Abstract
Diabetic neuropathy (DN) is a serious and debilitating complication of both type 1 and type 2 diabetes. Despite intense research efforts into multiple aspects of this complication, including both vascular and neuronal metabolic derangements, the only treatment remains maintenance of euglycemia. Basic research into the mechanisms responsible for DN relies on using the most appropriate animal model. The advent of genetic manipulation has moved mouse models of human disease to the forefront. The ability to insert or delete genes affected in human patients offers unique insight into disease processes; however, mice are still not humans and difficulties remain in interpreting data derived from these animals. A number of studies have investigated and described DN in mice but it is difficult to compare these studies with each other or with human DN due to experimental differences including background strain, type of diabetes, method of induction and duration of diabetes, animal age and gender. This review describes currently used DN animal models. We followed a standardized diabetes induction protocol and designed and implemented a set of phenotyping parameters to classify the development and severity of DN. By applying standard protocols, we hope to facilitate the comparison and characterization of DN across different background strains in the hope of discovering the most human like model in which to test potential therapies.
Keywords: NOD, Akita, ob/ob, db/db, outbred mice, nerve conduction velocity, intraepidermal nerve fiber density
DIABETES MELLITUS
The term “diabetes mellitus” describes a number of conditions in which blood glucose is elevated including insulin dependent diabetes mellitus (IDDM) [1], non-insulin dependent diabetes mellitus (NIDDM) and gestational diabetes. Data gathered by the American Diabetes Association (http://www.ada.org) in 2005 estimate that 14.6 million people were diagnosed with diabetes while 6.2 million are currently undiagnosed in the United States. Today, over twenty million Americans are diabetic and the incidence is increasing by 5% per year.
The high level of blood glucose that defines diabetes is also the leading factor in cellular dysregulation and destruction resulting in the macro- and microvascular complications of this disease. Macrovascular complications lead to atherosclerosis and dilated cardiomyopathy [2], while microvascular complications include diabetic nephropathy [3, 4] retinopathy [5, 6] and neuropathy [7-10]. The most common of these complications is diabetic neuropathy (DN) occurring in approximately 60% of diabetic patients [11].
Beyond effective control of glucose levels, there are no treatments to prevent or cure DN. Numerous factors have impeded laboratory investigations aimed at the development of effective therapies, including an incomplete analysis of existing animal models that develop DN, lack of consistency among mouse models (diabetes induction and genetics) and lack of consensus concerning phenotyping methods. The AMDCC was formed by the NIH to develop new animal models of diabetes and its complications; its goal is to identify the most appropriate animal models to study the etiology, prevention and treatment of diabetic complications. The group has reported its findings comparing the effects of diabetes on cardiovascular disease [2], and nephropathy [12] in inbred mice from different genetic background strains and maintains a website containing its recommended phenotyping protocols. AMDCC investigators continue to refine genetic and dietary factors that affect the development of diabetes and its complications, potential interactions and overlap of complications within a genetic model, and common pathways that may influence treatment paradigms in human patients.
In order to provide a consistent method of examining DN in diabetic mouse models, our laboratory implemented a standardized diabetes induction protocol used by the AMDCC (http://www.amdcc.org) and developed an independent set of phenotyping parameters to classify and categorize mouse models of diabetes with regard to the development and severity of DN [13, 14]. The current review suggests a definition of murine DN, summarizes the published data on mouse models of DN and recommends a set of standardized criteria to define DN in mice. These criteria, along with detailed instructions on the methodologies used to phenotype mice for the presence of DN are available at our website at http://www.pnrd.umich.edu.
DIABETIC NEUROPATHY (DN): DEFINITION AND PREVALENCE
DN is defined as “the presence of symptoms and/or signs of peripheral nerve dysfunction in people with diabetes after the exclusion of other causes” [15] and is the most common complication of diabetes. Statistics gathered in 2005 indicate that 60-70% of diabetic patients have mild to severe DN (http://www.ada.org). DN is characterized by progressive, length dependent sensory loss [10]. DN first affects the longest axons innervating the feet; by the time symptoms reach the knees, the fingers are often affected [10]. Sensory symptoms correlate with abnormal nerve conduction studies [10, 16, 17], decreased myelinated fiber density (MFD) [10, 17-19] and decreased intraepidermal nerve fiber (IENF) densities [20-22]. Regenerating axons are present in human sural nerve [23, 24] and in skin biopsies [25], but over the course of the disease, regeneration fails. While maintenance of euglycemia may slow peripheral nerve deterioration [26, 27], no treatment is currently available to reverse the loss of nerve fibers and function in DN.
MOUSE MODELS OF DN
In human patients, the diagnosis of DN is made following the establishment of diabetes and ruling out other possible causes of neuropathy [7-10]. Methods used to assess neuropathy include a careful neurological examination of tendon reflexes, the ability to sense hot, cold or vibratory stimuli, and in select cases, nerve conduction studies using surface electrodes and skin biopsies [10, 13, 14, 28]. These measures have been successfully adapted and applied to mouse models of neuropathy including DN [13, 14, 29-32].
DN is described in mice with type 1 or 2 diabetes including spontaneous (genetic) and chemically induced (streptozotocin, STZ) models (Tables 1-3). The earliest reports of DN in mouse models centered on the BKS.Cg-m+/+Leprdb/J mouse (BKS-db/db) a spontaneous model of type 2 diabetes. These mice express a mutation in the leptin receptor resulting in hyperphagia, severe obesity, hyperinsulinemia and hyperglycemia beginning at approximately 4 weeks of age. DN is reported as early as 4 weeks post onset of diabetes as measured by an increase in thermal latency. Both sensory and motor nerve conduction velocities are decreased [13, 14, 33-36], axonal transport is slowed [37, 38] and both neurotransmitter levels [39] and IENF densities are decreased [13, 14] (Table 1). A second spontaneous model of type 2 diabetes is the B6.V-Lepob/J or B6-ob/ob mouse. The B6-ob/ob mouse lacks leptin and is similar to the BKS-db/db mouse with regard to the development of type 2 diabetes. These mice also develop DN 7 weeks post onset of hyperglycemia [40, 41], characterized by increased thermal latency, decreased mechanical response and decreased motor and sensory nerve conduction velocities [40, 41]. Type 2 diabetes is inducible in the C57BL/6J mouse by feeding a diet high in fat (45-60% of calories derived from fat) [42, 43]. Under these conditions, C57BL/6J mice develop hyperglycemia and insulin insensitivity. The first examination of DN in this model was published by Obrosova and colleagues, who reported decreased motor and sensory nerve conduction velocities, increased thermal latencies and mechanical allodynia, similar to DN observed in spontaneous type 2 diabetes [43].
