Abstract
Antineoplastic chemotherapeutic agents may indirectly activate dendritic cells (DCs) by inducing the release of “danger” signals from dying tumor cells. Whereas the direct cytotoxic or inhibitory effect of conventional chemotherapy on DCs has been reported, modulation of DC function by chemotherapeutic agents in low noncytotoxic concentrations has not yet been investigated. We have tested the effects of different classes of antineoplastic chemotherapeutic agents used in low noncytotoxic concentrations on the Ag-presenting function of DCs. We revealed that paclitaxel, doxorubicin, mitomycin C, and methotrexate up-regulated the ability of DCs to present Ags to Ag-specific T cells. Stimulation of DC function was associated with the up-regulation of expression of Ag-processing machinery components and costimulatory molecules on DCs, as well as increased IL-12p70 expression. However, the ability of DCs treated with paclitaxel, methotrexate, doxorubicin, and vinblastine to increase Ag presentation to Ag-specific T cells was abolished in DCs generated from IL-12 knockout mice, indicating that up-regulation of Ag presentation by DCs is IL-12-dependent and mediated by the autocrine or paracrine mechanisms. At the same time, IL-12 knockout and wild-type DCs demonstrated similar capacity to up-regulate OVA presentation after their pretreatment with low concentrations of mitomycin C and vincristine, suggesting that these agents do not utilize IL-12-mediated pathways in DCs for stimulating Ag presentation. These findings reveal a new mechanism of immunopotentiating activity of chemotherapeutic agents—a direct immunostimulatory effect on DCs (chemomodulation)—and thus provide a strong rationale for further assessment of low-dose chemotherapy given with DC vaccines for cancer treatment.
Antineoplastic chemotherapeutic drugs induce cell cycle arrest and apoptosis not only in tumor cells, but also in nonmalignant cells, including those of the immune system. However, early preclinical clinical studies have highlighted an important phenomenon; that is, although vaccines are less effective in patients heavily pretreated with chemotherapy, no detrimental effects in immune responses to vaccines occur in patients when the vaccine is given in combination with certain chemotherapeutic agents, for example, 5-fluorouracil and docetaxel (1). Recent findings suggest that some chemotherapeutic agents can improve the efficacy of immunotherapy by different means (2, 3).
Chemotherapy might be associated with lymphodepletion, which can lead to the elimination of regulatory T cells and poorly functional antitumor T cells (2). It has been shown that, in moderate doses, cyclophosphamide acts via the elimination of regulatory T cells (4–6), while gemcitabine attenuates the tumor-suppressive environment by eliminating myeloid-derived suppressor cells (7, 8). Tumor cell death induced by chemotherapeutic drugs might be accompanied by the release of multiple “danger” signals and facilitate the engulfment of tumor Ags (9). For instance, doxorubicin, 5-fluorouracil, gemcitabine, and paclitaxel induce apoptosis of tumor cells, resulting in the accessibility of tumor Ags, increased immunogenicity of tumor cells, and up-regulation of CD8+ T cell function (10–13). Dendritic cells (DCs)3 can use chemotherapy-induced apoptosis of tumor cells to acquire Ags from dying cells (14–16). In fact, cyclophosphamide augmented the antitumor effects of DC vaccines in melanoma and colon carcinoma models (6, 17) and gemcitabine increased the survival of tumor-bearing mice treated with DC vaccines in a pancreatic tumor model (18).
The molecular pathways of certain “immunogenic” forms of cancer chemotherapy that cause DC activation have recently been defined. Anthracyclins, DNA-damaging compounds, have been shown to induce the translocation of calreticulin to the surface of dying tumor cells promoting phagocytosis by DCs (19). High-mobility group box 1 (HMGB1) alarmin protein secreted by dying tumor cells interacts with TLR4 on DCs to cause immunogenic cross-presentation (20). Also, many chemotherapeutic agents induce production of uric acid caused by tumor cell death, which may activate DCs, thereby promoting tumor rejection (21). Thus, the efficacy of successful combinations of conventional chemotherapy and DC vaccines was in part due to the accessibility of Ags, expression of calreticulin, and release of alarmin and uric acid from dying tumor cells, that is, a reliance on the cytotoxic effect of chemotherapy on tumor cells (6, 21, 22). However, the direct effects of chemotherapeutics in low noncytotoxic doses on DCs have not yet been established.
Chao et al. reported that DCs had different sensitivities to conventional cytotoxic agents (23). Thus, DCs were sensitive to doxorubicin and vinblastine, but relatively resistant to etoposide and 5-fluorouracil, while cisplatin and vincristine triggered DC apoptosis both in vitro and in vivo (24, 25). Docetaxel suppressed the motility of DCs toward MIP-1α and MIP-3β (26), while mitomycin C down-regulated expression of CD80 and CD86 on DCs (25, 27). In vivo, the levels of conventional DC subsets were markedly decreased in the bone marrow of cancer patients receiving cytostatic treatments (28), and the myeloid-to-plasmacytoid DC ratio was significantly lower in ovarian cancer patients receiving chemotherapy (29). Similarly, compared with untreated patients, chemotherapy-treated colorectal cancer patients had 30% fewer DC subsets and fewer monocyte-derived DCs (30).
However, while antineoplastic agents in conventional and moderately low doses were shown to suppress DC function and longevity, a recent study reported that paclitaxel in low nontoxic concentrations could up-regulate DC function in vitro (31). Furthermore, it was demonstrated that several antineoplastic chemotherapeutic agents in low concentrations did not induce apoptosis of DCs, but activated small Rho GTPases in DCs, which play a key role in regulating cell motility, endocytosis, and cell-cell interaction (32). These results raise the question whether common chemotherapeutic drugs in low, noncytotoxic concentrations might directly modulate functional activity of DCs.
In the present study, we have tested the effect of different classes of antineoplastic chemotherapeutic agents used in low, noncytotoxic concentrations on the Ag-presenting function of DCs. We revealed that paclitaxel, doxorubicin, mitomycin C, and methotrexate directly up-regulated the ability of DCs to present Ags to Ag-specific T cells in vitro. This was associated with the up-regulation of Ag-processing machinery (APM) proteins and costimulatory molecules on DCs, that is, signals 1 and 2 for T-dependent immune responses. Recently, a class of third signals, often delivered by DCs, has been shown to greatly enhance immune responses, especially against tumors (33, 34). Among signal 3 factors, IL-12 is particularly effective (35, 36). We have demonstrated that the addition of low concentrations of paclitaxel, doxorubicin, methotrexate, and vinblastine to DCs resulted in increased IL-12p70 expression in DCs. DCs isolated from IL-12 knockout mice and treated with paclitaxel, methotrexate, doxorubicin, and vinblastine, but not mytomycin C and vincristine, did not display the ability to up-regulate presentation of OVA Ag to OVA-specific CD8+ T cells. In summary, these findings revealed a new mechanism of the immunopotentiating activity of chemotherapeutic agents—a direct stimulatory effect on DCs or chemomodulation of DCs—and thus provide a strong rationale for further assessment of low-dose chemotherapy given with DC vaccines for cancer treatment.
