Abstract
Despite the emerging role of mitochondria in immunity, a link between bioenergetics and the immune response in autism has not been explored. Mitochondrial outcomes and phorbol 12-myristate 13-acetate (PMA)–induced oxidative burst were evaluated in granulocytes from age-, race-, and gender-matched children with autism with severity scores of ≥7 (n = 10) and in typically developing (TD) children (n = 10). The oxidative phosphorylation capacity of granulocytes was 3-fold lower in children with autism than in TD children, with multiple deficits encompassing ≥1 Complexes. Higher oxidative stress in cells of children with autism was evidenced by higher rates of mitochondrial reactive oxygen species production (1.6-fold), higher mitochondrial DNA copy number per cell (1.5-fold), and increased deletions. Mitochondrial dysfunction in children with autism was accompanied by a lower (26% of TD children) oxidative burst by PMA-stimulated reduced nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase and by a lower gene expression (45% of TD children's mean values) of the nuclear factor erythroid 2–related factor 2 transcription factor involved in the antioxidant response. Given that the majority of granulocytes of children with autism exhibited defects in oxidative phosphorylation, immune response, and antioxidant defense, our results support the concept that immunity and response to oxidative stress may be regulated by basic mitochondrial functions as part of an integrated metabolic network.
Keywords: mitochondria, autism, NFE2L2, Nrf2, oxidative stress, immune response, bioenergetics, NADPH oxidase
A higher incidence of mitochondrial dysfunction,1 altered immune response,2–4 increased cellular oxidative damage1,5,6 and decreased antioxidant defenses5,7–11 has been reported in autism, possibly contributing to its etiology and/or morbidity. Thus, we explored the possibility that these pathways could be conceived as part of a common, integrated mechanism in which basic mitochondrial functions would play a central role.12–15 To this end, oxidative phosphorylation (OXPHOS) capacity, immune response to phorbol 12-myristate 13-acetate (PMA), and markers of oxidative stress (reactive oxygen species [ROS] production, mitochrondrial DNA [mtDNA] deletions) were evaluated in granulocytes from children with autism and typically developing (TD) age- and gender-matched children. Although the choice of studying peripheral blood mononuclear cells (PBMCs) as a biological material could be criticized in the context of autism research, several reports have confirmed the use of PBMC gene expression as a valid surrogate for gene expression in the brain.16–23 Furthermore, the novel role for Toll-like receptor 9 (TLR9) in energy metabolism and cellular protection has reinforced the conservation of the innate immunity pathway in nonimmune cells.24
The immune response was tested by following the activation of granulocytes by PMA. This soluble stimulus activates the protein kinase C (PKC)–dependent phosphorylation of cytosolic components of reduced nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase, eliciting the translocation of cytosolic components to the plasma membrane, assembly of a functional NADPH oxidase, and the ensuing “respiratory burst”25–27. In addition, gene expression of nuclear factor erythroid 2–related factor 2 (NFE2L2) was also evaluated because NFE2L2-mediated phase II antioxidant defenses seem critical for protecting memory T cells, increasing neuronal glutathione levels,28 decreasing the damage mediated by inhibitors of mitochondrial Complexes I and II,29,30 and modulating the cellular response to common environmental allergens.31
Case Report
Granulocytes from 10 children with autism with severity scores of ≥7 (median [quartile1, quartile 3]: 9 [8, 9]32) and 10 age-, gender-, and race-matched TD children were sampled from individuals previously described1 and enrolled in the CHildhood Autism Risks from Genetics and Environment (CHARGE) Study at the University of California, Davis. All details on patient selection, diagnosis, demographic characteristics, and clinical data of the study groups used in this study were published previously1 and are summarized in the Supplemental Information. Detailed descriptions of sample collection, cell isolation, and evaluation of outcomes shown in this study are included in the Supplemental Information.
To evaluate the OXPHOS capacity of granulocytes, resting oxygen uptake rates were evaluated with intact, not activated, cells suspended in 5 mM glucose in Hanks buffer saline solution (Table 1). For both diagnostic groups, >85% of the oxygen uptake rate was sensitive to 0.2 nmol × (mg protein)−1 oligomycin (not shown), a specific ATPase inhibitor,33 suggesting that most, if not all, oxygen uptake observed under these conditions is tightly coupled to OXPHOS. Granulocytes from children with autism exhibited a lower OXPHOS capacity than did those from TD children, characterized by a 2.9-fold lower oxygen uptake under resting conditions.
