Synopsis
During cardiac remodeling, the heart generates higher levels of reactive species; yet an intermediate “compensatory” stage of hypertrophy is associated with a greater ability to withstand oxidative stress. The mechanisms underlying this protected myocardial phenotype are poorly understood. We examined how a cellular model of hypertrophy deals with electrophilic insults, such as would occur upon ischemia or in the failing heart. For this, we measured energetics in control and phenylephrine (PE)-treated rat neonatal cardiac myocytes (NRCMs) under basal conditions and when stressed with 4-hydroxynonenal (HNE). PE treatment caused hypertrophy as indicated by augmented atrial natriuretic peptide and increased cellular protein content. Hypertrophied myocytes demonstrated a 2.5-fold increase in ATP-linked oxygen consumption and a robust augmentation of oligomycin-stimulated glycolytic flux and lactate production. Hypertrophied myocytes displayed a protected phenotype that was resistant to HNE-induced cell death and a unique bioenergetic response characterized by a delayed and abrogated rate of oxygen consumption and a twofold increase in glycolysis upon HNE exposure. This augmentation of glycolytic flux was not due to increased glucose uptake, suggesting that electrophile stress results in utilization of intracellular glycogen stores to support the increased energy demand. Hypertrophied myocytes also had an increased propensity to oxidize HNE to 4-hydroxynonenoic acid and sustained less protein damage due to acute HNE insults. Inhibition of aldehyde dehydrogenase resulted in bioenergetic collapse when myocytes were challenged with HNE. The integration of electrophile metabolism with glycolytic and mitochondrial energy production appears to be important for maintaining myocyte homeostasis under conditions of increased oxidative stress.
Keywords: cardiac hypertrophy, mitochondria, glycolysis, aldehyde dehydrogenase, extracellular flux, 4-hydroxynonenal
Introduction
Electrophilic aldehydes generated from the oxidation of lipids have been detected in nearly all tissues that have experienced oxidative injury. In the heart, they are found in the context of ischemia [1–3], hypertrophy [4–6], and failure [7, 8] and have been classically used as indicators of oxidative stress. The presence of protein adducts with 4-hydroxynonenal (HNE) correlate well with the severity of diastolic dysfunction [9], are localized to myocytes [8], and are consistently elevated and associated with impairment of left ventricular contractile function in heart failure patients [7]. These findings suggest that aldehydes may be active instruments of injury rather than passive footprints of oxidative damage. Nevertheless, how the heart deals with such electrophilic stress is not well understood.
General oxidative stress characterized by increased dihydroethidium staining and the presence of protein-HNE adducts are found relatively early after suprarenal aortic constriction [4]. Similarly, volume overload induced by aortocaval shunting in pigs results in an early increase in aldehydes such as malondialdehyde (MDA) and HNE (peaking at 48 h of overload) [5]. Interestingly, this initial onslaught of reactive species appears to be followed by a transient cardiac phenotype that is relatively protected from redox stress. For example, a model of stable hypertrophy was shown to resist injury caused by ischemia-reperfusion [10, 11] or by perfusion with reactive oxygen species (ROS) [12]; this protection was shown to be due, in part, to heightened antioxidant defenses [10, 11]. The failing heart, however, appears to have lost this adaptation to redox stress, as implicated by a considerable increase in reactive species, oxidative protein damage, and functional decline [7–9, 13].
Remarkable changes in myocardial energetics also occur in the course of cardiac remodeling (reviewed in [14, 15]); however, little is known about how the remodeled myocyte responds to the reactive species generated in the context of ischemia or failure. Understanding this response and how it changes in the course of disease is therefore important because it could lend insight into the mechanisms of myocardial dysfunction and failure. Based on our previous studies, we posit that a major factor underlying aldehyde toxicity stems from their effects on cellular bioenergetics. Isolated cardiomyocytes treated with HNE and subjected to extracellular flux analyses were shown to utilize more oxygen than untreated or nonanal (a non-reactive analog of HNE)-treated cells [16], which matches the pattern of increased oxygen consumption occurring in the post-ischemic [17, 18], hypertrophic, and failing heart [19–21]. This increase in oxygen consumption was due to proton leak as well as ATP-linked processes, suggesting that myocytes respond to aldehydic insults by attempting to increase ATP generation [16]. Thus, it appears that HNE increases cardiomyocyte energy demand and decreases mitochondrial efficiency, which could contribute significantly to cardiomyopathy.
A particularly intriguing hypothesis states that the increased demands of the failing heart lead to a state of energy depletion, through an initial compensatory phase of increased oxygen utilization [22, 23]. With respect to this hypothesis, it is not well understood how mounting oxidative stress or acute insults are handled in the hypertrophied or otherwise “pre-failing” heart. This led us to question how the hypertrophied myocyte handles products generated during oxidative stress, such as HNE. In the present study, we examined energetics in a phenylephrine (PE)-induced cardiomyocyte model of hypertrophy and measured HNE metabolism and the bioenergetic responses of the hypertrophied myocytes to HNE. Our findings suggest that PE-induced cardiomyocyte hypertrophy prevents HNE-induced damage through mechanisms relating to an increased glycolytic reserve and an augmented ability to detoxify lipid-derived aldehydes.
Experimental
Materials
Antimycin A, FCCP (carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone), oligomycin, phenylephrine, mammalian protease inhibitor cocktail and cyanamide were from Sigma (St. Louis, MO, USA) and of the highest grade offered. HNE and 3H-HNE were prepared on site as previously described [24]. Koningic acid was from TMS Co., Ltd. (Tokyo, Japan). Tritiated 2-deoxyglucose was from Moravek Biochemicals (Brea, CA). The DC Lowry Protein Assay® was from Biorad (Hercules, CA, USA).
Cardiac myocyte isolation and culture
Neonatal rat cardiac myocytes (NRCM) were isolated from 1- or 2-day–old Sprague-Dawley rats as described [25–28]. The myocytes were then treated with 100 μM phenylephrine (PE) for 48 h to induce hypertrophy [29, 30].
Reverse transcriptase PCR and quantitative real-time PCR
Atrial natriuretic protein (ANP) expression was measured by PCR as described in [31]. Total RNA from isolated cardiomyocytes was extracted with Trizol (Invitrogen, Carlsbad, CA). Total RNA levels were quantified using the ratio of absorbance at 260 nm to 280 nm with the NanoDrop™ 1000 Spectrophotometer (Thermo Scientific). To verify purity of the sample the absorbance ratio from 260 nm to 230 nm (A260/A230) was used. We limited the use of RNA with 260/230 ratio greater than 1.8. Total RNA (1 μg) then was subjected to reverse transcriptase (20 μl final volume) reaction for 30 minutes to synthesize cDNA using IScript™ cDNA synthesis kit (BioRad, Herculus, CA). The sequences used include: ANP sense cctgtgtacagtgcggtgtc; ANP anti-sense aagctgttgcagcctagtcc; ribosomal 18s sense aaacggctaccacatccaag; and ribosomal 18s anti-sense cctccaatggatcctcgtta. The relative level of mRNA transcripts was quantified by real-time PCR using SYBR® Green (BioRad, Herculus, CA). The data generated was normalized to 18s ribosomal RNA threshold cycle (CT) values using the ΔΔCT comparative method.
Measurement of cellular energetics using the XF24 extracellular flux analyzer
Cellular energetics were measured in intact NRCMs using a Seahorse Bioscience XF24 extracellular flux analyzer (Billerica, MA) as described previously [16]. To allow comparison between experiments, data are expressed as the rate of oxygen consumption (OCR) in pmol/min and the rate of proton production (PPR) in H+/min. For glycolysis assays, we used koningic acid (KA) to ensure that changes in extracellular acidification were due to glycolysis. For this, three baseline measurements were recorded, followed by the sequential injection of oligomycin (1 μg/ml) and KA (10 μg/ml). Glycolytic flux data were then reported as KA-inhibitable PPR. In some experiments, the data were normalized to the amount of protein present in the well of the microplate. For this, cells were lysed in the 24-well XF plates using 20 μl/well of lysis buffer containing 20 mM HEPES, pH 7.0, 1 mM EDTA, 1% NP-40, 0.1% SDS and protease inhibitor cocktail followed by protein assay.
