Abstract
The development of biomaterials for myocardial tissue engineering requires a careful assessment of their performance with regards to functionality and biocompatibility, including the immune response. Poly(3-hydroxybutyrate) (PHB), poly(e-caprolactone) (PCL), silk, poly-lactic acid (PLA), and polyamide (PA) scaffolds were generated by electrospinning, and cell compatibility in vitro, and immune response and cardiac function in vitro and in vivo were compared with a noncrosslinked collagen membrane (Col) control material. Results showed that cell adhesion and growth of mesenchymal stem cells, cardiomyocytes, and cardiac fibroblasts in vitro was dependent on the polymer substrate, with PHB and PCL polymers permitting the greatest adhesion/growth of cells. Additionally, polymer substrates triggered unique expression profiles of anti- and pro-inflammatory cytokines in human peripheral blood mononuclear cells. Implantation of PCL, silk, PLA, and PA patches on the epicardial surface of healthy rats induced a classical foreign body reaction pattern, with encapsulation of polymer fibers and induction of the nonspecific immune response, whereas Col and PHB patches were progressively degraded. When implanted on infarcted rat heart, Col, PCL, and PHB reduced negative remodeling, but only PHB induced significant angiogenesis. Importantly, Col and PHB modified the inflammatory response to an M2 macrophage phenotype in cardiac tissue, indicating a more beneficial reparative process and remodeling. Collectively, these results identify PHB as a superior substrate for cardiac repair.
Introduction
Myocardial infarction (MI) is a leading cause of death and disability throughout the western world. MI results in the irreversible loss of cardiomyocytes, and triggers a constellation of responses, including inflammation and cytokine activation, which results in fibrotic scar deposition. Compensatory mechanisms to maintain cardiac output in damaged myocardium ultimately lead to progressive left ventricular (LV) remodeling and impairment of LV function. In addition to traditional therapeutic interventions to limit myocardial damage, tissue engineering is a promising new method to counter LV dilation. Indeed, polymeric materials are used increasingly for surgical reconstruction of cardiovascular tissues and several studies indicate the benefits of biomaterials (BMs) by reducing remodeling after MI, and inducing stem cell function in the heart [1,2].
Categories of BMs used for cardiac regeneration include injectable polymers, porous scaffolds, and electrospun polymeric sheets [3–5]. Polymer electrospinning is a technique that uses high voltage to produce fibers on a submicron scale. The advantages of electrospinning include easy manipulation and control of mesh composition and configuration (aligned or random fibers), in addition to regulation of density and length of fibers, to better accommodate the reconstruction of a specific tissue [6,7]. Moreover, depending on the polymer used, electrospun mesh can be extremely elastic and easy to suture for implantation on tissues, for example, the epicardial surface of heart. BMs used to construct scaffolds can be largely divided into classes of natural or synthetic origin [8–11]. These scaffolds can be used directly, or after seeding with cells prior to implantation [12–14]. Predictably, the choice of BMs is an important consideration in tissue engineering [15–17]. Host response is influenced by the physicochemical properties of scaffolds, including degradability, crosslinking or plasma activation of polymeric surface, the source of raw material, and the nature of the polymer itself (natural or synthetic). Foreign material triggers immune responses driven by inflammatory mediators, including cytokines, and diverse immune cells, including macrophages, neutrophils, T and B cells, and dendritic cells. Thus, an understanding of the immune response to polymers is important for the design of implantable patches or devices [18]. For example, the implantation of degradable BMs, rather than nondegradable BMs, will diminish risk of infection [19]. The immune response is also influenced by the body implantation site; subcutaneous implantation often leads to encapsulation, and the host reaction may be limited to a foreign body response. However, epicardial implantation needs to preserve the geometry of the heart. Moreover, in case of injury (ie, MI), implanted BMs should prevent the decline of cardiac function. Thus, a suitable polymeric scaffold for cardiac tissue engineering should demonstrate an appropriate biodegradation life-time while, at the same time, promote wall motion recovery and induce restorative processes (angiogenesis and accelerated healing). In this context, a BM that induces a shift in the balance of infiltrating macrophages to an M2 phenotype would be preferred, since the activation of an M1 macrophage response is typically associated with transplant rejection and chronic inflammation, while M2 macrophages are thought to participate in tissue remodeling and transplant tolerance [20].
In this study, we compared a range of polymer scaffolds for some of these attributes. Through this comparison, we found that poly(3-hydroxybutyrate) (PHB) is a superior substrate for cardiac tissue implantation.
Materials and Methods
Electrospinning and scanning electron microscopy
Polymers were electrospun as previously reported [21], but in each case electrospinning parameters were determined empirically. PHB (400,000 g/mol) was dissolved in CHCl3 at 60°C and electrospun using a Nanospider instrument (Elmarco). NaCl (0.2 wt%) was added to the solution in order to increase the conductivity. Polymer solution (7 wt%) was loaded into a Nanospider sink and connected to a high-voltage supply. The voltage used for electrospinning was 81 kV and the collection distance was fixed at 11 cm. Poly(e-caprolactone) (PCL), poly-lactic acid (PLA), silk, and polyamide (PA) were electrospun using a hand-made laboratory electrospinning apparatus consisting of a high-voltage power supply (Spellman), syringe pump (Kd Scientific), 5 mL syringe (BD Plastipak), 0.6-mm needle (BD Microlance), and a grounded metal collector covered by a paper/nonwoven sheet. Polymer solutions were electrospun at room temperature. Polymer concentrations, solvents used, and electrospinning conditions are listed in Table 1. Silk fibroin was obtained from silkworm cocoons as described previously [22]. Polymer meshes (PHB, PCL, PLA, and PA) were washed with deionized water to remove organic solvents. Silk meshes were crosslinked by washing with methanol. After drying at room temperature, nanofiber meshes were activated with N2 plasma for 20 s to introduce polar groups onto the surface and increase the surface hydrophilicity [23]. Electrospun sheets were sterilized by UV radiation for 60 min before cell culture and in vivo applications. The diameter of fibers was determined by scanning electron microscopy (SEM) imaging. Briefly, electrospun scaffolds were coated with gold (Polaron SC7620 “Mini” Sputter Coater) and fiber structures were examined with a microscope (FEI Company) at an accelerating voltage of 25 kV. Average fiber diameter and size distributions were determined by measuring >100 fibers selected randomly from the SEM images, using ImageJ analysis software. Bovine-derived collagen type-I (Viscofan SA), at a thickness of 20 μm, was used as a control scaffold for all experimental procedures. Features of this material have been reported previously [24].
Table 1.
