Abstract
The aim of our investigation was to verify whether phage therapy (PT) can induce antiphage antibodies. The antiphage activity was determined in sera from 122 patients from the Phage Therapy Unit in Wrocław with bacterial infections before and during PT, and in sera from 30 healthy volunteers using a neutralization test. Furthermore, levels of antiphage antibodies were investigated in sera of 19 patients receiving staphylococcal phages and sera of 20 healthy volunteers using enzyme-linked immunosorbent assay. The phages were administered orally, locally, orally/locally, intrarectally, or orally/intrarectally. The rate of phage inactivation (K) estimated the level of phages' neutralization by human sera. Low K rates were found in sera of healthy volunteers (K≤1.73). Low K rates were detected before PT (K≤1.64). High antiphage activity of sera K>18 was observed in 12.3% of examined patients (n=15) treated with phages locally (n=13) or locally/orally (n=2) from 15 to 60 days of PT. High K rates were found in patients treated with some Staphylococcus aureus, Pseudomonas aeruginosa, and Enterococcus faecalis phages. Low K rates were observed during PT in sera of patients using phages orally (K≤1.04). Increased inactivation of phages by sera of patients receiving PT decreased after therapy. These results suggest that the antiphage activity in patients' sera depends on the route of phage administration and phage type. The induction of antiphage activity of sera during or after PT does not exclude a favorable result of PT.
Introduction
Since bacteriophages were discovered almost 100 years ago, there have been attempts to make use of their properties for therapeutic purposes. Nowadays, due to the antibiotic treatment crisis, there is growing interest in phage therapy (PT), and therefore it is necessary to conduct investigations, including those that go beyond only the antibacterial effect of phages.
Although phage investigations have been conducted for a long time, nowadays there are only a few research centers in the world where PT is applied in humans. The interactions between phage preparations and the human immune system have become an interesting subject of investigations in recent years, but only a few articles describe antibacteriophage activity of human serum of patients during phage treatment and healthy subjects (3,4,5,9). With regard to the small number of patients with high activity in their sera, it is difficult to define the relationship between serum antiphage activity and effectiveness of the PT.
Data from animal experiments indicate that phages can induce antibodies that neutralize their antibacterial activity (1,12,13). This phenomenon is especially relevant in PT, where it can interfere with its clinical efficacy. Studies between the way of phage application and their neutralization by antiphage sera in patients subjected to PT can bring enormous practical insight to the effect of the PT. In this study, we aimed to verify whether PT can induce antiphage activity in sera (AAS) from patients with bacterial infections treated at the Phage Therapy Unit (PTU) in Wrocław (measured by a neutralization test, which has been conducted since 2010 as a standard procedure in our PT patients) and whether PT can cause increased levels of antiphage antibodies in patients' sera (measured spectrophotometrically by enzyme-linked immunosorbent assay [ELISA]).
Materials and Methods
Adult patients with various infections (genitourinary tract infections, prostatitis, soft tissue infections, bone infection, and upper and lower respiratory tract infections) resistant to antibiotic treatment received phage treatment under the therapeutic protocol entitled “Experimental phage therapy of drug-resistant bacterial infections, including MRSA infections” treated at the PTU in Wrocław (10).
A total of 122 patients were studied, who received the phages Staphylococcus aureus (n=70), Enterococcus faecalis (n=8), Escherichia coli (n=16), Pseudomonas aeruginosa (n=14), Klebsiella pneumoniae (n=5), Serratia marcescens (n=1), Enterobacter cloacae (n=1), E. coli/E. faecalis (n=3), E. coli/K. pneumoniae (n=1), E. faecalis/Morganella morganii (n=1), or S. aureus/P. aeruginosa (n=2). Specific phage lysates were administered orally (n=27), locally (n=56), orally and locally (n=21), intrarectally (n=16), or orally and intrarectally (n=2) for 7–91 days. Blood samples were collected from 30 voluntary blood donors of the Blood Transfusion Center in Wrocław. Blood samples were also collected from each patient before and during PT and from some patients after PT. The sera were separated by centrifugation at 1,500 g for 10 min and stored at −70°C. The study was approved by the Bioethics Committee at the Wrocław Medical University and was conducted in accordance with the Helsinki Declaration of 1964 as revised in 2008. Each patient gave his informed consent prior beginning the treatment. The study was performed from 2010 to 2013. All sera were tested sequentially over several years in a neutralization test immediately after blood collection. After examination of the neutralization test, sera were frozen at −70°C and tested after thawing using ELISA.