Radiolabeled Glucose Uptake
Glucose uptake in intact NRCMs was determined using 3H-2-deoxy-D-glucose (3H-DOG). After 48 h of incubation in the absence or presence of PE, the medium was removed and replaced with fresh, serum-free DMEM (5.5 mM glucose) containing 3H-DOG (2 μCi/ml) and vehicle (ethanol), HNE (20 μM), or oligomycin (1 μg/ml). The NRCMs were then incubated at 37°C for 3 h. Following incubation, the cells were washed with ice-cold PBS six times to remove adherent radioactivity. The cells were then lysed with lysis buffer containing 20 mM HEPES, pH 7.0, 1 mM EDTA, 1% NP-40, 0.1% SDS, and centrifuged at 13,000xg for 10 min. The supernatant was collected and a small amount was used to determine the protein concentration by the Lowry DC method. The radioactivity was then measured by scintillation counting and normalized to protein.
Lactate Assay
Following 48 h of culture in the absence or presence of PE, the medium was removed and replaced with fresh, serum-free DMEM (5.5 mM glucose) containing vehicle (ethanol), HNE (20 μM), or oligomycin (1 μg/ml). The NRCMs were then incubated at 37°C for 3 h. Following incubation, 50 μl of medium was used to measure lactate levels using a Lactate Assay Kit (Eton Bioscience Inc., San Diego, CA).
Metabolism of HNE in NRCMs
Three to five days after isolation, NRCMs were exposed for 1 h to 3H-HNE (15 μM HNE) in modified Kreb’s Hensleit (KH) buffer, pH 7.4, containing in mM: 10 HEPES, 111 NaCl, 4.7 KCl, 2.0 MgSO4, 1.2 Na2HPO4, 1.2 CaCl2, 5.5 D-glucose, 1.0 pyruvate. The incubation medium was then filtered through a 0.2 μM filter and unmetabolized HNE and metabolic products of HNE in the incubation medium were resolved by HPLC and quantified by scintillation counting essentially as described in [3, 24]. To determine protein-bound HNE, the cells were scraped in 6% perchloric acid and the protein pellets were obtained by centrifugation at 13,000×g for 10 min. The pellets were then washed with acetone until no radioactivity was detected in the wash. Finally, the protein was resuspended in 100 μl 0.5 M Tris, pH 8.8, containing 1% SDS, the protein concentration was measured by the Lowry DC method, and the radioactivity in the protein sample was measured by scintillation counting.
Statistical analyses
Data are reported as means ± SEM. Comparisons between two groups were performed with Student’s t-test. Multigroup comparisons were performed using one-way ANOVA, with Bonferroni correction. The null hypothesis was rejected when p < 0.05.
Results
Effects of cardiomyocyte hypertrophy on cellular energetics
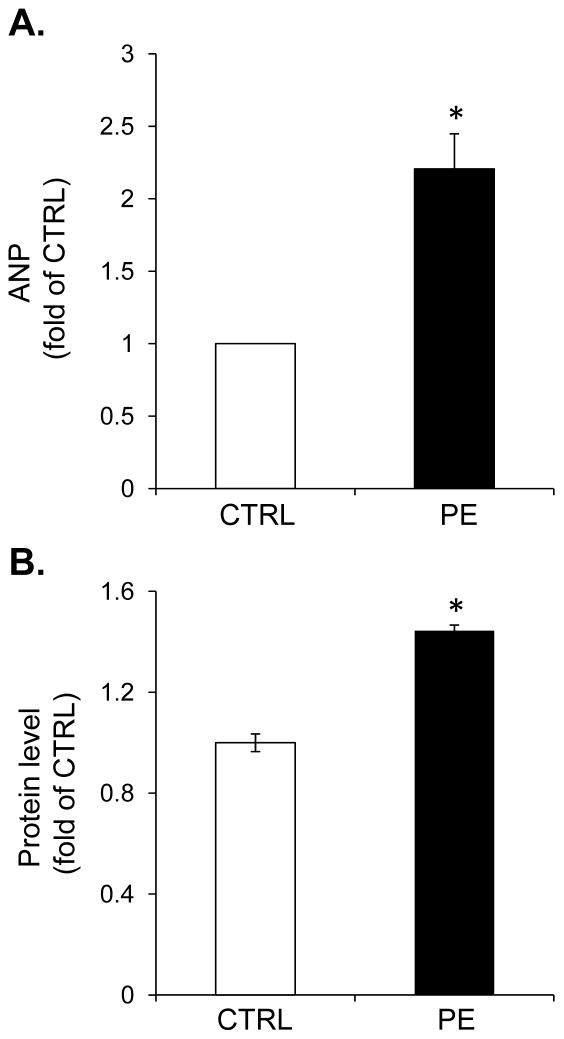
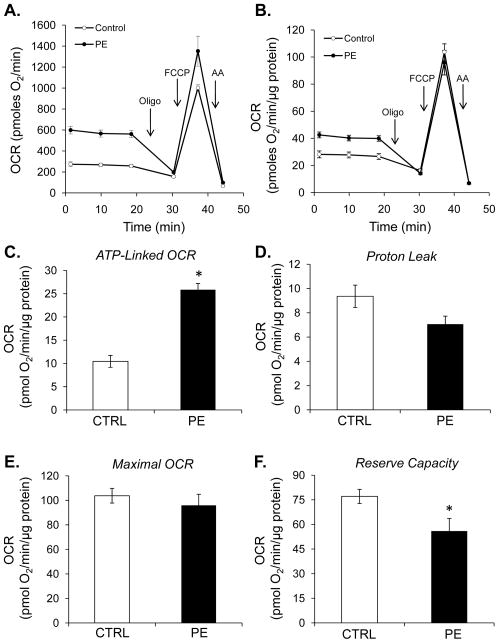
Extracellular flux analyses were used to determine the effects of phenylephrine (PE)-induced hypertrophy on cardiomyocyte energetics. NRCMs were treated for 48 h with phenylephrine to induce hypertrophy. As shown in Fig. 1A and B, PE increased transcription of a marker of hypertrophy, atrial natriuretic peptide (ANP), and increased total cellular protein content. After PE exposure, the cells were washed with DMEM and the energetic assays were performed as described in [16]. As shown in Fig. 2A and B, cells treated with PE consumed significantly more oxygen than controls cells at baseline. Addition of oligomycin was used to determine whether this was due to ATP-linked or non-ATP-linked changes in oxygen consumption. As shown in Fig. 2C, the increase in oxygen consumption was due to a 2.5-fold increase in ATP-linked oxygen demand. Non-ATP-linked oxygen consumption, which is likely due to proton leak [16, 32], was not significantly changed by PE treatment (Fig. 2D). While maximal oxygen consumption rates were not changed with PE (Figs. 2B and 2E), the reserve capacity was decreased (Fig. 2F), which appears to be due to the increase in ATP-linked oxygen demand.
Figure 1. Markers of myocyte hypertrophy in control and phenylephrine-treated myocytes.
Isolated cardiomyocytes were exposed to phenylephrine (PE, 100 μM) for 48 h, and markers of hypertrophy were measured. (A) Atrial natriuretic peptide (ANP) message as detected by pPCR. (B) Total cellular protein normalized to control cells. n = 3 pergroup, *p<0.05.
Figure 2. Hypertrophied myocytes demonstrate an increase in ATP-linked oxygen consumption.