Materials and Methods
Animals
Six- to 8-wk-old male C57BL/6 (Taconic), IL-12 knockout, and control WT mice (The Jackson Laboratory) were housed in a pathogen-free facility under controlled temperature, humidity, and 12-h light/dark cycle with food and water available ad libitum. Mice were acclimatized for at least 2 wk before the experiments.
Chemotherapeutic agents, Abs, and cytokines
Chemotherapeutic agents vinblastine, vincristine, paclitaxel, methotrexate, mitomycin C, doxorubicin, and bleomycin were from Calbiochem. FITC-labeled anti-mouse I-Ab, CD86, CD80, CD40, PE-labeled anti-mouse CD11c, FITC- or PE-labeled isotype-matched controls, and PE-labeled anti-mouse IL-12 (clone C15.6, p40/p70) mAbs were from BD Pharmingen. Monensin, an inhibitor of intracellular protein transport, was from eBioscience. Abs to APM components including anti-low molecular mass polypeptide (LMP)7, -LMP10, -delta, -MB1, -tapasin, -TAP, and -calnexin were generated and characterized as described previously (37) and were provided by Dr. S. Ferrone (University of Pittsburgh Cancer Institute, Pittsburgh, PA). The recombinant mouse IL-4 and GM-CSF were from PeproTech.
DC cultures and cell analysis
Bone marrow cells were isolated from the femur and tibia, depleted of erythrocytes, CD4+, CD8+, and B220+ cells, and cultured overnight in a complete RPMI 1640 medium supplemented with 10% FBS, 100 U/100 µg/ml penicillin/streptomycin, 1 mM sodium pyruvate, 2 mM l-glutamine, and 0.1 mM nonessential amino acids (Invitrogen) as described earlier (37). The nonadherent cells were collected and seeded in complete medium containing 1000 U/ml GM-CSF and IL-4 for 6 days. The chemotherapeutics were added to DC cultures on day 1 or 5 and on day 6 DCs were harvested and analyzed as stated below. The low noncytotoxic concentrations of cytotoxic agents that do not induce apoptosis of tumor cell lines and bone marrow-derived DCs were selected as described earlier (32) and are shown in Tables I and II.
Table I.
Regulation of APM protein expression in DCs by chemotherapeutic agents in low concentrationsa
| Chemotherapeutic Agents | LMP7 | LMP10 | Delta | MB1 | Tapasin | TAP | Calnexin |
|---|---|---|---|---|---|---|---|
| Antimicrotubule agents | |||||||
| Vinblastine | |||||||
| 1 nM | 119.7 ± 10.8 | 96.0 ± 1.4 | 124.4 ± 6.8 | 82.1 ± 1.8 | 93.0 ± 0.9 | 121.8 ± 11.4 | 101.8 ± 4.8 |
| 0.2 nM | 141.2 ± 4.4 | 122.6 ± 6.5 | 173.8 ± 2.3 | 110.3 ± 12.2 | 121.0 ± 2.4 | 134.9 ± 4.4 | 133.5 ± 4.4 |
| Vincristine | |||||||
| 1 nM | 120.6 ± 4.4 | 179.1 ± 11.5 | 77.0 ± 4.6 | 81.1 ± 2.2 | 103.2 ± 1.8 | 104.6 ± 6.8 | 90.9 ± 5.6 |
| 0.2 nM | 104.2 ± 3.4 | 102.3 ± 8.4 | 92.1 ± 2.3 | 106.1 ± 4.4 | 113.9 ± 8.8 | 114.4 ± 5.8 | 116.5 ± 3.2 |
| Paclitaxel | |||||||
| 5 nM | 140.0 ± 15.1 | 174.5 ± 4.2 | 118.8 ± 8.6 | 143.9 ± 21.8 | 117.1 ± 17.5 | 113.5 ± 12.4 | 103.2 ± 4.5 |
| 1 nM | 113.5 ± 4.4 | 97.6 ± 7.5 | 99.4 ± 1.5 | 81.1 ± 1.8 | 107.0 ± 0.9 | 91.6 ± 3.4 | 77.6 ± 3.2 |
| Antimetabolites | |||||||
| Methotrexate | |||||||
| 5 nM | 121.1 ± 12.9 | 157.4 ± 15.8 | 148.6 ± 15.8 | 157.5 ± 16.7 | 186.8 ± 14.0 | 157.6 ± 6.9 | 131.7 ± 14.2 |
| 1 nM | 93.7 ± 4.4 | 86.0 ± 6.5 | 77.2 ± 8.4 | 85.1 ± 4.8 | 111.9 ± 4.6 | 129.1 ± 4.4 | 116.9 ± 4.4 |
| Alkylating agents | |||||||
| Mitomycin C | |||||||
| 50 nM | 149.3 ± 4.4 | 122.6 ± 6.5 | 172.2 ± 2.3 | 123.6 ± 1.8 | 150.0 ± 7.8 | 144.8 ± 4.4 | 88.3 ± 4.5 |
| 10 nM | 129.9 ± 4.4 | 101.9 ± 6.5 | 130.4 ± 5.4 | 107.5 ± 2.8 | 134.6 ± 5.6 | 130.2 ± 8.6 | 94.3 ± 4.8 |
| Topoisomerase inhibitors | |||||||
| Doxorubicin | |||||||
| 10 nM | 124.9 ± 12.9 | 108.1 ± 13.7 | 132.1 ± 15.8 | 130.4 ± 20.4 | 181.6 ± 16.9 | 117.5 ± 4.3 | 122.4 ± 8.6 |
| 2 nM | 127.9 ± 4.4 | 127.9 ± 6.5 | 99.2 ± 1.5 | 73.6 ± 1.8 | 140.0 ± 5.8 | 119.3 ± 3.4 | 96.7 ± 4.5 |
| Others | |||||||
| Bleomycin | |||||||
| 5 nM | 85.4 ± 4.4 | 107.5 ± 3.4 | 103.4 ± 2.3 | 98.1 ± 1.8 | 90.7 ± 0.99 | 132.1 ± 5.6 | 69.0 ± 4.5 |
| 1 nM | 131.5 ± 6.4 | 120.7 ± 6.5 | 110.5 ± 4.6 | 74.5 ± 1.8 | 86.0 ± 0.99 | 94.4 ± 4.4 | 112.8 ± 4.4 |
DC cultures were treated with different chemotherapeutic agents in indicated concentrations on day 1 for 48 h and intracellular expression of MHC class I AMP component proteins in DCs was assessed on day 6. The results were calculated on the basis of MFI values and are expressed as the percentage of MFI alterations in comparison to MFI of control untreated DCs. Increase in APM component expression >30% was considered biologically significant and is shown in boldface. The mean ± SEM values from three independent experiments are shown. LMP7 and LMP10 are the two subunits of immunoproteasome. Analyzed APM components include the following: constitutive β subunits of the proteolytic delta (or β1) and MB1 (β5); inducible proteasome (immunoproteasome) β-type subunits LMP7 and LMP10; peptide transporters TAP1 and TAP2; and endoplasmic reticulum chaperones calnexin and tapasin.
Table II.
