FIG. 7.
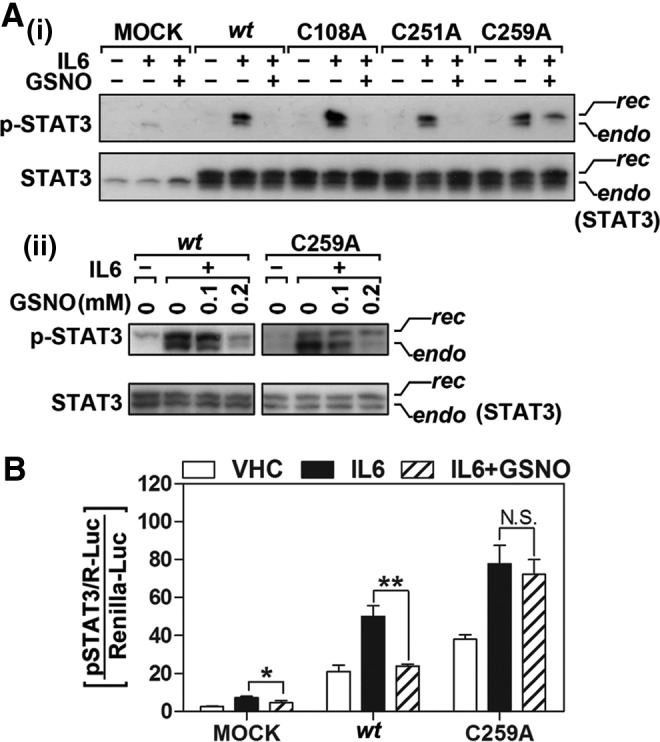
Point mutation of Cys259 to Ala abolishes the inhibitory action of GSNO on IL-6-induced STAT3 Tyr705 phosphorylation and its transactivity. (A) CHO cells were transfected and overexpressed with empty vector (MOCK), wt STAT3 or its point mutant (C108A, C251A, or C259). The cells were then pretreated with GSNO (300 μM for 2 h) followed by IL-6 (30 ng/ml) treatment for 30 min. The total and phospho-Tyr705 STAT3 levels were analyzed by Western analysis. The upper bands corresponds to recombinant STAT3 (rec), which is conjugated with myc and 6xHis tags; lower bands correspond to their endogenous counterparts (endo) (A-i). STAT3 phosphorylation in the cells expressing wtSTAT3 and mutant STAT3 (C259A) was also analyzed following the treatment of cells with lower concentration of GSNO (A-ii). (B) Effect of point mutation of Cys259 to Ala on the inhibitory role of GSNO in IL-6-induced STAT3 transactivation was examined by STAT3 responsive element luciferase assay. CHO cells transfected with STAT3-responsive luciferase construct (pSTAT3/R-Luc) and wt or C259A mutant STAT3 constructs were pretreated with GSNO (500 μM) for 2 h and then treated with IL-6 (30 ng/mL) for 24 h. STAT3 transactivities were analyzed by luciferase activity assay as described under experimental procedure. The renilla luciferase contruct (phRL-CMV) was used as a transfection control. The vertical lines in B indicate the standard error of mean; *p<0.05; **p<0.01; N.S.>0.05 compared with IL-6-treated groups.
