Abstract
Objective
Extracellular ubiquitin (Ub) is an immune modulator that plays a role in suppression of inflammation, organ injury, myocyte apoptosis and fibrosis. The purpose of this study was to investigate the effects of extracellular Ub on the process of cardiac angiogenesis.
Methods
Cardiac microvascular endothelial cells (CMECs) and aortic tissue were isolated from rats to measure changes in angiogenic protein levels and to assess angiogenic responses to extracellular Ub.
Results
In CMECs, extracellular Ub increased protein levels of vascular endothelial growth factor-A (VEGF-A) and matrix metalloproteinase-2 (MMP-2), known angiogenesis regulators. CMECs demonstrated enhanced rearrangement of fibrillar actin and migration in response to Ub treatment. Ub-treated CMECs demonstrated an increase in tube network formation which was inhibited by the CXCR4 receptor antagonist, AMD3100. Methylated Ub, unable to form polyubiquitin chains, enhanced tube network formation. Aortic ring sprouting assays demonstrated that Ub increases microvessel sprouting in the Matrigel.
Conclusion
The results of our study suggest a novel role for extracellular Ub in cardiac angiogenesis, providing evidence that extracellular Ub, at least in part acting via the CXCR4 receptor, has the potential to facilitate the process of angiogenesis in myocardial endothelial cells.
Keywords: Ubiquitin, Heart, Angiogenesis, Cardiac Microvascular Endothelial Cells, VEGF-A
Introduction
Angiogenesis, the formation of new blood vessels from pre-existing vessels, is a tightly regulated process that must adapt to many pathological conditions including ischemic heart disease (6). The ischemic environment created as a result of coronary artery occlusion during myocardial infarction (MI) injury is deficient of oxygen and nutrients and results in apoptotic and necrotic myocardial cell death (3; 13) New vessel formation is stimulated in pathological angiogenesis to bypass this occlusion and reestablish the vascular network. A number of angiogenic factors have been identified as regulators of angiogenesis including the CXC chemokine family (21; 31). It has been reported that the C-X-C chemokine receptor type 4/chemokine (C-X-C motif) ligand 12 (CXCR4/CXCL12) axis promotes vascular endothelial growth factor (VEGF)-mediated tumor angiogenesis (19). Interestingly, CXCR4 has recently been identified as a receptor for extracellular Ub (26).
Ubiquitin (Ub), a highly conserved 8.5 kDa protein is normally found inside all eukaryotic cells and is typically associated with tagging proteins for proteosomal degradation (11). Ub is also found in trace amounts extracellularly under basal conditions and elevated levels of plasma Ub have been identified in a number of diseases (1; 2; 4; 20; 24; 33). The β-adrenergic receptor (β-AR) agonist, isoproterenol (ISO), originally described by Rona et al. (25), induces cardiac dysfunction and myocardial ischemic injury. Previously our lab has shown that β-AR stimulation increases extracellular levels of Ub, and treatment of adult rat ventricular myocytes with Ub inhibits β-AR-stimulated apoptosis (30). Recently, our lab provided evidence that exogenous Ub plays an important role in β-AR-stimulated myocardial remodeling with effects on left ventricular function, fibrosis and myocyte apoptosis (10).
In the current study, we first investigated the angiogenic potential of extracellular Ub using cardiac microvascular endothelial cells (CMECs), a major cell type involved in the processes of angiogenesis in the heart. The angiogenic potential of extracellular Ub was further characterized using aortic ring assays. The data presented here suggest a role for extracellular Ub in the processes of angiogenesis.
Materials and Methods
Vertebrate Animals
All experiments and procedures were reviewed and approved by the East Tennessee State University Institutional Committee on Animal Care and conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Male Sprague-Dawley rats (average wt: 200–225g; Harlan, Indianapolis, IN) were used for isolation of microvascular cardiac endothelial cells and aortas. Rats were anesthetized using a mixture of isoflurane (2.5%) and oxygen (0.5 l/min) and the heart was excised following a bilateral cut in the diaphragm. Animals were euthanized by exsanguinations.
