Abstract
Although most mRNA molecules derived from protein-coding genes are destined to be translated into functional polypeptides, some are eliminated by cellular quality control pathways that collectively perform the task of mRNA surveillance. In the nonsense-mediated mRNA decay (NMD) pathway premature translation termination promotes the recruitment of a set of factors that destabilize a targeted mRNA. The same factors also appear to play key roles in repressing the translation of the mRNA, dissociating its terminating ribosome and mRNP proteins, promoting the degradation of its truncated polypeptide product, and possibly even feeding back to the site of transcription to interfere with splicing of the primary transcript.
Gene expression must maintain a high level of fidelity to ensure cell function and viability. To tackle this challenge, cells use multiple decay pathways to eliminate nonfunctional transcripts1,2. At the level of mRNA, three pathways operate during translation to protect the cell from the possible accumulation of aberrant mRNAs and potentially toxic proteins. These include: Non-Stop Decay (NSD), which detects and degrades mRNAs lacking a stop codon3-5, No-Go Decay (NGD), which targets mRNAs having ribosomes stalled in translation elongation6,7, and Nonsense-mediated mRNA Decay (NMD), which promotes the degradation of mRNAs undergoing premature translation termination8-11. While these pathways were originally thought to simply promote accelerated mRNA decay, recent studies suggest more complex post-transcriptional regulation12. In NMD, the Upf proteins, a set of conserved factors of which Upf1 is the central regulator, recruit decay enzymes to promote endonucleolytic cleavage, 5’ to 3’ decay, or 3’ to 5’ decay of prematurely terminating mRNAs. However, they must also discriminate between normal and premature termination events and conduct ancillary processes involving translational repression of mRNAs containing premature termination codons (PTCs), dissociate and properly recycle poorly dissociable PTC-bound ribosomes, and unwind the PTC-containing mRNP, all in the interest of facilitating rapid decay of the mRNA and reutilization of the components of the translational machinery. Upf1 also promotes proteolysis of the nascent polypeptide, presumably to ensure a minimal opportunity for that protein fragment to interfere in cellular processes. In this Review, we provide an overview of the NMD pathway, seeking unified explanations for its broad range of activities in all eukaryotes.
mRNA substrates for NMD
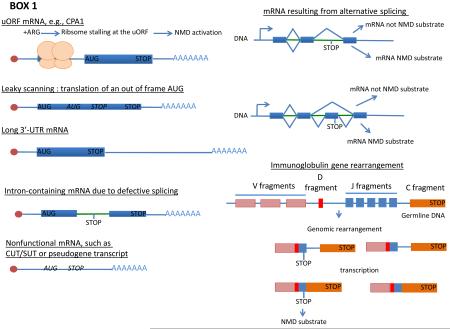
NMD targets transcripts in which translation is arrested by a premature termination codon (BOX 1). As such, the substrates of this pathway include transcripts derived from genes harboring nonsense mutations, inefficiently spliced pre-mRNAs that enter the cytoplasm with their introns intact, mRNAs in which the ribosome has bypassed the initiator AUG and commenced translation further downstream, some mRNAs that contain upstream open reading frames (uORFs), mRNAs that are subject to frameshifting or that are generated by certain alternative splicing events, bicistronic mRNAs, and transcripts of pseudogenes, transposable elements, and genes that are subject to programmed rearrangements13-16. NMD substrates also include mRNAs in which the translation termination codon occurs in an mRNA context characteristic of premature termination, e.g., at the 5’ end of an atypically long 3’-untranslated region (3’-UTR)17. The latter class includes mRNAs with normal or biologically regulated extensions of their 3'-UTRs.
BOX 1. Substrates of the NMD pathway.
In addition to mRNAs transcribed from genes harboring nonsense mutations, NMD also targets mRNAs in which translation termination occurs in a context that differs from conventional mRNAs. For example, some mRNAs containing one or more upstream ORFs (uORF), such as the yeast CPA1 mRNA31, are downregulated by NMD. With CPA1 mRNA, the nascent Arginine Attenuator Peptide (AAP) encoded by the uORF stalls the ribosome at the termination codon in the presence of arginine31. mRNAs subject to leaky scanning, which can result in translation initiation at an out of frame AUG (and thus lead to premature termination), are also targeted by NMD. Termination that triggers NMD can also result from an unconventional 3’-UTR that extends significantly longer than those present on most mRNAs, either normally127 or as a result of an mRNA processing error17. Intron-containing pre-mRNAs exported to the cytoplasm due to inefficient splicing, such as the CYH2 pre-mRNA in yeast are also NMD substrates, as well as non-functional mRNAs transcribed from pseudogenes, transposons, or intergenic regions, as long as these transcripts are exported to the cytoplasm169-171. Finally, in mammalian cells where alternative splicing is widespread, mRNA isoforms containing PTCs are an important physiological class of NMD substrates, as are mRNAs transcribed from unproductively rearranged immunoglobulin mRNAs and T-cell receptor mRNAs.
Gene expression profiling of yeast, fly, and human cells show that from 1 to 10% of cellular transcripts are up-regulated by NMD inactivation13,18-24. These experiments have demonstrated that NMD not only controls the expression of aberrant transcripts, but also that of many apparently wild-type mRNAs. Accordingly, NMD appears to have regulatory functions beyond mRNA surveillance25,26.
The role of translation termination
Destabilization of nonsense-containing mRNAs depends on recognition of the nonsense codon by the translational machinery27-31 and the ability of that machinery to recognize a stop codon as ‘premature.’ Thus, a key question is how do normal and premature translation termination differ.
Normal translation termination
The termination of protein synthesis at UAA, UAG, or UGA stop codons occurs in multiple steps and requires two classes of release factors (RFs). Class I RFs act like tRNAs in that they first bind to the ribosomal A site in response to the presence of nonsense codons. However, instead of promoting peptidyl transfer they trigger hydrolysis of the polypeptide chain attached to the P site tRNA. Class II RFs are GTPases that stimulate class I RFs32. Translation termination in eukaryotes is mediated by the class I factor eRF1 and the class II factor eRF3 (Sup45 and Sup35 in yeast)33-35. Following stop codon recognition by eRF1, its conserved GGQ motif activates the peptidyltransferase center of the ribosome to mediate peptide release36,37. eRF3 GTPase activity is dependent on ribosome-bound eRF1 and couples eRF1 recognition of a nonsense codon to efficient polypeptide release38. Dissociation and recycling of the termination complex is facilitated by the ABCE1 ATPase and initiation factors eIF3, eIF1, eIF1A, and eIF3j 39-42. ABCE1 interacts with eRF1 and this interaction is conserved by the ABCE1 homolog, Rli1, in yeast. Rli1 also interacts with subunit j of the initiation factor eIF3 (Hcr1p in yeast)43.
Other factors have been assigned possible roles in translation termination because alterations in their activity (or presence) affect nonsense codon readthrough. Notably, normal termination is thought to be stimulated by the interaction of the poly(A)-binding protein (Pab1 in yeast) with eRF344,45. Pab1 overexpression enhances termination efficiency in yeast45 and, reciprocally, deleting PABPC1 increases nonsense codon readthrough in human cells46. Downstream of a normal termination codon (NTC) eRF3-Pab1 interaction may orchestrate formation of an mRNP complex favorable to termination44-48. Additional proteins implicated in termination include the mRNA export factors encoded by NPL3, DBP5, and GLE149-51. While mutations in these genes lead to nonsense suppression and sensitivity to drugs affecting translation, it’s possible that these are indirect consequences of their effects on mRNP structure.
The aberrant nature of premature termination
Premature termination does not appear to be the mechanistic equivalent of normal termination. Toeprint analyses assaying the precise position of single ribosomes on mRNA failed to yield any toeprinting signals from normal yeast termination codons unless eRF1 was inactivated by a temperature-sensitive lesion. In contrast, ribosomes at PTCs yielded toeprint signals consistent with A-site occupancy even without eRF1 inactivation52. Similarly, a codon 39 PTC that targets human β-globin mRNA for NMD was found to yield a toeprint whereas the normal termination codon of β-globin mRNA did not53. These data indicate that, in both yeast and human cells translation termination at a PTC is less efficient than normal termination and leads to a pause of the ribosome at the nonsense codon. Premature termination should thus be considered aberrant. Independent evidence for mechanistic differences between premature and normal termination follows from the identification of PTC124 (ataluren), a novel compound that promotes the translational readthrough of premature but not normal termination codons in human cells54.
An unresolved question is which step in premature translation termination is affected. There is no evidence that peptide hydrolysis is slower at PTCs than at NTCs, thus implicating reductions in the rate of later steps in the termination process. Most evidence points to reductions in the efficiency of ribosome and mRNP dissociation subsequent to peptide hydrolysis. For example, the toeprinting experiments alluded to in the previous paragraph imply that ribosomes are dissociated much more slowly from PTCs than NTCs52,53 and translational reinitiation after premature termination is markedly reduced in vivo or in vitro when any one of the three yeast Upf proteins is absent52,55. Further, following translation termination at a PTC, yeast upf1Δ extracts manifest a ribosome recycling defect that can be complemented by purified Upf155. In human cells, the expression of ATPase- or helicase-deficient Upf1 leads to the accumulation of β-globin mRNA decay intermediates that are endonucleolytically cleaved by Smg-6, blocked in subsequent 5’ to 3’ exonucleolytic degradation of the 3’-cleavage product, and associated with Upf1 and the other Upfs in an mRNP that may also include the terminating ribosome56. Collectively, these results indicate that, in addition to promoting accelerated mRNA decay, the Upfs also play a role in disassembling the poorly dissociable post-premature termination mRNP48.
Factors required for NMD
NMD is primarily carried out by the Upf proteins, but other factors are also necessary to recruit and regulate the Upfs and to activate NMD
Upf proteins
The proteins encoded by UPF1 (also known as SMG2), UPF2 (also known as SMG3 and NMD2), and UPF3 (also known as SMG4) are the principal NMD regulators in eukaryotes57-62. Mutations in the UPF genes, or silencing of their expression, stabilize nonsense-containing mRNAs, and increase their relative abundance, but generally have little or no effect on most wild-type transcripts63. The three Upf proteins interact (FIG. 1a) with Upf2 acting as a bridge between Upf1 and Upf363-65.
Figure 1. Structures and consequences of interactions between factors involved in NMD.
a Interactions between the Upf proteins. Yeast amino acid numbering has been used to define the interaction domains and the size of the respective proteins. RNP= putative ribonucleoprotein domain, MIF4G= middle portion of eIF4G, CH= cysteine- and histidine-rich zinc-finger domain, 1B, 1C= additional regulatory domains. b Upf2 interaction switches Upf1 from closed (left) to open (right) conformation. The respective structures are of yeast Upf1 and human Upf1:Upf2, with coloring as indicated in the linear Upf1 model depicted in a. Yeast Upf1 (left) is shown bound to RNA-ADP:AIF4- (black). The Upf1:Upf2 heterodimer did not associate with RNA under the experimental conditions used for structure determination. Modified with permission from78,79.
Upf1 is a large cytoplasmic protein (109 kDa in yeast; 130 kDa in humans) with two principal domains. A cysteine- and histidine-rich zinc-finger domain (CH domain) located near the Upf1 N-terminus is followed by a flexible linker segment and the conserved motifs common to superfamily I (SF1) helicases9,66,67. The Upf1 helicase core includes two RecA-like domains common to all SFI helicases, and two regulatory domains (1B and 1C) specific to Upf168. Upf1 is able to bind RNA in the presence or absence of ATP64,69,70. Both the CH domain, and the RNA-dependent ATPase and RNA helicase activities are essential for NMD69-72. The CH domain also interacts with Upf2, an acidic 127 kD protein with three conserved MIF4G (middle portion of eIF4G) domains59,62,73 (FIG. 1a). It utilizes the third of these domains to interact with Upf3, a basic 45 kD protein with an RNP-type RNA-binding domain (RBD)63,74. The nonspecific RNA-binding activity of the resulting Upf2:Upf3 complex is thought to be a property of a conserved basic region of Upf2.
UPF1 is the key effector of NMD, with Upf2 and Upf3 regulating Upf1 function. This conclusion follows from biochemical analyses showing that maximal activation of Upf1’s ATPase activity in vitro requires both Upf2 and Upf364 and that overexpression of UPF1 can compensate for upf2Δ and upf3Δ mutations, but not vice versa75. This functional hierarchy is consistent with mechanistic analyses. Upf1’s CH domain can interact with a C-terminal domain of Upf2 and mutations affecting this interaction inhibit NMD63,73,76,77. In the absence of Upf2, Upf1 exists in a closed conformation in which the CH domain interacts with the RecA-like domains78 (FIG. 1b). In this conformation domain 1B binds to RNA, thus increasing the overall extent of RNA binding by Upf1 and decreasing its ATPase and helicase activities64,78,79. Upon binding to Upf2, the Upf1 closed state undergoes substantial conformational change, with the CH domain switching from a position near the RNA binding site to a more distal position physically disconnected from both the RNA and nucleotide binding sites (FIG. 1b). Mediated by high affinity Upf2:CH domain interaction, this “open” form binds RNA less extensively and has increased levels of ATPase and helicase activities, i.e., it switches from a state in which it is tightly bound to RNA to one in which it has the capability for RNA unwinding64,78. The effects of Upf2 interaction on helicase and ATPase activation can be mimicked by deletion of the Upf1 CH domain78.