TABLE 1.
Mitochondrial Outcomes in Granulocytes
| Outcome | Children With Autism | TD Children | P |
|---|---|---|---|
| Resting O2 uptake | 0.38 ± 0.09 | 1.1 ± 0.3 | .05 |
| NADH oxidase (95% CI) | 4.6 ± 3.1 (2–7) | 10.9 ± 4.9 (7–15) | .01 |
| Succinate oxidase (95% CI) | 1.6 ± 1.2 (1–2) | 5.2 ± 2.0 (5–13) | .001 |
| Glycerophosphate oxidoreductase (95% CI) | 6.7 ± 4.0 (4–10) | 13 ± 10 (3–23) | — |
| Cytochrome c oxidase (95% CI) | 9.9 ± 4.3 (7–13) | 16 ± 13 (5–26) | — |
| ATPase (95% CI) | 46 ± 28 (27–66) | 123 ± 54 (80–167) | .005 |
| Citrate synthase (95% CI) | 166 ± 37 (93–239) | 110 ± 28 (55–166) | — |
| Rate of H2O2 production | 0.11 ± 0.02 | 0.07 ± 0.02 | .04 |
| mtDNA deletions at CYTB | 0.54 ± 0.01 | 0.60 ± 0.02 | .03 |
| mtDNA deletions at ND4 | 0.43 ± 0.01 | 0.43 ± 0.02 | — |
| NFE2L2 gene expression level | 0.45 ± 0.01 | 1.00 ± 0.03 | .01 |
Data are presented as means ± SDs. All activities were expressed as nmol × (min × mg protein)−1, then normalized to citrate synthase also expressed as nmol × (min × mg protein)−1, and multiplied by 1000. CI, confidence interval; CYTB, cytochrome b; ND4 NADH dehydrogenase subunit 4, .
Various segments of the electron transport chain were tested in granulocytes, namely reduced nicotinamide-adenine dinucleotide (NADH) oxidase, succinate oxidase, α-glycerophosphate oxidoreductase, and cytochrome c oxidase activities, all normalized to citrate synthase activity, a marker of mitochondrial mass.34 NADH oxidase includes the transfer of electrons from NADH (derived from malate) to oxygen through a series of 3 carriers of the electron transport chain (Complex I, III, and IV) and finally to Complex V or ATPase. The rate of oxygen consumption of permeabilized granulocytes from children with autism under phosphorylating conditions (supplemented with malate-glutamate and adenosine diphosphate) was 42% of that of TD children (P < .01; see Table 1 for averages and Supplemental Table 3 for each individual proband). Similarly, succinate oxidase activity (which evaluates the segment encompassing Complex II, III, IV, and V) was 31% of that of TD children (P < .001; Table 1, Supplemental Table 3). Mean Complex V or ATPase activity was also significantly decreased (by 63%; P < .005) in children with autism (Table 1, Supplemental Table 3), whereas mean α-glycerophosphate oxidoreductase and cytochrome c oxidase activities were not significantly different between diagnostic groups.
The type of OXPHOS deficiencies and the incidence of individuals with autism with OXPHOS deficits followed the same trend as that reported for the lymphocytes from the same cohort of children (>60% for both NADH- and succinate-oxidase activities; >40% for ATPase1; Supplemental Table 3), suggesting that the observed effects are not cell-type specific. Indeed, all mitochondrial outcomes tested in lymphocytes correlated statistically with those obtained with granulocytes from the same individual (Supplemental Fig 2).
No differences in mitochondrial mass (as judged by the activity of citrate synthase34) were observed in granulocytes from children with autism and TD children. However, another putative marker for mitochondrial mass, mtDNA copy number per cell, was significantly increased in granulocytes from children with autism (1.5-fold of that from TD children1; Supplemental Table 4). Increased mtDNA copy number without increases in OXPHOS capacity and/or mitochondrial mass, as observed in this study, has been attributed to a cellular response to cope with oxidative stress35 in an attempt to sustain adequate levels of mitochondrial transcripts from wild-type mtDNA.36 Consistent with this view, an increased mean mitochondrial ROS production (1.6-fold; Table 1) and increased mtDNA deletions in the segment encoding for cytochrome b (CYTB) but not in that encoding for NADH dehydrogenase subunit 4 (ND4) were observed in granulocytes from children with autism (Table 1, Supplemental Table 4).
