Abstract
Hepatic encephalopathy (HE) is a common complication of chronic alcoholism and patients show neurological symptoms ranging from mild cognitive dysfunction to coma and death. The HE brain is characterized by glial changes, including microglial activation, but the exact pathogenesis of HE is poorly understood. During a study investigating cell proliferation in the subventricular zone of chronic alcoholics, a single case with widespread proliferation throughout their adjacent grey and white matter was noted. This case also had concomitant HE raising the possibility that glial proliferation might be a pathological feature of the disease. In order to explore this possibility fixed postmortem human brain tissue from chronic alcoholics with cirrhosis and HE (n = 9), alcoholics without HE (n = 4) and controls (n = 4) were examined using immunohistochemistry and cytokine assays. In total, 4/9 HE cases had PCNA- and a second proliferative marker, Ki-67-positive cells throughout their brain and these cells co-stained with the microglial marker, Iba1. These cases were termed ‘proliferative HE’ (pHE). The microglia in pHEs displayed an activated morphology with hypertrophied cell bodies and short, thickened processes. In contrast, the microglia in white matter regions of the non-proliferative HE cases were less activated and appeared dystrophic. pHEs were also characterized by higher interleukin-6 levels and a slightly higher neuronal density . These findings suggest that microglial proliferation may form part of an early neuroprotective response in HE that ultimately fails to halt the course of the disease because underlying etiological factors such as high cerebral ammonia and systemic inflammation remain.
Keywords: Alcoholism, human brain, encephalopathy, liver disease, microglial proliferation
Introduction
Alcohol is the most commonly used and abused drug in the world with 13% of all Australian adults indulging in high-risk alcohol consumption (Australian Bureau of Statistics 2006). One of the more serious consequences of chronic alcohol consumption is alcohol-related brain damage (ARBD). ARBD is characterized by impairment of cognitive functions including working memory (Green et al. 2010). Pathologically, ARBD is characterized by mild brain atrophy that is largely due to white matter (WM) loss and dendritic dearborization (Harper and Corbett 1990). Neuronal loss is confined to the pyramidal neurons of the prefrontal cortex in alcoholics free of liver pathology or thiamine deficiency (Kril et al. 1997). ARBD is likely to have a complex etiology due to the effects of alcohol on the liver, nutrition and risk-taking behavior potentially associated with head injury along with other lifestyle choices such as cigarette smoking (Brust 2010).
Hepatic encephalopathy (HE) is a worsening of brain function arising from acute and chronic liver failure, often as a result of chronic alcohol abuse. HE manifests clinically with mild cognitive symptoms such as poor judgment, confusion and forgetfulness to more severe symptoms such as significant tremor, coma and death. Excess brain ammonia levels have long been considered the main causative factor in the disease as this neurotoxin crosses the blood brain barrier (BBB) and is taken up by the astrocytes leading to cytotoxic edema and astrocyte dysfunction (Albrecht and Norenberg 2006; Butterworth et al. 1987). Despite this long-standing hypothesis there is no consistent relationship between the clinical severity of HE and serum ammonia levels or cerebral metabolites (Dam et al. 2013).
More recently it has been proposed that systemic inflammation, or septicemia in combination with high ammonia, are responsible for HE (Hung et al. 2013; Shawcross et al. 2010).
Pathologically HE is characterized by swollen and glassy astrocytic nuclei referred to as Alzheimer type II astrocytes (At2a). The At2a are largely found in the basal ganglia, thalamus and at the cortical grey matter (GM):WM junction but will be present throughout the brain in the most severe cases (Harris et al. 2008). Previous work in our laboratory suggests that, at autopsy, approximately 20% of chronic alcoholic brains have pathological evidence of HE(Sutherland et al. 2013).
Microglia are the resident immune cells in the brain. Under normal conditions, microglia have a highly ramified morphology, motile cell processes and actively survey their environment. They are a sensor of tissue threats and their monitoring function includes the integrity of synaptic contacts (Graeber 2010). When challenged by internal or external noxious stimuli these mesodermal-derived cells become activated, undergoing morphological changes, up-regulating molecules such as the phagocytosis-promoting CD68, and produce a wide range of cytokines (Kettenmann et al. 2011). Activation of microglia in response to acute injury is considered crucial for tissue repair, however some studies suggest that excessive or prolonged microglial activation is deleterious to surrounding cells, propagating further tissue damage and cell death. Indeed, the latter scenario has been proposed to account for neuronal loss in animal models of alcohol abuse (Zhao et al. 2013).
In line with this hypothesis, microglial activation has also been demonstrated in postmortem brain tissue of cirrhotic HE patients (Zemtsova et al. 2011) with a follow up transcriptomic study showing a dysregulation of genes involved in microglial activation and inflammation (Gorg et al. 2013). Although microglial activation can result in cell division, there is currently a paucity of data demonstrating microglial hyperplasia in the human brain under physiological or pathological conditions.
In this study we use immunohistochemistry and cytokine assays to explore the role of microglial activation in HE associated with chronic alcoholism.
Methods
Tissue collection
This study was approved by the University of Sydney's Human Research Ethics Committee (HREC #13027). Fixed and frozen tissue for the study was obtained from the New South Wales Tissue Resource Centre (NSW TRC), a member of the Australian Brain Bank Network and part-funded by the National Institute on Alcohol Abuse and Alcoholism (R24AA012725) to provide brain tissue for alcoholism research. NSW TRC supplied clinicopathological information including brain weight, post-mortem interval (PMI), brain pH, length of tissue fixation period, liver pathology and macro- and microscopic neuropathology, alcohol consumption and smoking history. NSW TRC exclusion criteria includes infectious diseases such as Hepatitis B and C. Mean daily and lifetime alcohol consumption was based on next-of-kin questionnaires and available medical records. Chronic alcoholics were subsequently defined as individuals who had consumed greater then 80g of alcohol per day for the majority of their adult life (usually > 30 years), while controls had consumed less than 20g of alcohol per day. Furthermore, the NSW TRC had previously examined hematoxylin and eosin stained sections from the basal ganglia and frontal cortex of all cases for evidence of HE. An experienced neuropathologist scored the severity of HE based on the number of At2a per 200× field as none, mild (1 or more At2a per 200x field), moderate (2–3 At2a per field) or severe (mostly At2a) (Harris et al. 2008). A full description of the NSW TRC banking procedure has previously described (Sheedy et al. 2008).
Fixed tissue blocks of the periventricular region incorporating the wall of the lateral ventricle, adjacent parenchyma, corpus callosum and head of the caudate nucleus were supplied from 4 controls, 4 chronic alcoholics with no evidence of HE and 9 chronic alcoholics with both hepatic cirrhosis and HE. These tissue blocks had been stored in formalin for varying lengths of time ranging from 3-155 months. 48μm thick sections were cut using a freezing microtome as previously described(Sutherland et al. 2013). Formalin fixed paraffin embedded (FFPE) 7μm sections and 500mg of frozen tissue were obtained from the superior frontal gyrus (SFG), the precentral gyrus (PCG) and cerebellum of the same cases. Unlike the thick sections, the FFPE sections were from tissue that had been fixed for only two weeks and therefore less affected by long-term fixation that can reduce the epitope availability of some antigens such as Ki-67 (Lyck et al. 2008).
Immunohistochemistry
For immunostaining of proliferating cell nuclear antigen (PCNA) antigen retrieval was performed on free-floating (48μm) sections in 5mL centrifuge tubes containing 10mM sodium citrate buffer (pH 8.5) in a 60°C water bath overnight. Immunostaining for Iba1 required no antigen retrieval. After allowing sections to return to room temperature (RT) sections were washed three times in 50% ethanol and incubated in 50% ethanol with 3% H2O2 (v/v) for 30 minutes to quench endogenous peroxidase activity. Sections were then blocked for 30 minutes with 10% normal horse serum (NHS; Gibco, Life Technologies Australia Pty Ltd., Mulgrave, Australia) prepared in 0.1M Tris/0.015M NaCl (TBS, pH 7.4) with 0.1% Triton-X-100. Sections were incubated in the primary antibody for one hour at RT and then 4°C overnight (details of primary antibodies are provided in Table 1). Primary and secondary antibodies were diluted in 1% NHS prepared using TBS with 0.1% Triton X-100, and TBS was used for all wash steps.