Patients received phage lysates in all cases. Phage preparations used in ELISA examinations were purified for greater reliability of the results due to the high sensitivity of ELISA. The purification of bacteriophages included three steps: phage propagation, phage concentration, and purification. Bacteriophages were propagated, and then phage lysates were concentrated using a Vivaflow 200 (tangential flow module) with a Hydrosart membrane (the cutoff is 30,000 MWCO). The next step was the removal of lysate contaminants from the bacteriophage suspensions by size exclusion chromatography. The concentrated bacteriophage suspension was chromatographed on a Sepharose 4B column (GE Life Sciences K 26/100; elution buffer 0.068 M phosphate buffer, pH 7.2; flow rate 0.3 mL/min; detection UV at 280 nm). To remove endotoxin from the bacteriophages, affinity chromatography was used with the following conditions: Chisso Corporation Mini-column Cellufine Sulfate (1 mL); adsorption buffer 0.01 M phosphate buffer; elution buffer 0.01 M phosphate buffer+1 M NaCl, pH 7.6; flow rate 5 mL/min; detection UV at 280 nm). The endotoxin assay was performed using the Endpoint Chromogenic Limulus Amebocyte Lysate (LAL) Test.
The specific phages were chosen on the basis of the phage typing procedure, which enabled us to determine the phage ability to lyse specific bacteria. The term “specific phage” means phage lysing a definite bacterial strain causing patients' illness. In most cases, phages used in therapy lyse a particular genus of bacteria, for example staphylococcal phages lyse the majority of Staphylococcus species. Phages are grouped into sets depending on their specificity (Klebsiella phages, Pseudomonas phages, etc.).
Sera from 30 healthy volunteers were examined in a neutralization test of S. aureus 676/Z phage and S. aureus A3/R phage. The neutralization of specific phage by patients' sera was estimated as the rate of phage inactivation (K; the formula for its calculation is shown below) during a 30 min reaction of the phage (50 μL) with diluted serum (450 μL) at 37°C according to the method described by Adams (2) and Pescovitz et al. (11) with some modification. The sera were tested up to dilution 1:1,500. The titer of phages used as antigens was 1×106 pfu/mL. After incubation, the mixture was diluted 100 times with cold broth and subjected to phage titer determination by the double-agar layer method of Gratia (6) as described by Adams (2). Statistical analysis between groups examined in the neutralization test was performed using the Mann–Whitney U-test (independent trials) or Wilcoxon rank sum test (dependent trials) (statistical significance was set at p<0.05). Results are presented as the mean phage inactivation rate K±standard deviation (SD; Table 3).
Table 3.
Mean Rates of Phage Inactivation Depending on the Route of Phage Administration (120 Patients)
| Route of phage administration | Mean K±SD before phage therapy | Mean K±SD during phage therapy | p (Wilcoxon test) |
|---|---|---|---|
| Oral (n=27) | 0.05±0.06 | 0.13±0.22 | 0.001* |
| Local (n=56) | 0.10±0.27 | 11.44±25.81 | 0.001* |
| Oral and local (n=21) | 0.04±0.05 | 12.06±47.82 | 0.0002* |
| Intrarectal (n=16) | 0.05±0.08 | 1.15±3.84 | 0.006* |
The K rate rose significantly (p<0.05, Wilcoxon test) in patients receiving phages orally, locally, orally and locally, or intrarectally. Mann–Whitney U-test revealed a statistically significant difference in the increase in the K index between orally and locally treated patients (mean increase in K=0.08 and 11.34 respectively; *p=0.001).
p<0.05.
SD, standard deviation; K, phage inactivation.
The rate of phage inactivation is:
 |
where D is the reciprocal of the serum dilution, T is the time in minutes during which the reaction occurred (30 min in this case), Po is the phage titer at the start of the reaction, and Pt is the phage titer at time T.
We also investigated the level of antiphage antibodies. Immune analysis was based on detection of specific antiphage antibodies in human sera reacting with phage antigens using indirect ELISA. Studies were examined by the method according to Waddell et al. (14) with some changes. Purified S. aureus phage preparations as antigens (108 pfu/mL), as well as 100-fold dilution of human sera as primary antibody, were used. To remove endotoxin from the bacteriophage preparations, affinity chromatography was used. The quantity of endotoxin in the phage preparations was <5 EU/mL in the final dilution. Any nonspecific binding sites on the surface were blocked with 1% casein (blocking protein). In the first stage, 96-well microplates were loaded with antigens. Next, primary antibodies were bound with antigens, making a complex. A specific antibody bound with antigen was detected using a secondary antibody (binding all human Igs) linked with the enzyme horseradish peroxidase (HRP) diluted 60,000× in phosphate buffered saline (PBS) with the addition of 0.05% Tween 20 and 1% blocking protein. The final step was to apply a chemical substrate (orthophenylenediamine) that is converted by the enzyme into a color measured spectrophotometrically at 450 nm to determine the presence and quantity of antiphage antibodies. To verify the reliability of the tests, a series of control samples were carried out to detect possible errors. The value of absorbance in control wells (no phage as antigens) was subtracted from the tested samples. Statistical analysis between groups examined in the ELISA test was performed using the Student's t-test (dependent trials) and Mann–Whitney U-test (independent trials; significance set at p<0 .05). The number of patients examined with the neutralization test does not correspond on the graphs with the number of patients examined with ELISA.
