Abstract
Ethanol is a known neuromodulatory agent with reported actions at a range of neurotransmitter receptors. Here, we used an indirect approach, measuring the effect of alcohol on metabolism of [3-13C]pyruvate in the adult Guinea pig brain cortical tissue slice and comparing the outcomes to those from a library of ligands active in the GABAergic system as well as studying the metabolic fate of [1,2-13C]ethanol. Ethanol (10, 30 and 60 mM) significantly reduced metabolic flux into all measured isotopomers and reduced all metabolic pool sizes. The metabolic profiles of these three concentrations of ethanol were similar and clustered with that of the α4β3δ positive allosteric modulator DS2 (4-Chloro-N-[2-(2-thienyl)imidazo[1,2a]-pyridin-3-yl]benzamide). Ethanol at a very low concentration (0.1 mM) produced a metabolic profile which clustered with those from inhibitors of GABA uptake, and ligands showing affinity for α5, and to a lesser extent, α1-containing GABA(A)R. There was no measureable metabolism of [1,2-13C]ethanol with no significant incorporation of 13C from [1,2-13C]ethanol into any measured metabolite above natural abundance, although there were measurable effects on total metabolite sizes similar to those seen with unlabeled ethanol.
The reduction in metabolism seen in the presence of ethanol is therefore likely to be due to its actions at neurotransmitter receptors, particularly α4β3δ receptors, and not because ethanol is substituting as a substrate or because of the effects of ethanol catabolites acetaldehyde or acetate. We suggest that the stimulatory effects of very low concentrations of ethanol are due to release of GABA via GAT1 and the subsequent interaction of this GABA with local α5-containing, and to a lesser extent, α1-containing GABA(A)R.
Keywords: alcohol, metabonomics, NMR spectroscopy, 13C metabolism, GABA(A)
Introduction
Ethanol, one of the most commonly used (and abused) drugs in the Western world, is a known sedative and depressant acting on a wide range of targets including several neurotransmitter receptors and ion channels in the central nervous system. Physicochemical characteristics of ethanol molecule assure that it readily passes blood brain barrier (Gratton et al. 1997) and, despite earlier conflicting data (for a review see (Phillips 1981)), recent evidence favours the view that ethanol can actually damage and/or selectively lower blood brain barrier via specific mechanisms (Ehrlich et al. 2012, Muneer et al. 2011).
Until about two or three decades ago, most of the attempts to explain pharmacological actions of ethanol were based on interactions between ethanol and the lipid components of biological membranes presumably resulting in non-specific alterations of membrane fluidity (Spanagel 2009). Such explanations were, however, untenable because the membrane lipids are not significantly perturbed until concentrations of ethanol reach levels about one to two orders of magnitude greater than those encountered during mild to medium alcohol intoxication in human subjects (Spanagel 2009). Consequently, the membrane lipid theory of ethanol actions might perhaps help to explain the lethality of very high doses of alcohol (leading to concentrations ≫100 mM in situ) but cannot account for the variety of specific effects of alcohol on brain and behaviour at concentrations resulting from common alcohol self-administration (typically 5 to 40 mM). More recent studies (for a review see (Spanagel 2009)), though not yet identifying any specific “ethanol receptors”, point to several potential targets, mainly neurotransmitter receptors and ion channels, which could selectively respond to low and medium doses of alcohol (review Hodge 2006).
Molecules sensitive to low concentrations of ethanol include glycine receptors (Perkins et al. 2010, Engblom & Akerman 1991), NMDA receptors (Allgaier 2002, Lovinger et al. 1990), L-type Ca2+-channels and G-protein coupled inwardly-rectifying potassium channels (GIRK; functionally altered by as low as 1 mM ethanol) (Lewohl et al. 1999, Ikeda et al. 2002).
GABA-A receptors have also been considered as potential ethanol targets. Interestingly, the most abundant synaptic GABA-A receptors consisting mainly from α1, β2 and γ2 subunits are practically non-responsive to ethanol (Mori et al. 2000) while those containing α4β3δ (and α6 in cerebellum) and thought to be located mostly extrasynaptically, are about as ethanol-sensitive as NMDA receptors (except for being activated rather than inhibited by ethanol; (Wallner et al. 2006); see also (Kaur et al. 2009, Lovinger & Homanics 2007)). The GABAergic inhibitory system can also be influenced by ethanol via additional mechanisms such as potentiation of GABA release at GABAergic synapses (Roberto et al. 2004, Roberto et al. 2003).
Ethanol has dramatic effects on brain energy metabolism, particularly in terms of D-glucose utilization. Ethanol reduces D-glucose uptake and metabolism (Pawlosky et al. 2010, Volkow et al. 2006) and increases the metabolism of acetate (Wolkow 2013).
We use a cortical tissue slice system in vitro where metabolism of [3-13C]pyruvate is used as a marker of drug effects by measuring resultant isotopomer and total metabolite pools following a period of incubation both with and without the drug (Nasrallah et al. 2010b, Rae et al. 2009). This approach is particularly suitable for investigating specific effects of alcohol on brain tissue. It circumvents the possible confounding involvement of blood brain barrier as mentioned above (there is neither blood brain barrier nor blood circulation in our model) and eliminates actions of ethanol metabolites as alcohol is not metabolised by brain to any significant extent (Mukherji et al. 1975, Xiang & Shen 2011). The resulting metabolic profiles were then compared with our extensive database describing effects, respectively, of various neurotransmitter (GABA) concentrations and activators/inhibitors of particular GABA receptors or transporters by specific drugs. This approach has been used successfully in the past to identify possible sites of action for the party drug γ-hydroxybutyrate (Nasrallah et al. 2010b), sites which were subsequently confirmed by others (Absalom et al. 2012). Here, we have explored the effects of a range of ethanol concentrations (0.1 ≥ ≤ 60 mM) on brain metabolism in vitro.
We have also taken advantage of the brain slice as a model of brain metabolism free of peripheral interference to examine the issue of whether or not ethanol itself is metabolized in the brain by studying potential incorporation of 13C from [1,2-13C]ethanol by brain cortical tissue slices.
Methods
Materials
Female Guinea pigs (Dunkin-Hartley), weighing 400–800 g, were fed ad libitum on standard Guinea pig/rabbit pellets, with fresh carrots and lucerne hay roughage. Animals were maintained on a 12 h light/dark cycle. All experiments were conducted in accordance with the guidelines of the National Health and Medical Research Council of Australia and were approved by the institutional (UNSW) Animal Care Ethics Committee.
