S4C). These results suggest that constitutive β-catenin stabilization in McSCs induces premature McSC differentiation and their eventual loss of self-renewing capacity, yielding premature hair graying.
β-Catenin Is Required for McSC Differentiation
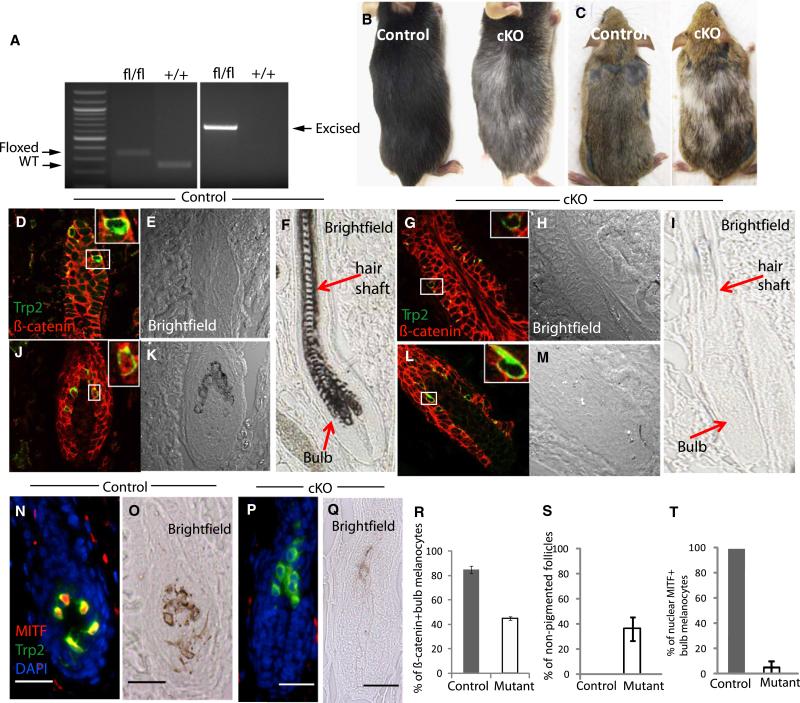
To further examine the importance of Wnt signaling in McSC regulation, we depleted β-catenin gene expression using a conditional knockout (cKO) mouse (Tyr-rtTA;tetO-Cre; β-cateninfl/fl) that cannot express functional β-catenin protein (Brault et al., 2001) in melanocytes upon doxycycline (Dox) treatment.
We maintained mice on Dox-containing food beginning at the first telogen. In the following second hair cycle, we did not detect any measurable changes in melanocyte number or their expression of differentiation markers in the bulge or in the hair bulb. However, continued induction past the second telogen and into the next hair cycle eventually resulted in gray hair coats on the cKO mice (Figure 3B). Unlike control bulb melanocytes, the mutant bulb melanocytes display a clear lack of nuclear β-catenin (Figures 3J–3M and 3R). These bulb melanocytes have defects in pigment production and fail to express melanocyte differentiation markers like MITF that are normally found in bulb melanocytes (Figures 3N–3Q and 3T and Figure S5). Furthermore, HFs had markedly reduced numbers of bulb melanocytes. We found that bulge McSCs and their descendant bulb melanocytes in the cKO mice incorporate BrdU about two-fold less than control melanocytes during anagen (Figure S5I). However, bulge melanocytes persist and remain unpigmented, similar to control mice (Figures 3D, 3E, 3G, and 3H). We did not detect expression of cleaved caspase-3, an apoptosis marker, in cKO bulb melanocytes (Figure S6), suggesting that the reduced number of bulb melanocytes is largely due to a proliferation defect. Our data show that loss of β-catenin in melanocytes inhibits follicular melanocyte proliferation and differentiation during anagen, resulting in the formation of unpigmented hair (Figure 3I and Figure S3). These results show that McSCs, similar to EpSCs (Lowry et al., 2005), utilize Wnt signaling to stimulate their initial differentiation during anagen. Therefore, the coactivation of Wnt signaling in EpSCs and McSCs at anagen onset (Figure 1) suggests that both stem cell populations become coordinately committed to the generation of a pigmented HF within a shared niche.
Figure 3. Wnt/β-Catenin Signaling Is Required for Repopulation of Mature Pigment-Producing Melanocytes in the Hair Bulb.
(A) PCR genotyping of dorsal skin shows bands for the floxed β-catenin gene (left) and the cre-recombination-induced excised gene (right).
(B) Treatment of Tyr-rtTA;TetO-Cre;β-cateninfl/fl mice with Dox from their first telogen onward results in gray hair coats in the third hair cycle, both spontaneous and induced. More than 15 mice were analyzed from five litters.
(D–Q) Immunohistochemistry of the indicated markers during third anagen, 8 days after depilation.
(R–T) Quantification of histological and histochemical analyses at third anagen. Data are reported as average ± SD.
(C) Tyr-CreERT2;β-cateninfl/fl mice produced similar results after TAM treatment for 5 days during second and third early anagen. See also Figures S5 and S6.