Electrospinning Conditions of Different Polymer-Based Scaffolds
| Solution | Concentration (w/t) | Solvent | Solvent proportion | Additives | Distance between electrodes (cm) | Needle diameter (mm) | Feed rate (mL/h) | Voltage (kV) |
|---|---|---|---|---|---|---|---|---|
| PHB | 7% | CHCl3 | — | NaCl 0.2%wt | 11 | — | — | 81 |
| PCL | 10% | DMF/CHCl3 | 1:8 | — | 13 | 0.6 | 14 | |
| PLA | 10% | THF/DMSO | 8:2 | — | 11 | 0.6 | 9 | |
| Silk | 10% | HFIP | — | — | 10 | 0.6 | 11 | |
| PA | 12% | HCOOH/CH3COOH | 1:2 | — | 13 | 0.6 | 14 |
PHB, poly(3-hydroxybutyrate); PCL, poly(e-caprolactone); PLA, poly-lactic acid; PA, polyamide; HFIP, hexafluoroisopropanol; DMF, dimethylformamide; THF, tetrahydrofuran.
Cell culture and polymer mesh seeding
The mouse cardiac HL-1 cell line [25] was grown in Claycomb medium (Sigma) supplemented with 10% fetal bovine serum, 2% L-glutamine, and 1% penicillin/streptomycin. Cardiac fibroblasts were obtained from digestion of neonatal hearts from 1–3-day-old pups. Briefly, hearts were excised and digested as described previously [26]. Briefly, hearts were excised, auricles were removed, and ventricles were minced and digested with collagenase type II (4 mg/mL) in DMEM supplemented with 10% fetal calf and horse serum (4:1), for 1 h with gentle shaking. After centrifugation at 800 g, the pellet was recovered in HBSS and digested with Dispase II (2.4 U/mL; Roche) and DNAase I (1,000 U/mL; Roche) for 20 min at 37°C. Cells were collected by centrifugation and seeded at 100,000 cells/cm2 and cultured for 60 min to recover fibroblasts, which were used before passage 3. Mesenchymal stem cells (MSCs) from dental pulp were purchased from Inbiomed (Inbiobank) and cultured in DMEM supplemented with 10% fetal calf serum. To visualize cell spreading, 20 μL of cell suspension was pipetted onto the dry surface of polymer meshes and cells were allowed to adhere for 20 min. Thereafter, meshes were placed into 24-well plates filled with culture media, and incubated for a further 48 h. Next, medium was then removed and meshes were washed with phosphate-buffered saline (PBS), fixed with 2% paraformaldehyde (PFA), washed again with PBS, labeled with 4′,6-diamidino-2-phenylindole (DAPI), and visualized with a fluorescent microscope.
Cell-seeding assay
To measure cell attachment to electrospun sheets, HL-1 cells, cardiac fibroblasts, and MSCs (105 cells/mL) were added to wells containing 1 cm2 BM sheets. Cells were allowed to attach to polymeric meshes for 1 h. Thereafter, sheets were thoroughly washed with PBS, fixed with 2% PFA, washed again with PBS, and stained with DAPI, and cells were counted under a microscope at 200× magnification. The experiment was performed four times.
MTT proliferation assay
HL-1 cells, cardiac fibroblasts, and MSCs were plated at 5×104, 6×104, and 3×104 cells/cm2, respectively, onto polymeric sheets in the appropriate medium. Proliferation was measured after 96 h using thiazolyl blue tetrazolium bromide (MTT assay; Sigma-Aldrich), following the manufacturer's instructions. Absorbance was measured at 550 nm using a microplate reader (Victor3 1420 Multilabel Counter; PerkinElmer, Inc.). The experiment was performed three times.
Pyrogen test and real-time polymerase chain reaction
Blood from healthy donors was obtained from the Valencian Blood Tissue Bank, after informed consent. Peripheral blood mononuclear cells (PBMNCs) were isolated by Ficoll density gradient centrifugation, and incubated with polymeric scaffolds (1 cm2 patches), at 1×106 cells/mL for 5 h. To measure pro-inflammatory cytokine gene expression following attachment to scaffolds, PBMNCs were washed with PBS, and RNA was extracted using TRIzol Reagent (Invitrogen). Purification of RNA was carried out with the RNeasy Plus Mini Kit (Qiagen). RNA was quantified by spectrometry (NanoDrop ND-1000; NanoDrop Technologies), and integrity/purity was assessed by agarose gel electrophoresis and the 260/280 nm absorbance ratio. Complementary DNA was synthesized using M-MLV Reverse Transcriptase (Invitrogen). Real-time polymerase chain reaction (PCR) cycling was carried out on a LightCycler 480 (Roche), using LightCycler 480 SYBR Green I Master (Roche Molecular Biochemical). Quantitative PCR was performed under the following conditions: 95°C at 10 min, 40 cycles at 95°C, 15 s; 58°C, 10 s; and 72°C, 20 s. Specificity of the primers was tested by melting-curve analysis using the following conditions: 95°C, 5 s; 60°C, 15 s; and 97°C continuous, one cycle. Primers used to analyze the expression of common pro-inflammatory cytokines were designed using the Primer-Blast online tool oligonucleotide: IL-β (forward AGGCACAAGGCACAACAGGCT; reverse AACAACTGACGCGGCCTGCC, 277-bp amplicon), IL-4 (forward TCTGTGCACCGAGTTGACCGT; reverse TGCTGTGCAGTCGCACCCAG, 150-bp amplicon), IL-6 (forward CATTCTGCCCTCGAGCCCACC; reverse GGCAGCAGGCAACACCAGGA, 139-bp amplicon), IL-10 (forward AGACCCAGACATCAAGGCGCA; reverse TCGATGACAGCGCCGTAGCC, 82-bp amplicon), IL-13 (forward GGCATGTACTGTGCAGCCCTGG; reverse GTGCGGGCAGAATCCGCTCA, 96-bp amplicon), and IFN-γ (forward GAGTGTGGAGACCATCAAGGA; reverse CTGTTTTAGCTGCTGGCGAC, 176-bp amplicon). Actin B (ACT-β; forward AGAGCCTCGCCTTTGCCGATCC; reverse CATGCCGGAGCCGTTGTCGAC, 101-bp amplicon) was used as a housekeeping gene for internal normalization.
Animal model
Adult male Wistar rats (Charles River Laboratories, Inc.) weighing 200–250 g were maintained under standard laboratory conditions. The number of animals used in the study was 95. Mortality rate due to surgical procedures was about 10% in all groups. All procedures were approved by institutional ethical and animal care committees.