Smg factors
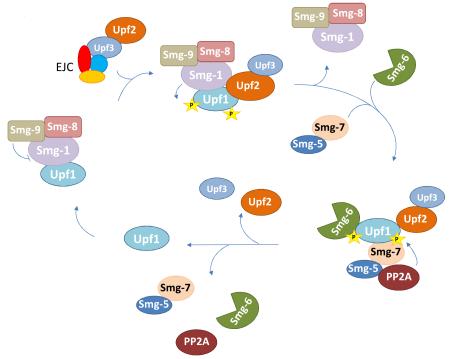
In worms and other multicellular organisms, NMD is also regulated by Smg factors, proteins that are involved in a cycle of Upf1 phosphorylation and dephosphorylation (BOX 2). This cycle is required for NMD activation and regulates protein-protein interactions that recruit specific mRNA decay enzymes (see below). There’s no evidence that Upf1 ATPase and helicase activities depend on the protein’s phosphorylation status, but dephosphorylation has been shown to allow recycling of Upf1, including its exit from P-bodies80 (BOX 2). The Upf1 phosphorylation/dephosphorylation cycle does not appear to be restricted to multicellular organisms. In yeast, Upf1 and Upf2 are also phosphorylated81. Ebs1 has been identified as a weak homologue to Smg-7 and has been implicated in the Upf1 phosphorylation/dephosphorylation cycle in yeast. However, deletion of EBS1 has only a moderate effect on NMD82.
BOX 2. Phosphorylation cycle of UPF1.
Factors Smg-1, Smg-5, Smg-6, Smg-7, Smg-8, and Smg-9, are regulators of the phosphorylation status of Upf196,172,173. Phosphorylation of Upf1 on multiple serine residues present in N- and C-terminal domains of the protein is catalyzed by Smg-1, a protein kinase related to the phosphatidylinositol 3 kinase-related kinase (PIKK) superfamily96,173-176. Smg-1 activity is regulated by Smg-8 and Smg-9, which form a complex with Smg-1 and prevent inappropriate phosphorylation of Upf196,177. In addition to Smg-1, phosphorylation of Upf1 also requires Upf2 and Upf3, confirming that the formation of a trimeric Upf1:Upf2:Upf3 complex triggers NMD175. Phosphorylated Upf1 can interact with the Smg-5:Smg-7 complex, and Smg-6, in turn promoting Upf1 dephosphorylation by recruiting the protein phosphatase PP2A96,150,172,175,178-180. The recruitment of Smg-5:Smg-7 and Smg-6 to phosphorylated Upf1 is thought to be direct, through a 14-3-3-like fold present in the three factors110,150. Additional interactions may also be pertinent as Smg-7 is detected in the Smg-1–Upf1–Upf2–EJC complex and Smg-6 interacts with the EJC complex77,91. Dephosphorylation has been shown to allow recycling of Upf1, including its exit from P-bodies80.
The EJC
In mammalian cells, NMD is generally splicing dependent and usually requires that a PTC be localized at least 50-55 nt upstream of an exon-exon junction8. This phenomenon relies on the exon-junction complex (EJC), a group of proteins deposited on an mRNA during splicing, 20-24 nt 5’ of an exon-exon boundary83,84 (FIG. 2). The composition of the EJC is dynamic, and includes at least the core proteins Y14, Magoh, Barentz, and eIF4AIII, and one effector of NMD, Upf3. The EJC travels with an mRNA into the cytoplasm, where it acts as an effector for events in mRNA metabolism, including the activation of translation, mRNA localization, and NMD85-87. Upf3 binds to the EJC during splicing and Upf2 associates with EJC-bound Upf3 in the cytoplasm85,86,88-90. The EJC also plays a role in the recruitment of Smg-6, a factor implicated in Upf1’s phosphorylation cycle as well in the endonucleolytic cleavage of PTC-containing mRNAs. Smg-6 and Upf3 share the same binding site and compete for EJC interaction, suggesting that the EJC is rearranged during NMD activation or that the NMD pathway can, in some instances, function independently of Upf391. Indeed, NMD activation independent of Upf3, Upf2, or some EJC components has been reported, suggesting that NMD in mammals is not restricted to a single pathway92,93.
Figure 2. Activation of metazoan NMD by EJC-dependent interactions.
The exon-junction complex (EJC) is a group of proteins deposited on an mRNA during splicing, 20-24 nt 5’ of an exon-exon boundary83,84,86,108. The composition of the EJC is dynamic, and includes at least the core proteins Y14, Magoh, Barentz, and eIF4AIII, and one effector of NMD, Upf385,86,88,181-183. In mammalian cells, Upf3 is loaded onto mRNAs during splicing and binds to a composite site comprised of parts of Y14, Magoh, and eIF4AIII89. Upf2 is thought to join the complex in the cytoplasm via Upf3 binding, after mRNA export from the nucleus (top image). In parallel, Upf1 associates with eRF1-bound eRF3, and Smg-1, Smg-8, and Smg-9 associate with Upf1, collectively forming the SURF complex96,177. If premature translational termination leads to retention of the downstream EJC then Upf1:Upf2 interaction is facilitated, leading to the formation of the DECID complex and to Upf1 phosphorylation and ATPase activation (middle image). Activation of NMD independently of Upf2, Upf3, or some EJC components has been described, suggesting that alternative pathways may also exist92,93. Upf1 phosphorylation inhibits translation in cis and promotes its interaction with Smg-6, an endonuclease that can cleave the mRNA, and with the Smg-5:Smg-7 complex, which appears to promote deadenylation and decapping (bottom image)56,147,148,150,151,172,184. Mago=Magoh; BTZ=Barentz; R1=eRF1; R3=eRF3. Modified with permission from91
Release factors
The dependence of NMD on translation termination is reinforced by experiments demonstrating that the Upf proteins bind the release factors. In humans and yeast, Upf1 interacts with eRF346,77,94,95, an interaction thought to recruit Upf1 to the termination complex. Support for this notion comes from identification of a complex between Upf1 and the release factors in human cells, i.e, the SURF (Smg-1-Upf1-Release Factors) complex (FIG. 2)77,96. In yeast, Upf2 and Upf3 also interact with eRF3, and Upf1 can also interact with eRF1. eRF3 seems to have a unique Upf1-binding site located in its GTPase domain46,97, but Upf2, Upf3, and eRF1 all compete for binding to eRF3 and might share a common interaction domain97. Upf1 is thought to interact with eRF3 through its CH-domain46, but this result is controversial95,98. Regardless of the definition of the respective interaction sites, the binding of eRF1 and eRF3 to Upf1 inhibits Upf1’s ATPase activity94, an observation consistent with the notion that Upf1 is initially recruited to the premature termination complex in an inactive form that must then be activated by the Upf2:Upf3 complex64.
In yeast, mutations in the UPF genes promote nonsense suppression66,70,75,97,99,100. The initial interpretation of this observation, based in part on the interactions of the UPF proteins with the release factors, was that the Upfs played a direct role in maintaining the fidelity of translation termination. However, RNAi depletion of human Upf1 was shown to decrease nonsense codon readthrough, a surprising result considering the conservation of the termination and NMD machinery46. These discrepancies were resolved recently by the identification of mutations that reverse the readthrough phenotype in a yeast upf1Δ strain101. This study showed that the mRNA encoding yeast’s principal Mg2+ transporter, Alr1, contained upstream ORFs and was an endogenous NMD substrate. When NMD was inactivated ALR1 mRNA was stabilized, in turn raising cellular levels of Alr1. As a consequence, intracellular levels of Mg2+ also increased, thus reducing the fidelity of translation termination101. Thus, although Upf1 interacts with the release factors, the ALR1 results suggest that, at least in yeast, its effects on termination fidelity are largely indirect.
NMD activation and regulation
As noted above, considerable information is available on the structures, activities, and interactions of the factors that regulate NMD. Below we discuss how these factors might operate during premature translation termination to activate NMD. We attempt to reconcile the different models of NMD activation while keeping in mind two key challenges to the initiation of this process: the specific targeting of the Upfs to an mRNA engaged in terminating translation prematurely and the subsequent activation of the ATPase and helicase activities of Upf1 by Upf2 and Upf3.
The mRNA marking models
Studies in yeast first suggested a mechanism for discriminating normal from premature termination by virtue of a protein mark that would be present only on prematurely terminated mRNAs102-104. Evidence that the Upf proteins interacted with eRF1 and eRF394,103,105 led to the notion of a termination ‘surveillance complex’ that could detect the presence of specific proteins 3’ to the termination codon102-104. Yeast factors that interacted with the surveillance complex to activate NMD were thought to be mRNA-binding proteins such as Hrp1 that exited with the transcript from the nucleus, and were not removed by a translating ribosome because termination occurred prematurely103,104
A related model has also been proposed for mammalian cells, where NMD often depends on the presence of an intron downstream of the PTC, a link that reflects a role for the exon junction complex (EJC)106-108. This model109 posits that Upf2 and Upf3 bind to the EJC core whereas Upf1 and its regulator Smg-1 are recruited to the termination complex by the release factors, with the latter forming the SURF complex with Smg-1 regulators Smg-8 and Smg-977,96 (BOX 2 and FIG. 2). If a ribosome stops at a PTC before it displaces the downstream EJC, then SURF-associated Upf1 is thought to be able to interact with Upf2:Upf3 bound to the EJC. In turn, this can activate Upf1 phosphorylation by Smg-1, forming the DECID (DECay InDucing) complex, and activate the Upf1 ATPase and helicase activities, leading to mRNA degradation through interaction of Upf1 with Smg 5-7 and Smg-6110. However, this proposed mechanism fails to be a suitable explanation for PTC-containing mRNAs derived from intron-less precursors or those mRNAs that are subject to NMD without prior splicing, proper EJC spacing, or EJC components111-115.
The faux-UTR model
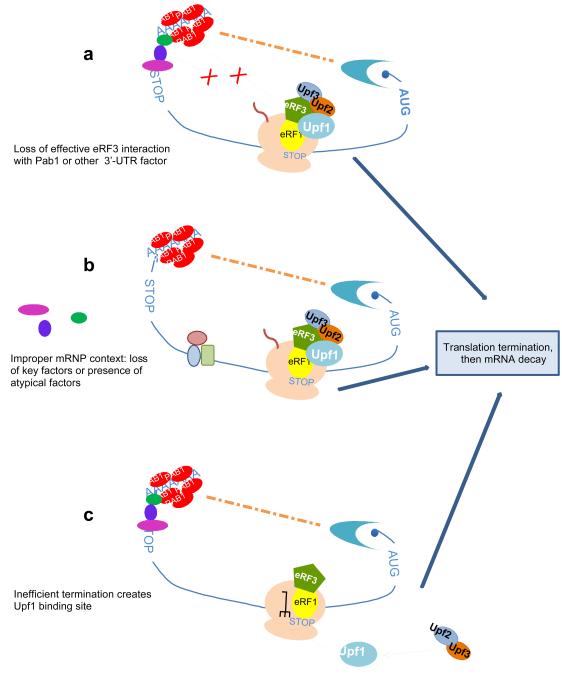
In yeast and C. elegans, mRNP immunoprecipitation experiments show that Upf1 preferentially associates with mRNAs that are NMD substrates18,116. However, the mechanism of its selective recruitment or selective retention on ribosomes engaged in premature termination is unknown. The same concern applies to mammalian SURF assembly. Thus, an alternate explanation for the initiation of NMD takes into account the nature of the mRNP 3’ to a nonsense codon. Studies in yeast have shown that deleting the coding region downstream of a PTC, thus bringing the normal 3’-UTR close to the premature termination codon, will stabilize an otherwise unstable mRNA, as well as eliminate the toeprint associated with premature termination52,117,118. Likewise, in human cells, an mRNA containing synthetic sequences allowing the 3’-UTR to fold back and be positioned near a PTC also becomes resistant to NMD119. Reciprocally, mRNAs with substantially extended 3’-UTRs are NMD subtrates in yeast, human, Drosophila, and plant cells95,120-129. Collectively, these observations indicate that normal rates of mRNA decay, and most likely normal termination, depend on the mRNP structure downstream of a termination codon. As postulated by the faux-UTR model, the 3’-UTR created by a PTC must lack termination regulatory factors present on a normal 3’-UTR, leading to aberrant translation termination and subsequent NMD activation130. In this model, proper translation termination and normal mRNA decay are postulated to depend in part on interactions between Pab1 and eRF3 (FIG. 3). Aspects of this model have been validated46,52,95,119, but others have not. Notably, the cellular absence of Pab1 or the deletion of the respective interacting domains on Pab1 do not convert wild-type mRNAs to NMD substrates131,132. These results do not negate the general notions of the faux-UTR model, but they do indicate that the termination role of the 3’-mRNP is more complex than the sum of the functions of just Pab1 and eRF3.