Table 1.
Primary antibodies
| Primary Antibody | Company | Species raised in | Catalogue Number | Section thickness (μm) | Heat-Induced Epitope Retrieval | Antibody dilution |
|---|---|---|---|---|---|---|
| Proliferating Cell Nuclear Antigen | Santa Cruz Biotechnology, California, USA | Mouse monoclonal | sc-56 | 48 | 10mM sodium citrate buffer pH 8.5 at 60°C overnight | 1:750 |
| 7 | 10mM Tris 1mM EDTA pH 9.0 at 95°C for 30 minutes | 1:500 | ||||
| Ionized calcium binding adaptor molecule 1 | Wako Pure Chemicals, Osaka, Japan | Rabbit polyclonal | 019-19741 | 48 | None required | 1:1000 |
| 7 | 10mM Tris 1mM EDTA pH 9.0 at 95°C for 30 minutes | 1:1000 | ||||
| Ki-67 (Clone MIB-1) | Dako, Denmark | Mouse monoclonal | M7240 | 7 | 10mM Tris 1mM EDTA pH 9.0 at 110°C for 30 minutes | 1:500 |
| Beta III Tubulin | Abcam, Cambridge, USA | Rabbit polyclonal | ab18207 | 7 | 10mM Tris 1mM EDTA pH 9.0 at 110°C for 30 minutes | 1:200 |
| GFAP | Dako, Denmark | Rabbit polyclonal | Z0334 | 7 | 10mM Tris 1mM EDTA pH 9.0 at 110°C for 30 minutes | 1:500 |
| NeuN | Millipore, Billerica, USA | Rabbit polyclonal | ABN78 | 7 | 10mM Tris 1mM EDTA pH 9.0 at 110°C for 30 minutes | 1:200 |
Sections were incubated in a biotinylated secondary antibody (biotinylated anti-mouse or anti-rabbit IgG (H+L); 1:200, Vector Laboratories, Burlingame, California, USA) for one hour, then with an avidin-biotin-peroxidase complex (Vectastain Elite ABC, Universal, 1:100) for one hour. Immunostaining was visualized with 3,3'-diaminobenzidine (DAB) in the presence of 5% H2O2 for 2-10 minutes. Sections were mounted and dried, then counterstained with hematoxylin using standard protocols.
Antigen retrieval for the 7μm FFPE sections was performed in a decloaking chamber (BioCare medical DC2002, Concord, USA) for 30 minutes using 10 mM Tris, 1 mM EDTA (TE) buffer pH 9.0 (different antigen retrieval regimens are outlined in Table 1). Sections were washed in 0.05M Tris/0.15M NaCl/0.05% Tween-20 (TBST, pH 7.4) and incubated for 10 minutes in 3% H2O2 in methanol, followed by 15 minutes in 10% NHS before overnight incubation in primary antibody at 4°C (Table 1). Primary and secondary antibodies were diluted in 1% NHS prepared in TBST. The slides were incubated in the secondary antibody (1:200) for 30 minutes then with an avidin-biotin-peroxidase complex (1:100) for 30 minutes. Visualization was with DAB in 5% H2O2 for 2-10 minutes with hematoxylin counterstaining.
Quantification
PCNA- and Iba1-positive cells were counted manually in the GM and WM in thick (48μm) sections of the periventricular region. Counts were performed using an eyepiece graticule at 400x magnification in three randomly selected regions. For GM counts, four contiguous graticules were counted at ventral, middle and dorsal sites in the caudal nucleus. Similarly, four contiguous graticules were counted in two sites in the corpus callosum and a third site in the corona radiata. PCNA-positive (+) cells were counted if they had a prominently stained nucleus and were not associated with blood vessels. A clearly defined nucleus was deemed necessary for Iba1+ cells. The mean cell counts per mm2 were converted to mm3 by dividing by the thickness of the sections (approximately 0.05mm). PCNA+ and Ki-67+ cells (mm2) were counted manually at 400x magnification in the GM and WM of the 7μm SFG and PCG sections of pHE cases. Ki-67+ cells were counted for all individuals in the SFG and PCG.
Immunofluorescence
Double immunofluorescence co-localization studies were performed on FFPE sections. Antigen retrieval was performed as outlined in Table 1. Sections were then washed in 0.01M phosphate buffered saline (Bioline, Alexandria, Australia) with 0.1% Tween-20 (PBST), blocked for 20 minutes in 10% normal goat serum (NGS) and incubated overnight in a primary antibody cocktail containing two antibodies raised in different species (Table 1). Sections were then moved into an opaque slide box to protect them from UV light and incubated in a secondary antibody cocktail (1:200: AlexaFluor 488, goat anti-rabbit IgG, A11008; AlexaFluor 594, goat anti-mouse IgG, A11004) for 30 minutes. Tissue autofluorescence was quenched using 0.1% Sudan black B (B.D.H Laboratory Chemicals Group, United Kingdom) in 70% ethanol for 4 minutes, before counterstaining with 50μg/ml DAPI (Invitrogen, D306). Sections were cover slipped with Aquatex mounting media (VWR international Ltd.), sealed and viewed using confocal microscopy (Leica SPE-II, Leica Microsystems, Wetzlar, Germany) at the Advanced Microscope Facility of the Bosch Institute (University of Sydney).
Cytokine analysis
100mg of fresh frozen tissue samples from the SFG and PCG were homogenized using a motorized overhead stirrer at 500 rpm (RW digital 20, Ika® Works, Selangor, Malaysia) in 2mL of the following lysis buffer: Tris 5mM, NaCl 15mM, 1% Triton-X-100, protease inhibitors (Roche diagnostics, Castle Hill, Australia), pH 7.4. Homogenates were snap frozen in liquid nitrogen and stored at −80°C. The frozen homogenates were then thawed before being placed on ice on a shaker for 90 minutes and centrifuged at 2000g for 10 minutes at 4°C. Supernatants were collected and stored at −80°C until analysis. Total protein content in the supernatants was determined using a BioRad DC protein assay (500-0011, Bio-Rad Laboratories, Gladesville, Australia) according to the manufacturer's instructions and absorbance read with a plate reader (FLUOstar Omega; BMG Labtech, Ortenberg, Germany) at the Bosch Institute's Molecular Biology Facility (University of Sydney). Protein concentrations were determined using MARS data analysis software (v1.10, BMG Labtech).
The levels of IL-4, IL-6, IL-10 and IFN-γ in the tissue supernatants from the SFG and PCG were measured using a multiplex ELISA (Cat No. 112551HU, Quansys Biosciences, Logan, Utah 84321, USA) according to the manufacturer's instructions and imaged using a BioRad ChemiDoc MP at the Bosch Molecular Biology Facility. These images were then imported and analyzed in Q-View software (v2.16) before being normalized to total protein content.
Cresyl violet staining for neuronal counts
7μm FFPE sections of the SFG and PCG were stained with cresyl violet according to standard protocols. Neuronal counts were performed on an Olympus BX51 microscope at 200x magnification using an eyepiece graticule (0.01mm2). The total number of neurons was counted in three cortical strips from the pial surface to the GM:WM junction in each region of each case (Kril et al. 1997).