MI and surgical procedures
Permanent ligation of the left anterior coronary artery was performed as described previously [27]. Immediately after ligation, 1 cm2 of acellular UV-sterilized patches was attached to the epicardial surface with two points of suture (polybrene 6.0). Infarcted animals without patch implantation were used as the control group. Patches were also sutured to the epicardial surface of noninfarcted animals to study host body response.
Morphometric analysis
Infarct size was measured in 8–12 transverse sections of 7 μm (one slice in each 200 μm section of tissue), from apex to base. Sections were fixed with 2% PFA and stained with Masson's trichrome. Fibrotic area was determined by computer planimetry of photo-images corresponding to entire sections (Image Proplus 7.1 software). Infarct size was expressed as the percentage of total LV area and as a mean of all slices from each heart.
Echocardiography
To evaluate heart function, transthoracic echocardiography was performed in rats under inhalatory anesthesia (Sevorane) using an echocardiographic system (Vivid 5; General Electrics) equipped with a 10-MHz linear-array transducer, as previously reported [27]. Measurements were taken in infarcted rats, with or without epicardial implantation of BMs (n=6 in each group), at baseline and 2 weeks post-transplantation. LV dimensions in end diastole (LVDd) and end systole (LVDs), anterior and posterior wall (AW and PW) thickness in diastole and systole, and end-diastolic area (EDA) and end-systolic area (ESA) were measured. Fractional area change (FAC) was calculated as [(EDA – ESA)/EDA]×100. Fractional shortening (FS) was calculated as [(LVDd – LVDs)/LVDd]×100. Changes in AW were calculated as (AWs – AWd/AWd)×100.
Immunohistochemistry
Immunohistochemical analysis was performed 4 weeks after implantation. Animals were euthanized and the hearts were removed, washed with PBS, and fixed in 2% PFA. Heart tissue sections were prepared for immunohistochemistry as described previously [28].
Analysis of vascular density
Immunohistochemical detection of vessels was performed with anti-rat caveolin (Chemicon International). Vessels were counted inside each polymeric patch (three random sections of each patch) implanted on the epicardial surface of healthy or infarcted rats, 2 weeks after BM implantation (n=4 animals in each group). Vessels were counted in four random fields of each section under ×200 magnification (12 photos per animal), and the number of vessels per area unit (mm2) was scored using a light microscope and Image Proplus 7.1 software.
Analysis of macrophage M1/M2 inflammatory response
Heart sections were stained for CD68, CCR7, and CD163 to quantify the macrophage phenotype surrounding the implant site. Immunolabeling was performed with antibodies against CD68 (1:200 dilution; Serotec) and CD163 (1:100 dilution; Serotec), followed by detection with an Alexa 594-conjugated secondary antibody (1:200 dilution; Invitrogen); and against CCR7 (1:1000 dilution; Epitomics) followed by detection with an FITC-conjugated secondary antibody (1:200 dilution; Jackson Immunology). Quantification was performed in healthy and infarcted rats sacrificed at 2 weeks post-transplantation. A total of six serial heart sections were prepared and two images of the zone surrounding the implant were taken per section with a 20× objective. Immunofluorescent images were acquired with an MR3 AxioCam camera (Zeiss) adapted to the M1 AxioImager Zeiss microscope (Zeiss). Immunopositive cells were quantified using ImageJ software. The ratio of M1 and M2 macrophages was calculated by quantifying the number of CCR7+/CD68+ cells (M1) and CD163+/CD68+ cells (M2) in each field.
Statistical analysis
Data are expressed as mean±SEM (standard, error of measurement). Comparisons between control and experimental groups were performed with the Wilcoxon W test or the Kruskal–Wallis test, as appropriate. Analyses were conducted with SPSS and GraphPad Prism 5 software. Differences were considered statistically significant at P<0.05 with a 95% confidence interval.
Results
Characterization of electrospun meshes and cell-spreading profile
Polymer substrates were electrospun under similar conditions of voltage, flow rate, and concentration, and the nanometric fibers were collected as a heterogeneous mesh and examined by SEM. Bovine-derived nonporous collagen (Col) was used as a control scaffold to compare to the electrospun polymer scaffolds. These scaffolds are biocompatible, support cell growth, and have been validated in cardiac regenerative therapies [24]. The relative frequencies of fiber diameter are shown in Supplementary Figure S1 (Supplementary Data are available online at www.liebertpub.com/scd). All BMs were composed of a dense network of randomly interwoven fibers that formed highly porous three-dimensional structures (Fig. 1A, top). Among the scaffolds tested, PCL and PA were more manageable, whereas silk was rather fragile and showed limited deformation capacity. To monitor cell compatibility with scaffolds, cardiac fibroblasts were seeded onto dry polymer meshes, followed by culture for 4 days. As visualized by nuclear staining, fibroblasts displayed a spreading behavior that was dependent on the polymer substrate. Whereas seeding onto Col, PCL, and PLA allowed extensive and homogeneous cell growth, fibroblasts seeded onto PHB, silk, and PA displayed a limited cell-spreading phenotype (Fig. 1A, bottom).
FIG. 1.
Characterization of polymer meshes. (A) Scanning electron microscopy of BMs: Col, PHB, PCL, Silk, PLA, and PA (upper panels), and nuclear staining of cardiac fibroblasts seeded onto BMs and cultured for 4 days (lower panels; magnification 200×). (B) Adhesion of MSCs, cardiac fibroblasts, and HL-1 cells to different polymer meshes relative to control collagen. (C) Proliferation of MSCs, cardiac fibroblasts, and HL-1 cells on different BMs assessed by MTT assay. The colorimetric reaction was measured at 550 nm, and expressed as arbitrary units of optical density. Scale bar in panel A=10 μm; *P<0.05, †P<0.01, ‡P<0.001. BMs, biomaterials; Col, collagen membrane; MSCs, mesenchymal stem cells; PA, polyamide; PCL, poly(e-caprolactone); PHB, poly(3-hydroxybutyrate); PLA, poly-lactic acid. Color images available online at www.liebertpub.com/scd
Attachment, viability, and inflammatory response of cells cultured on electrospun meshes
MSCs, cardiac fibroblasts, and HL-1 myocytes were incubated with polymer sheets, and attachment was evaluated after 60 min by nuclear (DAPI) staining of PFA-fixed cells. Whereas attachment of MSCs and fibroblasts to silk, PLA, and PA polymers was comparable to the control Col substrate, PHB and PCL polymers contained significantly greater numbers of both cell types (Fig. 1B). A similar result was noted with HL-1 cells, which also attached in greater numbers to PLA polymers (Fig. 1B). This result was not surprising as nonporous sheets have, in general, a limited surface area in comparison to porous sheets generated by electrospinning (Fig. 1A). After an extended culture of 4 days, PCL polymer sheets contained significantly greater numbers of all three cell types compared to Col, whereas silk supported greater growth of MSCs only (Fig. 1C).
