Abstract
Communication and swallowing deficits are common in Parkinson’s disease (PD). Evidence indicates that voice and speech dysfunction manifest early, prior to motor deficits typically associated with striatal dopamine loss. Unlike deficits in the extremities, cranial sensorimotor deficits are refractory to standard dopamine-related pharmacological and surgical interventions, thus the mechanisms underlying vocal deficits are unclear. While neurotoxin models have provided some insight, they typically model nigrostriatal dopamine depletion and are therefore limited. Widespread alpha-synuclein (aSyn) pathology is common to familial and sporadic PD, and transgenic mouse models based on aSyn over-expression present a unique opportunity to explore vocalization deficits in relation to extra-striatal, non-dopaminergic pathologies. Specifically, mice over-expressing human wild-type aSyn under a broad neuronal promoter (Thy1-aSyn) present early, progressive motor and non-motor deficits starting at 2–3 months, followed by parkinsonism with dopamine loss at 14 months. We recorded ultrasonic vocalizations from Thy1-aSyn mice and wild-type (WT) controls at 2–3, 6–7 and 9 months. Thy1- aSyn mice demonstrated early, progressive vocalization deficits compared to WT. Duration and intensity of calls were significantly reduced and call profile was altered in the Thy1-aSyn mice, particularly at 2–3 months. Call rate trended towards a more drastic decrease with age in the Thy1-aSyn mice compared to WT. Alpha-synuclein pathology is present in the periaqueductal gray and may underlie the manifestation of vocalization deficits. These results indicate that aSyn over-expression can induce vocalization deficits at an early age in mice and provides a new model for studying the mechanisms underlying cranial sensorimotor deficits and treatment interventions for PD.
Keywords: alpha-synuclein, Parkinson’s disease, ultrasonic vocalization, cranial sensorimotor deficits, mouse
Introduction
Cranial sensorimotor dysfunction, such as speech, voice and swallowing deficits, are common in Parkinson’s disease (PD) affecting up to 90 % of individuals and are associated with negative consequences to quality of life (Fuh et al., 1997; Ho, Iansek, Marigliani, Bradshaw, & Gates, 1998; Marras et al., 2008; Plowman-Prine, Sapienza, et al., 2009). Specifically, deficits may include a hoarse, breathy voice quality, a variable rate of speech often characterized by short rushes of speech, reductions in loudness and pitch variability (Darley, Aronson, & Brown, 1968, 1969; Hammer & Barlow, 2010; Ho et al., 1998; Plowman-Prine, Okun, et al., 2009), as well as reduced harmonics to noise ratio, and irregular vibratory patterns in sustained phonation (Rusz, Cmejla, Ruzickova, & Ruzicka, 2011) all of which may affect communication ability (Darley et al., 1968, 1969; Ho et al., 1998; Plowman-Prine, Okun, et al., 2009). Importantly, voice changes may manifest as one or more of the previously mentioned deficits and there is evidence that the onset of cranial sensorimotor deficits occurs early in the disease process, prior to the onset of cardinal signs (resting tremor, bradykinesia, muscle rigidity) typically associated with striatal dopamine loss (B. Harel, Cannizzaro, & Snyder, 2004; B. T. Harel, Cannizzaro, Cohen, Reilly, & Snyder, 2004; Postuma, Lang, Gagnon, Pelletier, & Montplaisir, 2012; Stewart et al., 1995; Sung et al., 2010), suggesting that voice deficits could provide an early biomarker for diagnosis. The underlying mechanisms and the role of nigrostriatal dopamine loss on vocal deficits remain unclear. Cranial sensorimotor deficits are not fully amenable to traditional pharmacological and surgical interventions that are predicated on nigrostriatal dopamine deficiency and are aimed at restoring dopamine (e.g., carbidopa/ levodopa) or at modulating the basal ganglia circuits affected by dopamine loss (e.g., deep brain stimulation) (Ciucci, Barkmeier-Kraemer, & Sherman, 2008; D'Alatri et al., 2008; Pinto et al., 2004). Studies aimed at gaining a better understanding of the onset and neurodegenerative processes underlying cranial sensorimotor deficits are indicated and can facilitate the development of improved treatment and potentially earlier diagnosis.
Germane to studying cranial sensorimotor deficits are rodent models of vocalization. Rodents produce ultrasonic vocalizations (USV), which are analogous to human vocalizations in several aspects. The origin of rodent USV is the larynx and vocalizations are produced via modulation of egressive airflow (Johnson, Ciucci, Russell, Hammer, & Connor, 2010; Riede, 2011). USVs are communicative in nature, and are capable of eliciting a response from the listener (Brudzynski, 2005; Brudzynski & Ociepa, 1992; Brudzynski & Pniak, 2002; McGinnis & Vakulenko, 2003). These vocalizations are vulnerable to nigrostriatal dopamine loss in a rat model of PD. Unilateral infusions of 6-hydroxydopamine (6-OHDA) into the medial forebrain bundle results in USV deficits (Ciucci et al., 2009; Ciucci et al., 2007) that are analogous to vocal deficits seen in humans, such as reduced frequency variability and reduced intensity (Darley et al., 1968, 1969; Hammer & Barlow, 2010; Ho et al., 1998; Plowman-Prine, Okun, et al., 2009). However, the 6- OHDA model is limited in that it represents an acute, non-progressive neuronal loss and a limited pathology (nigrostriatal dopamine depletion), that does not encompass the full disease process (Chesselet, 2008).
While the etiology of idiopathic PD remains unknown, genetics of familial cases have provided some insight (Martin, Dawson, & Dawson, 2010) and build the foundation for development of transgenic animal models. Alpha-Synuclein (aSyn), a vesicular membrane binding protein, in particular, has been implicated in the pathology of PD for several reasons. In addition to being one of the primary components of the inclusion bodies that characterize the brains of most individuals with PD (Braak et al., 2003; Jensen & Gai, 2001; Perrin, Woods, Clayton, & George, 2000; Spillantini et al., 1997), overexpression or mutation of aSyn in humans results in PD (Chartier-Harlin et al., 2004; Ibanez et al., 2004; Singleton et al., 2003; Zarranz et al., 2004), and recent studies have identified genetic variability in the gene for aSyn as a risk factor for sporadic PD (Hardy, 2010). Based on this, transgenic mouse models have been developed that exploit the relationship between aSyn and PD and result in varying degrees of sensorimotor and behavioral deficits as well as neuropathological changes (Chesselet & Richter, 2011). Specifically, transgenic mice overexpressing human wild-type aSyn under the Thy-1 promoter (Thy1-aSyn) develop motor deficits beginning as early as 2 months of age, and striatal dopamine loss at 14 months of age (Chesselet et al., 2012; Fleming et al., 2004b; Lam et al., 2011). However, cranial sensorimotor deficits have not been characterized in this model. Thus, the purpose of this study is to determine if Thy1-aSyn mice demonstrate early and progressive vocalization deficits in addition to fine limb motor deficits. Here we report that Thy1-aSyn mice demonstrate decreased duration of multiple call types as well as an altered call profile compared to wild-type (WT) littermates at 2–3 months of age and that vocalization deficits are progressive in nature. Importantly, aSyn aggregates are evident in the periaqueductal gray (PAG), a region of the brainstem important for the coordination of vocal behaviors (Ennis, Xu, & Rizvi, 1997), indicating a possible mechanism for vocalization deficits. Thy1-aSyn mice represent the first genetic model available to study the pathogenesis of and potential therapeutic interventions for cranial sensorimotor deficits in PD.