Extracellular flux analysis of NRCMs: (A) Oxygen consumption rates (OCR) in control and PE-treated cells. After three baseline measurements, oligomycin (Oligo; 1 μg/ml), FCCP (1 μM), and antimycin A (AA; 10 μM) were injected to assess mitochondrial efficiency, maximal respiratory rate, and non-mitochondrial oxygen consumption. Panel B shows data normalized to total protein. (C) Measurements of ATP-linked oxygen consumption rate (OCR), (D) proton leak, (E) maximal OCR, and (F) mitochondrial reserve capacity. n = 5 per group, *p<0.05.
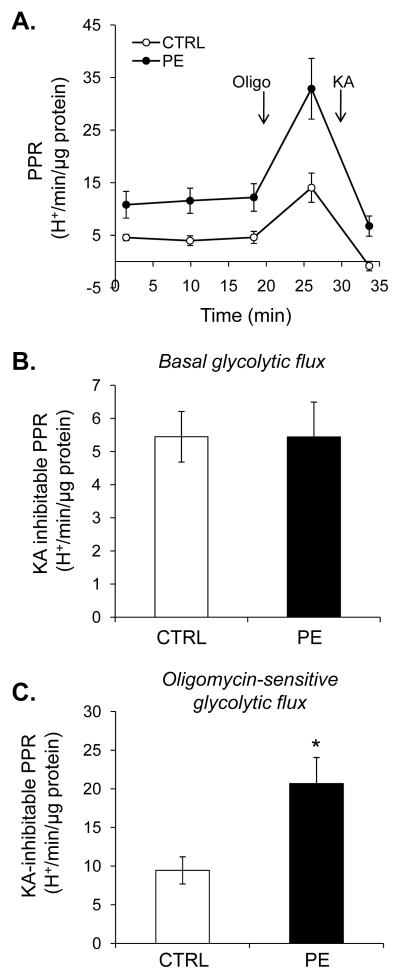
To measure changes in glycolysis, the proton production rate (PPR) was used as a surrogate marker of glycolytic flux [33]. To aid in confirming that the changes in PPR were representative of glycolysis, we incorporated the use of koningic acid (KA) in our glycolysis assays. Briefly, KA is a sesquiterpene antibiotic that forms a covalent adduct with Cys-149 of glyceraldehyde-3-phosphate dehydrogenase (GAPDH), thereby inhibiting the enzyme and glycolysis [34–36]. For these assays, three baseline PPR measurements were recorded, followed by addition of oligomycin. After one measurement, koningic acid was then injected to inhibit glycolysis, and one more PPR was recorded (Fig. 3A). KA-inhibitable PPR was then used to measure changes in glycolytic flux. Although hypertrophied myocytes showed no increase in basal, KA-inhibitable glycolysis (Fig. 3B), PPR was increased more than twofold when mitochondrial ATP production was inhibited with oligomycin (Figs. 3C).
Figure 3. Myocyte hypertrophy results in an increased ability to augment glycolysis.
Extracellular flux analysis of NRCMs: (A) Proton production rates (PPR) of control and PE-treated cells. After three baseline measurements, oligomycin (Oligo; 1 μg/ml) and koningic acid (KA; 10 μg/ml) were injected to assess KA-inhibitable basal (panel B) and oligomycin-stimulated (panel C) glycolytic flux. n = 5 per group, *p<0.05
Cell death induced by electrophile stress
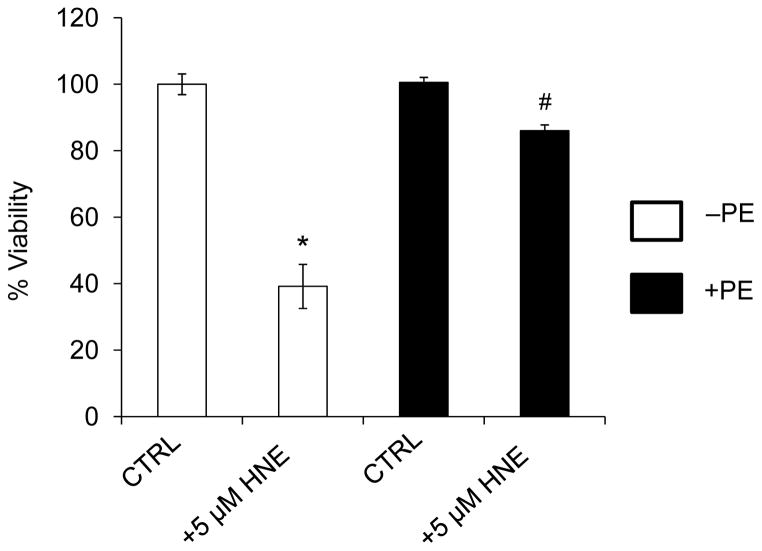
In previous studies, we examined cell death in NRCMs exposed to HNE and found that incubation of cells with 5 μM HNE for 16 h resulted in ~50% cell death [16]. Using identical conditions, HNE-induced cell death was examined in control and hypertrophied myocytes. As shown in Fig. 4, 16 h of HNE treatment resulted in ~60% loss in cell viability in control myocytes; however, PE-treated myocytes showed a marked protection against cell death caused by HNE.
Figure 4. Phenylephrine-hypertrophied myocytes are protected from HNE-induced cell death.
NRCMs were treated without or with PE (100 μM) for 48 h followed by exposure to HNE (5 μM) for 16 h. Cell viability was then measured by MTT assay. n = 5 per group, *p<0.05.
Energetic responses of hypertrophied myocytes to HNE
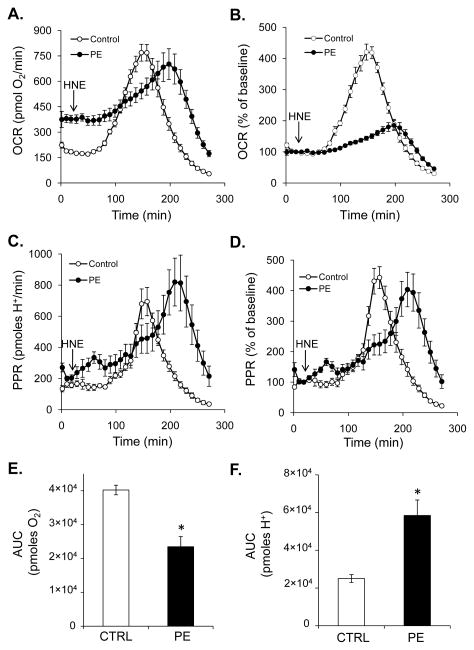
Because hypertrophied myocytes had a significantly different energetic profile compared to normal myocytes (Figs. 2 and 3), we next questioned how energetics would change when these myocytes are challenged with HNE. After three baseline measurements, control and PE-hypertrophied myocytes were treated with HNE and the rates of oxygen consumption and proton production (PPR) were measured in time. As shown in Fig. 5A and C, HNE led to a transient, robust increase in OCR and PPR which was followed by rates that fell below baseline. Interestingly, the response of NRCMs that were treated with PE for 48 h differed both temporally and in a manner of extent. The bioenergetic rates in PE-treated myocytes exposed to HNE peaked 40–50 min after that of non-hypertrophied cells (Fig. 5A and C). Shown in Fig. 5B and D are OCR and ECAR traces normalized to baseline energetic rates. Interestingly, the extent to which HNE increased oxygen consumption was largely diminished, but the increase in glycolysis appeared to be conserved. To quantify the overall changes in respiratory activity and glycolysis in control and PE-treated myocytes, the area under the curve (AUC) from baseline was determined. As shown in Fig. 5E and F, PE-treated myocytes used almost twofold less oxygen in the response to HNE and increased their overall rate of glycolytic flux after HNE exposure, as indicated by a 2.3-fold increase in proton production. Pretreatment of control and hypertrophied NRCMs with koningic acid prior to HNE exposure inhibited HNE-induced changes in oxygen consumption and glycolysis (Supplementary Fig. 1).
