Abstract
Polypyrimidine tract binding protein (PTB) is a major hnRNP protein with multiple roles in mRNA metabolism, including regulation of alternative splicing and internal ribosome entry site-driven translation. We show here that a fourfold overexpression of PTB results in a 75% reduction of mRNA levels produced from transfected gene constructs with different polyadenylation signals (pA signals). This effect is due to the reduced efficiency of mRNA 3′ end cleavage, and in vitro analysis reveals that PTB competes with CstF for recognition of the pA signal's pyrimidine-rich downstream sequence element. This may be analogous to its role in alternative splicing, where PTB competes with U2AF for binding to pyrimidine-rich intronic sequences. The pA signal of the C2 complement gene unusually possesses a PTB-dependent upstream sequence, so that knockdown of PTB expression by RNA interference reduces C2 mRNA expression even though PTB overexpression still inhibits polyadenylation. Consequently, we show that PTB can act as a regulator of mRNA expression through both its negative and positive effects on mRNA 3′ end processing.
Between the branch point and the 3′ splice site (3′SS) of metazoan introns lies a pyrimidine-rich sequence which is critical for efficient splicing (49). Initial analysis of factors that recognize this sequence identified an hnRNP-like protein called polypyrimidine tract binding protein (PTB) or hnRNP I (20, 22, 23, 40). PTB has strong RNA binding activity, since it possesses four tandem RNA recognition motif domains (42). In vitro RNA binding analysis (SELEX) revealed its preferred RNA binding site as UCUU flanked by pyrimidines rather than a nonspecific pyrimidine sequence (41). Subsequent studies indicated that far from being a positively acting splicing factor, PTB actually acts as a selective splicing repressor (55, 57). The splicing factor responsible for recognition of the 3′SS pyrimidine tract and AG dinucleotide is the dimeric U2 auxiliary factor protein or U2AF (7). The U2AF 65-kDa subunit interacts with the pyrimidine tract (63), while the smaller 35-kDa subunit directly contacts the 3′SS sequence (34). Recognition of the pyrimidine tract by U2AF has been identified as a major site of splicing regulation. This can be modulated in a positive fashion through interaction with splicing-regulatory proteins bound to adjacent exon enhancer sequences (4, 51) or in a negative fashion by competition for binding with PTB (57). In many cases the pyrimidine tract of PTB-regulated exons contains high-affinity binding sites for PTB (41), and direct competition between PTB and U2AF65 for binding to the pyrimidine tract can lead to exon skipping (28, 50). However, regulation by PTB often requires additional PTB-binding elements remote from the 3′SS pyrimidine tract. These may mediate cooperative binding of PTB (11), which can interfere with binding of U2AF as well as other splicing factors (57). Furthermore, PTB exists in several different isoforms generated by alternative splicing (PTB1—referred to throughout the paper as PTB, PTB2, and PTB4), which result in the insertion of 19 or 26 amino acids between the second and third RNA recognition motifs (22, 23). These three isoforms have differential activity for some splicing events (61), as does the brain-specific nPTB, which is expressed from a separate gene (31).
A second role for PTB in mRNA processing has been uncovered in its recognition of polyadenylation signals (pA signals). In general, pA signals comprise an AAUAAA sequence 20 to 30 nucleotides (nt) upstream of the cleavage site to which the poly(A) tail is added by poly(A) polymerase (44). AAUAAA is recognized by the multimeric protein cleavage polyadenylation stimulatory factor (CPSF), and its interaction is cooperatively enhanced by the binding of a second protein, cleavage stimulatory factor (CstF), to a G/U- or U-rich downstream sequence element (DSE) present immediately following the cleavage site. CstF is a heterotrimeric protein (77-, 64-, and 50-kDa subunits) whose 64-kDa polypeptide directly interacts with the DSE (10, 64). The cooperative binding of CPSF and CstF promotes recruitment of two further factors, CFI and CFII, and the combined protein complex so formed mediates cleavage at the polyadenylation site (pA site). This is then coupled to polyadenylation of the free 3′ terminus. In addition to DSEs, pA sites may also possess U-rich upstream sequence elements (USEs) required for full activity. Several viral pA sites possess such USEs, including sites of human immunodeficiency virus type 1 (6, 9, 13, 24, 56), Moloney murine leukemia virus (18), simian virus 40 (8), and adenovirus (45). Two mammalian genes are also known to possess USEs: B1 lamin (5) and C2 complement (36, 37). The latter pA signal has an unusual structure which includes a USE but lacks any clear DSE (37). The C2 USE has been shown to specifically bind PTB, which is required for its efficient polyadenylation. The same USE also recruits CstF, which in this instance appears to activate polyadenylation rather than 3′ end cleavage (36).
The above-described role of PTB in regulating mRNA splicing prompted us to investigate the possible general role of PTB in polyadenylation. Since both CstF64 and PTB recognize related pyrimidine-rich sequence motifs, we reasoned that PTB might antagonize the recognition of pA sites in a fashion analogous to its effect on the recognition of the 3′SS. The data presented in these studies demonstrate that PTB can indeed compete with CstF64 for binding to the pyrimidine-rich DSE. As a result of this competition, PTB inhibits 3′ end cleavage of the mRNA, leading to a significant down-regulation of polyadenylated message and a corresponding increase in unprocessed “read-through” message. We also show for the C2 pA signal (by RNA interference [RNAi]-mediated depletion) that PTB is required for its activity in vivo even though PTB overexpression still results in its repression. Therefore, for some pA signals, such as C2, PTB may have a dual role in both activation and repression of polyadenylation. This raises the possibility that PTB functions as a modulating influence in mRNA 3′ end processing. In the case of splicing, PTB acts to repress the inclusion of specific exons within the final mRNA. In the case of polyadenylation, PTB may operate in some situations to reduce the overall levels of mRNA synthesized and so reduce protein expression from a given gene.
MATERIALS AND METHODS
Plasmid constructions.
A 526-nt fragment containing the cytomegalovirus (CMV) promoter (3) was isolated by PCR using the forward (CCACATGTTTACATAACTTACGGTAAATG) and reverse (GAGTACAGGCAAAAAGCAGAG) oligonucleotide primers (CMV fragment). The β-globin gene (described below) was isolated by PCR using the forward (CATGGTGCACCTGACTCCTGAGGAGAAGTC) and reverse (TCCAGATGCTCAAGGCCCTTCATAATATCC) oligonucleotide primers. These two PCR products were then blunt-end ligated, reamplified, and cut with ApaI (CMV-β-globin fragment).