Cell Isolation and culture
Adult rat CMECs were isolated as described (22; 36), with minor modifications. Briefly, hearts from adult male Sprague-Dawley rats were removed under sterile conditions and perfused with DMEM supplemented with 0.1% penicillin-streptomycin (PS). After removing atria, visible connective tissue, valvular tissue, and the right ventricle, the left ventricle was immersed in 70% ethanol for 20 sec to devitalize epicardial mesothelial and endocardial endothelial cells. After cutting away the outer one-third of the ventricular wall, the remaining tissue was washed in Hanks' balanced salt solution (HBSS). The tissue was finely minced and digested in 15 ml HBSS containing 30 mg collagenase at 37°C with gentle shaking for 20 min. After a second digestion under the same conditions with the addition of 3 mg trypsin, the solution was passed through an 80 μm nylon mesh to remove undigested tissue. The dissociated cells were pelleted at 1,050 rpm for 5 min, washed in HBSS, resuspended in DMEM supplemented with 0.2% PS and 20% heat-inactivated FBS, and plated on laminin (10 μg/ml) coated dishes or coverslips. The culture medium was replaced after 1 h to remove nonadherent cells. Using Griffonia Simplicifolia Lectin-1 (GSL-1) cytochemical staining we found that the CMECs culture was ≥95% pure.
Cell treatment
Confluent cultures of CMECs were serum starved for 24 h followed by treatment with Ub (20 μg/ml) for indicated time-points. Ub concentration used in this study was selected based on a dose response study where the 20 μg/ml concentration of Ub worked the best (data not shown). AMD+Ub cells were pretreated with AMD3100 (100 μM) for 30 min prior to Ub treatment. In a few experiments, methylated Ub (20 μg/ml) was used to examine the effect of monoubiquitination and CXCL12 (1nM) was used as a positive control for CXCR4 activation. Serum contains many angiogenic factors including ubiquitin (1; 2). DMEM supplemented with 20% Fetal bovine serum (FBS) served as a positive control in angiogenesis assays.
Western Blot Analysis
Total cell lysates were prepared in lysis buffer (10 mM Tris pH 7.5, 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 0.5% Nonidet P-40, 0.4 mM phenylmethylsulfonyl fluoride), and protein contents were measured using the Bradford assay (Bio-Rad Laboratories, Inc., Hercules, CA). Equal amounts of proteins (25 μg) were resolved by 10% SDS–PAGE and electrophoretically transferred to PVDF membranes. After blocking, the membranes were probed with VEGF-A (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), MMP-2 (Millipore, Billerica, MA), MMP-9 and actin (Millipore) antibodies. The membranes were then incubated with appropriate secondary antibodies, and immune complexes were detected using chemiluminescence and autoradiography. Band intensities were quantified using Kodak photodocumentation system (Eastman Kodak Co.).
Migration Assay
Movement of cells through a wound introduced in a cell monolayer was determined as described (12). Briefly, CMECs were grown as a confluent monolayer. After cells were made quiescent in serum free medium for 24 h, a wound was created in the center of the cell monolayer by gentle removal of attached cells using a sterile plastic pipette tip. Cell debris was removed by a PBS wash and images of the wound were acquired (0 h) using a Nikon TE-2000 microscope with a Retiga-1300 color cooled camera. The cells were then incubated in serum-free DMEM containing Ub for 24 h and 48 h. Images were again acquired using the setup as described above. The wound area was measured using Bioquant Image Analysis software (Bioquant Image Analysis Corp., Nashville, TN). The ability of cells to migrate into the wounded area was assessed by comparing micrographs at time zero, 24 h and 48 h along the wounded area. The percentage of non-recovered wound area was calculated by dividing the wound area after 24 h or 48 h by the initial wound area at time zero, multiplied by 100.
Actin Polymerization Assay
Serum starved confluent cultures of CMECs were treated with Ub for 24 h. Cells were washed twice with PBS, fixed in 3.7% formaldehyde solution in PBS for 10 min at room temperature, and permeabilized with 0.1% Triton X-100. Nonspecific binding was blocked by incubating slides for 20 min at room temperature in blocking solution (1% BSA in PBS). Cells were stained with Phalloidin-FITC (1U/slide in blocking solution; Sigma-Aldrich, St. Louis, MO) for 20 min at room temperature in the dark. After washing, the slides were mounted with SlowFade (Invitrogen Corp., Carlsbad, CA) and visualized under fluorescence microscopy using a rhodamine filter. Images were acquired using a Nikon TE-2000 microscope with a Retiga-1300 color cooled camera. The actin structure was analyzed using Bioquant Image Analysis software.
In Vitro Tubule Formation Assay
Three-dimensional cultures of CMECs were established as described (15) with minor modifications. Twenty four well plates were coated with 250 μl of Matrigel (growth factor reduced BD Matrigel Matrix, BD Biosciences, San Jose, CA) and placed in a humidified CO2 incubator at 37°C for 15 min to allow them to solidify. 6 × 104 CMECs in DMEM (250 μl) supplemented with 0.1% PS with or without Ub, methylated Ub, CXCL12, AMD+Ub or FBS were then added to each well. CMECs were allowed to form tubular structures for 6 h in culture. Images were acquired using a Nikon TE-2000 microscope with a Retiga-1300 color cooled camera. The percent area occupied by tubule formation was analyzed using Bioquant Analysis software and calculated by dividing the area covered by tubule formation by the background area after 6 h, multiplied by 100.