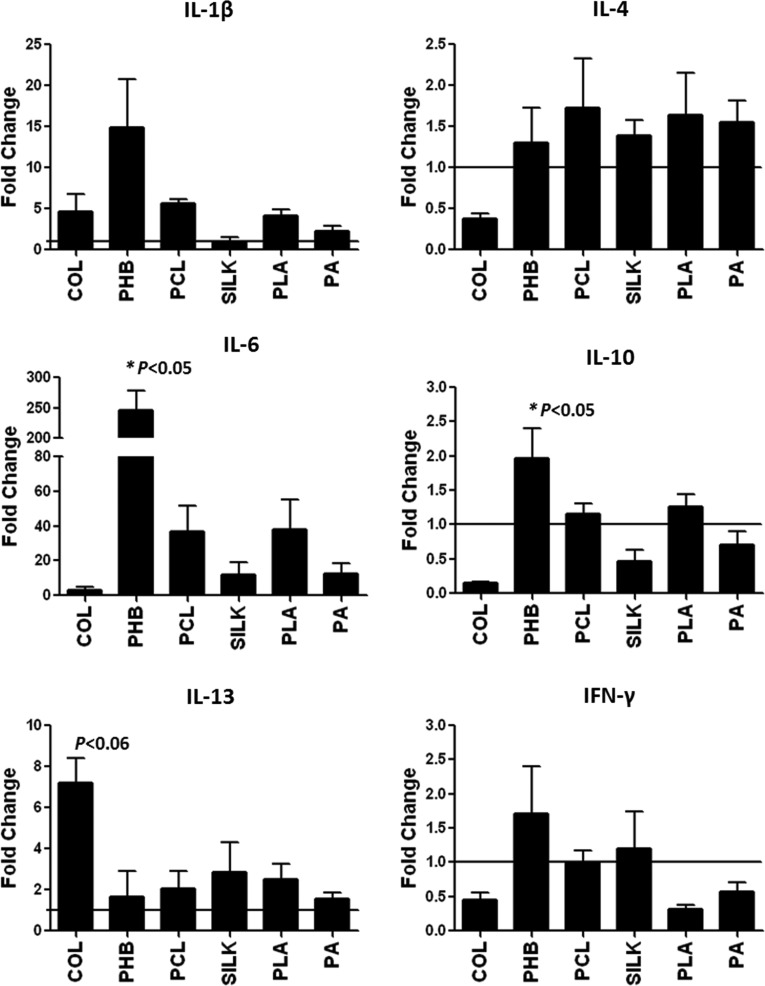
To examine the acute inflammatory response induced by electrospun meshes, freshly isolated PBMNCs from healthy subjects were cultured with polymeric scaffolds for 5 h and RNA was extracted for gene expression analysis. Results showed that IL-1β expression was induced to similar levels in PBMNCs after culture with Col, PHB, PCL, PLA, and PA polymers, but not silk, while expression levels of IL-4 were unaffected by any polymer substrate (Fig. 2 and Supplementary Table S1). Additionally, IL-6 expression was induced after cell contact with Col (3.00±1.69-fold), PCL (36.71±14.77-fold), silk (11.86±7.07-fold), PLA (37.79±17.43-fold), PA (12.29±6.36-fold), and significantly with PHB (246.0±32.15-fold; P<0.05 in PHB vs. Col, PCL, silk, PLA, and PA). Notably, PHB alone significantly induced IL-10 expression (P<0.05 in PHB vs. Col). Moreover, IL-13 expression was induced significantly by collagen (P<0.06 in Col vs. PHB). Collectively, these results reveal a complex pattern of cytokine expression that is dependent on the scaffold material.
FIG. 2.
Quantification of gene transcription levels of IL-1, IL-4, IL-6, IL-10, IL-13, and IFN-γ in PBMNCs incubated for 5 h with polymer meshes of the indicated BMs. Data are expressed as relative fold over control (normalized values represented as mean±SEM (standard error of measurement) of three independent experiments; *P<0.05). PBMNCs, peripheral blood mononuclear cells.
Cardiac biocompatibility of polymer meshes
We evaluated degradation of scaffold materials 2 and 8 weeks after implantation onto the epicardial surface of healthy rats, and analyzed the foreign body response with respect to inflammation, granulomatous reaction, vascular congestion, hemorrhage, and epicardial fibrosis (Table 2). All implanted materials were visible at 8 weeks postimplantation, with partial degradation of Col and PHB patches, and no macroscopic degradation observed with PCL, PA, PLA, and silk patches (Fig. 3A). Degradation of Col patches was more rapid than PHB, as deduced both by the absence of large fragments of polymer at 2 weeks, and the absence of phagocytic multinucleated giant cells (Supplementary Fig. S1). Additionally, PLA scaffolds displayed a prominent increase in volume shortly after implantation that persisted throughout the time period analyzed. This event was accompanied by exacerbated cell infiltration, composed predominantly of mononuclear cells (Fig. 3B and Supplementary Fig. S2). In contrast, PCL, silk, and PA patches were quickly encapsulated and degraded following a pattern of foreign body reaction, with the accumulation of multinucleated giant cells (Supplementary Fig. S1). Further, PBH sheets and collagen control membranes provoked a mild inflammatory response that was mostly resolved at 8 weeks, consistent with graft acceptance (Table 1). Of note, vascular congestion induced by sheet implantation (indicative of angiogenesis) was particularly evident in PHB-implanted animals (Table 1).
Table 2.
Histopathological Analysis of Biomaterials Implanted in the Rat Heart
| COL | PHB | PCL | Silk | PLA | PA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factors evaluated | 2 weeks | 8 weeks | 2 weeks | 8 weeks | 2 weeks | 8 weeks | 2 weeks | 8 weeks | 2 weeks | 8 weeks | 2 weeks | 8 weeks |
| Biomaterial integrity | ++ | − | + | − | + | + | ++ | ++ | + | ++ | − | − |
| Granulomatous reaction | + | − | + | − | ++ | ++ | + | + | +++ | +++ | +++ | ++ |
| Inflammatory infiltrate | ++ | − | + | + | ++ | + | ++ | ++ | + | + | ++ | − |
| Vascular congestion | + | − | +++ | ++ | − | ++ | + | ++ | ++ | ++ | − | − |
| Hemorrhage | − | − | − | − | − | − | − | − | − | − | − | − |
| Epicardial fibrosis | − | − | + | − | ++ | − | + | − | − | − | − | − |
The study was performed at 2 and 8 weeks.