Effects of chemotherapeutic agents in low, nontoxic concentrations on the phenotypic maturation of DCsa
| Chemotherapeutics | CD80 (%) | CD86 (%) | CD40 (%) | MHC Class II (%) |
|---|---|---|---|---|
| Control nontreated DCs | 40.1 ± 2.4 | 21.7 ± 1.6 | 8.6 ± 0.8 | 47.2 ± 2.4 |
| Vinblastine | ||||
| 1 nM | 42.7 ± 3.4 | 22.5 ± 1.8 | 10.4 ± 0.9 | 44.3 ± 2.3 |
| 0.2 nM | 45.7 ± 4.4 | 21.7 ± 2.2 | 8.1 ± 1.0 | 43.1 ± 3.2 |
| Vincristine | ||||
| 1 nM | 40.8 ± 3.8 | 23.4 ± 2.4 | 10.4 ± 0.8 | 55.2 ± 2.5 |
| 0.2 nM | 40.3 ± 2.4 | 23.1 ± 1.8 | 8.8 ± 0.9 | 54.9 ± 3.9 |
| Paclitaxel | ||||
| 5 nM | 53.6 ± 3.2 | 31.1 ± 2.8 | 14.0 ± 1.2 | 65.4 ± 4.8 |
| 1 nM | 47.3 ± 2.8 | 27.4 ± 1.6 | 11.3 ± 0.9 | 63.0 ± 5.2 |
| Methotrexate | ||||
| 5 nM | 72.3 ± 4.2 | 48.5 ± 3.6 | 19.9 ± 1.8 | 78.8 ± 5.4 |
| 1 nM | 49.2 ± 2.6 | 24.6 ± 2.4 | 10.4 ± 0.9 | 59.5 ± 4.6 |
| Mitomycin C | ||||
| 50 nM | 52.4 ± 3.2 | 24.7 ± 2.4 | 12.5 ± 1.2 | 47.3 ± 2.8 |
| 10 nM | 42.8 ± 2.6 | 23.5 ± 1.6 | 10.6 ± 0.8 | 46.6 ± 3.4 |
| Doxorubicin | ||||
| 10 nM | 54.5 ± 4.2 | 29.0 ± 1.8 | 11.1 ± 1.0 | 59.0 ± 4.8 |
| 2 nM | 48.1 ± 2.4 | 26.1 ± 2.8 | 11.1 ± 0.9 | 57.5 ± 3.6 |
| Bleomycin | ||||
| 5 nM | 44.8 ± 3.6 | 20.0 ± 1.8 | 9.7 ± 0.7 | 51.2 ± 4.2 |
| 1 nM | 46.1 ± 4.4 | 23.6 ± 2.2 | 10.1 ± 0.9 | 46.4 ± 2.8 |
DC cultures were treated with different chemotherapeutic agents in indicated concentrations on day 1 and DC phenotype was assessed on day 6. The results are expressed as the percentage of CD80+, CD86+, CD40+, and MHC class II+ cells within the CD11c+ DC population. The means ± SEM values are shown. The table summarizes the results of three independent experiments. The values of marker expression on treated DCs that are significantly higher or lower (p < 0.05, Student’s t test, n = 3) than the values of control nontreated DCs are shown in boldface.
Ag presentation assay
Presentation of OVA Ags by DCs was assessed as described (38). Briefly, control DCs and DCs treated with chemotherapeutic agents (0.5 × 106 cells/ml) were loaded with dialyzed OVA (1 mg/ml; Sigma-Aldrich) overnight. Then, DCs were washed and mixed (1 × 104 cells) with syngeneic B3Z86/90.14 (B3Z) OVA-specific T cells (2 × 104) in triplicate in 96-well plates. After 48 h, cell-free culture supernatants were assessed for IL-2 production by ELISA (Endogen) according to the manufacturer’s instructions. B3Z cells obtained from B3Z86/90.14 transgenic mice (H-2Kb, I-Ab) were provided by Dr. B. A. Osborne (University of Massachusetts, Amherst, MA).
Flow cytometry
For the phenotypic analysis, DCs were incubated with Abs for 30 min and washed in PBS containing 0.1% BSA and 0.1% sodium azide. For the intracellular staining of APM protein expression, DCs were washed in PBS with 0.5% BSA, fixed in 2% paraformaldehyde, and permeabilized in PBS containing 1% BSA and 0.1% saponin (37). Then, DCs were stained with Abs to individual APM components followed by incubation with FITC-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories). Murine IgG was used as a control. To examine intracellular IL-12p70 expression, DCs were treated with 2 µM monensin for 5 h, washed, fixed in 2% paraformaldehyde, and permeabilized with 0.1% saponin (Sigma-Aldrich) in PBS with 0.5% BSA. Cells were then stained with 0.06 µg of PE-conjugated rat anti-mouse IL-12 Ab. Cells were analyzed on FACScan flow cytometer using the CellQuest software (BD Biosciences). The results are expressed as the percentage of positive cells or the mean fluorescence intensity (MFI).
Data analysis
Statistical analysis of experimental data was performed with software package SigmaStat (STSS). For all analyses, the level of significance was set at probability of p < 0.05. ANOVA was used for comparison of multiple groups. For single comparison of two groups, the Student t test was used after evaluation of normality. Data are presented as the means ± SEM.