Table 1. Please Provide Table Heading.
| Model | Age (weeks) |
Behavior | NCV | Anatomy | Biochemistry | Glucose | Weight grams |
First Author Citation |
|---|---|---|---|---|---|---|---|---|
| Type 1 | ||||||||
| Ins.Dd1 | 28 | NA | ↓CMAP, ↓sensory NCV |
↓MFD sural nerve |
NA | > 250 mg/dL | NA | Elias Diabetes 47:1637-642, 1998 |
|
C57BL/
6J X Ins2C96Y (Akita) |
31 | ↑gait disturbance, Morris water maze |
↓sensory NCV | NA | NA | ~ 31 mmol/L | ~ 22 | Choeiri Behav Brain Res 157:31-38, 2005 |
|
C57BL/
6-Ins2Akita/J (Akita) |
28 | no change in thermal latency |
no change in motor or sensory NCV |
NA | NA | 584 mg/dL | Sullivan Neurobiol Dis 28:276-285, 2007 |
|
| NOD | 18 | ↑TF latency | NA | NA | NA | 19.6 mmol/L | No Change | Obrosova FASEB J 19:401-403, 2005 |
| 23 to 25 | NA | NA | sympathetic neuritic dystrophy |
NA | >600 mg% | NA | Schmidt Am J. Pathol 163:2077-2091, 2003 |
|
| NA | thermal hyperalgesia |
NA | NA | NA | <300 mg/dL | 20-30 | Davar Neurosci Lett 190:171-174, 1995 |
|
| NA | thermal hyperalgesia |
NA | NA | NA | 19.6 mmol/L | NA | Gabra Eur J Pharmacol 514:61-67, 2005 |
|
| Type 2 | ||||||||
|
BKS.Cg-m+/
+Leprdb/J (BKS-db/db) |
33 | NA | ↓motor NCV | NA | NA | 350 mg% (fasting) |
> 50 | Sima Acta Neuropath 40:85-89, 1978 |
| 33 | NA | NA | transmission elec- tron microscopy, severe axonal and Schwann cell abnormalities |
NA | NA | NA | Sima Lab Invest 40:627-632, 1979 |
|
| 56 | NA | NA | transmission elec- tron microscopy, severe axonal and Schwann cell abnormalities |
NA | NA | NA | Carson Neuropathol App Neurobiol 6:361-374, 1980 |
|
| 25 | NA | ↓motor NCV and distal motor latency |
NA | NA | HbAI, 4.48% 532.1 mg/100mL (fasting) |
41.7 | Moore Exp Neurol 70:548-555, 1980 |
|
| 33 | NA | ↓motor NCV | ↓axonal number ↓axonal size |
NA | 156 mg% (fasting) |
2 standard deviations above control values |
Robertson Diabetes 29:60-67, 1980 |
|
| 25 | NA | NA | NA | ↓fucose incorporation ↓eucine incorporation ↓ |
NA | NA | Chez Neurochem Res 8:465-472, 1983 |
|
| 60 | NA | NA | ↓myelinated fiber area ↓axon area ↓index of circularity |
NA | 34 mmol/L | 22 | Sharma Diabetes 32:1152-1161, 1983 |
|
| 31 | NA | NA | NA | ↓slow transport of AChE |
NA | NA | Vitadello Exp Neurol 82:143-147, 1983 |
|
| ~57 | hearing loss of 64% at 180 days of age |
↓NCV | ↓axon size | ↓slow transport of AChE |
NA | NA | Gorio Adv Exp med Biol 174:549-564, 1984 |
|
| 40 | NA | NA | ↓axonal area ↓NCV |
NA | 424 mg/ 100mL |
54 | Norido Exp Neurol 83:221-223, 1984 |
|
| 17 | NA | NA | NA | ↓transport of actin and tubulin |
NA | NA | Vitadello J Neurochem 45:860-868, 1985 |
|
| 40 | NA | NA | ↓density of particles P face freeze fracture |
NA | 539 mg/dL | 55 | Antonella J Neurol Sci 69:301-317, 1985 |
|
| 20 | NA | no change in motor NCV |
NA | NA | 19 mmol/L | 34 | Whiteley Exp Neurol 89:314-321, 1985 |
|
| 2.5-17 months |
NA | NA | ↓varicosity and size of Meissner corpuscles |
NA | 240-400 mg/dL | NA | Ras Exp Neurol 91:488-501, 1986 |
|
| 38 | NA | NA | NA | ↑32P phospholipid incoporation |
393 mg/dL | 57 | Berti-Mattera Diabetes 37:1703-1707, 1988 |
|
| 14-17 | NA | NA | NA | ↑fructose in sciatic nerve |
37 mmol/dL | 39 | Calcutt Metabolism 37:450-453, 1988 |
|
| 25-30 | NA | NA | NA | ↑glucose in sciatic nerve ↓accumulation of PFK and ChAT |
26 mmol/L | 48 | Calcutt Muscle Nerve 11:1206-1210, 1988 |
|
| 40 | NA | NA | NA | no change in ATPase activity |
~25 mmol/L >6 %, BAIC |
~50 | Bianchi Diabetologia 33:131-136, 1990 |
|
| NA | NA | NA | ↓number of CGRP-IR DRG neurons |
RIA ↓CGRP no change SP |
15 mmol/L | NA | Schmidt Exp Neurol 132:16-23, 1995 |
|
| 12 | NA | NA | ↓IENF | NA | NA | NA | Gibran J Surg Res 108:122-128, 2002 |
|
| 8 | ↑temperature (decreased sensitivity) |
↓sensory NCV ↓motor NCV |
↓vascularity of vasa nervorum in sciatic nerve |
NA | ~540 mg/dL | NA | Ii Circulation 112:93-102, 2005 |
|
| 32 | ↑thermal latency | Ex vivo CAP no change |
NA | NA | NA | 25-44 | Walwyn Exp Neurol 198:60-270, 2006 |
|
| 28 | thermal hypoalgesia |
↓sensory NCV ↓motor NCV |
↓IENF | NA | 378 mg/dL | Sullivan Neurobiol Dis 28: 276-285,2007 |
||
|
B6.V Lepob/J
(B6-ob/ob) |
11 | ↑hind paw latency ↓mechanical response |
↓sensory NCV ↓motor NCV |
↓IENF ↑NT+ DRG neurons ↑PAR+ nuclei |
NA | 17.6 mmol/L | 57 | Drel Diabetes 55:3335-3343, 2006 |
| 11 | ↑hind paw latency ↓mechanical response |
↓sensory NCV ↓motor NCV |
↓IENF ↑NT+ DRG neurons ↑PAR+ nuclei |
NA | 17.6 mmol/L | 57 | Vareniuk Exp Neurol 205:425-436, 2007 |
|
Methods for measuring blood glucose vary across laboratories and are reported as milligrams per deciliter (mg/dL), milligrams percent (mg %) and as millimoles per liter (mmol/L). AChE = acetylcholine esterase, CAP = compound action potential, CGRP = calcitonin gene-related peptide, ChAT = choline acetyltransferase, (fasting) = fasting blood glucose levels, IENF = intraepidermal nerve fiber, MFD = myelinated fiber density, NA = not reported, NCV = nerve conduction velocity, NT = nitrotyrosine immunohistochemistry, PAR = poly(ADP-ribose), PFK = 6-phosphofructokinase, RIA = radioimmunoassay, SP = substance P.