Aortic Ring Sprouting Assay
Aortic ring assays were performed as described (23) with minor modifications. Aortas were removed from adult male Sprague-Dawley rats (200–225 g) and immediately placed in ice cold DMEM supplemented with 0.1% PS. Aortas were cleaned of surrounding connective tissue and sliced into 1 mm thick rings under a dissecting microscope in sterile conditions. Ninety-six well plates were coated with 60 μl of Matrigel and placed at 37°C for 10 min to gel. Aortic rings were placed in wells, sealed in place with a 20 μl Matrigel overlay, and again placed at 37°C for 10 min to gel. Serum free DMEM (100 μl) containing Ub was added. Medium alone and medium supplemented with 20% heat-inactivated FBS served as controls. Aortic rings were incubated at 37°C in 5% CO2 for 11 days to allow microvessel sprouting. Medium and treatments were replaced every 48 hr. Images were acquired using a Nikon TE-2000 microscope with a Retiga-1300 color cooled camera. Sprouting was analyzed by calculating the percent of arterial ring circumference (exterior and interior) occupied by microvessel sprouts.
Statistical analysis
All data are reported as mean ± SEM. Statistical analyses were performed using Student's t-test or one-way analysis of variance (ANOVA) followed by the Student-Newman-Keuls test. Probability (P) values of <0.05 were considered to be significant.
Results
Extracellular Ub increases VEGF-A and MMP-2 protein levels in CMECs
Angiogenesis is a multi-step process that begins with the reception of angiogenic signals from its surroundings by endothelial cells, followed by the proteolytic degradation of extracellular matrix (ECM) to allow for migration and proliferation of ECs (8). Western blot analyses showed that treatment with extracellular Ub (24 h) increases VEGF-A protein levels by 2.04±0.34-fold vs. control (CTL; *P<0.05 vs. CTL; n=4; Fig. 1A). Ub treatment (24 h) also increased MMP-2 protein levels (1.87±0.39-fold vs. CTL; *P<0.05 vs. CTL; n=5; Fig. 1B), while it had no effect on MMP-9 protein levels (Fig 1C).
Figure 1.


Treatment with Ub increases expression of VEGF-A and MMP-2. CMECs were treated with Ub (20 μg/ml) for 24 h. Cell lysates (25 μg) were analyzed by western blot using VEGF-A (A), MMP-2 (B) or MMP-9 antibodies (C). The lower panels demonstrate quantitative analysis of band optical density. Values represent mean data normalized to total actin. *P<0.05 vs. control (CTL); n=4–5.
Extracellular Ub enhances migration of CMECs in vitro
To examine the functional effects of extracellular Ub on endothelial cell migration, an essential early event in angiogenesis, we used an in vitro migration (scratch) assay as previously described (12). Extracellular Ub treatment of wound gaps in established CMEC cultures accelerated cell migration (Fig. 2A). A trend towards decreased percentage of non-recovered area was observed at 24 h. However, the percent non-recovered wound area (mean data ±SEM) was significantly smaller than CTL (CTL, 99.5±4.0, Ub, 74.9±6.2*; *P<0.05 vs. CTL; n=4; Fig. 2B) at 48 h. FBS, used as a positive control, showed significantly smaller percent non-recovered area at both time points. Similar results were seen in human umbilical vein endothelial cells (HUVECs; data not shown). Methylated Ub was also tested to establish whether polyubiquitination was required for the Ub enhanced cell migration. Treatment with methylated Ub also enhanced migration of cells into the wound (data not shown).
Figure 2.
Extracellular Ub stimulates wound healing. Confluent CMEC monolayers were wounded using a sterile pipette tip and treated with Ub (20 μg/ml). DMEM supplemented with 20% FBS served as a positive control. The migration of the cells into the wound was assessed at 24 and 48 h after treatment. Panel A demonstrates the wound area at 0 h, 24 h and 48 h after treatment. Panel B represents wound healing which was assessed by calculating percent of non-recovered wound area. *P<0.05 vs. CTL; n=4.
Extracellular Ub induces rearrangement of cytoskeleton
The rearrangement of actin in endothelial cells is one of the driving forces of migration in angiogenesis (16; 17). Phalloidin-FITC staining of CMECs after 24 h treatment with extracellular Ub indicated that Ub promotes rearrangement of fibrillar actin in the cytoskeleton, a prerequisite for cell migration and tube formation. The actin structures associated with migration, including stress fibers (yellow arrows), lamellipodia (white arrow), and filopodia (red arrow) were observed in the extracellular Ub treated CMECs (Fig. 3).