Results
Regulation of Ag-presenting function of DCs by low concentrations of chemotherapeutic agents
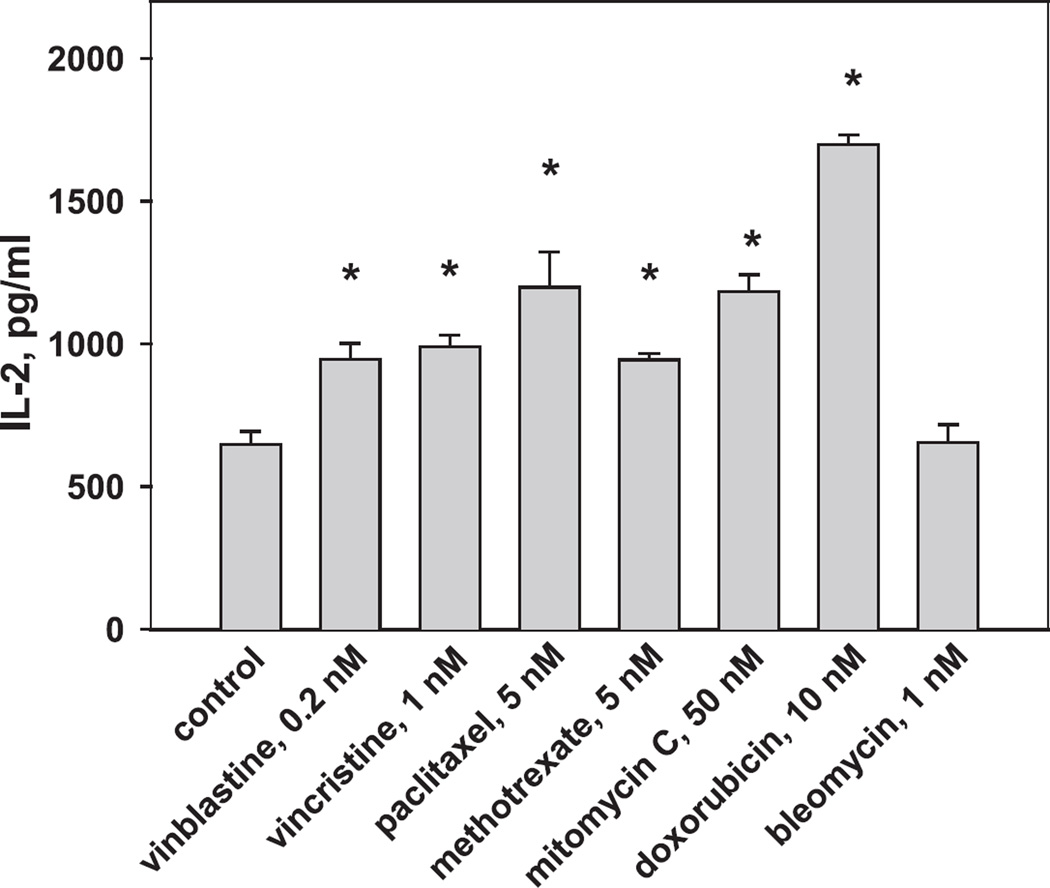
To determine how chemotherapeutic agents alter Ag processing and presentation by DCs, we have utilized OVA as a model Ag given that antigenic epitopes of this Ag have been well characterized. Concentrations of tested drugs were selected based on our previous data and were 10- to 15-fold lower than the lowest concentrations that induced apoptosis of DCs and tumor cells in vitro (32). From 13 initially selected antineoplastic chemotherapeutic agents, only 7 were chosen for these studies as demonstrating a well-displayed dose-dependent inhibition of DC viability in the annexin V/propidium iodide assay (32). DC cultures were treated with antineoplastic drugs on day 1, harvested on day 6, loaded with OVA, and mixed with B3Z CD8+ T cell clone specific for the H-2Kb-restricted SIINFEKL peptide of OVA. Production of IL-2, which reflects T cell activation upon recognition of the OVA epitope 257–264 in the context of the H-2Kb molecule, is shown in Fig. 1. As this figure depicts, vinblastine (0.2 nM), vincristine (1 nM), and methotrexate (5 nM) up-regulated OVA Ag presentation by DCs to Ag-specific T cells by up to 150% (p < 0.01, ANOVA). Paclitaxel (5 nM), mitomycin C (50 nM), and doxorubicin (10 nM) had even stronger effects and increased OVA presentation from 648.0 ± 45.5 pg/ml in control DCs to 1198.4 ± 123.0, 1184.6 ± 56.6, and 1698.4 ± 32.8 pg/ml in treated DCs, respectively (p < 0.01). Bleomycin had no effect on DCs in this assay.
FIGURE 1.
Up-regulation of Ag-presenting function of DCs by chemotherapeutic agents. DC cultures were initiated from the bone marrow precursors and treated with low concentrations of selected chemotherapeutics on day 1 for 48 h. Day 6 DCs were loaded with OVA overnight and cocultured with B3Z CD8+ T cell clone specific for the H-2Kb-restricted SIINFEKL peptide of OVA. Production of IL-2, which reflects T cell activation upon recognition of the OVA epitope 257–264 in the context of the H-2Kb molecule, was determined by ELISA. *, p < 0.01 vs control, ANOVA. Shown are the results of one representative experiment of three independent experiments with similar results. Data are expressed as the mean ± SEM. Control, nontreated DCs.
Treatment of DCs with chemotherapeutic drugs in low, noncytotoxic concentrations added to DC cultures on day 5 resulted in similar alterations of Ag-presenting function of DCs, although the magnitude of the effect was slightly smaller (data not shown). Taken together, these data demonstrate that selected chemotherapeutic drugs in low, noncytotoxic concentrations are able to increase Ag presentation by DCs. Because up-regulation of APC function of DCs is usually associated with cell maturation, the results in Fig. 1 raise the question whether the up-regulation of APC function of DCs by chemotherapeutic drugs correlates with the developmentally regulated expression of MHC class I Ag-processing machinery components.
Effect of chemotherapeutic agents on Ag-processing machinery in DCs
Next, we determined if chemotherapeutics alter Ag processing in DCs. Using Abs specific for APM components that have been tested in our laboratory (39, 40), we demonstrated that chemotherapeutic agents could up-regulate expression of key APM proteins in DCs in vitro (Table I), which has been shown to correlate with peptide-MHC class I complex expression and Ag presentation to T cells (37). For instance, methotrexate (5 nM) was a strong up-regulator of immunoproteasome, constitutive proteasome, and chaperone proteins in DCs (p < 0.05), while bleomycin (5 nM) had no significant effect on APM in DCs with only a slight down-regulation of calnexin expression. Mitomycin C (50 nM), doxorubicin (10 nM), paclitaxel (5 nM), and vinblastine (0.2 nM) increased expression of several APM proteins in DCs, while vincristine (1 nM) elevated only LMP10. The results in Table I were calculated on the basis of MFI values and are expressed as the percentage of MFI alterations in comparison to MFI in untreated DCs (n = 3). Similar results were obtained when the percentage of positive cells was assessed by flow cytometry. For instance, Fig. 2 shows the data from a representative experiment focusing on TAP binding protein (tapasin), which mediates the interaction between newly assembled MHC class I molecules and TAP. As shown, methotrexate and doxorubicin increased MFI values of tapasin expression in DCs by 3- and 2-fold, respectively. The percentage of positive cells also increased (Fig. 2). In contrast, bleomycin displayed a slight down-regulation of APM protein expression in DCs in vitro. Thus, chemotherapeutic agents, including methotrexate, paclitaxel, and doxorubicin, in low, noncytotoxic concentrations directly up-regulate expression of Ag-processing machinery components in DCs, which may represent the first mechanism (i.e., signal 1) of stimulation of APC function of DCs by these agents.
FIGURE 2.
Chemotherapeutic agents regulate expression of APM components in DCs. Bone marrow-derived DCs were treated with medium (control) or chemotherapeutic agents, collected, washed, fixed, permeabilized, and subjected to intracellular staining for different APM proteins as described in Materials and Methods. Staining for tapasin, TAP binding protein, which mediates the interaction between newly assembled MHC class I molecules and TAP, is shown as an example. Data from one representative experiment from three independent studies are shown; y-axis, event count; x-axis, fluorescence intensity; isotype control, open field. Chemotherapeutic agents were used in the following concentrations: methotrexate, 5 nM; doxorubicin, 10 nM; and bleomycin, 5 nM.