The respiratory burst in TD granulocytes, evaluated as the oxygen consumption to produce superoxide anion after PMA addition, was comparable to reported control values (1.1 to 7.737 vs 4 nmol O2.− × [106 polymorphonuclear cells (PMN) × minute]−1; Table 2, Supplemental Table 5). Upon activation, the maximum oxidative burst rate and the total amount of oxygen consumed in 5 minutes by granulocytes from children with autism was 24% and 40% of that from TD children, respectively (P < .05; Table 2, Supplemental Table 5). A longer interval between the addition of PMA and the start of the oxidative burst (also called latency) was observed in 7 of 9 children with autism (1.4-fold; Table 2, Supplemental Table 5), which is suggestive of a delayed or defective signal transduction pathway involving PKC alone or in combination with other pathways38 (eg, NFE2L2).
TABLE 2.
Oxygen Uptake of PMA-Stimulated Granulocytes
| Outcome | Children With Autism (n = 10) | TD Children (n = 10) | P |
|---|---|---|---|
| Latency, s | 42 ± 5 | 30 ± 6 | .07 |
| Maximum rate of O2 uptake, (nmol O2) × (min × mg protein)−1 | 1.9 ± 0.5 | 8 ± 1 | .003 |
| Total O2 consumed in 5 minutes, (nmol O2) × (mg protein)−1 | 28 ± 8 | 70.5 ± 0.4 | .005 |
Oxygen uptake rates were evaluated in 5 mM glucose-, calcium-, and magnesium-supplemented Hanks buffer saline solution without phenol red (20°–22°C) before and after adding 20 mg PMA × mL−1 and are presented as means ± SDs.
In search of a common mechanism that would explain the above results, we focused on NFE2L2 because this nuclear transcription factor regulates clusters of genes that control cellular antioxidants,39–41 modulate both innate and adaptive immune responses,42 and has a strong association with mitochondrial function, glucose and fatty acid homeostasis, and immune response via peroxisome proliferator-activated receptor γ (PPARγ).43–46 Consistent with this hypothesis, the transcript levels of NFE2L2 (normalized to glyceraldehyde 3-phosphate dehydrogenase [GAPDH]) evaluated by quantitative polymerase chain reaction were 45% of those of TD children (Table 1).
Discussion
This study performed with granulocytes from children with autism confirms and extends that previously obtained with lymphocytes from the same cohort of children.1 Deficits in OXPHOS were accompanied by higher oxidative stress (increased ROS production, increased mtDNA deletions, and higher mtDNA copy number) in both cell types, suggesting that these features are not cell specific. This report broadens our knowledge because it includes studies on the immune response of granulocytes from probands. These cells presented a lower PMA-mediated oxidative burst with a longer latency to trigger this response. Taken together, these findings are in agreement with those reporting immune dysregulation, mitochondrial dysfunction, increased oxidative stress, and decreased antioxidant or repair capacity in some cases of autism.1–11,47–50
Several studies suggest that pre- or perinatal exposure to certain triggers might imprint a state of both dysregulated immune response and mitochondrial dysfunction in the progeny as a result of the integration of basic mitochondrial functions with the immune response and antioxidant defense mechanisms.51–55 Furthermore, maternal exposure during pregnancy to various pathogens and/or the maternal immune response (fever, inflammation) have been associated with significant increased risk of autism spectrum disorder (ASD).56–59 In this regard, gestational exposure to the viral mimetic poly(I:C) in rodents resulted in ASD-like behavioral abnormalities in the progeny,60 and immune cells from the same animals had OXPHOS deficits still present in adulthood.61 These findings are consistent with the current view of chronic inflammation in which the proinflammatory-phase