Table 3. Outbred STZ-Treated Mouse Models of DN.
| Model | Duration (weeks) |
STZ mg/kg Days |
Behavior | NCV | Anatomy | Biochemistry Gene Expression Other |
Glucose | Weight grams |
First Author Citation |
|---|---|---|---|---|---|---|---|---|---|
| CD-1 | 1 | 200/1 | thermal hyperalgesia (allodynia) |
NA | NA | NA | < 20 mmol/L | 25-30 | Gabra Eur J Pharm 457:115-124, 2002 |
| CD-1 | 11 days | 200/1 | thermal hyperalgesia (allodyia) |
NA | NA | NA | < 20 mmol/L | 25-30 | Gabra Peptides 24:1131-1139, 2003 |
| ddY | 2 | 150/1 | ↓nociceptive threshold (pressure) |
NA | NA | NA | < 400 mg/dL | 25 | Suzuki Jpn J Pharm 79:169-175, 1999 |
| ddY | 4 | 200/1 | thermal and mechanical hyperalgesia |
NA | ↑VR1 in DRG neurons |
NA | 599.6 mg/dL | 31.5 | Rashid J Pharm Exp Ther 306:709-717, 2003 |
| ddY | 8 | 200/1 | ↑mechanical threshold |
NA | NA | NA | < 16.7 mmol/L | > 35 | Murakami J Gene Med 8:773-781, 2006 |
| ICR | 2 | 200/1 | altered response to formalin induced nociception |
NA | NA | first phase longer, second phase abolished |
> 400 mg/dL | ~20 | Kamei Brain Res 862:257-261, 2000 |
| ICR | 2 | 200/1 | ↓mechanical nociceptive threshold |
NA | NA | NA | > 400 mg/dL | ~20 | Onodera Jpn J Pharm 85:335-337, 2001 |
| ICR | 3 | 200/1 | ↓mechanical threshold |
↑C-fiber wind up |
NA | NA | < 350 mg/dL | 31-42 | Kimura J Pharm Sci 97:195-202, 2005 |
| Laka | 4 | 200/1 | thermal hyperalgesia |
NA | NA | NA | 293 mg/dL | 19.5 | Anjaneyulu Progress Neuro- Psycopharm Bio Psych 27:1001-1005, 2003 |
| Laka | 4 | 200/1 | thermal hyperalgesia |
NA | NA | NA | 465.26 mg/dL | 17.4 | Anjaneyulu Eur J Pharm 497:285-292, 2004 |
| Laka | 4 | 200/1 | thermal hyperalgesia |
NA | NA | NA | 412 mg/dL | 17.42 | Sharma Phytother Res 21:278-283, 2007 |
| Swiss Wistar |
14 | 50/3 | NA | ↓MCV | no change in MFD |
delayed nerve regeneration |
41.1 mmol/L | 36.2 | Kennedy Brain 123:2118-2129, 2000 |
| Swiss- Webster |
6-8 | 100/2 | NA | ↓FSAs | NA | NA | 22.9 mml/L | 31 | Goss Diabetes 51:2227-2232, 2002 |
| Swiss mice | 8 | 3/70 | NA | no siginificant change in SCV |
NA | NA | 12.8 mmol/L | 32 | Zochodne Brain 127:2193-2200, 2004 |
| Swiss Wistar |
36 | 85, 70, 55 | ↓MCV ↓SCV ↓M wave |
↓IENF ↓DRG neurons |
NA | 31.9 mmol/L | 34.5 | Kennedy Diabetes 54:830-837, 2005 |
|
| Swiss NIH albino mice |
12 | 50/5 | ↓thermal latency ↑pressure latency |
NA | ↓MFD ↓Schwann cells/mm2 |
↓NGF serum ↓NGF sciatic nerve |
308 mg/dL | no change from control |
Arrieta EurJ Clin Invest 35:201-207, 2005 |
| Swiss Mice | 8 | 200/1 | ↑thermal latency) | ↓MNCV | NA | ↓blood flow | 486 mg/dL | 21 | Demiot Diabetes 55:1478-1483, 2006 |
| Swiss Webster |
24 | 100/2 | ↑thermal latency | ↓sensory amp ↓SCV, ↓MCV and motor amp |
↓sweat gland innervation |
↓pilocarpin-induced sweating |
22 mmol/L | 26 | Chattopadhyay Diabetologia 50:1550-1558, 2007 |
IENF = intraepidermal nerve fiber, GSH = glutathione S-transferase, MDA = malondialdehyde, MFD = myelinated fiber density, MCV = motor conduction velocity, NA = not reported, NCV. = nerve conduction velocity, NGF = nerve growth factor, PCr/Cr = phosphocreatinine/creatinine ratio, SCV = senory conduction velocity, SOD = superoxide dismutase, VR1 = Vanilloid receptor 1.
Models of spontaneous type 1 diabetes have resulted from insulin mutations (Table 1). Mutations in the insulin 1 (Ins.Dd1) and insulin 2 (C57BL/6-Ins2Akita/J) genes result in hypoinsulinemia and hyperglycemia [44, 45]. Sensory nerve conduction velocities are reduced in both types of mice following 20-24 weeks of diabetes (Table 1). Ins.Dd1 mice also demonstrate reduced myelinated fiber density (MFD) in the sural nerve [44]. DN was also examined in another model of spontaneous type 1 diabetes, the non-obese diabetic (NOD) mouse. Similar to human patients, in these mice type 1 diabetes develops following immune destruction of pancreatic beta cells. Sympathetic neuritic dystrophy is reported as early as 3 weeks post onset of diabetes [46]. Examination of thermal thresholds reveals both thermal hypoalgesia, 13 weeks post onset of diabetes [47], and hyperalgesia, 14 and 21 weeks post onset of diabetes [48, 49]. Measures of MFD and nerve conduction velocities are not reported in the NOD mice and IENF has yet to be analyzed in these spontaneous type 1 models (Table 1) [50].
C57BL/6J is the most commonly used strain of inbred laboratory mice and form the foundation for most gene targeting studies. Type 1 diabetes may be induced in this background strain by a high or low dose regimen of streptozotocin (STZ) injection (Table 2). Both paradigms result in hyperglycemia; however, toxicity (high post-injection mortality) is often higher with single, high dose STZ treatment. Low dose STZ treatment results in a slow destruction of beta cells and immune activation resembling autoimmune induced type 1 diabetes [51]. Low dose STZ-treatment of short duration (6-12 weeks) results in decreased motor and sensory nerve conduction velocity, [52-54] and increased thermal latency [52]. Long term examinations of C57BL/6J mice (24 weeks) reveal no changes in nerve conduction, sensory function or IENF in this model [13, 14]. In these studies, DN was not assessed until 12 weeks post STZ treatment. While fasting blood glucose and glycated hemoglobin (GHb) were significantly elevated 24 weeks post STZ-treatment and the mice displayed significant weight loss, all hallmarks of type 1 diabetes in rodents, there was no behavioral or electrophysiological evidence of DN. This discrepancy between short term and long term assessments of DN in STZ-treated C57BL/6J mice awaits further study. This issue will only be clarified by serial assessment of DN immediately following STZ-treatment, with special attention to insulin levels, diet and gender.
Table 2. Table Inbred C57BL/6J High and Low Dose STZ Models of DN.