Statistical analysis
The software program JMP 8 (SAS Institute Inc, Cary, US) was used to perform all statistical analyses with a p-value < 0.05 accepted as the level of significance with no corrections made for multiple comparisons. Group differences were determined using analysis of variance or chi2 analysis. Potential confounders were explored using linear regression with a stepwise model.
Results
Our laboratory has an interest in cell proliferation in ARBD. During a study investigating proliferation in the SVZ of chronic alcoholics, a case with PCNA-positive (+) cells throughout the GM and WM adjacent to the SVZ was discovered. As this case had a pathological diagnosis of mild HE, the possibility that widespread cell proliferation is a feature of HE was investigated.
Quantification of proliferating cells in HE alcoholics
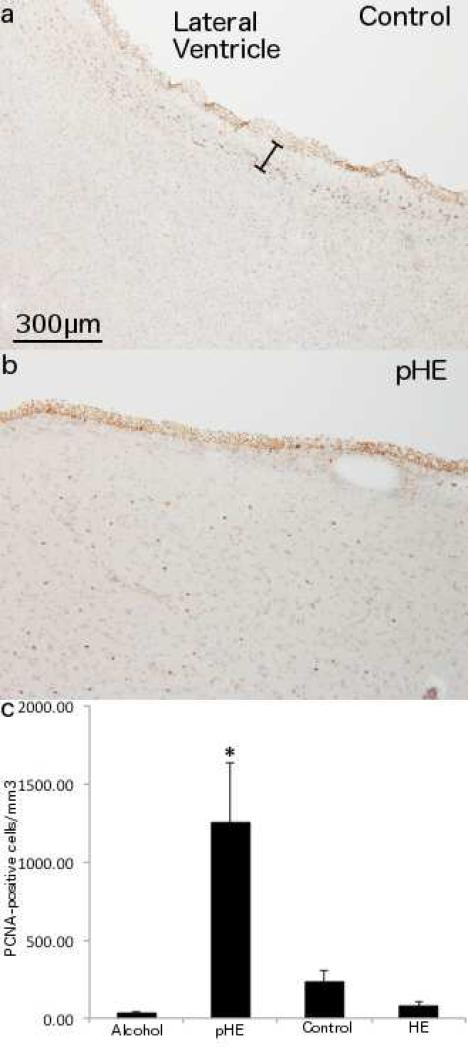
PCNA-immunopositive cells were quantified in 48μm fixed sections of the periventricular region from controls (n = 4) (Fig. 1a), chronic alcoholics (n = 4) and chronic alcoholics with both cirrhosis and a pathological diagnosis of HE (n = 9). Four HE cases had greater than 400 PCNA+ cells/mm3 (mean = 1248.8 ± S.D. = 761.3 cells/mm3) (Fig. 1b), while PCNA+ cells were low in the remaining 5 HE cases (77.3 ± 72.1 cells/mm3) (Fig 1c). There was also a low number of immunopositive cells present in all controls (mean = 233.3 ± 151.4 cells/mm3) and alcoholic cases (30.0 ± 22.8 cells/mm3) (Fig 1c). The cases with prominent PCNA-positivity were referred to as proliferative HE cases (pHEs). These pHEs had PCNA+ cells distributed throughout their GM and WM (Fig. 1b). These cells had predominantly small round nuclei although there were also some with more irregularly shaped nuclei and occasional cells with both nuclear and cytoplasmic PCNA staining.
Fig. 1. Proliferation in HE alcoholics with cirrhosis.
PCNA immunostaining of 48μm sections of the rostral caudate nucleus shows (a) a typical staining pattern in a neurologically normal individual with immunopositive cells in the ependymal layer and subventricular zone (SVZ) but not the underlying parenchyma. The approximate width of the SVZ is indicated (black spacer). (b) a pHE case with PCNA+ cells throughout the caudate nucleus underlying the SVZ. (c) a significantly higher number of PCNA+ cells in pHEs compared to all other groups. 200x magnification.
All four pHEs were chronic alcoholics with liver cirrhosis and varying degrees of HE (one mild, two moderate and one severe). This combination of HE and cirrhosis was not unique to the pHEs but the other five alcoholics with cirrhosis and varying degrees of HE had PCNA staining patterns similar to the controls. Other clinical and pathological data including mean daily alcohol consumption and co-morbidities were compared between the groups. Two HE-alcoholics had sepsis and one, a pHE case, displayed chronic hepatic inflammation with liver necrosis. An additional pHE case had a history of seizures but overall there appeared to be no common clinical or pathological thread to link these four individuals (Table 2).
Table 2.
Clinical and demographic comparisons
| Parameter [±S.D.] | pHEs [n = 4] | Cirrhotic HE [n = 5] | Alcoholics [n = 4] | Controls [n = 4] |
|---|---|---|---|---|
| Mean age (years) | 55 [7.5] | 57 [11.6] | 53 [7.5] | 59 [11.6] |
| Gender (M/F) | 3/1 | 2/3 | 3/1 | 3/1 |
| Mean PMI | 22 [5.6] | 34 [19.9] | 24 [6.6] | 20 [9.5] |
| Mean brain weight (g) | 1287 [86] | 1269 [124] | 1392 [52] | 1372 [135] |
| Mean brain pH | 6.36 [0.20] | 6.44 [0.37] | 6.70 [0.23] | 6.61 [0.23] |
| Mean Fixation period (months) | 84.5 [53.8]* | 56.8 [20.9] | 15.7 [9.5]* | 17.8 [22.5]* |
| Severity of liver pathology (0,1,2,3)B | 0,0,0,4 | 0,0,0,5 | 1,1,2,0 | 1,2,0,0A |
| Mean daily alcohol consumption (g/day)C,D | 211 [114.8] | 206 [87.4] | 183 [41.0] | 5 [6.2]* |
| Smoking (Ever/Never)C | 3/1 | 3/2 | 3/1 | 1/2A |
| Smoking (Mean pack years)C | 25.3 [22.1]A | 26.7 [25.1] | 27.5 [22.2] | 1.9 [2.8]A |
significant difference, p-value < 0.05
Data missing
liver pathology: 0 = nil or congestion, 1 = mild steatosis, 2 = severe steatosis/mild fibrosis; 3 = cirrhosis or severe inflammation
estimated from medical history and next of kin questionnaires.
calculated by mean daily consumption × 365 × years drinking [excluding periods of abstinence].
PCNA has other cellular functions apart from its role in proliferation such as DNA repair (Stoimenov and Helleday 2009). Prominent PCNA nuclear staining is also seen in the supposedly post-mitotic ependymal layer of the adult brain (Curtis et al. 2005; Sutherland et al. 2013; van den Berge et al. 2011). Therefore to confirm that the PCNA+ cells had indeed undergone proliferation 7μm FFPE periventricular sections all pHEs were stained with Ki-67 as an additional proliferative marker. Furthermore, to determine the extent of proliferation three other regions that have been intensively studied in ARBD, the prefrontal cortex (SFG), primary motor cortex (PCG), and cerebellum were stained with Ki-67. Neuronal loss has been previously demonstrated in the SFG but not the PCG (Kril et al. 1997). Similarly, the cerebellum, in the absence of thiamine deficiency, does not show neuronal loss in ARBD.