−, not detected; +, slight presence; ++, moderate presence; +++, strong presence.
FIG. 3.

(A) Macroscopic examination of BMs on the epicardial surface of healthy rats, 8 weeks after implantation. (B) Cross-section of heart tissue stained with hematoxylin and eosin showing cardiac patches at 8 weeks after implantation. Inset, detail of BM region at 400× magnification. Color images available online at www.liebertpub.com/scd
Neoangiogenesis in the border zone of implanted patches
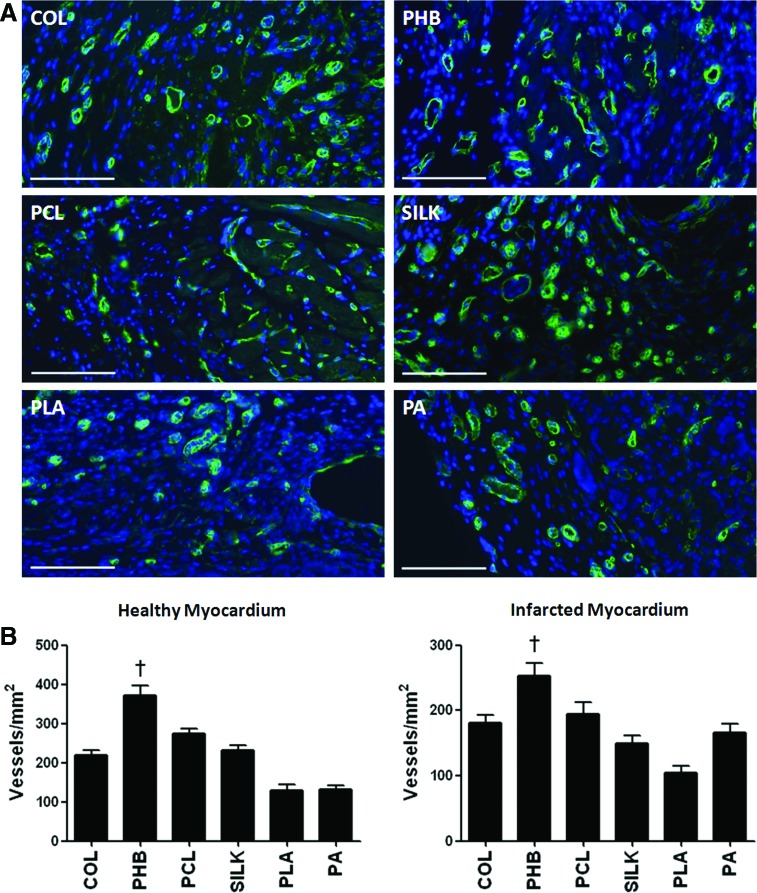
Two weeks after implantation, angiogenesis in scaffolds was quantified by scoring the number of capillaries and arterioles with an antibody to caveolin (Fig. 4A). When scaffolds were implanted in healthy rats, vessel density in tissue sections of implanted materials was 219.8±12.39 for Col, 372.4±24.90 for PHB, 275.2±10.63 for PCL, 230.6±12.75 for silk, 129.2±14.32 for PLA, and 131.1±9.52 for PA (P<0.05 for PHB vs. Col, PCL, silk, PLA, and PA; Fig. 4B). When scaffolds were implanted in rats with permanent LDA occlusion, vessel density within implanted materials was 181.4±11.56 for Col, 253.4±18.73 for PHB, 194.8±17.11 for PCL, 149.4±12.15 for silk, 105.1±9.5 for PLA, and 166.0±12.95 for PA (P<0.05 for PHB vs. Col, PCL, silk, PLA, and PA; Fig. 4B). Thus, implantation of PHB scaffold resulted in significantly increased neoangiogenesis, in both healthy and infarcted myocardium.
FIG. 4.
Vascularization of polymer meshes in the healthy heart 2 weeks after implantation. (A) Representative immunofluorescence images of sprouting vessels inside BMs, stained for caveolin-1 (green) and nuclei (DAPI, blue), in healthy hearts. (B) Quantification of the total number of vessels in healthy and infarcted rats, 2 weeks after implantation (n=6 in each group; †P<0.01). DAPI, 4′,6-diamidino-2-phenylindole. Color images available online at www.liebertpub.com/scd
LV remodeling and cardiac function
Having shown that polymer scaffolds could modify the inflammatory response of host tissue, and direct angiogenesis, we next questioned whether this could lead to improved heart function after MI. Interestingly, epicardial implantation of Col, PHB, and PCL sheets, but not silk, PLA, and PA, significantly reduced scar tissue and prevented negative remodeling after MI (Fig. 5A). Percentage of scar tissue was 27.84%±1.55% in control group (n=9), 16.93%±1.80% in Col group (n=7), 17.47%±2.26% in PHB group (n=6), 13.86%±1.09% in PCL group (n=4), 19.14%±1.27% in silk group (n=4), 24.31%±5.24% in PLA group (n=4), and 22.39%±4.11% in PA group (n=4) (P<0.01 in Col and PCL, vs. control group; P<0.05 in PHB vs. control group).
FIG. 5.
Effect of cell transplantation on infarct size and cardiac function 2 weeks after implantation. (A) Representative heart sections of sham or infarcted rats implanted or not with cardiac patches. Fibrotic area in the left ventricle was calculated by quantification of Masson's trichrome-stained sections in each group and compared with a group of infarcted rats without BM implantation (*P<0.05 in PHB vs. control; †P<0.01 in Col and PCL vs. control). (B) Measurement of LV function in control and treated animals. Quantified values of fractional area change (FAC), fractional shortening (FS), and anterior wall thickening (AWT) are given. No significant differences were obtained between control group and groups implanted with different BMs (n=6). LV, left ventricular. Color images available online at www.liebertpub.com/scd
To determine whether polymer implantation improved cardiac function in infarcted animals, noninvasive echocardiography was performed 2 weeks after transplantation. None of the polymers implanted resulted in a significant recovery of systolic function in rats, as calculated by percentage of FAC (36.52%±0.79% in control group, 44.45%±4.18% in Col, 39.83%±9.20% in PHB, 44.62%±4.00% in PCL, 40.11±9.64 in silk, 42.68±7.21 in PLA, and 44.42±2.84 in PA), FS (24.82%±3.49% in control group, 25.72%±3.26% in Col, 25.80±2.00 in PHB, 28.14±3.11 in PCL, 26.61±0.18 in silk, 25.65%±3.59% in PLA, and 27.71±2.69 in PA), and anterior wall thickening (AWT: 25.41%±3.27% in control group, 38.06%±1.33% in Col, 32.33±1.87 in PHB, 31.54±2.14 in PCL, 34.64±1.44 in silk, 30.05%±1.50% in PLA, and 30.54±1.06 in PA) (Fig. 5B). These results are in agreement with a previous report [29] and indicate that although implantation of naked polymer meshes does not improve cardiac function, they appear not to be detrimental for cardiac performance when applied to the epicardial surface of infarcted hearts. Indeed, implantation of polymer meshes in noninfarcted rats was similarly not deleterious for cardiac wall motion.