Figure 5. Energetic responses of normal and hypertrophied myocytes to HNE.
Extracellular flux analyses of NRCMs treated with HNE. After three baseline measurements, the cells were exposed to HNE (20 μM) for the time shown. (A and B) Oxygen consumption rates (OCR) of NRCMs: panel A, raw OCR data; panel B, data shown as percent of baseline. (C and D) Proton production rates (PPR) indicative of glycolysis in NRCMs: panel C, raw PPR data; panel D, PPR shown as percent of baseline values. (E and F) Area under the curve (AUC) analyses of oxygen consumption (panel E) and proton production (panel F) were used to determine the amount of oxygen consumed and protons produced with each treatment. n = 5 per group, *p<0.05.
Effects of HNE on glycolysis and glucose uptake in control and hypertrophied myocytes
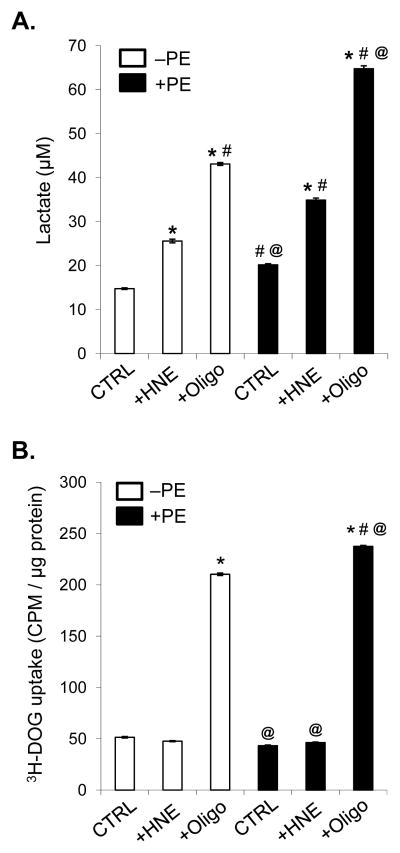
To further determine whether the apparent increase in glycolysis as measured by PPR in Fig. 5B, D, and F was due to increased glycolysis, we measured lactate production in cells treated for 3 h with HNE. As shown in Fig. 6A, HNE treatment led to a 1.7-fold increase in lactate production in control cells. Addition of oligomycin to control cells for the 3 h period resulted in a nearly threefold increase in lactate production; this finding is in agreement with the increase in PPR upon oligomycin treatment shown in Fig. 3. Hypertrophied myocytes showed only a modest increase in lactate production compared with controls cells; however, lactate production was increased by 2.4-fold in hypertrophied cells treated with HNE, affirming that hypertrophied myocytes, as compared with control myocytes, utilize glycolysis to a greater extent when challenged with HNE. Oligomycin treatment of hypertrophied cells resulted in a 4.4-fold increase in lactate production over control (non-PE-treated) myocytes, and a 1.5-fold increase in lactate production over oligomycin-treated, non-hypertrophied cells.
Figure 6. Lactate production and 2-deoxyglucose uptake in NRCMs.
NRCMs were treated without (white bars) or with PE (100 μM; black bars) for 48 h followed by incubation in DMEM (5.5 mM D-glucose) in the absence or presence of HNE (20 μM; +HNE) or oligomycin (1 μg/ml; +Oligo). (A) Lactate concentration in the medium after 3 h of incubation. (B) 3H-2-deoxyglucose (3H-DOG) uptake in NRCMs: NRCMs were treated identically to cells in panel A, and 3H-DOG (0.2 μCi/ml) was included in the medium. Radioactivity in the cells was then measured and normalized to total protein. n = 3–4 per group; P<0.0001 (one-way ANOVA); *p<0.05 vs. CTRL (white bar); #p<0.05 vs. +HNE (white bar); @p<0.05 vs. +Oligo (white bar).
To determine if HNE and oligomycin increase glucose uptake by NRCMs, we measured 3H-DOG uptake in non-hypertrophied and hypertrophied myocytes treated without or with HNE or oligomycin. Interestingly, HNE treatment did not affect 3H-DOG uptake in either control or PE-treated NRCMs (Fig. 6B); however, oligomycin treatment in both non-PE and PE-treated cells increased 3H-DOG uptake by more than fourfold.
Metabolism of HNE in hypertrophied NRCMs
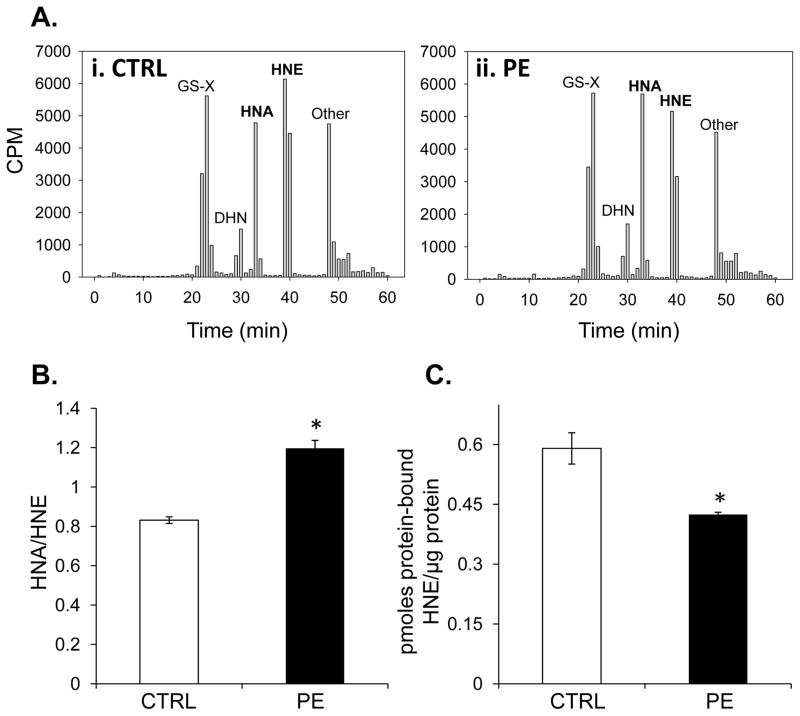
Next, we examined HNE metabolism in control and hypertrophied myocytes. As shown in Table 1 and Fig. 7A, the largest route for HNE metabolism in control and PE-treated myocytes was via glutathione conjugation, followed by oxidation to HNE and reduction to DHN. The most striking difference between control and PE-treated myocytes was the formation of HNA, such that the HNA to HNE ratios were significantly different between the groups (Fig. 7B). Interestingly, this difference in metabolism was associated with a reduction in the amount of protein-bound HNE in the hypertrophied myocytes (Fig. 7C), suggesting that PE-hypertrophied myocytes incur less aldehyde-induced protein damage, which may be attributed to greater dehydrogenase-mediated HNE oxidation.
Table 1.
HNE metabolism in control and phenylephrine-treated myocytes1.
| Metabolite2 | Control myocytes (% metabolism) | Hypertrophied myocytes (% metabolism) |
|---|---|---|
| GS-X | 30.0±1.1 | 31.1±1.0 |
| DHN | 6.7±0.5 | 7.7±0.5 |
| HNA | 17.5±1.4 | 21.0±1.4 |
| HNE | 24.9±2.4 | 20.9±1.9 |
| Other | 20.9±0.5 | 19.3±0.9 |
NRCMs were treated for 48 h in the absence or presence of 100 μM phenylephrine to induce hypertrophy. The cells were then exposed to 15 μM 3H-HNE for 1h at 37°C. Metabolites in the media were then resolved by HPLC and quantified by scintillation counting. n = 4 (separate isolations per group) ± SEM.