Regulation of DC maturation/activation by chemotherapeutic agents
Next, we tested how chemotherapeutic drugs in low, noncytotoxic concentrations affect DC maturation and expression of costimulatory molecules, that is, signal 2 for T cell activation. The results were expressed as the percentage of CD80+, CD86+, CD40+, and MHC class II+ cells within the CD11c+ DC population. Table II summarizes the results of three independent experiments and shows that paclitaxel (5 and 1 nM), methotrexate (5 and 1 nM), and doxorubicin (10 and 2 nM) significantly up-regulated expression of CD80, CD86, CD40, and MHC class II molecules on immature DCs, while mitomycin C (50 nM) significantly increased CD80 and CD40 expression (p < 0.05 vs control nontreated DCs). Vinblastine, vincristine, and bleomycin did not alter expression of CD80, CD86, CD40, and MHC class II molecules in tested concentrations (Table II). A representative example of actual flow cytometry data is shown in Fig. 3 and demonstrates that methotrexate (5 nM) shifted phenotypically immature DCs to a more mature phenotype; paclitaxel (5 nM) doubled the percentage of CD11c+CD86+ cells and increased the percentage of CD11c+MHC class II+ cells from 16% in control to 24% in treated group. Vincristine (1 nM) had no significant effect on DC phenotype. Analysis of MFI data confirmed these results and also demonstrated that paclitaxel, methotrexate, and doxorubicin increased expression of MHC II and costimulatory molecules on DCs. For instance, in the experiment shown in Fig. 3, MFI values for MHC class II expression on nontreated DCs was 219.8; on methotrexate-, paclitaxel-, and vincristine-treated DCs MFI values were 276.9, 320.3, and 224.3, respectively. These data were reproduced in three independent studies and shown in Table II. The data in Table II also demonstrate that paclitaxel, methotrexate, and doxorubicin shifted DCs to more mature phenotypes. For example, analysis of expression of costimulatory molecules on the total CD11c+ population assessed that in the presence of methotrexate, the expression of CD40 and MHC class II on DCs increased from 8.6% to 19.9% and from 47.2% to 78.8%, respectively (p < 0.05, Table II). Importantly, if chemotherapeutic agents were added to DC cultures on day 5, the results were similar, although to a lesser extent. Therefore, these data demonstrate that paclitaxel, methotrexate, and doxorubicin in low concentrations up-regulate the expression of costimulatory molecules on DCs and support DC maturation. Increase of costimulatory molecules on DCs (i.e., signal 2 for T cell activation) might represent the second mechanism of augmentation of Ag-presenting capacity of DCs by chemotherapeutic agents used in low, nontoxic concentrations.
FIGURE 3.
Phenotype of DCs treated with anticancer chemotherapeutic agents. Immature DCs were generated from the bone marrow hematopoietic precursors in cultures supplemented with GM-CSF and IL-4 as described in Materials and Methods. Chemotherapeutic agents were added on day 1 for 48 h in indicated concentrations. Treatment with medium served as a control. DCs were harvested and analyzed on day 6 by FASCan. The percentage of CD11c+MHC class II+ (A) and CD11c+CD86+ (B) DCs are shown. The data shown are representative of three independent experiments.
Chemotherapeutic agents in low concentrations increase IL-12 production by DCs
The strength of T cell activation is determined by the level of MHC-peptide complexes on APCs (signal 1), the expression of costimulatory molecules (signal 2), and a third signal delivered by IL-12, which is essential for the clonal expansion, effector function, and memory phenotype of T cells (34, 41, 42). Consequently, to explain the up-regulation of APC function of DCs by chemotherapeutics, we examined whether IL-12 expression could be modified in DCs by tested antineoplastic drugs. The results revealed that paclitaxel (5 nM) was the strongest inducer of IL-12 expression in DCs (p < 0.001, ANOVA). Vinblastine (0.2 nM), methotrexate (5 nM), mitomycin C (50 nM), and doxorubicin (10 nM) increased IL-12 expression by up to 75% (p < 0.05, ANOVA). For example, spontaneous intracellular IL-12 levels in DCs corresponded to 4.2 ± 0.3, 13.9 ± 0.8, 7.4 ± 0.7, and 5.7 ± 0.5 MFI in control, paclitaxel-, methotrexate-, and doxorubicin-treated DCs, respectively (p < 0.05 for all treated cells vs control). Vincristine (1 nM) and bleomycin (1 nM) had no effect on spontaneous IL-12 production (Fig. 4). Thus, these results demonstrate that paclitaxel, vinblastine, methotrexate, mitomycin C, and doxorubicin are able to induce IL-12 expression in nonactivated DCs and raise the next question whether the increased expression of IL-12 in DCs treated with chemotherapeutics is involved in upregulation of their Ag-presenting function.
FIGURE 4.
Regulation of IL-12 expression in DCs by chemotherapeutic drugs. DCs were differentiated from the bone marrow hematopoietic precursors in cultures supplemented with GM-CSF and IL-4 and treated with indicated concentrations of cytotoxic drugs for 48 h. DCs were collected on day 6 and intracellular staining for IL-12 was performed without any additional DC activators as described in Materials and Methods. The results are expressed as MFI. The data shown are the means ± SEM of three independent experiments. Control, nontreated DC; *, p < 0.05 vs control, ANOVA.
IL-12−/− DCs treated with low concentrations of paclitaxel, methotrexate, and doxorubicin lack their ability to up-regulate OVA peptide presentation to Ag-specific T cells
To reveal the role of IL-12 in augmenting the Ag-presenting function of DCs by low concentrations of cytotoxic drugs, DC cultures were initiated from the bone marrow precursors obtained from IL-12 knockout and control wild-type mice. DCs were then treated with chemotherapeutic agents and tested for their potential to present OVA Ag as above. Fig. 5 presents the results of these studies shown as the percentage of activation or inhibition of OVA presentation by drug-treated DCs relative to the control nontreated DCs. As expected, Ag presentation in wild-type DCs was up-regulated by all tested drugs by up to ~150–250% with the exception of bleomycin, confirming the data presented in Fig. 1. For example, Ag presentation by DCs treated with 5 nM paclitaxel and 10 nM doxorubicin was increased to 184.9 ± 12.4% and 186.3 ± 15.7%, respectively (p < 0.05, ANOVA, n = 3) when compared with the control nontreated DCs from wild-type mice. However, the ability of DCs treated with paclitaxel, methotrexate, doxorubicin, and vinblastine to increase Ag presentation to Ag-specific T cells was abolished in DCs generated from IL-12 knockout mice (Fig. 5). For instance, OVA Ag presentation by paclitaxel- and doxorubicin-treated DCs was 82.5 ± 7.8% and 70.1 ± 5.6% of control IL-12−/− DCs, respectively. This indicates that up-regulation of Ag presentation by DCs induced by paclitaxel, methotrexate, doxorubicin, and vinblastine is IL-12-dependent and most likely mediated by autocrine or paracrine mechanisms. At the same time, IL-12 knockout and wild-type DCs demonstrated similar capacity to up-regulate OVA presentation after their pretreatment with low concentrations of mitomycin C (50 nM) and vincristine (1 nM) (p < 0.05) (Fig. 5), suggesting that these agents do not utilize IL-12-mediated pathways in DCs for stimulating their APC function.
FIGURE 5.
IL-12 mediates chemotherapy-induced up-regulation of OVA Ag presentation by DCs. Bone marrow hematopoietic precursors were isolated from IL-12 knockout and control wild-type mice and treated with chemotherapeutics on day 1 for 48 h. Day 6 DCs were washed, pulsed with OVA (1 mg/ml, overnight), and cultured with B3Z CD8+ T cell clone specific for the H-2Kb-restricted SIINFEKL peptide. Production of IL-2 by T cells, which reflects T cell activation, was assessed by ELISA. The levels of OVA presentation by DCs from wild-type and IL-12 knockout mice after pretreatment with chemotherapeutic agents were compared. The data are presented as the percentage of activation or inhibition of OVA presentation by chemotherapeutic agent-treated DCs relative to control untreated DCs. The results of one representative experiment out of three independent experiments are shown; *, p < 0.05 wild type vs IL-12 knockout (one-way ANOVA, n = 3).
