Abstract
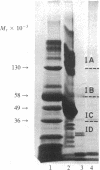
Human myelogeneous leukemia cells in liquid culture can be induced to mature along the monocyte/macrophage pathway by a maturation inducer derived from the conditioned medium of activated human T lymphocytes. Serum-free conditioned medium was used for the isolation of the T-cell lymphokine. The maturation inducer was purified approximately equal to 6000-fold by ammonium sulfate precipitation, low-salt elution from DEAE-Sepharose CL-6B, gel filtration on Bio-Gel A-0.5m, and NaDodSO4/PAGE under nonreducing conditions. The molecular weight of the maturation inducer was 36,000-58,000 on NaDodSO4/PAGE. Terminal differentiation associated with inhibition of leukemia cell proliferation and expression of mature cell properties was observed with the isolated maturation inducer, identical to the activity observed with the unfractionated conditioned medium. Cell-cycle analysis revealed that the proportion of replicating S-phase cells was reduced from 40% to 7% after initial interaction of the maturation inducer with cells. The differentiating cells simultaneously acquired monocyte antigen, membrane complement receptors, phagocytic function, and monocyte/macrophage morphology. The maturation-inducing activity is dose-dependent, with more inducer causing the development of more mature cells in a shorter time period. The maturation inducer was shown to be stable after pH 2 treatment, independent of interleukin 2 and colony-stimulating factor, devoid of alpha-, beta-, and gamma-interferon, and not affected by antibody to interferon. The maturation inducer may play a role as a physiological regulator of monocytic and leukemia cell development.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chiao J. W., Andreeff M., Freitag W. B., Arlin Z. Induction of in vitro proliferation and maturation of human aneuploid myelogenous leukemic cells. J Exp Med. 1982 May 1;155(5):1357–1369. doi: 10.1084/jem.155.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiao J. W., Freitag W. F., Steinmetz J. C., Andreeff M. Changes of cellular markers during differentiation of HL-60 promyelocytes to macrophages as induced by T lymphocyte conditioned medium. Leuk Res. 1981;5(6):477–489. doi: 10.1016/0145-2126(81)90118-1. [DOI] [PubMed] [Google Scholar]
- Chiao J. W., Pahwa R. N., Good R. A. Human T lymphocytes and myeloid colony forming cells share common antigen. Exp Hematol. 1980 Jan;8(1):6–15. [PubMed] [Google Scholar]
- Chiao J. W., Wang C. Y. Differentiation antigens of HL-60 promyelocytes during induced maturation. Cancer Res. 1984 Mar;44(3):1031–1033. [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Elias L., Wogenrich F. J., Wallace J. M., Longmire J. Altered pattern of differentiation and proliferation of HL-60 promyelocytic leukemia cells in the presence of leucocyte conditioned medium. Leuk Res. 1980;4(3):301–307. doi: 10.1016/0145-2126(80)90037-5. [DOI] [PubMed] [Google Scholar]
- Hozumi M. Fundamentals of chemotherapy of myeloid leukemia by induction of leukemia cell differentiation. Adv Cancer Res. 1983;38:121–169. doi: 10.1016/s0065-230x(08)60189-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Regulation of normal differentiation in mouse and human myeloid leukemic cells by phorbol esters and the mechanism of tumor promotion. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5158–5162. doi: 10.1073/pnas.76.10.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson I. L., Sarngadharan M. G., Breitman T. R., Gallo R. C. Isolation and characterization of a T lymphocyte-derived differentiation inducing factor for the myeloid leukemic cell line HL-60. Blood. 1984 Mar;63(3):510–517. [PubMed] [Google Scholar]
- Olsson I., Olofsson T., Mauritzon N. Characterization of mononuclear blood cell-derived differentiation inducing factors for the human promyelocytic leukemia cell line HL-60. J Natl Cancer Inst. 1981 Dec;67(6):1225–1230. [PubMed] [Google Scholar]
- Perussia B., Dayton E. T., Fanning V., Thiagarajan P., Hoxie J., Trinchieri G. Immune interferon and leukocyte-conditioned medium induce normal and leukemic myeloid cells to differentiate along the monocytic pathway. J Exp Med. 1983 Dec 1;158(6):2058–2080. doi: 10.1084/jem.158.6.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike B. L., Robinson W. A. Human bone marrow colony growth in agar-gel. J Cell Physiol. 1970 Aug;76(1):77–84. doi: 10.1002/jcp.1040760111. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Tomida M., Hozumi M. Production by mouse spleen cells of factors stimulating differentiation of mouse myeloid leukemic cells that differ from the colony-stimulating factor. Cancer Res. 1980 Dec;40(12):4804–4809. [PubMed] [Google Scholar]
- Yen A., Chiao J. W. Control of cell differentiation during proliferation. I. Monocytic differentiation of HL-60 promyelocytes. Exp Cell Res. 1983 Jun;146(1):87–93. doi: 10.1016/0014-4827(83)90327-0. [DOI] [PubMed] [Google Scholar]