Analysis of M1/M2 inflammatory response
Finally, given their importance as modulators of post-MI wound healing, including angiogenesis [30], we analyzed the polarization profile of infiltrating macrophages in response to scaffold implantation. M1 macrophages, characterized by the cell surface markers CD68 and CD80, and M2 macrophages, characterized by CD163 in rats, were measured by immunohistochemistry of inflammatory infiltrates at the border zone with implanted materials (Fig. 6A). Quantitative analysis of the macrophage phenotype in noninfarcted rats at 2 weeks postimplantation showed that whereas PCL, silk, and PA implantation triggered an M1 macrophage infiltration, Col, PHB, and PLA implantation led to the infiltration of M2 macrophages (Fig. 6B). With the exception of silk polymer, a similar pattern of polarization was found in the infarcted-rat group (Fig. 6C). Indeed, this M2 phenotype was in agreement with the finding of increased vessel formation with PBH scaffolds (Fig. 4), and is a desirable attribute of BMs with tissue-regenerative potential.
FIG. 6.
Modulation of M1/M2 phenotype by BM implantation. Representative immunofluorescence images of healthy and infarcted rat hearts stained for CD68 and CD163 (red) and CCR7 (green). Nuclei were stained with DAPI (blue). Ratio, M1 (CCR7+/CD68+)/M2 (CD163+/CD68+), of macrophages present in the biomaterial-surrounding region 2 weeks after the biomaterial implantation in noninfarcted and infarcted rats. Scale bar=50 μm. Color images available online at www.liebertpub.com/scd
Discussion
Together with providing mechanical support for tissue replacement, and a degree of elasticity compatible with native heart beating, engineered constructs for cardiac repair should ideally possess a chemical composition that allows safe biodegradation while limiting the inflammatory response [13]. In an effort to identify materials that fulfill some of these criteria, we chose five commonly used polymers [1,7] and one less-studied substrate (PHB), and compared their suitability for repair by evaluating biological responses in vitro and cardiac physiological responses in vivo. Angiogenic capacity, biodegradation, modulation of host immune response, and prevention of LV remodeling, among other parameters, were analyzed. As expected, all polymers supported cell adhesion and growth [31–35], although some (Col, PCL, and PLA) stimulated cell spreading while others (PHB, silk, and PA) prevented cell movement. Interestingly, although PHB and PCL induced cell adhesion to a similar degree, the physicochemical properties of PHB prevented cell spreading (Fig. 1A)—a feature that could be advantageous to meet safety requirements in clinical protocols. Indeed, differential adhesion of cells to electrospun PHB can be achieved by modulating the composition of pure and blended PHB [36].
A major obstacle to the application of synthetic scaffolds for reparative surgery is the foreign body response of the host to polymers and products of biodegradation, and several studies have addressed the use of anti-inflammatory agent-eluting scaffolds to overcome negative body reactions [37]. However, the controlled release of therapeutic molecules is complex and not always long lasting [38]. For this reason, the identification of matrix scaffolds that minimize this reaction is of great interest for tissue engineering modalities. Whereas Col and PHB were mostly degraded after 8 weeks of implantation, other materials tested (PCL, silk, PLA, and PA) showed minimal scaffold degradation, and were encapsulated or produced an exacerbated inflammatory reaction. Activation of Th1 lymphocytes produces inflammatory cytokines, such as IL-2, TNF-α, and IFN-γ, whereas Th2 lymphocytes produce IL-4, IL-6, and IL-10 that do not prime macrophages and are associated with transplant tolerance. Notably, PHB polymer induced the expression of IL-6 and IL-10 in PBMNCs, suggesting a posterior Th2 response and graft acceptance [8]. Additionally, from the scaffolds tested, PHB polymer induced the greatest degree of vessel growth in both healthy and infarcted myocardium. This may be associated with its ability to induce IL-6 expression in immune cells, exerting paracrine actions in areas surrounding the implanted patch [20].
Several natural or synthetic BMs have been shown to improve cardiac function after implantation [24,39,40]. However, of the cardiac patches that improved wall motion and restored cardiac function, most were used in combination with cells or growth factors [24,29,32,41,42]. In our study, the biocompatibility and elastic properties of Col, PHB, and PCL polymers prevented ventricular dilatation and reduced scar tissue formation, in accordance with previous reports [32,39,43]. Perhaps not surprisingly, these beneficial effects were not sufficient to improve functional parameters in terms of area fractional change, FS, and AWT (Fig. 5A).
An understanding of the immune response of host cardiac tissue to BMs is a prerequisite for successful implantation in myocardial restorative protocols. However, few studies have compared biological responses to different polymer substrates, which is essential to improve safety and efficacy of devices that utilize such materials. A major finding of this study was the demonstration that the inflammatory profile of infiltrating macrophages was dependent on the polymer substrate. Implantation of Col, PHB, and PLA polymers resulted in the enrichment of M2-restorative macrophages in healthy and infarcted myocardium, and was consistent with the observation that Col and PHB induced expression of the M2 cytokines IL-13 and IL-10, respectively, in PBMNCs. This polymer-specific induction of macrophage polarization is clearly important since substrates that a priori might appear suitable for use in cardiac tissue engineering—for example, PCL—may not fulfill safety criteria formulated as pure scaffolds if they provoke a chronic M1 response [20]. Indeed, pure PCL electrospun sheets are less favorable than optimized PCL–polyethylene glycol polymers for maintenance of embryonic stem cell viability and cardiomyocyte differentiation [16]. As yet, we cannot explain why silk patches implanted in healthy myocardium shift the macrophage balance to an M1 phenotype, while the same patches implanted in infarcted rats direct macrophages toward an M2 phenotype. This phenomenon warrants further investigation.