Figure 3.
Extracellular Ub stimulates the polymerization of actin into structures. Semi-confluent cultures of CMECs were treated with Ub (20 μg/ml) for 24 h, stained with Phalloidin-FITC (IU), and visualized under fluorescence microscopy. Representative images from A: CTL (20X), B: Ub (20X) and C: Ub (100X) are shown. The white arrow indicates a thick cortical network of actin filaments called lamellipodia that are found at the leading edge of migrating cells. The yellow arrows indicate bundles of actin filaments called stress fibers that are required for the traction of the rear of the cells toward the leading edge during migration. The red arrow indicates filamentous membrane projections called filopodia that act as sensors of motile stimuli.
Extracellular Ub enhances In Vitro Tube Formation
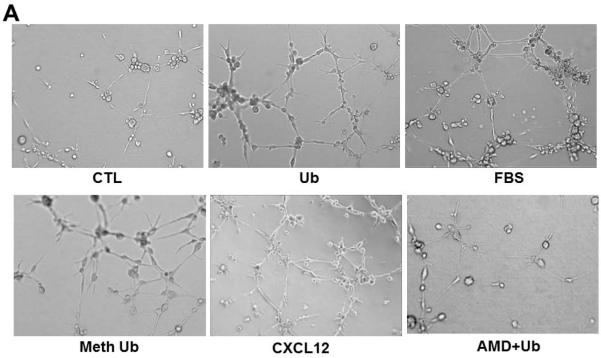
The Matrigel angiogenesis assay has proved to be a valuable tool for studying the alignment of endothelial cells into a network of tubes, which is essential for the ultimate establishment of blood flow. The formation of this network involves endothelial cell migration, alignment, sprouting and branching. Figure 4A shows an increase in the network formation at 6 h of extracellular Ub treatment (seen at 10X) compared to control. Increased network formation was also observed using methylated Ub and CXCL12 (a known ligand for CXCR4 receptor). Pretreatment with AMD3100 (CXCR4 antagonist) resulted in inhibition of the Ub-mediated increases in the network formation. FBS also enhanced the sprouting of the individual CMECs (Fig 4A). Figure 4B depicts increased sprouting of the individual CMECs (arrows) following Ub treatment compared to control at a higher magnification (20X). These sprouts convert into endothelial tubules and form connections with other tubules to establish branch points. The percent area occupied by tube formation (mean data ±SEM) was significantly higher in Ub-treated samples than CTL (CTL, 16.8±1.7, Ub, 24.1±2.8*; *P<0.05 vs. CTL; n=3). Percent area occupied by the tube formation was comparable to that with FBS treatment (Fig 4C). Similar results were observed with methylated Ub (CTL, 16.8±1.7, Meth Ub, 23.7±0.9*; *P<0.05 vs. CTL; n=3). AMD significantly blocked the increased network formation seen by Ub (Ub, 24.1±2.8, AMD+Ub, 16.6±0.9&; &P<0.05 vs. Ub; n=3).
Figure 4.
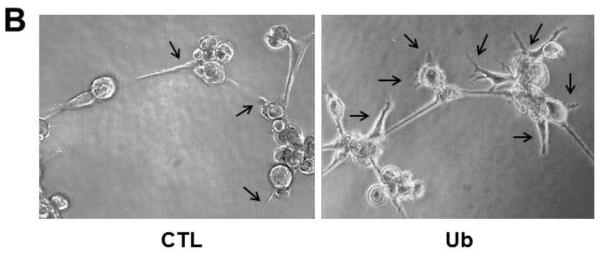
Extracellular Ub enhances three-dimensional tubule formation. CMECs were plated onto Matrigel in 24-well plates (6 × 104 cells/well) and treated with Ub (20 μg/ml), Meth Ub (20 μg/ml) or CXCL12 (1 nM). AMD+Ub cells were pretreated with AMD3100 (AMD; 100 μM) for 30 min prior to Ub treatment. DMEM supplemented with 20% FBS served as a positive control. Endothelial cell tubule formation was assessed following 6 h after treatment. Panels A and B demonstrate the tubular structures at 10X and 20X, respectively. Arrows indicate sprouting. Panel C represents quantitative analysis of tube formation assessed by calculating percent area occupied by the tubular network. *P<0.05 vs. CTL; &P<0.5 vs. Ub; n=3.