Phage treatment
Table 1 lists the type of infection, the route of phage administration, and the type of phages used in the treatment. Phage preparations (lysates) were used by patients orally, locally, or intrarectally. The local route of administration of phage preparations (twice daily) included gargling, fistula irrigation, irrigation of the abscess cavity, sitz baths, wet compresses, nose drops, ear drops, vaginal irrigation, and inhalations. The oral route of treatment was 10–20 mL three times daily, at least 30 min before meals. Ten mL of oral suspension of dihydroxyaluminium sodium carbonate (68 mg/mL) was used by these patients up to 20 min prior to a phage preparation to protect the phages against inactivation by gastric juices. The intrarectal route of treatment was 10–20 mL twice daily. Phage administration took place for 12 weeks, but this could be extended for up to 12 more weeks in cases when infection remained (10). The S. aureus phage cocktail MS-1 was administered for 6 weeks.
Table 1.
Patients with Different Types of Infection Treated with Phages
| Type of infectiona | Oral phage administration phage type | Local phage administration phage type | Oral and local phage administration phage type | Intrarectal phage administration phage type | Oral and intrarectal phage administration phage type |
|---|---|---|---|---|---|
| 1 |
E. coli (n=4) K. pneumoniae (n=1) |
E. coli (n=1) E. coli/E. faecalis (n=2) |
E. coli (n=2) E. coli/E. faecalis (n=1) |
E. faecalis (n=5) E. coli (n=3) P. aeruginosa (n=1) K. pneumoniae (n=2) E. coli/K. pneumoniae (n=1) E. faecalis/M. morganii (n=1) |
E. coli (n=1) K. pneumoniae (n=1) |
| 2 |
E. faecalis (n=1) E. coli (n=2) |
||||
| 3 |
S. aureus (n=8) E. coli (n=3) |
S. aureus (n=11) P. aeruginosa (n=7) S. marcescens (n=1) |
S. aureus (n=6) E. cloacae (n=1) |
||
| 4 |
S. aureus (n=4) P. aeruginosa (n=1) |
S. aureus (n=22) E. faecalis (n=1) P. aeruginosa (n=1) S. aureus/P. aeruginosa (n=1) |
S. aureus (n=2) E. faecalis (n=1) |
||
| 5 |
S. aureus (n=3) P. aeruginosa (n=1) |
S. aureus (n=7) K. pneumoniae (n=1) |
S. aureus (n=7) | ||
| 6 | P. aeruginosa (n=2) | S. aureus/P. aeruginosa (n=1) | P. aeruginosa (n=1) | ||
| Total no. patients | 27 | 56 | 21 | 16 | 2 |
Type of infection: 1, genitourinary tract infection; 2, prostatitis; 3, soft tissue infection (skin, fistula); 4, bone infection; 5, upper respiratory tract infection (rhinitis, pharyngitis, sinusitis, otitis, laryngitis); 6, lower respiratory tract infection (bronchitis, cystic fibrosis).
Results
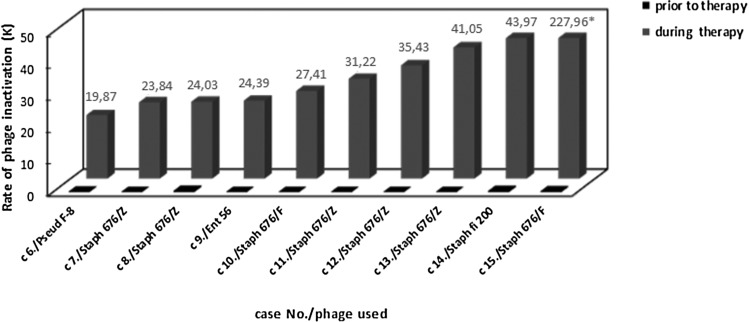
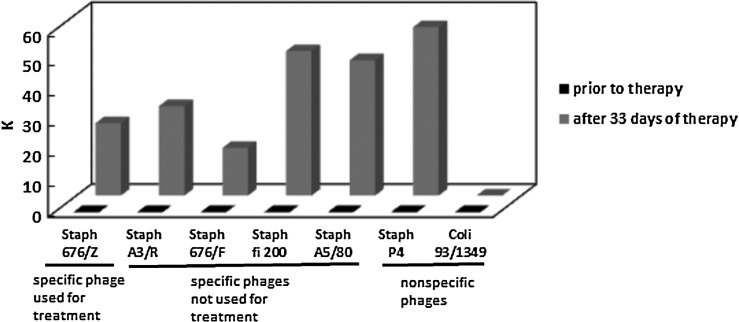
Sera from healthy volunteers were controls in the study of the presence of antiphage antibodies in sera of patients undergoing PT. Sera of 30 healthy volunteers caused weak neutralization of S. aureus 676/Z phage (K≤0.68) and S. aureus A3/R phage (K≤1.73). Antiphage activity of sera from the 122 patients receiving PT, depending on the route of phage administration, is shown in Table 2. High levels of AAS (at least 50% neutralization at serum dilution ≥1:800) were associated with K>18. Medium levels of AAS (between >50% neutralization at serum dilution ≥1:200 and <50% neutralization at serum dilution ≤1:800) were estimated with K=5–18. Low levels of AAS (≤50% neutralization at serum dilution ≤1:200) were associated with K<5 (Table 2). All patients had very weak AAS before PT (mean K=0.07±0.19), while during treatment it rose significantly (mean K=7.0±24.72; p<0.05, Wilcoxon test). All patients treated with phage orally (n=27) had low K (≤1.04) during the phage treatment. The analysis of the reaction of sera from 56 patients who applied phages locally showed that, in 13 patients, high AAS was observed (K>18), whereas in five patients, medium AAS was observed (K=5–18), and the remaining 38 patients had low AAS (K≤3.7). Only 2 out of 21 patients treated orally/locally had high AAS (K>18), and the remaining 19 patients had low AAS (K≤4.53). Only 1 out of 16 patients treated intrarectally had medium AAS (K=16.4), whereas the remaining 15 patients had low AAS (K≤1.82). Patients treated orally/intrarectally had low AAS (K≤0.1). In summary, out of 122 patients treated with phages, 15 (12.3%) had high AAS (K>18) for 15–60 days of PT, 6 (4.9%) had medium AAS (K=5–18) for 7–91 days of PT, and 101 (82.8%) had low AAS (K<5). High antiphage activity of sera was observed only in some patients treated with phages locally, while orally treated patients had low AAS. In patients who applied phages locally or locally/orally with high AAS, we found only those treated with P. aeruginosa (n=1), S. aureus (n=13), or E. faecalis (n=1) phages who applied them locally or locally/orally was the AAS high (K>18) (Figs. 1 and 2). Some phages were more immunogenic: P. aeruginosa F-8, S. aureus 676/Z, S. aureus 676/F, S. aureus fi 200, and E. faecalis Ent 56 (Fig. 2). Of the 10 patients with K>18, shown in Figure 2, eight had a bone infection. Only case 8 had an upper respiratory tract infection, and case 13 had a soft tissue infection. The difference in K value between cases 10 and 15 (bone infections treated with the same phage; Fig. 2) may be caused by the different time of blood sampling (later in case 15). Furthermore, individual differences in immune response to phage for both patients cannot be excluded. High phage inactivation by sera from five patients receiving the S. aureus MS-1 phage cocktail (A5/80, 676/Z, P4) locally was observed (Fig. 1). The S. aureus MS-1 phage cocktail is composed of S. aureus phages: A5/80, 676/Z, and P4. All patients with K>18 treated locally with the S. aureus MS-1 phage cocktail had a bone infection. Phages S. aureus A5/80 and S. aureus P4 from the S. aureus MS-1 phage cocktail were more immunogenic than phage S. aureus 676/Z. Low phage inactivation by sera from six patients treated locally with the S. aureus MS-1 phage cocktail (A5/80, 676/Z, P4) was detected (data not presented). The K rate rose significantly (p<0.05, Wilcoxon test) in patients receiving phages orally, locally, orally and locally, or intrarectally (Table 3). The Mann–Whitney U-test revealed a statistically significant difference for the K index between orally and locally treated patients (mean increase in K was 0.08 and 11.34 respectively; p=0.001). Medium and high inactivation of phages by sera of patients receiving PT decreased after therapy. In a patient using S. aureus P4 phage locally for 91 days, we observed a reduction in the K rate 40 days after completion of therapy. In another patient using S. aureus fi 200 phage locally for 80 days, there a decrease in the K rate was detected 4 months after completion of therapy. In a patient using E. faecalis Ent 15/P phage intrarectally for 65 days, a decrease in the K rate was detected 7 days after completion of therapy. We also tested the possibility of cross-reaction of sera from seven patients treated with a specific staphylococcal phage used for treatment with other staphylococcal and coli phages from our collection (which were not administered to the patient). In the sera of four patients, we observed high AAS with phages used for treatment, in contrast to low AAS with other phages (the results for one patient are presented in Fig. 3). In three patients, we detected high AAS with the staphylococcal phage used or not used for treatment and low AAS with the coli phage (the results for one patient are presented in Fig. 4).
Table 2.