Sodium [3-13C]pyruvate, sodium [13C]formate and [1,2-13C]ethanol were purchased from Cambridge Isotope Laboratories Inc (Andover, MA, USA). 4-Chloro-N-[2-(2-thienyl)imidazo[1,2a]-pyridin-3-yl]benzamide (DS2, positive allosteric modulator of δ subunit-containing GABA(A) receptors (Wafford et al. 2009)), (R)-1-(1-Phenylethyl)-1H-imidazole-5-carboxylic acid ethyl ester (etomidate; interacts with β2 and β3-containing subunits of GABA(A) receptors (Sanna et al. 1997, Uchida et al. 1995)), 8-Azido-5,6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a][1,4]benzodiazepine-3-carboxylic acid ethyl ester (RO-15-4513, highly active benzodiazepine ligand, antagonises effects of ethanol (Harris & Lal 1988)) and 5,6-Dihydro-5-methyl-6-oxo-4H-imidazo[1,5a]thieno[2,3-f][1,4]diazepine-3-carboxylic acid 1,1-dimethylethyl ester (RO 19-4603; benzodiazepine inverse agonist, antagonises locomotor effects of ethanol (Suzdak et al. 1988) were purchased from Tocris Cookson (Bristol, UK). 7-Ethynyl-1-methyl-5-phenyl-1,3-dihydro-2H-1,4-benzodiazepin-2-one (QH-ii-066 ; α5-selective agonist (Huang et al. 2000)) was custom synthesised as described previously (Huang et al. 1996). Ethanol (HPLC Grade) was obtained from Merck (Merck Australia, Kilsyth Vic, Australia).
Modulation of metabolic activity by ethanol and related ligands
Guinea pig cortical slices were made and prepared as described previously (Nasrallah et al. 2010b). To determine the metabolic effects of modulation of metabolism by ethanol, slices were incubated for 1 h with 2 mmol/L sodium [3-13C]pyruvate (control) and a range of concentrations of ethanol: 0.1, 1.0, 10, 30.0, 60.0 and 100 mmol/L.
We studied whether ethanol itself was used as a substrate by slices by incubating slices for 1 h with 2 mM sodium pyruvate (control) and 1.0 and 10 mmol/L [1,2-13C]ethanol.
We also studied the effects of various ethanol-related ligands by incubating slices with 2 mmol/L sodium [3-13C]pyruvate (control) and 1.0 and 10 nmol/L RO 19-4603, 0.1 and 1.0 nmol/L RO 15-4516, 2 and 20 μmol/L etomidate, 0.1 and 1.0 μmol/L DS2 or 4 and 40 nmol/L QH-ii-066.
The number of samples was N = 4 in all cases.
Preparation of samples and NMR analysis
On completion of the incubation period, slices were removed from the incubation buffer by rapid filtration and extracted in methanol/chloroform according to the method of Le Belle (Le Belle et al. 2002). Extracts were lyophilized, and the pellet retained for protein estimation by the Lowry technique. Lyophilized supernatants were stored at −20 °C until required for acquisition of NMR spectra. This was conducted as described previously and included acquisition of fully-relaxed 1H, 1H{13C-decoupled} and 13C{1H-decoupled spectra. {Nasrallah, 2010 #2593}. In the case of the experiments with [1,2-13C]ethanol and QH-ii-066, spectra were acquired on a Bruker AVANCE III HD 600 spectrometer fitted with a cryoprobe (TCI) and refrigerated sample changer. 1H{13C-decoupled} spectra were acquired using bilev composite pulse decoupling across an effective bandwidth of 48000 Hz during the acquisition time, on a 30s duty cycle, while 13C{1H-decoupled} spectra were acquired on a 4 s duty cycle using continuous WALTZ-65 decoupling.
Experimental data (N = 4) are given as means (standard deviation). Statistical analysis was done using ANOVA for comparing ligand-treated metabolism at each receptor with control (N = 4, followed, only where statistical significance was indicated by Scheffe F-test, by a nonparametric (Mann-Whitney U) test (Statview Student)). The statistical analysis was performed on the raw experimental values, not the relative differences as shown in the graph. Significance was assumed at α = 0.05. Data are shown graphically as change in each variable relative to the mean of that variable in the control experiment in order more clearly to show the metabolic “pattern” generated by the ligand relative to control.
Pattern recognition of the data
Multivariate statistical analysis used the Simca-P+ software package (v11.5, Umetrics, Umeå, Sweden). Each dataset for a particular manipulation was imported as the relative change from the average value obtained from the control group for that particular experiment. Data were univariance scaled to standardize variance between the high and low concentration metabolites (Wold et al. 1998), to ensure that the 13C labeling and steady state pool size concentrations equally contributed to the model.
Here, we included the data from experiments acquired under identical conditions using a range of ligands at GABA(A)R (Rae et al. 2009), GABA(B)R (Nasrallah et al. 2007) and GABA(C)R (Nasrallah et al. 2010b), inhibitors of GABA uptake (GAT inhibitors) (Nasrallah et al. 2010a), exogenous GABA (Nasrallah et al. 2009 ) and experiments where the GABA-transaminase inhibitor vigabatrin was also incubated with activators of slice activity (Nasrallah et al. 2011) into SIMCA P+ along with data acquired in this work. The data were then subject to principal components analysis to identify common patterns of activation between ethanol and other compounds active in the GABAergic system.
Results
Effects of ethanol on metabolism of [3-13C]pyruvate
The metabolic profiles of the effects of each concentration of ethanol on metabolism are shown in Fig. 1. The figure shows the change in concentration for each variable relative to the control average for that variable. The statistically significant changes shown were calculated using the total values of metabolic flux or metabolic pools (i.e.”raw data” as opposed to the values of changes relative to controls). The different concentrations of ethanol produced different results, with the lower two concentrations (0.1 and 1.0 mM) producing profiles distinct from one another and distinct from those of 10, 30 and 60 mM ethanol, which were all broadly similar. The lowest concentration of ethanol (0.1 mM) produced a decrease in net flux into all measured isotopomers apart from Asp C2 and C3, a decrease in the pool size of lactate and Gln but produced significant increases in the total metabolite pools of Glu, GABA, Asp and Ala. Higher concentrations of ethanol produced significant decreases in metabolite pools and isotopomer net fluxes.
Figure 1. Relative effect of different concentrations of exogenous ethanol on net flux of 13C and on total metabolite pool sizes in brain cortical tissue slices incubated 1 h with sodium [3-13C]pyruvate.

Data are shown as relative to the control mean, with control metabolism centered at zero. Error bars represent standard deviations. Statistically significant changes (calculated on the raw data not the relative change in flux or pool size, see Methods) are indicated by * (P < 0.05, different to control).
Incubation of brain cortical tissue slices with sodium pyruvate and [1,2-13C]ethanol
No carbon label was detected in 1H spectra and no label was detected in 13C{1H-decoupled} spectra above natural abundance level. There was no evidence for double label (due to adjacent 13C nuclei) in any of the resonances. In addition, no [1,2-13C]ethanol was observed in any of the spectra. To determine whether this was because of loss of ethanol in the freeze drying process, we ran spectra of the extract prior to the freeze drying process and observed the expected peaks from [1,2-13C]ethanol. This extract was then subjected to freeze drying, resuspended in D2O and another 13C spectrum acquired. As expected, there was no resonance from [1,2-13C]ethanol (spectra supplied as supplementary data). From this we concluded that [1,2-13C]ethanol was not metabolized by brain cortical slices to any significant extent and that the residual ethanol was removed by lyophilisation.