Extracellular Ub stimulates microvessel outgrowth from aortic rings
Angiogenesis involves a range of cell types. Therefore, the aortic ring assay provides a more physiologically relevant in vitro model for angiogenesis. Our analysis demonstrates that the Ub enhances microvessel sprouting from the cultured aortic rings (Fig 5A). The microvessel sprouting was significantly higher with Ub and FBS treatments (percent atrial ring circumference occupied; CTL, 0; Ub, 48.9±7.8*; FBS, 46.8±7.1*; *P<0.05 vs. CTL; n=4–5; Fig. 5B).
Figure 5.


Extracellular Ub enhances microvessel sprouting from aortic rings. Aortas were removed from adult male Sprague-Dawley rats and immediately sliced into 1 mm thick rings. Rings were embedded in Matrigel, treated with Ub (20 μg/ml) and cultured for 11 days. DMEM supplemented with 20% FBS served as a positive control. Panel A demonstrates representative images of microvessel sprouting at 4X. Panel B represents quantitative data assessed by calculating percent of arterial ring circumference occupied by sprouts. The values are mean data ±SEM. *P<0.05 vs. CTL; n=4–5.
Discussion
Cardiovascular diseases (CVD) are the leading cause of mortality globally, resulting from necrosis of the heart muscle due to blockage in the coronary arteries (35). We previously reported that extracellular Ub plays a cardioprotective role by modulating β-AR-stimulated myocardial function and inhibiting myocardial fibrosis and cardiac myocyte apoptosis (30). The mechanisms that mediate the role of extracellular Ub in suppression of inflammation and organ injury remain unresolved. The major findings of this study are – 1) extracellular Ub increases VEGF-A and MMP-2 protein levels; 2) Ub treatment enhances migration of endothelial cells into a wound area, and increases tubule formation in three dimensional culture; 3) CXCR4 antagonist, AMD3100, inhibits Ub-stimulated increases in tubule formation; and 4) extracellular Ub induces sprouting from the aortic tissue. Collectively, these studies suggest that extracellular Ub has the potential to stimulate angiogenesis in the ischemic myocardium.
Angiogenesis is a multi-step process. It starts with the stimulation of endothelial cells with angiogenic factors such as VEGF. These angiogenic factors may originate from the cells themselves or from the surrounding tissue. The activated cells secrete enzymes such as MMPs to degrade the basement membrane and the extracellular matrix. The activation and degradation of extracellular matrix help endothelial cells to migrate towards the angiogenic factors (8). Here we show that treatment of CMECs with Ub significantly increases the expression of VEGF-A and MMP-2. VEGF-A is suggested to increase the release of MMP-2 in human dermal microvascular endothelial cells (18). On the other hand, MMPs are suggested to induce the release of VEGF by ovarian cancer cells (5). Whether increased expression of VEGF-A induces MMP-2 expression or vice versa requires further investigation. Previously we have shown that infusion of Ub alone or in the presence of ISO enhanced expression of MMP-2 in the heart (10). In isolated cardiac fibroblasts, Ub alone failed to increase the expression of MMP-2. However, it enhanced ISO-mediated increases in MMP-2 protein levels (10). These studies suggest cell-type specific roles of extracellular Ub.
Migration is an important step during angiogenesis. During migration, a cell has to free itself from the extracellular matrix and the neighboring cells. MMPs promote degradation of extracellular matrix. Here we show that Ub facilitates the migration of cells within the wounded area. This increase in cell motility could be due, at least in part, to increased expression of MMP-2. Actin remodeling plays an essential role in migration of endothelial cells. Re-arrangement of actin into filopodia, lamellopodia and stress fibers plays an essential role in cell migration (17). Using phalloidin-FITC staining of actin, we observed formation of filopodia, lamellipodia and stress fibers in response to Ub treatment. While extracellular matrix degradation and actin rearrangements are essential steps in the angiogenesis, new basement membrane components must be deposited to support the newly formed blood vessel maturation. Here we provide evidence that Ub treatment increases the formation of tubular structure by CMECs in Matrigel. Similar observations were made using HUVECs (data not shown). These findings were further supported by ex vivo aortic ring assay where Ub enhanced microvessel sprouting from aortic rings.