Antiphage Activity of Sera from 122 Patients Receiving Phage Therapy
| Route of phage administration | Percentage of patients with low levels of AAS during phage therapy (K<5) | Percentage of patients with medium levels of AAS during phage therapy (K = 5–18) | Percentage of patients with high levels of AAS during phage therapy (K>18) |
|---|---|---|---|
| Oral (n = 27) | 100% (n = 27) | 0% (n = 0) | 0% (n = 0) |
| Before therapy K = 0.00–0.33 | |||
| During therapy K = 0.00–1.04 | |||
| Local (n = 56) | 67.8% (n = 38) | 9% (n = 5) | 23.2% (n = 13) |
| Before therapy K = 0.00–1.64 | Before therapy K = 0.01–0.13 | Before therapy K = 0.00–0.53 | |
| During therapy K = 0.00–3.70 | During therapy K = 5.70–16.53 | During therapy K = 19.87–148.61 | |
| Oral and local (n = 21) | 90.5% (n = 19) | 0% (n = 0) | 9.5% (n = 2) |
| Before therapy K = 0.00–0.10 | Before therapy K = 0.03–0.25 | ||
| During therapy K = 0.00–4.53 | During therapy K = 41.05–227.96 | ||
| Intrarectal (n = 16) | 93.8% (n = 15) | 6.2% (n = 1) | 0% (n = 0) |
| Before therapy K = 0.00–0.35 | Before therapy K = 0.02 | ||
| During therapy K = 0.00–1.82 | During therapy K = 16.40 | ||
| Oral and intrarectal (n = 2) | 100% (n = 2) | 0% (n = 0) | 0% (n = 0) |
| Before therapy K = 0.00–0.10 | |||
| During therapy K = 0.002–0.10 | |||
| Total (n = 122) | 82.8% (n = 101) | 4.9% (n = 6) | 12.3% (n = 15) |
| Before therapy K = 0.00–1.64 | Before therapy K = 0.01–0.13 | Before therapy K = 0.00–0.53 | |
| During therapy K = 0.00–4.53 | During therapy K = 5.70–16.53 | During therapy K = 19.87–227.96 |
K<5 was associated with ≤50% neutralization at serum dilution ≤1:200. K = 5–18 was estimated between >50% neutralization at serum dilution ≥1:200 and <50% neutralization at serum dilution ≤1:800. K > 18 was estimated as ≥50% neutralization at serum dilution ≥1:800.
AAS, antiphage activity of sera.
FIG. 1.
Patients treated locally with Staphylococcus aureus MS-1 phage cocktail: (A) case 1, (B) case 2, (C) case 3, (D) case 4, and (E) case 5. The rates of phage inactivation (K) by sera were determined in the neutralization test. S. aureus MS-1 phage cocktail is composed of three phages: S. aureus A5/80, S. aureus 676/Z, and S. aureus P4.
FIG. 2.
High phage inactivation by sera from patients receiving Pseudomonas, Staphylococcus, or Enterococcus phages. Cases 6–12 and 14, local administration; cases 13 and 15, local and oral administration. The rates of phage inactivation (K) by sera were determined in the neutralization test. The height of the last bar does not correspond to the value written on the bar.
FIG. 3.
Rates of phage inactivation (K) by serum of patient treated locally with S. aureus 676/Z phage (case 11). Specific phage means phage lysing definite bacterial strain causing patients' illness. The rates of phage inactivation (K) by sera were determined in the neutralization test.
FIG. 4.
Rates of K by serum of patient treated locally with S. aureus 676/Z phage (case 7). Specific phage means phage lysing definite bacterial strain causing patients' illness. The rates of K by sera were determined in the neutralization test.
Interestingly, preliminary analysis performed in patients with high antiphage serum activity (K>18) showed that, in seven cases, a favorable response to PT (categories A–C) was observed, whereas there was an unfavorable response in eight others (categories D–G). The scale of the PT results was elaborated and presented in detail by Międzybrodzki et al. (10). This observation suggests that induction of antiphage activity of serum during or after PT does not exclude the good outcome of PT in general.