Methods
Animals
Transgenic mice used in this study were previously created to overexpress the human wild-type aSyn under the Thy-1 promoter (Rockenstein et al., 2002) on a mixed C57BL/6-DBA/2 background. Animals were maintained for more than 10 generations on the hybrid C57BL/6-DBA/2 background by mating female heterozygous for the transgene with male wild type (WT) mice on the hybrid background obtained from Charles River Laboratories, Inc. (Wilmington, MA; (Fernagut et al., 2007b; Fleming et al., 2004a; Fleming et al., 2006)). Only male mice were recorded and analyzed, as Thy1-aSyn mice carry the transgene on the X chromosome, and female mice show a much reduced phenotype or no phenotype in some measures, likely due to the reduced expression pattern of the transgene as a consequence of random inactivation of one X chromosome. USV were collected from a total of 46 male Thy1- aSyn and 36 male WT littermates between the ages of 2 and 9 months. However, given that not all mice vocalized (see Results; Figure 2), only 36 Thy1-aSyn and 22 WT were used in the acoustic analysis. WT females between the ages of 2 and 6 months were used to elicit vocalizations. Vocalizations were analyzed at 3 different ages: 2–3 months (14 Thy1-aSyn, 12 WT), 6–7 months (7 Thy1-aSyn, 7 WT), and 9 months (5 Thy1-aSyn, 3 WT). Animal care was conducted in accordance with the United States Public Health Service Guide for the Care and Use of Laboratory Animals, and procedures were approved by the Institutional Animal Care and Use Committee at the University of California Los Angeles (UCLA). Animals were maintained on a reverse light/dark cycle with lights off at 10 am and all testing was performed between 12 and 4 pm during the dark cycle under low light. Food and water were available ad libitum. All animals were housed socially and were sexually experienced in the same manner.
Figure 2. Percent of mice that vocalized.
The percent of mice that called in the Thy1-aSyn condition trended towards reductions at 6–7 months and 9 months compared to 2–3 months. In the WT condition, the percent of mice that vocalized increased at 6–7 months compared to 2–3 months, but was comparable to the Thy1-aSyn condition at 9 months. The total number of animals in each group at each time point is displayed on the bars.
Behavioral Tests
USV recording
Vocalizations were recorded with an ultrasonic microphone with a flat frequency response up to 150 kHz and a working frequency response range of 10–180 kHz (CM16, Avisoft, Germany), 16-bit depth and sampled at 250,000 Hz. The microphone was fixed to a tripod and placed in a cage with clean bedding. Males were isolated from their cage-mates and immediately placed in the cage with a sexually receptive female. Females were judged to be sexually receptive by the presence of the following behaviors: ear wiggling, darting, and lordosis. The male was allowed to explore until he demonstrated interest in the female (sniffing, mounting, vocalizing). The female was then removed, in order to ensure that all subsequent vocalizations originated from the male, and recording began immediately. If the male did not call after the female, bedding from a female cage was placed in the cage to elicit vocalizations, particularly with Thy1-aSyn and WT mice above 3 months of age, which called less readily. Although we did not specifically test or control for this variable and it is possible that call structure and profile varied subtly with the addition of soiled bedding, there did not appear to be any differences. In some instances the female had urinated and/or defecated in the cage of the male, leaving behind this stimulus after she had been removed. Adding soiled bedding from the female in the cage, where no or less of such a marker was left behind, reduced variability by presenting the male mice with comparable stimulating environment. Recording lasted between 1–6 minutes per animal, depending on how readily the animal called.
Analysis
All analyses were performed with the investigator masked to experimental condition.
USV Analysis
Acoustic analysis was performed offline with a customized automated program, SASLab Pro (Avisoft, Germany). Spectrograms were built from each waveform with a frequency resolution set to a fast Fourier transform (FFT) of 512 points, frame size of 100% with a flat top window, and the temporal resolution was set to display 75% overlap. The percent of mice that vocalized as well as call rate (calls per second for the first 60 seconds after vocalizing commenced) were quantified and are described below. Mice produce multiple types of vocalizations, or calls, that are visually and acoustically distinct based on the manner of frequency modulation (Figure 1). There are multiple ways to classify and analyze rodent ultrasonic vocalizations and, notably, there is not one universally accepted method (Ciucci et al., 2009; Ciucci et al., 2007; Ey et al., 2013; Hammerschmidt, Radyushkin, Ehrenreich, & Fischer, 2012; Mahrt, Perkel, Tong, Rubel, & Portfors, 2013; Portfors, 2007; Scattoni, Gandhy, Ricceri, & Crawley, 2008; Wright, Gourdon, & Clarke, 2010). Given this, the purpose of this experiment was to evaluate and characterize vocalizations in a pre-manifest rodent model of PD, allowing for comparisons with other PD models of vocalization deficits (Ciucci et al., 2009; Ciucci et al., 2007) as well as to vocal deficits experienced by individuals with PD. The classification scheme used by Portfors (2007) is similar to that used previously for analysis of rat vocalizations (Ciucci et al., 2009; Ciucci et al., 2007), therefore, calls were first classified into 9 categories (adapted from Portfors, 2007) to ensure that values for acoustic parameters were detectable and not masked due to combination of inherently (acoustically) disparate call types. The proportion of each call type was calculated to determine an overall call profile (Wright et al., 2010). Thus, the call profile was calculated as the percent of each call type relative to all the calls a given mouse produced.
Figure 1. Call types.
USV were classified as one of 9 call types (adapted from (Portfors, 2007).
The following acoustic measures were examined within each of the 9 call types: average duration (start minus end time) in seconds, average bandwidth (highest minus lowest frequency) in kiloHertz (kHz) and intensity range (highest minus lowest intensity within the sample). Intensity range in decibels (dB), rather than an average intensity value, was used in order to control for variability in mouth to microphone distance as the mouse moved around the cage during the recording.
Immunohistochemistry
Male Thy1-aSyn and WT littermates aged 5 months (WT n = 5; Thy1-aSyn n = 7) were used. Mice were deeply anesthetized with pentobarbital (100 mg/kg, ip) and intracardially perfused with 0.1M phosphate buffered saline (PBS, pH 7.4) at room temperature, followed by 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4). Brains were quickly removed, post-fixed in the same fixative overnight at 4°C, cryoprotected in 30% sucrose in 0.1M PBS, frozen on powdered dry ice and stored at −80°C. Coronal brain sections (40 µm) were cut on a Leica CM 1800 cryostat (Deerfield, IL) and processed for aSyn immunostaining as in Fernagut et al., 2007(Fernagut et al., 2007a). Sections were washed in phosphate buffered saline (PBS, pH 7.4) then incubated with 5µg/ml proteinase K (Invitrogen, Carlsbad, CA, USA) for 10 minutes (min). Endogenous peroxidase activity was blocked with 0.5% H2O2 in PBS for 15 min. Nonspecific binding was blocked with mouse IgG blocking reagent M.O.M kit (Vector Laboratories, Burlingame, CA, USA) for the primary antibody to aSyn made in mouse for 1 hour at room temperature. Sections were incubated with mouse anti-aSyn which recognizes both mouse and human aSyn (1:250, Cat # 610787, BD Biosciences, San Jose, CA) at 4°C overnight. After PBS washes, sections were incubated with secondary antibody biotinylated goat anti-mouse IgG (1:200, Vector Laboratories, CA) for 2 hours. The avidin-biotin complex method was used to detect the secondary antibody (ABC elite kit, Vector laboratories, Burlingame, CA) and the reaction product was visualized by 3, 3’-diaminobenzidine tetrachloride (DAB, Sigma, St Louis, MO). Sections were dehydrated and cleared with xylene, mounted with Eukit mounting medium (Calibrated Instruments, Hawthorne, NY), and examined under bright-field illumination with a Zeiss Axioskop microscope (Thornwood, NY). Digital images were captured by a Spot digital camera (Sterling Heights, MI). Control sections were incubated with mouse IgG1 (1 µg/ml, Sigma) at the same concentration correlated to the primary antibody used in experimental sections.