In human myeloid cell lines, CXCR4 is identified as a receptor for extracellular ubiquitin (26). The chemokine CXCL12, also known as stromal cell-derived factor-1α, mediates its effects through the CXCR4 receptor. CXCL12/CXCR4 axis is suggested to play a role in blood vessel growth and development. Mice lacking CXCL12 or CXCR4 exhibit defective formation of the large vessels supplying the gastrointestinal tract (32). The CXCL12/CXCR4 axis is shown to promote VEGF-mediated tumor angiogenesis (19). In endothelial cells, CXCL12 alone or in combination with VEGF significantly enhanced cell survival and migration. In aortic ring assay, CXCR4 antagonist AMD3100 inhibited CXCL-12-mediated increases in vascular sprouting (7). In the heart, CXCL12/CXCR4 axis is reported to play a role in improving cardiac function predominantly by affecting myocyte survival and angiogenesis (14; 29). The Ub/CXCR4 axis is also shown to induce chemotaxis, although its efficacy is lower than that of CXCL12 (26–28; 34). The Ub/CXCR4 axis is recently suggested to play a role in acute lung infection-enhanced lung tumor metastasis (37). Cardiac myocytes and non-myocyte cell populations of the heart express CXCR4 receptors (9). Previously, we have shown that extracellular Ub plays an anti-apoptotic role in β-AR-stimulated cardiac myocyte apoptosis (30). Recently, we provided evidence that exogenous Ub plays a cardioprotective role by modulating β-AR-stimulated myocardial function, and inhibiting myocardial fibrosis and cardiac myocyte apoptosis (10). In this study, we provide evidence that extracellular Ub enhances migration of endothelial cells in the wound and stimulates the capillary network formation. CXCL12 also enhanced tubular network formation inmatrigel. The CXCR4 antagonist, AMD3100, almost completely inhibited Ub-stimulated increases in capillary network formation. Methylated Ub, in which seven lysine residues and the alpha-amino group are reductively dimethylated, showed the same effect as native Ub. Therefore, it is likely that the effects of extracellular Ub involve the CXCR4 receptor for intracellular signaling in endothelial cells of the heart.
Perspectives
The formation of new blood vessels is essential for repair and maintenance of normal cardiac function. Here we demonstrated that extracellular Ub induces an angiogenic effect in cardiac microvascular endothelial cells. Ub promotes actin rearrangement and polymerization, endothelial cell migration and tubule formation. The data presented here suggest an important role for extracellular Ub/CXCR4 axis in myocardial angiogenesis. Identification of signaling mechanisms leading to Ub-mediated regulation of processes of angiogenesis may uncover novel strategies to promote vascular remodeling and improved cardiac function.
Acknowledgement
This work is supported by a Merit Review award (number BX000640) from the Biomedical Laboratory Research and Development Service of the Veterans Affairs Office of Research and Development, National Institutes of Health (Grant numbers R21HL-091405 and R21HL-092459), and institutional Research and Improvement funds to KS.
Abbreviations
- ANOVA
analysis of variance
- β-AR
β-adrenergic receptor
- CMECs
cardiac microvascular endothelial cells
- ECs
endothelial cells
- CO2
carbon dioxide
- CTL
control
- CVD
cardiovascular diseases
- CXCL12
chemokine (C-X-C motif) ligand 12
- CXCR4
C-X-C chemokine receptor type 4
- DMEM
Dulbecco's modified eagle medium
- ECM
extracellular matrix
- EDTA
ethylenediaminetetraacetic acid
- EGTA
ethylene glycol tetraacetic acid
- FBS
fetal bovine serum
- FITC
fluorescein isothiocyanate
- HBSS
Hanks' balanced salt solution
- HCl
Hydrogen chloride
- HUVECs
human umbilical vein endothelial cells
- kDa
kiloDalton
- MI
myocardial infarction
- MMP-2
matrix metalloproteinase-2
- NaCl
sodium chloride
- PBS
phosphate-buffered saline
- PS
penicillin-streptomycin
- PVDF
polyvinylidene difluoride
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- SEM
standard error of the mean
- Ub
Ubiquitin
- VEGF-A
vascular endothelial growth factor-A
Footnotes
Disclosures No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- 1.Akarsu E, Pirim I, Capoglu I, Deniz O, Akcay G, Unuvar N. Relationship between electroneurographic changes and serum ubiquitin levels in patients with type 2 diabetes. Diabetes Care. 2001;24:100–103. doi: 10.2337/diacare.24.1.100. [DOI] [PubMed] [Google Scholar]