Figure 3. Alternative models for NMD activation by premature termination.
Not all mRNAs require an EJC for NMD activation, particularly in lower eukaryotes. The Upf proteins may associate with a prematurely terminating ribosome because essential interactions between Pab1 (or other 3’-UTR-associated proteins) and eRF3 have been disrupted (a), the mRNP context is non-accommodating for termination, i.e., critical proteins have not been added to or removed from the mRNP (a variation of the EJC model) (b), or the inefficiency of premature termination has left the ribosome in an atypical conformation (c). In all three situations the altered state is thought to allow Upf1 association with the eRFs and the ribosome, followed shortly by binding of the Upf2:Upf3 complex to Upf1 and the activation of mRNA decay.
In a variation of the faux-UTR model Hogg and Goff have reported the differential binding of human Upf1 as a direct function of mRNA 3’-UTR length133. They suggest that the apparent correlation of binding preference with NMD sensitivity implicates 3’-UTR length as a primary association determinant sensed by Upf1. This confirms the role of the 3’-UTR downstream of the PTC as a major determinant in NMD activation. Two aspects of the observed binding of Upf1 to 3’-UTRs are not easily reconciled with earlier studies. First, binding takes place when translation is inhibited, which is surprising because NMD depends on nonsense codon recognition by the ribosome (see above). Second, it is unclear how human Upf1, a protein quite closely related to its yeast counterpart, might be able to distinguish a completely different spectrum of UTR lengths than occur in yeast.
Reconciling mRNA targeting models
Although multiple aspects of the NMD pathway are understood in detail, there remains considerable controversy over its precise mechanism of action. This is particularly true if one attempts to reconcile models of NMD in lower and higher eukaryotes. That said, gene expression pathways in lower and higher eukaryotes are so substantially similar that it seems at least some unitary NMD characteristics should emerge.
A feature common to the prevailing models is that activation of NMD might depend on an inappropriate mRNP structure neighboring the PTC. This situation would hold whether Pab1 or other 3’-UTR factors are absent, or because an EJC is present, and could certainly accommodate organisms such as plants, in which NMD can be activated by either the presence of an exon-exon junction downstream of a PTC, or by an extended 3’-UTR126. An accommodating mRNP structure might provide sufficient specificity for Upf1 interaction with eRF3. The predicted competition of Upf1 and Pab1 for binding to eRF3 that was not manifested in vitro132 may work in the context an RNP (FIG. 3). This would explain Upf1 specificity for premature termination, but leaves open the mechanism of Upf2 and Upf3 recruitment as well as the basis for Upf1 association with the 40S ribosomal subunit in yeast55. Clearly, 40S association could reflect a cause or consequence of ribosome dissociation. Another important consideration in any mRNP-centric model is that the presence or absence of certain proteins, for example the components of the EJC, could be seen not as crucial NMD factors but as activators of translation, a step critical to PTC recognition. In this regard, it should be noted that EJC proteins also have important roles in determining the ability of a mature mRNP to be translated134-136.
A completely different way to consider this problem is from the perspective of the different efficiencies of termination. If normal termination is enhanced by interactions between the release factors and proteins associated with a normal 3’-UTR then perhaps the inefficiency of premature termination is initially attributable to poor release factor binding at the A-site of the ribosome or to slow dissociation of the termination complex after peptide hydrolysis. The latter is a more likely possibility because the toeprinting differences between normal and premature terminators indicate that a ribosome is stalled on the PTC and because there is no evidence for differences in peptide hydrolysis rates in normal and premature termination. However, either inefficiency might create a Upf1 binding site on the ribosome that is distinct from the A- or P-sites, but dependent on an atypical conformational state assumed by one of them; for example, the P site may retain a deacylated tRNA. Interaction between Upf1 and the eRFs could still occur, but the order of the hypothesized binding to the termination complex would now be reversed. This altered site model (FIG. 3) addresses specificity for premature termination, and Upf1:eRF and Upf1:ribosome interactions, but, as with the altered mRNP model discussed above, still leaves open the question of how Upf2 and Upf3 are recruited to activate Upf1. In cryoelectron microscopy reconstructions of a eukaryotic ribosome recycling complex only the structural homologue of eRF1 (Dom34) and the recycling factor ABCE1 (Rli1 in yeast) are present. This indicates that dissociation of eRF3 precedes ABCE1/Rli1 binding for ribosome recycling42. This result prompts us to propose that an aberrant step in premature translation termination could be the inefficient release of eRF3, possibly linked to the absence of Pab1. The presence of eRF3 on a terminating ribosome could recruit Upf1 to mediate an alternative mode of ribosome recycling.
Consequences of activating NMD
Activation of NMD has multiple ramifications. After recognition of the termination event as premature, and formation of the trimeric Upf1:Upf2:Upf3 complex, several parallel and interrelated events ensue. The targeted mRNA is subject to translational repression, and activation of the Upf1 ATPase and helicase activities plays a critical role in promoting dissociation and recycling of the ribosome and other associated factors, steps that are likely to prepare the mRNA for additional events in decay. NMD activation also promotes overall downregulation of expression of the products of the targeted allele, including degradation of the nascent polypeptide fragment and inhibition of splicing of the respective pre-mRNA.
Translational repression
Muhlrad and Parker123 observed that the presence of a PTC in a CUP1 fusion mRNA led to an apparent decrease in its translational efficiency. This observation led to the hypothesis that nonsense-containing mRNAs must be translationally repressed prior to their degradation (FIG. 4 pathway A), a proposal for which P-body localization of NMD-targeted mRNAs was considered substantial support137,138. However, as discussed below, reductions in the level of polypeptides produced by a PTC-containing mRNA can also be explained by the existence of a proteasome-dependent proteolytic pathway that targets such proteins139. That said, independent evidence for translational repression of NMD substrates has been obtained in mammalian cells. Isken et al.25 have observed that phosphorylated Upf1 can interact with eIF3 and interfere with translation initiation in cis. Strong support for this possibility came from experiments showing that comparable effects were not observed in mRNAs employing the eIF3-independent CrPV IRES25.
Figure 4. Ancillary processes that accompany NMD.
The association of the Upf proteins with a prematurely terminating ribosome has multiple consequences for the expression of the respective mRNA, including: A, translational repression of the mRNA, most likely at the level of initiation; B, disassembly of the mRNP and dissociation of the inefficiently terminating ribosome; C1, accelerated decapping and/or poly(A) shortening of the targeted mRNA or C2, endonucleolytic cleavage of the targeted mRNA; D, degradation of the nascent polypeptide; and E, feedback to the site of transcription, leading to the inhibition of splicing of the nascent pre-mRNA. The order of the different events is arbitrary, but is intended to imply that translational repression and mRNP disassembly may be logical prerequisites to the onset of mRNA decay. 4E=eIF4E; P=proteasome; AAA=poly(A) tail; R1=eRF1; R3=eRF3; 4G=eIF4G; 3=eIF3; 4A=eIF4A; structure marked “C”=Ccr4/Not deadenylase complex; Dcp1/Dcp2=decapping enzyme.
Ribosome release and recycling
Activation of the Upf1 ATPase and helicase activities appears to play a critical role in preparing the mRNA for decay by dissociating the ribosome from the termination site, possibly starting with the 60S subunit, and unwinding other components of the mRNP55,56 (FIG. 4 pathway B). This conclusion follows from an understanding of the aberrant nature of premature termination and from studies of the consequences of inactivating Upf1. Thus, addition of cycloheximide to yeast in vitro translation reactions was shown to allow detection of additional toeprints in close proximity to PTCs52. These toeprints were derived from post-termination ribosomes that failed to be released at premature terminators and were able to scan both 3’ and 5’ from the PTC and reinitiate translation at nearby AUGs. In eRF1-defective extracts, these toeprints were eliminated and replaced by toeprints corresponding to ribosomes stalled with the relevant stop codon in their A-sites. This indicated that, prior to any reinitiation event, a PTC must be recognized by eRF1 and peptide hydrolysis must be triggered. Importantly, the reinitiation toeprints were eliminated in extracts lacking one of the Upf proteins. This observation not only linked the reinitiation toeprints to NMD, but also reinforced the notion of inefficient premature termination and suggested that the Upf proteins might influence the extent to which prematurely terminating ribosomes remain associated with an mRNA. This possibility was underscored by the demonstration that reinitiation downstream of a PTC was also eliminated in upf-deletion strains in vivo55.
A further understanding of the post-termination function of the Upf proteins followed from additional in vitro translation analyses in yeast extracts55 and from studies in human cells expressing ATPase- or helicase-deficient Upf156. A yeast in vitro assay that monitored ribosomes undergoing premature termination indicated that extracts lacking Upf1 not only fail to reinitiate, but also appear to have a 60S joining defect in conventional initiation. The latter defect is thought to be a consequence of inadequate dissociation and recycling of ribosomes at a prior premature termination event. The inability of upf1Δ extracts to recycle ribosomes efficiently from a nonsense-containing mRNA is consistent with other experiments indicating links between termination and initiation40,43 and with prior studies showing genetic interactions between Upf1 and eIF1 or eIF3, initiation factors that stimulate the in vitro dissociation of post-termination 80S complexes16,32,39. In human cells Franks et al.56 showed that endonucleolytically cleaved and partially degraded nonsense-containing β-globin mRNA accumulates in the presence of ATPase- or helicase-deficient Upf1. The decay intermediate appeared in a complex that included all three Upfs and, possibly, the ribosome. In short, a combination of experiments in yeast and human cells indicate that at least one inefficient step in premature termination occurs at post-termination ribosome release, and Upf1 (and presumably the other Upfs) plays an important role in the latter release event, including the activation of its ATPase and helicase activities to couple release and mRNP disassembly to a step enabling ribosome reutilization. A role for the Upfs in the disassembly of a poorly dissociable termination complex is reminiscent of the function of Dom34 and Hbs1 in No-Go Decay6.
Accelerated mRNA decay
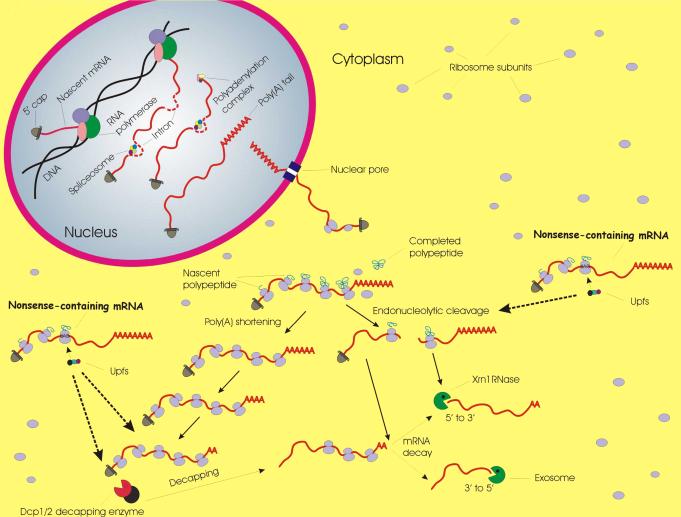
Upf1 interactions with several factors appear to play a key role in promoting the degradation of NMD substrates by multiple decay pathways in yeast and mammalian cells (FIG. 4, pathways C1 and C2, and FIG. 5)13,140-142. In yeast and human cells Upf1 interacts with the Dcp1/Dcp2 decapping enzyme, the exoribonuclease Xrn1, and various exosome components, suggesting a direct recruitment of the decay enzymes59,140-143. In addition, PNRC2 (Proline rich nuclear receptor co-regulatory protein 2) interacts with hyperphosphorylated Upf1 and Dcp1a, providing an additional link between the NMD and decapping machineries144. However, the notion of direct interactions between Upf1 and decay enzymes may be too simplistic. For example, it has been suggested that interactions between yeast Upf1 and Dcp2 may be indirect consequences of Upf1 interaction with the decapping activators Edc3 and Pat159,145,146.
Figure 5. After interaction with the NMD and Smg factors nonsense-containing mRNAs are degraded by conventional cellular mRNA decay pathways.