Wnt Ligands Expressed by EpSCs Are Capable of Activating Wnt Signaling in Neighboring Melanocytes at Anagen Onset
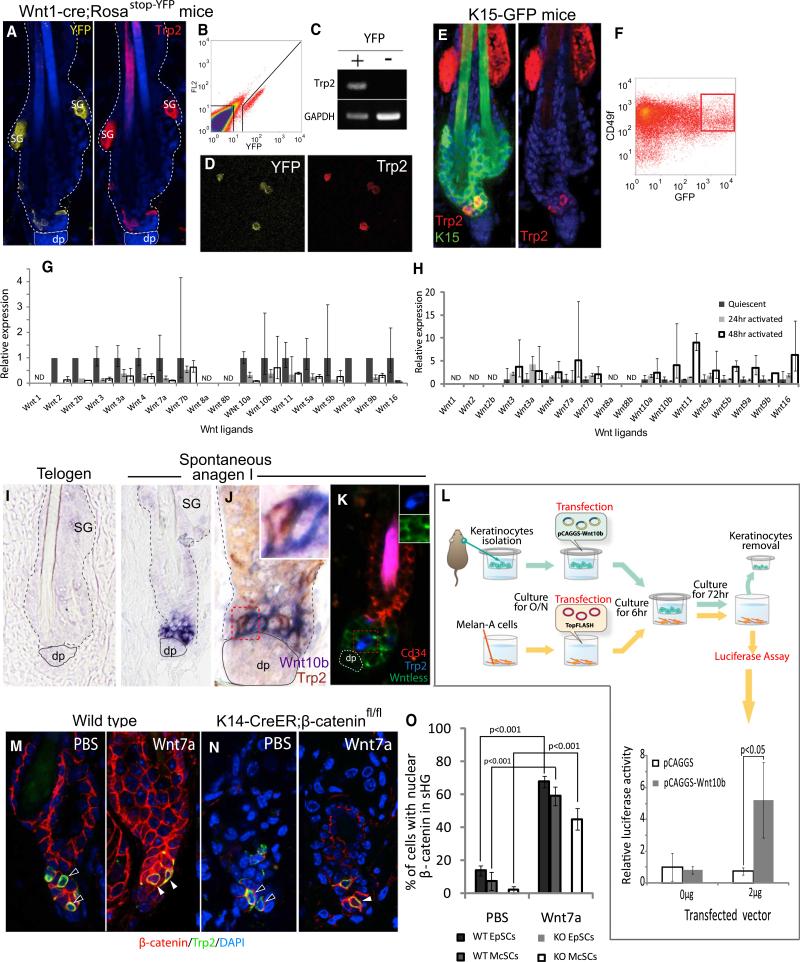
In an effort to characterize the Wnt ligand(s) responsible for β-catenin stabilization in McSCs at anagen onset, we isolated McSCs using double transgenic Wnt1-Cre;Rosastop-YFP mice. In these mice, all neural crest-derived melanocytes in skin express YFP (Lang, et al., 2005). Immunohistochemistry showed that 100% of Trp2+ McSCs express YFP (Figure 4A). Cytospin analysis of isolated YFP+ cells confirmed that 100% of YFP+ cells express Trp2 (Figure 4D). We then performed qPCR to compare the expression levels of Wnt ligands in McSCs isolated at telogen and anagen onset (depilation-induced). However, we found that no Wnt ligand is significantly upregulated in McSCs at 24 or 48 hr into anagen onset (Figure 4G).
Figure 4. Isolation of McSCs and Identification of Wnt Ligands in the Niche.
(A) Trp2 immunofluorescence on skin from Wnt1-Cre;Rosastop-YFP mice.
(B–D) YFP+ cells were isolated with FACS and assessed for purity by RT-PCR and cytospin analysis.
(E) Trp2 immunofluorescence on skin from K15-GFP mice.
(F) GFP+ CD49f+ EpSCs were isolated from K15-GFP mice, as described (Morris et al., 2004).
(G) Wnt ligand qPCR of McSCs isolated from 8-week-old mice at telogen, 24 and 48 hr after depilation.
(H) qPCR of EpSCs isolated at identical time points.
(I) In situ hybridization for Wnt10b at telogen and spontaneous anagen onset (postnatal day 24: P24).
(J) Detection of Wnt10b by in situ hybridization (purple) and Trp2 (brown) by immunohistochemistry.
(K) Triple immunofluorescence of CD34 (Trempus et al., 2003), Trp2, and Wntless. Wntless expression is confined to CD34– sHG EpSCs at anagen onset, excluding Trp2+ McSCs (inset).
(L) Coculture of primary keratinocytes and mouse melanocytes (melan-a cells), transfected with pCAGGS-Wnt10b and TopFLASH vector, respectively.
(M and N) Intradermal inoculation of Wnt7a or PBS (control) soaked beads into 8-week-old telogen skin of wild-type (M) or K14-CreER;β-cateninfl/fl (N) mice. In (N), HFs that exhibited complete deletion of β-catenin in sHG EpSCs (55% ± 2.7%) were analyzed for nuclear β-catenin positivity in McSCs.
(O) Quantification of nuclear β-catenin positivity in EpSCs and McSCs in the sHG at 5 days after Wnt7a-bead inoculations.
Data are reported as average ± SD. SG, sebaceous gland; dp, dermal papilla; open and filled arrowheads, Trp2+ McSCs without and with nuclear β-catenin, respectively. See also Figure S7.
How do McSCs activate Wnt signaling without producing an endogenous Wnt ligand at anagen onset? The stem cell theory suggests that stem cells are regulated by both intrinsic programs and by extrinsic signals derived from neighboring niche cells (Schofield, 1978). To begin to examine the possibility of an extrinsic regulator of McSC Wnt signal activation, we focused on K15+ EpSCs (Liu et al., 2003), niche cells that neighbor McSCs (Tanimura et al., 2011). A previous report demonstrated by in situ hybridization that Wnt 10b is the only detectable Wnt ligand in the lowermost permanent portion of follicular epithelium, corresponding to the sHG (Reddy et al., 2001). More recently, microarray data indicating that several Wnts are upregulated in sHG EpSCs at anagen onset was reported (Greco et al., 2009). To confirm Wnt ligand expression by K15+ EpSCs, we isolated them using K15-GFP transgenic mice that express GFP in K15+ EpSCs (Morris et al., 2004) (Figure 4F). qPCR confirmed upregulation of several Wnts in K15+ EpSCs at anagen onset, consistent with a previous report (Figure 4H). We also confirmed that Wnt 10b, was expressed in the sHG (Figure 4J). Additionally, Wntless, known to be required for Wnt ligand secretion (Fu et al., 2009), is strongly expressed in sHG EpSCs but not in McSCs at anagen onset (Figure 4K). These data indicate that, in contrast to McSCs, EpSCs can express and secrete canonical Wnt ligands in a hair cycle-dependent manner and may serve as a cellular source of β-catenin stabilization during anagen onset.