- 2.Akarsu E, Pirim I, Selcuk NY, Tombul HZ, Cetinkaya R. Relation between serum ubiquitin levels and KT/V in chronic hemodialysis patients. Nephron. 2001;88:280–282. doi: 10.1159/000046005. [DOI] [PubMed] [Google Scholar]
- 3.Anversa P, Cheng W, Liu Y, Leri A, Redaelli G, Kajstura J. Apoptosis and myocardial infarction. Basic Res Cardiol. 1998;93(Suppl 3):8–12. doi: 10.1007/s003950050195. [DOI] [PubMed] [Google Scholar]
- 4.Asseman C, Pancre V, Delanoye A, Capron A, Auriault C. A radioimmunoassay for the quantification of human ubiquitin in biological fluids: application to parasitic and allergic diseases. J Immunol Methods. 1994;173:93–101. doi: 10.1016/0022-1759(94)90286-0. [DOI] [PubMed] [Google Scholar]
- 5.Belotti D, Paganoni P, Manenti L, Garofalo A, Marchini S, Taraboletti G, Giavazzi R. Matrix metalloproteinases (MMP9 and MMP2) induce the release of vascular endothelial growth factor (VEGF) by ovarian carcinoma cells: implications for ascites formation. Cancer Res. 2003;63:5224–5229. [PubMed] [Google Scholar]
- 6.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 7.Carr AN, Howard BW, Yang HT, Eby-Wilkens E, Loos P, Varbanov A, Qu A, DeMuth JP, Davis MG, Proia A, Terjung RL, Peters KG. Efficacy of systemic administration of SDF-1 in a model of vascular insufficiency: support for an endothelium-dependent mechanism. Cardiovasc Res. 2006;69:925–935. doi: 10.1016/j.cardiores.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Castro MM, Tanus-Santos JE. Inhibition of matrix metalloproteinases (MMPs) as a potential strategy to ameliorate hypertension-induced cardiovascular alterations. Curr Drug Targets. 2013;14:335–343. doi: 10.2174/1389450111314030005. [DOI] [PubMed] [Google Scholar]
- 9.Dai S, Yuan F, Mu J, Li C, Chen N, Guo S, Kingery J, Prabhu SD, Bolli R, Rokosh G. Chronic AMD3100 antagonism of SDF-1alpha-CXCR4 exacerbates cardiac dysfunction and remodeling after myocardial infarction. J Mol Cell Cardiol. 2010;49:587–597. doi: 10.1016/j.yjmcc.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daniels CR, Foster CR, Yakoob S, Dalal S, Joyner WL, Singh M, Singh K. Exogenous ubiquitin modulates chronic beta-adrenergic receptor-stimulated myocardial remodeling: role in Akt activity and matrix metalloproteinase expression. Am J Physiol Heart Circ Physiol. 2012;303:H1459–H1468. doi: 10.1152/ajpheart.00401.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 12.Hochman E, Castiel A, Jacob-Hirsch J, Amariglio N, Izraeli S. Molecular pathways regulating pro-migratory effects of Hedgehog signaling. J Biol Chem. 2006;281:33860–33870. doi: 10.1074/jbc.M605905200. [DOI] [PubMed] [Google Scholar]
- 13.Kajstura J, Cheng W, Reiss K, Clark WA, Sonnenblick EH, Krajewski S, Reed JC, Olivetti G, Anversa P. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest. 1996;74:86–107. [PubMed] [Google Scholar]
- 14.Kanki S, Segers VF, Wu W, Kakkar R, Gannon J, Sys SU, Sandrasagra A, Lee RT. Stromal cell-derived factor-1 retention and cardioprotection for ischemic myocardium. Circulation: Heart Failure. 2011;4:509–518. doi: 10.1161/CIRCHEARTFAILURE.110.960302. [DOI] [PubMed] [Google Scholar]
- 15.Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988;107:1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lamalice L, Houle F, Jourdan G, Huot J. Phosphorylation of tyrosine 1214 on VEGFR2 is required for VEGF-induced activation of Cdc42 upstream of SAPK2/p38. Oncogene. 2004;23:434–445. doi: 10.1038/sj.onc.1207034. [DOI] [PubMed] [Google Scholar]
- 17.Lamalice L, Le Boeuf F, Huot J. Endothelial cell migration during angiogenesis. Circ Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 18.Lamoreaux WJ, Fitzgerald ME, Reiner A, Hasty KA, Charles ST. Vascular endothelial growth factor increases release of gelatinase A and decreases release of tissue inhibitor of metalloproteinases by microvascular endothelial cells in vitro. Microvasc Res. 1998;55:29–42. doi: 10.1006/mvre.1997.2056. [DOI] [PubMed] [Google Scholar]