The nuclear biogenesis (upper left) and cytoplasmic translation and decay (center) of normal cellular mRNAs are depicted. Degradation of these mRNAs is initiated by exonucleolytic shortening of the poly(A) tail, catalyzed by the Ccr4:Caf1 and Pan2:Pan3 complexes2,138,185-188 (complexes not shown), or by endonucleolytic cleavage. Subsequent to poly(A) shortening, mRNAs are usually degraded by the decapping-dependent 5’ to 3’ pathway or by the 3′ to 5′ exosome-dependent pathway. The latter pathways are also utilized to eliminate the 5’ and 3’ mRNA fragments resulting from endonucleolytic cleavage. Nonsense-containing mRNAs recruit the Upf factors (or the Upf and Smg factors) to the prematurely terminating ribosome, resulting in interactions that promote accelerated entry of the mRNA into the poly(A)-shortening or decapping pathways, or lead to endonucleolytic cleavage. Products of endonucleolytic are then degraded by the standard 5’ to 3’ and 3’ to 5’ pathways. Some steps of the 5’ to 3’ pathway, including translational repression, may take place in cytoplasmic P-bodies (GW-bodies in mammalian and Drosophila cells; not shown), sites where the Dcp1/Dcp2 decapping enzyme, the Xrn1 5’-3’ exonuclease, and the Pat1, Dhh1, and Lsm1–7 decapping activators can accumulate185,186. Evidence showing that decapped decay intermediates can be polyribosome-associated187 suggests that assembly of these structures is not essential for mRNA decay, at least in yeast. Moreover, in both yeast and metazoan cells, disruption of P bodies by depletion of core P body components failed to alter decay phenotypes for several different mRNAs138,185,188.
As with the activation of Upf1 by Upf2:Upf3, the assembly of decay complexes in multicellular organisms appears to be a bit more straightforward and to include important roles for the non-Upf Smg factors. Two pathways have been described, which involve the interaction of phosphorylated Upf1 with either Smg-6 or Smg-5:Smg-7 (FIG. 2). Smg-6, by virtue of its PIN domain, has an endonuclease activity with which it initiates mRNA decay near the PTC in Drososphila and human cells56,147,148. The resulting 5’ and 3’ fragments are then degraded by the exosome and Xrn1, respectively148,149. Recruitment of Smg-5:Smg-7 is proposed to promote 5’ to 3’ and 3’ to 5’ decay of the targeted mRNA150. This is consistent with the absence or inactivity of PIN domains in both proteins and with experiments showing that tethering of Smg-7 to an otherwise normal mRNA is sufficient to promote its degradation, a function carried by its C-terminal domain150-152. Interestingly, it has been suggested that the choice between the two pathways in some organisms may reflect genomic differences153. For example, D. melanogaster lacks Smg-7 and its nonsense-containing mRNAs are thought to only initiate decay by Smg-6-mediated endonucleolytic cleavage154, a conclusion rendered somewhat complicated by the recent observation that Drosophila NMD remains active in flies harboring SMG6 mutations155.
Concomitant targeting of the nascent polypeptide
At least in yeast, NMD also activates the rapid degradation of the truncated polypeptide by the proteasome139,156, underscoring the biological importance of minimizing the accumulation of potentially toxic partially-synthesized proteins (FIG. 4, pathway D). Proteolysis depends on Upf1, and utilizes the ubiquitin-proteasome pathway, but its timing relative to other steps in NMD is unknown. Upf1 function in nonsense-mediated polypeptide decay may include a role as an E3 ubiquitin ligase: its N-terminal CH domain is distantly related to classical E3-RING finger domains, and it could interact with an E2 (Ubc3), and Upf1 could self-ubiquitinate in vitro in a Upf3-dependent reaction156. Although it is tempting to speculate that such modification of Upf1 may be crucial to its role in promoting proteolysis, we cannot rule out the possibility that ubiquitination, like phosphorylation, controls the ability of Upf1 to interact with critical mRNA decay factors. The targeted degradation of the nascent nonsense polypeptide is reminiscent of both the co-translational decay of the polypeptide generated by mRNAs subject to NSD as well as the bacterial tmRNA system, which disassembles elongation-stalled ribosomes while adding an ssrA tag to the associated polypeptide to promote its rapid decay157,158. In all of these circumstances mRNA decay, peptide decay, and ribosome recycling appear to be highly coordinated.
Feedback to the site of transcription
The evidence is fairly strong that, in all genera, NMD is a cytoplasmic, translation-dependent process. However, an increasing number of studies has shown that pre-mRNAs derived from some PTC-containing genes, for example, those encoding Ig-μ and T cell receptor (TCR)-β, accumulate at or near their respective sites of transcription. This surprising down-regulation of expression is gene- and allele-specific, that is, it is not detected with frameshift or missense alleles of the same genes, it is Upf1- and Smg-6-dependent, and the pre-mRNAs which accumulate at the transcription site are unspliced159-161 (FIG. 4, pathway E). As there is no current evidence for a direct link between NMD and gene-specific silencing mechanisms, it is useful to consider alternative, indirect explanations for this phenomenon. For example, it is well known that pre-mRNAs with defective 3' processing are often retained at their site of transcription162. Further, the Ccr4/Not complex, which is involved in cytoplasmic mRNA deadenylation and includes components that cycle to the nucleus, has been implicated in quality control mechanisms that stall transcripts in the nucleus163. Hence, it is possible that the rapid deadenylation of some mammalian NMD substrates may yield a post-translationally modified factor from the "accelerated" Ccr4/Not deadenylation complex in the cytoplasm that feeds back to its nuclear counterpart, with the latter stalling late events in nuclear processing and export164. Although the mechanism for allele specificity in such a model is unclear evidence that transcription may influence mRNA decay, and possibly vice versa, is starting to accumulate165-167.
Conclusions
The NMD pathway, one of several cytoplasmic quality control mechanisms ensuring the fidelity of gene expression, minimizes the accumulation of potentially toxic polypeptides that arise as products of premature translation termination, or termination that mimics at least some regulatory features characteristic of premature termination. The substrates of this pathway include the transcripts of genes harboring nonsense mutations or processing errors, pre-mRNAs that have entered the cytoplasm, mRNAs from rearranged genes, edited transcripts, mRNAs with uORFs, pseudogene mRNAs, and others. Collectively, NMD substrates may account for as much as 10% of the transcriptome in exponentially growing undifferentiated cells, including some mRNAs that encode normal proteins. It remains to be established whether all of these mRNAs contain PTCs or PTC-like features.
The three Upf proteins are the key NMD factors in all eukaryotes. Of these, Upf1, a superfamily I RNA helicase, is the central regulator. A detailed understanding of Upf1’s specificity for binding to a ribosome translating a PTC-containing mRNA remains to be elucidated, but it may depend on interaction with eRF3 and/or eRF1, an accommodating mRNP structure downstream of the PTC, and the local absence of Pab1. In mammalian cells, activation of the Upf1 ATPase and helicase activities is often a consequence of interaction with Upf2 and Upf3 that is associated with a downstream EJC. In transcripts without EJCs the mode of Upf1 association with Upf2:Upf3 is presently unclear and raises the possibility that a unitary NMD model may be elusive. Interaction with Upf2:Upf3 not only activates Upf1’s enzymatic activities, but also leads to its phosphorylation and its interaction with several other proteins including several Smg factors. Activated and phosphorylated Upf1 has multiple activities and much needs to be learned about the relative order of these activities and their coordinattion. Upf1 and its interactors appear to recruit decay enzymes that promote endonucleolytic cleavage, 5’ to 3’ decay, or 3’ to 5’ decay, but the mechanism of this recruitment as well as the means for selecting a particular decay pathway are far from clear. Upf1 can also repress the translation of a PTC-containing mRNA, dissociate and properly recycle its poorly dissociable PTC-bound ribosome, and unwind the PTC-containing mRNP, all in the interest of facilitating rapid decay of the mRNA and reutilization of the components of the translational machinery. Presumably to ensure that the nascent polypeptide has a minimal opportunity to interfere in cellular processes, Upf1 also promotes its proteolysis. It remains to be established whether all of these activities require all three Upfs, thus raising the question of whether mRNAs that associate with only one or two of these factors are subject to only a subset of their collective functions.
Almost all NMD-related activities are cytoplasmic. However, the extensive network of factors that carry out all the ramifications of NMD includes proteins that cycle between the cytoplasm and the nucleus. Accordingly, some cytoplasmic NMD events may provide feedback to nuclear steps in gene expression. Collectively, the set of events triggered by premature translational termination extend far beyond merely enhancing the rate of decay of a nonsense-containing mRNA. The resulting all encompassing shut-down of expression helps to explain why nonsense alleles are effectively null alleles and suggests potential avenues for enhancing the effects of therapeutic nonsense suppression54,168.
Finally, studies of NMD have underscored how little is known about translation termination in eukaryotes, at least when compared to our understanding of initiation. Not very long ago termination appeared to depend on just a nonsense codon and the eRFs. This parts list has grown, but a thorough understanding of the termination mechanism awaits resolution of the complexities implied by the indirect effects of mRNP proteins, the dissimilarities of normal and premature termination, and the possible complications of sequence context.
Definitions
Nonsense codon
The original name for UAA, UAG, or UGA, the codons that provide a stop signal for the elongation of protein synthesis when the ribosome reaches the normal end of an mRNA’s open reading frame. When they occur prior to that site they promote premature translational termination. Prevalent in all non-coding regions, such as introns, thus explaining why transcripts encompassing in-frame non-coding segments often become NMD substrates.
Upf (Up-frameshift) factors
The core regulators of the NMD pathway. These factors were first identified in yeast genetic screens seeking alleles that affected the consequences of frameshiting, the process wherein the ribosome can shift the translational reading frame during protein synthesis. Some yeast mRNAs undergoing frameshifting are known to be substrates for NMD.
Smg (Suppressor with morphogenetic effects on genitalia) factors
Additional effectors of the NMD decay pathway in multicellular organisms. Their name refers to the unusal phenotype they manifest in Caenorhabditis elegans, the organism in which they were first identified by genetic screens. SMG2, SMG3, and SMG4 are orthologues of the UPF1, UPF2/NMD2 and UPF3 genes, respectively.
Zinc-finger domain (CH domain)
A protein structural motif that coordinates zinc ions with cysteine and histidine residues. This motif is often central to protein modules that serve as interaction domains or binding sites for DNA or RNA. In Upf1, this domain encompasses a unique combination of three zinc-binding motifs arranged into two tandem modules.
RNA helicase
An enzyme harboring ATPase and RNA unwinding activity. Cells contain many different RNA helicases that are respectively involved in a wide spectrum of gene expression functions, including pre-mRNA splicing, protein synthesis, and mRNA decay.
P (Processing) bodies
Cytoplasmic foci, also known as GW-bodies and decapping bodies, that contain high concentrations of factors involved in 5’ to 3’ mRNA decay, such as the decapping complex, Dcp1-Dcp2, and the exoribonuclease, Xrn1, as well as the machinery of miRNA-mediated mRNA decay in multicelular organisms. The role of P-bodies in mRNA decay has been controversial because these structures are not essential for mRNA decay or miRNA silencing, and mRNAs that enter P-bodies are not necessarily destined for degradation and can exit these foci and reinitiate translation.
Cycloheximide
An inhibitor of protein synthesis in eukaryotes that blocks the translocation of the ribosome during the elongation step. When added to cells or extracts in which translation is underway this drug will stall elongating ribosomes on the mRNA.
Ribosomal A, P, and E sites
Ribosomes, the cellular machines catalyzing protein synthesis from an mRNA template, harbor these three sites to accomodate tRNAs. Aminoacyl-tRNA binds in the A site, peptidyl-tRNA binds in the P site, and deacylated tRNA binds in the E site. During translational elongation, tRNAs move successively from the A to the P to the E site, always in response to specific codon:anticodon pairing.
PIN domain
A protein domain of approximately 130 amino acids that can function as a ribonuclease and cleave single stranded RNA. The name is derived from its identification in the N-terminus of the PilT protein.
Translational readthrough
Termination at a nonsense codon is not absolute. A near-cognate aminoacyl-tRNA, i.e., one which can base pair with a stop codon at two of the three positions of a codon–anticodon complex, is a competitor for eRF1 binding in the A site. If the stop codon is “read” by this competing tRNA, the ribosome will insert an amino acid residue and continue to translate sequences downstream of the nonsense codon, usually until it reaches the next stop codon. This readthrough event is also known as nonsense suppression and its frequency is often monitored as an indicator for the fidelity and efficiency of translation termination.
Toeprinting
This method identifies the position of ribosomes on an mRNA. The technique is based on the inhibition of primer extension by reverse transcriptase (RT). Using a labeled oligonucleotide that hybridizes 3' to the region of interest of an mRNA, reverse transcriptase extends the primer and synthesizes complementary DNA from the site of primer binding toward the 5' end of the mRNA. When the reverse transcriptase encounters the ribosome (or associated factor) bound to the mRNA, polymerization is halted and a cDNA toeprint fragment is generated. Its size is then determined on high-resolution polyacrylamide gels. At termination codons, the cessation of reverse transcription can be detected approximately 13 nt downstream of the U of the termination codon. This spacing is consistent with ribosomes that have paused with the termination codon in the ribosome’s A site.