The α-globin poly(A) sequence was isolated by PCR by using the forward (TTCTGCCTAAGGGCCCTCCTCCCCT) and reverse (CGACGTCTCTCCAGGAACCAGACTC) oligonucleotide primers. The product was digested with ApaI, ligated to the CMV-β-globin fragment, reamplified, cut with AflIII and XbaI, and ligated to pUC 18 cut with AflIII/XbaI (α). The C2 complement poly(A) sequence (36) was isolated using forward (TTGGCCTGTCCCCAGATTCCTTCCCT) and reverse (CAAGGCCAGCCCTACCTGGCC) oligonucleotide primers. The product was digested with ApaI, ligated to the CMV-β-globin fragment, reamplified, cut with AflIII and XbaI, and ligated to pUC 18 cut with AflIII/XbaI (C2). The β-globin gene and the β-globin poly(A) sequence have been described previously (14) (β). The CMV fragment together with the β-globin gene's exon 1 was amplified by PCR using the forward (CGTTACATAACTTACGGTAAATG) and the reverse (CTGCCCAGGGCCTCACCACCAACT) oligonucleotide primers (CMV-exon1 fragment). The β-globin gene's exon 2 was amplified by PCR using the forward (GCTGCTGGTGGTCTACCCTTGGACC) and the reverse (GGACTTCAAGAGTCCTAG) oligonucleotide primers (exon2 fragment). These two PCR products were then ligated and reamplified with the 3′ primer (CMV-exon1-exon2 fragment). The β-globin gene's exon 3 and the β-globin pA signal were isolated by PCR using the forward CTC (CTGGGCAACGTGCTGGTCTG) and the reverse (TTCGAACGTACGGACGTCCGT) primers. This PCR product was ligated to the CMV-exon1-exon2 fragment, reamplified, digested with AflIII/HindIII, and ligated to pUC 18 cut with AflIII/HindIII (β cDNA).
PTB plasmids were described previously (61). The Va plasmid, which expresses the adenovirus VA1 gene, was previously described (14).
Cell culture, transfection, and RNA isolation.
Subconfluent HeLa cells were transfected as previously described (18) using 3 μg of either α, β, C2, or cDNA plasmids, 1.5 μg of each PTB plasmid, and 0.5 μg of Va plasmid.
Cytoplasmic RNA was isolated as previously described (15); nuclear and total RNAs were isolated using the hot-phenol method (18). Pellets were resuspended in 40 to 90 μl of R loop buffer (2).
RNA analysis.
S1 nuclease analysis was performed as described previously (2), using the EcoRI-linearized (β) (β cDNA) or C2 construct end-filled with [α-32P]dATP and the Bsp1201-linearized (α) construct end-filled with [α-32P]dGTP as probes.
Nuclear run-on (NRO) analysis.
Nuclear isolation and run-on analysis were performed as previously described (19, 43). Probes BE, BE2, and B3 for the NRO analysis were described previously (43).
Control probes for M13 (background), Va, and HIS (adenovirus VA1 gene and mouse histone H4, respectively) have also been described (1).
Western blot analysis.
Cell lysates were prepared using RIPA buffer (12), boiled, and quantified using the Bradford assay, and 5 μg of each lysate was separated by sodium dodecyl sulfate (SDS)-polyacrylamide electrophoresis and transferred to a Hybond pure nitrocellulose membrane (Amersham). Membranes were immunoblotted using anti-PTB (1:2,000) and antiactin (1:2,000) antibodies by standard procedures (30).
In vitro transcription and in vitro cleavage analysis.
The α pA signal was amplified by PCR using the forward (AGATTTAGGTGACACTATACCTGGCCCACAAGTAT; containing an SP6 promoter sequence) and the reverse (CTGCAGAGAGGTCCTTGGTCTGAGACA) oligonucleotide primers. The β pA signal was amplified by PCR using the forward (AGATTTAGGTGACACTATACCTGGCCCACA AGTAT; containing an SP6 promoter sequence) and the reverse (GTTTGAACTAGCTCTTCATTTCTTTATG) oligonucleotide primers. These pA signals were in vitro transcribed, and cleavage reactions were carried out as previously described (36).
Ultraviolet (UV) cross-linking competition assays.
The 32P-labeled RNA substrate was incubated with 10 ng of a CstF purified protein fraction (or a mixture of CPSF and CstF enriched fractions) and various amounts of rPTB (10, 50, 100, 250, or 500 ng) (36).
RNAi assays.
For RNAi, HeLa cells were plated to a density of 10,000 cells per well in a 24-well plate, and assays were performed as previously described (58) with minor modifications. On day 6, cells were transfected with 1 μg of each reporter and 0.5 μg of Va plasmid.
RESULTS
PTB overexpression reduces mRNA levels.
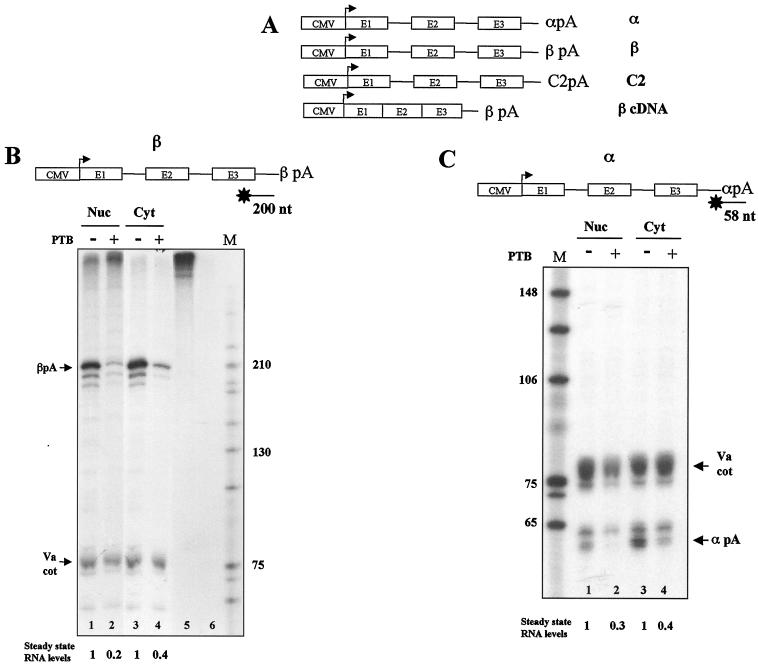
To investigate the potential effect of PTB on polyadenylation, a transient-transfection assay was employed using different β-globin gene constructs with transcription driven by the strong CMV promoter and one of three alternative pA signals: α-globin, β-globin, or C2 complement (α, β, or C2) (Fig. 1A). A further construct was created that lacked introns (β cDNA). The β and α constructs were cotransfected into HeLa cells with or without a PTB expressing plasmid (at a ratio of 2:1, β/α plasmid to PTB plasmid), and both nuclear and cytoplasmic RNA fractions were purified (Fig. 1B and C). Each transfection included a third plasmid containing the adenovirus Va gene. Since the Va gene is efficiently transcribed by RNA polymerase III (Pol III), its expression provides an internal transfection efficiency and loading control. The RNA samples were subjected to S1 nuclease mapping using either β or α 3′-end-labeled DNA probes, together with a Va-specific probe. Compared to the relatively constant Va signal (75 nt), both the β and α transcript 3′ signals showed a significant reduction (about threefold) in mRNA levels when the transfection included the PTB plasmid. This effect was particularly marked in the nuclear fractions. It should be noted that the α and β signals break up into several closely spaced bands which reflect the ability of S1 nuclease to partially degrade duplex structures near the 3′ terminus, especially when the sequence is AT rich. Overexpression of PTB had a similar effect on both weak pA (α) and strong pA (β) signals, suggesting a very efficient activity for PTB. The different efficiencies of the α and β pA signals have been previously described (17). Note that in Fig. 1, where the Va, β, and α S1 probes showed nearly equal specific activities, it is clear that the construct containing the weaker α pA resulted in significantly less cytoplasmic mRNA than the construct with the stronger β pA (compare Fig. 1B and C, lanes 3).