Quantification of proteinase K resistant aSyn in periaquaduct gray (PAG)
Quantification was performed on brain sections at the level of Bregma −3.40 mm from WT (n = 3) and transgenic mice (n = 4). The contour of the PAG was delineated with a 5× objective using the Stereo Investigator software (MicroBrightField, Colchester, VT) coupled to a Leica DM-LB microscope with a Ludl XYZ motorized stage and z-axis microcator (MT12, Heidenheim, Traunreut, Germany). The contour was then divided into dorsal and ventral PAG by the midline of aqueduct. Two images from dorsal or ventral PAG in each animal were acquired using Stereo Investigator at 40×. Images of the PAG were transformed to 8 bit files using ImageJ software (ImageJ software, version 1.38×, National Institutes of Health). In order to perform the particle analysis in ImageJ the threshold was set manually to ensure the inclusion of all aggregates. The area occupied by aggregates in the set threshold was measured using Image J and percentage of area occupied by proteinase K resistant aSyn aggregates was calculated. The mean of WT animals (background) was subtracted from values measured in the Thy1-aSyn mice (WT dorsal PAG 0.32 ± 0.005, WT ventral 0.34 ± 0.023, Mean ± SEM, n=3).
Statistical Analysis
As reported in the Results section, few mice in both the WT and Thy1-aSyn groups called at 9 months of age. As such, Analysis of Variance was not possible. Alternatively, we tested with a priori planned comparisons between groups at each time point. Descriptive data for the 9 month old mice is also provided. To test for significant differences between Thy1-aSyn and WT mice on call complexity, duration, bandwidth and intensity for each call type, we used a two-sample t-test (unequal variance) to compare the Thy1-aSyn and WT mice at 2–3 months and 6–7 months. The alpha level was set a priori as less than 0.05 and corrections for multiple comparisons within each call type were made, resulting in an alpha of 0.0125. However, given the exploratory nature of this study and the associated risk of beta error, interpretation of p-values for both corrected and uncorrected alpha levels are presented.
Results
Acoustic analysis of the vocalizations revealed significant differences between the Thy1-aSyn and WT controls as well as several trends. Descriptive statistics for percent call type, duration, bandwidth and intensity are displayed in Table 1 for Thy1-aSyn and WT mice at 2–3, 6–7 and 9 months.
Table 1.
Summary of dependent variables.
| Condition | Age (months) |
Call Type Mean (SEM) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Simple | Upsweep | Downsweep | Jump Up | Jump down | Harmonic | Half Cycle | Cycle | Two Cycle | |||
| Percentage Call Type | Wild-type | 2–3 | 8.62(0.94) | 25.65(4.39) | 1.13(0.33) | 1.91(0.72) | 17.16(4.34) | 24.21(7.13) | 16.65(2.62) | 4.06(1.45) | 2.18(0.86) |
| 6–7 | 14.08(3.76) | 44.96(5.24) | 2.28(1.23) | 5.08(2.55) | 6.60(1.91) | 10.30(1.94) | 13.92(2.44) | 2.01(0.85) | 0.69(0.46) | ||
| 9 | 15.34(9.94) | 14.87(10.10) | 7.22(3.44) | 3.33(2.17) | 6.95(4.34) | 32.77(11.88) | 13.55(2.24) | 3.06(0.66) | 2.90(2.90)^ | ||
| Thy1-aSyn | 2–3 | 11.51(2.55) | 32.95(5.66) | 1.73(0.88) | 1.79(0.55) | 20.86(5.10) | 16.44(4.51) | 13.08(1.73) | 1.45(0.22) | 0.18(0.10) | |
| 6–7 | 7.24(2.18) | 29.82(12.27) | 1.60(0.80) | 1.04(0.45) | 27.78(8.63) | 8.88(4.54) | 20.37(6.67) | 2.73(1.16) | 0.54(0.42) | ||
| 9 | 8.24(3.55) | 41.61(10.48) | 1.17(0.94) | 3.63(1.74) | 20.07(11.24) | 4.12(3.55) | 20.12(7.76) | 0.22(0.22)^ | 0.22(0.22)^ | ||
| Duration (Sec) | Wild-Type | 2–3 | 0.0263(0.0019) | 0.0312(0.0019) | 0.0472(0.0121) | 0.0285(0.0238) | 0.0440(0.0035) | 0.698(0.0004) | 0.0532(0.0025) | 0.0826(0.0076) | 0.1034(0.0076) |
| 6–7 | 0.0206(0.0032) | 0.0263(0.0017) | 0.0473(0.0070) | 0.0259(0.0016) | 0.0377(0.0037) | 0.0593(0.0074) | 0.0500(0.0014) | 0.0792(0.0054) | 0.0823(0.0170) | ||
| 9 | 0.0236(0.0069) | 0.0236(0.0065) | 0.0639(0.0149) | 0.0388(0.0110) | 0.0468(0.0017) | 0.0884(0.0234) | 0.0590(0.0025) | 0.0779(0.0108) | 0.1331(N/A)^ | ||
| Thy1-asyn | 2–3 | 0.0235(0.0020) | 0.0293(0.0014) | 0.0339(0.0054) | 0.0272(0.0018) | 0.0323(0.0010) | 0.0515(0.0029) | 0.0450(0.0025) | 0.0580(0.0054) | 0.0947(0.0037) | |
| 6–7 | 0.0235(0.0020) | 0.0293(0.0014) | 0.0339(0.0054) | 0.02772(0.0018) | 0.0323(0.0010) | 0.0515(0.0029) | 0.0450(0.0025) | 0.0580(0.0054) | 0.0947(0.0037) | ||
| 9 | 0.0224(0.0043) | 0.0263(0.0035) | 0.0300(0.0016) | 0.0194(0.0036) | 0.0334.(0.0045) | 0.0592(0.0137) | 0.0438(0.0048) | 0.0637(N/A)^ | 0.0875(N/A)^ | ||
| Bandwidth (Hz) | wild-type | 2–3 | 7016(323) | 21558(1001) | 18891(3215) | 20665(4096) | 29919(2667) | 29441(2234) | 29768(2586) | 32134(3191) | |
| 6–7 | 6103(792) | 25301(2231) | 21946(4129) | 26596(4989) | 26324(3116) | 28067(3122) | 31501(5293) | 35640(2240) | |||
| 9 | 15820(8474) | 24552(5704) | 29736(6281) | 29580(1030) | 30105(4299) | 36766(6017) | 57141(13916) | 53050(N/A)^ | |||
| Thy1-asyn | 2–3 | 7294(442) | 20969(1134) | 17548(3640) | 21985(4205) | 23337(2461) | 26956(1724) | 22174(2680) | 30997(2714) | ||
| 6–7 | 6861(806) | 22731(2004) | 18400(4950) | 31487(3834) | 30197(510) | 35111(3191) | 36653(3782) | 58685(14265) | |||
| 9 | 6621(1590) | 22107(3238) | 15689(989) | 25338(5554) | 28741(17156) | 27990(2710) | 36925(N/A)^ | 45425(N/A)^ | |||
| Intensity Range (dB) | wild-type | 2–3 | 16.26(2.70) | 23.37(3.00) | 8.42(1.26) | 10.40(1.74) | 18.40(3.12) | 18.65(4.23) | 22.65(1.69) | 14.80(2.46) | 12.13(5.08) |
| 6–7 | 23.02(4.10) | 27.87(3.12) | 18.78(3.38) | 12.59(5.13) | 19.51(3.14) | 28.41(3.71) | 27.02(1.96) | 16.22(3.60) | 20.46(7.71) | ||
| 9 | 21.03(5.58) | 22.67(10.47) | 13.26(5.88) | 14.71(1.04) | 16.96(5.38) | 29.73(2.18) | 21.71(2.44) | 21.66(4.00) | 2.97(N/A)^ | ||
| Thy1-asyn | 2–3 | 16.76(1.65) | 23.78(1.27) | 10.78(3.69) | 9.98(2.48) | 23.10(2.77) | 18.74(2.37) | 22.97(1.45) | 11.63(3.87) | 7.29(1.36) | |
| 6–7 | 10.34(1.23) | 19.21(2.59) | 15.52(7.84) | 9.79(2.91) | 26.23(4.76) | 24.15(2.00) | 29.29(3.22) | 15.61(3.12) | 10.44(6.43) | ||
| 9 | 6.91(4.77) | 17.82(3.55) | 18.64(N/A)^ | 7.33(1.11) | 20.68(7.66) | 21.65(10.13) | 15.35(5.15) | 4.66(N/A)^ | 2.97(N/A)^ | ||
Data are expressed as mean and SEM of call type (percentage), duration (seconds), bandwidth (Hz), and intensity range (dB) for WT and Thy1-aSyn at 2–3 months, 6–7 months, and 9 months.