Acknowledgments
Research in the authors’ laboratories was supported by grants from the U.S. National Institutes of Health, the Human Frontier Science Program, and the US-Israel Binational Science Foundation. We thank Jeremie Piton for help with drafting Figure 1 and Feng He for useful comments on the manuscript.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Ghosh S, Jacobson A. RNA decay modulates gene expression and controls its fidelity. WIREs RNA. 2010;1:351–361. doi: 10.1002/wrna.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garneau NL, Wilusz J, Wilusz CJ. The highways and byways of mRNA decay. Nat. Rev. Mol. Cell. Biol. 2007;8:113–126. doi: 10.1038/nrm2104. [DOI] [PubMed] [Google Scholar]
- 3.Frischmeyer PA, et al. An mRNA surveillance mechanism that eliminates transcripts lacking termination codons. Science. 2002;295:2258–2261. doi: 10.1126/science.1067338. [DOI] [PubMed] [Google Scholar]
- 4.Schaeffer D, van Hoof A. Different nuclease requirements for exosome-mediated degradation of normal and nonstop mRNAs. Proc. Natl. Acad. Sci. USA. 2011;108:2366–2371. doi: 10.1073/pnas.1013180108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klauer AA, van Hoof A. Degradation of mRNAs that lack a stop codon: a decade of nonstop progress. Wiley Interdiscip. Rev. RNA. 2012;3:649–660. doi: 10.1002/wrna.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shoemaker CJ, Eyler DE, Green R. Dom34:Hbs1 promotes subunit dissociation and peptidyl-tRNA drop-off to initiate no-go decay. Science. 2010;330:369–372. doi: 10.1126/science.1192430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doma MK, Parker R. Endonucleolytic cleavage of eukaryotic mRNAs with stalls in translation elongation. Nature. 2006;440:561–564. doi: 10.1038/nature04530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isken O, Maquat LE. Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function. Genes Dev. 2007;21:1833–1856. doi: 10.1101/gad.1566807. [DOI] [PubMed] [Google Scholar]
- 9.Jacobson A, Izaurralde E. Nonsense-mediated mRNA decay: from yeast to metazoans. In: Mathews MB, Sonenberg N, Hershey JWB, editors. in Translational Control in Biology and Medicine. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2007. pp. 659–691. [Google Scholar]
- 10.Nicholson P, Muhlemann O. Cutting the nonsense: the degradation of PTC-containing mRNAs. Biochem. Soc. Trans. 2010;38:1615–1620. doi: 10.1042/BST0381615. [DOI] [PubMed] [Google Scholar]
- 11.Rebbapragada I, Lykke-Andersen J. Execution of nonsense-mediated mRNA decay: what defines a substrate? Curr. Opin. Cell Biol. 2009;21:394–402. doi: 10.1016/j.ceb.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Shoemaker CJ, Green R. Translation drives mRNA quality control. Nature Struct. Mol. Biol. 2012;19:594–601. doi: 10.1038/nsmb.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He F, et al. Genome-wide analysis of mRNAs regulated by the nonsense-mediated and 5' to 3' mRNA decay pathways in yeast. Mol. Cell. 2003;12:1439–1452. doi: 10.1016/s1097-2765(03)00446-5. [DOI] [PubMed] [Google Scholar]
- 14.He F, Peltz SW, Donahue JL, Rosbash M. Jacobson, A. Stabilization and ribosome association of unspliced pre-mRNAs in a yeast upf1- mutant. Proc. Natl. Acad. Sci. USA. 1993;90:7034–7038. doi: 10.1073/pnas.90.15.7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Vock VM, Li S, Olivas OR, Wilkinson MF. A quality control pathway that down-regulates aberrant T-cell receptor (TCR) transcripts by a mechanism requiring UPF2 and translation. J. Biol. Chem. 2002;277:18489–18493. doi: 10.1074/jbc.M111781200. [DOI] [PubMed] [Google Scholar]
- 16.Welch EM, Jacobson A. An internal open reading frame triggers nonsense-mediated decay of the yeast SPT10 mRNA. EMBO J. 1999;18:6134–6145. doi: 10.1093/emboj/18.21.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muhlrad D, Parker R. Aberrant mRNAs with extended 3' UTRs are substrates for rapid degradation by mRNA surveillance. RNA. 1999;5:1299–1307. doi: 10.1017/s1355838299990829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johansson MJ, He F, Spatrick P, Li C, Jacobson A. Association of yeast Upf1p with direct substrates of the NMD pathway. Proc. Natl. Acad. Sci. U S A. 2007;104:20872–20877. doi: 10.1073/pnas.0709257105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendell JT, Sharifi NA, Meyers JL, Martinez-Murillo F, Dietz HC. Nonsense surveillance regulates expression of diverse classes of mammalian transcripts and mutes genomic noise. Nature Genet. 2004;36:1073–1078. doi: 10.1038/ng1429. [DOI] [PubMed] [Google Scholar]
- 20.Rehwinkel J, Letunic I, Raes J, Bork P, Izaurralde E. Nonsense-mediated mRNA decay factors act in concert to regulate common mRNA targets. RNA. 2005;11:1530–1544. doi: 10.1261/rna.2160905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wittmann J, Hol EM, Jack HM. hUPF2 silencing identifies physiologic substrates of mammalian nonsense-mediated mRNA decay. Mol. Cell. Biol. 2006;26:1272–1287. doi: 10.1128/MCB.26.4.1272-1287.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guan Q, et al. Impact of nonsense-mediated mRNA decay on the global expression profile of budding yeast. PLoS Genet. 2006;2:e203. doi: 10.1371/journal.pgen.0020203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Viegas MH, Gehring NH, Breit S, Hentze MW, Kulozik AE. The abundance of RNPS1, a protein component of the exon junction complex, can determine the variability in efficiency of the Nonsense Mediated Decay pathway. Nucleic Acids Res. 2007;35:4542–4551. doi: 10.1093/nar/gkm461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yepiskoposyan H, Aeschimann F, Nilsson D, Okoniewski M, Muhlemann O. Autoregulation of the nonsense-mediated mRNA decay pathway in human cells. RNA. 2011;17:2108–2118. doi: 10.1261/rna.030247.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isken O, Maquat LE. The multiple lives of NMD factors: balancing roles in gene and genome regulation. Nat. Rev. Genet. 2008;9:699–712. doi: 10.1038/nrg2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rehwinkel J, Raes J, Izaurralde E. Nonsense-mediated mRNA decay: Target genes and functional diversification of effectors. Trends Biochem. Sci. 2006;31:639–646. doi: 10.1016/j.tibs.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, et al. Polysome-associated mRNAs are substrates for the nonsense-mediated mRNA decay pathway in Saccharomyces cerevisiae. RNA. 1997;3:234–244. [PMC free article] [PubMed] [Google Scholar]
- 28.Gozalbo D, Hohmann S. Nonsense suppressors partially revert the decrease of the mRNA level of a nonsense mutant allele in yeast. Curr. Genet. 1990;17:77–79. doi: 10.1007/BF00313252. [DOI] [PubMed] [Google Scholar]
- 29.Losson R, Lacroute F. Interference of nonsense mutations with eukaryotic messenger RNA stability. Proc. Natl. Acad. Sci. USA. 1979;76:5134–5137. doi: 10.1073/pnas.76.10.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Belgrader P, Cheng J, Maquat LE. Evidence to implicate translation by ribosomes in the mechanism by which nonsense codons reduce the nuclear level of human triosephosphate isomerase mRNA. Proc. Natl. Acad. Sci. USA. 1993;90:482–486. doi: 10.1073/pnas.90.2.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaba A, Jacobson A, Sachs MS. Ribosome occupancy of the yeast CPA1 upstream open reading frame termination codon modulates nonsense-mediated mRNA decay. Mol. Cell. 2005;20:449–460. doi: 10.1016/j.molcel.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 32.Jackson RJ, Hellen CU, Pestova TV. Termination and post-termination events in eukaryotic translation. Adv. Protein Chem. Struct. Biol. 2012;86:45–93. doi: 10.1016/B978-0-12-386497-0.00002-5. [DOI] [PubMed] [Google Scholar]
- 33.Frolova L, et al. A highly conserved eukaryotic protein family possessing properties of polypeptide chain release factor. Nature. 1994;372:701–703. doi: 10.1038/372701a0. [DOI] [PubMed] [Google Scholar]
- 34.Stansfield I, et al. The products of the SUP45 (eRF1) and SUP35 genes interact to mediate translation termination in Saccharomyces cerevisiae. EMBO J. 1995;14:4365–4373. doi: 10.1002/j.1460-2075.1995.tb00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhouravleva G, et al. Termination of translation in eukaryotes is governed by two interacting polypeptide chain release factors, eRF1 and eRF3. EMBO J. 1995;14:4065–4072. doi: 10.1002/j.1460-2075.1995.tb00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng Z, et al. Structural insights into eRF3 and stop codon recognition by eRF1. Genes Dev. 2009;23:1106–1118. doi: 10.1101/gad.1770109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alkalaeva EZ, Pisarev AV, Frolova LY, Kisselev LL, Pestova TV. In vitro reconstitution of eukaryotic translation reveals cooperativity between release factors eRF1 and eRF3. Cell. 2006;125:1125–1136. doi: 10.1016/j.cell.2006.04.035. [DOI] [PubMed] [Google Scholar]
- 38.Salas-Marco J, Bedwell DM. GTP hydrolysis by eRF3 facilitates stop codon decoding during eukaryotic translation termination. Mol. Cell. Biol. 2004;24:7769–7778. doi: 10.1128/MCB.24.17.7769-7778.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pisarev AV, Hellen CU, Pestova TV. Recycling of eukaryotic posttermination ribosomal complexes. Cell. 2007;131:286–299. doi: 10.1016/j.cell.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pisarev AV, et al. The role of ABCE1 in eukaryotic posttermination ribosomal recycling. Mol. Cell. 2010;37:196–210. doi: 10.1016/j.molcel.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- References 39 and 40 demonstrate the first in vitro post-termination recycling of the eukaryotic ribosome and elucidate the roles of initiation factors and the ABCE1 ATPase in this process.
- 41.Barthelme D, et al. Ribosome recycling depends on a mechanistic link between the FeS cluster domain and a conformational switch of the twin-ATPase ABCE1. Proc. Natl. Acad. Sci. USA. 2011;108:3228–3233. doi: 10.1073/pnas.1015953108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becker T, et al. Structural basis of highly conserved ribosome recycling in eukaryotes and archaea. Nature. 2012;482:501–506. doi: 10.1038/nature10829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This study utilizes cryoelectron microscopy to derive structures of the yeast ribosome containing the recycling factor Rli1 (a homologue of mammalian ABCE1) and Dom34, a homologue of eRF1 implicated in the No-Go Decay pathway. Rli1 is shown to interact with and stabilize Dom34 in a conformation presumably adopted by eRF1 to activate the peptidyl transferase center of the ribosome. This suggests that Hbs1 and its homologue, eRF3, might only be required to bring Dom34 or eRF1 to the A site of the ribosome, without active roles in peptide release (for eRF1-eRF3) or ribosome dissociation (for both)
- 43.Khoshnevis S, et al. The iron-sulphur protein RNase L inhibitor functions in translation termination. EMBO Rep. 2010;11:214–219. doi: 10.1038/embor.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoshino S, Imai M, Kobayashi T, Uchida N, Katada T. The eukaryotic polypeptide chain releasing factor (eRF3/GSPT) carrying the translation termination signal to the 3'-poly(A) tail of mRNA. Direct association of erf3/GSPT with polyadenylate-binding protein. J. Biol. Chem. 1999;274:16677–16680. doi: 10.1074/jbc.274.24.16677. [DOI] [PubMed] [Google Scholar]
- 45.Cosson B, et al. Poly(A)-binding protein acts in translation termination via eukaryotic release factor 3 interaction and does not influence PSI(+) propagation. Mol. Cell. Biol. 2002;22:3301–3315. doi: 10.1128/MCB.22.10.3301-3315.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- References 44 and 45 show that eRF3 and Pab1 interact and play a role in translation termination.
- 46.Ivanov PV, Gehring NH, Kunz JB, Hentze MW, Kulozik AE. Interactions between UPF1, eRFs, PABP and the exon junction complex suggest an integrated model for mammalian NMD pathways. EMBO J. 2008;27:736–747. doi: 10.1038/emboj.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- References 46, 95, and 119 provide evidence that the distance between a premature termination event and the poly(A)-binding protein, Pab1, influences NMD activation in mammalian cells and could reflect competition between Pab1 and Upf1 for binding to eRF3.