| Model | Duration (weeks) |
STZ mg/kg |
Behavior | NCV | Anatomy | Biochemistry Gene Expression |
Glucose | Weight grams |
First Author Citation |
|---|---|---|---|---|---|---|---|---|---|
| High Dose | |||||||||
| C57BL/6J | 6 | 200 | NA | NA | ↓peptidergic input to spinal cord lamina IIi |
NA | ~25 mmol/L | ↓30% | Akkina Exp Neurol 167:173-182, 2001 |
| 7 | 180 | NA | NA | ↓IENF density | NA | 405 mg/dL | 21 | Christianson Exp Neurol 179:188-199, 2003 |
|
| 9 | 180 | no difference thermal or chemogenic stimuli; mechanical hypoalgesia |
NA | NA | NA | 292 mg/dL | 21 | Christianson J Pain 4:493-504, 2003 |
|
| 9 | 150 | NA | ↓MNCV ↓SNCV |
NA | ↓PCr/Cr ↑glucose ↑sorbitol ↑fructose |
23.4 mmol/L | 24.9 | Obrosova FASEB J 19:401-420, 2004 |
|
| 6 | 180 | no difference in mechanosensory function |
NA | ↓DRG neuronal size |
↑ATF3 expression |
26.6 mmol/L | 22.33 | Wright J Periph Nerv Syst 9:242-254, 2004 |
|
| 1 | 200 | ↓thermal sensitivity | NA | NA | NA | > 20 mmol/L | NA | Gabra Reg Pep 127:245-248, 2005 |
|
| 8 | 180 | ↓mechanical sensitivity | NA | ↓IENF | NA | 366 mg/dL | 21.3 | Christianson Neuroscience 145:303-313, 2007 |
|
| 7 | 180 | ↓formalin-induced pain behavior |
NA | ↓number of Fos positive neurons in dorsal horn |
NA | 20 mmol/L | 20 | Johnson J Pain 8:637-649, 2007 |
|
| 6 | 100 | ↓thermal sensitivity, ↓me- chanical sensitivity (pressure), ↑mechanical sensitivity (non Frey filament) |
NA | ↓IENF ↑NT IHC ↑PAR IHC |
NA | 33 mmol/L | 25 | Drel Europ J Pharm 569: 48-58, 2007 |
|
| Low Dose | |||||||||
| C57BL/6J | 12 | 40 /5 | ↑hind paw latency | NA | NA | ↓mitochondrial activity |
26.4 mmol/dL | 19.3 | Tam FASEB J 18:1767-1769, 2004 |
|
129/Sv X
C57Bl6 |
~7 | 40 / 7 | NA | ↓motor and sensory NCV |
↓nerve blood flow |
↓PCr, PCr/Cr | 22.7 mmol/L | 24.5 | Obrosova Diabetes 53:711-720, 2004 |
| B6/129S7 | 6 | 40 / 5 | NA | ↓motor and sensory NCV |
NA | ↓GSH, ↓SOD, ↑MDA, ↑catalase |
329 mg/dL | 19.7 | Kellogg Antiox and Redox Sig 7:1521-1529, 2005 |
| C57BL/6J | NA | 50 / 5 | NA | NA | NA | prolonged Ca++ elevation in DRG neurons |
21.15 mmol/L | NA | Kostyuk Neuroscience 90:535-541, 1999 |
| C57BL/6J | 24 | 50 / 5 | no change in thermal latency |
no change in NCV |
no change in IENF |
no change in GSH, SOD, MDA, Catalase |
366 mg/dL | Vincent Exper Neurol 208:216-227, 2007 |
|
| C57BL/6J | 24 | 50 / 5 | no change in thermal latency |
no change in NCV |
no change in TUNEL+ cells or IENF |
NA | 466 mg/dL | Sullivan Neurobiol Dis 28:276-285, 2007 |
|
ATF3 = activating transcription factor 3, IENF = intraepidermal nerve fiber, IHC = immunohistochemistry, GSH = glutathione S-transferase, MDA = malondialdehyde, NCV. = nerve conduction velocity, NT = nitrotyrosine, PAR = poly(ADP-ribose), PCr/Cr = phosphocreatinine/creatinine ratio, SOD = superoxide dismutase.
DN was also examined in C57BL/6J mice following a single high dose of STZ (Table 2) [30, 47, 55-59]. Similar to the majority of reports in low dose STZ-treated mice, DN was examined following diabetes duration of 1-9 weeks. There is one report of a decrease in motor and sensory nerve conduction velocities along with elevations of glucose, sorbitol and fructose within the sciatic nerve [60] (Table 2). Investigations of DN in this model by Wright and co-workers reveal decreased mechanical sensitivity at 8 and 9 weeks post STZ-treatment with reduced IENF densities but no changes in thermal thresholds. Nerve conduction velocities were not measured (Table 2) [30, 55-57, 59]. Thus, while there is no doubt that the C57BL/6J mouse develops profound diabetes in response to single high dose STZ treatment, evidence of DN is again only described in short term diabetes. Similar to the results after low dose STZ-induction of diabetes, two studies of diabetes of 24 weeks [13, 14] found no evidence of DN in C57BL/6J animals after single high dose STZ-treatment. It is also evident from the reports summarized in Table 2 that few studies examine all 3 aspects of DN; therefore, the degree of DN remains to be fully characterized.
The outbred Swiss albino mouse (a.k.a. Swiss Webster, Swiss Wistar, Swiss NIH Albino) is the most thoroughly characterized model of DN (Table 3). Diabetes was induced by both high and low STZ treatment with similar results (Table 3). Decreased motor [61-63] and sensory [62, 64] nerve conduction velocities are reported following 8-36 weeks of diabetes. Studies vary in the response to a thermal stimulus. Arrieta et al. [65] report thermal allodynia 12 weeks post STZ-treatment while Demiot et al. [63] report thermal hypoalgesia 8 weeks post STZ-treatment. IENF density [62] and MFD [65] are reduced in diabetic Swiss mice, as is nerve blood flow, levels of nerve growth factor (NGF) in sciatic nerve and serum, and size of dorsal root ganglia (DRG) neurons [62]. Yet even in this model, there is no comprehensive report of DN including behavior, nerve function and peripheral nerve/DRG anatomy.
Other outbred strains include CD-1/ICR, ddY and Laka mice. CD-1/ICR mice demonstrate thermal allodynia following 7 to 11 days of diabetes [66-69]. Similarly, allodynia is reported in short term diabetes (2 and 4 weeks) [70, 71]. Single high dose STZ-treatment used to induce diabetes and DN in ddY and Laka mice (Table 3) resulted in hypoalgesia in ddY mice. DN characterized by hyperalgesia was documented in Laka mice [72-74] (Table 3).
DN SCREENING
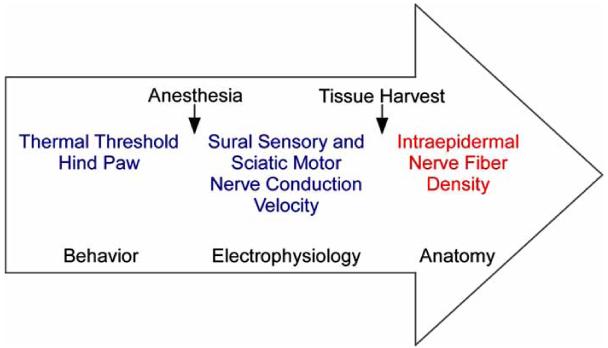
As demonstrated above, there is no single protocol for inducing diabetes or a standard set of tests used to define DN. We designed a set of assays to screen for the presence of DN, as well as a set of advanced measures to fully characterize DN. Mice are screened for DN by first establishing that they become and remain hyperglycemic using AMDCC guidelines. We adapted current methodologies used to assess neuropathy in human patients to establish the presence of DN in mice. These methods include using heat/pain thresholds to assess sensory function (hyper- or hypoalgesia), measuring nerve conduction velocities to establish the status of nerve electrophysiology (NCV) and examination of an anatomical marker of end organ innervation (IENF density). Initial screening of a new mouse model includes nerve conduction studies, behavioral measures of sensory function, and assessment of IENF density (Fig. 1). This panel of tests is performed at 3 and 6 months following induction of diabetes. If the presence of DN is verified, shorter and longer time points may be added to characterize the progression of DN.
Fig. (1). Time Line of Neuropathy Screening.
Behavioral assessment of sensory function is completed prior to anesthesia. NCV are measured in anesthetized mice followed by immediate foot pad dissection.