Comparisons were made using a two-sample t-test (unequal variance);
Based on data from one animal.
Shaded regions indicate that measurements were not calculated.
Percent of mice that called
Overall, mice in the Thy1-aSyn condition vocalized less frequently as they aged, with fewer mice vocalizing at 6–7 months and 9 months relative to 2–3 months. While the percent of WT mice followed a similar trend as the Thy1-aSyn at 2–3 months and 9 months, more WT mice called at 6–7 months compared to Thy1-aSyn mice (Figure 2).
Call rate
Both the Thy1-aSyn and WT mice had similar call rates at 2–3 months, at approximately 2.5 calls per second. There was a trend towards a decrease in call rate in both groups over time, but to a greater degree in the Thy1-aSyn. Thy1-aSyn mice demonstrated a 50 % decrease at 6–7 months and a 62 % decrease at 9 months, while mice in the WT condition only decreased by 18% and 38 % at the 6–7 and 9 month time points, respectively (Figure 3).
Figure 3. Call Rate.
Data are expressed as mean and SEM. The trend towards reduced call rates at 6–7 and 9 months was more pronounced in the Thy1-aSyn condition compared to WT. Total number of animals in each group at each time point is displayed on the bars.
Call Profile
The overall composition of call types was different for Thy1-aSyn compared to WT controls at 2- 3 months and 6–7 months. The percent of the two cycle call type at 2–3 months was significantly reduced (almost 12-fold) in the Thy-aSyn condition compared to WT prior to correcting for multiple comparisons (t(8.21)=−2.3143, p=0.0486). The percent of the jump down call type at 6–7 months was also significantly reduced in the WT condition compared to Thy1-aSyn prior to correcting for multiple comparisons (t(6.811)=2.7047, p=0.0312), a 4-fold difference between the genotypes (Figure 4).
Figure 4. Distribution of Call Types.
Data are expressed as mean and SEM. The percent of two-cycle calls was significantly reduced in the Thy1-aSyn condition at 2–3 months compared to WT, while the percent of jump down calls was significantly reduced in the WT condition at 6 –7 months compared to the Thy1-aSyn condition. Comparisons were made using a two-sample t-test (unequal variance); horizontal bars indicate significant differences between conditions, *p-value < 0.05 and ^p-value < 0.0125 compared to WT at same age.
Duration
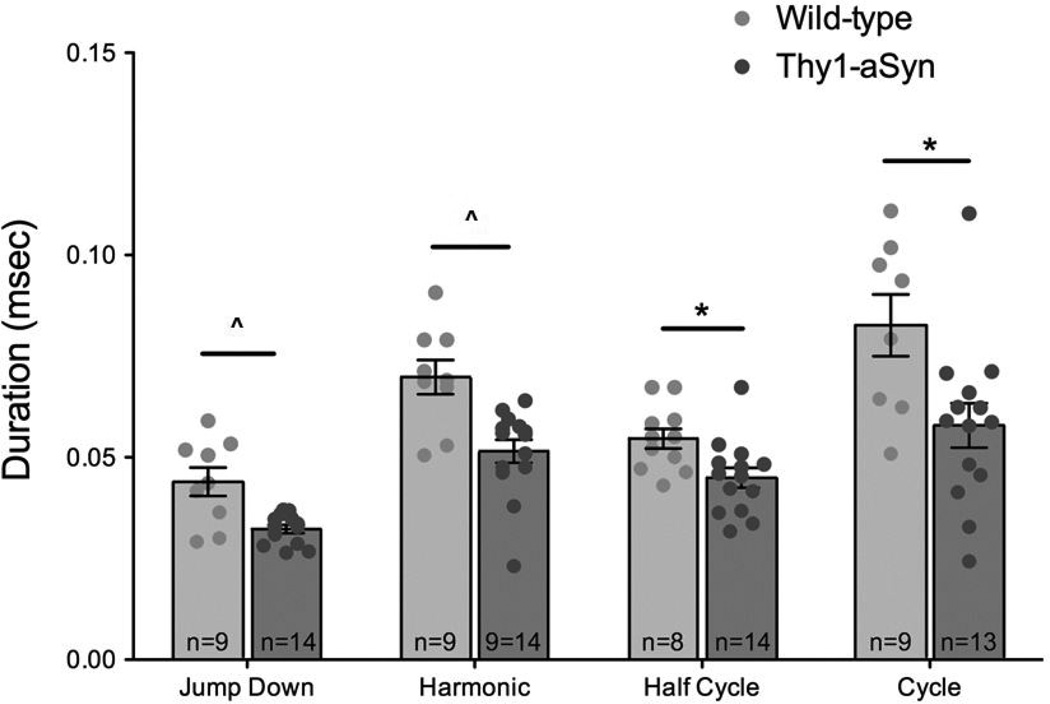
At 2–3 months, average duration was significantly decreased in the Thy1-aSyn condition compared to the WT for harmonic calls (t(15.179)=−3.5936, p=0.0026) and jump down calls (t(9.402)=−3.177, p=0.0106), 26 % decreases for both call types. In addition, duration was decreased in Thy1-aSyn mice by 15 % in half cycle calls (t(19.516)=−2.337), p=0.0302) and 30 % in cycle calls (t(14.056)=−2.6272, p=0.0198), prior to correcting for multiple comparisons (Figure 5 – 6). At 6–7 months, the average duration was not significantly different.
Figure 5. Duration at 2–3 Months.
Data are expressed as mean and SEM. Average duration was significantly reduced in Thy1-aSyn condition at 2–3 months compared to WT for jump down, harmonic, half cycle, and cycle calls. Comparisons were made using a two-sample t-test (unequal variance); horizontal bars indicate significant differences between conditions, *p-value < 0.05 and ^p-value < 0.0125 compared to WT at same age. The total number of animals in each group at each time point is displayed on the bars.
Figure 6. Duration at 2 –3 Months, Sample Calls.
Examples of Jump Down (left), Half Cycle, Cycle and Harmonic calls from WT (top row) and Thy1-aSyn mice (bottom row). Frequency in kilohertz is on the yaxis, time in seconds on the x-axis.
Intensity
The intensity range of simple calls was significantly reduced by more than 50 % in the Thy1-aSyn condition at 6–7 months compared to WT prior to correcting for multiple comparisons (t(5.545)=−2.974, p=0.0273) (Figure 7).
Figure 7. Intensity Range of Simple Calls.