GS-X represents the glutathione conjugates of HNE (including GS-HNE and GS-DHN); DHN represents dihydroxynonene, HNA represent 4-hydroxynonenoic acid, and HNE is unmetabolized 4-hydroxynonenal.
Figure 7. HNE metabolism in control and hypertrophied cardiomyocytes.
Isolated NRCMs were exposed to 3H-HNE (15 μM; ~70,000 CPM/well) for 1 h and the metabolites in the medium were resolved by HPLC and quantified by scintillation counting. (A) Representative radioplots of metabolites extruded by NRCMs treated with radiolabeled HNE: panel i, control NRCMs and panel ii, phenylephrine (PE; 100 μM)-treated myocytes. (B) Ratio of HNA to HNE from group data. N = 4/group, *p<0.05. (C) Protein-bound 3H-HNE quantified from protein isolated from 3H-HNE myocytes. n = 4 per group, *p<0.05.
To test whether loss of aldehyde dehydrogenase activity would affect the bioenergetic response to HNE, we performed experiments using HNE-treated NRCMs in the absence or presence of cyanamide, an inhibitor of aldehyde dehydrogenase. Three baseline oxygen consumption and extracellular acidification rates were recorded, followed by addition of vehicle or cyanamide to the cells. Two more rates were recorded, and then vehicle or HNE was added to the cells for the indicated time. As shown in Supplementary Fig. 2A, addition of cyanamide alone had a modest effect on OCR, but a much greater effect was observed after HNE addition. As previously shown [16], HNE increased the rate of oxygen consumption by more than twofold. Pretreatment of the cells with cyanamide, however, hastened the increase in oxygen consumption and promoted a sudden bioenergetic collapse. Analogous results are shown in the glycolytic flux assays shown in Supplementary Fig. 2B. Interestingly, cyanamide alone increased glycolytic flux by approximately threefold. An even higher transient increase in extracellular acidification rate is shown in the cyanamide+HNE trace; this was followed by an abrupt loss of all energetic activity.
Discussion
Cardiac hypertrophy is an important compensatory response to physiologic and pathological stimuli. A variety of hormonal and hemodynamic stimuli drive the hypertrophic program, leading to increases in myofibrillar content (rather than proliferation) and changes in metabolic phenotype. In particular, the hypertrophy of myocytes occurs concomitantly with an increase in glucose uptake and glycolysis and a later decrease in overall fatty acid metabolism [14, 37]. This switch mimics the metabolic profile observed in the fetal heart [38]. However, little is known about how metabolism changes in the hypertrophied heart under conditions of increased stress caused by reactive species such as oxidants and electrophiles. As a first step to addressing this, we examined energetics and HNE metabolism in control and PE-hypertrophied myocytes. The major findings of this study are: (1) hypertrophied myocytes utilize more oxygen than control myocytes; (2) hypertrophy increases the ability of the myocyte to utilize glycolysis during stress; (3) hypertrophied myocytes are better able to survive electrophilic insults; and (4) hypertrophied myocytes increase their apparent aldehyde dehydrogenase activity and concomitantly demonstrate lower levels of protein-HNE adducts.
In our study, we utilized a well-studied PE model of cardiomyocyte hypertrophy [29, 30]. Phenylephrine is an α1-adrenergic agonist that can be used to regulate cardiac metabolism and function. α-adrenergic stimulation produces both inotropic [39] and chronotropic [40] myocardial effects associated acutely with an increase in Ca2+ flux [41], phophatidylinositol turnover [41], and inhibition of cAMP phosphodiesterase activity [42]. These effects are followed by an increase in the synthetic rates of contractile proteins, brain natriuretic peptide (BNP) and atrial natriuretic protein (ANP) expression, and increases in cellular volume and protein content [29, 30, 43, 44]. Interestingly, PE has been shown to precondition the heart [45–47] and protect mitochondria from calcium overload [48]. In our study, we used ANP message and protein content as positive indicators of the PE-induced hypertrophic response prior to examining energetics and HNE metabolism (Fig. 1A and B).
Using sequential addition of oligomycin, FCCP, and antimycin A in extracellular flux assays, we were able to examine energetics in hypertrophied myocytes and quantify several distinct metabolic parameters (see [16] for further information on extracellular flux assays in primary cardiomyocytes). Inhibition of mitochondrial ATP synthesis with oligomycin was used to determine the extent of ATP-linked and non-ATP-linked utilization, the uncoupling agent FCCP was used to determine the maximal respiratory and reserve capacities, and antimycin A was used to inhibit all electron flux and measure non-mitochondrial oxygen consumption. Interestingly, we found that the increase in basal oxygen consumption in PE-hypertrophied myocytes was due to an increase in ATP demand, with no contribution of proton leak (Fig. 2). While it has been shown that PE increases myocardial oxygen utilization in the nonfailing heart [49], whether this leads to efficient energy production is unknown. Our finding suggests that PE-treated myocytes utilize oxygen in an efficient manner during the hypertrophic response. There was no increase in maximal respiratory capacity in hypertrophied myocytes and a net decrease in the mitochondrial reserve capacity. Consistent with previous studies [50], PE-mediated hypertrophy augmented the rate of glycolysis when oxidative phosphorylation was inhibited (Fig. 3). This suggests that the hypertrophied myocyte is better able to call upon glycolysis when mitochondrial ATP synthesis is lost. Collectively, these data suggest that PE-induced hypertrophy decreases the mitochondrial reserve capacity (due to an increase in basal oxygen consumption) while augmenting the apparent glycolytic reserve.
There may be several reasons for this increase in glycolytic capacity. It has been shown that stimulation of α-adrenergic receptors increases the activity of phosphofructokinase (PFK) in perfused rat hearts [50, 51], suggesting that this rate-limiting enzyme could have been affected in hypertrophied myocytes. Furthermore, in the pressure-overloaded heart, the levels of PFK activators are elevated tenfold, leading to an increase in glycolytic flux [52], and the activity and/or expression of several glycolytic enzymes have been shown to be increased in the hypertrophied heart [14, 53, 54]. Interestingly, HNE exposure increased glycolytic flux to a greater extent in hypertrophied myocytes compared with non-hypertrophied myocytes. Although lactate production mirrored the HNE-induced changes in glycolysis measured by extracellular flux analyses, 3H-deoxyglucose (3H-DOG) uptake was unchanged by HNE exposure, indicating that the glucose oxidized by the cell under electrophile stress was derived from intracellular stores—likely glycogen. That glucose derived from glycogen is preferentially oxidized in the heart compared with exogenous glucose [55, 56] therefore suggests that glycogen may be the depot that is first utilized by the heart under conditions of electrophile stress. Although glycogen metabolism in the hypertrophied heart does not differ from the non-hypertrophied heart under non-ischemic conditions [55], glycogen turnover is accelerated in hypertrophied hearts exposed to severe ischemia [57]—a stress where a marked increase in electrophiles such as HNE is observed [2, 3]. Oligomycin, however, significantly increased 3H-DOG uptake, indicating that overt loss of mitochondrial ATP synthesis does result in an increased capacity of the cell to import extracellular glucose. Taken together, these findings imply that HNE could promote glycogen turnover, which is then used to provide fuel for the myocyte to survive under conditions of electrophilic stress.