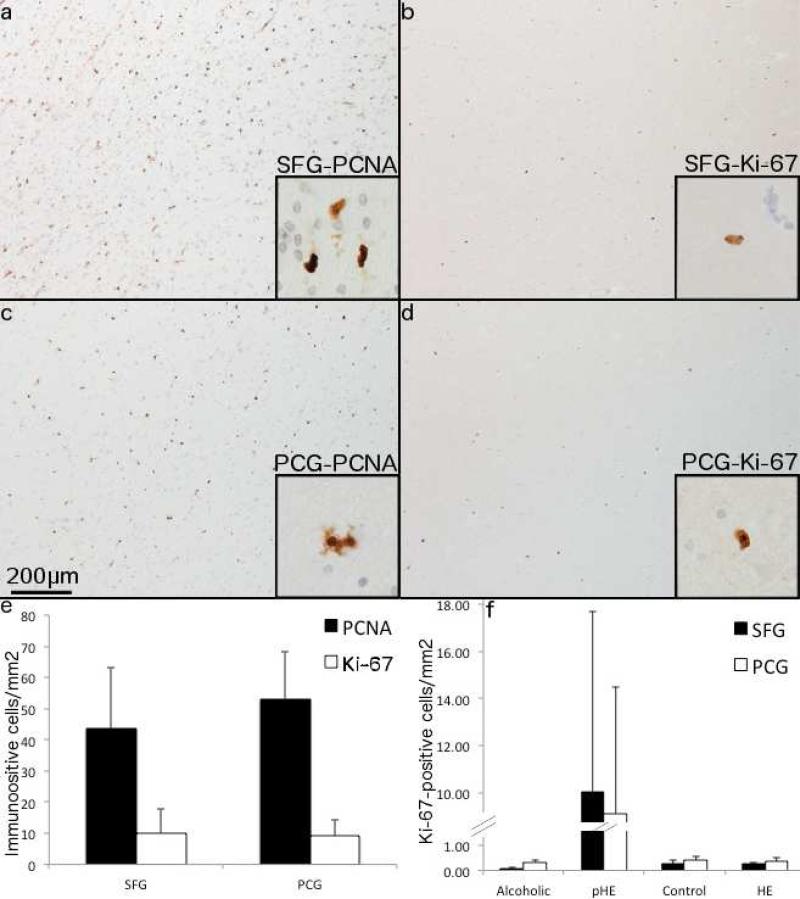
Both PCNA+ and Ki-67+ cells were found in the SFG and PCG of all pHEs (Fig. 2a-e). There was a similar number of PCNA+ cells present in the SFG (43.6 ± 39.3 cells/mm2) and PCG (53.2 ± 30.1 cells/mm2), but considerably fewer Ki-67+ cells in both the SFG (10.1 ± 15.2 cells/mm2) and PCG (9.1 ± 10.6 cells/mm2) (Fig. 2e). There was no difference in the number of immunopositive cells between the SFG and PCG for either PCNA (p = 0.62) or Ki-67 (p = 0.96) (Fig. 2e). Similarly, Ki-67+ and PCNA+ cells were found throughout the cerebellum of all pHEs however these were not quantified. In contrast Ki-67+ cells were rare in both the SFG and PCG of the non-proliferative HE cases, non-HE alcoholics and controls (Fig. 2f).
Fig. 2. PCNA labels a greater number of cells in pHEs compared to Ki-67.
7μm serial sections of the SFG (a and b) and PCG (c and d) of a pHE case show PCNA+ cells (a and c) and Ki-67+ cells (b and d). 200× magnification and 600× for insets. (e) Quantification of Ki-67+ and PCNA+ cells in pHE cases shows a greater number of PCNA+ cells compared to Ki-67 in both the SFG and PCG. (f) pHEs have substantially more Ki-67+ cells in both regions compared to the other three groups.
Co-localization studies
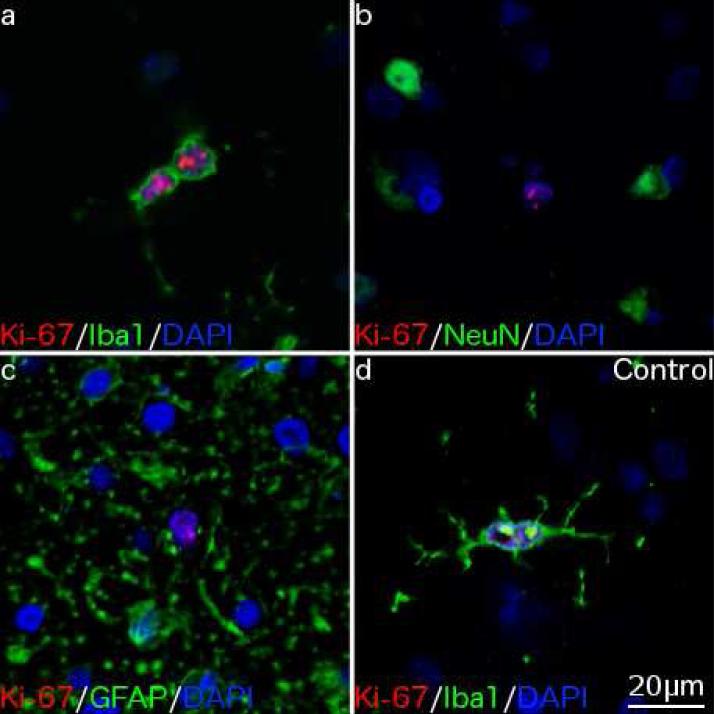
Double labeling immunofluorescence staining was used to determine the phenotype of Ki-67+ cells. In 3/4 pHEs, all Ki-67+ cells co-localized with the microglial marker Iba1 (Fig. 3a), but not the neuronal marker NeuN (Fig. 3b), the astrocytic marker GFAP (Fig. 3c) or the microtubule associated neuronal marker beta III tubulin (data not shown). One pHE had Ki-67+/Iba1− cells in addition to Ki-67+/Iba1+ cells, but to date the identity of these additional proliferative cells remains unknown. In all other alcoholics and controls examined the rare Ki-67+ cells seen all co-localized with Iba1 (Fig. 3d). This suggests that a low level of microgliogenesis occurs in the adult human brain.
Fig. 3. Phenotyping proliferative cells.
Immunofluorescence double labeling shows (a) Ki-67+ cells in a pHE case co-localized with the microglial marker Iba1 but not (b) the neuronal marker NeuN or (c) the astrocytic marker GFAP. (d) A rare Ki-67+ cell in a neurologically normal individual co-localizes with Iba1. 400× magnification.
Microglial morphology in HE
Thick sections of human postmortem brain tissue are particularly well suited to visualizing microglial morphology (Streit et al. 2009). Microglia in the periventricular region of controls had a typical ramified appearance, with a small cell body and long, fine processes.
There were distinct morphological differences between GM and WM microglia with the former having a round cell body and fine branching processes extending radially in all directions (Fig. 4a) whereas the latter were orientated along the WM tracts (Fig. 4b).
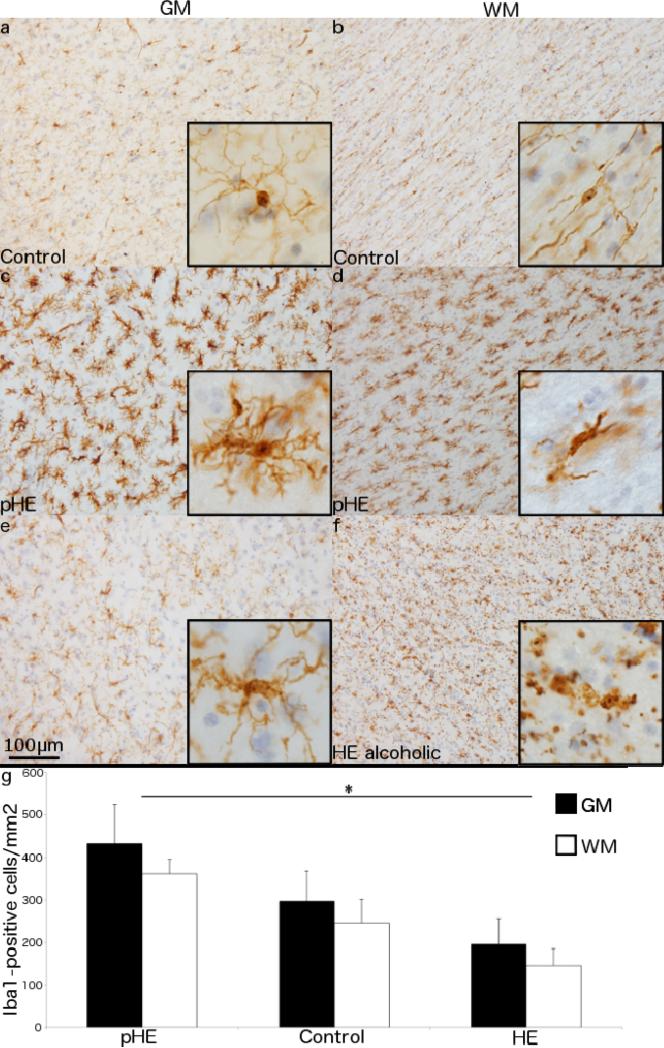
Fig. 4. Morphological differences in Iba1+ cells in HE.