response (mainly fueled by ATP generated in glycolysis13) predominates and persists unless external changes are implemented.62 In our study, the frequency of children (for whom all outcomes were available) having both autism and concurrent deficits in OXPHOS, immune response, and antioxidant response (considering values outside the 95% confidence interval) was 6 out of 8, supporting the concept that immunity and response to oxidative stress are interconnected and that they may be regulated by basic mitochondrial functions as part of an integrated metabolic network.14
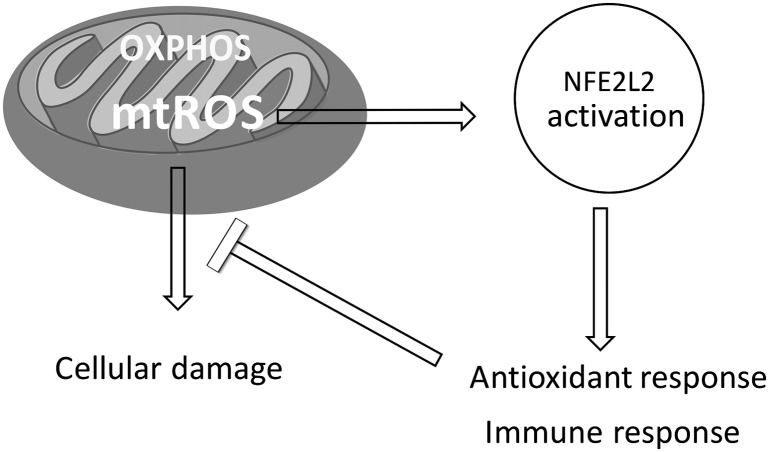
Given the critical role of NFE2L2 as regulator of the antioxidant response, alone or in combination with PKC,38 and its strong association with mitochondrial morphology,63 glucose and fatty acid homeostasis, and immune response via PPARγ,43–46 it is likely that a dysregulation in the NFE2L2 pathway may contribute to a state of chronic inflammation with a diminished capacity to compensate for conditions of increased oxidative stress,64 including exposure to environmental triggers,65,66 thereby limiting the mitochondrial switch to a phase with increased OXPHOS and more reparative features and lower inflammatory/cytotoxic responses14,15 (Fig 1). Our findings should be interpreted with caution because this is a case-control study, in which blood samples were collected postdiagnosis in a small number of probands.
FIGURE 1.
Molecular network linking NFE2L2-mediated antioxidant and immune responses and mitochondrial (mt) OXPHOS and ROS. A disruption of this network starting either with a functional disruption of NFE2L2 or OXPHOS deficits may contribute to a state of chronic inflammation, accompanied by a diminished capacity to compensate for conditions of increased oxidative stress, including exposure to environmental triggers, possibly contributing to the etiology and/or morbidity of autism.
Although we cannot exclude other mechanisms that could account for our findings, comparisons between our study and others reporting a downregulation in the expression of genes encoding for Complexes I, III, IV, and V67,68 or the occurrence of pathogenic mtDNA mutations69–72 are limited because of the following issues: (1) the age of the individuals used spanned from 2 to 60 years old,67,68 above the age range of our subjects (2–5 years)1; (2) levels of transcripts from postmortem (frozen and with varied post-mortem intervals) brain samples67,68 are likely not comparable to OXPHOS activities obtained with freshly obtained PBMCs; (3) messenger RNA expression does not necessarily predict protein expression and/or activity73–75; and (4) sequencing of mtDNA segments of our samples did not reveal a high incidence of any pathogenic mutation6 as observed by others.76 Although some of these observations could be reconciled considering differences between tissues or proportions of mutant mtDNA, no study has evaluated the gene expression for NFE2L2 in children with autism. Thus, we cannot exclude the possibility that the reported downregulation could be downstream from NFE2L2.
In conclusion, this is the first report to our knowledge suggesting a molecular network linking mitochondrial OXPHOS and the inflammation/immune response, opening new doors for future studies and pharmacologic targets. In this regard, activation of the NFE2L2 pathway has been reported as being beneficial at decreasing the behavioral abnormalities and brain pathology in a murine model of Huntington disease.77
Supplementary Material
Acknowledgments
We thank all of the families involved in this study, Ms Alicja Omanska-Klusek and Ms Catherine Ross-Inta for their technical expertise, and Ms Melissa Rose, for clinical coordination in obtaining and transporting specimens from CHARGE Study participants.