- 47.Hilleren P, Parker R. mRNA surveillance in eukaryotes: kinetic proofreading of proper translation termination as assessed by mRNP domain organization? RNA. 1999;5:711–719. doi: 10.1017/s1355838299990519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amrani N, Sachs MS, Jacobson A. Early nonsense: mRNA decay solves a translational problem. Nat. Rev. Mol. Cell. Biol. 2006;7:415–425. doi: 10.1038/nrm1942. [DOI] [PubMed] [Google Scholar]
- 49.Estrella LA, Wilkinson MF, Gonzalez CI. The shuttling protein Npl3 promotes translation termination accuracy in Saccharomyces cerevisiae. J. Mol. Biol. 2009;394:410–422. doi: 10.1016/j.jmb.2009.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gross T, et al. The DEAD-box RNA helicase Dbp5 functions in translation termination. Science. 2007;315:646–649. doi: 10.1126/science.1134641. [DOI] [PubMed] [Google Scholar]
- 51.Bolger TA, Folkmann AW, Tran EJ, Wente SR. The mRNA export factor Gle1 and inositol hexakisphosphate regulate distinct stages of translation. Cell. 2008;134:624–633. doi: 10.1016/j.cell.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Amrani N, et al. A faux 3'-UTR promotes aberrant termination and triggers nonsense-mediated mRNA decay. Nature. 2004;432:112–118. doi: 10.1038/nature03060. [DOI] [PubMed] [Google Scholar]
- Toeprinting and protein-tethering experiments which showed that normal termination and premature termination are functionally different events, and that poly(A)-binding protein that is localized close to a premature termination codon can mimic a normal 3'-UTR and promote mRNA stabilization.
- 53.Peixeiro I, et al. Interaction of PABPC1 with the translation initiation complex is critical to the NMD resistance of AUG-proximal nonsense mutations. Nucleic Acids Res. 2011;40:1160–1173. doi: 10.1093/nar/gkr820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toeprinting experiments on normal or premature terminators in mammalian cells that confirm the abberant nature of translation termination at a PTC and a role for Pab1 in this process.
- 54.Welch EM, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- This paper describes the identification and initial characterization of PTC124 (ataluren), a novel compound that promotes readthrough of premature but not normal termination codons in human cells. The compound underscores the mechanistic differences between premature and normal termination and has the potential to be a clinically useful therapeutic for a broad range of genetic disorders caused by nonsense mutations.
- 55.Ghosh S, Ganesan R, Amrani N, Jacobson A. Translational competence of ribosomes released from a premature termination codon is modulated by NMD factors. RNA. 2010;16:1832–1847. doi: 10.1261/rna.1987710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This paper provides two lines of evidence that post-termination ribosome dissociation is altered at premature termination: reinititaion after termination at a PTC is shown to be affected in yeast cells lacking any of the Upf factors, and ribosome reutilization in vitro is shown to be less efficient after premature termination than it is after normal termination.
- 56.Franks TM, Singh G, Lykke-Andersen J. Upf1 ATPase-dependent mRNP disassembly is required for completion of nonsense- mediated mRNA decay. Cell. 2010;143:938–950. doi: 10.1016/j.cell.2010.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The first paper to demonstrate that the Upf1 ATPase activity disassembles an mRNP substrate prior to promoting mRNA decay and Upf1 recycling.
- 57.Hodgkin J, Papp A, Pulak R, Ambros V, Anderson P. A new kind of informational suppression in the nematode Caenorhabditis elegans. Genetics. 1989;123:301–313. doi: 10.1093/genetics/123.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leeds P, Peltz SW, Jacobson A, Culbertson MR. The product of the yeast UPF1 gene is required for rapid turnover of mRNAs containing a premature translational termination codon. Genes Dev. 1991;5:2303–2314. doi: 10.1101/gad.5.12a.2303. [DOI] [PubMed] [Google Scholar]
- 59.He F, Jacobson A. Identification of a novel component of the nonsense-mediated mRNA decay pathway by use of an interacting protein screen. Genes Dev. 1995;9:437–454. doi: 10.1101/gad.9.4.437. [DOI] [PubMed] [Google Scholar]
- 60.Perlick HA, Medghalchi SM, Spencer FA, Kendzior RJ, Jr, Dietz HC. Mammalian orthologues of a yeast regulator of nonsense transcript stability. Proc. Natl. Acad. Sci. USA. 1996;93:10928–32. doi: 10.1073/pnas.93.20.10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conti E, Izaurralde E. Nonsense-mediated mRNA decay: molecular insights and mechanistic variations across species. Curr. Opin. Cell Biol. 2005;17:316–325. doi: 10.1016/j.ceb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 62.Cui Y, Hagan KW, Zhang S, Peltz SW. Identification and characterization of genes that are required for the accelerated degradation of mRNAs containing a premature translational termination codon. Genes Dev. 1995;9:423–436. doi: 10.1101/gad.9.4.423. [DOI] [PubMed] [Google Scholar]
- 63.He F, Brown AH, Jacobson A. Upf1p, Nmd2p, and Upf3p are interacting components of the yeast nonsense-mediated mRNA decay pathway. Mol. Cell. Biol. 1997;17:1580–1594. doi: 10.1128/mcb.17.3.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chamieh H, Ballut L, Bonneau F, Le Hir H. NMD factors UPF2 and UPF3 bridge UPF1 to the exon junction complex and stimulate its RNA helicase activity. Nat. Struct. Mol. Biol. 2008;15:85–93. doi: 10.1038/nsmb1330. [DOI] [PubMed] [Google Scholar]
- The first paper to demonstrate that the ATPase and helicase activities of Upf1 are stimulated by the binding of Upf2 and Upf3.
- 65.Serin G, Gersappe A, Black JD, Aronoff R, Maquat LE. Identification and characterization of human orthologues to Saccharomyces cerevisiae Upf2 protein and Upf3 protein (Caenorhabditis elegans SMG-4) Mol. Cell. Biol. 2001;21:209–23. doi: 10.1128/MCB.21.1.209-223.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leeds P, Wood JM, Lee BS, Culbertson MR. Gene products that promote mRNA turnover in Saccharomyces cerevisiae. Mol. Cell. Biol. 1992;12:2165–2177. doi: 10.1128/mcb.12.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Altamura N, Groudinsky O, Dujardin G, Slonimski PP. NAM7 nuclear gene encodes a novel member of a family of helicases with a Zn-ligand motif and is involved in mitochondrial functions in Saccharomyces cerevisiae. J. Mol. Biol. 1992;224:575–587. doi: 10.1016/0022-2836(92)90545-u. [DOI] [PubMed] [Google Scholar]
- 68.Cheng Z, Muhlrad D, Lim MK, Parker R, Song H. Structural and functional insights into the human Upf1 helicase core. EMBO J. 2007;26:253–264. doi: 10.1038/sj.emboj.7601464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bhattacharya A, et al. Characterization of the biochemical properties of the human Upf1 gene product that is involved in nonsense-mediated mRNA decay. RNA. 2000;6:1226–1235. doi: 10.1017/s1355838200000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weng Y, Czaplinski K, Peltz SW. Genetic and biochemical characterization of mutations in the ATPase and helicase regions of the Upf1 protein. Mol. Cell. Biol. 1996;16:5477–5490. doi: 10.1128/mcb.16.10.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Czaplinski K, Weng Y, Hagan KW, Peltz SW. Purification and characterization of the Upf1 protein: a factor involved in translation and mRNA degradation. RNA. 1995;1:610–623. [PMC free article] [PubMed] [Google Scholar]
- 72.Weng Y, Czaplinski K, Peltz SW. ATP is a cofactor of the Upf1 protein that modulates its translation termination and RNA binding activities. RNA. 1998;4:205–214. [PMC free article] [PubMed] [Google Scholar]
- 73.Mendell JT, Medghalchi SM, Lake RG, Noensie EN, Dietz HC. Novel Upf2p orthologues suggest a functional link between translation initiation and nonsense surveillance complexes. Mol. Cell. Biol. 2000;20:8944–8957. doi: 10.1128/mcb.20.23.8944-8957.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kadlec J, Izaurralde E, Cusack S. The structural basis for the interaction between nonsense-mediated mRNA decay factors UPF2 and UPF3. Nature Struct. Mol. Biol. 2004;11:330–337. doi: 10.1038/nsmb741. [DOI] [PubMed] [Google Scholar]
- 75.Maderazo AB, He F, Mangus DA, Jacobson A. Upf1p control of nonsense mRNA translation is regulated by Nmd2p and Upf3p. Mol. Cell. Biol. 2000;20:4591–4603. doi: 10.1128/mcb.20.13.4591-4603.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He F, Brown AH, Jacobson A. Interaction between Nmd2p and Upf1p is required for activity but not for dominant-negative inhibition of the nonsense-mediated mRNA decay pathway in yeast. RNA. 1996;2:153–170. [PMC free article] [PubMed] [Google Scholar]
- 77.Kashima I, et al. Binding of a novel SMG-1-Upf1-eRF1-eRF3 complex (SURF) to the exon junction complex triggers Upf1 phosphorylation and nonsense-mediated mRNA decay. Genes Dev. 2006;20:355–367. doi: 10.1101/gad.1389006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A demonstration that NMD activation in mammalian cells involves an interaction of the SURF (Smg-1, Upf1 and release factors 1 and 3) complex located at a premature terminator and Upf2:Upf3 bound to the EJC.
- 78.Chakrabarti S, et al. Molecular mechanisms for the RNA-dependent ATPase activity of Upf1 and its regulation by Upf2. Mol. Cell. 2011;41:693–703. doi: 10.1016/j.molcel.2011.02.010. [DOI] [PubMed] [Google Scholar]
- The first structure of Upf1 with both the CH and the helicase domain. This structure shows that Upf1 can switch from a closed-conformation to an open and active conformation upon Upf2 binding to the Upf1 N-terminal zinc-finger domain.
- 79.Clerici M, et al. Unusual bipartite mode of interaction between the nonsense-mediated decay factors, UPF1 and UPF2. EMBO J. 2009;28:2293–2306. doi: 10.1038/emboj.2009.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Durand S, et al. Inhibition of nonsense-mediated mRNA decay (NMD) by a new chemical molecule reveals the dynamic of NMD factors in P-bodies. J. Cell. Biol. 2007;178:1145–1160. doi: 10.1083/jcb.200611086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang W, Cajigas IJ, Peltz SW, Wilkinson MF, Gonzalez CI. A role for Upf2p phosphorylation in Saccharomyces cerevisiae nonsense-mediated mRNA decay. Mol. Cell. Biol. 2006;26:3390–4000. doi: 10.1128/MCB.26.9.3390-3400.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Luke B, et al. Saccharomyces cerevisiae Ebs1p is a putative ortholog of human Smg7 and promotes nonsense-mediated mRNA decay. Nucleic Acids Res. 2007;35:7688–7697. doi: 10.1093/nar/gkm912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Le Hir H, Moore MJ, Maquat LE. Pre-mRNA splicing alters mRNP composition: evidence for stable association of proteins at exon-exon junctions. Genes Dev. 2000;14:1098–1108. [PMC free article] [PubMed] [Google Scholar]
- References 83, 85, 86, 88, and 89 demonstrate that hUpf2 and hUpf3 are associated with the components of the exon junction complex and provide a link between NMD activation and intron location in mammalian cells.
- 84.Sun X, Maquat LE. mRNA surveillance in mammalian cells: the relationship between introns and translation termination. RNA. 2000;6:1–8. doi: 10.1017/s1355838200991660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lykke-Andersen J, Shu MD, Steitz JA. Communication of the position of exon-exon junctions to the mRNA surveillance machinery by the protein RNPS1. Science. 2001;293:1836–1839. doi: 10.1126/science.1062786. [DOI] [PubMed] [Google Scholar]
- 86.Le Hir H, Gatfield D, Izaurralde E, Moore MJ. The exon-exon junction complex provides a binding platform for factors involved in mRNA export and nonsense-mediated mRNA decay. EMBO J. 2001;20:4987–4997. doi: 10.1093/emboj/20.17.4987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lejeune F, Ishigaki Y, Li X, Maquat LE. The exon junction complex is detected on CBP80-bound but not eIF4E-bound mRNA in mammalian cells: dynamics of mRNP remodeling. EMBO J. 2002;21:3536–3545. doi: 10.1093/emboj/cdf345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim VN, Kataoka N, Dreyfuss G. Role of the nonsense-mediated decay factor hUpf3 in the splicing- dependent exon-exon junction complex. Science. 2001;293:1832–1836. doi: 10.1126/science.1062829. [DOI] [PubMed] [Google Scholar]
- 89.Buchwald G, et al. Insights into the recruitment of the NMD machinery from the crystal structure of a core EJC-UPF3b complex. Proc. Natl. Acad. Sci. USA. 2010;107:10050–10055. doi: 10.1073/pnas.1000993107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Melero R, et al. The cryo-EM structure of the UPF-EJC complex shows UPF1 poised toward the RNA 3' end. Nat. Struct. Mol. Biol. 2012;19:498–505. doi: 10.1038/nsmb.2287. [DOI] [PubMed] [Google Scholar]
- 91.Kashima I, et al. SMG6 interacts with the exon junction complex via two conserved EJC-binding motifs (EBMs) required for nonsense-mediated mRNA decay. Genes Dev. 2010;24:2440–2450. doi: 10.1101/gad.604610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chan WK, et al. An alternative branch of the nonsense-mediated decay pathway. EMBO J. 2007;26:1820–1830. doi: 10.1038/sj.emboj.7601628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gehring NH, et al. Exon-junction complex components specify distinct routes of nonsense-mediated mRNA decay with differential cofactor requirements. Mol. Cell. 2005;20:65–75. doi: 10.1016/j.molcel.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 94.Czaplinski K, et al. The surveillance complex interacts with the translation release factors to enhance termination and degrade aberrant mRNAs. Genes Dev. 1998;12:1665–1677. doi: 10.1101/gad.12.11.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- References 94 and 97 demonstrate direct interactions between Upf1 and the release factors.