In this study, we also observed that the hypertrophic myocyte responds differently to HNE, i.e., it uses less oxygen after being exposed to HNE than a non-hypertrophied myocyte and appears to rely more on glycolysis during the electrophilic encounter (Fig. 5). This may be due to the fact that, in the hypertrophied heart, glucose oxidation is not in correspondence with glycolytic rates and that mitochondrial glucose oxidation may actually be lower than that found in the non-hypertrophied heart (reviewed in [14]). Hence, it appears that under conditions of electrophile stress, the hypertrophied myocyte utilizes anaerobic glycolytic pathways to a greater extent than the non-hypertrophied myocyte. The bioenergetic response to HNE, in general, was also delayed temporally with respect to that of a normal myocyte. This latency could be explained by differences in how the cell metabolizes electrophiles such as HNE. HNE has been shown to be detoxified by several pathways including oxidation to 4-hydroxynonenoic acid (HNA), reduction to dihydroxynonene (DHN), and conjugation to glutathione [24]. In the rat heart, it was shown that the predominant route of metabolism for HNE is via NAD+-dependent oxidation to HNA and that mitochondria are centers for such HNE metabolism [3]. HNE metabolism in control and hypertrophied myocytes appeared similar (Table 1) with the exception of differences in the quantities of HNA and HNE, leading to an increased HNA/HNE ratio and decreased protein-HNE adducts in hypertrophied myocytes (Fig. 7). It is intriguing to speculate that the bioenergetic phenomena observed in the PE-hypertrophied myocytes exposed to HNE may be due to better metabolism of toxic aldehydes, leading to preservation of myocardial energetics. Aldehyde dehydrogenase 2, a mitochondrial localized ALDH isoform, has been shown to attenuate myocardial ischemia-reperfusion injury [58, 59] and plays an important role in detoxifying aldehydes in the heart [3, 24]. In our study, ALDH2 expression was not increased over control levels in the hypertrophied myocytes (data not shown), indicating that other factors relating to the activity of the enzyme, such as phosphorylation or other post-translational protein modifications, may be responsible for the observed effects. Indeed, protein kinase C epsilon (PKCε) has been shown to phosphorylate and activate ALDH2 [60], and phenylephrine and other adrenergic agonists activate PKCε and promote their translocation to membrane fractions [61–63].
We propose that the increase in apparent aldehyde dehydrogenase activity may be responsible for the attenuation of protein damage by HNE and could, in part, underlie the energetic changes and protection against cell death observed in PE-treated myocytes after HNE exposure (Fig. 4 and 5). Our finding that ALDH activity is important for maintaining energetic integrity in myocytes exposed to electrophile stress (Supplementary Fig. 2) further supports this concept. An additional factor unveiled by these studies is the glycolytic reserve, which was increased in the hypertrophied myocyte. Although the mitochondrial response to HNE appeared to be diminished, the enhanced ability of PE-treated myocytes to utilize glycolysis may be important during conditions of increased oxidative stress. Taken as a whole, we conclude that HNE metabolism and the ability to sustain glycolysis may be linked and integral conduits used by myocytes to maintain energetic poise in the face of electrophilic insults. Understanding how each of these factors change during the course of myocardial remodeling should yield further insights into the mechanisms of heart failure.
Supplementary Material
Acknowledgments
Funding: SPJ was supported by grants from the NIH-NHLBI (R01 HL083320, R01 HL094419), American Heart Association National Center Scientist Development Grant (0535270N), NIH-NCRR (P20 RR024489), and Kentucky Science and Engineering Foundation grant (KSEF-1677-RDE-011). BGH was supported by a grant from the NIH-NCRR (P20 RR024489) and a University of Louisville Clinical and Translational Science Pilot Program grant (20020).
Abbreviations
- ALDH
aldehyde dehydrogenase
- ANP
atrial natriuretic peptide
- BNP
brain natriuretic peptide
- DHN
dihydroxynonene
- DMEM
Dulbecco’s modified Eagle’s medium
- ECAR
extracellular acidification rate
- FCCP
carbonyl cyanide p-trifluoromethoxyphenylhydrazone
- HNA
4-hydroxynonenoic acid
- HNE
4-hydroxynonenal
- MDA
malondialdehyde
- NRCM
neonatal rat cardiomyocyte
- OCR
oxygen consumption rate
- PE
phenylephrine
- PKC
protein kinase C
- PPR
proton production rate
- GS-X
glutathione conjugates of HNE, including GS-HNE and GS-DHN
- XF
extracellular flux
Footnotes
Author contributions: B.E.S. designed and performed experiments, analyzed data, and helped write the paper; D.W.G. performed experiments and analyzed data; R.E.B. performed experiments and analyzed data; J.K.S. performed experiments and analyzed data; S.P.J. contributed reagents/materials, helped design experiments, analyzed data, and helped write the paper; and B.G.H. designed/performed experiments, analyzed data, and wrote the paper.
References
- 1.Roth E, Torok B, Zsoldos T, Matkovics B. Lipid peroxidation and scavenger mechanism in experimentally induced heart infarcts. Basic Res Cardiol. 1985;80:530–536. doi: 10.1007/BF01907916. [DOI] [PubMed] [Google Scholar]
- 2.Eaton P, Li JM, Hearse DJ, Shattock MJ. Formation of 4-hydroxy-2-nonenal-modified proteins in ischemic rat heart. Am J Physiol. 1999;276:H935–943. doi: 10.1152/ajpheart.1999.276.3.H935. [DOI] [PubMed] [Google Scholar]
- 3.Hill BG, Awe SO, Vladykovskaya E, Ahmed Y, Liu SQ, Bhatnagar A, Srivastava S. Myocardial ischaemia inhibits mitochondrial metabolism of 4-hydroxy-trans-2-nonenal. Biochem J. 2009;417:513–524. doi: 10.1042/BJ20081615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kai H, Mori T, Tokuda K, Takayama N, Tahara N, Takemiya K, Kudo H, Sugi Y, Fukui D, Yasukawa H, Kuwahara F, Imaizumi T. Pressure overload-induced transient oxidative stress mediates perivascular inflammation and cardiac fibrosis through angiotensin II. Hypertens Res. 2006;29:711–718. doi: 10.1291/hypres.29.711. [DOI] [PubMed] [Google Scholar]
- 5.Fiorillo C, Nediani C, Ponziani V, Giannini L, Celli A, Nassi N, Formigli L, Perna AM, Nassi P. Cardiac volume overload rapidly induces oxidative stress-mediated myocyte apoptosis and hypertrophy. Biochim Biophys Acta. 2005;1741:173–182. doi: 10.1016/j.bbadis.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava S, Chandrasekar B, Gu Y, Luo J, Hamid T, Hill BG, Prabhu SD. Downregulation of CuZn-superoxide dismutase contributes to beta-adrenergic receptor-mediated oxidative stress in the heart. Cardiovasc Res. 2007;74:445–455. doi: 10.1016/j.cardiores.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Mak S, Lehotay DC, Yazdanpanah M, Azevedo ER, Liu PP, Newton GE. Unsaturated aldehydes including 4-OH-nonenal are elevated in patients with congestive heart failure. J Card Fail. 2000;6:108–114. [PubMed] [Google Scholar]