FIG. 1.
Overexpression of PTB reduces steady-state mRNA levels. (A) Gene constructs used to transfect HeLa cells. Each construct is driven by the CMV promoter and uses the β-globin gene as a reporter gene. The α-globin poly(A) signal (α), the β-globin poly(A) signal (β), and the C2 complement poly(A) signal (C2) were placed downstream of the β-globin gene. The β cDNA construct lacks introns. (B) Overexpression of PTB reduces steady-state β mRNA levels up to fivefold. S1 analysis of nuclear and cytoplasmic RNA isolated from HeLa cells transiently transfected with the β construct alone (lanes 1 and 3) or cotransfected with a PTB-expressing plasmid (lanes 2 and 4) followed by denaturing polyacrylamide gel electrophoresis is shown. Lanes 5 (no RNA, no S1 nuclease) and 6 (no RNA) are controls. The VA I adenovirus gene (Va) was used as a cotransfection control (Va cot), confirming equal transfection efficiency and loading of the RNA. Arrows indicate the positions of the pA and Va control bands. Lane M, size markers (in nucleotides). (C) Overexpression of PTB reduces steady-state α mRNA levels up to threefold. S1 analysis of nuclear and cytoplasmic RNA isolated from HeLa cells transiently transfected with the α construct (lanes 1 and 3) or cotransfected with a PTB-expressing plasmid (lanes 2 and 4) is shown. Quantitation of detected β or α signal is shown below gels, with values for transfections without PTB cotransfection set as 1.
Control experiments.
Since the PTB-mediated reduction in mRNA levels could result from a range of different mechanisms, a series of controls was devised to rule out effects other than the inhibition of 3′ end processing. As shown in Fig. 2A, Western blotting indicates that PTB transfection significantly increases PTB protein levels. Considering that transfection efficiency approaches 50%, this suggests that the transfected HeLa cell population is overexpressing PTB by approximately fourfold. Furthermore, mutation of the PTB-expressing plasmid by introducing a frame-shift mutation near the N terminus of the gene shows a loss of the PTB inhibitory effect on β mRNA levels (using the same transfection and RNA mapping analysis as shown in Fig. 1B), demonstrating that PTB protein expression is required for the mRNA reduction effect. This result also excludes the possibility that there is competition for transcription factors between the CMV promoters of the cotransfected plasmids (Fig. 2B). In view of PTB's known effect on splicing, it was further possible that the reduction in mRNA levels was caused by failure to splice efficiently. However, transfection of the β cDNA construct with each of the different PTB isoforms (PTB1, -2 and -4) still showed a marked reduction in mRNA levels (with all three PTB isoforms) as shown by S1 mapping using the 3′ β probe (Fig. 2C, β pA band). Note that the expression levels are much lower with this cDNA gene construct, since intron sequences are required for efficient transcription (19) and export of mRNA (47). The 60-nt band in the lane lacking PTB cotransfection (lane 1) may reflect a cryptic splicing event, since a potential 5′SS sequence is present at this location. Interestingly, this product is also repressed by PTB overexpression. As a final control, we tested whether PTB overexpression might be affecting overall transcription levels. Nuclear run-on analysis was performed on the β construct with or without cotransfected PTB (Fig. 2D). Quantitative comparison of β nascent transcription with or without PTB cotransfection revealed no significant change compared to the cotransfection control Va transcript, which is transcribed by Pol III instead of Pol II. Note that the overall NRO signals are twofold lower in the β plus PTB sample than with β alone, as judged by the endogenous histone gene signal. This may reflect lower RNA recovery in this experiment. Overall, these data indicate that the threefold reduction in mRNA levels caused by PTB overexpression cannot be attributed to effects on transcription.
FIG. 2.
Reduction in steady-state RNA levels requires PTB expression and is independent of splicing and nascent transcription. (A) Overexpression of PTB in HeLa cells. Western blot analysis of HeLa cell nuclear extract using anti-PTB antibody (diluted 1:2,000) after transfection with a PTB-expressing plasmid (lane 1) or in its absence (lane 2). A control Western blot with actin antibody confirmed equal protein loading (data not shown). (B) Expression of nonsense PTB message has no effect on steady-state RNA levels, while overexpression of PTB reduces the levels threefold. Lane 1, S1 analysis of cytoplasmic RNA isolated from HeLa cells transfected with the β construct; lanes 2 and 3, β cotransfected with a PTB-expressing plasmid (lane 2) or a plasmid carrying a nonsense PTB message (lane 3); lanes 4 and 5, controls (no RNA with or without S1 nuclease treatment), and Va was used as a cotransfection control (Va cot). (C) Reduction in steady-state RNA levels is independent of splicing. S1 analysis of total RNA isolated from HeLa cells transfected with the βcDNA construct (lane 1) is shown. β cDNA cotransfected with plasmids expressing PTB 1 (lane 2), PTB 2 (lane 3), or PTB 4 (lane 4) are shown. Lanes 5 and 6 are controls (no RNA with or without S1), and the Va was used as a cotransfection control. Lane M, size markers; *, 60-nt band. Quantitation for panels B and C is as in Fig. 1. (D) Overexpression of PTB does not affect nascent transcription levels. Nuclear run-on analysis was carried out with β-transfected HeLa cells (β) with β plus cotransfected PTB1 expression plasmid (β+PTB) or with empty pUC18 vector (control). B2, BE2, and B3 are single-stranded M13 probes containing antisense sequences from the β-globin gene as indicated. The histone H4 probe (His) acts as an internal control for Pol II transcription. M13 indicates the probe background. Nylon filters of probe slot blots hybridized to 32P nuclear RNA were subjected to phosphorimage analysis and show no significant effect of PTB expression on the levels of nascent transcription.