Effects of related ligands
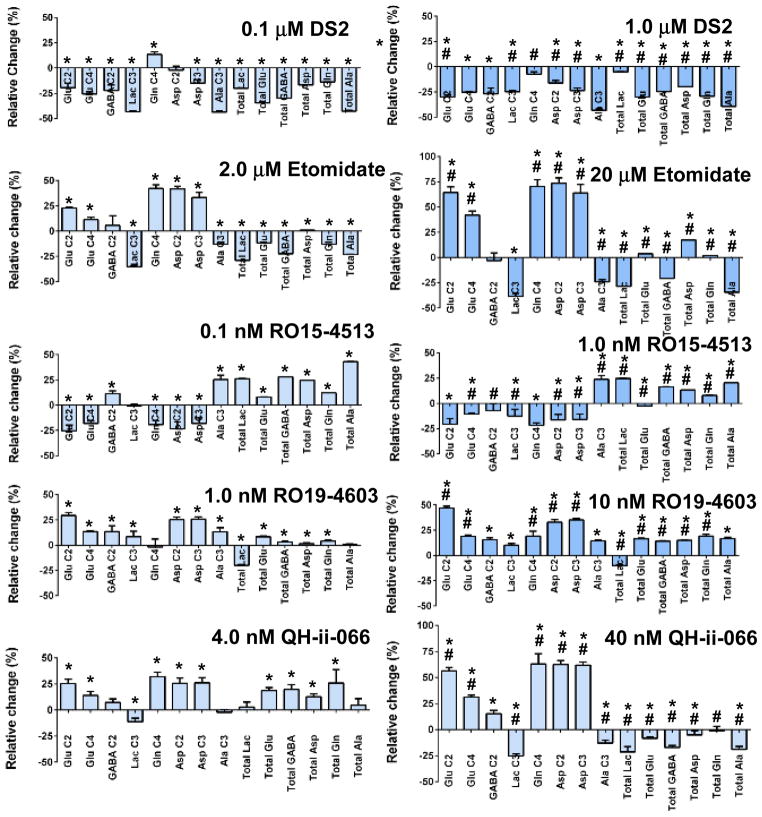
The metabolic profiles of the effects of DS2, a positive allosteric modulator of δ-subunit containing GABA(A) receptors, are shown in Fig. 2. Low (0.1 μM) concentrations of DS2 produced significant decreases in net flux into all isotopomers measured, apart from Gln C4 which increased and Asp C2 which was not changed. The total metabolite pool size of all measured metabolites was also decreased. When the concentration of DS2 was increased to 1.0 μM all net fluxes and metabolite pool sizes were decreased (Fig. 2).
Figure 2. Relative effect of ligands with relevance to ethanol on net flux of 13C and on total metabolite pool sizes in brain cortical tissue slices incubated 1 h with sodium [3-13C]pyruvate.
Data are shown as relative to the control mean, with control metabolism centered at zero. Error bars represent standard deviations. Statistically significant changes (calculated on the raw data not the relative change in flux or pool size, see Methods) are indicated by * (P < 0.05, different to control) or # (P < 0.05, different to other concentration of same ligand).
The β-subunit selective activator etomidate (2 μM) significantly increased net flux into all Krebs cycle related metabolites, had no effect on flux into GABA C2 and decreased net flux into glycolysis byproducts Lac C3 and Ala C3. All metabolite pools were significantly decreased. Increasing the concentration to 20 μM resulted in further significant increases in net flux into the isotopomers of Glu, Gln and Asp. The total metabolic pool of Asp was also significantly increased, as was Glu (Fig. 2).
RO15-4513 at 0.1 nM resulted in significant increases in the pool sizes of all metabolites measured, as well as increased in the net fluxes into Ala C3 and GABA C2. All other net fluxes measured were decreased apart from Lac C3 which was unaffected. Increasing the concentrations to 1.0 nM produced similar metabolite pool increases and increased net flux into Ala C3 but there was no change in GABA C2 in this case.
RO19-4603 ( 1.0 nM) strongly stimulated net flux into most isotopomers (Fig. 2), except for Gln C4, which was not affected. Increasing the concentration tenfold further increased net fluxes into many isotopomers and also increased net flux into Glu C4. Pool sizes, apart from that of lactate, which was decreased, were also increased (Fig. 2). This metabolic pattern is indicative of increased net flux into the Krebs cycle, with increased pyruvate clearance.
QH-ii-066 at 4 nmol/L had strongly stimulatory effects on net flux through the Krebs cycle with increased incorporation of 13C into Glu C2, C4, Asp C2 and C3, along with relative decreases in incorporation into Lactate C3. The incorporation of 13C into Gln C4 was also increased. The pool sizes of Glu, GABA, Asp and Gln were significantly increased. These changes in 13C incorporation were amplified with 40 nmol/L QH-ii-066, with increased labelling of GABA C2 and further decreases in labeling of Lactate C3 and Ala C3. The metabolic pools of lactate, Glu, GABA, Asp and Ala were significantly reduced compared to control (Fig. 2).
Principal components analysis of the data
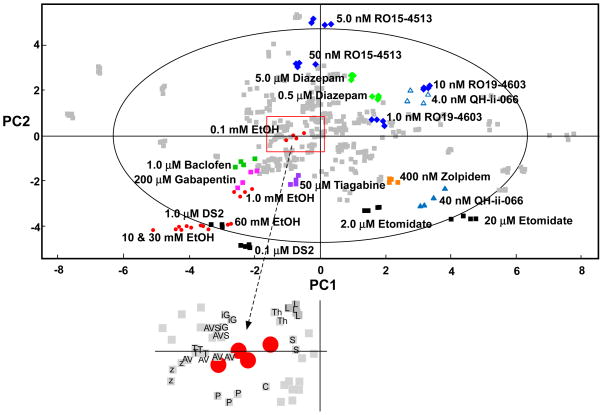
Principal components analysis of the data generated a three component model accounting for 82 % of the variance in the data (PC1 = 47%, PC2 = 26% and PC3 = 9%; Q2 = 70%, where Q2 is the fraction of the total variation in the data which can be explained by a component. A rule of thumb is that values of Q2 > 50% are considered a good fit (Eriksson et al. 2006). The first two (major) components of the model are shown in Fig. 3 with the concentrations of ethanol each shown in red. The high (10, 30 and 60 mM) concentrations of ethanol cluster near the bottom left hand corner of the plot along with 1.0 μM DS2. The 1.0 mM cluster of ethanol is weighted less heavily on PC1 and PC2 and lies in closer proximity to a cluster of compounds whose common factor is their activity of “mainstream” GABAergic synapses. The cluster includes 1.0 μM Baclofen (GABA(B)R agonist, green squares in Fig. 3), 200 μM Gabapentin (pink squares; (Sills 2006)) and 50 μM tiagabine (purple squares, inhibitor at GAT1 (Borden et al. 1994)). By contrast the 0.1 mM ethanol cluster (see inset to Fig. 3) is located closest to data derived from an experiment using both the GABA-T inhibitor vigabatrin (100 μM) and the glutamate receptor agonist 5 μM AMPA (AV). It is also near to 40 nM zolpidem (z), 10 μM isoguvacine (G), 10 μM SGS-742 (S), 5 μM picrotoxin (P), 0.1 nM L655–708 (L) and the GAT1 inhibitor CI966 (C) as well as an experiment where vigabatrin (100 μM) was incubated with AMPA (5 μM) and the GAT blocker SKF-89976A.