In summary, the principal findings of this article are as follows: (i) among the synthetic polymers analyzed, PHB shows the greatest biocompatibility in terms of degradation in vivo, angiogenic capacity, and prevention of negative remodeling in infarcted tissue; (ii) PHB induced a significant expression of IL-6 in PBMNCs, which might explain the increased angiogenic capacity of PHB meshes and the polarization of immune response toward graft acceptance; and (iii) PCL is the most suitable polymer for cell retention and spreading although, given its marked ability to induce inflammatory responses, it is probably not suitable for cardiac repair when used as pure electrospun sheets. Thus, through a direct comparison of polymer substrates widely used in tissue engineering, we provide the first demonstration that poly(hydroxybutyrate) is a superior scaffold for cardiac repair. The main attributes of this material include increased cell affinity, induction of cytokine production, a permissive inflammatory response, and stimulation of tissue remodeling. Further investigations will be required to determine whether PHB patches, perhaps seeded with cells or therapeutic drugs, provide a greater alternative to existing methods. Nevertheless, our results identify PHB as a capable substrate for therapeutic intervention.
Supplementary Material
Acknowledgments
This study was supported in part by grants from Aitex, and RETICS program from Instituto de Salud Carlos III (RD12/0019/0025). D.C. is supported by an INNPACTO grant (Ministerio de Ciencia e Innovacion, Spain and European Union (FEDER). P.S. acknowledges support from Miguel Servet I3 SNS Program (ISCIII) and PI13/0414 grant. B.P. acknowledges support from ISCIII (PI10/01621, CP09/00333). The authors thank R. Carrero and L. Pardo for technical assistance, and A. Hernández at the Core facility of confocal microscopy-Centro de Investigación Príncipe Felipe. We thank Dr. Claycomb (Louisiana State University Medical Center, United States) for HL-1 cell line donation. We are grateful to all patients and our clinical colleague Dr. Luis Larrea, who donated or collected clinical samples.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Rane AA. and Christman KL. (2011). Biomaterials for the treatment of myocardial infarction: a 5-year update. J Am Coll Cardiol 58:2615–2629 [DOI] [PubMed] [Google Scholar]
- 2.Pok S. and Jacot JG. (2011). Biomaterials advances in patches for congenital heart defect repair. J Cardiovasc Transl Res 4:646–654 [DOI] [PubMed] [Google Scholar]
- 3.Kofidis T, Lebl DR, Martinez EC, Hoyt G, Tanaka M. and Robbins RC. (2005). Novel injectable bioartificial tissue facilitates targeted, less invasive, large-scale tissue restoration on the beating heart after myocardial injury. Circulation 112:I173–I177 [DOI] [PubMed] [Google Scholar]
- 4.Ozawa T, Mickle DA, Weisel RD, Koyama N, Wong H, Ozawa S. and Li RK. (2002). Histologic changes of nonbiodegradable and biodegradable biomaterials used to repair right ventricular heart defects in rats. J Thorac Cardiovasc Surg 124:1157–1164 [DOI] [PubMed] [Google Scholar]
- 5.Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, Cohen S. and Leor J. (2008). Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation 117:1388–1396 [DOI] [PubMed] [Google Scholar]
- 6.Orlova Y, Magome N, Liu L, Chen Y. and Agladze K. (2011). Electrospun nanofibers as a tool for architecture control in engineered cardiac tissue. Biomaterials 32:5615–5624 [DOI] [PubMed] [Google Scholar]
- 7.Sill TJ. and von Recum HA. (2008). Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 29:1989–2006 [DOI] [PubMed] [Google Scholar]
- 8.Badylak SF. (2007). The extracellular matrix as a biologic scaffold material. Biomaterials 28:3587–3593 [DOI] [PubMed] [Google Scholar]
- 9.Kadner K, Dobner S, Franz T, Bezuidenhout D, Sirry MS, Zilla P. and Davies NH. (2012). The beneficial effects of deferred delivery on the efficiency of hydrogel therapy post myocardial infarction. Biomaterials 33:2060–2066 [DOI] [PubMed] [Google Scholar]
- 10.Miyagi Y, Chiu LL, Cimini M, Weisel RD, Radisic M. and Li RK. (2011). Biodegradable collagen patch with covalently immobilized VEGF for myocardial repair. Biomaterials 32:1280–1290 [DOI] [PubMed] [Google Scholar]
- 11.Patra C, Talukdar S, Novoyatleva T, Velagala SR, Muhlfeld C, Kundu B, Kundu SC. and Engel FB. (2012). Silk protein fibroin from Antheraea mylitta for cardiac tissue engineering. Biomaterials 33:2673–2680 [DOI] [PubMed] [Google Scholar]
- 12.Karam JP, Muscari C. and Montero-Menei CN. (2012). Combining adult stem cells and polymeric devices for tissue engineering in infarcted myocardium. Biomaterials 33:5683–5695 [DOI] [PubMed] [Google Scholar]
- 13.Segers VF. and Lee RT. (2011). Biomaterials to enhance stem cell function in the heart. Circ Res 109:910–922 [DOI] [PubMed] [Google Scholar]
- 14.Habib M, Shapira-Schweitzer K, Caspi O, Gepstein A, Arbel G, Aronson D, Seliktar D. and Gepstein L. (2011). A combined cell therapy and in-situ tissue-engineering approach for myocardial repair. Biomaterials 32:7514–7523 [DOI] [PubMed] [Google Scholar]
- 15.Liu Z, Wang H, Wang Y, Lin Q, Yao A, Cao F, Li D, Zhou J, Duan C, Du Z. and Wang C. (2012). The influence of chitosan hydrogel on stem cell engraftment, survival and homing in the ischemic myocardial microenvironment. Biomaterials 33:3093–3106 [DOI] [PubMed] [Google Scholar]
- 16.Gupta MK, Walthall JM, Venkataraman R, Crowder SW, Jung DK, Yu SS, Feaster TK, Wang X, Giorgio TD, et al. (2011). Combinatorial polymer electrospun matrices promote physiologically-relevant cardiomyogenic stem cell differentiation. PLoS One 6:e28935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozawa T, Mickle DA, Weisel RD, Koyama N, Ozawa S. and Li RK. (2002). Optimal biomaterial for creation of autologous cardiac grafts. Circulation 106:I176–I182 [PubMed] [Google Scholar]
- 18.Gardner AB, Lee SK, Woods EC. and Acharya AP. (2013). Biomaterials-based modulation of the immune system. BioMed Res Int 2013:732182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daghighi S, Sjollema J, van der Mei HC, Busscher HJ. and Rochford ET. (2013). Infection resistance of degradable versus non-degradable biomaterials: an assessment of the potential mechanisms. Biomaterials 34:8013–8017 [DOI] [PubMed] [Google Scholar]