PTB overexpression inhibits mRNA 3′ end cleavage.
Many of the mechanisms that might account for the observed reduction in mRNA levels caused by PTB overexpression are ruled out in the above experiments (Fig. 2). We therefore directly tested the possible effect of PTB overexpression on mRNA 3′ end processing both in vivo and in vitro. For in vivo 3′-end analysis, a specific 3′-end S1 probe was designed that can distinguish between polyadenylated transcripts (pA transcripts) and uncleaved (read-through) transcripts extending beyond the normal pA site. The ratio of pA to read-through bands gives a measure of the efficiency of mRNA 3′ end processing. As shown in Fig. 3, cytoplasmic RNA from β transfections in the absence of cotransfected PTB gave virtually no read-through signal, indicating the high efficiency of the β-globin pA site. However, when any of the three PTB isoforms was cotransfected with β, a threefold reduction in the β pA band was observed with a commensurate fivefold increase in the read-through signal. These results strongly suggest that the reductions in mRNA levels mediated by PTB overexpression are the result of impaired mRNA 3′ end processing.
FIG. 3.
Overexpression of PTB reduces the efficiency of mRNA 3′ end processing in vivo. Lane 1, S1 analysis of total RNA isolated from HeLa cells transiently transfected with the β construct; lanes 2 to 4, β cotransfected with PTB1 (lane 2), PTB2 (lane 3), or PTB4 (lane 4); lane 5 (no RNA), negative control. Va was used as a cotransfection control. Arrows indicate the positions of the read-through (RT), pA, and Va cot bands. Quantitation is as for Fig. 1.
To obtain direct evidence for PTB inhibition of mRNA 3′ end processing, in vitro 3′ processing reactions were carried out using synthetic 32P-labeled β or α pA site RNA substrates, synthesized by SP6 RNA polymerase (Fig. 4, input band). 3′ processing reactions were performed using nuclear extracts under standard conditions that allow 3′ processing but not polyadenylation to occur (36). Either recombinant PTB (0.25 μM) or an equivalent weight of bovine serum albumin (BSA) was added to the reactions as indicated. Since the amount of PTB in the unsupplemented nuclear extracts was measured at ∼0.2 μM, the levels of PTB were increased about twofold. For both the β and α substrates, the 5′ and 3′ products of cleavage are clearly visible (lane 1), though for β the 5′ product is a doublet band due to heterogeneity in the actual site of cleavage. Importantly, PTB but not BSA addition caused a clear threefold reduction in the level of 3′ end cleavage. These results demonstrate that increasing the levels of PTB twofold causes a severe inhibition of the 3′ cleavage reaction.
FIG. 4.
PTB inhibits cleavage of the α-globin and β-globin poly(A) signals in vitro. In vitro cleavage reactions were performed using SP6 synthetic RNA substrates containing either the β-globin (β-pA) or the α-globin pA signal (α-pA) followed by fractionation using denaturing polyacrylamide gel electrophoresis. The addition of 150 ng of recombinant PTB to the reaction mixture resulted in a threefold inhibition of cleavage (lanes 2). BSA (150 ng) was added to the reaction mixture as a control (lanes 3), or no nuclear extract was added (lanes 4). The position of uncleaved pre-mRNA substrate (input), upstream cleavage products (5′), and downstream cleavage products (3′) are indicated by arrows. Size markers are indicated. In the data quantitation, the proportion of 3′-end-processed RNA for lanes 1 is set as 1.
PTB competes with CstF64 binding to α and β pA signals.
It seemed plausible that the capacity of PTB to inhibit the 3′ cleavage reaction might result from competitive binding of PTB and CstF to the α and β DSEs, since both of these sequences are pyrimidine rich. This would be analogous to the competitive binding of U2AF and PTB at the 3′SS pyrimidine tract as described above. To investigate this possibility, the competitive binding of α and β pA site RNAs to CstF and PTB was monitored by UV cross-linking analysis (Fig. 5). Each RNA was incubated with purified CstF fractions followed by the addition of increasing amounts of recombinant PTB. Both the α and β RNAs gave a single cross-linking protein band of 64 kDa with CstF alone, corresponding in size to CstF64, the subunit known to directly bind the DSE of the pA signal (25, 52, 53, 60). However, as PTB was titrated into the binding reactions, a lower-mass (57 kDa) band corresponding to PTB became apparent. Furthermore, as the PTB concentration was increased, the higher CstF64 band appeared to be displaced. Interestingly, with the α RNA, complete displacement of CstF occurred at 250 ng of PTB, while for β RNA the larger amount of 500 ng was required to displace CstF64. This probably reflects the relative binding strength of these two pA sites for CstF, since α has a short GU-rich DSE with a lower affinity for CstF64 while β has a longer GU- and U-rich DSE with a higher affinity for CstF64. These data provide a clear molecular explanation for the inhibitory effect of PTB on the mRNA 3′-end cleavage reaction. As with PTB competing for the binding of U2AF at 3′SS pyrimidine tracts, so too PTB can block the binding of CstF to pA site DSEs.
FIG. 5.
PTB and CstF64 compete for the same pre-mRNA binding sites in a UV cross-linking competition assay. (α-pA) 0, 10, 50, 100, or 250 ng of rPTB was added to a CstF purified protein fraction. Following UV cross-linking, labeled proteins were fractionated by SDS-polyacrylamide gel electrophoresis. As described above, PTB and CstF64 compete for binding to α-globin pA pre-mRNA. (β-pA) 0, 10, 50, 100, 250, or 500 ng of rPTB was added to a CstF purified protein fraction as for α-pA. Again, PTB and CstF64 compete for binding to the β-globin pA pre-mRNA.
Reducing PTB levels by RNAi has a selective effect on pA site recognition.