Figure 3. Principal components analysis of ethanol and ligand data shown against metabolic fingerprint data from selected ligands active at GABA(A), GABA(B) and GABA(C) receptors, GAT inhibitors and exogenous GABA.
These data generated a three component model accounting for 82% of the variance in the data (47, 26 and 9%, respectively), the major two components of which are shown in this diagram. Ethanol data are shown as red circles, DS2 and etomidate as black squares, RO15-4513 and RO19-4603 as blue diamonds, Gabapentin as pink squares, tiagabine as purple squares, diazepam as green diamonds, QH-ii-066 as blue triangles, Zolpidem as orange squares and baclofen as green squares. All other data are represented as grey squares. The large outer ellipse represents the 95% confidence interval (Hotelling score). The inset to the figure shows an enlargement of the area of the PCA plot immediately surrounding the low (0.1 mM) concentration of ethanol. Key T, 10 μM tiagabine; Th, 10 10 μM THIP; G, 10 μM isoguvacine; S, 10 μM SGS-742; AV, 100 μM vigabatrin with 5 μM AMPA; z, 40 nM zolpidem; C, CI966; L, L655–708; AVS 100 μM vigabatrin + 5 μM AMPA + 10 μM SKF-89976A; P, picrotoxin.
RO15-4513 at 0.1 nM clusters outside the Hotelling circle (x,y coordinates, ~ 0,5; Fig. 3) with the only other ligand in the vicinity (−0.5, 5) the ρ-subunit specific antagonist (+)-(S)-4-amino-1-cyclopent-1-enyl(butyl)phosphinic acid (Kumar et al. 2008). At this concentration RO15-4513 should be reasonable specific for benzodiazepine insensitive GABA(A)R; i.e. α4 and α6-containing GABA(A)R which also have a γ subunit. At 1.0 nM RO15-4513 clusters within the Hotelling circle (−0.5,3) with nearby ligands being the GABA(B)R agonist Baclofen (10 μM), the GABA(B)R antagonist phaclofen (100 μM) and the α5-specific inverse agonist L655–708.
The other ligand used as an alcohol antagonist, RO19-4603, clusters in the top right hand quadrant of the Hotelling circle (2, 1) at 1.0 nM (Fig. 3). Nearby ligands include GABA (1.0 μM), γ-hydroxybutyrate (1 μM) and the potent GABA(B)R agonist SKF-97541 (0.2 μM). Increasing the concentration of RO19-4603 to 10 nM shifts the cluster (3.5, 2) close to the α5-specific agonist QH-ii-066 (4 nmol/L).
Discussion
Ethanol and brain metabolism
Ethanol at all concentrations used from 0.1 to 60 mM had significant effects on brain metabolism. At concentrations >1.0 mM these effects were fairly uniform and resulted in decreased net flux into the Krebs cycle along with decreased net flux into the glycolytic byproducts lactate and alanine as well as decreased total metabolite pool sizes. Ethanol has previously been reported to decrease GABA (Gomez et al. 2012) and aspartate levels (Biller et al. 2009) when administered acutely but there is little data available at concentrations equivalent to the lowest ones used here (0.1 mM).
Decreased glucose metabolism in the brain in the presence of ethanol is a consistently reported finding in the literature (Volkow et al. 1990, Handa et al. 2000, Volkow et al. 2006). The question as to whether this is caused by substitution of glucose as a fuel source by ethanol has been dealt with by the finding that ethanol is not significantly metabolized in the brain (Mukherji et al. 1975, Xiang & Shen 2011) although uptake varies regionally (Li et al. 2012). In the brain cortical slice, there is no peripheral metabolism or blood brain barrier to complicate the analysis, showing that here, ethanol is unlikely to act as a metabolic fuel and the results are not influenced by significant levels of acetaldehyde or acetate produced from ethanol metabolism elsewhere in the body (Pawlosky et al. 2010). Our results suggest that ethanol at higher concentrations (10, 30 and 60 mM) is producing similar metabolic outcomes and is likely acting at α4β3δ-containing receptors. This conclusion is based on the fact that the metabolic profiles of these concentrations of ethanol cluster with that from 1.0 μM DS2 (Fig. 3), which is a positive allosteric modulator showing specificity for α4β3δ-containing receptors (Wafford et al. 2009). These concentrations of alcohol show the same weighting on PC2 as etomidate, which is specific for β-containing receptors, although it has slightly higher affinity for β3 than β4 and less specificity for δ vs γ (Sanna et al. 1997). This is in keeping with reports from other laboratories that α4β3δ receptors are sensitive to alcohol (Sundstrom-Poromaa et al. 2002, Wallner et al. 2003). These authors have reported activity at concentrations ≥ 3 mM but other authors have reported effects of alcohol at lower (1–3 mM) concentrations (Sundstrom-Poromaa et al. 2002). This difference in concentration has been explained as being due to the timecourse of exposure to ethanol such that higher concentrations were shown to have a larger effect when ethanol at lower concentrations was not preapplied (Smith & Gong 2007). This effect is also seen here where the concentrations of ethanol were applied for 40 min without pre-exposure; ethanol at concentrations of 10 mM and above showed strong clustering with the metabolic profile generated by DS2, but ethanol at 1 mM or less did not (Fig. 3).
To really nail the question as to whether ethanol is serving as a significant substrate in the brain, we performed the reverse experiment, supplying 2 mM pyruvate as substrate and studying the flux of label supplied as [1,2-13C]ethanol. No incorporation of 13C label into any metabolic intermediate was observed following a 1 h incubation, with no label observed in acetate above natural abundance levels and no detection of carbon-carbon coupling in any sample. While, as expected, ethanol was removed by the freeze-drying process, inspection of the aqueous phase of the extracted tissue revealed [1,2-13C]ethanol but no significant incorporation of label into any other metabolite. While we cannot rule out metabolism of ethanol at levels below the detection limit of NMR spectroscopy, we can categorically say that ethanol metabolism is not responsible for the significant decrease in label incorporation that is seen from [3-13C]pyruvate in the presence of unlabeled ethanol (Fig. 1).