- 8.Nakamura K, Kusano K, Nakamura Y, Kakishita M, Ohta K, Nagase S, Yamamoto M, Miyaji K, Saito H, Morita H, Emori T, Matsubara H, Toyokuni S, Ohe T. Carvedilol decreases elevated oxidative stress in human failing myocardium. Circulation. 2002;105:2867–2871. doi: 10.1161/01.cir.0000018605.14470.dd. [DOI] [PubMed] [Google Scholar]
- 9.Asselin C, Shi Y, Clement R, Tardif JC, Des Rosiers C. Higher circulating 4-hydroxynonenal-protein thioether adducts correlate with more severe diastolic dysfunction in spontaneously hypertensive rats. Redox Rep. 2007;12:68–72. doi: 10.1179/135100007X162202. [DOI] [PubMed] [Google Scholar]
- 10.Kirshenbaum LA, Singal PK. Antioxidant changes in heart hypertrophy: significance during hypoxia-reoxygenation injury. Can J Physiol Pharmacol. 1992;70:1330–1335. doi: 10.1139/y92-186. [DOI] [PubMed] [Google Scholar]
- 11.Kirshenbaum LA, Singal PK. Increase in endogenous antioxidant enzymes protects hearts against reperfusion injury. Am J Physiol. 1993;265:H484–493. doi: 10.1152/ajpheart.1993.265.2.H484. [DOI] [PubMed] [Google Scholar]
- 12.Singal PK, Gupta M, Randhawa AK. Reduced myocardial injury due to exogenous oxidants in pressure induced heart hypertrophy. Basic Res Cardiol. 1991;86:273–282. doi: 10.1007/BF02190607. [DOI] [PubMed] [Google Scholar]
- 13.Wang G, Hamid T, Keith RJ, Zhou G, Partridge CR, Xiang X, Kingery JR, Lewis RK, Li Q, Rokosh DG, Ford R, Spinale FG, Riggs DW, Srivastava S, Bhatnagar A, Bolli R, Prabhu SD. Cardioprotective and antiapoptotic effects of heme oxygenase-1 in the failing heart. Circulation. 2010;121:1912–1925. doi: 10.1161/CIRCULATIONAHA.109.905471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sambandam N, Lopaschuk GD, Brownsey RW, Allard MF. Energy metabolism in the hypertrophied heart. Heart Fail Rev. 2002;7:161–173. doi: 10.1023/a:1015380609464. [DOI] [PubMed] [Google Scholar]
- 15.Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovasc Res. 2009;81:412–419. doi: 10.1093/cvr/cvn301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hill BG, Dranka BP, Zou L, Chatham JC, Darley-Usmar VM. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem J. 2009;424:99–107. doi: 10.1042/BJ20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sako EY, Kingsley-Hickman PB, From AH, Foker JE, Ugurbil K. ATP synthesis kinetics and mitochondrial function in the postischemic myocardium as studied by 31P NMR. J Biol Chem. 1988;263:10600–10607. [PubMed] [Google Scholar]
- 18.Smith DR, Stone D, Darley-Usmar VM. Stimulation of mitochondrial oxygen consumption in isolated cardiomyocytes after hypoxia-reoxygenation. Free Radic Res. 1996;24:159–166. doi: 10.3109/10715769609088013. [DOI] [PubMed] [Google Scholar]
- 19.Strauer BE. Cardiac energetics in clinical heart disease. Basic Res Cardiol. 1987;82(Suppl 2):389–402. doi: 10.1007/978-3-662-11289-2_38. [DOI] [PubMed] [Google Scholar]
- 20.Gunning JF, Coleman HN., 3rd Myocardial oxygen consumption during experimental hypertrophy and congestive heart failure. J Mol Cell Cardiol. 1973;5:25–38. doi: 10.1016/0022-2828(73)90033-3. [DOI] [PubMed] [Google Scholar]
- 21.Gunning JF, Cooper Gt, Harrison CE, Coleman HN., 3rd Myocardial oxygen consumption in experimental hypertrophy and congestive heart failure due to pressure overload. Am J Cardiol. 1973;32:427–436. doi: 10.1016/s0002-9149(73)80033-5. [DOI] [PubMed] [Google Scholar]
- 22.Katz AM. Is the failing heart energy depleted? Cardiol Clin. 1998;16:633–644. viii. doi: 10.1016/s0733-8651(05)70040-0. [DOI] [PubMed] [Google Scholar]
- 23.Katz AM. Evolving concepts of heart failure: cooling furnace, malfunctioning pump, enlarging muscle. Part II: Hypertrophy and dilatation of the failing heart. J Card Fail. 1998;4:67–81. doi: 10.1016/s1071-9164(98)90509-7. [DOI] [PubMed] [Google Scholar]
- 24.Srivastava S, Chandra A, Wang LF, Seifert WE, Jr, DaGue BB, Ansari NH, Srivastava SK, Bhatnagar A. Metabolism of the lipid peroxidation product, 4-hydroxy-trans-2-nonenal, in isolated perfused rat heart. J Biol Chem. 1998;273:10893–10900. doi: 10.1074/jbc.273.18.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akao M, Ohler A, O’Rourke B, Marban E. Mitochondrial ATP-sensitive potassium channels inhibit apoptosis induced by oxidative stress in cardiac cells. Circ Res. 2001;88:1267–1275. doi: 10.1161/hh1201.092094. [DOI] [PubMed] [Google Scholar]
- 26.Jones SP, Teshima Y, Akao M, Marban E. Simvastatin attenuates oxidant-induced mitochondrial dysfunction in cardiac myocytes. Circ Res. 2003;93:697–699. doi: 10.1161/01.RES.0000097262.21507.DF. [DOI] [PubMed] [Google Scholar]
- 27.Jones SP, Zachara NE, Ngoh GA, Hill BG, Teshima Y, Bhatnagar A, Hart GW, Marban E. Cardioprotection by N-acetylglucosamine linkage to cellular proteins. Circulation. 2008;117:1172–1182. doi: 10.1161/CIRCULATIONAHA.107.730515. [DOI] [PubMed] [Google Scholar]
- 28.Ngoh GA, Facundo HT, Hamid T, Dillmann W, Zachara NE, Jones SP. Unique hexosaminidase reduces metabolic survival signal and sensitizes cardiac myocytes to hypoxia/reoxygenation injury. Circ Res. 2009;104:41–49. doi: 10.1161/CIRCRESAHA.108.189431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simpson P. Stimulation of hypertrophy of cultured neonatal rat heart cells through an alpha 1-adrenergic receptor and induction of beating through an alpha 1- and beta 1-adrenergic receptor interaction. Evidence for independent regulation of growth and beating. Circ Res. 1985;56:884–894. doi: 10.1161/01.res.56.6.884. [DOI] [PubMed] [Google Scholar]
- 30.Lee HR, Henderson SA, Reynolds R, Dunnmon P, Yuan D, Chien KR. Alpha 1-adrenergic stimulation of cardiac gene transcription in neonatal rat myocardial cells. Effects on myosin light chain-2 gene expression. J Biol Chem. 1988;263:7352–7358. [PubMed] [Google Scholar]
- 31.Watson LJ, Facundo HT, Ngoh GA, Ameen M, Brainard RE, Lemma KM, Long BW, Prabhu SD, Xuan YT, Jones SP. O-linked beta-N-acetylglucosamine transferase is indispensable in the failing heart. Proc Natl Acad Sci U S A. 2010;107:17797–17802. doi: 10.1073/pnas.1001907107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Echtay KS, Esteves TC, Pakay JL, Jekabsons MB, Lambert AJ, Portero-Otin M, Pamplona R, Vidal-Puig AJ, Wang S, Roebuck SJ, Brand MD. A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. EMBO J. 2003;22:4103–4110. doi: 10.1093/emboj/cdg412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrick DA, Neilson A, Beeson C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today. 2008;13:268–274. doi: 10.1016/j.drudis.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 34.Endo A, Hasumi K, Sakai K, Kanbe T. Specific inhibition of glyceraldehyde-3-phosphate dehydrogenase by koningic acid (heptelidic acid) J Antibiot (Tokyo) 1985;38:920–925. doi: 10.7164/antibiotics.38.920. [DOI] [PubMed] [Google Scholar]