Glossary
- ASD
autism spectrum disorder
- mtDNA
mitochondrial DNA
- NADH
reduced nicotinamide-adenine dinucleotide
- NADPH
reduced nicotinamide-adenine dinucleotide phosphate
- NFE2L2
nuclear factor erythroid 2–related factor 2
- OXPHOS
oxidative phosphorylation
- PBMC
peripheral blood mononuclear cell
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- ROS
reactive oxygen species
- TD
typically developing
Footnotes
Dr Giulivi conceptualized and designed the study, drafted the initial manuscript, and contributed to intellectual content; Dr Napoli carried out data analysis, contributed to biochemical data analyses and interpretation, and revised the manuscript; Ms Wong performed all experiments on molecular biology, contributed to the analysis and interpretation of data, and drafted part of the manuscript; Dr Hertz-Picciotto reviewed the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This study was performed with funding from the Simons Foundation (SFARI 271406 to Dr Giulivi) and NIEHS R01-ES011269, R01-ES015359, and R01-ES020392. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Giulivi C, Zhang YF, Omanska-Klusek A, et al. Mitochondrial dysfunction in autism. JAMA. 2010;304(21):2389–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Onore C, Careaga M, Ashwood P. The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immun. 2012;26(3):383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goines P, Haapanen L, Boyce R, et al. Autoantibodies to cerebellum in children with autism associate with behavior. Brain Behav Immun. 2011;25(3):514–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gregg JP, Lit L, Baron CA, et al. Gene expression changes in children with autism. Genomics. 2008;91(1):22–29 [DOI] [PubMed] [Google Scholar]
- 5.James SJ, Rose S, Melnyk S, et al. Cellular and mitochondrial glutathione redox imbalance in lymphoblastoid cells derived from children with autism. FASEB J. 2009;23(8):2374–2383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Napoli E, Wong S, Giulivi C. Evidence of reactive oxygen species-mediated damage to mitochondrial DNA in children with typical autism. Mol Autism. 2013;4(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ming X, Johnson WG, Stenroos ES, Mars A, Lambert GH, Buyske S. Genetic variant of glutathione peroxidase 1 in autism. Brain Dev. 2010;32(2):105–109 [DOI] [PubMed] [Google Scholar]
- 8.Golse B, Debray-Ritzen P, Durosay P, Puget K, Michelson AM. Alterations in two enzymes: superoxide dismutase and glutathion peroxidase in developmental infantile psychosis (infantile autism) [in French ]. Rev Neurol (Paris). 1978;134(11):699–705 [PubMed] [Google Scholar]
- 9.Tórsdóttir G, Hreidarsson S, Kristinsson J, Snaedal J, Jóhannesson T. Ceruloplasmin, superoxide dismutase and copper in autistic patients. Basic Clin Pharmacol Toxicol. 2005;96(2):146–148 [DOI] [PubMed] [Google Scholar]
- 10.Frustaci A, Neri M, Cesario A, et al. Oxidative stress-related biomarkers in autism: systematic review and meta-analyses. Free Radic Biol Med. 2012;52(10):2128–2141 [DOI] [PubMed] [Google Scholar]
- 11.Parellada M, Moreno C, Mac-Dowell K, et al. Plasma antioxidant capacity is reduced in Asperger syndrome. J Psychiatr Res. 2012;46(3):394–401 [DOI] [PubMed] [Google Scholar]
- 12.Sena LA, Li S, Jairaman A, et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity. 2013;38(2):225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu TF, Brown CM, El Gazzar M, et al. Fueling the flame: bioenergy couples metabolism and inflammation. J Leukoc Biol. 2012;92(3):499–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnoult D, Soares F, Tattoli I, Girardin SE. Mitochondria in innate immunity. EMBO Rep. 2011;12(9):901–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cloonan SM, Choi AM. Mitochondria: commanders of innate immunity and disease? Curr Opin Immunol. 2012;24(1):32–40 [DOI] [PubMed] [Google Scholar]
- 16.Sullivan PF, Fan C, Perou CM. Evaluating the comparability of gene expression in blood and brain. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(3):261–268 [DOI] [PubMed] [Google Scholar]