To understand how IL-12 regulates Ag presentation in DCs after chemomodulation, we also analyzed the expression of MHC and costimulatory molecules on wild-type and IL-12−/− DCs pretreated with chemotherapeutic drugs in low concentrations. The capacity of paclitaxel and doxorubicin to up-regulate MHC, CD80, CD86, and CD40 molecules on DCs were completely or significantly abrogated in the absence of IL-12 production by DCs (data not shown). This suggests that induced expression of IL-12 in paclitaxel- and doxorubicin-treated DCs plays a principal role in stimulating DC maturation and Ag processing and, in turn, in Ag presentation.
In summary, our results revealed that several chemotherapeutic agents are able to up-regulate DC maturation and Ag presentation when used in low nontoxic concentrations. These effects were mediated in the autocrine or paracrine manner by DC-derived IL-12, which was induced by pretreatment with paclitaxel, methotrexate, doxorubicin, and vinblastine, but not mytomycin C and vincristine.
Discussion
The way certain antineoplastic chemotherapeutic agents and dying tumor cells interact with the cells of the immune system determines the development of antitumor immune response and the efficacy of subsequent immunotherapy in chemo-immunotherapeutic protocols (19, 43, 44). Recent findings demonstrate that dying tumor cells could release “danger” signals (damage-associated molecular pattern, DAMP) that lead to the activation of DCs and may facilitate tumor Ag engulfing and processing (45, 46). Several DAMP molecules have been identified including HMGB1, heat shock proteins, S100 proteins, uric acid, and adenosine triphosphate (47, 48) (20, 46, 49–51). However, although conventional chemotherapy is capable of triggering the release of danger signals from dying tumor cells, these doses of cytotoxic drugs might be not optimal for DC function and longevity. DCs are sensitive to doxorubicin in 0.1–50 µM and vinblastine in 0.025–250 µM concentration range (23). Cisplatin and vincristine might trigger DC apoptosis in a wide range of concentrations (24, 25). We have recently evaluated the effect of low, nontoxic concentrations of paclitaxel in a murine lung carcinoma model and reported that it protects DCs from tumor-induced immunosuppression, stimulates DC maturation, and augments the antitumor efficacy of DC vaccines (31). It is possible that direct effects of paclitaxel on DCs play an important role in immune potentiating activities of low-dose chemotherapy in vivo. To pursue this possibility, it was our goal to verify direct immunostimulatory effects of different classes of chemotherapeutic agents, so-called chemomodulation, on DCs in vitro in the present study. The low concentrations of different types of chemotherapeutic drugs that are noncytotoxic for DCs have been recently reported (32). We took advantage of these data and determined the effects of several antineoplastic chemotherapeutic drugs on Ag-presenting properties of DCs.
We demonstrated that chemotherapeutic agents, if used in low noncytotoxic concentrations, up-regulated APC function of DCs: DCs pretreated with paclitaxel, methotrexate, doxorubicin, vinblastine, vincristine, and mitomycin C, but not bleomycin, displayed an increased potential to present the OVA Ag to Ag-specific CD8+ T cells. This immunopotentiating effect of selected drugs on the APC activity of DCs could be mediated by several pathways. T cell activation primarily depends on the expression of costimulatory molecules and MHC-peptide complexes on APCs, that is, signals 1 and 2 (52). Therefore, by analyzing the effect of low concentrations of chemotherapeutics on the expression of Ag-processing machinery components, we determined that increased Ag-presenting abilities of chemotherapy-treated DCs correlates with the up-regulation of MHC class I APM components including immunoproteasome, constitutive proteasome, and chaperone proteins for methotrexate ≫ vinblastine ≫ mitomycin C ≫ paclitaxel ≫ doxorubicin. At the same time, methotrexate ≫ paclitaxel ≫ doxorubicin ≫ mitomycin C significantly up-regulated expression of CD80, CD86, CD40, and MHC class II molecules on DCs. Taken together, these results suggest that the stimulation of DC maturation and Ag processing might be involved in the upregulation of APC function of DCs by selected chemotherapeutic agents. Furthermore, IL-12 might also play an important role in this phenomenon as a signal 3 molecule (34, 53), and, indeed, we revealed that paclitaxel ≫ methotrexate ≫ vinblastine ≫ mitomycin C ≫ doxorubicin increased spontaneous IL-12 expression in treated DCs, as assessed by intracellular staining. These results were confirmed by the Luminex multiplex-based technique in an independent study, where we demonstrated that paclitaxel and methotrexate were strong inducers of IL-12 production by DCs (54). In the same study, we showed that production of IL-10 was decreased by 30–40% in DCs after their pretreatment with low concentrations of paclitaxel, methotrexate, vinblastine, and mitomycin C. Thus, the results of cytokine production by DCs after chemomodulation are in agreement with the results showing an increased Ag-presenting capability of these DCs.
To further elucidate the role of DC-derived IL-12, cells were prepared from IL-12−/− precursors and tested in the same functional and phenotypic assays. We showed that the ability of DCs treated with paclitaxel, methotrexate, doxorubicin, and vinblastine to increase Ag presentation to Ag-specific T cells was abolished in DCs generated from IL-12 knockout mice. Interestingly, in the absence of IL-12, the APC potential of DCs treated with mitomycin C and vincristine was similar to that of wild-type DCs (i.e., was IL-12-independent). Comparative analysis of these results suggests that the tested drugs utilize different chemomodulatory mechanisms to stimulate Ag presentation by DCs. For instance, the moderate stimulation of OVA presentation by DCs induced by mitomycin C is primarily mediated by augmentation of Ag processing in DCs and to a lesser extent by increased expression of costimulatory molecules, with IL-12 playing no role. Paclitaxel and doxorubicin are likely to up-regulate APC activity of DCs by raising the level of IL-12 (at least for paclitaxel), stimulating expression of CD86, CD80, and CD40 and increasing Ag processing. Importantly, IL-12 played a fundamental role in Ag presentation since no amplification of OVA presentation was seen in IL-12−/− DCs.
Collectively, our data provide direct evidence of DC-stimulating activity of chemotherapeutic agents, with paclitaxel, methotrexate, and doxorubicin demonstrating the strongest effect on DCs in different assays; mitomycin C, vinblastine, and vincristine showing moderate to low effects; and bleomycin indicating no ability to change DC function in tested concentrations. Importantly, paclitaxel, methotrexate, doxorubicin, and mitomycin C represent different groups of antineoplastic chemotherapeutic agents (antimicrotubule, anti-metabolites, topoisomerase inhibitors, and alkylating agents, respectively; see Table I) with different mechanisms of cytotoxic and cytostatic effects on cells when used in conventional concentrations. Our results suggest that chemotherapeutic agents in low concentrations may operate through unusual signal transduction pathways in DCs. Indeed, we have recently reported that different classes of chemotherapeutic drugs in low, noncytotoxic concentrations regulate the activity of the small Rho GTPase family members, including Rac, RhoA, and RhoE in DCs (32). Thus, it is likely that among the three strongest stimulators of Ag presentation by DCs (doxorubicin, paclitaxel and mitomycin C; see Fig. 1), IL-12-dependent effect of doxorubicin and paclitaxel is mediated by STAT4, while the IL-12-independent effect of mitomycin C is mediated by activation of small Rho GTPases. These studies are in progress in our laboratory.