It can therefore be concluded that the decreased metabolism seen previously in brains exposed to typical concentrations (5–40 mM) of ethanol is most likely due to effects at neurotransmitter receptors, particularly α4β3δ-containing GABA(A)R.
Ethanol effects at GABA receptors
Ethanol has long been known to have biphasic effects (Pohorecky 1977b) with excitatory (stimulatory) effects as well as “relaxing” effects reported at low concentrations. Indeed, the effects of low concentrations of alcohol have been suggested to underpin its pleasurable and addictive actions (Pohorecky 1977a, Learn et al. 2003). Ethanol at 0.1 mM produced a metabolic profile different to those produced by higher concentrations, with the main difference being increases in the total metabolite pools of Glu, GABA, Asp and Ala (Fig. 1). Total net flux was decreased compared to control, indicating that overall metabolism was still depressed, even with this low concentration of ethanol. The small increase in metabolic pool size, however, suggests that a metabolic pool was activated by alcohol.
The metabolic patterns generated by 0.1 mM ethanol clustered with those of a range of other ligands. There are two major classes of drugs in this cluster.
The cluster of related metabolic profiles includes those from the GABA transporter blockers CI-966 and tiagabine, experiments combining the GABA-T inhibitor vigabatrin (100 μM) with the glutamate receptor agonist AMPA (5 μM), both with and without the GAT1 channel blocker SKF89976A.
Drugs which are agonists at GABA(A)R including isoguvacine, THIP (4,5,6,7-Tetrahydroisoxazolo[5,4-c]pyridin-3-ol hydrochloride) and zolpidem plus inverse agonists or antagonists L655–708 and picrotoxin.
We have previously examined the effects of inhibition of GABA uptake on metabolism (Nasrallah et al. 2010a). In general, inhibition of the GAT1 transporters produces metabolic profiles which are not similar to those of ethanol, being located in the bottom right hand quadrant of the PCA plot shown in Fig. 3. However, a number of GAT inhibitors do show strong similarity to the metabolic profile of 0.1 mM ethanol. Tiagabine is a potent and specific inhibitor of GABA uptake showing more than 300 fold specificity for GAT1 over GAT2/3 (Borden et al. 1994). Its efficacy in reducing ethanol-related reward has been studied, with mixed results (Rimondini et al. 2002, Nguyen et al. 2005, Fehr et al. 2007). CI-966 is also a specific GAT1 blocker, being around 200 times more potent at GAT1 than GAT2 or GAT3.
Vigabatrin, which is an irreversible inhibitor of GABA-transaminase (Lippert et al. 1977), increases GABA levels in a dose dependent manner (Jung et al. 1977). When incubated with slices, 100 μM vigabatrin increases GABA levels and increases net flux into GABA C2 as well as increasing net flux into Glu C4 while decreasing glutamate/glutamine cycling (decreasing net flux into Gln C4 and Ala C3) (Nasrallah et al. 2011). When the slices are activated in some fashion, such as by addition of AMPA, the presence of 400 μM vigabatrin results in significantly decreased net activity in the slice. We interpreted this as resulting from increased inhibition upon slice activation, possibly due to efflux of GABA from GATs (Nasrallah et al. 2011). In the presence of only 100 μM vigabatrin, the consequences of activating slices are much milder but are likely to arise from a similar mechanism. Ethanol has been shown to result in GABA release (Roberto et al. 2004, Criswell et al. 2008) possibly through a protein-kinase C coupled mechanism (Kelm et al. 2010). If we accept that ethanol is inducing a localized GABA release then the resulting metabolic pattern is likely due to its action at associated nearby GABA receptors.
Isoguvacine is an agonist at GABA(A) and is also active at ρ1-containing GABA(A)R (Kusama et al. 1993). It is as potent as GABA at α5β1-containing receptors. L655–708 is an inverse agonist, selective for α5-containing GABA(A) receptors where it binds to the benzodiazepine binding site, located between the α and γ subunits of αγ-containing receptor subtypes (Quirk et al. 1996). THIP displays a “feeble” affinity for ρ1-containing GABA(A)R but is potent at α5β3γ2 (Ebert et al. 1994). The affinity of THIP for receptors is dramatically increased in the presence of a δ- subunit, but at concentrations much lower than that used here (submicromolar vs 10 μM). Picrotoxin is a relatively nonspecific noncompetitive antagonist at GABA(A) receptors (Inoue & Akaike 1988) and since it is turning off inhibition, it is difficult to draw conclusions about its mode of action. Zolpidem binds to the benzodiazepine site but shows specificity for α1-containing receptors (Puia et al. 1991).
Taken together, it would seem that the action of the ethanol-stimulated released GABA may be at α5βγ receptors and to a lesser extent at α1βγ. Blockade of these receptors has been reported to attenuate the abuse of ethanol in squirrel monkeys (Platt et al. 2005). However, an agonist at these receptors with reported specificity, QH-ii-066 {Huang, 2000 #4636} when stimulated below the IC50 for α5β2γ2 (6.8 nM) does not cluster in the vicinity of 0.1 mM ethanol (Fig. 3) but in another quadrant of the Hotelling circle near to RO19-4603, Ethanol at 1.0 mM produced a metabolic profile that clustered part way between those at 10 mM+ concentrations at that at 0.1 mM (Fig. 3) and probably represents a composite metabolic outcome. Nearby ligands included gabapentin (200 μM). Gabapentin, a GABA mimetic, has a plethora of pharmacological actions, including at Ca2+ channels (Sills 2006). Notably, it has shown utility in reducing alcohol consumption and cravings (Anton et al. 2011, Furieri & Nakamura-Palacios 2007). Other nearby ligands (Fig. 3) include tiagabine (50 μM) a GAT1 inhibitor which has also shown some utility in reducing alcohol consumption (Myrick et al. 2005, Nguyen et al. 2005) although the results have been somewhat mixed (Rimondini et al. 2002, Fehr et al. 2007). Neither gabapentin nor tiagabine (Kastberg et al. 1998) interact directly with ethanol. The only other nearby ligand, Baclofen (1.0 μM), the typical agonist at GABA(B)R, is also of utility in alcohol dependence (Bucknam 2007) where it has been suggested to “substitute” for alcohol, although it has little impact on alcohol’s motivational effects (Maccioni et al. 2008).
While we have focused in this work on the effect of alcohol in the GABAergic system an important caveat is that some of the drugs to which the effects of alcohol were compared may also act on other alcohol targets such as GIRK, L-type Ca2+ channels or glycine receptors. For example, etomidate interacts with glycine (and several other) receptors but has no effect on GIRK. Little is known of DS2 or RO19-4603 or RO15-4513 effects on those targets.