To test whether a canonical Wnt ligand secreted by epithelial cells can influence Wnt signaling activity in adjacent melanocytes, we cultured Wnt10b-transfected epithelial cells with melanocytes that contain a Wnt-responsive luciferase reporter (Figure 4L). In this assay, Wnt activity was upregulated about 5-fold in melanocytes, suggesting that Wnt10b secreted by epithelial cells can activate Wnt signaling in adjacent melanocytes in vitro. To determine whether an extrinsic canonical Wnt ligand can activate McSCs in vivo, we introduced beads soaked in either PBS or Wnt7a, another Wnt found upregulated in EpSCs, into the dorsal dermis of wild-type mice at the second telogen. Within 5 days of the initial inoculation, nuclear β-catenin was observed in McSCs specifically in HFs adjacent to the Wnt7a-soaked beads, demonstrating that Wnt activation occurs precociously in sHG McSCs when exposed to exogenous Wnt ligands (Figures 4M and 4O). Consequently, these McSCs upregulated melanocyte differentiation markers including MITF (Figure S7). Not surprisingly, EpSCs in the sHG of these same HFs also exhibited precocious β-catenin stabilization in this experiment (Figures 4M and 4O). To exclude possible secondary effects of β-catenin stabilization in EpSCs and concomitant anagen onset, we introduced Wnt7a-beads into K14-CreER; β-cateninfl/fl mice that do not enter anagen upon TAM induction (Lowry et al., 2005). The EpSCs in these arrested telogen follicles failed to express nuclear β-catenin, as expected. Within 5 days after Wnt7a inoculation, McSCs in lowest parts of the follicles surrounding Wnt7a-beads still exhibited expression of nuclear β-catenin (Figures 4M and 4O), demonstrating that an exogenous canonical Wnt ligand is sufficient to activate Wnt signaling within McSCs, independent of hair cycle effects. Taken together with the data showing that Trp2+ McSCs lie adjacent to K15+ EpSCs that secrete canonical Wnt ligands at anagen onset (Figure 4E), our results suggest that Wnts produced by EpSCs may comprise one of the mechanisms that result in the activation of Wnt signaling in McSCs and EpSCs in their shared niche.
β-Catenin Stabilization in EpSCs Promotes Expansion of Melanocytes
To explore the physiological implication of Wnt signaling coactivation, we asked how activation of Wnt signaling (β-catenin stabilization) in EpSCs affects McSC behavior. We used double-transgenic K15-crePR1;β-cateninfl(ex3)/+ mice in which the constitutively active form of β-catenin can be induced in K15+ EpSCs upon RU486 treatment (Morris et al., 2004). After RU486 treatment, β-catenin stabilization is observed in the bulge epithelial cells, producing irregular buds arising from the bulge area, consistent with previous studies (Figures 5B and 5C) (Gat et al., 1998; Silva-Vargas et al., 2005). This leads to an abnormal enlargement of the bulge area (Figures 5A and 5B), containing Trp2+ melanocytes that localize specifically to areas of nuclear β-catenin-positive epithelial cells (Figures 5B and 5C). McSCs showed increased proliferation, leading to a dramatic increase in their numbers in the outer root sheath (ORS) (Figure 5C). Cells of the upper follicle and interfollicular epidermis are not derived from K15+ EpSCs during homeostasis (Ito et al., 2005). These areas did not exhibit nuclear β-catenin positivity and lacked melanocytes (Figure 5D).
Figure 5. β-Catenin Activation in EpSCs Directs McSC Proliferation and Localization of Melanocyte Progeny.
(A–D) K15-CrePR1;β-cateninfl(ex3)/+ mice and littermates were topically treated with 1% RU486 for 10 days beginning at P20, and then skin samples were subjected to immunofluorecence. The inset in (C) shows that Trp2+ melanocytes display the proliferation marker, Ki67, in a serial section of the boxed area.
(E and F) K14-CreER;β-cateninfl(ex3)/+ mice and littermates were treated with TAM for 7 days beginning at P20, and then skin samples were subjected to immunofluorecence.
(G and H) Quantification of melanocytes in each mouse model. In (G), results are representative of 50 skin sections analyzed with Image J (NIH). Data are reported as average ± SD.
(I) Schematic representation of the melanocyte expansion upon forced activation of Wnt signaling in epithelilal cells.
Arrows, Ki67+ melanocytes; arrowheads, melanocyte. The scale bar represents 100 μm.
To confirm the correlation of epithelial β-catenin activation and melanocyte expansion, we used K14-CreER;β-cateninfl(ex3)/+ mice in a complementary experiment. In these mice, the TAM-inducible CreER gene is driven by a K14 promoter that is active in the entire basal layer of all skin epithelial compartments, including the upper follicle and interfollicular epidermis (Vasioukhin et al., 1999). After TAM treatment, we found expanded numbers of Trp2+ melanocytes ectopically located in all compartments within the skin epithelium (Figure 5F). These results suggest that β-catenin stabilization in epithelial cells promotes melanocyte proliferation.