- 20.Freytes DO, Santambrogio L. and Vunjak-Novakovic G. (2012). Optimizing dynamic interactions between a cardiac patch and inflammatory host cells. Cells Tissues Organs 195:171–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reneker DH. and Chun I. (1996). Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 7:216–223 [Google Scholar]
- 22.Aznar-Cervantes S, Roca MI, Martinez JG, Meseguer-Olmo L, Cenis JL, Moraleda JM. and Otero TF. (2012). Fabrication of conductive electrospun silk fibroin scaffolds by coating with polypyrrole for biomedical applications. Bioelectrochemistry 85:36–43 [DOI] [PubMed] [Google Scholar]
- 23.Sanchis RM, Calvo O, Sánchez L, García D. and Balart R. (2007). Enhancement of wettability in low density polyethylene films using low pressure glow discharge N2 plasma. J Polym Sci Part B 45:2390–2399 [Google Scholar]
- 24.Arana M, Pena E, Abizanda G, Cilla M, Ochoa I, Gavira JJ, Espinosa G, Doblare M, Pelacho B. and Prosper F. (2013). Preparation and characterization of collagen-based ADSC-carrier sheets for cardiovascular application. Acta Biomater 9:6075–6083 [DOI] [PubMed] [Google Scholar]
- 25.Claycomb WC, Lanson NA, Jr., Stallworth BS, Egeland DB, Delcarpio JB, Bahinski A. and Izzo NJ., Jr. (1998). HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A 95:2979–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arminan A, Gandia C, Bartual M, Garcia-Verdugo JM, Lledo E, Mirabet V, Llop M, Barea J, Montero JA. and Sepulveda P. (2009). Cardiac differentiation is driven by NKX2.5 and GATA4 nuclear translocation in tissue-specific mesenchymal stem cells. Stem Cells Dev 18:907–918 [DOI] [PubMed] [Google Scholar]
- 27.Gandia C, Arminan A, Garcia-Verdugo JM, Lledo E, Ruiz A, Minana MD, Sanchez-Torrijos J, Paya R, Mirabet V, et al. (2008). Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells 26:638–645 [DOI] [PubMed] [Google Scholar]
- 28.Arminan A, Gandia C, Garcia-Verdugo JM, Lledo E, Trigueros C, Ruiz-Sauri A, Minana MD, Solves P, Paya R, Montero JA. and Sepulveda P. (2010). Mesenchymal stem cells provide better results than hematopoietic precursors for the treatment of myocardial infarction. J Am Coll Cardiol 55:2244–2253 [DOI] [PubMed] [Google Scholar]
- 29.Cortes-Morichetti M, Frati G, Schussler O, Duong Van Huyen JP, Lauret E, Genovese JA, Carpentier AF. and Chachques JC. (2007). Association between a cell-seeded collagen matrix and cellular cardiomyoplasty for myocardial support and regeneration. Tissue Eng 13:2681–2687 [DOI] [PubMed] [Google Scholar]
- 30.Harel-Adar T, Ben Mordechai T, Amsalem Y, Feinberg MS, Leor J. and Cohen S. (2011). Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc Natl Acad Sci U S A 108:1827–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin M, Ishii O, Sueda T. and Vacanti JP. (2004). Contractile cardiac grafts using a novel nanofibrous mesh. Biomaterials 25:3717–3723 [DOI] [PubMed] [Google Scholar]
- 32.Piao H, Kwon JS, Piao S, Sohn JH, Lee YS, Bae JW, Hwang KK, Kim DW, Jeon O, et al. (2007). Effects of cardiac patches engineered with bone marrow-derived mononuclear cells and PGCL scaffolds in a rat myocardial infarction model. Biomaterials 28:641–649 [DOI] [PubMed] [Google Scholar]
- 33.Min BM, Lee G, Kim SH, Nam YS, Lee TS. and Park WH. (2004). Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials 25:1289–1297 [DOI] [PubMed] [Google Scholar]
- 34.Hong Y, Huber A, Takanari K, Amoroso NJ, Hashizume R, Badylak SF. and Wagner WR. (2011). Mechanical properties and in vivo behavior of a biodegradable synthetic polymer microfiber-extracellular matrix hydrogel biohybrid scaffold. Biomaterials 32:3387–3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nikolovski J. and Mooney DJ. (2000). Smooth muscle cell adhesion to tissue engineering scaffolds. Biomaterials 21:2025–2032 [DOI] [PubMed] [Google Scholar]
- 36.Asran A, Razghandi K, Aggarwal N, Michler GH. and Groth T. (2010). Nanofibers from blends of polyvinyl alcohol and polyhydroxy butyrate as potential scaffold material for tissue engineering of skin. Biomacromolecules 11:3413–3421 [DOI] [PubMed] [Google Scholar]
- 37.Heo DN, Lee JB, Bae MS, Hwang YS, Kwon KH. and Kwon IK. (2011). Development of nanofiber coated indomethacin-eluting stent for tracheal regeneration. J Nanosci Nanotechnol 11:5711–5716 [DOI] [PubMed] [Google Scholar]
- 38.Vacanti NM, Cheng H, Hill PS, Guerreiro JD, Dang TT, Ma M, Watson S, Hwang NS, Langer R. and Anderson DG. (2012). Localized delivery of dexamethasone from electrospun fibers reduces the foreign body response. Biomacromolecules 13:3031–3038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujimoto KL, Tobita K, Merryman WD, Guan J, Momoi N, Stolz DB, Sacks MS, Keller BB. and Wagner WR. (2007). An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction. J Am Coll Cardiol 49:2292–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chachques JC, Trainini JC, Lago N, Cortes-Morichetti M, Schussler O. and Carpentier A. (2008). Myocardial assistance by grafting a new bioartificial upgraded myocardium (MAGNUM trial): clinical feasibility study. Ann Thorac Surg 85:901–908 [DOI] [PubMed] [Google Scholar]
- 41.Tan MY, Zhi W, Wei RQ, Huang YC, Zhou KP, Tan B, Deng L, Luo JC, Li XQ, Xie HQ. and Yang ZM. (2009). Repair of infarcted myocardium using mesenchymal stem cell seeded small intestinal submucosa in rabbits. Biomaterials 30:3234–3240 [DOI] [PubMed] [Google Scholar]
- 42.Danoviz ME, Nakamuta JS, Marques FL, dos Santos L, Alvarenga EC, dos Santos AA, Antonio EL, Schettert IT, Tucci PJ. and Krieger JE. (2010). Rat adipose tissue-derived stem cells transplantation attenuates cardiac dysfunction post infarction and biopolymers enhance cell retention. PLoS One 5:e12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Callegari A, Bollini S, Iop L, Chiavegato A, Torregrossa G, Pozzobon M, Gerosa G, De Coppi P, Elvassore N. and Sartore S. (2007). Neovascularization induced by porous collagen scaffold implanted on intact and cryoinjured rat hearts. Biomaterials 28:5449–5461 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.