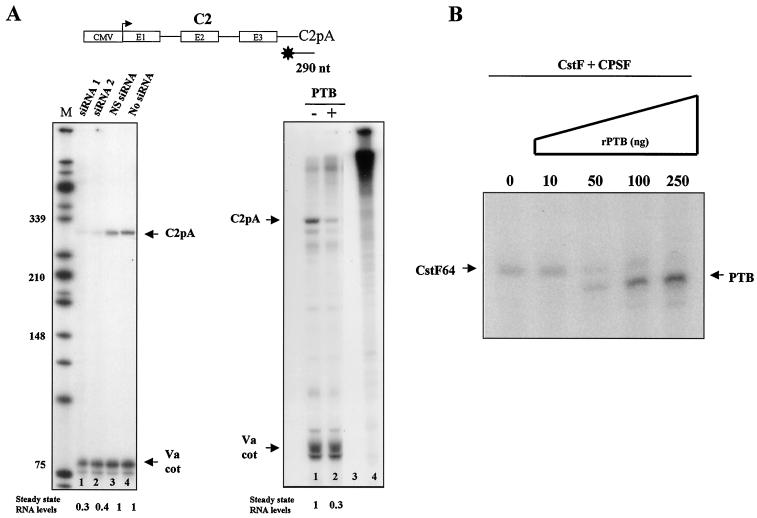
The advent of RNAi technology has allowed the possibility of reducing expression of specific proteins by transfecting tissue culture cells with short duplex RNAs corresponding in sequence to target mRNAs (16, 27). This technique has recently been applied to down-regulate PTB expression and resulted in the alteration of alternative splicing profiles for the FGF-R2 gene, known to be regulated by PTB (58). We wished to apply this same procedure to study PTB regulation of mRNA 3′-end cleavage as demonstrated in these studies. Two different small interfering RNAs (siRNAs), known to partially or more completely oblate PTB expression, were utilized (Fig. 6 and 7). As indicated by Western blot analysis, PTB levels were differentially reduced following HeLa cell transient transfection with these two PTB-specific siRNAs compared to results with an unrelated siRNA preparation (Fig. 6A, upper panel). As an internal control the level of actin expression was also measured and remained unchanged, indicating the specificity of the siRNAs. As shown in Fig. 6B and C, siRNA-induced knockdown of PTB had no effect on the levels of mRNA expressed from either the β or α construct. In contrast, levels of mRNA from the C2 construct were found to be highly sensitive to PTB knockdown (Fig. 7A). The human C2 pA signal (as described above) is unusual in possessing a USE but no DSE. Previous in vitro studies indicated that PTB is required for the positive activity of the C2 USE on mRNA 3′ end processing (36). Consistent with these studies, the C2 pA site (unlike α and β globin) does show sensitivity to the PTB-specific siRNAs. As shown in Fig. 7A, a stepwise decrease in C2 mRNA is clearly evident, with C2-transfected HeLa cells previously transfected with the two PTB-specific siRNAs. Furthermore, this effect is not seen with HeLa cells transfected by the unrelated siRNA. Using a C2 S1 3′-end probe, a 290-nt band corresponding to the C2 mRNA is detected, and this signal diminishes (up to threefold) compared to the Va control signal with the PTB-specific-siRNA-treated cells.
FIG. 6.
In vivo knockdown of PTB by RNAi has no significant effect on efficiency of α-globin and β-globin polyadenylation. (A) Western blot analysis of HeLa cells treated with PTB siRNA 1 (lane 1) and siRNA 2 (lane 2), both of which specifically target PTB RNA. NS siRNA is a nonspecific RNAi duplex (lane 3), and in lane 4 no siRNA was transfected into the cells. PTB expression was dramatically reduced, as shown in lanes 1 and 2. Actin was used as an internal control, and its expression remains unchanged (lanes 1 to 4, lower panel). Depletion of PTB in HeLa cells has no effect on the efficiency of the β-globin pA signal (B) or α-globin pA signal (C). S1 analysis of cytoplasmic RNA isolated from HeLa cells transiently transfected with β (B) or α (C) constructs and treated with either siRNA 1 (lanes 1) or siRNA 2 (lanes 2) are analyzed. Cells were treated with an NS siRNA duplex (lanes 3) or with no siRNA (lanes 4). Va was used as a cotransfection control. Lanes M, size markers. Quantitation showed no significant decrease in the levels of steady-state RNA.
FIG. 7.
Both overexpression and in vivo knockdown of PTB by RNAi significantly reduce the C2 complement pA site use. (A) Depletion of PTB in HeLa cells cotransfected with the construct C2 reduces the levels of steady-state RNA up to threefold, while overexpression of PTB also reduces steady-state RNA levels up to threefold. S1 analysis of cytoplasmic RNA isolated from HeLa cells transiently transfected with the C2 construct and treated with either siRNA 1 (lane 1) or siRNA 2 (lane 2) is shown. For lanes 3 and 4, cells were treated with an NS siRNA duplex (lane 3) or with no duplex (lane 4). Va was used as a cotransfection control. Lane M, size markers. On the right panel, an S1 analysis of cytoplasmic RNA isolated from HeLa cells transiently transfected with the C2 construct (lane 1) and cotransfected with a PTB-1-expressing plasmid (lane 2) is shown. Lanes 3 and 4 are controls, minus RNA ± S1 nuclease. Va was used as a cotransfection control (Va cot). (B) PTB and CstF64 compete for the same pre-mRNA binding sites in a UV cross-linking competition assay. Zero, ten, fifty, one hundred, or two hundred fifty nanograms of rPTB was added to a CstF and CPSF purified protein fraction. PTB and CstF64 compete for the C2 complement pA pre-mRNA, as shown by SDS-polyacrylamide gel electrophoresis.
Although PTB is shown here to act as a positive activator of the C2 pA signal, it is also known that CstF is required for the recognition and polyadenylation of the C2 pA site (36). Consistent with this fact is the observation that overexpression of PTB blocks mRNA expression from the C2 gene construct, just as we observed for the α and β gene constructs (Fig. 7A). Thus, HeLa cells transfected with the C2 construct showed reduced 3′-end signals when cotransfected with a PTB expression plasmid. To verify that PTB was able to bind the C2 USE in competition with CstF64, we performed UV cross-linking experiments with a synthetic C2 pA signal RNA. The pA signal was first incubated with CstF64, and then increasing amounts of recombinant PTB were added to the reaction. Figure 7B shows that PTB was able to efficiently compete with CstF64 for binding to the C2 USE. This result confirms that PTB can act as a negative regulator of both DSE-dependent and USE-dependent pA sites by blocking the binding of CstF64 and thereby inhibiting 3′-end processing.