- 17.Washizuka S, Iwamoto K, Kakiuchi C, Bundo M, Kato T. Expression of mitochondrial complex I subunit gene NDUFV2 in the lymphoblastoid cells derived from patients with bipolar disorder and schizophrenia. Neurosci Res. 2009;63(3):199–204 [DOI] [PubMed] [Google Scholar]
- 18.Coppola G, Karydas A, Rademakers R, et al. Gene expression study on peripheral blood identifies progranulin mutations. Ann Neurol. 2008;64(1):92–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Padmos RC, Hillegers MH, Knijff EM, et al. A discriminating messenger RNA signature for bipolar disorder formed by an aberrant expression of inflammatory genes in monocytes. Arch Gen Psychiatry. 2008;65(4):395–407 [DOI] [PubMed] [Google Scholar]
- 20.Scherzer CR, Eklund AC, Morse LJ, et al. Molecular markers of early Parkinson’s disease based on gene expression in blood. Proc Natl Acad Sci USA. 2007;104(3):955–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong SW, Collins CD, Shimizu-Motohashi Y, et al. Characteristics and predictive value of blood transcriptome signature in males with autism spectrum disorders. PLoS ONE. 2012;7(12):e49475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glatt SJ, Tsuang MT, Winn M, et al. Blood-based gene expression signatures of infants and toddlers with autism. J Am Acad Child Adolesc Psychiatry. 2012;51(9):934–944, e932 [DOI] [PMC free article] [PubMed]
- 23.Jasinska AJ, Service S, Choi OW, et al. Identification of brain transcriptional variation reproduced in peripheral blood: an approach for mapping brain expression traits. Hum Mol Genet. 2009;18(22):4415–4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shintani Y, Kapoor A, Kaneko M, et al. TLR9 mediates cellular protection by modulating energy metabolism in cardiomyocytes and neurons. Proc Natl Acad Sci USA. 2013;110(13):5109–5114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397(2):342–344 [DOI] [PubMed] [Google Scholar]
- 26.Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109(1):33–44 [DOI] [PubMed] [Google Scholar]
- 27.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4(3):181–189 [DOI] [PubMed] [Google Scholar]
- 28.Escartin C, Won SJ, Malgorn C, et al. Nuclear factor erythroid 2-related factor 2 facilitates neuronal glutathione synthesis by upregulating neuronal excitatory amino acid transporter 3 expression. J Neurosci. 2011;31(20):7392–7401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JM, Shih AY, Murphy TH, Johnson JA. NF-E2-related factor-2 mediates neuroprotection against mitochondrial complex I inhibitors and increased concentrations of intracellular calcium in primary cortical neurons. J Biol Chem. 2003;278(39):37948–37956 [DOI] [PubMed] [Google Scholar]
- 30.Calkins MJ, Jakel RJ, Johnson DA, Chan K, Kan YW, Johnson JA. Protection from mitochondrial complex II inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc Natl Acad Sci USA. 2005;102(1):244–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rangasamy T, Guo J, Mitzner WA, et al. Disruption of Nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J Exp Med. 2005;202(1):47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. J Autism Dev Disord. 2009;39(5):693–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lardy HA, Connelly JL, Johnson D. Antibiotic studies. I. Inhibition of phosphoryl transfer in mitochondria by oligomycin and aurovertin. Biochemistry. 1964;3:1961–1968 [DOI] [PubMed] [Google Scholar]
- 34.Ramachandran A, Ceaser E, Darley-Usmar VM. Chronic exposure to nitric oxide alters the free iron pool in endothelial cells: role of mitochondrial respiratory complexes and heat shock proteins. Proc Natl Acad Sci USA. 2004;101(1):384–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Napoli E, Ross-Inta C, Wong S, et al. Altered zinc transport disrupts mitochondrial protein processing/import in fragile X-associated tremor/ataxia syndrome. Hum Mol Genet. 2011;20(15):3079–3092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Attardi G, Yoneda M, Chomyn A. Complementation and segregation behavior of disease-causing mitochondrial DNA mutations in cellular model systems. Biochim Biophys Acta. 1995;1271(1):241–248 [DOI] [PubMed] [Google Scholar]