Melanocyte Expansion by β-Catenin Stabilization in EpSCs Is Mediated by Edn Receptor B Signaling
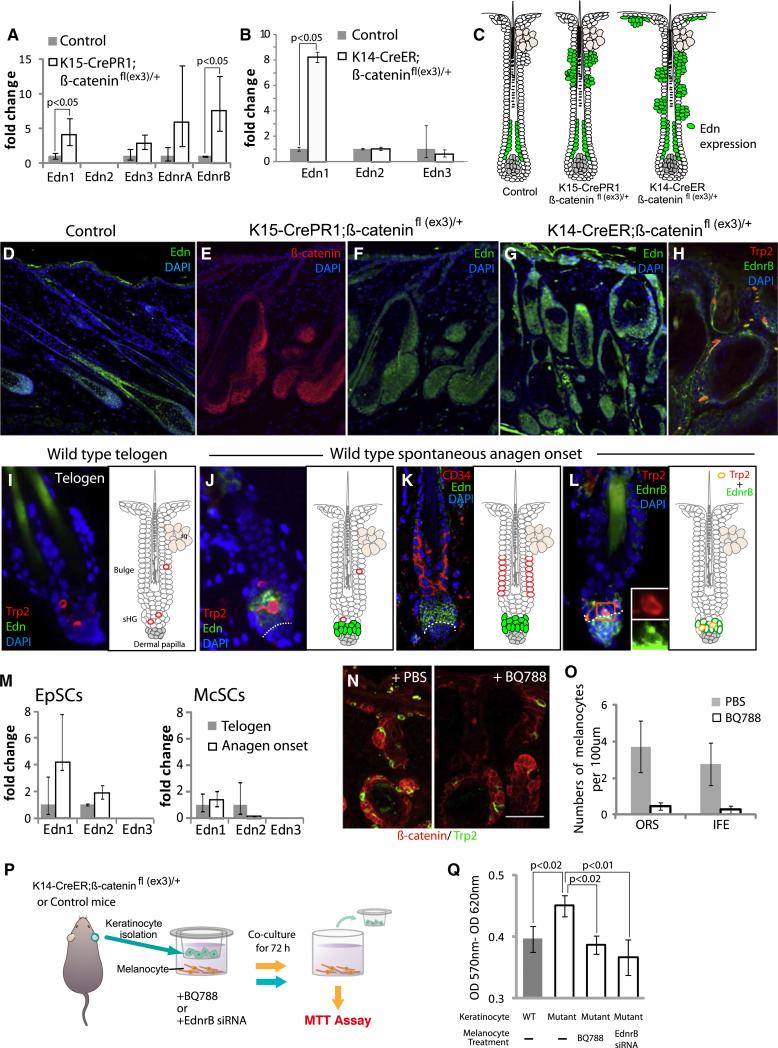
To understand how β-catenin stabilization within epithelial cells results in melanocyte expansion, we performed a microarray analysis using complementary DNA (cDNA) prepared from whole skin samples harvested from the above described K15-crePR1; β-cateninfl(ex3)/+ mice (Figure 5). The analysis (data not shown) and qPCR show that Edn1, a direct target of Wnt signaling (Kim et al., 2005) and a potent mitogen for melanocytes (Sal-dana-Caboverde and Kos, 2010), is markedly upregulated in mutant skin (Figure 6A). Immunohistochemical detection of Edns on skin sections further confirmed enhanced levels of Edns in the ORS (Figure 6F), remarkably corresponding to areas of Wnt-activated epithelial cells in these mutant mice (Figure 6E). In contrast, Edn expression in control anagen HFs was largely confined to the matrix and inner parts of the hair (Figure 6D). Similarly, TAM-induced K14-creER;β-cateninfl(ex3)/+ mice show expansion of Edn expression beyond the HF and into the interfollicular epidermis as well, coinciding with areas of forced β-catenin stabilization (Figures 6G and 5I). The upregulation of Edn1 transcripts was also confirmed by qPCR with cDNA obtained from skin epidermis isolated from K14-creER;β-cateninfl(ex3)/+ mice (Figure 6B).
Figure 6. Wnt-Activated EpSCs Promote McSC Proliferation by Secretion of Edns.
(A) qPCR of Edns and Ednr with cDNA from K15-CrePR1;β-cateninfl(ex3)/+ mouse skin, harvested after 10 days of RU486 treatment beginning at P20.
(B) qPCR confirmation of Edn1 upregulation with cDNA obtained from epithelial sheets from K14-CrePR1;β-cateninfl(ex3)/+ mice after 7 days of TAM treatment beginning at P20.
(D–L) Immunofluorescence for indicated markers on skin sections of indicated mice.
(C) Summary of Edn expression.
(M) qPCR for Edns with EpSCs and McSCs, purified from K15-GFP and Wnt1-Cre;Rosastop-YFP mice, respectively.
(N and O) In vivo BQ788, EdnrB antagonist, reduces melanocyte proliferation in K14-CreER;β-cateninfl(ex3)/+ mouse skin.
(P and Q) In vitro coculture of K14-CreER;β-cateninfl(ex3)/+ or control keratinocytes and melanocytes. BQ788 and siRNA against EdnrB neutralize melanocyte hyperproliferation with mutant keratinocytes.
Data are reported as average ± SD. dp, dermal papilla. See also Figures S8 and S9.
To examine whether Edns are upregulated in EpSCs at anagen onset, when Wnt signaling is activated in EpSCs of wild-type mice, we sorted EpSCs from K15-GFP mice (Figure 2E). qPCR analysis shows that Edn 1 and 2 are upregulated at anagen onset (Figure 6M). Immunohistochemistry reveals that Edns can be detected in CD34– sHG cells at the onset of anagen (Figures 6J and 6K), in a pattern similar to that seen for β-catenin stabilization (Figure 1). We also isolated McSCs as described above (Figures 4A and 4B) and found that McSCs do not upregulate any Edns at anagen (Figure 6M) but do express Edn receptor B (EdnrB) that binds to Edn 1, 2, and 3 (Sakurai et al., 1990) (Figures 6H and 6L), suggesting that McSCs are capable of transducing Edn ligand signals expressed within the niche at anagen onset (Figure 6K). EdnrA, another receptor for Edns, was not detected in McSCs at either time point analyzed.
To determine whether Edns, which are upregulated upon β-catenin stabilization in EpSCs, may contribute to McSC proliferation, we used a potent and specific antagonist of EdnrB, BQ788. We injected BQ788, or vehicle (PBS) into TAM-induced 21d old K14-creER;β-cateninfl(ex3)/+ mice and analyzed their skin 7 days later. PBS injection resulted in the expected expansion of melanocytes. However, administration of BQ788 resulted in marked abrogation of this expansion (Figures 6N and 6O). We did not detect cell death in the skin injected with PBS or BQ788 (data not shown).
To further investigate the interaction between Wnt-activated epithelial cells and melanocytes, we utilized a coculture model (Figure 6P). We isolated epithelial cells from TAM-induced K14-CreER;β-catenin fl(ex3)/+ or control mice and seeded them in a transwell insert to culture with mouse melanocytes (melana cells). After 72 hr of coculture, melanocytes cultured with epithelial cells expressing the stabilized β-catenin exhibited significantly increased proliferation compared to those cultured with wild-type epithelial cells. This effect was neutralized by the addition of BQ788 or by short interfering RNA (siRNA)-mediated knockdown of EdnrB in melanocytes (Figure 6Q and Figure S8). These results show that Wnt-activated epithelial cells upregulate expression of Edn ligands and can promote the proliferation of melanocytes in an EdnrB-dependent manner.