Data are expressed as mean and SEM. Intensity range was significantly reduced at 6–7 months in the Thy1-aSyn condition compared to the WT. Comparisons were made using a two-sample t-test (unequal variance); horizontal bars indicate significant differences between conditions, ^p-value < 0.0125 to WT at same age. Total number of animals in each group at each time point is displayed on the bars.
Bandwidth
There were no significant differences in bandwidth at 2–3 months or 6–7 months for any call type (Table 1).
Immunohistochemistry
We analyzed proteinase K resistant aSyn aggregates in the periaqueductal gray (PAG), which has been implicated in the coordination of vocal behaviors (Jurgens, 2009; Jürgens, 2002; Larson & Kistler, 1986). These aggregates were present in the PAG of 5 months old Thy1-aSyn mice (Figure 8 E–L), but not in WT controls (Figure 8 A–D), as illustrated with microscopic images for the lateral PAG (LPAG), dorsal lateral PAG (DLPAG) and dorsal medial PAG (DMPAG). Percentage of surface area occupied by aSyn aggregates in the dorsal and ventral PAG is shown in Figure 8 M.
Figure 8. Proteinase K resistant aSyn immunostaining in periaquaductal gray (PAG) area.
from a WT mouse (A–C) and two Thy1-aSyn mice (D–I) at the age of 5 months. No proteinase K resistant aSyn specific staining was observed in PAG from the WT mouse. Proteinase K resistant aSyn aggregates were observed in lateral PAG (LPAG), dorsal lateral PAG (DLPAG) and dorsal medial PAG (DMPAG) in the Thy1-aSyn mouse brain. Scale bar: 20 µm. Quantification of proteinase K resistant aSyn aggregates in dorsal and ventral PAG (M). Percentage of surface area occupied by proteinase K resistant aSyn aggregates was measured in Thy1-aSyn mice at 5 months of age (n = 4). The mean of staining in WT animals (n=3) was subtracted from values measured in the Thy1-aSyn mice to account for background.
Discussion
Thy1-aSyn mice demonstrate early, progressive vocalization deficits compared to WT controls. Figure 9 summarizes the results in relationship to the progressive phenotype of the mouse model. Duration and intensity were significantly decreased in the Thy1-aSyn mice compared to WT controls and the overall call profile was altered. In addition, Thy1-aSyn mice had lower call rates at 6–7 months and 9 months compared to WT. While call rates were lower in both groups at the later time points, it was more pronounced in the Thy1-aSyn condition compared to WT. In addition, although call rate appeared to change as a function of time and condition, the percent of mice that called followed similar trends in both groups with age. As expected from the spectrum of communication deficits in PD patients, the acoustic parameters evaluated in this study do not appear to be equally vulnerable across all call types in the Thy1-aSyn mice. Importantly, for those call types that were vulnerable, vocalization deficits are reminiscent of the communication deficits experienced by individuals with PD. For example, individuals with PD experience significantly reduced loudness and reduced variability of pitch and loudness (Darley et al., 1968, 1969; Ho et al., 1998; Plowman-Prine, Okun, et al., 2009). The call profile and duration of Thy1-aSyn mice, in particular, were affected at an early age (2–3 months), indicating that this model may be appropriate for studying cranial sensorimotor deficits which are thought to develop early in the disease progression (B. Harel et al., 2004; B. T. Harel et al., 2004; Postuma et al., 2012; Rusz et al., 2011; Stewart et al., 1995) and remain refractory to standard treatments based on nigrostriatal dopamine depletion (Ciucci et al., 2008; D'Alatri et al., 2008; Pinto et al., 2004). Importantly, broad neuronal aSyn overexpression starts only at about 2 weeks after birth in Thy1-aSyn mice and does therefore not interfere with embryonic and early postnatal development of the peripheral structures involved in speech and breathing. At 2 months of age transgenic mice are indistinguishable from wild-type mice in general health, gross anatomy and body weight. This supports that specific dysfunction of the central and/or peripheral nervous system is underlying observed early deficits in vocalization in these mice. Previous studies have shown that over-expression of wild-type aSyn under the Thy1 promoter results in a broad distribution of aSyn aggregates in the brain of Thy1-aSyn mice from 2 months of age with little variation with increasing age (Chesselet et al., 2012). The presence of aSyn aggregates in the PAG of Thy1-aSyn mice indicates a possible mechanism for vocalization deficits in this pre-manifest Parkinson’s disease model (Figure 8).
Figure 9. Timeline of vocalization deficits in Thy1-aSyn mice.
Figure depicts the timeline of vocalization deficits described in this manuscript in relation to other previously published phenotypic markers in the Thy1-aSyn mice (for review see Chesselet et al., 2012). Reduced duration of several call types and differences in call profile at 2–3 months of age coincide with aSyn overexpression, formation of aSyn aggregation, early sensorimotor deficits and non motor-deficits as well as high extracellular dopamine in the striatum of Thy1-aSyn mice. Reduced intensity range at 6–7 months of age coincides with high extracellular dopamine (DA) levels (filled circles mark main time points of measurements). PAG, periaqueductal gray
Call profile and duration were both affected at 2–3 months in the Thy1-aSyn mice compared to WT controls, whereas the intensity range of simple calls was reduced at 6–7 months in the Thy1-aSyn mice. Given that two cycle calls are characteristically the longest of the cycle-like calls (Figure 1), with a correspondingly longer duration than half cycle or cycle calls, it is not surprising that Thy1-aSyn mice demonstrate reductions in duration at 2–3 months and produce a smaller percentage of these calls compared to their WT littermates. As the PAG has been implicated in the coordination of vocal behaviors (Jurgens, 2009; Jürgens, 2002; Larson & Kistler, 1986), vocalization deficits may manifest as a result of vocalization related deficits in respiratory control. However, pulmonary function was not measured directly in this study, and awaits further investigation. In transgenic Tau.P301L mice, decreased duration was observed in combination with tauopathy in pontine, medullary and midbrain structures, including the PAG, retroambiguus nuclei, and Kolliker-Fuse nuclei (Menuet et al., 2011). Importantly, these regions are implicated in the coordination of vocalizations (Jurgens, 2009; Jürgens, 2002; Larson & Kistler, 1986). While the underlying pathology of these two mouse models is different, the findings support the theory that decreased duration may be related to dysfunction of the PAG. Importantly, respiration subserving speech may be compromised in PD (Huber & Darling, 2011) and sustained phonation may be decreased (Canter, 1965). Future lines of research aimed at characterizing cranial sensorimotor deficits in this model should incorporate measures of pulmonary function to better understand the physiological components underlying vocalization deficits.
In general, the percentage of mice that vocalized and their call rate tended to decrease as the mice approached 9 months of age in both Thy1-aSyn and WT conditions (Figure 2), however this was not tested with parametric statistics because of the low number of mice that vocalized at 9 months of age. Whether males vocalized less because the females were not interested or vice versa, there is evidence that females prefer males that vocalize (Pomerantz, Nunez, & Bean, 1983) and that males prefer environments associated with mating opportunities (Kudwa, Dominguez-Salazar, Cabrera, Sibley, & Rissman, 2005; Pankevich, Cherry, & Baum, 2006; Pierman, Tirelli, Douhard, Baum, & Bakker, 2006; Popik, Wrobel, Rygula, Bisaga, & Bespalov, 2003). Failure to vocalize in the presence of females may represent a communication/motivation deficit, which effectively renders the Thy1-aSyn mice disadvantaged relative to the WT littermates when with mating opportunities. Similar to the Thy1-aSyn mice in the current study, in a Tau.P301L mouse model of tauopathy, call rate decreased in all mice with age, but to a lesser degree in controls (Menuet et al., 2011). Thus it appears that while these components of communicative behavior in mice may decline with age, mice experiencing various pathologies, in this case aSyn pathology, may experience more severe deficits. How this translates to humans with PD is unclear, although speech rate (among other communication deficits) is frequently affected in PD (Darley et al., 1969), and may be related to similar underlying mechanisms.