In summary, the effects of ethanol at concentrations of 10 mM and above appear to be mediated via GABA(A) receptors, specifically α4β3δ-containing GABA(A)R. The action of alcohol at these receptors causes a direct reduction in metabolic activity which can be attributed solely to the actions of ethanol, not acetaldehyde or acetate, nor to substrate substitution by ethanol. Very low concentrations of ethanol (0.1 mM) generate a metabolic similar to that of low concentrations of GABA released via GAT reversal. This GABA may act at GABA receptors such as α5 (or to a lesser extent) α1- containing βγ GABA(A)R. A role for GABA(A)rho receptors in this effect is also a possibility.
Supplementary Material
Acknowledgments
The staff of the UNSW Analytical Centre are thanked for expert technical support. This work was supported by the Australian National Health and Medical Research Council (Grant 568767 to CR and VJB, and Fellowship to CR).
Abbreviations used in text
- GIRK
G-protein coupled inwardly rectifying potassium channels
- GABA(A)R
GABA-A receptors
- GABA(B)R
GABA-B receptors
- DS2
4-Chloro-N-[2-(2-thienyl)imidazo[1,2a]-pyridin-3-yl]benzamide
- GAT1
GABA transporter 1
- NMDA
N-methyl-D-aspartate
- THIP
4,5,6,7-Tetrahydroisoxazolo[5,4-c]pyridin-3-ol hydrochloride
Footnotes
The authors have no conflict of interest to declare.
References
- Absalom N, Eghorn LF, Villumsen IS, et al. alpha 4 beta delta GABA(A) receptors are high-affinity targets for gamma-hydroxybutyric acid (GHB) Proc Natl Acad Sci USA. 2012;109:13404–13409. doi: 10.1073/pnas.1204376109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allgaier C. Ethanol sensitivity of NMDA receptors. Neurochem Int. 2002;41:377–382. doi: 10.1016/s0197-0186(02)00046-3. [DOI] [PubMed] [Google Scholar]
- Anton RF, Myrick H, Wright TM, Latham PK, Baros AM, Waid LR, Randall PK. Gabapentin Combined With Naltrexone for the Treatment of Alcohol Dependence. Am J Psychiatr. 2011;168:709–717. doi: 10.1176/appi.ajp.2011.10101436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biller A, Bartsch AJ, Homola G, Solymosi L, Bendszus M. The effect of ethanol on human brain metabolites longitudinally characterized by proton MR spectroscopy. J Cereb Blood Flow Metab. 2009;29:891–902. doi: 10.1038/jcbfm.2009.12. [DOI] [PubMed] [Google Scholar]
- Borden LA, Murali Dhar TG, Smith KE, Weinshank RL, Branchek TA, Gluchowski C. Tiagabine, SK&F 89976-A, CI-966, and NNC-711 are selective for the cloned GABA transporter GAT-1. Eur J Pharmacol. 1994;269:219–224. doi: 10.1016/0922-4106(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Bucknam W. Suppression of symptoms of alcohol dependence and craving using high dose baclofen. Alcohol and Alcoholism. 2007 doi: 10.1093/alcalc/agl091. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Ming Z, Kelm MK, Breese GR. Brain regional differences in the effect of ethanol on GABA release from presynaptic terminals. J Pharmacol Exp Therap. 2008;326:596–603. doi: 10.1124/jpet.107.135418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert B, Wafford KA, Whiting PJ, Krogsgaardlarsen P, Kemp JA. Molecular pharmacology of gammaaminobutyric acid type A receptor agonists and partial agonists in oocytes injected with different alpha-receptor, beta-receptor and gamma-receptor subunit combinations. Mol Pharmacol. 1994;46:957–963. [PubMed] [Google Scholar]
- Ehrlich D, Pirchl M, Humpel C. Effects of longterm moderate ethanol and cholesterol on cognition, cholinergic neurons, inflammation and vascular impairment in rats. Neurosci. 2012;205:154–166. doi: 10.1016/j.neuroscience.2011.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engblom AC, Akerman KEO. Effect of ethanol on gammaaminobutyric acid and glycine receptor coupled Cl fluxes in rat brain synaptoneurosomes. J Neurochem. 1991;57:384–390. doi: 10.1111/j.1471-4159.1991.tb03764.x. [DOI] [PubMed] [Google Scholar]
- Eriksson L, Johansson E, Kettaneh-Wold N, Trygg J, Wikstrom C, Wold S. Multi- and megavariate data analysis, Part 1 Basic Principles and Applications. Umetrics AB; Umea, Sweden: 2006. [Google Scholar]
- Fehr C, Hohmann N, Grunder G, et al. Tiagabine does not attenuate alcohol-induced activation of the human reward system. Psychopharmacol. 2007;191:975–983. doi: 10.1007/s00213-006-0696-5. [DOI] [PubMed] [Google Scholar]
- Furieri FA, Nakamura-Palacios EA. Gabapentin reduces alcohol consumption and craving: A randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychiatry. 2007;68:1691–1700. doi: 10.4088/jcp.v68n1108. [DOI] [PubMed] [Google Scholar]
- Gomez R, Behar KL, Watzl J, et al. Intravenous Ethanol Infusion Decreases Human Cortical gamma-Aminobutyric Acid and N-Acetylaspartate as Measured with Proton Magnetic Resonance Spectroscopy at 4 Tesla. Biol Psychiatr. 2012;71:239–246. doi: 10.1016/j.biopsych.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton JA, Abraham MH, Bradbury MW, Chadha HS. Molecular factors influencing drug transfer across the blood-brain barrier. J Pharm Pharmacol. 1997;49:1211–1216. doi: 10.1111/j.2042-7158.1997.tb06072.x. [DOI] [PubMed] [Google Scholar]
- Handa RK, DeJoseph MR, Singh LD, Hawkins RA, Singh SP. Glucose transporters and glucose utilization in rat brain after acute ethanol administration. Metabolic Brain Dis. 2000;15:211–222. doi: 10.1007/BF02674530. [DOI] [PubMed] [Google Scholar]
- Harris CM, Lal H. Central nervous system effects of the imidazodiazepine RO-15-4513. Drug Development Research. 1988;13:187–203. [Google Scholar]
- Huang Q, He XH, Ma CR, Liu RY, Yu S, Dayer CA, Wenger GR, McKernan R, Cook JM. Pharmacophore/receptor models for GABA(A)/BzR subtypes (alpha 1 beta 3 gamma 2, alpha 5 beta 3 gamma 2, and alpha 6 beta 3 gamma 2) via a comprehensive ligand-mapping approach. J Med Chem. 2000;43:71–95. doi: 10.1021/jm990341r. [DOI] [PubMed] [Google Scholar]