DISCUSSION
The results presented in this paper suggest an unanticipated role for PTB in mRNA 3′-end processing. We show that increasing the concentration of PTB in cells results in the inhibition of mRNA 3′-end processing through the direct competition of CstF and PTB for binding to the pyrimidine-rich downstream sequence elements commonly present in metazoan pA signals. PTB may thus play an analogous role in alternative splicing and 3′-end processing; in both cases PTB acts as a negative regulator by competing for binding sites with an activating factor. In the case of splicing it is the U2AF protein; in the case of 3′-end processing it is the CstF complex. There is one significant difference between the two pathways, however. In splicing PTB can select different exons for alternative splicing but doesn't normally have any effect on the overall level of gene expression (51, 55). A caveat to this is that if PTB-selected alternative splicing leads to inclusion of a premature stop codon in the final mRNA, then nonsense-mediated decay will be induced and so cause reduced mRNA levels (35, 59). Indeed, such an effect occurs with the autoregulation of PTB itself. Here alternative splicing of PTB mRNA is induced by PTB overexpression, causing inclusion of premature stop codons in the mRNA and subsequent mRNA degradation (62). In contrast, PTB-mediated inhibition of 3′-end processing leads directly to a reduction in gene expression. The less efficiently an mRNA is polyadenylated, the fewer translatable mRNAs will be produced, and consequently protein production decreases (21, 46).
In the particular case of the C2 pA signal, the USE contains a high-affinity binding site for PTB. Previous in vitro experiments indicated that PTB is required for full activity of the pA site (36). Our results confirm these data in vivo by showing that PTB knockdown (by RNAi) significantly reduces the activity of the C2 pA signal (Fig. 7). It is possible that the binding of PTB to the C2 USE aids recruitment of either or both CPSF and CstF, so activating C2 polyadenylation. However, the C2 pA signal, like the α and β pA sites, is also repressed by higher levels of PTB, suggesting that the C2 gene might be particularly sensitive to changes in the cellular PTB concentration. CstF was previously shown to be required for in vitro polyadenylation of the C2 pA site following 3′ end cleavage. This unusual role of CstF may reflect the absence of a clear DSE for the human C2 pA signal. It is, however, interesting that some mammalian C2 pA signals (notably rabbit) (37) possess clear DSE sequences in addition to the conserved USE. Whether CstF activates 3′ end cleavage or polyadenylation of C2, it is still likely that CstF will interact with either or both the USE and potential weak DSE sequences and so activate C2 cleavage and polyadenylation. This interaction with potentially weak CstF RNA binding sites may well be outcompeted by PTB binding. Thus, the inhibition of C2 polyadenylation when PTB is overexpressed is clearly explicable.
The data presented in this study suggest that a modest variation in PTB protein concentration can significantly reduce the efficiency of polyadenylation. Thus, in the in vitro 3′ end cleavage assays (Fig. 4), a twofold increase in the level of PTB caused a threefold reduction in 3′ end processing. Similarly, we have shown in vivo that a fourfold increase in PTB caused a 75% reduction in mRNA levels. Interestingly, it has been previously demonstrated that the levels of PTB mRNA can vary at least 10-fold between different tissues at different developmental stages (40). In Drosophila, the PTB homologue is almost exclusively expressed in the male fly and may play a specific role in sex determination (48). The possibility therefore exists that physiological variations in PTB levels could significantly alter the level of specific mRNA classes in different cell types by a mechanism involving inhibition of 3′ end processing. This may be especially significant for mRNAs that lack strong DSEs (CstF binding sites) so that PTB competition with CstF can occur at lower PTB concentrations.
Our results showing a direct role for PTB in pA site recognition extend our understanding of the interplay between mRNA splicing and polyadenylation. Previously it has been shown both in vivo and in vitro that polyadenylation is strongly activated by splicing of the terminal intron (14, 38, 39). The molecular details of this process are known to involve the recognition of the terminal intron 3′SS by U2AF. This promotes recruitment of pA factors to the downstream pA signals through interaction between U2AF65 and the C-terminal domain of poly(A) polymerase (32, 54). Interestingly, PTB can prevent terminal exon definition in two ways. First, it can block U2AF binding to the polypyrimidine tract in the 3′SS. Second, as shown in these studies, it can block binding of CstF64 to the pA site and thereby inhibit 3′ end cleavage and processing. A direct role for PTB in 3′ exon definition has also been shown in the regulated alternative splicing of the calcitonin gene exon 4. Here a downstream intronic enhancer element was shown to bind PTB in competition with U2AF. This causes inclusion of exon 4 as the terminal exon by activating an otherwise inactive intronic pA signal (29).
In conclusion, it is clear that the abundant hnRNP protein PTB has the potential to modulate a number of different steps on the road to gene expression. While its effect on alternative splicing is now well recognized, PTB has also been shown to play a role in selecting internal ribosome entry sites to aid translation of particular mRNA coding sequences (26, 33). We now add to this list of PTB functions the ability to modulate 3′ end processing. In view of both the general down-regulation of pA site use by PTB overexpression, as well as its specific effect on C2 and presumably other related pA signals, we predict that PTB may play an important role in modulating protein expression levels through its pA-specific effects.
Acknowledgments
We thank the members of the N.J.P. lab for helpful suggestions throughout these studies. CstF and CPSF purified protein fractions were the generous gifts of Kevin Ryan and James L. Manley.
P.C.-B. was supported by the Portuguese Fundação para a Ciência e Tecnologia. This work was supported by an MRC project grant to N.J.P. and Wellcome Programme grants to N.J.P. and C.S.
REFERENCES
- 1.Ashe, H. L., J. Monks, M. Wijgerde, P. Fraser, and N. J. Proudfoot. 1997. Intergenic transcription and trans-induction of the human β-globin locus. Genes Dev. 11:2494-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ashe, M. P., P. Griffin, W. James, and N. J. Proudfoot. 1995. Poly(A) site selection in the HIV-1 provirus: inhibition of promoter-proximal polyadenylation by the downstream major splice donor site. Genes Dev. 9:3008-3025. [DOI] [PubMed] [Google Scholar]
- 3.Ashe, M. P., A. Furger, and N. J. Proudfoot. 2000. Poly(A) site occlusion in the 5′LTR of the HIV-1 provirus principally depends on the close proximity of U1 snRNP, stem loop 1. RNA 6:170-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black, D. L. 2003. Mechanisms of alternative pre-messenger RNA splicing. Annu. Rev. Biochem. 72:291-336. [DOI] [PubMed] [Google Scholar]
- 5.Brackenridge, S., and N. J. Proudfoot. 2000. Recruitment of a basal polyadenylation factor by the upstream sequence element of the human lamin B2 polyadenylation signal. Mol. Cell. Biol. 20:2660-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, P. H., L. S. Tiley, and B. R. Cullen. 1991. Efficient polyadenylation within the human immunodeficiency virus type 1 long terminal repeat requires flanking U3-specific sequences. J. Virol. 65:3340-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burge, C. B., T. Tuschl, and P. A. Sharp. 1999. Splicing of precursors to mRNAs by the spliceosomes. In R. F. Gesteland, T. R. Cech, and J. F. Atkins (ed.), The RNA world, p. 525-560. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 8.Carswell, S., and J. C. Alwine. 1989. Efficiency of utilization of the simian virus 40 late polyadenylation site: effects of upstream sequences. Mol. Cell. Biol. 9:4248-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cherrington, J., and D. Ganem. 1992. Regulation of polyadenylation in HIV: contributions of promoter proximity and upstream sequences. EMBO J. 11:1513-1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colgan, D., and J. L. Manley. 1997. Mechanism and regulation of mRNA polyadenylation. Genes Dev. 11:2755-2766. [DOI] [PubMed] [Google Scholar]
- 11.Chou, M.-Y., J. G. Underwood, J. Nikolic, M. H. T. Luu, and D. L. Black. 2000. Multisite RNA binding and release of polypyrimidine tract binding protein during the regulation of c-src neural-specific splicing. Mol. Cell 5:949-950. [DOI] [PubMed] [Google Scholar]
- 12.Davis, L., M. Kuehl, and J. Battey. 1994. Basic methods in molecular biology. Appleton & Lange, Norwalk, Conn.