- 37.Wolach B, Gavrieli R, de Boer M, et al. Chronic granulomatous disease in Israel: clinical, functional and molecular studies of 38 patients. Clin Immunol. 2008;129(1):103–114 [DOI] [PubMed] [Google Scholar]
- 38.Rushworth SA, Chen XL, Mackman N, Ogborne RM, O’Connell MA. Lipopolysaccharide-induced heme oxygenase-1 expression in human monocytic cells is mediated via Nrf2 and protein kinase C. J Immunol. 2005;175(7):4408–4415 [DOI] [PubMed] [Google Scholar]
- 39.Tsuji G, Takahara M, Uchi H, et al. Identification of ketoconazole as an AhR-Nrf2 activator in cultured human keratinocytes: the basis of its anti-inflammatory effect. J Invest Dermatol. 2012;132(1):59–68 [DOI] [PubMed] [Google Scholar]
- 40.Lee JM, Li J, Johnson DA, et al. Nrf2, a multi-organ protector? FASEB J. 2005;19(9):1061–1066 [DOI] [PubMed] [Google Scholar]
- 41.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116 [DOI] [PubMed] [Google Scholar]
- 42.Johnson DA, Amirahmadi S, Ward C, Fabry Z, Johnson JA. The absence of the pro-antioxidant transcription factor Nrf2 exacerbates experimental autoimmune encephalomyelitis. Toxicol Sci. 2010;114(2):237–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pi J, Leung L, Xue P, et al. Deficiency in the nuclear factor E2-related factor-2 transcription factor results in impaired adipogenesis and protects against diet-induced obesity. J Biol Chem. 2010;285(12):9292–9300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho HY, Gladwell W, Wang X, et al. Nrf2-regulated PPARγ expression is critical to protection against acute lung injury in mice. Am J Respir Crit Care Med. 2010;182(2):170–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ikeda Y, Sugawara A, Taniyama Y, et al. Suppression of rat thromboxane synthase gene transcription by peroxisome proliferator-activated receptor gamma in macrophages via an interaction with NRF2. J Biol Chem. 2000;275(42):33142–33150 [DOI] [PubMed] [Google Scholar]
- 46.Vats D, Mukundan L, Odegaard JI, et al. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell Metab. 2006;4(1):13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57(1):67–81 [DOI] [PubMed] [Google Scholar]
- 48.Croonenberghs J, Bosmans E, Deboutte D, Kenis G, Maes M. Activation of the inflammatory response system in autism. Neuropsychobiology. 2002;45(1):1–6 [DOI] [PubMed] [Google Scholar]
- 49.Molloy CA, Morrow AL, Meinzen-Derr J, et al. Elevated cytokine levels in children with autism spectrum disorder. J Neuroimmunol. 2006;172(1-2):198–205 [DOI] [PubMed] [Google Scholar]
- 50.Ashwood P, Wakefield AJ. Immune activation of peripheral blood and mucosal CD3+ lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms. J Neuroimmunol. 2006;173(1-2):126–134 [DOI] [PubMed] [Google Scholar]
- 51.Suliman HB, Welty-Wolf KE, Carraway MS, Schwartz DA, Hollingsworth JW, Piantadosi CA. Toll-like receptor 4 mediates mitochondrial DNA damage and biogenic responses after heat-inactivated E. coli. FASEB J. 2005;19(11):1531–1533 [DOI] [PubMed] [Google Scholar]
- 52.Sasai M, Shingai M, Funami K, et al. NAK-associated protein 1 participates in both the TLR3 and the cytoplasmic pathways in type I IFN induction. J Immunol. 2006;177(12):8676–8683 [DOI] [PubMed] [Google Scholar]
- 53.Seth RB, Sun L, Chen ZJ. Antiviral innate immunity pathways. Cell Res. 2006;16(2):141–147 [DOI] [PubMed] [Google Scholar]
- 54.Shi HX, Liu X, Wang Q, et al. Mitochondrial ubiquitin ligase MARCH5 promotes TLR7 signaling by attenuating TANK action. PLoS Pathog. 2011;7(5):e1002057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carta S, Tassi S, Delfino L, et al. Deficient production of IL-1 receptor antagonist and IL-6 coupled to oxidative stress in cryopyrin-associated periodic syndrome monocytes. Ann Rheum Dis. 2012;71(9):1577–1581 [DOI] [PubMed] [Google Scholar]
- 56.Patterson PH. Maternal infection and immune involvement in autism. Trends Mol Med. 2011;17(7):389–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patterson PH. Maternal infection and autism. Brain Behav Immun. 2012;26(3):393. [DOI] [PubMed] [Google Scholar]
- 58.Atladóttir HO, Thorsen P, Østergaard L, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40(12):1423–1430 [DOI] [PubMed] [Google Scholar]