Thy1-aSyn mice demonstrate widespread aSyn pathology including in areas such as the substantia nigra related to PD associated sensorimotor deficits (Chesselet & Richter, 2011; Chesselet et al., 2012; Fernagut et al., 2007a; Fleming et al., 2004b). As nuclei central to the modulation and coordination of vocalization and respiration lie within the brainstem (Bianchi & Gestreau, 2009; Jürgens, 2002; Larson & Kistler, 1986), it is possible that duration, complexity, and intensity deficits present in Thy1-aSyn mice are related to pathology in these areas. Our immunohistochemical findings support this interpretation. Thy1-aSyn mice demonstrated aSyn aggregates in the PAG (Figure 8 E–M). The PAG is thought to play a critical role in vocalization behaviors via direct and indirect connections with cortical and brainstem regions, such as the cingulate cortex, basal ganglia, cerebellum, and importantly, laryngeal motor nuclei (nucleus ambiguus) (Jurgens, 2009; Jürgens, 2002; Larson & Kistler, 1986; Van Daele & Cassell, 2009). Disruptions to these pathways likely underlie the early and progressive vocalization deficits observed in the present study. In brains of PD patients, aSyn pathology appears early in the lower brainstem and spreads to midbrain and cortical areas as the disease progresses (Braak et al., 2003). Early and progressive communication deficits in PD may therefore be related to aSyn pathology in humans and Thy1-aSyn mice.
This study represents the first characterization of the pathogenesis of cranial sensorimotor deficits in the Thy1-aSyn transgenic mouse model. By comparison, vocalization deficits have been explored within a 6-OHDA neurotoxin model of nigrostriatal dopamine depletion in rats and include both similarities and differences to acoustic deficits found in this transgenic mouse model (Ciucci et al., 2009; Ciucci et al., 2007). As in the 6-OHDA neurotoxin model, call profile and intensity were vulnerable in the Thy1-aSyn model, suggesting that these acoustic components may be dependent on an intact dopamine system. While striatal dopamine loss is not observed until 14 months in the Thy1-aSyn model, there are disruptions in dopamine transmission as early as 6 months (Lam et al., 2011), which may account for the reduction in intensity at the 6–7 month cohort. However, given the drastic reduction in the percent of mice that called with age (particularly at 9 months), evaluating vocalization deficits out to 14 months would be difficult. Duration of calls is not affected in a 6-OHDA model, suggesting degradation of dopaminergic neurons in the nigrostriatal pathway is not sufficient to affect this parameter. In contrast, bandwidth was not affected in the Thy1-aSyn model, but was significantly impaired in the 6-OHDA model (Ciucci et al., 2009; Ciucci et al., 2007), indicating that this deficit is observed in relation to the development of dopamine-dependent parkinsonian symptoms. While the mechanisms underlying durational deficits in the calls of the Thy1-aSyn model and bandwidth deficits in the neurotoxin model are not well understood, one hypothesis is that they are related to differences between the two models, and associated pathologies. Whereas the 6-OHDA model is characterized by a rapid and complete loss of nigrostriatal dopamine neurons, the Thy1-aSyn model encompasses more widespread, progressive aSyn pathology, beyond the nigrostriatal dopamine system, and involving other cortical and brainstem substrates (Chesselet et al., 2012; Fernagut et al., 2007a; Rockenstein et al., 2002) similar to PD in humans (Braak et al., 2003) including the PAG, which is important for the coordination laryngeal control and vocalizations (Jurgens, 2009; Larson & Kistler, 1986). Importantly, the similarities and differences between these two models suggest that while acoustic deficits, such as duration, may be related to extra-striatal pathology, others, such as bandwidth may be dopamine dependent. Thus comparison of USVs in these two models may shed light on the mechanisms underlying the variety of communication deficits that may manifest in isolation or in combination and include reductions in loudness (intensity), pitch (frequency) variability, and pulmonary deficits (Darley et al., 1968, 1969; Ho et al., 1998; Huber & Darling, 2011; Plowman-Prine, Okun, et al., 2009; Solomon & Hixon, 1993). Further characterization of mechanisms underlying the various voice deficits in transgenic rodent models may lead to the development of novel and targeted treatments to improve vocal function, communication and quality of life for individuals with PD.
Our findings provide specific evidence for vocalization deficits in this model. Deficits represent biologically meaningful changes in mice over-expressing aSyn and the observed differences reflect vocalization deficits observed in humans with PD. These findings further our understanding of the pathology underlying voice deficits in humans.
Conclusion
Overall, Thy1-aSyn mice exhibited early, progressive vocalization deficits compared to WT controls. Call duration and intensity were reduced and call profile was altered in the transgenic mice compared to WT controls, particularly at 2–3 months and call rate trended towards more drastic reductions with age in the Thy1-aSyn mice compared to WT. Several important themes emerge from these findings. These deficits may be related to aSyn pathology in the PAG, a region of the midbrain critical to vocalization function. Early and progressive deficits are present in Thy1-aSyn mice, suggesting this can be an appropriate model for studying cranial sensorimotor deficits. Moreover, vocalization deficits present in Thy1-aSyn mice were not all present in a 6-OHDA model of PD, complementing the nigrostriatal dopamine deficiency literature and opening the door for future studies investigating vocalization deficits in a broader, progressive model of the disease pathology. Although the underlying mechanisms are still not completely understood, deficits observed in Thy1-aSyn mice are comparable to some voice and speech deficits in humans with PD. Most notably, deficits do not manifest equally and universally across all call types or acoustic parameters of the ultrasonic vocalizations, just as voice deficits in individuals with PD do not necessarily interfere will all acoustic properties of the speech signal. Lastly, these data hint at a role for other cortical and brainstem regions in mediating cranial sensorimotor deficits. Given the early onset of voice deficits in humans with PD, which remain refractory to traditional treatment, the data reported here offer an important step towards a better understanding of the mechanisms underlying cranial sensorimotor deficits, thus providing new targets and a model to test novel therapeutic strategies.
Acknowledgements
We thank the following people for their contributions to this project: Krystal De La Rosa for help with the recordings, Jaime Basken for help with data analysis, Eileen Torres for help with immunohistochemical labeling and Dr. Glen Leverson for statistical consultation. We acknowledge the following funding sources. NIDCD P30 DC 010754 (National Institutes of Health) (Ciucci); PHS grants NS-P50 NS38367 (UCLA Morris K. Udall Parkinson Disease Research Center of Excellence), and gifts to the Center for the Study of Parkinson’s Disease at UCLA (Chesselet); RO1 MH070712 (National Institutes of Health) (White); T32 DC009401 (NIDCD, National Institutes of Health) (Grant).