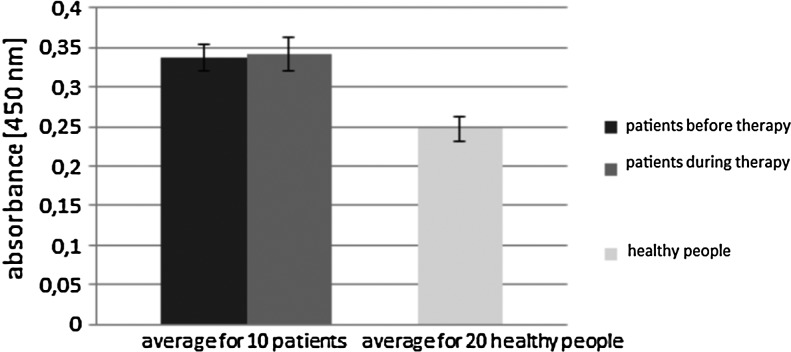
Our preliminary results from ELISA showed that sera of examined patients undergoing PT had higher levels of antiphage antibodies during treatment (oral and/or local administration) in about half of the cases. Furthermore, in some cases, that level was even insignificantly lower than before treatment. Estimation of the level of phage antibodies in sera of 10 patients treated with S. aureus A3/R phage is shown in Figure 5. Sera of four patients showed a higher immune response (higher level of antiphage antibodies). These results did not correspond with the route of administration (oral or local) (Fig. 5). Patient 3 who applied phage orally had a lower level of antiphage antibodies during phage treatment, and patient 6 had a higher level than before treatment. Patients treated with S. aureus 676/Z phage generally were characterized by a lower level of antibodies in sera (Fig. 6), but six of them showed a greater immune response during PT than those treated with phage A3/R (all patients applied phages locally). Statistical analysis of absorbance values in the ELISA test for two examined groups (receiving S. aureus A3/R and S. aureus 676/Z phages) was performed using Student's t-test (significance set at p<0.05). Results are presented in Table 4. Sera of three patients with K>18 during treatment with S. aureus 676/Z phage (measured by neutralization test) also indicated the highest increase in value of absorbance detected by ELISA assay. In the other patients investigated by ELISA, K<6. Statistical analysis of patients' K rate investigated using the neutralization test was performed using Wilcoxon test, shown in Table 5 (these patients were also examined by ELISA test). Comparison of the level of antiphage antibodies in human sera from patients treated with PT and healthy subjects detected by ELISA is shown in Figures 7 and 8. The results from ELISA indicate that the level of antiphage antibodies in sera of healthy subjects was only insignificantly lower than that in patients' sera (during treatment); for the group of patients receiving S. aureus A3/R phage, p>0.05 (Student's t-test), and for patients receiving S. aureus 676/Z phage, p>0.05 (Mann–Whitney U-test), in comparison to healthy controls. These results indicate that phages, which are well known for their vast abundance in the environment, can induce antibody production in healthy subjects.
FIG. 5.
Patients treated with S. aureus A3/R phage (local administration, patients 3 and 6 oral administration, 100-fold dilution of human sera). The level of antiphage antibodies (all human Igs) was determined in enzyme-linked immunosorbent assay (ELISA). Purified S. aureus phage preparations as antigens (108 pfu/mL) were used.
FIG. 6.
Patients treated with S. aureus 676/Z phage (local administration, 100-fold dilution of human sera). The level of antiphage antibodies (all human Igs) was determined in ELISA. Purified S. aureus phage preparations as antigens (108 pfu/mL) were used.
Table 4.
Mean Level of Absorbance Depending on the Type of Phage Used in Phage Therapy (Patients' Sera Investigated by ELISA Test)
| Type of phage used in PT | Mean absorbance±SD before phage therapy | Mean absorbance±SD during phage therapy | p (Student's t-test) |
|---|---|---|---|
| S. aureus A3/R (n=10) | 0.35±0.08 | 0.35±0.07 | 0.94 |
| S. aureus 676/Z (n=9) | 0.10±0.01 | 0.14±0.04 | 0.03* |
p<0.05.
ELISA, enzyme-linked immunosorbent assay.
Table 5.
Mean Rates of K Depending on the Type of Phage Used in Phage Therapy (Patients' Sera Investigated by Neutralization Test); These Patients Were Also Examined by ELISA
| Type of phage used in PT | Mean K±SD before phage therapy | Mean K±SD during phage therapy | p (Wilcoxon test) |
|---|---|---|---|
| S. aureus A3/R (n=10) | 0.39±0.04 | 0.97±2.00 | 0.01* |
| S. aureus 676/Z (n=9) | 0.08±0.11 | 8.07±11.11 | 0.01* |
p<0.05.
FIG. 7.
Patients treated with S. aureus A3/R phage and healthy people (100-fold dilution of human sera). The level of antiphage antibodies (all human Igs) was determined in ELISA. Purified S. aureus phage preparations as antigens (108 pfu/mL) were used.
FIG. 8.
Patients treated with S. aureus 676/Z phage and healthy people (100-fold dilution of human sera). The level of antiphage antibodies (all human Igs) was determined in ELISA. Purified S. aureus phage preparations as antigens (108 pfu/mL) were used.