- 35.Sakai K, Hasumi K, Endo A. Inactivation of rabbit muscle glyceraldehyde-3-phosphate dehydrogenase by koningic acid. Biochim Biophys Acta. 1988;952:297–303. doi: 10.1016/0167-4838(88)90130-6. [DOI] [PubMed] [Google Scholar]
- 36.Sakai K, Hasumi K, Endo A. Identification of koningic acid (heptelidic acid)-modified site in rabbit muscle glyceraldehyde-3-phosphate dehydrogenase. Biochim Biophys Acta. 1991;1077:192–196. doi: 10.1016/0167-4838(91)90058-8. [DOI] [PubMed] [Google Scholar]
- 37.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 38.Lopaschuk GD, Jaswal JS. Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation. J Cardiovasc Pharmacol. 2010;56:130–140. doi: 10.1097/FJC.0b013e3181e74a14. [DOI] [PubMed] [Google Scholar]
- 39.Govier WC. A positive inotropic effect of phenylephrine mediated through alpha adrenergic receptors. Life Sci. 1967;6:1361–1365. doi: 10.1016/0024-3205(67)90182-8. [DOI] [PubMed] [Google Scholar]
- 40.Steinberg SF, Drugge ED, Bilezikian JP, Robinson RB. Acquisition by innervated cardiac myocytes of a pertussis toxin-specific regulatory protein linked to the alpha 1-receptor. Science. 1985;230:186–188. doi: 10.1126/science.2994230. [DOI] [PubMed] [Google Scholar]
- 41.Amitai G, Brown RD, Taylor P. The relationship between alpha 1-adrenergic receptor occupation and the mobilization of intracellular calcium. J Biol Chem. 1984;259:12519–12527. [PubMed] [Google Scholar]
- 42.Buxton IL, Brunton LL. Action of the cardiac alpha 1-adrenergic receptor. Activation of cyclic AMP degradation. J Biol Chem. 1985;260:6733–6737. [PubMed] [Google Scholar]
- 43.Meidell RS, Sen A, Henderson SA, Slahetka MF, Chien KR. Alpha 1-adrenergic stimulation of rat myocardial cells increases protein synthesis. Am J Physiol. 1986;251:H1076–1084. doi: 10.1152/ajpheart.1986.251.5.H1076. [DOI] [PubMed] [Google Scholar]
- 44.Hanford DS, Thuerauf DJ, Murray SF, Glembotski CC. Brain natriuretic peptide is induced by alpha 1-adrenergic agonists as a primary response gene in cultured rat cardiac myocytes. J Biol Chem. 1994;269:26227–26233. [PubMed] [Google Scholar]
- 45.Banerjee A, Locke-Winter C, Rogers KB, Mitchell MB, Brew EC, Cairns CB, Bensard DD, Harken AH. Preconditioning against myocardial dysfunction after ischemia and reperfusion by an alpha 1-adrenergic mechanism. Circ Res. 1993;73:656–670. doi: 10.1161/01.res.73.4.656. [DOI] [PubMed] [Google Scholar]
- 46.Tsuchida A, Liu Y, Liu GS, Cohen MV, Downey JM. alpha 1-adrenergic agonists precondition rabbit ischemic myocardium independent of adenosine by direct activation of protein kinase C. Circ Res. 1994;75:576–585. doi: 10.1161/01.res.75.3.576. [DOI] [PubMed] [Google Scholar]
- 47.Cohen MV, Yang XM, Liu GS, Heusch G, Downey JM. Acetylcholine, bradykinin, opioids, and phenylephrine, but not adenosine, trigger preconditioning by generating free radicals and opening mitochondrial K(ATP) channels. Circ Res. 2001;89:273–278. doi: 10.1161/hh1501.094266. [DOI] [PubMed] [Google Scholar]
- 48.Gao H, Chen L, Yang HT. Activation of alpha1B-adrenoceptors alleviates ischemia/reperfusion injury by limitation of mitochondrial Ca2+ overload in cardiomyocytes. Cardiovasc Res. 2007;75:584–595. doi: 10.1016/j.cardiores.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 49.Nikolaidis LA, Hentosz T, Doverspike A, Huerbin R, Stolarski C, Shen YT, Shannon RP. Catecholamine stimulation is associated with impaired myocardial O(2) utilization in heart failure. Cardiovasc Res. 2002;53:392–404. doi: 10.1016/s0008-6363(01)00490-4. [DOI] [PubMed] [Google Scholar]
- 50.Clark MG, Patten GS. Adrenergic regulation of glucose metabolism in rat heart. A calcium-dependent mechanism mediated by both alpha- and beta-adrenergic receptors. J Biol Chem. 1984;259:15204–15211. [PubMed] [Google Scholar]
- 51.Patten GS, Filsell OH, Clark MG. Epinephrine regulation of phosphofructokinase in perfused rat heart. A calcium ion-dependent mechanism mediated via alpha-receptors. J Biol Chem. 1982;257:9480–9486. [PubMed] [Google Scholar]
- 52.Nascimben L, Ingwall JS, Lorell BH, Pinz I, Schultz V, Tornheim K, Tian R. Mechanisms for increased glycolysis in the hypertrophied rat heart. Hypertension. 2004;44:662–667. doi: 10.1161/01.HYP.0000144292.69599.0c. [DOI] [PubMed] [Google Scholar]
- 53.Taegtmeyer H, Overturf ML. Effects of moderate hypertension on cardiac function and metabolism in the rabbit. Hypertension. 1988;11:416–426. doi: 10.1161/01.hyp.11.5.416. [DOI] [PubMed] [Google Scholar]
- 54.Keller A, Rouzeau JD, Farhadian F, Wisnewsky C, Marotte F, Lamande N, Samuel JL, Schwartz K, Lazar M, Lucas M. Differential expression of alpha- and beta-enolase genes during rat heart development and hypertrophy. Am J Physiol. 1995;269:H1843–1851. doi: 10.1152/ajpheart.1995.269.6.H1843. [DOI] [PubMed] [Google Scholar]
- 55.Allard MF, Henning SL, Wambolt RB, Granleese SR, English DR, Lopaschuk GD. Glycogen metabolism in the aerobic hypertrophied rat heart. Circulation. 1997;96:676–682. doi: 10.1161/01.cir.96.2.676. [DOI] [PubMed] [Google Scholar]
- 56.Henning SL, Wambolt RB, Schonekess BO, Lopaschuk GD, Allard MF. Contribution of glycogen to aerobic myocardial glucose utilization. Circulation. 1996;93:1549–1555. doi: 10.1161/01.cir.93.8.1549. [DOI] [PubMed] [Google Scholar]
- 57.Wambolt RB, Henning SL, English DR, Dyachkova Y, Lopaschuk GD, Allard MF. Glucose utilization and glycogen turnover are accelerated in hypertrophied rat hearts during severe low-flow ischemia. J Mol Cell Cardiol. 1999;31:493–502. doi: 10.1006/jmcc.1998.0804. [DOI] [PubMed] [Google Scholar]
- 58.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma H, Guo R, Yu L, Zhang Y, Ren J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur Heart J. 2010 doi: 10.1093/eurheartj/ehq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Churchill EN, Disatnik MH, Mochly-Rosen D. Time-dependent and ethanol-induced cardiac protection from ischemia mediated by mitochondrial translocation of varepsilonPKC and activation of aldehyde dehydrogenase 2. J Mol Cell Cardiol. 2009;46:278–284. doi: 10.1016/j.yjmcc.2008.09.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Puceat M, Hilal-Dandan R, Strulovici B, Brunton LL, Brown JH. Differential regulation of protein kinase C isoforms in isolated neonatal and adult rat cardiomyocytes. J Biol Chem. 1994;269:16938–16944. [PubMed] [Google Scholar]
- 62.Clerk A, Bogoyevitch MA, Anderson MB, Sugden PH. Differential activation of protein kinase C isoforms by endothelin-1 and phenylephrine and subsequent stimulation of p42 and p44 mitogen-activated protein kinases in ventricular myocytes cultured from neonatal rat hearts. J Biol Chem. 1994;269:32848–32857. [PubMed] [Google Scholar]
- 63.Rao VU, Shiraishi H, McDermott PJ. PKC-epsilon regulation of extracellular signal-regulated kinase: a potential role in phenylephrine-induced cardiocyte growth. Am J Physiol Heart Circ Physiol. 2004;286:H2195–2203. doi: 10.1152/ajpheart.00475.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.