- 13.DeZazzo, J. D., J. E. Kilpatrick, and M. J. Imperiale. 1991. Involvement of long terminal repeat U3 sequences overlapping the transcription control region in human immunodeficiency virus type 1 mRNA 3′ end formation. Mol. Cell. Biol. 11:1624-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dye, M. J., and N. J. Proudfoot. 1999. Terminal exon definition occurs co- transcriptionally and promotes termination of RNA polymerase II. Mol. Cell 3:371-378. [DOI] [PubMed] [Google Scholar]
- 15.Eggermont, J., and N. J. Proudfoot. 1993. Poly(A) signals and transcriptional pause sites combine to prevent interference between RNA polymerase II promoters. EMBO J. 12:2539-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elbashir, S. M., J. Harborth, W. Lendeckel, A. Yalcin, K. Weber, and T. Tuschl. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411:494-498. [DOI] [PubMed] [Google Scholar]
- 17.Enriquez-Harris, P., N. Levitt, D. Briggs, and N. J. Proudfoot. 1991. A pause site for RNA polymerase II is associated with termination of transcription. EMBO J. 10:1833-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furger, A., J. Monks, and N. J. Proudfoot. 2001. The retroviruses human immunodeficiency virus type 1 and Moloney murine leukemia virus adopt radically different strategies to regulate promoter-proximal polyadenylation. J. Virol. 75:11735-11746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furger, A., J. M. O'Sullivan, A. Binnie, B. A. Lee, and N. J. Proudfoot. 2002. Promoter proximal splice sites enhance transcription. Genes Dev. 16:2792-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia-Blanco, M. A., S. F. Jamison, and P. A. Sharp. 1989. Identification and purification of a 62,000-dalton protein that binds specifically to the polypyrimidine tract of introns. Genes Dev. 3:1874-1886. [DOI] [PubMed] [Google Scholar]
- 21.Gehring, N. H., U. Frede, G. Neu-Yilik, P. Hundsdoerfer, B. Vetter, M. W. Hentze, and A. E. Kulozik. 2001. Increased efficiency of mRNA 3′ end formation: a new genetic mechanism contributing to hereditary thrombophilia. Nat. Genet. 28:389-392. [DOI] [PubMed] [Google Scholar]
- 22.Ghetti, A., S. Pinol-Roma, W. M. Michael, C. Morandi, and G. Dreyfuss. 1992. HnRNP I, the polypyrimidine tract binding protein: distinct nuclear localization and association with hnRNAs. Nucleic Acids Res. 20:3671-3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gil, A., P. A. Sharp, S. F. Jamison, and M. A. Garcia-Blanco. 1991. Characterization of cDNAs encoding the polypyrimidine tract-binding protein. Genes Dev. 5:1224-1236. [DOI] [PubMed] [Google Scholar]
- 24.Gilmartin, G. M., E. S. Fleming, and J. Oetjen. 1992. Activation of HIV-1 pre-mRNA 3′ processing in vitro requires both an upstream element and TAR. EMBO J. 11:4419-4428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilmartin, G. M., and J. R. Nevins. 1991. Molecular analyses of two poly(A) site-processing factors that determine the recognition and efficiency of cleavage of the pre-mRNA. Mol. Cell. Biol. 11:2432-2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gosert, R., K. H. Chang, R. Rijnbrand, M. Yi, D. V. Sangar, and S. M. Lemon. 2000. Transient expression of cellular polypyrimidine-tract binding protein stimulates cap-independent translation directed by both picornaviral and flaviviral internal ribosome entry sites in vivo. Mol. Cell. Biol. 20:1583-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hannon, G. J. 2002. RNA interference. Nature 418:244-251. [DOI] [PubMed] [Google Scholar]
- 28.Lin, C. H., and J. G. Patton. 1995. Regulation of alternative 3′ splice site selection by constitutive splicing factors. RNA 1:234-245. [PMC free article] [PubMed] [Google Scholar]
- 29.Lou, H., D. M. Helfman, R. F. Gagel, and S. M. Berget. 1999. Polypyrimidine tract binding protein positively regulates inclusion of an alternative 3′ terminal exon. Mol. Cell. Biol. 19:78-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 31.Markovtsov, V., J. M. Nikolic, J. A. Goldman, C. W. Turck, M. Y. Chou, and D. L. Black. 2000. Cooperative assembly of an hnRNP complex induced by a tissue-specific homolog of polypyrimidine tract binding protein. Mol. Cell. Biol. 20:7463-7479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Millevoi, S., F. Geraghty, B. Idowu, J. L. Y. Tam, M. Antoniou, and S. Vagner. 2002. A novel function for the U2AF65 splicing factor in promoting pre-mRNA 3′-end processing. EMBO Rep. 3:869-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell, S. A., K. A. Spriggs, M. J. Coldwell, R. J. Jackson, and A. E. Willis. 2003. The Apaf-1 internal ribosome entry segment attains the correct structural conformation for function via interactions with PTB and unr. Mol. Cell 11:757-771. [DOI] [PubMed] [Google Scholar]
- 34.Moore, M. J. 2000. Intron recognition comes of age. Nat. Struct. Biol. 7:14-16. [DOI] [PubMed] [Google Scholar]
- 35.Moore, M. J. 2002. Nuclear RNA turnover. Cell 108:431-434. [DOI] [PubMed] [Google Scholar]
- 36.Moreira, A., Y. Takagaki, S. Brackenridge, M. Wollerton, J. L. Manley, and N. J. Proudfoot. 1998. The upstream sequence element of the C2 complement poly(A) signal activates mRNA 3′ end formation by two distinct mechanisms. Genes Dev. 12:2522-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreira, A., M. Wollerton, J. Monks, and N. J. Proudfoot. 1995. Upstream sequence elements enhance poly(A) site efficiency of the C2 complement gene and are phylogenetically conserved. EMBO J. 14:3809-3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niwa, M., C. C. MacDonald, and S. M. Berget. 1992. Are vertebrate exons scanned during splice site selection? Nature 360:277-280. [DOI] [PubMed] [Google Scholar]