Discussion
The examinations performed on healthy volunteers and on patients with bacterial infections indicated that bacteriophages may have an influence on induction of antiphage antibody production (9,11). A safety test of PT performed by Bruttin and Brüssow (3) on 15 adult healthy volunteers using E. coli T4 phage orally indicated no increase in level of antiphage IgG, IgM, and IgA in the serum after 1 month of the study. Our study of S. aureus 676/Z and S. aureus A3/R phage neutralization by sera of healthy volunteers indicated low inactivation of phages (K≤0.68 and K≤1.73 respectively; serum dilution up to 1:100). Kamme (8) also examined the level of antiphage antibodies in sera of 100 healthy volunteers, which showed a low level of antiphage antibodies for S. aureus phages mostly up to serum dilution 1:80. Pescovitz et al. (11) performed a study of 52 healthy volunteers who received the E. coli φX174 phage intravenously. E. coli φX174 phage was in the body for 3–4 days after administration. After this time, the production of IgM antibodies was induced, which removed E. coli φX174 phage before the 7th day. High levels of IgG antibodies were detected in the secondary response. The mean K was 9 on the 7th day after the first immunization, with the range between 1.5 and 50 (11). Kucharewicz-Krukowska and Ślopek (9) examined sera of 57 patients with bacterial infections in neutralization of phages used orally. Patients had mainly monoinfections (40 cases), and the other 17 cases had polyinfections. The predominating group of bacteria was Staphylococci, which caused 29/40 monoinfections. Also in our group of patients, the predominating group of bacteria was S. aureus, which caused 70/115 monoinfections. A low level of antiphage antibodies in serum dilution up to 1:40 with two exceptions was observed before PT in the studies by Kucharewicz-Krukowska and Ślopek (9). On the 10th day of oral PT, high AAS was observed in three cases (serum dilution 1:320–1:1,280), while in the other cases (n=54), AAS was low—serum dilution <1:160 (9). In our study, before PT, mean K=0.07±0.19 (serum dilution up to 1:100), while during therapy, it rose significantly (mean K=7.0±24.72; p<0.05, Wilcoxon test). In our study, out of 122 patients treated with phages, 15 (12.3%) had high AAS (K>18, serum dilution ≥1:800, ≥50% neutralization) for 15–60 days of PT; 6 (4.9%) had medium AAS (K=5–18, serum dilution between 1:200 [>50% neutralization] and 1:800 [<50% neutralization]) for 7–91 days of PT; and 101 (82.8%) had low AAS (K<5, serum dilution ≤1:200, <50% neutralization). In patients who applied phages locally or locally/orally with high AAS (K>18), we found only those treated with P. aeruginosa (n=1), S. aureus (n=13), or E. faecalis (n=1) phages who applied them locally or locally/orally was the AAS high (K>18) (Figs. 1 and 2). Patients treated orally had low AAS (K≤1.04, serum dilution up to 1:100). A uniform pattern for antiphage activity of sera after PT is hard to establish because it varies from patient to patient. It reflects different immunogenicity of phages, total doses, and other factors. The results presented in the work (e.g., antiphage activity of sera after PT) are completely new observations and cannot be compared to previous research due to the lack of publications in this field. A correlation between K rate and the level of absorbance (K>18 and high antibody production) was noted in three patients treated with S. aureus 676/Z phage. Such a correlation was not found in the group of patients treated with S. aureus A3/R phage and healthy subjects.
Kamme (8) examined antiphage antibodies in the course of staphylococcal infection of 68 patients. Kamme supposed that lysogenic bacterial strains may produce phages, which induce antiphage antibodies. An increased level of antiphage antibodies was observed between 6 and 14 days of the disease course. Also, for 20 patients with staphylococcal infections, antiphage antibody titers during exacerbation and regression of the infection were examined (7). Kamme concluded that the level of antiphage antibodies depended on the form of infections and intensity of the disease.
Kucharewicz-Krukowska and Ślopek (9) analyzed the influence of high antiphage activity of sera of two patients on the result of PT. In one case, they observed negative results, and in the other, transient improvement. However, the weak effect of PT could be connected to different factors. Our observation of 15 patients with high antiphage activity of sera indicated that there was clinical improvement in seven patients, while in the remaining eight patients, therapy was without clear effect. This suggests that the induction of AAS during or after PT does not exclude a positive result of PT. The different immunogenicity of phages, even those infecting the same host, suggests that the high variability of protein sequences in phage structural proteins may be responsible for the observed differences in immune response. Statistical analysis (Wilcoxon test) of our results indicated a significant increase in the K rate in patients receiving phages orally, locally, orally and locally, or intrarectally. The Mann–Whitney U-test revealed a statistically significant difference in the increase in K rate during PT between orally and locally treated patients.
Conclusions
Our results indicate that low levels of antiphage activity of patients' sera were detected before PT (K≤1.64). Sera of healthy volunteers caused low neutralization of phages (K≤1.73). The results from ELISA indicate that levels of antiphage antibodies in sera of healthy subjects were only insignificantly lower than those in patients' sera (during treatment).