- Huang Q, Zhang WJ, Liu RY, McKernan RM, Cook JM. Benzo-fused benzodiazepines employed as topological probes for the study of benzodiazepine receptor subtypes. Medicinal Chemistry Research. 1996;6:384–391. [Google Scholar]
- Ikeda K, Kobayashi T, Kumanishi T, Yano R, Sora I, Niki H. Molecular mechanisms of analgesia induced by opioids and ethanol: is the GIRK channel one of the keys? Neurosci Res. 2002;44:121–131. doi: 10.1016/s0168-0102(02)00094-9. [DOI] [PubMed] [Google Scholar]
- Inoue M, Akaike N. Blockade of gammaaminobutyric acid gated chloride channels in frog sensory neurons by picrotoxin. Neurosci Res. 1988;5:380–394. doi: 10.1016/0168-0102(88)90024-7. [DOI] [PubMed] [Google Scholar]
- Jung MJ, Lippert B, Metcalf BW, Bohlen P, Schechter PJ. Gamma-vinyl GABA (4-amino-hex-5-enoic acid) a new selective irreversible inhibitor of GABA-T - effects on brain GABA metabolism in mice. J Neurochem. 1977;29:797–802. doi: 10.1111/j.1471-4159.1977.tb10721.x. [DOI] [PubMed] [Google Scholar]
- Kastberg H, Jansen JA, Cole G, Wesnes K. Tiagabine: absence of kinetic or dynamic interactions with ethanol. Drug metabolism and drug interactions. 1998;14:259–273. doi: 10.1515/dmdi.1998.14.4.259. [DOI] [PubMed] [Google Scholar]
- Kaur KH, Baur R, Sigel E. Unanticipated Structural and Functional Properties of delta-Subunit-containing GABA(A) Receptors. J Biol Chem. 2009;284:7889–7896. doi: 10.1074/jbc.M806484200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm MK, Weinberg RJ, Criswell HE, Breese GR. The PLC/IP3R/PKC pathway is required for ethanol-enhanced GABA release. Neuropharmacol. 2010;58:1179–1186. doi: 10.1016/j.neuropharm.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RJ, Chebib M, Hibbs DE, Kim HL, Johnston GAR, Salam NK, Hanrahan JR. Novel gamma-aminobutyric acid rho(1) receptor antagonists; Synthesis, pharmacological activity and structure-activity relationships. J Med Chem. 2008;51:3825–3840. doi: 10.1021/jm7015842. [DOI] [PubMed] [Google Scholar]
- Kusama T, Spivak CE, Whiting P, Dawson VL, Schaeffer JC, Uhl GR. Pharmacology of GABA Rho-1 and GABA alpha/beta receptors expressed in xenopus oocytes and cos cells. Br J Pharmacol. 1993;109:200–206. doi: 10.1111/j.1476-5381.1993.tb13554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Belle JE, Harris NG, Williams SR, Bhakoo KK. A comparison of cell and tissue extraction techniques using high-resolution 1H NMR spectroscopy. NMR Biomed. 2002;15:37–44. doi: 10.1002/nbm.740. [DOI] [PubMed] [Google Scholar]
- Learn JE, Smith DG, McBride WJ, Lumeng L, Li TK. Ethanol effects on local cerebral glucose utilization in high-alcohol-drinking and low-alcohol-drinking rats. Alcohol. 2003;29:1–9. doi: 10.1016/s0741-8329(02)00323-3. [DOI] [PubMed] [Google Scholar]
- Lewohl JM, Wilson WR, Mayfield RD, Brozowski SJ, Morrisett RA, Harris RA. G-protein-coupled inwardly rectifying potassium channels are targets of alcohol action. Nat Neurosci. 1999;2:1084–1090. doi: 10.1038/16012. [DOI] [PubMed] [Google Scholar]
- Li ZZ, Xu YW, Warner D, Volkow ND. Alcohol ADME in Primates Studied with Positron Emission Tomography. PLoS One. 2012:7. doi: 10.1371/journal.pone.0046676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippert B, Metcalf BW, Jung MJ, Casara P. 4-Amino-Hex-5-enoic acid, a selective catalytic inhibitor of 4-aminobutyric acid aminotransferase in mammalian brain. European Journal of Biochemistry. 1977;74:441–445. doi: 10.1111/j.1432-1033.1977.tb11410.x. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, Homanics GE. Tonic for what ails us? high-affinity GABA(A) receptors and alcohol. Alcohol. 2007;41:139–143. doi: 10.1016/j.alcohol.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibition of neuronal glutamate receptor function. Ann Med. 1990;22:247–252. doi: 10.3109/07853899009148935. [DOI] [PubMed] [Google Scholar]
- Maccioni P, Fantini N, Froestl W, Carai MAM, Gessa GL, Colombo G. Specific reduction of alcohol’s motivational properties by the positive allosteric modulator of the GABA(B) receptor, GS39783 - Comparison with the effect of the GABA(B) receptor direct agonist, baclofen. Alcoholism-Clinical and Experimental Research. 2008;32:1558–1564. doi: 10.1111/j.1530-0277.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- Mori T, Aistrup GL, Nishikawa K, Marszalec W, Yeh JZ, Narahashi T. Basis of variable sensitivities of GABA(A) receptors to ethanol. Alcoholism-Clinical and Experimental Research. 2000;24:965–971. [PubMed] [Google Scholar]
- Mukherji B, Kashiki Y, Ohyanagi H, Sloviter HA. Metabolism of ethanol and acetaldehyde by isolated perfused rat brain. J Neurochem. 1975;24:841–843. [PubMed] [Google Scholar]
- Muneer PMA, Alikunju S, Szlachetka AM, Haorah J. Inhibitory effects of alcohol on glucose transport across the blood-brain barrier leads to neurodegeneration: preventive role of acetyl-l-carnitine. Psychopharmacol. 2011;214:707–718. doi: 10.1007/s00213-010-2076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Taylor B, Larowe S, Nguyen S, Boyle E, Cochran K, Malcolm R. Retrospective chart review comparing tiagabine and benzodiazepines for the treatment of alcohol withdrawal. J Psychoact Drugs. 2005;37:409–414. doi: 10.1080/02791072.2005.10399814. [DOI] [PubMed] [Google Scholar]
- Nasrallah F, Griffin JL, Balcar VJ, Rae C. Understanding your inhibitions. Modulation of brain cortical metabolism by GABA-B receptors. J Cereb Blood Flow Metab. 2007;27:1510–1520. doi: 10.1038/sj.jcbfm.9600453. [DOI] [PubMed] [Google Scholar]
- Nasrallah F, Griffin JL, Balcar VJ, Rae C. Understanding your inhibitions. Effects of GABA and GABAA receptors on brain cortical metabolism. J Neurochem. 2009;108:57–71. doi: 10.1111/j.1471-4159.2008.05742.x. [DOI] [PubMed] [Google Scholar]
- Nasrallah FA, Balcar VJ, Rae C. A metabonomic study of inhibition of GABA uptake in the cerebral cortex. Metabolomics. 2010a;6:67–77. [Google Scholar]
- Nasrallah FA, Balcar VJ, Rae CD. Activity dependent GABA release controls brain cortical tissue slice metabolism. Journal of Neuroscience Research. 2011;89:1935–1945. doi: 10.1002/jnr.22649. [DOI] [PubMed] [Google Scholar]