Finally, we asked whether Wnt signaling in EpSCs is required for their upregulation of Edn expression and associated McSC proliferation seen in early anagen. To address this question, we depleted β-catenin in K15+ EpSCs by utilizing K15-CrePR1; β-cateninfl/fl mice. Upon RU486 treatment, we observed the expected depletion of β-catenin specifically in EpSCs (Figure S9B). We then used a potent anagen induction stimulus (depilation) and carefully examined Edn expression in EpSCs. After depilation of induced mice, we observed the expected hair cycle arrest (Figure S9F). In these arrested follicles, the EpSCs lacked any detectable expression of Edns (Figure S9C). qPCR with cDNA prepared from epithelial sheets, including HFs, confirmed the lack of Edn upregulation in the cKO samples, in contrast to the controls (Figure S9D). Trp2+ McSCs failed to proliferate after depilation, while McSCs of control mice initiated proliferation normally (Figures S9E and S9F). Our results show that the induction of Edn in the sHG and the initiation of McSC proliferation ultimately requires EpSC Wnt/β-catenin signaling, either directly or indirectly through mechanisms associated with anagen onset.
DISCUSSION
The organized assembly of different cell populations is fundamental to adult organ regeneration. The remarkable phenomenon has been documented in the regeneration of complex organs, including limbs, heart or brain, in only a few animal species, such as the newt (Brockes and Kumar, 2005). In mammals, regeneration of simple stratified epithelia such as skin and cornea from their constituent stem cells was accomplished over a decade ago (Doran et al., 1980; Pellegrini et al., 1997). However, attempts to regenerate complex organs composed of multiple cell types, such as limbs, have been largely ineffective. For example, we previously demonstrated that adult mouse skin can regenerate HFs de novo after wounding (Ito et al., 2007). Yet these new HFs develop without the efficient recruitment of melanocytes, resulting in the production of unpigmented hair. The lack of clinical therapies to effectively regenerate a complete functional organ partly reflects our incomplete understanding of the cellular and molecular mechanisms that orchestrate the dynamic spatiotemporal interactions between constituent progenitor populations during adult tissue/organ regeneration. To approach this issue, we turned to the cycling HF, a unique example of spontaneous regeneration in mammals. In this miniature organ, EpSCs and McSCs coordinately proliferate and differentiate to produce the pigmented HF with each hair cycle. Using lineage-specific promoters in mutant mouse models, our study demonstrated that Wnt signaling is a key pathway for not only intrinsic but also extrinsic regulations of McSCs by EpSCs in their shared niche and plays critical roles in coupling their behavior during pigmented HF regeneration.
Wnt Signaling within McSCs Is Critical for McSC Differentiation and Hair Pigmentation but Ultimately Promotes McSC Exhaustion
Despite the well-known role for Wnt signaling in multiple stem cell populations, including EpSCs and intestinal stem cells (Haegebarth and Clevers, 2009), and its implication in tumorigenesis, including melanoma (O'Connell and Weeraratna, 2009), the involvement of this pathway in melanocytes in vivo was undertermined. We showed that the Wnt signaling in McSCs is critical for McSC differentiation and hair pigmentation. However, forced activation of Wnt signaling in McSCs ultimately resulted in the premature differentiation of McSCs and accelerated McSC exhaustion (Figure S4). These results may be consistent with the previously proposed theory that the untimely differentiation of McSCs eventually leads to their decreased numbers in the bulge (Inomata et al., 2009; Nishimura et al., 2005).
We also showed that Wnt suppression is necessary to maintain McSCs in an undifferentiated state. Our analysis of nuclear β-catenin localization shows that Wnt signaling is normally inactive in bulge McSCs in both mice and humans (Figure 1 and Figure S1). These data agree with past studies, showing that both follicular EpSCs and McSCs express high levels of secreted Wnt inhibitors that inhibit melanocyte differentiation in vitro (Morris et al., 2004; Nishikawa and Osawa, 2007; Tumbar et al., 2004; Yamaguchi et al., 2004). Together, these results suggest that Wnt inhibitors expressed in the stem cell niche may play an important role in the maintenance of undifferentiated McSCs. Clinically, the regulation of Wnt signaling in McSCs may offer strategies to preserve McSCs, which have been shown to be exhausted during aging as a result of inappropriate differentiation (Nishimura et al., 2005).
Synchronized Wnt Activation of McSCs and EpSCs in the Shared Niche Underlies their Coordinated Initiation of Differentiation at Anagen Onset
Our study showed a coordinated activation of Wnt signaling in both EpSCs and McSCs in the sHG at anagen onset. Intriguingly, McSCs do not upregulate Wnt ligands, which initiate the Wnt signaling pathway, at anagen onset. Nevertheless, our results suggest that McSCs as well as EpSCs in the sHG have the ability to respond to available Wnt ligands, as demonstrated by injection of a canonical Wnt ligand during telogen phase. Consistent with previous studies, we found that canonical Wnt ligand expression is upregulated by EpSCs at anagen onset (Greco et al., 2009; Reddy et al., 2001), providing a potential source of Wnt stimulation for EpSCs and McSCs. We propose that the coordinated activation of Wnt signaling in both EpSCs and McSCs within the sHG at anagen onset may, at least in part, be coupled by Wnt ligands secreted by EpSCs (Figure 7, stem cell coactivation model, step1). As activation of Wnt signaling drives both McSC and EpSC differentiation, our data illustrate a possible molecular mechanism that initiates the coordinated differentiation in each of these two distinct stem cells.
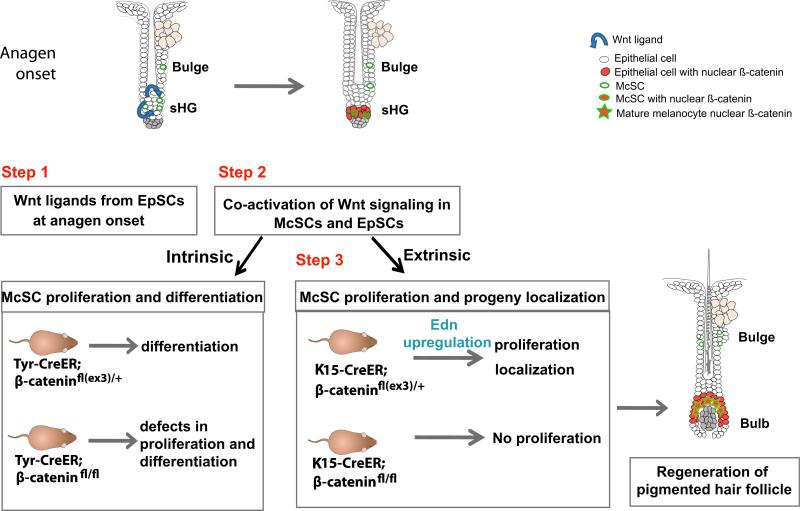
Figure 7. Proposed Model: Stem Cell Coactivation in a Shared HF Niche.