GUIDELINES FOR DISEASE DEFINITION
Age related normal data for motor and sensory nerve conduction velocities are available for human patients; unfortunately, this type of information is not available for mice. Baseline control values for nerve conduction velocity in mice vary across age and genetic background as do the relative decreases following diabetes. This variability is in part due to diabetes duration, type of diabetes, background strain, gender and diet. Decreases in motor nerve conduction velocity range from 13% in C57BL/6J mice diabetic for 8 weeks [60] to a 50% reduction in the BKS-db/db mouse following 21 weeks of diabetes [13, 33]. Percent decreases in sensory nerve conduction velocities vary from 10% in the Swiss Wistar mouse following 36 weeks of diabetes to 53% in the BKS-db/db mouse 4 weeks after the onset of diabetes [36]. In the literature and in our own laboratory, the BKS-db/db mouse model of type 2 diabetes exhibits the most robust changes in both motor and sensory nerve conduction velocities with no less than a 20% decrease in either measure [13, 34, 36]. In models of type 1 diabetes, both inbred and outbred strains, induced or spontaneous, larger decreases are noted in motor (~24%) than sensory (~20%) conduction velocities. Because the duration of diabetes has a profound effect on nerve conduction velocity, we suggest that a 10 to 15% reduction in conduction velocity in animals with less than 8 weeks diabetes and a 15 to 20% reduction in mice diabetic for over 12 weeks be considered a robust model of DN.
Changes in sensory behavior are equally variable and time dependent, as many mouse models exhibit hyperalgesia and allodynia [48, 72, 75] in the early phases of neuropathy and hypoalgesia [13, 40] in later stages. Allodynia and thermal hyperalgesia are most often reported in mice with diabetes duration of ~4 weeks [48, 72, 75]. The percent change in thermal threshold in these studies ranged from 29-47%. Hypoalgesia is commonly reported following 8 or more weeks of diabetes and the percent increase in threshold is reported to be as high as 88% [40]. It is often the case that the mice fail to respond within a set of temperature cut off points established to prevent injury. It is reasonable that a 20% change in mechanical (allodynia) or thermal (hypo- or hyperalgesia) thresholds would be indicative of DN.
IENF density is a newly established parameter for measuring DN in mice. Two papers by Christianson [30, 59] report a minimum decrease of 50% in C57BL/6J mice following 7 weeks of diabetes. Our laboratory published similar findings in the BKS-db/db mouse following 24 weeks of diabetes [13]. As more reports of DN are published in mice of different background strains and with diabetes of longer duration, a minimum percent decrease may be established for this measure.
ADVANCED NEUROPATHY PHENOTYPING
Once the presence of DN is verified, advanced neuropathy phenotyping protocols are used to further characterize DN severity, progression, and potential mechanisms (Fig. 2). These procedures are more time-consuming and are not required to establish the presence of DN. For example, nerve conduction assays are performed at 4 week intervals rather than only at 3 and 6 month time points. Assessments of MFD of the sural nerve and/or quantitation using electron microscopy of thinly myelinated and unmyelinated fibers yield additional anatomical confirmation of DN [35, 44]. Biochemical measures for oxidative stress adducts and enzymes are performed in plasma and tissue extracts of DRG and peripheral nerve [47, 54, 76-78]. Additional anatomical endpoints include assays of cell death (TUNEL) [77, 78], localization of oxidative stress adducts in DRG and peripheral nerve [13, 40, 47, 79] and classification of different populations of IENF based on neurotransmitter expression [80]. These biochemical and anatomical assessments are used to further understand the mechanisms underlying the development DN; they are not used to establish the presence of DN. For this reason, no reference ranges for normal and abnormal findings of these multiple assays are suggested in the overview.
Fig. (2). Advanced Neuropathy Phenotyping.
As with neuropathy screening, behavioral assays are performed in conscious mice. Multiple tissues are harvested and analyzed immediately or stored for future use.
AN EXAMPLE OF MURINE PHENOTYPING
Our laboratory has phenotyped multiple mouse models for the presence of DN [13]. The BKS-db/db mouse is an example of a spontaneous model of type 2 diabetes that develops a robust DN. Previous work in this model demonstrated clear deficits in NCV [33, 34, 81-85] and fiber density and/or morphology [33, 35, 81-83, 86-88] following the development of diabetes. BKS-db/db mice develop hyperglycemia between 3-4 weeks of age (http://jaxmice.jax.org/) and a profound neuropathy at 24 weeks post onset of diabetes [13]. At 24 weeks, BKS-db/db developed significantly elevated fasting glucose, GHb and body weights compared to their nondiabetic (BKS-db+) littermates (Table 1).
We utilized the criteria outlined above to confirm and extend the phenotype of these animals regarding the presence of DN. Behavioral testing of the BKS-db/db mice demonstrated elevated tail flick latencies indicating sensory loss (nondiabetic 4.8 sec ± 0.14 and diabetic 9.7 sec ± 0.07, p<0.001). The BKS-db/db mice also demonstrated profound changes in NCV with decreased tail sensory NCV (nondiabetic 23.8 m/sec ± 0.2, diabetic 17.4 m/sec ± 0.3, p < 0.001), increased tail distal motor latencies (nondiabetic 2.97 msec ± 0.02, diabetic 4.7 msec ± 0.07, p < 0.001), and decreased sciatic motor NCV (nondiabetic 38.8 m/sec ± 0.8, diabetic 26.7 m/sec ± 1.2, p < 0.05). To complete guideline criteria, IENF densities were measured in BKS-db/db mice including in the foot pad. IENF density was significantly reduced at 24 weeks post onset of diabetes (nondiabetic 37.7 fibers/mm ± 2.6, diabetic 21.0 fibers/mm ± 2.0, p < 0.01) in the BKS-db/db mice (Fig. 3).
Fig. (3). IENF measures functional innervation of the skin.
A) PGP9.5 immunofluorescence within a nondiabetic (n = 5) BKS-db+ foot pad demonstrating a normal pattern of innervation (dots). B) PGP9.5 immunofluorescence within a diabetic (n = 5) BKS-db/db foot pad demonstrating a decrease in the number of fibers (dots). C) Quantitation of IENF is presented as the number of fibers/linear mm of epidermis. d = dermis, e = epidermis, dots = intraepidermal nerve fiber, arrowhead = dermal fiber bundles. γp>0.01. Bar = 100 m. Open bars represent the nondiabetic measurements and the black bars represent the diabetic measurements. (Reprinted with permission of from Elsevier, Neurobiol Dis 2007 (13)).
We also completed measurements of advanced neuropathy phenotyping. TUNEL assessment of fragmented DNA was used as a marker of glucose-mediated injury in DRG neurons. An increase in the number of TUNEL positive sensory neurons was detected at 24 weeks in the DRG of BKS-db/db mice (nondiabetic 13.7% ± 1.4, diabetic 21.3% ± 2.0, p < 0.01) [13]. Nitrotyrosine immunoreactivity is another marker of oxidative/nitrosative stress [47, 89]. An increase in the number and intensity of nitrotyrosine positive sensory neurons was detected at 24 weeks in the DRG of BKS-db/db mice (pixel intensity nondiabetic 48.11 ± 3.7, diabetic 84.53 ± 6.1, p < 0.01) [13] (Fig. 4). In summary, behavioral, electrophysiological and anatomical assessments of DN are profoundly impaired in the BKS-db/db mice. Our published data [13] agree with previous reports of DN in this model [33, 34, 81-85].
Fig. (4). Damaged DNA was measured by TUNEL staining.