High antiphage activity of sera (K>18) during PT was observed in some patients (12.3%) receiving phages locally (n=13) or locally/orally (n=2) for 15–60 days of PT, while oral administration of phages weakly induced a humoral response (K≤1.04). As mentioned above, sera of three patients, treated with S. aureus 676/Z phage, with K>18 (during therapy), also showed a high immune response detected by ELISA, measured by the increase in value of absorbance (p<0.05, Student's t-test). In the rest of the patients from the same group investigated by ELISA, K<6, and we did not observe such a correlation (the level of absorbance during therapy was lower or higher than before treatment).
In some patients treated with P. aeruginosa (n=1), S. aureus (n=13), or E. faecalis (n=1) phages who applied them locally or locally/orally, we found high antiphage activity of sera. Medium or high inactivation of phages by sera of patients receiving PT decreased after therapy.
Our preliminary observations performed in 15 patients with high antiphage activity of sera suggest that the induction of antiphage activity of patients' sera during or after PT does not exclude a good result of PT.
These results suggest that the antiphage activity in patients' sera depends on the route of phage administration (high AAS was observed in some patients treated with phages locally, while orally treated patients had low AAS), phage type, and possibly the immune status of the patient (5), while the clinical significance of antiphage antibodies appearing in sera of patients receiving PT needs to be further investigated.
Acknowledgments
This study was supported by the grant Operational Programme—Innovative Economy 2007–2013 (OP IE) “Optimization of the characteristics and production of therapeutic bacteriophage” (POIG.01.03.01-00-003/08).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Adams MH. Antigenic properties. In: Bacteriophages. New York: Interscience, 1959a:96–119 [Google Scholar]
- 2.Adams MH. Methods of study of bacterial viruses. In: Bacteriophages. New York: Interscience, 1959b:443–522 [Google Scholar]
- 3.Bruttin A, and Brüssow H. Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrob Agents Chemother 2005;49:2874–2878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Górski A, Borysowski J, Międzybrodzki R, and Weber-Dąbrowska B. Bacteriophages in medicine. In: McGrath S. and van Sinderen D, eds. Bacteriophage. Genetics and Molecular Biology. Norfolk, United Kingdom: Caister Academic Press, 2007:125–157 [Google Scholar]
- 5.Górski A, Międzybrodzki R, Borysowski J, et al. Phage as a modulator of immune responses: practical implications for phage therapy. Adv Virus Res 2012;83:41–71 [DOI] [PubMed] [Google Scholar]
- 6.Gratia A. Des relations numeriques entre bacteries lysogenes et particles de bacteriophage. Ann Inst Pasteur 1936;57:652–676 [Google Scholar]
- 7.Hedström SA, and Kamme C. Antibodies against staphylococcal bacteriophages in human sera. II. Assay of antibodies in exacerbation and regression of chronic staphylococcal osteomyelitis. Acta Pathol Microbiol Scand B Microbiol Immunol 1973;81:749–752 [PubMed] [Google Scholar]
- 8.Kamme C. Antibodies against staphylococcal bacteriophages in human sera. I. Assay of antibodies in healthy individuals and in patients with staphylococcal infections. Acta Pathol Microbiol Scand B Microbiol Immunol 1973;81:741–748 [PubMed] [Google Scholar]
- 9.Kucharewicz-Krukowska A, and Ślopek S. Immunogenic effect of bacteriophage in patients subjected to phage therapy. Arch Immunol Ther Exp 1987;35:553–561 [PubMed] [Google Scholar]
- 10.Międzybrodzki R, Borysowski J, Weber-Dąbrowska B, et al. Clinical aspects of phage therapy. Adv Virus Res 2012;83:73–121 [DOI] [PubMed] [Google Scholar]
- 11.Pescovitz MD, Torgerson TR, Ochs HD, et al. Effect of rituximab on human in vivo antibody immune responses. J Allergy Clin Immunol 2011;128:1295–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stashak PW, Baker PJ, and Roberson BS. The serum antibody response to bacteriophage phiX174 in germ-free and conventionally reared mice. Immunology 1970;18:307–317 [PMC free article] [PubMed] [Google Scholar]
- 13.Sulakvelidze A, and Barrow P. Phage therapy in animals and agribusiness. In: Kutter E. and Sulakvelidze A, eds. Bacteriophages. Biology and Applications. Boca Raton, FL: CRC Press, 2005:335–380 [Google Scholar]
- 14.Waddell TE, Franklin K, Mazzocco A, and Johnson RP. Preparation and characterization of anti-phage serum. In: Clokie MRJ. and Kropinski AM, eds. Bacteriophages: Methods and Protocols. Vol. 1. Isolation, Characterization, and Interactions. New York: Humana Press, 2009:287–292 [DOI] [PubMed] [Google Scholar]