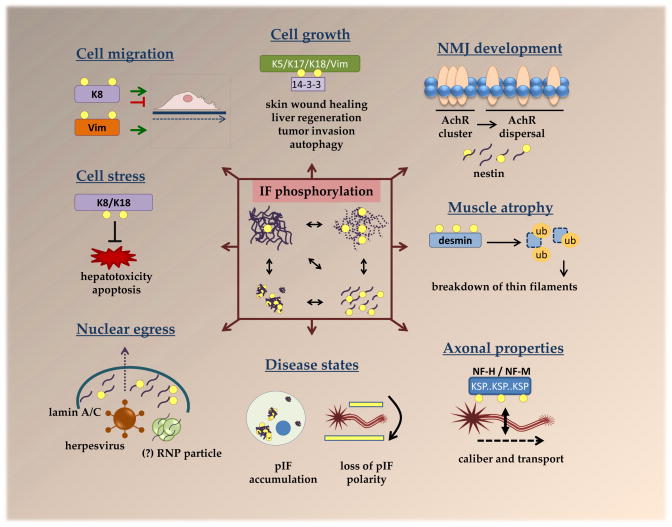
Figure 2. Multifunctional roles for IF protein phosphorylation.
Numerous cellular properties are affected by IF phosphorylation, which, as depicted in the central box, leads to filament reorganization, disassembly and aggregation. Phosphorylation of K5, K17, K18 and vimentin facilitates their association with 14-3-3 to promote cellular growth via several mechanisms, including regulation of 14-3-3 localization and its association with other proteins, such as the autophagy regulator Beclin 117–19,77. Keratin phosphorylation has been shown to both inhibit and promote cell migration, depending on the type of cell and stimulus, whereas vimentin phosphorylation is strongly associated with promoting cell migration34. phosphorylation by host and viral kinases facilitates nuclear egress of herpesviruses64. A similar nuclear egress mechanism may be utilized by RNPs68, although this has not been fully confirmed with respect to the requirement for lamin phosphorylation Phosphorylation-mediated dissasembly of myoblast nestin IFs appears to be important for neuromuscular junction development by facilitating acetylcholine receptor dispersal69. Fasting-induced desmin phosphorylation leads to its subsequent ubiquitination and proteasomal degradation, which, in turn, results in the breakdown of thin filaments to promote muscle atrophy73. While phosphorylation of most IFs is low basally, but triggered by stress or physiological stimuli, neurofilaments (NF-M and NF-H) are heavily phosphorylated under basal conditions within tail-domain K-S-P repeats10. This is thought to regulate axonal stability, caliber, and transport51,52. Hyperphosphorylated IF aggregates are present in various diseases and, in neurodegenerative diseases, hyperphosphorylated NF aggregates appear in neuronal cell bodies, leading to loss in the polarity of phosphorylated NFs50.