- 39.Niwa, M., S. D. Rose, and S. M. Berget. 1990. In vitro polyadenylation is stimulated by the presence of an upstream intron. Genes Dev. 4:1552-1559. [DOI] [PubMed] [Google Scholar]
- 40.Patton, J. G., S. A. Mayer, P. Tempst, and B. Nadal Ginard. 1991. Characterization and molecular cloning of polypyrimidine tract-binding protein: a component of a complex necessary for pre-mRNA splicing. Genes Dev. 5:1237-1251. [DOI] [PubMed] [Google Scholar]
- 41.Perez, I., C. H. Lin, J. G. McAfee, and J. G. Patton. 1997. Mutation of PTB binding sites causes misregulation of alternative 3′ splice site selection in vivo. RNA 3:764-778. [PMC free article] [PubMed] [Google Scholar]
- 42.Perez, I., J. G. McAfee, and J. G. Patton. 1997. Multiple RRMs contribute to RNA binding specificity and affinity for polypyrimidine tract binding protein. Biochemistry 36:11881-11890. [DOI] [PubMed] [Google Scholar]
- 43.Plant, K. E., S. J. Routledge, and N. J. Proudfoot, N. J. 2001. Intergenic transcription in the human beta-globin gene cluster. Mol. Cell. Biol. 21:6507-6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Proudfoot, N. J. 1991. Poly(A) signals. Cell 64:671-674. [DOI] [PubMed] [Google Scholar]
- 45.Proudfoot, N. J. 1996. Ending the message is not so simple. Cell 87:779-781. [DOI] [PubMed] [Google Scholar]
- 46.Proudfoot, N. J. 2001. Genetic dangers in poly(A) signals. EMBO Rep. 2:891-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reed, R., and E. Hurt. 2002. A conserved mRNA export machinery coupled to pre-mRNA splicing. Cell 22:523-531. [DOI] [PubMed] [Google Scholar]
- 48.Robida, M. D., and R. Singh. 2003. Drosophila polypyrimidine-tract binding protein (PTB) functions specifically in the male germ line. EMBO J. 22:2924-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ruskin, B., and M. R. Green. 1985. Role of the 3′ splice site consensus sequence in mammalian pre-mRNA splicing. Nature 317:732-734. [DOI] [PubMed] [Google Scholar]
- 50.Singh, R., J. Valcarcel, and M. R. Green. 1995. Distinct binding specificities and functions of higher eukaryotic polypyrimidine tract-binding proteins. Science 268:1173-1176. [DOI] [PubMed] [Google Scholar]
- 51.Smith, C. W., and J. Valcarcel. 2000. Alternative pre-mRNA splicing: the logic of combinatorial control. Trends Biochem. Sci. 25:381-388. [DOI] [PubMed] [Google Scholar]
- 52.Takagaki, Y., and J. Manley. 1997. RNA recognition by the human polyadenylation factor CstF. Mol. Cell. Biol. 17:3907-3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takagaki, Y., J. L. Manley, C. C. MacDonald, J. Wilusz, and T. A. Shenk. 1990. A multisubunit factor, CstF, is required for polyadenylation of mammalian pre-mRNAs. Genes Dev. 4:2112-2120. [DOI] [PubMed] [Google Scholar]
- 54.Vagner, S., C. Vagner, and I. W. Mattaj. 2000. The carboxyl terminus of vertebrate poly(A) polymerase interacts with U2AF 65 to couple 3′-end processing and splicing. Genes Dev. 14:403-413. [PMC free article] [PubMed] [Google Scholar]
- 55.Valcarcel, J., and F. Gebauer. 1997. Post-transcription regulation: the dawn of PTB. Curr. Biol. 7:705-708. [DOI] [PubMed] [Google Scholar]
- 56.Valsamakis, A., S. Zeichner, S. Carswell, and J. C. Alwine. 1991. The human immunodeficiency virus type 1 polyadenylation signal: a 3′ long terminal repeat element upstream of the AAUAAA necessary for efficient polyadenylation. Proc. Natl. Acad. Sci. USA 88:2108-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagner, E. J., and M. A. Garcia-Blanco. 2001. Polypyrimidine tract binding protein antagonizes exon definition. Mol. Cell. Biol. 10:3281-3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wagner, E. J., and M. A. Garcia-Blanco. 2002. RNAi-mediated PTB depletion leads to enhanced exon definition. Mol. Cell 10:943-949. [DOI] [PubMed] [Google Scholar]
- 59.Wilusz, C. J., W. Wang, and S. W. Peltz. 2001. Curbing the nonsense: the activation and regulation of mRNA surveillance. Genes Dev. 15:2781-2785. [DOI] [PubMed] [Google Scholar]
- 60.Wilusz, J., and T. Shenk. 1988. A 64 kd nuclear protein binds to RNA segments that include the AAUAAA polyadenylation motif. Cell 52:221-228. [DOI] [PubMed] [Google Scholar]
- 61.Wollerton, M. C., C. Gooding, F. Robinson, E. C. Brown, R. J. Jackson, and C. W. J. Smith. 2001. Differential alternative splicing activity of isoforms of polypyrimidine tract binding protein (PTB). RNA 7:819-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wollerton, M. C., C. Gooding, E. J. Wagner, M. A. Garcia-Blanco, and C. W. J. Smith. 2004. Autoregulation of polypyrimidine tract binding protein by alternative splicing leading to nonsense-mediated decay. Mol. Cell 13:91-100. [DOI] [PubMed] [Google Scholar]
- 63.Zamore, P. D., J. G. Patton, and M. R. Green. 1992. Cloning and domain structure of the mammalian splicing factor U2AF. Nature 355:609-614. [DOI] [PubMed] [Google Scholar]
- 64.Zhao, J., L. Hyman, and C. Moore. 1999. Formation of mRNA 3′ ends in eukaryotes: mechanism, regulation, and interrelationships with other steps in mRNA synthesis. Microbiol. Mol. Biol. Rev. 63:405-445. [DOI] [PMC free article] [PubMed] [Google Scholar]