At anagen onset, EpSCs, adjacent to McSCs, secrete Wnt ligands for HF regeneration (step 1). Concomitantly, McSCs and EpSCs coactivate Wnt signaling (step 2). Wnt activation within melanocytes results in their differentiation, while Wnt activation in EpSCs results in secretion of Edns which are received by McSCs expressing EdnrB (step 3). Continued Wnt activation in EpSC progeny in the growing edge of the follicle attracts and guides McSC progeny to the Wnt-activated bulb, site of hair differentiation and pigment incorporation into growing hair.
Interestingly, unlike McSCs in the sHG (CD34– area), McSCs within the bulge (CD34+ area) did not activate Wnt signaling at anagen onset and even after injection of a canonical Wnt ligand. Our data suggest that the initial coordinated activation of the two stem cells involving Wnt signaling may occur in the sHG compartment. It is currently unknown whether there is any intrinsic heterogeneity among follicular McSCs (i.e., bulge versus sHG McSCs). One may speculate that the differential responsiveness of bulge and sHG McSCs to Wnt ligands is due to the differential expression of additional factors between bulge and sHG EpSCs that contribute to regulation of Wnt signaling. For example, Wnt inhibitors are shown to be upregulated in bulge EpSCs compared to sHG EpSCs (Greco et al., 2009). Inhibition of these various factors in location-specific cell populations will be required to fully evaluate these possibilities.
Wnt Signaling in EpSCs Stimulates EdnrB-Dependent Melanocyte Proliferation
We showed that forced β-catenin stabilization in EpSCs dramatically enhanced McSC proliferation. In addition, our results demonstrate that the lack of β-catenin in EpSCs not only arrested EpSC proliferation but also blocked McSC proliferation (Figure 5). From these data, we propose that signaling crosstalk occurs between these two activated stem cell types, subsequent to Wnt coactivation, to influence McSC proliferation (Figure 7, stem cell coactivation model, steps 2 and 3). One intriguing interpretation for these findings is that McSC proliferation is tightly coupled to that of Wnt-activated EpSCs to ensure that sufficient McSC progeny are generated to populate the hair bulb, where Wnt signaling is active in differentiating matrical epithelial cells and where pigment transfer occurs.
Our study definitively demonstrates that a specific modulation of gene expression in EpSCs altered the behavior of McSCs. Our data demonstrate the dominant influence of EpSCs on McSC proliferation. These results may agree with a recent study suggesting that EpSCs provide a niche for McSCs. In that study, EpSC depletion was induced by germline loss of Col17A1, which is expressed in the basal layerof the epidermis including the bulge, and resulted in the McSC exhaustion (Tanimura et al., 2011).
We found that EpSCs with forced β-catenin stabilization promoted proliferation of McSCs and identified Edn signaling as a mechanism behind this non-cell-autonomous role of epithelial β-catenin. A recent study showed that forced activation of Wnt signaling in embryonic skin is accompanied by epidermal hyperpigmentation and upregulation of SCF, Adamts20, and Foxn1, genes known to regulate pigmentation (Zhang et al., 2008). We did not detect increased expression of any of these genes after forced activation of Wnt signaling in EpSCs by qPCR or microarray (data not shown).
By disrupting the Edn/EdnrB signaling by administration of BQ788, in both in vivo and in vitro experimental models, we showed that melanocytes react to Wnt-activated EpSCs through EdnrB signaling (Figure 6). These results are consistent with the well-established role for Edns in proliferation of embryonic melanoblasts or human melanocytes in multiple experimental models (Imokawa, 2004). It is unknown whether Edn1 is a direct target of Wnt signaling in the adult HF system as shown in colon cancer cells (Kim et al., 2005). Elucidation of the molecular mechanisms regulating Edn expression in EpSCs will further delineate the molecular networks leading to McSC proliferation.
The collaboration between distinct stem cell types has been well illustrated in studies of the Drosophila ovary, in which germ line and escort stem cells are physically associated and mutually comprise a niche for each other (Kirilly and Xie, 2007). This allows them to divide synchronously so that the germline progeny can be encased by escort progeny during division and maturation, to coordinately form a functional cyst in the ovary. We have demonstrated that EpSCs not only serve as the source for the epithelial components of the follicle, but also regulate proliferation and differentiation of McSCs, which in turn provide pigment to the epithelial cells during HF formation. Our study suggests that mammalian tissue/organ regeneration may apply the same principle as in Drosophila ovary to ensure maximum efficiency in the formation of a complex organ. Such conserved mechanisms may underlie the extraordinarily high regenerative capacity of the adult HF.
Collectively, our study demonstrated that Wnt signaling is a key pathway for both intrinsic and extrinsic regulation of McSCs in their niche. We expect that not only Wnt signaling but other signaling pathways active in EpSCs may influence McSCs as well and serve as additional means of communication between these two cell types. Investigation of McSC behavior and function following modulation of additional signaling pathways in EpSCs will further characterize the nature of communication between these distinct stem cell populations.
EXPERIMENTAL PROCEDURES
Mice
All protocols involving mice (Supplemental Experimental Procedures) were approved by the Institutional Animal Care and Use Committee at NYU School of Medicine and are detailed in Supplemental Experimental Procedures.
Immunofluorescence
We performed immunohistochemistry on dorsal skin tissue sections. (Supplemental Experimental Procedures and Table S2).
Isolation of McSCs
For isolation of YFP+ McSCs, epidermal single-cell suspension including follicular keratinocytes were sorted on the Dako MoFlo cell sorter (details are in the Supplemental Experimental Procedures).