Further understanding of the molecular regulation of DCs by chemotherapeutic agents will allow us to modulate DC function by a single agent or their combinations in the most appropriate way. For instance, our new data demonstrate that chemomodulation of DCs increases their resistance to tumor-induced immunosuppression and might convert tolerogenic DCs into immunostimulatory DCs (M. R. Shurin et al., manuscript in preparation). Thus, modulation of DC function by noncytotoxic chemotherapy, that is, chemomodulation of DCs, might serve as a new, powerful tool to improve the efficacy of DC vaccines in the tumor microenvironment. Although the combination of chemotherapy and immunotherapy is not new, the development of a combinatorial chemo-immunotherapy based on direct DC-stimulating effects of selected antineoplastic agents used in very low, noncytotoxic doses is a novel direction and one with high translational potential.
Footnotes
These studies were supported by National Institutes of Health Grant RO1 CA084270 (to M.R.S.).
Abbreviations used in this paper: DC, dendritic cell; APM, Ag-processing machinery; LMP, low molecular mass polypeptide; MFI, mean fluorescence intensity.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Schlom J, Arlen PM, Gulley JL. Cancer vaccines: moving beyond current paradigms. Clin. Cancer Res. 2007;13:3776–3782. doi: 10.1158/1078-0432.CCR-07-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nowak AK, Lake RA, Robinson BW. Combined chemoimmunotherapy of solid tumours: improving vaccines? Adv. Drug Deliv. Rev. 2006;58:975–990. doi: 10.1016/j.addr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Lake RA, Robinson BW. Immunotherapy and chemotherapy: a practical partnership. Nat. Rev. Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 4.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4+25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105:2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 5.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur. J. Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 6.Liu JY, Wu Y, Zhang XS, Yang JL, Li HL, Mao YQ, Wang Y, Cheng X, Li YQ, Xia JC, et al. Single administration of low dose cyclophosphamide augments the antitumor effect of dendritic cell vaccine. Cancer Immunol. Immunother. 2007;56:1597–1604. doi: 10.1007/s00262-007-0305-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin. Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 8.Ko HJ, Kim YJ, Kim YS, Chang WS, Ko SY, Chang SY, Sakaguchi S, Kang CY. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 2007;67:7477–7486. doi: 10.1158/0008-5472.CAN-06-4639. [DOI] [PubMed] [Google Scholar]
- 9.Nowak AK, Lake RA, Marzo AL, Scott B, Heath WR, Collins EJ, Frelinger JA, Robinson BW. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J. Immunol. 2003;170:4905–4913. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 10.Ehrke MJ, Verstovsek S, Maccubbin DL, Ujhazy P, Zaleskis G, Berleth E, Mihich E. Protective specific immunity induced by doxorubicin plus TNF-α combination treatment of EL4 lymphoma-bearing C57BL/6 mice. Int. J Cancer. 2000;87:101–109. doi: 10.1002/1097-0215(20000701)87:1<101::aid-ijc15>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Emens LA, Jaffee EM. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res. 2005;65:8059–8064. doi: 10.1158/0008-5472.CAN-05-1797. [DOI] [PubMed] [Google Scholar]
- 13.Coleman S, Clayton A, Mason MD, Jasani B, Adams M, Tabi Z. Recovery of CD8+ T-cell function during systemic chemotherapy in advanced ovarian cancer. Cancer Res. 2005;65:7000–7006. doi: 10.1158/0008-5472.CAN-04-3792. [DOI] [PubMed] [Google Scholar]
- 14.O’Neill DW, Bhardwaj N. Exploiting dendritic cells for active immunotherapy of cancer and chronic infections. Mol. Biotechnol. 2007;36:131–141. doi: 10.1007/s12033-007-0020-6. [DOI] [PubMed] [Google Scholar]
- 15.Shu S, Zheng R, Lee WT, Cohen PA. Immunogenicity of dendritic-tumor fusion hybrids and their utility in cancer immunotherapy. Crit. Rev. Immunol. 2007;27:463–483. doi: 10.1615/critrevimmunol.v27.i5.50. [DOI] [PubMed] [Google Scholar]
- 16.Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. Immunol. Rev. 2007;220:129–150. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- 17.Tong Y, Song W, Crystal RG. Combined intratumoral injection of bone marrow-derived dendritic cells and systemic chemotherapy to treat pre-existing murine tumors. Cancer Res. 2001;61:7530–7535. [PubMed] [Google Scholar]
- 18.Bauer C, Bauernfeind F, Sterzik A, Orban M, Schnurr M, Lehr HA, Endres S, Eigler A, Dauer M. Dendritic cell-based vaccination combined with gemcitabine increases survival in a murine pancreatic carcinoma model. Gut. 2007;56:1275–1282. doi: 10.1136/gut.2006.108621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, Castedo M, Mignot G, Panaretakis T, Casares N, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 20.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 21.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Yu B, Kusmartsev S, Cheng F, Paolini M, Nefedova Y, Sotomayor E, Gabrilovich D. Effective combination of chemotherapy and dendritic cell administration for the treatment of advanced-stage experimental breast cancer. Clin. Cancer Res. 2003;9:285–294. [PubMed] [Google Scholar]
- 23.Chao D, Bahl P, Houlbrook S, Hoy L, Harris A, Austyn JM. Human cultured dendritic cells show differential sensitivity to chemotherapy agents as assessed by the MTS assay. Br. J. Cancer. 1999;81:1280–1284. doi: 10.1038/sj.bjc.6694366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perrotta C, Bizzozero L, Falcone S, Rovere-Querini P, Prinetti A, Schuchman EH, Sonnino S, Manfredi AA, Clementi E. Nitric oxide boosts chemoimmunotherapy via inhibition of acid sphingomyelinase in a mouse model of melanoma. Cancer Res. 2007;67:7559–7564. doi: 10.1158/0008-5472.CAN-07-0309. [DOI] [PubMed] [Google Scholar]
- 25.Shin JY, Lee SK, Kang CD, Chung JS, Lee EY, Seo SY, Lee SY, Baek SY, Kim BS, Kim JB, Yoon S. Antitumor effect of intratumoral administration of dendritic cell combination with vincristine chemotherapy in a murine fibrosarcoma model. Histol. Histopathol. 2003;18:435–447. doi: 10.14670/HH-18.435. [DOI] [PubMed] [Google Scholar]
- 26.Nakashima H, Tasaki A, Kubo M, Kuroki H, Matsumoto K, Tanaka M, Nakamura M, Morisaki T, Katano M. Effects of docetaxel on antigen presentation-related functions of human monocyte-derived dendritic cells. Cancer Chemother. Pharmacol. 2005;55:479–487. doi: 10.1007/s00280-004-0918-7. [DOI] [PubMed] [Google Scholar]