A) TUNEL positive sensory neurons (arrows) were detected in the lumbar DRG of BKS-db/db. B) Increased number of TUNEL labeled DRG in BKS-db/db mice at 24 weeks, λp < 0.05. Five animals per group and > 150 neurons per animal were counted. Results are expressed as the percent TUNEL positive cells of total neurons counted. Localization of nitrated proteins was measured by nitrotyrosine immunofluorescence (NT-immunofluorescence). C) NT-immunofluorescence reveals an increase in nitrated proteins within DRG neurons (arrows) from BKS-db/db compared to BKS-db+ mice [nuclei stained with DAPI]. D) Histograms of the fluorescence signal indicate a relative increase in the intensity of NT-immunofluorescence in DRG from BKS-db+ versus BKS-db/db mice (γp<0.01). Bar = 20 m. Open bars represent the nondiabetic measurements and the black bars represent the diabetic measurements. (Reprinted with permission of from Elsevier, Neurobiol Dis 2007 (13)).
SUMMARY AND FUTURE DIRECTIONS
If the scientific community agrees upon standardized definitions of DN in mouse models of type 1 and 2 diabetes, mouse models could provide a more effective means for preclinical testing of potential therapies. The lack of a uniform definition of murine DN makes comparisons across different drug-intervention trials difficult at best. For example, our results point to the BKS-db/db mouse as a robust working model of DN in type 2 diabetes. If a therapy proved effective in the treatment of DN in this model, the use of standardized definitions of DN would allow confirmation of this beneficial effect in other mouse models of DN. Treatments that have a positive effect on DN across different mouse strains and different types of diabetes are much more likely to yield a meaningful clinical effect in man. It is clear that the lack of uniformity in diagnosing and monitoring DN in murine models is limiting needed therapeutic advances for patients. This review suggests a set of standardized phenotyping criteria for the diagnosis of DN. These criteria were extracted from the currently published literature and could serve as a “talking point” for the formation of standardized murine guidelines for the diagnosis of DN.
ACKNOWLEDGEMENTS
The authors wish to acknowledge Ms. Julie Erwin for expert manuscript preparation and Dr. Andrea Vincent for expert editorial advice. The authors also acknowledge the expertise of Mr. John M. Hayes and Ms. Carey Backus with regard to animal models of diabetes and neuropathy phenotyping methods. These studies were conducted within the Morphometry Core of the JDRF Center for the Study of Complications in Diabetes and the Michigan Diabetes Research and Training Center (NIH5P60 DK20572).
Grant support: This work was supported by the National Institutes of Health (NS38849, and DK60994), the Juvenile Diabetes Research Foundation Center for the Study of Complications in Diabetes, and the Program for Neurology Research and Discovery (www.pfund.umich.edu).
REFERENCES
- [1].Eurodiab ICG. Diabetologia. 1994;37:278–285. doi: 10.1007/BF00398055. [DOI] [PubMed] [Google Scholar]
- [2].Hsueh W, Abel ED, Breslow JL, Maeda N, Davis RC, Fisher EA, Dansky H, McClain DA, McIndoe R, Wassef MK, Rabadan-Diehl C, Goldberg IJ. Circ. Res. 2007;100:1415–27. doi: 10.1161/01.RES.0000266449.37396.1f. [DOI] [PubMed] [Google Scholar]
- [3].Forbes JM, Fukami K, Cooper ME. Exp. Clin. Endocrinol. Diabetes. 2007;115:69–84. doi: 10.1055/s-2007-949721. [DOI] [PubMed] [Google Scholar]
- [4].Wolf G, Ziyadeh FN. Nephron Physiol. 2007;106:26–31. doi: 10.1159/000101797. [DOI] [PubMed] [Google Scholar]
- [5].Yam JC, Kwok AK. Hong Kong Med. J. 2007;13:46–60. [PubMed] [Google Scholar]
- [6].Gardiner TA, Archer DB, Curtis TM, Stitt AW. Microcirculation. 2007;14:25–38. doi: 10.1080/10739680601072123. [DOI] [PubMed] [Google Scholar]
- [7].Said G. Nat. Clin. Pract. Neurol. 2007;3:331–40. doi: 10.1038/ncpneuro0504. [DOI] [PubMed] [Google Scholar]
- [8].Feldman EL. J. Clin. Invest. 2003;111:431–433. doi: 10.1172/JCI17862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Feldman EL, Stevens MJ, Russell JW, Greene DA, Porte D, Jr., Sherwin RS, Baron A. Ellenberg and Rifkin’s Diabetes Mellitus. 6th. McGraw Hill; 2003. pp. 771–788. [Google Scholar]
- [10].Feldman EL, Stevens MJ, Russell JW, Peltier A, Inzucchi S, Porte JD, Sherwin RS, Baron A. The Diabetes Mellitus Manual. 6th. McGraw-Hill; 2005. pp. 366–384. [Google Scholar]
- [11].Vincent AM, Feldman EL. Rev. Endo. Metabol. Dis. 2004;5:227–236. doi: 10.1023/B:REMD.0000032411.11422.e0. [DOI] [PubMed] [Google Scholar]
- [12].Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, Breyer MD. Diabetes. 2005;54:2628–2637. doi: 10.2337/diabetes.54.9.2628. [DOI] [PubMed] [Google Scholar]
- [13].Sullivan KA, Hayes JM, Wiggin TD, Backus C, Su Oh S, Lentz SI, Brosius F, 3rd, Feldman EL. Neurobiol. Dis. 2007;28:276–285. doi: 10.1016/j.nbd.2007.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Vincent AM, Russell JW, Sullivan KA, Backus C, Hayes JM, McLean LL, Feldman EL. Exp. Neurol. 2007;208:216–227. doi: 10.1016/j.expneurol.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D. Diabetes Care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- [16].Daube JR, Dyck PJ, Thomas PK. Diabetic Neuropathy. 2nd. W.B. Saunders Company; Philadelphia: 1999. pp. 222–237. [Google Scholar]
- [17].Russell J, Karnes J, Dyck P. J. Neurol. Sci. 1996;135:114–117. doi: 10.1016/0022-510x(95)00243-u. [DOI] [PubMed] [Google Scholar]
- [18].Dyck PJ, Giannini C, Lais A, Dyck PJ, Thomas PK, Griffin JW, Low PA, Poduslo JF. Peripheral neuropathy. W.B. Saunders Company; Philadelphia: 1993. pp. 514–595. [Google Scholar]