- 59.Zerbo O, Iosif AM, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. J Autism Dev Disord. 2013;43(1):25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwartzer JJ, Careaga M, Onore CE, Rushakoff JA, Berman RF, Ashwood P. Maternal immune activation and strain specific interactions in the development of autism-like behaviors in mice. Transcult Psychiatry. 2013;3:e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Giulivi C, Napoli E, Schwartzer J, Careaga M, Ashwood P. Gestational exposure to a viral mimetic poly(i:C) results in long-lasting changes in mitochondrial function by leucocytes in the adult offspring. Mediators Inflamm. 2013;2013:609602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McCall CE, El Gazzar M, Liu T, Vachharajani V, Yoza B. Epigenetics, bioenergetics, and microRNA coordinate gene-specific reprogramming during acute systemic inflammation. J Leukoc Biol. 2011;90(3):439–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jin YN, Yu YV, Gundemir S, et al. Impaired mitochondrial dynamics and Nrf2 signaling contribute to compromised responses to oxidative stress in striatal cells expressing full-length mutant Huntingtin. PLoS ONE. 2013;8(3):e57932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pickering AM, Linder RA, Zhang H, Forman HJ, Davies KJ. Nrf2-dependent induction of proteasome and Pa28αβ regulator are required for adaptation to oxidative stress. J Biol Chem. 2012;287(13):10021–10031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry. 2011;68(11):1095–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Napoli E, Hung C, Wong S, Giulivi C. Toxicity of the flame-retardant BDE-49 on brain mitochondria and neuronal progenitor striatal cells enhanced by a PTEN-deficient background. Toxicol Sci. 2013;132(1):196–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anitha A, Nakamura K, Thanseem I, et al. Downregulation of the expression of mitochondrial electron transport complex genes in autism brains. Brain Pathol. 2013;23(3):294–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ginsberg MR, Rubin RA, Falcone T, Ting AH, Natowicz MR. Brain transcriptional and epigenetic associations with autism. PLoS ONE. 2012;7(9):e44736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Graf WD, Marin-Garcia J, Gao HG, et al. Autism associated with the mitochondrial DNA G8363A transfer RNA(Lys) mutation. J Child Neurol. 2000;15(6):357–361 [DOI] [PubMed] [Google Scholar]
- 70.Connolly BS, Feigenbaum AS, Robinson BH, Dipchand AI, Simon DK, Tarnopolsky MA. MELAS syndrome, cardiomyopathy, rhabdomyolysis, and autism associated with the A3260G mitochondrial DNA mutation. Biochem Biophys Res Commun. 2010;402(2):443–447 [DOI] [PubMed] [Google Scholar]
- 71.Piryaei F, Houshmand M, Aryani O, Dadgar S, Soheili ZS. Investigation of the mitochondrial ATPase 6/8 and tRNA(Lys) genes mutations in autism. Cell J. 2012;14(2):98–101 [PMC free article] [PubMed] [Google Scholar]
- 72.Weissman JR, Kelley RI, Bauman ML, et al. Mitochondrial disease in autism spectrum disorder patients: a cohort analysis. PLoS ONE. 2008;3(11):e3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cole AR, Ji H, Simpson RJ. Proteomic analysis of colonic crypts from normal, multiple intestinal neoplasia and p53-null mice: a comparison with colonic polyps. Electrophoresis. 2000;21(9):1772–1781 [DOI] [PubMed] [Google Scholar]
- 74.Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol Cell Biol. 1999;19(3):1720–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anderson L, Seilhamer J. A comparison of selected mRNA and protein abundances in human liver. Electrophoresis. 1997;18(3-4):533–537 [DOI] [PubMed] [Google Scholar]
- 76.Hadjixenofontos A, Schmidt MA, Whitehead PL, et al. Evaluating mitochondrial DNA variation in autism spectrum disorders. Ann Hum Genet. 2013;77(1):9–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stack C, Ho D, Wille E, et al. Triterpenoids CDDO-ethyl amide and CDDO-trifluoroethyl amide improve the behavioral phenotype and brain pathology in a transgenic mouse model of Huntington’s disease. Free Radic Biol Med. 2010;49(2):147–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.