- 27.Jiga LP, Bauer TM, Chuang JJ, Opelz G, Terness P. Generation of tolerogenic dendritic cells by treatment with mitomycin C: inhibition of allogeneic T-cell response is mediated by downregulation of ICAM-1, CD80, and CD86. Transplantation. 2004;77:1761–1764. doi: 10.1097/01.tp.0000131165.37177.6e. [DOI] [PubMed] [Google Scholar]
- 28.Laane E, Bjorklund E, Mazur J, Lonnerholm G, Soderhall S, Porwit A. Dendritic cell regeneration in the bone marrow of children treated for acute lymphoblastic leukaemia. Scand. J. Immunol. 2007;66:572–583. doi: 10.1111/j.1365-3083.2007.02007.x. [DOI] [PubMed] [Google Scholar]
- 29.Wertel I, Polak G, Barczynski B, Kotarski J. Subpopulations of peripheral blood dendritic cells during chemotherapy of ovarian cancer (in Polish) Ginekol. Pol. 2007;78:768–771. [PubMed] [Google Scholar]
- 30.Bellik L, Gerlini G, Parenti A, Ledda F, Pimpinelli N, Neri B, Pantalone D. Role of conventional treatments on circulating and monocyte-derived dendritic cells in colorectal cancer. Clin. Immunol. 2006;121:74–80. doi: 10.1016/j.clim.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Zhong H, Han B, Tourkova IL, Lokshin A, Rosenbloom A, Shurin MR, Shurin GV. Low-dose paclitaxel prior to intratumoral dendritic cell vaccine modulates intratumoral cytokine network and lung cancer growth. Clin. Cancer Res. 2007;13:5455–5462. doi: 10.1158/1078-0432.CCR-07-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shurin GV, Tourkova IL, Shurin MR. Low-dose chemotherapeutic agents regulate small Rho GTPase activity in dendritic cells. J. Immunother. 2008;31:491–499. doi: 10.1097/CJI.0b013e318176fae4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol. Today. 1999;20:561–567. doi: 10.1016/s0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 34.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J. Exp. Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valenzuela J, Schmidt C, Mescher M. The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. J. Immunol. 2002;169:6842–6849. doi: 10.4049/jimmunol.169.12.6842. [DOI] [PubMed] [Google Scholar]
- 36.Valenzuela JO, Hammerbeck CD, Mescher MF. Cutting edge: Bcl-3 up-regulation by signal 3 cytokine (IL-12) prolongs survival of antigen-activated CD8 T cells. J. Immunol. 2005;174:600–604. doi: 10.4049/jimmunol.174.2.600. [DOI] [PubMed] [Google Scholar]
- 37.Tourkova IL, Shurin GV, Ferrone S, Shurin MR. Interferon regulatory factor 8 mediates tumor-induced inhibition of antigen processing and presentation by dendritic cells. Cancer Immunol. Immunother. 2009;58:567–574. doi: 10.1007/s00262-008-0579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shurin MR, Potapovich AI, Tyurina YY, Tourkova IL, Shurin GV, Kagan VE. Recognition of live phosphatidylserine-labeled tumor cells by dendritic cells: a novel approach to immunotherapy of skin cancer. Cancer Res. 2009;69:2487–2496. doi: 10.1158/0008-5472.CAN-08-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tourkova IL, Shurin GV, Chatta GS, Perez L, Finke J, Whiteside TL, Ferrone S, Shurin MR. Restoration by IL-15 of MHC class I antigen-processing machinery in human dendritic cells inhibited by tumor-derived gangliosides. J. Immunol. 2005;175:3045–3052. doi: 10.4049/jimmunol.175.5.3045. [DOI] [PubMed] [Google Scholar]
- 40.Whiteside TL, Stanson J, Shurin MR, Ferrone S. Antigen-processing machinery in human dendritic cells: up-regulation by maturation and down-regulation by tumor cells. J. Immunol. 2004;173:1526–1534. doi: 10.4049/jimmunol.173.3.1526. [DOI] [PubMed] [Google Scholar]
- 41.Repnik U, Bergant M, Wraber B, Jeras M. Late dendritic cells are still able to evoke a potent alloreactive CTL response. Immunobiology. 2008;213:51–64. doi: 10.1016/j.imbio.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Curtsinger JM, Gerner MY, Lins DC, Mescher MF. Signal 3 availability limits the CD8 T cell response to a solid tumor. J. Immunol. 2007;178:6752–6760. doi: 10.4049/jimmunol.178.11.6752. [DOI] [PubMed] [Google Scholar]
- 43.Lake RA, van der Most RG. A better way for a cancer cell to die. N. Engl. J. Med. 2006;354:2503–2504. doi: 10.1056/NEJMcibr061443. [DOI] [PubMed] [Google Scholar]
- 44.Obeid M, Tesniere A, Panaretakis T, Tufi R, Joza N, van Endert P, Ghiringhelli F, Apetoh L, Chaput N, Flament C, et al. Ecto-calreticulin in immunogenic chemotherapy. Immunol. Rev. 2007;220:22–34. doi: 10.1111/j.1600-065X.2007.00567.x. [DOI] [PubMed] [Google Scholar]
- 45.Haynes NM, van der Most RG, Lake RA, Smyth MJ. Immunogenic anti-cancer chemotherapy as an emerging concept. Curr. Opin. Immunol. 2008;20:545–557. doi: 10.1016/j.coi.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 46.Tesniere A, Apetoh L, Ghiringhelli F, Joza N, Panaretakis T, Kepp O, Schlemmer F, Zitvogel L, Kroemer G. Immunogenic cancer cell death: a key-lock paradigm. Curr. Opin. Immunol. 2008;20:504–511. doi: 10.1016/j.coi.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 47.van der Most RG, Currie AJ, Robinson BW, Lake RA. Decoding dangerous death: how cytotoxic chemotherapy invokes inflammation, immunity or nothing at all. Cell Death Differ. 2008;15:13–20. doi: 10.1038/sj.cdd.4402255. [DOI] [PubMed] [Google Scholar]
- 48.Heath WR, Carbone FR. Immunology: dangerous liaisons. Nature. 2003;425:460–461. doi: 10.1038/425460a. [DOI] [PubMed] [Google Scholar]
- 49.Binder RJ, Srivastava PK. Peptides chaperoned by heat-shock proteins are a necessary and sufficient source of antigen in the cross-priming of CD8+ T cells. Nat. Immunol. 2005;6:593–599. doi: 10.1038/ni1201. [DOI] [PubMed] [Google Scholar]
- 50.Hu DE, Moore AM, Thomsen LL, Brindle KM. Uric acid promotes tumor immune rejection. Cancer Res. 2004;64:5059–5062. doi: 10.1158/0008-5472.CAN-04-1586. [DOI] [PubMed] [Google Scholar]
- 51.Apetoh L, Tesniere A, Ghiringhelli F, Kroemer G, Zitvogel L. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res. 2008;68:4026–4030. doi: 10.1158/0008-5472.CAN-08-0427. [DOI] [PubMed] [Google Scholar]
- 52.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat. Rev. Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 53.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol. Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 54.Shurin GV, Amina N, Shurin MR. Cancer therapy and dendritic cell immunomodulation. In: Shurin MR, Salter RD, editors. Dendritic Cells in Cancer. New York: Springer; 2009. pp. 201–218. [Google Scholar]