Iba1+ cells in 48μm sections of a neurologically normal individual have a small cell body with long, fine processes (a) extending radially in the GM and (b) tangentially along the WM tracts. A pHE case displays microglial hypertrophy and hyperplasia in both their (c) GM and (d) WM. A non-proliferative HE case with (e) a subtle increase in the size of the cell body in the GM but (f) extensive, widespread fragmentation of the microglial cells in the WM. (g) A comparison of Iba1+ cells shows a significant increase in both the GM (p = 0.04) and WM (p < 0.001) in pHEs compared to HE cases. Data represent the mean ± SEM of Iba1+ cells/mm2. 200× magnification with 600× insets.
The microglia in pHEs had increased Iba1 staining intensity and a distinct morphology with increased cell body size and short, thickened processes in both the GM (Fig. 4c) and WM (Fig. 4d). In the remaining (non-proliferative) HE cases microglia in the GM displayed only mild hypertrophy (Fig. 4e), while those in the WM were highly fragmented and appeared dystrophic (Streit et al. 2004)(Fig. 4f). Only one non-HE alcoholic case was examined, and their microglial morphology was similar to the controls.
To ascertain if microglial proliferation was influencing total microglial numbers the number of Iba1+ cells in the GM and WM was quantified. In the GM, there was no difference in the number of microglia in typical HE cases (3909.3 ± 2718.3 cells/mm3; p = 0.35) or pHEs (8640.0 ± 3676.6; p = 0.24) compared with controls (5933.3 ± 2850.1). However, pHEs did have significantly more microglia than the typical HE cases (p = 0.04). The same trend was present in the WM with significantly more microglia in pHEs (7220.0 ± 1350.4 cells/mm3) compared to HE cases (2916.9 ± 1753.3; p < 0.01) although neither group was different from the controls (4913.3 ± 2242.7) (Fig. 4g).
Lastly, despite the pHEs having a significantly longer mean fixation time, regression analysis suggested that this had no effect on the above results.
Microglial and neuronal relationships
Given the relative loss of neurons in the SFG of chronic alcoholics (Kril et al. 1997) we explored how microglial proliferation might impact on neuronal viability in the SFG and PCG of HE alcoholics.
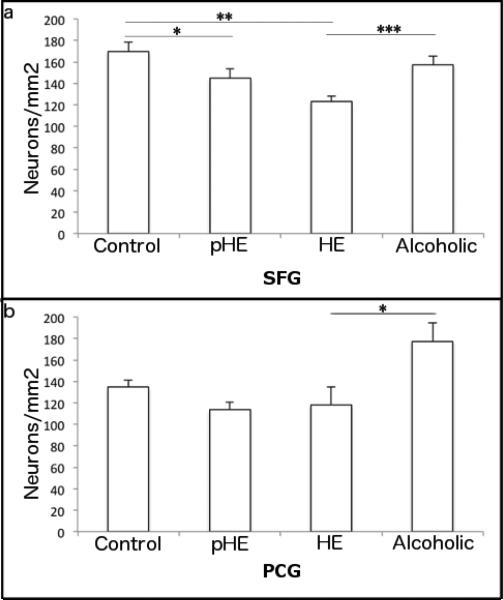
Neuronal counts of cresyl violet stained sections of the PCG and SFG were performed to determine if microglial proliferation or dystrophy were associated with neuronal loss. In the SFG there was no difference in mean neuronal density between the alcoholics (157.3 ± 16.5 neurons/mm2) and controls (169.3 ± 17.6 neurons/mm2; p = 0.30), however both pHEs (144.5 ± 17.8 neurons/mm2; p = 0.05) and typical HE cases (122.9 ± 11.8 neurons/mm2; p < 0.001) had significantly lower neuronal densities compared to controls. The difference between the two HE groups did not reach significance (p = 0.06) (Fig. 5a). These effects were not related to atrophy as the mean area counted in each SFG cortical strip was similar across all groups (1.23-1.41mm2) and normalizing for cortical thickness in the SFG did not alter the results.
Fig. 5. Reduction in neuronal density in the SFG of HE cases.
In comparison to controls there was a reduction of neuronal density in (a) the SFG of HE cases but not alcoholics (b) There was no difference in the neuronal density of HE cases compared to controls in the PCG. Data are presented as mean neuronal density/mm2 ± SEM.
In the PCG there was no difference in the neuronal density of controls, HE, or pHEs (134.7 ± 13.3, 118.0 ± 37.2 and 113.8 neurons/mm2, respectively). However, neuronal density was higher in alcoholics (144.5 ± 17.8 neurons/mm2) compared with pHE (p = 0.01) and HE cases (p = 0.02) (Fig. 5b). Unlike the SFG, there was cortical thinning present in the alcoholic group with a mean area of 1.13mm2 per cortical strip compared to 1.38mm2 in controls, 1.40mm2 in pHEs and 1.27mm2 in HE cases. When the counts were normalized for cortical thickness the difference in the alcoholic group was not retained (p = 0.2 and 0.08 for pHE and HE cases, respectively).
Cytokine levels in the cortex of HE patients
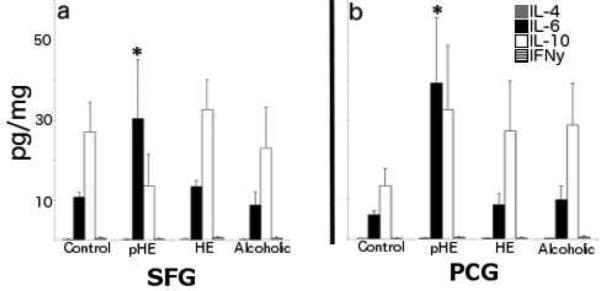
The levels of four different pro-inflammatory cytokines; IL-4, IL-6, IL-10 and IFN-λ, were quantified in frozen SFG and PCG and found to be at very low levels in all cases. Furthermore, there was no difference in the levels of IL-4, IL-10 or IFN-λ across all groups. However, there was a significant increase in the level of IL-6 in both the SFG (mean = 30.4 ± 29.7pg/mg) and PCG (mean = 39.3 ± 33.1pg/mg) of pHEs compared to all other groups (Fig. 6).
Fig. 6. IL-6 levels are elevated in the cortex of pHEs.
IL-6 levels were higher in pHEs in both (a) the SFG and (b) the PCG.
Discussion
Proliferative cells in the adult human brain are known to be rare outside of the two neurogenic niches: the SVZ and the subgranular zone of the hippocampus (SGZ) (Curtis et al. 2007; Low et al. 2011). Non-neuronal cell proliferation in the adult human neocortex has been described (Bhardwaj et al. 2006) but there is very little quantitative data on the normal turnover of glial cells. Here we provide evidence of both a low level of constitutive microglial proliferation in the aged human brain and widespread proliferation in a subset of patients with HE.