Quantitative RT-PCR
We obtained RNA from FACS-sorted cells or full thickness mouse dorsal skin with Trizol (Invitrogen, CA) and reverse transcribed with Superscript III First Strand Synthesis System (Invitrogen) for cDNA synthesis. Real-time amplification was carried out using the ABI 7900HT SDS system. The target gene transcripts were quantified relative to the housekeeping gene, GAPDH. Please see Table S1 for a list of primers used.
Quantification and Statistical Analysis
For quantification of immunohistochemical and histological analysis, more than 60 longitudinal HF sections from at least three different mice were analyzed. qPCRs were performed with duplicates, and two to three mice were used per genotype or time point. Results are representative of at least three independent experiments. A t test was used for statistical analyses.
Supplementary Material
ACKNOWLEDGMENTS
We thank funds from the Department of Dermatology and the Helen and Martin Kimmel Center for Stem Cell Biology at New York University (NYU), the Dermatology Foundation Career Development Award, and the New Scholar Award in aging from the Ellison Medical Foundation to M.I. We thank Tung-Tien Sun for critical reading of the manuscript. We thank Seth Orlow and Prashiela Manga for providing Melan-a cells and helpful advice. We thank our mentors George Cotsarelis and Sarah Millar for their helpful advice and constant encouragement. We thank Radhika Atit for her input and collaboration for this work. We thank Yan Deng and the Microscopy Core of NYU for use of confocal microscopes (grants NCRR S10 RR023704-01A1 and NCRR S10 RR024708).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Extended Experimental Procedures, nine figures, and two tables and can be found with this article online at doi:10.1016/j.cell.2011.05.004.
REFERENCES
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Dev. Cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Barker N. The canonical Wnt/beta-catenin signalling pathway. Methods Mol. Biol. 2008;468:5–15. doi: 10.1007/978-1-59745-249-6_1. [DOI] [PubMed] [Google Scholar]
- Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosenberg M, Muthusamy V, Curley DP, Wang Z, Hobbs C, Nelson B, Nogueira C, Horner JW, 2nd, Depinho R, Chin L. Characterization of melanocyte-specific inducible Cre recombinase transgenic mice. Genesis. 2006;44:262–267. doi: 10.1002/dvg.20205. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Kumar A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310:1919–1923. doi: 10.1126/science.1115200. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329–1337. doi: 10.1016/0092-8674(90)90696-c. [DOI] [PubMed] [Google Scholar]
- Delmas V, Beermann F, Martinozzi S, Carreira S, Ackermann J, Kumasaka M, Denat L, Goodall J, Luciani F, Viros A, et al. Beta-catenin induces immortalization of melanocytes by suppressing p16INK4a expression and cooperates with N-Ras in melanoma development. Genes Dev. 2007;21:2923–2935. doi: 10.1101/gad.450107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran TI, Vidrich A, Sun TT. Intrinsic and extrinsic regulation of the differentiation of skin, corneal and esophageal epithelial cells. Cell. 1980;22:17–25. doi: 10.1016/0092-8674(80)90150-6. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Moon RT, Raible DW. Control of neural crest cell fate by the Wnt signalling pathway. Nature. 1998;396:370–373. doi: 10.1038/24620. [DOI] [PubMed] [Google Scholar]
- Dunn KJ, Williams BO, Li Y, Pavan WJ. Neural crest-directed gene transfer demonstrates Wnt1 role in melanocyte expansion and differentiation during mouse development. Proc. Natl. Acad. Sci. USA. 2000;97:10050–10055. doi: 10.1073/pnas.97.18.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Jiang M, Mirando AJ, Yu HM, Hsu W. Reciprocal regulation of Wnt and Gpr177/mouse Wntless is required for embryonic axis formation. Proc. Natl. Acad. Sci. USA. 2009;106:18598–18603. doi: 10.1073/pnas.0904894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Greco V, Chen T, Rendl M, Schober M, Pasolli HA, Stokes N, Dela Cruz-Racelis J, Fuchs E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell. 2009;4:155–169. doi: 10.1016/j.stem.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegebarth A, Clevers H. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am. J. Pathol. 2009;174:715–721. doi: 10.2353/ajpath.2009.080758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, Taketo MM. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari L, Brault V, Kléber M, Lee HY, Ille F, Leimeroth R, Paratore C, Suter U, Kemler R, Sommer L. Lineage-specific requirements of beta-catenin in neural crest development. J. Cell Biol. 2002;159:867–880. doi: 10.1083/jcb.200209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Ikeya M, Lee SM, Johnson JE, McMahon AP, Takada S. Wnt signalling required for expansion of neural crest and CNS progenitors. Nature. 1997;389:966–970. doi: 10.1038/40146. [DOI] [PubMed] [Google Scholar]
- Imokawa G. Autocrine and paracrine regulation of melanocytes in human skin and in pigmentary disorders. Pigment Cell Res. 2004;17:96–110. doi: 10.1111/j.1600-0749.2003.00126.x. [DOI] [PubMed] [Google Scholar]
- Inomata K, Aoto T, Binh NT, Okamoto N, Tanimura S, Wakayama T, Iseki S, Hara E, Masunaga T, Shimizu H, Nishimura EK. Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell. 2009;137:1088–1099. doi: 10.1016/j.cell.2009.03.037. [DOI] [PubMed] [Google Scholar]
- Ito M, Kizawa K, Hamada K, Cotsarelis G. Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation. 2004;72:548–557. doi: 10.1111/j.1432-0436.2004.07209008.x. [DOI] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Kim TH, Xiong H, Zhang Z, Ren B. β-Catenin activates the growth factor endothelin-1 in colon cancer cells. Oncogene. 2005;24:597–604. doi: 10.1038/sj.onc.1208237. [DOI] [PubMed] [Google Scholar]
- Kirilly D, Xie T. The Drosophila ovary: an active stem cell community. Cell Res. 2007;17:15–25. doi: 10.1038/sj.cr.7310123. [DOI] [PubMed] [Google Scholar]