- 95.Singh G, Rebbapragada I, Lykke-Andersen J. A competition between stimulators and antagonists of Upf complex recruitment governs human nonsense-mediated mRNA decay. PLoS Biol. 2008;6:e111. doi: 10.1371/journal.pbio.0060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yamashita A, et al. SMG-8 and SMG-9, two novel subunits of the SMG-1 complex, regulate remodeling of the mRNA surveillance complex during nonsense-mediated mRNA decay. Genes Dev. 2009;23:1091–1105. doi: 10.1101/gad.1767209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang W, Czaplinski K, Rao Y, Peltz SW. The role of Upf proteins in modulating the translation read-through of nonsense-containing transcripts. EMBO J. 2001;20:880–890. doi: 10.1093/emboj/20.4.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kobayashi T, Funakoshi Y, Hoshino S, Katada T. The GTP-binding release factor eRF3 as a key mediator coupling translation termination to mRNA decay. J. Biol. Chem. 2004;279:45693–45700. doi: 10.1074/jbc.M405163200. [DOI] [PubMed] [Google Scholar]
- 99.Keeling KM, et al. Leaky termination at premature stop codons antagonizes nonsense-mediated mRNA decay in S. cerevisiae. RNA. 2004;10:691–703. doi: 10.1261/rna.5147804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weng Y, Czaplinski K, Peltz SW. Identification and characterization of mutations in the UPF1 gene that affect nonsense suppression and the formation of the Upf protein complex but not mRNA turnover. Mol. Cell. Biol. 1996;16:5491–5506. doi: 10.1128/mcb.16.10.5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Johansson MJ, Jacobson A. Nonsense-mediated mRNA decay maintains translational fidelity by limiting magnesium uptake. Genes Dev. 2010;24:1491–1495. doi: 10.1101/gad.1930710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A demonstration that the previously observed role of Upf1 in yeast termination fidelity is largely attributable to an indirect effect on the stability of the uORF-containing ALR1 mRNA, an endogenous NMD substrate that encodes a cellular transporter involved in the regulation of intracellular Mg2+ concentration.
- 102.Ruiz-Echevarria MJ, Peltz SW. The RNA binding protein Pub1 modulates the stability of transcripts containing upstream open reading frames. Cell. 2000;101:741–751. doi: 10.1016/s0092-8674(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 103.Gonzalez CI, Ruiz-Echevarria MJ, Vasudevan S, Henry MF, Peltz SW. The yeast hnRNP-like protein Hrp1/Nab4 marks a transcript for nonsense- mediated mRNA decay. Mol. Cell. 2000;5:489–499. doi: 10.1016/s1097-2765(00)80443-8. [DOI] [PubMed] [Google Scholar]
- 104.Gonzalez CI, Wang W, Peltz SW. Nonsense-mediated mRNA decay in Saccharomyces cerevisiae: a quality control mechanism that degrades transcripts harboring premature termination codons. Cold Spring Harb. Symp. Quant. Biol. 2001;66:321–328. doi: 10.1101/sqb.2001.66.321. [DOI] [PubMed] [Google Scholar]
- 105.Czaplinski K, Ruiz-Echevarria MJ, Gonzalez CI, Peltz SW. Should we kill the messenger? The role of the surveillance complex in translation termination and mRNA turnover. Bioessays. 1999;21:685–696. doi: 10.1002/(SICI)1521-1878(199908)21:8<685::AID-BIES8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 106.Carter MS, Li S, Wilkinson MF. A splicing-dependent regulatory mechanism that detects translation signals. EMBO J. 1996;15:5965–75. [PMC free article] [PubMed] [Google Scholar]
- 107.Thermann R, et al. Binary specification of nonsense codons by splicing and cytoplasmic translation. EMBO J. 1998;17:3484–94. doi: 10.1093/emboj/17.12.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Le Hir H, Izaurralde E, Maquat LE, Moore MJ. The spliceosome deposits multiple proteins 20-24 nucleotides upstream of mRNA exon-exon junctions. EMBO J. 2000;19:6860–6869. doi: 10.1093/emboj/19.24.6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schoenberg DR, Maquat LE. Regulation of cytoplasmic mRNA decay. Nature Rev. Genet. 2012;13:246–259. doi: 10.1038/nrg3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Okada-Katsuhata Y, et al. N- and C-terminal Upf1 phosphorylations create binding platforms for SMG-6 and SMG-5:SMG-7 during NMD. Nucleic Acids Res. 2012;40:1251–1266. doi: 10.1093/nar/gkr791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wen J, Brogna S. Splicing-dependent NMD does not require the EJC in Schizosaccharomyces pombe. EMBO J. 2010;29:1537–1551. doi: 10.1038/emboj.2010.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang J, Sun X, Qian Y, Maquat LE. Intron function in the nonsense-mediated decay of beta-globin mRNA: indications that pre-mRNA splicing in the nucleus can influence mRNA translation in the cytoplasm. RNA. 1998;4:801–15. doi: 10.1017/s1355838298971849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rajavel KS, Neufeld EF. Nonsense-mediated decay of human HEXA mRNA. Mol. Cell. Biol. 2001;21:5512–5519. doi: 10.1128/MCB.21.16.5512-5519.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.LeBlanc JJ, Beemon KL. Unspliced Rous sarcoma virus genomic RNAs are translated and subjected to nonsense-mediated mRNA decay before packaging. J. Virol. 2004;78:5139–5146. doi: 10.1128/JVI.78.10.5139-5146.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang J, Gudikote JP, Olivas OR, Wilkinson MF. Boundary-independent polar nonsense-mediated decay. EMBO Rep. 2002;3:274–279. doi: 10.1093/embo-reports/kvf036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Johns L, Grimson A, Kuchma SL, Newman CL, Anderson P. Caenorhabditis elegans SMG-2 selectively marks mRNAs containing premature translation termination codons. Mol. Cell. Biol. 2007;27:5630–5638. doi: 10.1128/MCB.00410-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Peltz SW, Brown AH, Jacobson A. mRNA destabilization triggered by premature translational termination depends on at least three cis-acting sequence elements and one trans-acting factor. Genes Dev. 1993;7:1737–1754. doi: 10.1101/gad.7.9.1737. [DOI] [PubMed] [Google Scholar]
- 118.Hagan KW, Ruiz-Echevarria MJ, Quan Y, Peltz SW. Characterization of cis-acting sequences and decay intermediates involved in nonsense-mediated mRNA turnover. Mol. Cell. Biol. 1995;15:809–823. doi: 10.1128/mcb.15.2.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eberle AB, Stalder L, Mathys H, Orozco RZ, Muhlemann O. Posttranscriptional gene regulation by spatial rearrangement of the 3' untranslated region. PLoS Biol. 2008;6:e92. doi: 10.1371/journal.pbio.0060092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Longman D, Plasterk RH, Johnstone IL, Caceres JF. Mechanistic insights and identification of two novel factors in the C. elegans NMD pathway. Genes Dev. 2007;21:1075–1085. doi: 10.1101/gad.417707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pulak R, Anderson P. mRNA surveillance by the Caenorhabditis elegans smg genes. Genes Dev. 1993;7:1885–1897. doi: 10.1101/gad.7.10.1885. [DOI] [PubMed] [Google Scholar]
- 122.Das B, Guo Z, Russo P, Chartrand P, Sherman F. The role of nuclear cap binding protein Cbc1p of yeast in mRNA termination and degradation. Mol. Cell. Biol. 2000;20:2827–2838. doi: 10.1128/mcb.20.8.2827-2838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Muhlrad D, Parker R. Recognition of yeast mRNAs as "nonsense containing" leads to both inhibition of mRNA translation and mRNA degradation: implications for the control of mRNA decapping. Mol. Biol. Cell. 1999;10:3971–3978. doi: 10.1091/mbc.10.11.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Behm-Ansmant I, Gatfield D, Rehwinkel J, Hilgers V, Izaurralde E. A conserved role for cytoplasmic poly(A)-binding protein 1 (PABPC1) in nonsense-mediated mRNA decay. EMBO J. 2007;26:1591–1601. doi: 10.1038/sj.emboj.7601588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kertesz S, et al. Both introns and long 3'-UTRs operate as cis-acting elements to trigger nonsense-mediated decay in plants. Nucleic Acids Res. 2006;34:6147–6157. doi: 10.1093/nar/gkl737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kerenyi Z, et al. Inter-kingdom conservation of mechanism of nonsense-mediated mRNA decay. EMBO J. 2008;27:1585–1595. doi: 10.1038/emboj.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kebaara BW, Atkin AL. Long 3'-UTRs target wild-type mRNAs for nonsense-mediated mRNA decay in Saccharomyces cerevisiae. Nucleic Acids Res. 2009;37:2771–2778. doi: 10.1093/nar/gkp146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Deliz-Aguirre R, Atkin AL, Kebaara BW. Copper tolerance of Saccharomyces cerevisiae nonsense-mediated mRNA decay mutants. Curr. Genet. 2011;57:421–430. doi: 10.1007/s00294-011-0356-0. [DOI] [PubMed] [Google Scholar]
- 129.Buhler M, Steiner S, Mohn F, Paillusson A, Muhlemann O. EJC-independent degradation of nonsense immunoglobulin μ mRNA depends on 3'-UTR length. Nature Struct. Mol. Biol. 2006;13:462–464. doi: 10.1038/nsmb1081. [DOI] [PubMed] [Google Scholar]
- 130.Amrani N, et al. Aberrant termination triggers nonsense-mediated mRNA decay. Biochem. Soc. Trans. 2006;34:39–42. doi: 10.1042/BST20060039. part 1. [DOI] [PubMed] [Google Scholar]
- 131.Meaux S, van Hoof A, Baker KE. Nonsense-mediated mRNA decay in yeast does not require PAB1 or a poly(A) tail. Mol. Cell. 2008;29:134–140. doi: 10.1016/j.molcel.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kervestin S, Li C, Buckingham R, Jacobson A. Testing the faux-UTR model for NMD: Analysis of Upf1p and Pab1p competition for binding to eRF3/Sup35p. Biochimie. 2012;94:1560–1571. doi: 10.1016/j.biochi.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hogg JR, Goff SP. Upf1 senses 3'UTR length to potentiate mRNA decay. Cell. 2010;143:379–389. doi: 10.1016/j.cell.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wiegand HL, Lu S, Cullen BR. Exon junction complexes mediate the enhancing effect of splicing on mRNA expression. Proc. Natl. Acad. Sci. USA. 2003;100:11327–11332. doi: 10.1073/pnas.1934877100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nott A, Le Hir H, Moore MJ. Splicing enhances translation in mammalian cells: an additional function of the exon junction complex. Genes Dev. 2004;18:210–222. doi: 10.1101/gad.1163204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gudikote JP, Imam JS, Garcia RF, Wilkinson MF. RNA splicing promotes translation and RNA surveillance. Nature Struct. Mol. Biol. 2005;12:801–819. doi: 10.1038/nsmb980. [DOI] [PubMed] [Google Scholar]
- References 135 and 136 demonstrate that components of the exon junction complex have important roles in the determination of mRNA translational efficiency.
- 137.Sheth U, Parker R. Targeting of aberrant mRNAs to cytoplasmic processing bodies. Cell. 2006;125:1095–1109. doi: 10.1016/j.cell.2006.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Stalder L, Muhlemann O. Processing bodies are not required for mammalian nonsense-mediated mRNA decay. RNA. 2009;15:1265–1273. doi: 10.1261/rna.1672509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kuroha K, Tatematsu T, Inada T. Upf1 stimulates degradation of the product derived from aberrant messenger RNA containing a specific nonsense mutation by the proteasome. EMBO Rep. 2009;10:1265–1271. doi: 10.1038/embor.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- References 139 and 156 reveal that Upf1 targets the polypeptide resulting from premature termination for degradation by the proteasome.