References
- Bianchi AL, Gestreau C. The brainstem respiratory network: an overview of a half century of research. Respiratory physiology & neurobiology. 2009;168(1–2):4. doi: 10.1016/j.resp.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiology of aging. 2003;24(2):197. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM. Principles of rat communication: quantitative parameters of ultrasonic calls in rats. Behavior genetics. 2005;35(1):85. doi: 10.1007/s10519-004-0858-3. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Ociepa D. Ultrasonic vocalization of laboratory rats in response to handling and touch. Physiology & Behavior. 1992;52(4):655. doi: 10.1016/0031-9384(92)90393-g. [DOI] [PubMed] [Google Scholar]
- Brudzynski SM, Pniak A. Social contacts and production of 50-kHz short ultrasonic calls in adult rats. Journal of comparative psychology (Washington, D.C.: 1983) 2002;116(1):73. doi: 10.1037/0735-7036.116.1.73. [DOI] [PubMed] [Google Scholar]
- Canter GJ. Speech Characteristics of Patients with Parkinson's Disease. Ii. Physiological Support for Speech. The Journal of speech and hearing disorders. 1965;30:44. doi: 10.1044/jshd.3001.44. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Kachergus J, Roumier C, Mouroux V, Douay X, Lincoln S, Destee A. Alpha-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet. 2004;364(9440):1167. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- Chesselet MF. In vivo alpha-synuclein overexpression in rodents: a useful model of Parkinson's disease? Experimental neurology. 2008;209(1):22. doi: 10.1016/j.expneurol.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesselet MF, Richter F. Modelling of Parkinson's disease in mice. Lancet neurology. 2011;10(12):1108. doi: 10.1016/S1474-4422(11)70227-7. [DOI] [PubMed] [Google Scholar]
- Chesselet MF, Richter F, Zhu C, Magen I, Watson MB, Subramaniam SR. A progressive mouse model of Parkinson's disease: the Thy1-aSyn ("Line 61") mice. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2012;9(2):297. doi: 10.1007/s13311-012-0104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci MR, Ahrens AM, Ma ST, Kane JR, Windham EB, Woodlee MT, Schallert T. Reduction of dopamine synaptic activity: degradation of 50-kHz ultrasonic vocalization in rats. Behavioral neuroscience. 2009;123(2):328. doi: 10.1037/a0014593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci MR, Barkmeier-Kraemer JM, Sherman SJ. Subthalamic nucleus deep brain stimulation improves deglutition in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2008;23(5):676. doi: 10.1002/mds.21891. [DOI] [PubMed] [Google Scholar]
- Ciucci MR, Ma ST, Fox C, Kane JR, Ramig LO, Schallert T. Qualitative changes in ultrasonic vocalization in rats after unilateral dopamine depletion or haloperidol: a preliminary study. Behavioural brain research. 2007;182(2):284. doi: 10.1016/j.bbr.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alatri L, Paludetti G, Contarino MF, Galla S, Marchese MR, Bentivoglio AR. Effects of bilateral subthalamic nucleus stimulation and medication on parkinsonian speech impairment. Journal of voice : official journal of the Voice Foundation. 2008;22(3):365. doi: 10.1016/j.jvoice.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Darley FL, Aronson AE, Brown JR. Motor speech signs in neurologic disease. The Medical clinics of North America. 1968;52(4):835. [PubMed] [Google Scholar]
- Darley FL, Aronson AE, Brown JR. Clusters of deviant speech dimensions in the dysarthrias. Journal of speech and hearing research. 1969;12(3):462. doi: 10.1044/jshr.1203.462. [DOI] [PubMed] [Google Scholar]
- Ennis M, Xu SJ, Rizvi TA. Discrete subregions of the rat midbrain periaqueductal gray project to nucleus ambiguus and the periambigual region. Neuroscience. 1997;80(3):829–845. doi: 10.1016/s0306-4522(97)00051-1. [DOI] [PubMed] [Google Scholar]
- Ey E, Torquet N, Le Sourd AM, Leblond CS, Boeckers TM, Faure P, Bourgeron T. The Autism ProSAP1/Shank2 mouse model displays quantitative and structural abnormalities in ultrasonic vocalisations. Behav Brain Res. 2013 doi: 10.1016/j.bbr.2013.08.031. [DOI] [PubMed] [Google Scholar]
- Fernagut PO, Hutson CB, Fleming SM, Tetreaut NA, Salcedo J, Masliah E, Chesselet MF. Behavioral and histopathological consequences of paraquat intoxication in mice: effects of alpha-synuclein over-expression. Synapse (New York, N.Y) 2007a;61(12):991. doi: 10.1002/syn.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernagut PO, Hutson CB, Fleming SM, Tetreaut NA, Salcedo J, Masliah E, Chesselet MF. Behavioral and histopathological consequences of paraquat intoxication in mice: effects of alpha-synuclein over-expression. Synapse. 2007b;61(12):991–1001. doi: 10.1002/syn.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, Chesselet MF. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J Neurosci. 2004a;24(42):9434–9440. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, Chesselet MF. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004b;24(42):9434. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Hutson CB, Rockenstein E, Masliah E, Levine MS, Chesselet MF. Behavioral effects of dopaminergic agonists in transgenic mice overexpressing human wildtype alpha-synuclein. Neuroscience. 2006;142(4):1245–1253. doi: 10.1016/j.neuroscience.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuh JL, Lee RC, Wang SJ, Lin CH, Wang PN, Chiang JH, Liu HC. Swallowing difficulty in Parkinson's disease. Clinical neurology and neurosurgery. 1997;99(2):106. doi: 10.1016/s0303-8467(97)00606-9. [DOI] [PubMed] [Google Scholar]
- Hammer MJ, Barlow SM. Laryngeal somatosensory deficits in Parkinson's disease: implications for speech respiratory and phonatory control. Experimental brain research.Experimentelle Hirnforschung.Experimentation cerebrale. 2010;201(3):401. doi: 10.1007/s00221-009-2048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt K, Radyushkin K, Ehrenreich H, Fischer J. The structure and usage of female and male mouse ultrasonic vocalizations reveal only minor differences. PLoS One. 2012;7(7):e41133. doi: 10.1371/journal.pone.0041133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy John. Genetic Analysis of Pathways to Parkinson Disease. Neuron. 2010;68(2):201. doi: 10.1016/j.neuron.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel B, Cannizzaro M, Snyder PJ. Variability in fundamental frequency during speech in prodromal and incipient Parkinson's disease: a longitudinal case study. Brain and cognition. 2004;56(1):24. doi: 10.1016/j.bandc.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Harel BT, Cannizzaro MS, Cohen H, Reilly N, Snyder PJ. Acoustic characteristics of Parkinsonian speech: a potential biomarker of early disease progression and treatment. Journal of Neurolinguistics. 2004;17(6):439–453. [Google Scholar]
- Ho AK, Iansek R, Marigliani C, Bradshaw JL, Gates S. Speech impairment in a large sample of patients with Parkinson's disease. Behavioural neurology. 1998;11(3):131. [PubMed] [Google Scholar]
- Huber JE, Darling M. Effect of Parkinson's disease on the production of structured and unstructured speaking tasks: respiratory physiologic and linguistic considerations. Journal of speech, language, and hearing research : JSLHR. 2011;54(1):33. doi: 10.1044/1092-4388(2010/09-0184). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez P, Bonnet AM, Debarges B, Lohmann E, Tison F, Pollak P, Brice A. Causal relation between alpha-synuclein gene duplication and familial Parkinson's disease. Lancet. 2004;364(9440):1169. doi: 10.1016/S0140-6736(04)17104-3. [DOI] [PubMed] [Google Scholar]
- Jensen PH, Gai WP. Alpha-synuclein. Axonal transport, ligand interaction and neurodegeneration. Advances in Experimental Medicine and Biology. 2001;487:129. [PubMed] [Google Scholar]