- Lang D, Lu MM, Huang L, Engleka KA, Zhang M, Chu EY, Lipner S, Skoultchi A, Millar SE, Epstein JA. Pax3 functions at a nodal point in melanocyte stem cell differentiation. Nature. 2005;433:884–887. doi: 10.1038/nature03292. [DOI] [PubMed] [Google Scholar]
- Larue L, Delmas V. The WNT/β-catenin pathway in melanoma. Front. Biosci. 2006;11:733–742. doi: 10.2741/1831. [DOI] [PubMed] [Google Scholar]
- Liu Y, Lyle S, Yang Z, Cotsarelis G. Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J. Invest. Dermatol. 2003;121:963–968. doi: 10.1046/j.1523-1747.2003.12600.x. [DOI] [PubMed] [Google Scholar]
- Lowry WE, Blanpain C, Nowak JA, Guasch G, Lewis L, Fuchs E. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama M, Osawa M, Mak SS, Ohtsuka T, Yamamoto N, Han H, Delmas V, Kageyama R, Beermann F, Larue L, Nishikawa S. Notch signaling via Hes1 transcription factor maintains survival of melanoblasts and melanocyte stem cells. J. Cell Biol. 2006;173:333–339. doi: 10.1083/jcb.200509084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Müller-Röver S, Handjiski B, van der Veen C, Eichmüller S, Foitzik K, McKay IA, Stenn KS, Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Invest. Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Osawa M. Generating quiescent stem cells. Pigment Cell Res. 2007;20:263–270. doi: 10.1111/j.1600-0749.2007.00388.x. [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Jordan SA, Oshima H, Yoshida H, Osawa M, Moriyama M, Jackson IJ, Barrandon Y, Miyachi Y, Nishikawa S. Dominant role of the niche in melanocyte stem-cell fate determination. Nature. 2002;416:854–860. doi: 10.1038/416854a. [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Granter SR, Fisher DE. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science. 2005;307:720–724. doi: 10.1126/science.1099593. [DOI] [PubMed] [Google Scholar]
- Nishimura EK, Suzuki M, Igras V, Du J, Lonning S, Miyachi Y, Roes J, Beermann F, Fisher DE. Key roles for transforming growth factor beta in melanocyte stem cell maintenance. Cell Stem Cell. 2010;6:130–140. doi: 10.1016/j.stem.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–527. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- O'Connell MP, Weeraratna AT. Hear the Wnt Ror: how melanoma cells adjust to changes in Wnt. Pigment Cell Melanoma Res. 2009;22:724–739. doi: 10.1111/j.1755-148X.2009.00627.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini G, Traverso CE, Franzi AT, Zingirian M, Cancedda R, De Luca M. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- Rawles ME. Origin of pigment cells from the neural crest in the mouse embryo. Physiol. Zool. 1947;20:248–266. doi: 10.1086/physzool.20.3.30151958. [DOI] [PubMed] [Google Scholar]
- Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, Millar SE. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech. Dev. 2001;107:69–82. doi: 10.1016/s0925-4773(01)00452-x. [DOI] [PubMed] [Google Scholar]
- Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543–557. doi: 10.1101/gad.1614408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- Saldana-Caboverde A, Kos L. Roles of endothelin signaling in melanocyte development and melanoma. Pigment Cell Melanoma Res. 2010;23:160–170. doi: 10.1111/j.1755-148X.2010.00678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Schouwey K, Delmas V, Larue L, Zimber-Strobl U, Strobl LJ, Radtke F, Beermann F. Notch1 and Notch2 receptors influence progressive hair graying in a dose-dependent manner. Dev. Dyn. 2007;236:282–289. doi: 10.1002/dvdy.21000. [DOI] [PubMed] [Google Scholar]
- Sharov A, Tobin DJ, Sharova TY, Atoyan R, Botchkarev VA. Changes in different melanocyte populations during hair follicle involution (catagen). J. Invest. Dermatol. 2005;125:1259–1267. doi: 10.1111/j.0022-202X.2005.23959.x. [DOI] [PubMed] [Google Scholar]
- Silva-Vargas V, Lo Celso C, Giangreco A, Ofstad T, Prowse DM, Braun KM, Watt FM. Beta-catenin and Hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Dev. Cell. 2005;9:121–131. doi: 10.1016/j.devcel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Takeda K, Yasumoto K, Takada R, Takada S, Watanabe K, Udono T, Saito H, Takahashi K, Shibahara S. Induction of melanocytespecific microphthalmia-associated transcription factor by Wnt-3a. J. Biol. Chem. 2000;275:14013–14016. doi: 10.1074/jbc.c000113200. [DOI] [PubMed] [Google Scholar]
- Tanimura S, Tadokoro Y, Inomata K, Binh NT, Nishie W, Yamazaki S, Nakauchi H, Tanaka Y, McMillan JR, Sawamura D, et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell. 2011;8:177–187. doi: 10.1016/j.stem.2010.11.029. [DOI] [PubMed] [Google Scholar]
- Trempus CS, Morris RJ, Bortner CD, Cotsarelis G, Faircloth RS, Reece JM, Tennant RW. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J. Invest. Dermatol. 2003;120:501–511. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Mater D, Kolligs FT, Dlugosz AA, Fearon ER. Transient activation of beta-catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev. 2003;17:1219–1224. doi: 10.1101/gad.1076103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc. Natl. Acad. Sci. USA. 1999;96:8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y, Itami S, Watabe H, Yasumoto K, Abdel-Malek ZA, Kubo T, Rouzaud F, Tanemura A, Yoshikawa K, Hearing VJ. Mesenchymal-epithelial interactions in the skin: increased expression of dickkopf1 by palmoplantar fibroblasts inhibits melanocyte growth and differentiation. J. Cell Biol. 2004;165:275–285. doi: 10.1083/jcb.200311122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Andl T, Yang SH, Teta M, Liu F, Seykora JT, Tobias JW, Piccolo S, Schmidt-Ullrich R, Nagy A, et al. Activation of β-catenin signaling programs embryonic epidermis to hair follicle fate. Development. 2008;135:2161–2172. doi: 10.1242/dev.017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YV, Cheong J, Ciapurin N, McDermitt DJ, Tumbar T. Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell. 2009;5:267–278. doi: 10.1016/j.stem.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.