The concept of proliferation in the adult human brain has recently emerged as an area of great interest in neuroscience. Much of this research focuses on the regenerative capacity of neurons in the SVZ and SGZ as this forms the biological basis for both therapeutic manipulation of endogenous neurogenesis and the likely success of stem cell or neuronal transplantation. The proliferative capacity of the glial cells in the human brain has not been as widely studied despite primary glial diseases such as HE and multiple sclerosis potentially benefitting from restorative therapies. In 1989, Brumback and Lapham demonstrated very rare astrocytic proliferation in the human brain, however there have been no further studies confirming their findings (Brumback and Lapham 1989). It has been suggested that oligodendrocytes may be replaced in pathological conditions such as epilepsy by their precursor, oligodendrocyte progenitor cells, although the quantity of these cells and even their existence in the normal human brain remains an area of ongoing debate (Chang et al. 2000; Geha et al. 2010; Rhee et al. 2009). Microglial proliferation has been demonstrated in young human brains but this was in pathological conditions such as epilepsy and cortical dysplasia (Wirenfeldt et al. 2009;Munakata et al. 2013). However in one study, Ki-67 events were also rarely detected in control tissues up to ten years of age (Munakata et al. 2013).. To the best of our knowledge microglial proliferation has not been previously shown in the adult human brain.
There are technical issues with all cell proliferation studies in human postmortem tissue including epitope availability and the sensitivity and specificity of endogenous proliferative markers. Along with other researchers, we have previously used PCNA because of its consistent staining in fixed brain tissue (Curtis et al. 2005; Sutherland et al. 2013; van den Berge et al. 2011). The current findings agree with our previous report that PCNA labels a greater number of cells in the adult human brain compared with Ki-67(Sutherland et al. 2013). PCNA is a nuclear marker that acts as a DNA clamp in the G1/S phases of the cell cycle; however, it is now known to have additional roles including DNA repair (Stoimenov and Helleday 2009). Surprisingly, PCNA occasionally labels cytoplasmic structures in addition to nuclear labeling. In contrast, Ki-67 staining was restricted exclusively to the nucleus. Although the longer half-life of PCNA (estimated to be 20 hours (Bravo and Macdonald-Bravo 1987)) compared with Ki-67 (one hour (Bruno and Darzynkiewicz 1992)) could account for the greater number of PCNA+ cells, the cytoplasmic staining of PCNA as well as its propensity to label the post-mitotic ependymal layer (Ihrie and Alvarez-Buylla 2011), raises serious questions over its specificity. In contrast, in tissue that has not undergone long fixation Ki-67 is both a sensitive and specific maker of proliferation.
Whilst microglial activation has been previously demonstrated in HE (Zemtsova et al. 2011), no studies have specifically addressed proliferation. Interestingly, in this study only a subset of HE cases had microglial proliferation. When all available clinical, demographic and pathological records were examined, no discerning features that separated the pHEs and those without proliferation were found. Variables included alcohol intake, age, severity of HE, toxicology, current medications and concomitant medical conditions. Limited clinical information and inter-person variability are inherent issues in postmortem human brain studies and it is possible that the proliferative response in pHEs is due to an undiagnosed co-morbidity or acute hypoxic event prior to death that is unrelated to HE. However, our laboratory has now examined proliferative markers in a total of 45 controls and chronic alcoholics with and without HE and have only seen this widespread proliferative response outside the SVZ in the four cases presented(Sutherland et al. 2013) (unpublished data). This indicates that HE is likely to be a major contributing factor to the proliferation seen in pHEs.
Interestingly, a recent genome-wide expression study in cirrhotic HE patients including those supplied from NSW TRC found upregulation of genes associated with the cell cycle providing further evidence for proliferation as an element of HE rather than being coincidental (Gorg et al. 2013).
In pathological conditions ‘reactive gliosis’ is often presumed to involve proliferation. However a recent systematic, stereological-based study in Alzheimer's disease demonstrated astrocytic and microglial activation without any change in cell numbers and minimal Ki-67 staining (Serrano-Pozo et al. 2013). This supports the premise that activation does not equate to proliferation and that microglial activation and proliferation should be seen as separate entities. Nevertheless, a second novel finding in this study is that a low level of microgliogenesis does occur in the normal aged human brain. Ki-67+ cells, although admittedly rare, were seen in all controls and non-pHE alcoholics and all co-localized with Iba1. A caveat here is that we only examined two controls in co-localization studies but low-level microgliogenesis is consistent with a previous study from our laboratory where Ki-67/Iba1 co-positive cells were demonstrated in the adult human olfactory bulb of a neurologically normal control (Sutherland et al. 2013).
Microglia can both produce and be stimulated by a host of cytokines. Here the pHEs all showed an increase in cerebral levels of the cytokine IL-6. Plasma levels of IL-6 are known to be elevated in HE patients (Shawcross et al. 2007), however it is unclear whether circulating IL-6 can enter the brain in HE as the BBB remains largely intact (Rangroo Thrane et al. 2012). Microglial proliferation can be stimulated in vitro by a number of cytokines such as M-CSF (Smith et al. 2013), MCP-1 (Hinojosa et al. 2011), GM-CSF and IL-3, however the addition of IL-6 to microglial cultures did not stimulate proliferation (Kloss et al. 1997). It seems more likely therefore that IL-6 up-regulation in pHEs is a by-product of microglial activation rather than stimulating proliferation. An important caveat here is that our exploration of proinflammatory cytokines was limited, excluding for example macrophage colony-stimulating factor (M-CSF) that was recently shown to stimulate proliferation in ex vivo human microglial cultures (Smith et al. 2013).
Along with cytokine production, there are morphological changes associated with the activation state of microglia. Here, there were significant morphological differences in the microglia from HE cases with and without proliferation. Traditionally, microglial activation has been defined by the expression of inflammatory molecules such as MHC class II or CD68, however it is important to make a distinction between inflammation and microglial activation. Using Iba1 stained thick sections allows visualization of the whole microglial cell in intricate detail to discriminate between ramified, activated and dystrophic microglia. The visible withdrawal and thickening of the processes in pHEs shown here is consistent with the morphological changes associated with microglial activation described by Yamada and Jinno in rodents (Yamada and Jinno 2013). Microglia are exceptionally dynamic, plastic cells, with a wide range of physiological functions beyond their phagocytic role such as monitoring the state of synapses (Wake et al. 2009). Determining the functional implications of these different microglial phenotypes in the normal and diseased brain however, requires state-specific markers and this remains an area of ongoing research.
The other novel finding in this study was microglial dystrophy in the WM of HE cases without proliferation. Microglial dystrophy is a recent concept, being first described in humans in 2004 (Streit et al. 2004). Streit and colleagues considered that microglial dystrophy rather than activation was associated with neuronal loss in Alzheimer's disease (Streit et al. 2009). More generally in neurodegenerative diseases, Graeber and Streit suggest that signals from damaged neurons activate microglia who then attempt to protect and recover the damaged cell. If this activation is insufficient to repair the damage, the neuron will continue to release activation signals resulting in chronic microglial activation, microglial fatigue and degeneration (Graeber and Streit 2010). Once these important cells have been lost, neurodegeneration follows.
The diagnosis of HE here was made by pathological examination so it was difficult to determine whether there had been any direct functional consequences associated with microglial proliferation, activation or dystrophy. As a potential correlate of neurocognitive status we used neuronal counts from the SFG, an area known to be susceptible to the toxic effects of alcohol as well as the PCG, an area whose neurons are preserved in chronic alcoholics (Kril and Harper 1989). SFG neuronal density was decreased in the SFG of all HE cases compared with controls, with a trend towards fewer neurons in the non-proliferative HE cases. We consider that the current findings are consistent with this neuroprotective hypothesis of microglia activation (Graeber and Streit 2010). Neuronal distress in HE may trigger an acute microglial response involving both activation and proliferation. If the precipitating factors of HE such as high ammonia levels and systemic inflammation are not resolved then the microglia stop proliferating and become dystrophic. The microglial dystrophy seen in the WM of the non-proliferative cases suggests that HE was more advanced in these cases.