- 19.Liang Z, Brooks J, Willard M, Liang K, Yoon Y, Kang S, Shim H. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem Biophys Res Commun. 2007;359:716–722. doi: 10.1016/j.bbrc.2007.05.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Majetschak M, King DR, Krehmeier U, Busby LT, Thome C, Vajkoczy S, Proctor KG. Ubiquitin immunoreactivity in cerebrospinal fluid after traumatic brain injury: clinical and experimental findings. Crit Care Med. 2005;33:1589–1594. doi: 10.1097/01.ccm.0000169883.41245.23. [DOI] [PubMed] [Google Scholar]
- 21.Moore BB, Keane MP, Addison CL, Arenberg DA, Strieter RM. CXC chemokine modulation of angiogenesis: the importance of balance between angiogenic and angiostatic members of the family. J Investig Med. 1998;46:113–120. [PubMed] [Google Scholar]
- 22.Mountain DJ, Singh M, Singh K. Downregulation of VEGF-D expression by interleukin-1beta in cardiac microvascular endothelial cells is mediated by MAPKs and PKCalpha/beta1. J Cell Physiol. 2008;215:337–343. doi: 10.1002/jcp.21315. [DOI] [PubMed] [Google Scholar]
- 23.Nicosia RF, Ottinetti A. Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab Invest. 1990;63:115–122. [PubMed] [Google Scholar]
- 24.Okada M, Miyazaki S, Hirasawa Y. Increase in plasma concentration of ubiquitin in dialysis patients: possible involvement in beta 2-microglobulin amyloidosis. Clin Chim Acta. 1993;220:135–144. doi: 10.1016/0009-8981(93)90042-3. [DOI] [PubMed] [Google Scholar]
- 25.Rona G, Chappel CI, Balazs T, Gaudry R. An infarct-like myocardial lesion and other toxic manifestations produced by isoproterenol in the rat. AMA Arch Pathol. 1959;67:443–455. [PubMed] [Google Scholar]
- 26.Saini V, Marchese A, Majetschak M. CXC chemokine receptor 4 is a cell surface receptor for extracellular ubiquitin. J Biol Chem. 2010;285:15566–15576. doi: 10.1074/jbc.M110.103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saini V, Marchese A, Tang WJ, Majetschak M. Structural determinants of ubiquitin-CXC chemokine receptor 4 interaction. J Biol Chem. 2011;286:44145–44152. doi: 10.1074/jbc.M111.298505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saini V, Staren DM, Ziarek JJ, Nashaat ZN, Campbell EM, Volkman BF, Marchese A, Majetschak M. The CXC chemokine receptor 4 ligands ubiquitin and stromal cell-derived factor-1alpha function through distinct receptor interactions. J Biol Chem. 2011;286:33466–33477. doi: 10.1074/jbc.M111.233742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saxena A, Fish JE, White MD, Yo S, Smyth JWP, Shaw RM, DiMaio JM, Srivastava D. Stromal cell-derived factor-1 alpha is cardioprotective after myocardial infarction. Circulation. 2008;117:2224–2231. doi: 10.1161/CIRCULATIONAHA.107.694992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh M, Roginskaya M, Dalal S, Menon B, Kaverina E, Boluyt MO, Singh K. Extracellular ubiquitin inhibits beta-AR-stimulated apoptosis in cardiac myocytes: role of GSK-3beta and mitochondrial pathways. Cardiovasc Res. 2010;86:20–28. doi: 10.1093/cvr/cvp402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strieter RM, Polverini PJ, Arenberg DA, Kunkel SL. The role of CXC chemokines as regulators of angiogenesis. Shock. 1995;4:155–160. doi: 10.1097/00024382-199509000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 33.Takagi M, Yamauchi M, Toda G, Takada K, Hirakawa T, Ohkawa K. Serum ubiquitin levels in patients with alcoholic liver disease. Alcohol Clin Exp Res. 1999;23:76S–80S. doi: 10.1111/j.1530-0277.1999.tb04539.x. [DOI] [PubMed] [Google Scholar]
- 34.Tripathi S, Saini V, Marchese A, Volkman BF, Tang WJ, Majetschak M. Modulation of the CXC chemokine receptor 4 agonist activity of ubiquitin through C-terminal protein modification. Biochemistry. 2013;52:4184–4192. doi: 10.1021/bi400254f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization . Global Atlas on cardiovascular disease prevention and control. WHO Press: 2011. [Google Scholar]
- 36.Xie Z, Pimental DR, Lohan S, Vasertriger A, Pligavko C, Colucci WS, Singh K. Regulation of angiotensin II-stimulated osteopontin expression in cardiac microvascular endothelial cells: role of p42/44 mitogen-activated protein kinase and reactive oxygen species. J Cell Physiol. 2001;188:132–138. doi: 10.1002/jcp.1104. [DOI] [PubMed] [Google Scholar]
- 37.Yan L, Cai Q, Xu Y. The ubiquitin-CXCR4 axis plays an impartant role in acute lung-infection-enhanced tumor metastasis. Clin Cancer Res. 2013;19:4706–4716. doi: 10.1158/1078-0432.CCR-13-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]