- Nasrallah FA, Maher AD, Hanrahan JR, Balcar VJ, Rae CD. γ-Hydroxybutyrate and the GABAergic footprint. A metabolomic approach to unpicking the actions of GHB. The Journal of Neurochemistry. 2010b;115:58–67. doi: 10.1111/j.1471-4159.2010.06901.x. [DOI] [PubMed] [Google Scholar]
- Nguyen SA, Deleon CP, Malcolm RJ, Middaugh LD. Tiagabine reduces ethanol reward in C57BL/6 mice under acute and chronic administration regimens. Synapse. 2005;56:135–146. doi: 10.1002/syn.20138. [DOI] [PubMed] [Google Scholar]
- Pawlosky RJ, Kashiwaya Y, Srivastava S, King MT, Crutchfield C, Volkow N, Kunos G, Li TK, Veech RL. Alterations in Brain Glucose Utilization Accompanying Elevations in Blood Ethanol and Acetate Concentrations in the Rat. Alcoholism-Clinical and Experimental Research. 2010;34:375–381. doi: 10.1111/j.1530-0277.2009.01099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins DI, Trudell JR, Crawford DK, Alkana RL, Davies DL. Molecular targets and mechanisms for ethanol action in glycine receptors. Pharmacol Ther. 2010;127:53–65. doi: 10.1016/j.pharmthera.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SC. Does ethanol damage the blood brain barrier? J Neurol Sci. 1981;50:81–87. doi: 10.1016/0022-510x(81)90043-5. [DOI] [PubMed] [Google Scholar]
- Platt DM, Duggan A, Spealman RD, Cook JM, Li XY, Yin WY, Rowlett JK. Contribution of alpha(1)GABA(A) and alpha(5)GABA(A) receptor subtypes to the discriminative stimulus effects of ethanol in squirrel monkeys. J Pharmacol Exp Therap. 2005;313:658–667. doi: 10.1124/jpet.104.080275. [DOI] [PubMed] [Google Scholar]
- Pohorecky LA. BIPHASIC ACTION OF ETHANOL. Biobehavioral Reviews. 1977a;1:231–240. [Google Scholar]
- Pohorecky LA. Biphasic action of ethanol. Biobehavioral Reviews. 1977b;1:231–240. [Google Scholar]
- Puia G, Vicini S, Seeburg PH, Costa E. Influence of recombinant gamma-aminobutyric acid-A receptor subunit composition on the action of allosteric modulators of gamma-aminobutyric acid-gated Cl- currents. Mol Pharmacol. 1991;39:691–696. [PubMed] [Google Scholar]
- Quirk K, Blurton P, Fletcher S, Leeson P, Tang F, Mellilo D, Ragan CI, McKernan RM. [3H]L-655,708, a novel ligand selective for the benzodiazepine site of GABAA receptors which contain the [alpha]5 subunit. Neuropharmacol. 1996;35:1331–1335. doi: 10.1016/s0028-3908(96)00061-5. [DOI] [PubMed] [Google Scholar]
- Rae C, Nasrallah FA, Griffin JL, Balcar VJ. Now I know my ABC. A systems neurochemistry and functional metabolomic approach to understanding the GABAergic system. J Neurochem. 2009;109 (Suppl 1):109–116. doi: 10.1111/j.1471-4159.2009.05803.x. [DOI] [PubMed] [Google Scholar]
- Rimondini R, Sommer W, Heilig M. Effects of tiagabine and diazepam on operant ethanol self-administration in the rat. Journal of Studies on Alcohol. 2002;63:100–106. [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci USA. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Murgia A, Casula A, Biggio G. Differential subunit dependence of the actions of the general anesthetics alphaxalone and etomidate at gamma-aminobutyric acid type A receptors expressed in Xenopus laevis oocytes. Mol Pharmacol. 1997;51:484–490. [PubMed] [Google Scholar]
- Sills GJ. The mechanisms of action of gabapentin and pregaballin. Curr Opin Pharmacol. 2006;6:108–113. doi: 10.1016/j.coph.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH. Ethanol effects on GABA-gated current in a model of increased alpha 4 beta delta GABA(A) receptor expression depend on time course and preexposure to low concentrations of the drug. Alcohol. 2007;41:223–231. doi: 10.1016/j.alcohol.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanagel R. Alcoholism: A Systems Approach From Molecular Physiology to Addictive Behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li XS, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated alpha(4)beta(2)delta GABA(A) receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzdak PD, Paul SM, Crawley JN. Effects of RO15-4513 and other benzodiazepine receptor inverse agonists on alcohol induced intoxication in the rat. J Pharmacol Exp Therap. 1988;245:880–886. [PubMed] [Google Scholar]
- Uchida I, Kamatchi G, Burt D, Yang J. Etomidate potentiation of GABA(A) receptor gated current depends on the subunit composition. Neurosci Lett. 1995;185:203–206. doi: 10.1016/0304-3940(95)11263-v. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wolf AP, et al. Acute effects of ethanol on regional brain glucose metabolism and transport. Psychiatry Res Neuroimaging. 1990;35:39–48. doi: 10.1016/0925-4927(90)90007-s. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Franceschi D, et al. Low doses of alcohol substantially decrease glucose metabolism in the human brain. Neuroimage. 2006;29:295–301. doi: 10.1016/j.neuroimage.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Wafford KA, van Niel MB, Ma QP, Horridge E, Herd MB, Peden DR, Belelli D, Lambert JJ. Novel compounds selectively enhance delta subunit containing GABA(A) receptors and increase tonic currents in thalamus. Neuropharmacol. 2009;56:182–189. doi: 10.1016/j.neuropharm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha(4)beta(3)delta and alpha(6)beta(3)delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Low dose acute alcohol effects on GABA(A) receptor subtypes. Pharmacol Ther. 2006;112:513–528. doi: 10.1016/j.pharmthera.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold S, Antti H, Lindgren F, Ohman J. Orthogonal signal correction of near-infrared spectra. Chemometrics and Intelligent Laboratory Systems. 1998;44:175–185. [Google Scholar]
- Xiang Y, Shen J. In vivo detection of intermediate metabolic products of 1-C-13 ethanol in the brain using C-13 MRS. NMR Biomed. 2011;24:1054–1062. doi: 10.1002/nbm.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.