- Johnson AM, Ciucci MR, Russell JA, Hammer MJ, Connor NP. Ultrasonic output from the excised rat larynx. The Journal of the Acoustical Society of America. 2010;128(2):EL75. doi: 10.1121/1.3462234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens U. The neural control of vocalization in mammals: a review. J Voice. 2009;23(1):1–10. doi: 10.1016/j.jvoice.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Jürgens Uwe. Neural pathways underlying vocal control. Neuroscience & Biobehavioral Reviews. 2002;26(2):235. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Kudwa AE, Dominguez-Salazar E, Cabrera DM, Sibley DR, Rissman EF. Dopamine D5 receptor modulates male and female sexual behavior in mice. Psychopharmacology. 2005;180(2):206. doi: 10.1007/s00213-005-2150-5. [DOI] [PubMed] [Google Scholar]
- Lam HA, Wu N, Cely I, Kelly RL, Hean S, Richter F, Maidment NT. Elevated tonic extracellular dopamine concentration and altered dopamine modulation of synaptic activity precede dopamine loss in the striatum of mice overexpressing human alpha-synuclein. Journal of neuroscience research. 2011;89(7):1091. doi: 10.1002/jnr.22611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson CR, Kistler MK. The relationship of periaqueductal gray neurons to vocalization and laryngeal EMG in the behaving monkey. Exp Brain Res. 1986;63(3):596–606. doi: 10.1007/BF00237482. [DOI] [PubMed] [Google Scholar]
- Mahrt EJ, Perkel DJ, Tong L, Rubel EW, Portfors CV. Engineered deafness reveals that mouse courtship vocalizations do not require auditory experience. J Neurosci. 2013;33(13):5573–5583. doi: 10.1523/JNEUROSCI.5054-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marras C, McDermott MP, Rochon PA, Tanner CM, Naglie G, Lang AE Parkinson Study Group, Datatop Investigators. Predictors of deterioration in health-related quality of life in Parkinson's disease: results from the DATATOP trial. Movement disorders : official journal of the Movement Disorder Society. 2008;23(5):653. doi: 10.1002/mds.21853. [DOI] [PubMed] [Google Scholar]
- Martin I, Dawson VL, Dawson TM. The impact of genetic research on our understanding of Parkinson's disease. Progress in brain research. 2010;183:21. doi: 10.1016/S0079-6123(10)83002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis Marilyn Y, Vakulenko M. Characterization of 50-kHz ultrasonic vocalizations in male and female rats. Physiology & Behavior. 2003;80(1):81. doi: 10.1016/s0031-9384(03)00227-0. [DOI] [PubMed] [Google Scholar]
- Menuet C, Cazals Y, Gestreau C, Borghgraef P, Gielis L, Dutschmann M, Hilaire G. Age-related impairment of ultrasonic vocalization in Tau.P301L mice: possible implication for progressive language disorders. PloS one. 2011;6(10):e25770. doi: 10.1371/journal.pone.0025770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankevich DE, Cherry JA, Baum MJ. Accessory olfactory neural Fos responses to a conditioned environment are blocked in male mice by vomeronasal organ removal. Physiology & Behavior. 2006;87(4):781. doi: 10.1016/j.physbeh.2006.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin RJ, Woods WS, Clayton DF, George JM. Interaction of human alpha-Synuclein and Parkinson's disease variants with phospholipids. Structural analysis using site-directed mutagenesis. The Journal of biological chemistry. 2000;275(44):34393. doi: 10.1074/jbc.M004851200. [DOI] [PubMed] [Google Scholar]
- Pierman S, Tirelli E, Douhard Q, Baum MJ, Bakker J. Male aromatase knockout mice acquire a conditioned place preference for cocaine but not for contact with an estrous female. Behavioural brain research. 2006;174(1):64. doi: 10.1016/j.bbr.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Pinto S, Ozsancak C, Tripoliti E, Thobois S, Limousin-Dowsey P, Auzou P. Treatments for dysarthria in Parkinson's disease. Lancet neurology. 2004;3(9):547. doi: 10.1016/S1474-4422(04)00854-3. [DOI] [PubMed] [Google Scholar]
- Plowman-Prine EK, Okun MS, Sapienza CM, Shrivastav R, Fernandez HH, Foote KD, Rosenbek JC. Perceptual characteristics of Parkinsonian speech: a comparison of the pharmacological effects of levodopa across speech and non-speech motor systems. NeuroRehabilitation. 2009;24(2):131. doi: 10.3233/NRE-2009-0462. [DOI] [PubMed] [Google Scholar]
- Plowman-Prine EK, Sapienza CM, Okun MS, Pollock SL, Jacobson C, Wu SS, Rosenbek JC. The relationship between quality of life and swallowing in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2009;24(9):1352. doi: 10.1002/mds.22617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz SM, Nunez AA, Bean NJ. Female behavior is affected by male ultrasonic vocalizations in house mice. Physiology & Behavior. 1983;31(1):91. doi: 10.1016/0031-9384(83)90101-4. [DOI] [PubMed] [Google Scholar]
- Popik P, Wrobel M, Rygula R, Bisaga A, Bespalov AY. Effects of memantine, an NMDA receptor antagonist, on place preference conditioned with drug and nondrug reinforcers in mice. Behavioural pharmacology. 2003;14(3):237. doi: 10.1097/00008877-200305000-00008. [DOI] [PubMed] [Google Scholar]
- Portfors CV. Types and functions of ultrasonic vocalizations in laboratory rats and mice. Journal of the American Association for Laboratory Animal Science : JAALAS. 2007;46(1):28. [PubMed] [Google Scholar]
- Postuma RB, Lang AE, Gagnon JF, Pelletier A, Montplaisir JY. How does parkinsonism start? Prodromal parkinsonism motor changes in idiopathic REM sleep behaviour disorder. Brain : a journal of neurology. 2012;135(Pt 6):1860. doi: 10.1093/brain/aws093. [DOI] [PubMed] [Google Scholar]
- Riede T. Subglottal pressure, tracheal airflow, and intrinsic laryngeal muscle activity during rat ultrasound vocalization. Journal of neurophysiology. 2011;106(5):2580. doi: 10.1152/jn.00478.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockenstein E, Mallory M, Hashimoto M, Song D, Shults CW, Lang I, Masliah E. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. Journal of neuroscience research. 2002;68(5):568. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- Rusz J, Cmejla R, Ruzickova H, Ruzicka E. Quantitative acoustic measurements for characterization of speech and voice disorders in early untreated Parkinson's disease. The Journal of the Acoustical Society of America. 2011;129(1):350. doi: 10.1121/1.3514381. [DOI] [PubMed] [Google Scholar]
- Scattoni ML, Gandhy SU, Ricceri L, Crawley JN. Unusual repertoire of vocalizations in the BTBR T+tf/J mouse model of autism. PLoS One. 2008;3(8):e3067. doi: 10.1371/journal.pone.0003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Gwinn-Hardy K. alpha-Synuclein locus triplication causes Parkinson's disease. Science (New York, N.Y.) 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Solomon NP, Hixon TJ. Speech breathing in Parkinson's disease. Journal of speech and hearing research. 1993;36(2):294. doi: 10.1044/jshr.3602.294. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Stewart C, Winfield L, Hunt A, Bressman SB, Fahn S, Blitzer A, Brin MF. Speech dysfunction in early Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 1995;10(5):562. doi: 10.1002/mds.870100506. [DOI] [PubMed] [Google Scholar]
- Sung HY, Kim JS, Lee KS, Kim YI, Song IU, Chung SW, Choi MG. The prevalence and patterns of pharyngoesophageal dysmotility in patients with early stage Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2010;25(14):2361. doi: 10.1002/mds.23290. [DOI] [PubMed] [Google Scholar]
- Van Daele DJ, Cassell MD. Multiple forebrain systems converge on motor neurons innervating the thyroarytenoid muscle. Neuroscience. 2009;162(2):501–524. doi: 10.1016/j.neuroscience.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JM, Gourdon JC, Clarke PB. Identification of multiple call categories within the rich repertoire of adult rat 50-kHz ultrasonic vocalizations: effects of amphetamine and social context. Psychopharmacology. 2010;211(1):1. doi: 10.1007/s00213-010-1859-y. [DOI] [PubMed] [Google Scholar]
- Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Annals of Neurology. 2004;55(2):164. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]