- [19].Perkins BA, Greene DA, Bril V. Diabetes Care. 2001;24:748–752. doi: 10.2337/diacare.24.4.748. [DOI] [PubMed] [Google Scholar]
- [20].Lauria G, Lombardi R, Borgna M, Penza P, Bianchi R, Savino C, Canta A, Nicolini G, Marmiroli P, Cavaletti G. J. Peripher. Nerv. Syst. 2005;10:202–208. doi: 10.1111/j.1085-9489.2005.0010210.x. [DOI] [PubMed] [Google Scholar]
- [21].Pittenger GL, Ray M, Burcus NI, McNulty P, Basta B, Vinik AI. Diabetes Care. 2004;27:1974–1979. doi: 10.2337/diacare.27.8.1974. [DOI] [PubMed] [Google Scholar]
- [22].Bianchi R, Brines M, Lauria G, Savino C, Gilardini A, Nicolini G, Rodriguez-Menendez V, Oggioni N, Canta A, Penza P, Lombardi R, Minoia C, Ronchi A, Cerami A, Ghezzi P, Cavaletti G. Clin. Cancer Res. 2006;12:2607–2612. doi: 10.1158/1078-0432.CCR-05-2177. [DOI] [PubMed] [Google Scholar]
- [23].Sullivan KA, Brown MS, Harmon L, Greene DA. J. Periph. Nerv. System. 2003;8:260–270. doi: 10.1111/j.1085-9489.2003.03030.x. [DOI] [PubMed] [Google Scholar]
- [24].Britland ST, Young RJ, Sharma AK, Clarke BF. Diabetes. 1990;39:898–908. doi: 10.2337/diab.39.8.898. [DOI] [PubMed] [Google Scholar]
- [25].Polydefkis M, Hauer P, Sheth S, Sirdofsky M, Griffin JW, McArthur JC. Brain. 2004;127:1606–1615. doi: 10.1093/brain/awh175. [DOI] [PubMed] [Google Scholar]
- [26].Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, Wilson DM, O’Brien PC, Melton Iii LJ. Neurology. 1993;43:817–824. doi: 10.1212/wnl.43.4.817. [DOI] [PubMed] [Google Scholar]
- [27].Dyck PJ, Davies JL, Wilson DM, Service FJ, Melton LJ, O’Brien PC. Diabetes Care. 1999;22:1479–1486. doi: 10.2337/diacare.22.9.1479. [DOI] [PubMed] [Google Scholar]
- [28].Lauria G, Lombardi R. BMJ. 2007;334:1159–62. doi: 10.1136/bmj.39192.488125.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zochodne DW, Verge VM, Cheng C, Sun H, Johnston J. Brain. 2001;124:2319–2334. doi: 10.1093/brain/124.11.2319. [DOI] [PubMed] [Google Scholar]
- [30].Christianson JA, Ryals JM, McCarson KE, Wright DE. J. Pain. 2003;4:493–504. doi: 10.1016/j.jpain.2003.07.002. [DOI] [PubMed] [Google Scholar]
- [31].Calcutt NA. J. Neurol. Sci. 2004;220:137–139. doi: 10.1016/j.jns.2004.03.015. [DOI] [PubMed] [Google Scholar]
- [32].Calcutt NA, Tomlinson DR, Biswas S. Diabetes. 1990;39:663–666. doi: 10.2337/diab.39.6.663. [DOI] [PubMed] [Google Scholar]
- [33].Sima AA, Robertson DM. Acta Neuropathol. (Berl) 1978;41:85–89. doi: 10.1007/BF00689757. [DOI] [PubMed] [Google Scholar]
- [34].Moore SA, Peterson RG, Felten DL, Cartwright TR, O’Connor BL. Exp. Neurol. 1980;70:548–555. doi: 10.1016/0014-4886(80)90181-8. [DOI] [PubMed] [Google Scholar]
- [35].Norido F, Canella R, Zanoni R, Gorio A. Exp. Neurol. 1984;83:221–232. doi: 10.1016/S0014-4886(84)90094-3. [DOI] [PubMed] [Google Scholar]
- [36].Ii M, Nishimura H, Kusano KF, Qin G, Yoon YS, Wecker A, Asahara T, Losordo DW. Circulation. 2005;112:93–102. doi: 10.1161/CIRCULATIONAHA.104.511964. [DOI] [PubMed] [Google Scholar]
- [37].Vitadello M, Couraud JY, Hassig R, Gorio A, Di GL. Exp. Neurol. 1983;82:143–147. doi: 10.1016/0014-4886(83)90249-2. [DOI] [PubMed] [Google Scholar]
- [38].Vitadello M, Filliatreau G, Dupont JL, Hassig R, Gorio A, Di Giamberardino L. J. Neurochem. 1985;45:860–868. doi: 10.1111/j.1471-4159.1985.tb04073.x. [DOI] [PubMed] [Google Scholar]
- [39].Schmidt Y, Unger JW, Bartke I, Reiter R. Exp. Neurol. 1995;132:16–23. doi: 10.1016/0014-4886(95)90054-3. [DOI] [PubMed] [Google Scholar]
- [40].Drel VR, Mashtalir N, Ilnytska O, Shin J, Li F, Lyzogubov VV, Obrosova IG. Diabetes. 2006;55:3335–3343. doi: 10.2337/db06-0885. [DOI] [PubMed] [Google Scholar]
- [41].Vareniuk I, Pavlov IA, Drel VR, Lyzogubov VV, Ilnytska O, Bell SR, Tibrewala J, Groves JT, Obrosova IG. Exp. Neurol. 2007;205:425–36. doi: 10.1016/j.expneurol.2007.03.019. [DOI] [PubMed] [Google Scholar]
- [42].Surwit RS, Feinglos MN, Rodin J, Sutherland A, Petro AE, Opara EC, Kuhn CM, Rebuffe-Scrive M. Metab. Clin. Exp. 1995;44:645–651. doi: 10.1016/0026-0495(95)90123-x. [DOI] [PubMed] [Google Scholar]
- [43].Obrosova IG, Ilnytska O, Lyzogubov VV, Pavlov IA, Mashtalir N, Nadler JL, Drel VR. Diabetes. 2007;56(10):2598–608. doi: 10.2337/db06-1176. [DOI] [PubMed] [Google Scholar]
- [44].Elias KA, Cronin MJ, Stewart TA, Carlsen RC. Diabetes. 1998;47:1637–1642. doi: 10.2337/diabetes.47.10.1637. [DOI] [PubMed] [Google Scholar]
- [45].Choeiri C, Hewitt K, Durkin J, Simard CJ, Renaud JM, Messier C. Behav. Brain Res. 2005;157:31–38. doi: 10.1016/j.bbr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- [46].Schmidt RE, Dorsey DA, Beaudet LN, Frederick KE, Parvin CA, Plurad SB, Levisetti MG. Am. J. Pathol. 2003;163:2077–2091. doi: 10.1016/S0002-9440(10)63565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Obrosova IG, Mabley JG, Zsengeller Z, Charniauskaya T, Abatan OI, Groves JT, Szabo C. FASEB J. 2005;19:401–403. doi: 10.1096/fj.04-1913fje. [DOI] [PubMed] [Google Scholar]
- [48].Gabra BH, Sirois P. Eur. J. Pharmacol. 2005;514:61–7. doi: 10.1016/j.ejphar.2005.03.018. [DOI] [PubMed] [Google Scholar]
- [49].Davar G, Waikar S, Eisenberg E, Hattori M, Thalhammer JG. Neurosci. Lett. 1995;190:171–4. doi: 10.1016/0304-3940(95)11532-2. [DOI] [PubMed] [Google Scholar]
- [50].McEvoy RC, Andersson J, Sandler S, Hellerstrom C. J. Clin. Invest. 1984;74:715–22. doi: 10.1172/JCI111487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Pereira L, Matthes J, Schuster I, Valdivia HH, Herzig S, Richard S, Gomez AM. Diabetes. 2006;55:608–615. doi: 10.2337/diabetes.55.03.06.db05-1284. [DOI] [PubMed] [Google Scholar]
- [52].Tam J, Rosenberg L, Maysinger D. FASEB J. 2004;18:1767–1769. doi: 10.1096/fj.04-1894fje. [DOI] [PubMed] [Google Scholar]