There are a number of caveats to this proposed mechanism; firstly there was no relationship between the pathological severity of HE and neuronal density, Ki-67+ cells or microglial activation. It is possible that a pathological diagnosis of HE does not reflect the clinical severity as the formation of At2a is directly correlated with ammonia levels (Norenberg et al. 1991), but serum ammonia levels are not related to the clinical severity of HE (Dam et al. 2013; Shawcross et al. 2011). The clinical diagnosis of HE requires careful psychometric testing and to date no studies, in humans or animals, have addressed whether disease severity is subsequently related to the extent of At2a in postmortem brain. In reality, despite the circumstantial evidence suggesting that variation in pathology is related to disease (HE) duration, we ultimately don't have the clinical data to confirm this hypothesis.
Overall, this work has demonstrated the novel finding of widespread microglial proliferation in postmortem brain tissue. The four cases involved all had a pathological diagnosis of HE but this was neither sufficient nor necessary for microgliogenesis. Current work continues to explore the impact of microglial proliferation in the overall context of ARBD and whether these “electricians” of the human brain are a potential therapeutic target for neurodegenerative disorders.
Acknowledgements
The authors would like to thank the donors and their families for their kind gift that has allowed this research to be undertaken and the New South Wales Tissue Resource Centre (NSW TRC) for providing tissue samples. We would like to acknowledge Dr. Louise Cole (Core Facilities Manager, Bosch Institute Advanced Microscopy Facility, The University of Sydney) for her support and assistance with the confocal microscopy and Dr. Donna Lai for her assistance with performing cytokine ELISAs. The NSW TRC is part of the NSW Brain Bank Network and Australian Brain Bank Network and is supported by the University of Sydney, National Health and Medical Research Council (NHMRC), Schizophrenia Research Institute and the National Institutes of Alcoholism and Alcohol Abuse (NIAAA). This work was supported by the NIAAA (R24 AA012725) and the NHMRC (grant #605210).
References
- Albrecht J, Norenberg MD. Glutamine: a Trojan horse in ammonia neurotoxicity. Hepatology. 2006;44(4):788–794. doi: 10.1002/hep.21357. doi:10.1002/hep.21357. [DOI] [PubMed] [Google Scholar]
- Australian Bureau of Statistics Alcohol Consumption in Australia: A Snapshot, 2004-05. [14th April 2012];Australian Bureau of Statistics. 2006 http://www.abs.gov.au/AUSSTATS/abs@.nsf/Previousproducts/4832.0.55.001Main Features99992004-05?opendocument&tabname=Summary&prodno=4832.0.55.001&issue=2004-05&num=&view=.
- Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Bjork-Eriksson T, Nordborg C, Gage FH, Druid H, Eriksson PS, Frisen J. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci U S A. 2006;103(33):12564–12568. doi: 10.1073/pnas.0605177103. doi:0605177103 [pii] 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R, Macdonald-Bravo H. Existence of two populations of cyclin/proliferating cell nuclear antigen during the cell cycle: association with DNA replication sites. J Cell Biol. 1987;105(4):1549–1554. doi: 10.1083/jcb.105.4.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumback RA, Lapham LW. DNA synthesis in Alzheimer type II astrocytosis. The question of astrocytic proliferation and mitosis in experimentally induced hepatic encephalopathy. Archives of neurology. 1989;46(8):845–848. doi: 10.1001/archneur.1989.00520440027016. [DOI] [PubMed] [Google Scholar]
- Bruno S, Darzynkiewicz Z. Cell cycle dependent expression and stability of the nuclear protein detected by Ki-67 antibody in HL-60 cells. Cell proliferation. 1992;25(1):31–40. doi: 10.1111/j.1365-2184.1992.tb01435.x. [DOI] [PubMed] [Google Scholar]
- Brust JC. Ethanol and cognition: indirect effects, neurotoxicity and neuroprotection: a review. International journal of environmental research and public health. 2010;7(4):1540–1557. doi: 10.3390/ijerph7041540. doi:10.3390/ijerph7041540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth RF, Giguere JF, Michaud J, Lavoie J, Layrargues GP. Ammonia: key factor in the pathogenesis of hepatic encephalopathy. Neurochemical pathology. 1987;6(1-2):1–12. doi: 10.1007/BF02833598. [DOI] [PubMed] [Google Scholar]
- Chang A, Nishiyama A, Peterson J, Prineas J, Trapp BD. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20(17):6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, Holtas S, van Roon- Mom WM, Bjork-Eriksson T, Nordborg C, Frisen J, Dragunow M, Faull RL, Eriksson PS. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315(5816):1243–1249. doi: 10.1126/science.1136281. doi:1136281 [pii] 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Penney EB, Pearson J, Dragunow M, Connor B, Faull RL. The distribution of progenitor cells in the subependymal layer of the lateral ventricle in the normal and Huntington's disease human brain. Neuroscience. 2005;132(3):777–788. doi: 10.1016/j.neuroscience.2004.12.051. doi:S0306-4522(05)00026-6 [pii] 10.1016/j.neuroscience.2004.12.051. [DOI] [PubMed] [Google Scholar]
- Dam G, Keiding S, Munk OL, Ott P, Vilstrup H, Bak LK, Waagepetersen HS, Schousboe A, Sorensen M. Hepatic encephalopathy is associated with decreased cerebral oxygen metabolism and blood flow, not increased ammonia uptake. Hepatology. 2013;57(1):258–265. doi: 10.1002/hep.25995. doi:10.1002/hep.25995. [DOI] [PubMed] [Google Scholar]
- Geha S, Pallud J, Junier MP, Devaux B, Leonard N, Chassoux F, Chneiweiss H, Daumas-Duport C, Varlet P. NG2+/Olig2+ cells are the major cycle-related cell population of the adult human normal brain. Brain Pathol. 2010;20(2):399–411. doi: 10.1111/j.1750-3639.2009.00295.x. doi:10.1111/j.1750-3639.2009.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorg B, Bidmon HJ, Haussinger D. Gene expression profiling in the cerebral cortex of patients with cirrhosis with and without hepatic encephalopathy. Hepatology. 2013;57(6):2436–2447. doi: 10.1002/hep.26265. doi:10.1002/hep.26265. [DOI] [PubMed] [Google Scholar]
- Graeber MB. Changing face of microglia. Science. 2010;330(6005):783–788. doi: 10.1126/science.1190929. doi:330/6005/783 [pii] 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 2010;119(1):89–105. doi: 10.1007/s00401-009-0622-0. doi:10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- Green A, Garrick T, Sheedy D, Blake H, Shores EA, Harper C. The effect of moderate to heavy alcohol consumption on neuropsychological performance as measured by the repeatable battery for the assessment of neuropsychological status. Alcohol Clin Exp Res. 2010;34(3):443–450. doi: 10.1111/j.1530-0277.2009.01108.x. doi:ACER1108 [pii] 10.1111/j.1530-0277.2009.01108.x. [DOI] [PubMed] [Google Scholar]
- Harper C, Corbett D. Changes in the basal dendrites of cortical pyramidal cells from alcoholic patients--a quantitative Golgi study. J Neurol Neurosurg Psychiatry. 1990;53(10):856–861. doi: 10.1136/jnnp.53.10.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris J, Chimelli L, Kril J, Ray D. Greenfield's Neuropathology. Hodder Arnold; United Kingdom: 2008. Nutritional deficiencies, metabolic disorders and toxins affecting the nervous system. pp. 675–731. [Google Scholar]