- 140.Lejeune F, Li X, Maquat LE. Nonsense-mediated mRNA decay in mammalian cells involves decapping, deadenylating, and exonucleolytic activities. Mol Cell. 2003;12:675–687. doi: 10.1016/s1097-2765(03)00349-6. [DOI] [PubMed] [Google Scholar]
- 141.Mitchell P, Tollervey D. An NMD pathway in yeast involving accelerated deadenylation and exosome-mediated 3'-->5' degradation. Mol. Cell. 2003;11:1405–1413. doi: 10.1016/s1097-2765(03)00190-4. [DOI] [PubMed] [Google Scholar]
- 142.Cao D, Parker R. Computational modeling and experimental analysis of nonsense-mediated decay in yeast. Cell. 2003;113:533–545. doi: 10.1016/s0092-8674(03)00353-2. [DOI] [PubMed] [Google Scholar]
- 143.Lykke-Andersen J. Identification of a human decapping complex associated with hUpf proteins in nonsense-mediated decay. Mol. Cell. Biol. 2002;22:8114–8121. doi: 10.1128/MCB.22.23.8114-8121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cho H, Kim KM, Kim YK. Human proline-rich nuclear receptor coregulatory protein 2 mediates an interaction between mRNA surveillance machinery and decapping complex. Mol. Cell. 2009;33:75–86. doi: 10.1016/j.molcel.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 145.Swisher KD, Parker R. Interactions between Upf1 and the decapping factors Edc3 and Pat1 in Saccharomyces cerevisiae. PLoS One. 2011;6:e26547. doi: 10.1371/journal.pone.0026547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Peltz SW, He F, Welch E, Jacobson A. Nonsense-mediated mRNA decay in yeast. Prog. Nucleic Acid Res. Mol. Biol. 1994;47:271–298. doi: 10.1016/s0079-6603(08)60254-8. [DOI] [PubMed] [Google Scholar]
- 147.Eberle AB, Lykke-Andersen S, Muhlemann O, Jensen TH. SMG6 promotes endonucleolytic cleavage of nonsense mRNA in human cells. Nat. Struct. Mol. Biol. 2009;16:49–55. doi: 10.1038/nsmb.1530. [DOI] [PubMed] [Google Scholar]
- 148.Huntzinger E, Kashima I, Fauser M, Sauliere J, Izaurralde E. SMG6 is the catalytic endonuclease that cleaves mRNAs containing nonsense codons in metazoan. RNA. 2008;14:2609–2617. doi: 10.1261/rna.1386208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- References 147 and 148 demonstrate that Smg-6 is the endonucleolytic enzyme in human and Drosophila NMD.
- 149.Gatfield D, Izaurralde E. Nonsense-mediated messenger RNA decay is initiated by endonucleolytic cleavage in Drosophila. Nature. 2004;429:575–578. doi: 10.1038/nature02559. [DOI] [PubMed] [Google Scholar]
- This paper demonstrates that NMD in Drosophila is initiated by endonucleolytic cleavage. This is the first demonstration that NMD could trigger endonucleolytic cleavage of the target mRNA.
- 150.Fukuhara N, et al. SMG7 is a 14-3-3-like adaptor in the nonsense-mediated mRNA decay pathway. Mol. Cell. 2005;17:537–547. doi: 10.1016/j.molcel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 151.Unterholzner L, Izaurralde E. SMG7 acts as a molecular link between mRNA surveillance and mRNA decay. Mol. Cell. 2004;16:587–596. doi: 10.1016/j.molcel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 152.Glavan F, Behm-Ansmant I, Izaurralde E, Conti E. Structures of the PIN domains of SMG6 and SMG5 reveal a nuclease within the mRNA surveillance complex. EMBO J. 2006;25:5117–5125. doi: 10.1038/sj.emboj.7601377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Muhlemann O, Lykke-Andersen J. How and where are nonsense mRNAs degraded in mammalian cells? RNA Biol. 2010;7:28–32. doi: 10.4161/rna.7.1.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gatfield D, Unterholzner L, Ciccarelli FD, Bork P, Izaurralde E. Nonsense-mediated mRNA decay in Drosophila: at the intersection of the yeast and mammalian pathways. EMBO J. 2003;22:3960–3970. doi: 10.1093/emboj/cdg371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Frizzell KA, Rynearson SG, Metzstein MM. Drosophila mutants show NMD pathway activity is reduced, but not eliminated, in the absence of Smg6. RNA. 2012;18:1475–1486. doi: 10.1261/rna.032821.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Takahashi S, et al. Upf1 potentially serves as a RING-related E3 ubiquitin ligase via its association with Upf3 in yeast. RNA. 2008;14:1950–1958. doi: 10.1261/rna.536308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ito-Harashima S, Kuroha K, Tatematsu T, Inada T. Translation of the poly(A) tail plays crucial roles in nonstop mRNA surveillance via translation repression and protein destabilization by proteasome in yeast. Genes Dev. 2007;21:519–524. doi: 10.1101/gad.1490207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Moore SD, Sauer RT. The tmRNA system for translational surveillance and ribosome rescue. Ann. Rev. Biochem. 2007;76:101–124. doi: 10.1146/annurev.biochem.75.103004.142733. [DOI] [PubMed] [Google Scholar]
- 159.Muhlemann O, et al. Precursor RNAs harboring nonsense codons accumulate near the site of transcription. Mol. Cell. 2001;8:33–43. doi: 10.1016/s1097-2765(01)00288-x. [DOI] [PubMed] [Google Scholar]
- 160.de Turris V, Nicholson P, Orozco RZ, Singer RH, Muhlemann O. Cotranscriptional effect of a premature termination codon revealed by live-cell imaging. RNA. 2011;17:2094–2107. doi: 10.1261/rna.02918111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- This paper utilizes an in situ hybridization approach to show that PTC+ transcripts are specifically retained at the site of transcription.
- 161.Buhler M, Mohn F, Stalder L, Muhlemann O. Transcriptional silencing of nonsense codon-containing immunoglobulin minigenes. Mol. Cell. 2005;18:307–317. doi: 10.1016/j.molcel.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 162.Hilleren P, McCarthy T, Rosbash M, Parker R, Jensen TH. Quality control of mRNA 3'-end processing is linked to the nuclear exosome. Nature. 2001;413:538–542. doi: 10.1038/35097110. [DOI] [PubMed] [Google Scholar]
- 163.Assenholt J, et al. Implication of Ccr4-Not complex function in mRNA quality control in Saccharomyces cerevisiae. RNA. 2011;17:1788–1794. doi: 10.1261/rna.2919911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen CY, Shyu AB. Rapid deadenylation triggered by a nonsense codon precedes decay of the RNA body in a mammalian cytoplasmic nonsense-mediated decay pathway. Mol. Cell. Biol. 2003;23:4805–4813. doi: 10.1128/MCB.23.14.4805-4813.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Trcek T, Larson DR, Moldon A, Query CC, Singer RH. Single-molecule mRNA decay measurements reveal promoter- regulated mRNA stability in yeast. Cell. 2011;147:1484–1497. doi: 10.1016/j.cell.2011.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Harel-Sharvit L, et al. RNA polymerase II subunits link transcription and mRNA decay to translation. Cell. 2010;143:552–563. doi: 10.1016/j.cell.2010.10.033. [DOI] [PubMed] [Google Scholar]
- 167.Bregman A, et al. Promoter elements regulate cytoplasmic mRNA decay. Cell. 2011;147:1473–1483. doi: 10.1016/j.cell.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 168.Keeling KM, Bedwell DM. Suppression of nonsense mutations as a therapeutic approach to treat genetic diseases. Wiley Interdiscip. Rev. RNA. 2011;2:837–852. doi: 10.1002/wrna.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mitrovich QM, Anderson P. Unproductively spliced ribosomal protein mRNAs are natural targets of mRNA surveillance in C. elegans. Genes Dev. 2000;14:2173–2184. doi: 10.1101/gad.819900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Marquardt S, Hazelbaker DZ, Buratowski S. Distinct RNA degradation pathways and 3' extensions of yeast non-coding RNA species. Transcription. 2011;2:145–154. doi: 10.4161/trns.2.3.16298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Thompson DM, Parker R. Cytoplasmic decay of intergenic transcripts in Saccharomyces cerevisiae. Mol. Cell. Biol. 2007;27:92–101. doi: 10.1128/MCB.01023-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ohnishi T, et al. Phosphorylation of hUPF1 induces formation of mRNA surveillance complexes containing hSMG-5 and hSMG-7. Mol. Cell. 2003;12:1187–1200. doi: 10.1016/s1097-2765(03)00443-x. [DOI] [PubMed] [Google Scholar]
- 173.Grimson A, O'Connor S, Newman CL, Anderson P. SMG-1 is a phosphatidylinositol kinase-related protein kinase required for nonsense-mediated mRNA Decay in Caenorhabditis elegans. Mol. Cell. Biol. 2004;24:7483–7490. doi: 10.1128/MCB.24.17.7483-7490.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pal M, Ishigaki Y, Nagy E, Maquat LE. Evidence that phosphorylation of human Upf1 protein varies with intracellular location and is mediated by a wortmannin-sensitive and rapamycin-sensitive PI 3-kinase-related kinase signaling pathway. RNA. 2001;7:5–15. doi: 10.1017/s1355838201000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Page MF, Carr B, Anders KR, Grimson A. Anderson, P. SMG-2 is a phosphorylated protein required for mRNA surveillance in Caenorhabditis elegans and related to Upf1p of yeast. Mol. Cell. Biol. 1999;19:5943–5951. doi: 10.1128/mcb.19.9.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Denning G, Jamieson L, Maquat LE, Thompson EA, Fields AP. Cloning of a novel phosphatidylinositol kinase-related kinase: characterization of the human SMG-1 RNA surveillance protein. J. Biol. Chem. 2001;276:22709–22714. doi: 10.1074/jbc.C100144200. [DOI] [PubMed] [Google Scholar]
- 177.Arias-Palomo E, et al. The nonsense-mediated mRNA decay SMG-1 kinase is regulated by large-scale conformational changes controlled by SMG-8. Genes Dev. 2011;25:153–164. doi: 10.1101/gad.606911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Anders KR, Grimson A, Anderson P. SMG-5, required for C.elegans nonsense-mediated mRNA decay, associates with SMG-2 and protein phosphatase 2A. EMBO J. 2003;22:641–650. doi: 10.1093/emboj/cdg056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cali BM, Kuchma SL, Latham J, Anderson P. smg-7 is required for mRNA surveillance in Caenorhabditis elegans. Genetics. 1999;151:605–616. doi: 10.1093/genetics/151.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chiu SY, Serin G, Ohara O, Maquat LE. Characterization of human Smg5/7a: a protein with similarities to Caenorhabditis elegans SMG5 and SMG7 that functions in the dephosphorylation of Upf1. RNA. 2003;9:77–87. doi: 10.1261/rna.2137903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Andersen CB, et al. Structure of the exon junction core complex with a trapped DEAD-box ATPase bound to RNA. Science. 2006;313:1968–1972. doi: 10.1126/science.1131981. [DOI] [PubMed] [Google Scholar]
- 182.Bono F, Ebert J, Lorentzen E, Conti E. The crystal structure of the exon junction complex reveals how it maintains a stable grip on mRNA. Cell. 2006;126:713–725. doi: 10.1016/j.cell.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 183.Tange TO, Nott A, Moore MJ. The ever-increasing complexities of the exon junction complex. Curr. Opin. Cell Biol. 2004;16:279–284. doi: 10.1016/j.ceb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 184.Isken O, et al. Upf1 phosphorylation triggers translational repression during nonsense-mediated mRNA decay. Cell. 2008;133:314–327. doi: 10.1016/j.cell.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Eulalio A, Behm-Ansmant I, Izaurralde E. P bodies: at the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell. Biol. 2007;8:9–22. doi: 10.1038/nrm2080. [DOI] [PubMed] [Google Scholar]
- 186.Parker R, Sheth U. P bodies and the control of mRNA translation and degradation. Mol. Cell. 2007;25:635–646. doi: 10.1016/j.molcel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 187.Hu W, Sweet TJ, Chamnongpol S, Baker KE, Coller J. Co-translational mRNA decay in Saccharomyces cerevisiae. Nature. 2009;461:225–229. doi: 10.1038/nature08265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Decker CJ, Teixeira D, Parker R. Edc3p and a glutamine/asparagine-rich domain of Lsm4p function in processing body assembly in Saccharomyces cerevisiae. J. Cell. Biol. 2007;179:437–449. doi: 10.1083/jcb.200704147. [DOI] [PMC free article] [PubMed] [Google Scholar]