- [53].Obrosova IG, Li F, Abatan OI, Forsell MA, Komjati K, Pacher P, Szabo C, Stevens MJ. Diabetes. 2004;53:711–720. doi: 10.2337/diabetes.53.3.711. [DOI] [PubMed] [Google Scholar]
- [54].Kellogg AP, Pop-Busui R. Antioxid. Redox. Signal. 2005;7:1521–1529. doi: 10.1089/ars.2005.7.1521. [DOI] [PubMed] [Google Scholar]
- [55].Akkina SK, Patterson CL, Wright DE. Exp. Neurol. 2001;167:173–182. doi: 10.1006/exnr.2000.7547. [DOI] [PubMed] [Google Scholar]
- [56].Christianson JA, Riekhof JT, Wright DE. Exp. Neurol. 2003;179:188–199. doi: 10.1016/s0014-4886(02)00017-1. [DOI] [PubMed] [Google Scholar]
- [57].Wright DE, Ryals JM, McCarson KE, Christianson JA. J. Peripher. Nerv. Syst. 2004;9:242–54. doi: 10.1111/j.1085-9489.2004.09404.x. [DOI] [PubMed] [Google Scholar]
- [58].Gabra BH, Merino VF, Bader M, Pesquero JB, Sirois P. Regul. Peptides. 2005;127:245–248. doi: 10.1016/j.regpep.2004.12.003. [DOI] [PubMed] [Google Scholar]
- [59].Christianson JA, Ryals JM, Johnson MS, Dobrowsky RT, Wright DE. Neuroscience. 2007;145:303–13. doi: 10.1016/j.neuroscience.2006.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Obrosova IG, Minchenko AG, Frank RN, Seigel GM, Zsengeller Z, Pacher P, Stevens MJ, Szabo C. Int. J. Mol. Med. 2004;14:55–64. [PubMed] [Google Scholar]
- [61].Kennedy JM, Zochodne DW. Brain. 2000;123(Pt 10):2118–2129. doi: 10.1093/brain/123.10.2118. [DOI] [PubMed] [Google Scholar]
- [62].Kennedy JM, Zochodne DW. Diabetes. 2005;54:830–837. doi: 10.2337/diabetes.54.3.830. [DOI] [PubMed] [Google Scholar]
- [63].Demiot C, Tartas M, Fromy B, Abraham P, Saumet JL, Sigaudo-Roussel D. Diabetes. 2006;55:1478–1483. doi: 10.2337/db05-1433. [DOI] [PubMed] [Google Scholar]
- [64].Goss JR, Goins WF, Lacomis D, Mata M, Glorioso JC, Fink DJ. Diabetes. 2002;51:2227–2232. doi: 10.2337/diabetes.51.7.2227. [DOI] [PubMed] [Google Scholar]
- [65].Arrieta O, Garcia-Navarrete R, Zuniga S, Ordonez G, Ortiz A, Palencia G, Morales-Espinosa D, Hernandez-Pedro N, Sotelo J. Eur. J. Clin. Invest. 2005;35:201–207. doi: 10.1111/j.1365-2362.2005.01467.x. [DOI] [PubMed] [Google Scholar]
- [66].Gabra BH, Sirois P. Eur. J. Pharmacol. 2002;457:115–24. doi: 10.1016/s0014-2999(02)02658-4. [DOI] [PubMed] [Google Scholar]
- [67].Gabra BH, Sirois P. Peptides. 2003;24:1131–9. doi: 10.1016/j.peptides.2003.06.003. [DOI] [PubMed] [Google Scholar]
- [68].Onodera K, Zushida K, Kamei J. Jpn. J. Pharmacol. 2001;85:335–7. doi: 10.1254/jjp.85.335. [DOI] [PubMed] [Google Scholar]
- [69].Kimura S, Tanabe M, Honda M, Ono H. J. Pharmacol. Sci. 2005;97:195–202. doi: 10.1254/jphs.fp0040785. [DOI] [PubMed] [Google Scholar]
- [70].Suzuki Y, Goto K, Ishige A, Komatsu Y, Kamei J. Jpn. J. Pharmacol. 1999;79:169–75. doi: 10.1254/jjp.79.169. [DOI] [PubMed] [Google Scholar]
- [71].Rashid MH, Inoue M, Bakoshi S, Ueda H. J. Pharmacol. Exp. Ther. 2003;306:709–17. doi: 10.1124/jpet.103.050948. [DOI] [PubMed] [Google Scholar]
- [72].Anjaneyulu M, Chopra K. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27:1001–1005. doi: 10.1016/S0278-5846(03)00160-X. [DOI] [PubMed] [Google Scholar]
- [73].Anjaneyulu M, Chopra K. Eur. J. Pharmacol. 2004;497:285–292. doi: 10.1016/j.ejphar.2004.06.063. [DOI] [PubMed] [Google Scholar]
- [74].Sharma S, Chopra K, Kulkarni SK. Phytother. Res. 2007 doi: 10.1002/ptr.2070. [DOI] [PubMed] [Google Scholar]
- [75].Rashid MH, Inoue M, Bakoshi S, Ueda H. J. Pharmacol. Exp. Ther. 2003;306:709–717. doi: 10.1124/jpet.103.050948. [DOI] [PubMed] [Google Scholar]
- [76].Brownlee M. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- [77].Russell JW, Golovoy D, Vincent AM, Mahendru P, Olzmann JA, Mentzer A, Feldman EL. FASEB J. 2002;16:1738–1748. doi: 10.1096/fj.01-1027com. [DOI] [PubMed] [Google Scholar]
- [78].Vincent AM, McLean LL, Backus C, Feldman EL. FASEB J. 2005;19:638–640. doi: 10.1096/fj.04-2513fje. [DOI] [PubMed] [Google Scholar]
- [79].Drel VR, Pacher P, Vareniuk I, Pavlov I, Ilnytska O, Lyzogubov VV, Tibrewala J, Groves JT, Obrosova IG. Eur. J. Pharmacol. 2007;569:48–58. doi: 10.1016/j.ejphar.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gibran NS, Jang YC, Isik FF, Greenhalgh DG, Muffley LA, Underwood RA, Usui ML, Larsen J, Smith DG, Bunnett N, Ansel JC, Olerud JE. J. Surg. Res. 2002;108:122–128. doi: 10.1006/jsre.2002.6525. [DOI] [PubMed] [Google Scholar]
- [81].Robertson DM, Sima AAF. Diabetes. 1980;29:60–67. doi: 10.2337/diab.29.1.60. [DOI] [PubMed] [Google Scholar]
- [82].Brown MR, Dyck PJ, McClearn GE, Sima AA, Powell HC, Porte D., Jr. Diabetes. 1982;31:65–70. doi: 10.2337/diab.31.1.s65. [DOI] [PubMed] [Google Scholar]
- [83].Gorio A, Aporti F, DiGregorio F, Schiavinato A, Siliprandi R, Vitadello M. Adv. Exp. Med. Biol. 1984;174:549–564. doi: 10.1007/978-1-4684-1200-0_46. [DOI] [PubMed] [Google Scholar]
- [84].Whiteley SJ, Tomlinson DR. Exp. Neurol. 1985;89:314–321. doi: 10.1016/0014-4886(85)90092-5. [DOI] [PubMed] [Google Scholar]
- [85].Walwyn WM, Matsuka Y, Arai D, Bloom DC, Lam H, Tran C, Spigelman I, Maidment NT. Exp. Neurol. 2006;198:260–270. doi: 10.1016/j.expneurol.2005.12.006. [DOI] [PubMed] [Google Scholar]
- [86].Sima AA, Robertson DM. Lab. Invest. 1979;40:627–632. [PubMed] [Google Scholar]
- [87].Carson KA, Bossen EH, Hanker JS. Neuropathol. App. Neurobiol. 1980;6:361–374. doi: 10.1111/j.1365-2990.1980.tb00672.x. [DOI] [PubMed] [Google Scholar]
- [88].Sharma AK, Thomas PK, Gabriel G, Stolinski C, Dockery P, Hollins GW. Diabetes. 1983;32:1152–1161. doi: 10.2337/diab.32.12.1152. [DOI] [PubMed] [Google Scholar]
- [89].Ilnytska O, Lyzogubov VV, Stevens MJ, Drel VR, Mashtalir N, Pacher P, Yorek MA, Obrosova IG. Diabetes. 2006;55:1686–1694. doi: 10.2337/db06-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]