- Hinojosa AE, Garcia-Bueno B, Leza JC, Madrigal JL. CCL2/MCP-1 modulation of microglial activation and proliferation. Journal of neuroinflammation. 2011;8:77. doi: 10.1186/1742-2094-8-77. doi:10.1186/1742-2094-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung TH, Lay CJ, Chang CM, Tsai JJ, Tsai CC, Tsai CC. The effect of infections on the mortality of cirrhotic patients with hepatic encephalopathy. Epidemiology and infection. 2013:1–8. doi: 10.1017/S0950268813000186. doi:10.1017/S0950268813000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihrie RA, Alvarez-Buylla A. Lake-front property: a unique germinal niche by the lateral ventricles of the adult brain. Neuron. 2011;70(4):674–686. doi: 10.1016/j.neuron.2011.05.004. doi:S0896- 6273(11)00382-5 [pii] 10.1016/j.neuron.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiological reviews. 2011;91(2):461–553. doi: 10.1152/physrev.00011.2010. doi:10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- Kloss CU, Kreutzberg GW, Raivich G. Proliferation of ramified microglia on an astrocyte monolayer: characterization of stimulatory and inhibitory cytokines. Journal of neuroscience research. 1997;49(2):248–254. [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, Cartwright H. The cerebral cortex is damaged in chronic alcoholics. Neuroscience. 1997;79(4):983–998. doi: 10.1016/s0306-4522(97)00083-3. doi:S0306-4522(97)00083-3 [pii] [DOI] [PubMed] [Google Scholar]
- Kril JJ, Harper CG. Neuronal counts from four cortical regions of alcoholic brains. Acta Neuropathol. 1989;79(2):200–204. doi: 10.1007/BF00294379. [DOI] [PubMed] [Google Scholar]
- Low VF, Dragunow M, Tippett LJ, Faull RL, Curtis MA. No change in progenitor cell proliferation in the hippocampus in Huntington's disease. Neuroscience. 2011;199:577–588. doi: 10.1016/j.neuroscience.2011.09.010. doi:10.1016/j.neuroscience.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Lyck L, Dalmau I, Chemnitz J, Finsen B, Schroder HD. Immunohistochemical markers for quantitative studies of neurons and glia in human neocortex. J Histochem Cytochem. 2008;56(3):201–221. doi: 10.1369/jhc.7A7187.2007. doi:jhc.7A7187.2007 [pii] 10.1369/jhc.7A7187.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg MD, Baker L, Norenberg LO, Blicharska J, Bruce-Gregorios JH, Neary JT. Ammonia-induced astrocyte swelling in primary culture. Neurochemical research. 1991;16(7):833–836. doi: 10.1007/BF00965694. [DOI] [PubMed] [Google Scholar]
- Rangroo Thrane V, Thrane AS, Chang J, Alleluia V, Nagelhus EA, Nedergaard M. Real-time analysis of microglial activation and motility in hepatic and hyperammonemic encephalopathy. Neuroscience. 2012;220:247–255. doi: 10.1016/j.neuroscience.2012.06.022. doi:10.1016/j.neuroscience.2012.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee W, Ray S, Yokoo H, Hoane ME, Lee CC, Mikheev AM, Horner PJ, Rostomily RC. Quantitative analysis of mitotic Olig2 cells in adult human brain and gliomas: implications for glioma histogenesis and biology. Glia. 2009;57(5):510–523. doi: 10.1002/glia.20780. doi:10.1002/glia.20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Pozo A, Gomez-Isla T, Growdon JH, Frosch MP, Hyman BT. A phenotypic change but not proliferation underlies glial responses in Alzheimer disease. The American journal of pathology. 2013;182(6):2332–2344. doi: 10.1016/j.ajpath.2013.02.031. doi:10.1016/j.ajpath.2013.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawcross DL, Shabbir SS, Taylor NJ, Hughes RD. Ammonia and the neutrophil in the pathogenesis of hepatic encephalopathy in cirrhosis. Hepatology. 2010;51(3):1062–1069. doi: 10.1002/hep.23367. doi:10.1002/hep.23367. [DOI] [PubMed] [Google Scholar]
- Shawcross DL, Sharifi Y, Canavan JB, Yeoman AD, Abeles RD, Taylor NJ, Auzinger G, Bernal W, Wendon JA. Infection and systemic inflammation, not ammonia, are associated with Grade 3/4 hepatic encephalopathy, but not mortality in cirrhosis. Journal of hepatology. 2011;54(4):640–649. doi: 10.1016/j.jhep.2010.07.045. doi:10.1016/j.jhep.2010.07.045. [DOI] [PubMed] [Google Scholar]
- Shawcross DL, Wright G, Olde Damink SW, Jalan R. Role of ammonia and inflammation in minimal hepatic encephalopathy. Metabolic brain disease. 2007;22(1):125–138. doi: 10.1007/s11011-006-9042-1. doi:10.1007/s11011-006-9042-1. [DOI] [PubMed] [Google Scholar]
- Sheedy D, Garrick T, Dedova I, Hunt C, Miller R, Sundqvist N, Harper C. An Australian Brain Bank: a critical investment with a high return. Cell Tissue Bank. 2008;9(3):205–216. doi: 10.1007/s10561-008-9076-1. doi:10.1007/s10561-008-9076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Gibbons HM, Oldfield RL, Bergin PM, Mee EW, Curtis MA, Faull RL, Dragunow M. M-CSF increases proliferation and phagocytosis while modulating receptor and transcription factor expression in adult human microglia. J Neuroinflammation. 2013;10:85. doi: 10.1186/1742-2094-10-85. doi:10.1186/1742-2094-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoimenov I, Helleday T. PCNA on the crossroad of cancer. Biochem Soc Trans. 2009;37(Pt 3):605–613. doi: 10.1042/BST0370605. doi:10.1042/BST0370605. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Braak H, Xue QS, Bechmann I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease. Acta neuropathologica. 2009;118(4):475–485. doi: 10.1007/s00401-009-0556-6. doi:10.1007/s00401-009-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004;45(2):208–212. doi: 10.1002/glia.10319. doi:10.1002/glia.10319. [DOI] [PubMed] [Google Scholar]
- Sutherland GT, Sheahan PJ, Matthews J, Dennis CV, Sheedy DS, McCrossin T, Curtis MA, Kril JJ. The effects of chronic alcoholism on cell proliferation in the human brain. Exp Neurol. 2013;247:9–18. doi: 10.1016/j.expneurol.2013.03.020. doi:10.1016/j.expneurol.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berge SA, van Strien ME, Korecka JA, Dijkstra AA, Sluijs JA, Kooijman L, Eggers R, De Filippis L, Vescovi AL, Verhaagen J, van de Berg WD, Hol EM. The proliferative capacity of the subventricular zone is maintained in the parkinsonian brain. Brain. 2011;134(Pt 11):3249–3263. doi: 10.1093/brain/awr256. doi:10.1093/brain/awr256. [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(13):3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. doi:10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada J, Jinno S. Novel objective classification of reactive microglia following hypoglossal axotomy using hierarchical cluster analysis. J Comp Neurol. 2013;521(5):1184–1201. doi: 10.1002/cne.23228. doi:10.1002/cne.23228. [DOI] [PubMed] [Google Scholar]
- Zemtsova I, Gorg B, Keitel V, Bidmon HJ, Schror K, Haussinger D. Microglia activation in hepatic encephalopathy in rats and humans. Hepatology. 2011;54(1):204–215. doi: 10.1002/hep.24326. doi:10.1002/hep.24326. [DOI] [PubMed] [Google Scholar]
- Zhao YN, Wang F, Fan YX, Ping GF, Yang JY, Wu CF. Activated microglia are implicated in cognitive deficits, neuronal death, and successful recovery following intermittent ethanol exposure. Behavioural brain research. 2013;236(1):270–282. doi: 10.1016/j.bbr.2012.08.052. doi:10.1016/j.bbr.2012.08.052. [DOI] [PubMed] [Google Scholar]