Abstract
Microglia are resident immune cells of brain and activated by peripheral tissue injury. In the present study, we investigated the possible induction of several microglial surface immunomolecules in the spinal cord, including leukocyte common antigen (LCA/CD45), MHC class I antigen, MHC class II antigen, Fc receptor, and CD11c following formalin injection into the rat’s hind paw. CD45 and MHC class I were upregulated in the activated microglia, which was evident on day 3 with the peak expression on day 7 following peripheral formalin injection. There was a very low basal expression of MHC class II, CD11c, and the Fc receptor, which did not change after the formalin injection. These results, for the first time, indicate that peripheral formalin injection can induce phenotypic changes of microglia with distinct upregulation of CD45 and MHC class I antigen. The data suggest that phenotypic changes of the activated microglia may be a unique pattern of central changes following peripheral tissue injury.
Keywords: Microglia, Formalin, CD45, OX-42, MHC class I, MHC class II
There is increasing evidence that activated glia (microglia and astrocytes) in the spinal cord are key players in the induction and maintenance of pathological pain [18,22,31,32]. Interestingly, although microglial activation usually occurs in parallel with astrocyte activation, microglia demonstrate more complicated features of activation than astrocytes. For example, peripheral nerve injury induced spinal cord microglial proliferation on the ipsilateral side, beginning at day 1 and peaking on day 3 after injury. Astrocyte proliferation was rare, or nonexistent [5]. Moreover, microglial activation was often accompanied by a series of sterotypical morphological changes, evident at 2–3 days after peripheral tissue injury [3,10,12,13,15, 20, 23]. However, such microglial morphological changes were not induced by CFA (Complete Freund’s Adjuvant)-induced inflammation [2,15,20,33]. Microglial cells are generally considered immune cells (central macrophages) of the CNS. In the normal brain, microglia display a ramified morphology with a relatively small cell body and weakly expressing molecules normally expressed by other haematopoietic lineages, such as CD45, MHC class II antigens, CD11c, CD40 and CD86 costimulatory molecules [11,25]. Microglial cells respond to central and peripheral tissue injury (microglial activation) and activated cells display these very same molecules that are either significantly upregulated or changed in their morphology [21,27]. The induced expression of the aforementioned immunomolecules in microglia has been observed in a variety of experimental situations, including peripheral nerve injury [16,19,29], brain injury [4,6,24] and an animal model of multiple sclerosis [27]. The cell phenotypes and morphological changes may be regular and joint characteristics for microglial activation. The changes seem to occur in a progressive manner, reflecting different stages of activated microglia.
We previously reported robust microglial activation with morphological changes in the spinal cord dorsal horn on the side ipsilateral to the formalin injection into the rat’s hind paw, starting on day 1–3 and peaking on day 7 post-injection [10,20]. However, microglia have been reported to be activated rapidly in carrageenan- and formalin-induced pain models without any morphological change [17,30]. It appears that even when microglia have a resting morphology, they are functionally active [17,26], which suggests that microglial activation in animal pain models may have different stages, with and without morphological and cell phenotypic changes, and may also have different functions in nociceptive modulation within the spinal cord.
In the present study, we activated microglia with peripheral formalin injection into the rat’s hindpaw, and used immunolabeling to measure immunomolecules on microglia in the spinal cord. The measured immunomolecules were CD45, MHC class I antigen, MHC class II antigen, Fc receptor, CD11c, and as well as CD11b. The results show that peripheral tissue injury induced microglia to acquire phenotypic changes, including the upregulated expression of CD45 and MHC class I antigen, along with morphological changes.
Adult male Sprague–Dawley rats weighing 200–225 gm (Charles River Laboratories, Wilmington, MA) were used. The animal room was artificially lighted from 7:00 a.m. until 7:00 p.m. The experimental protocol was approved by our Institutional Animal Care and Use Committee. Experimental rats received subcutaneous injections of 100 µl 5% formalin (diluted in saline) into the plantar surface of the right hind paw. Rats in the control group were injected with 100 µl 0.9% saline instead of formalin, and 4 rats served as normal controls and received no treatment. Survival times were 2 h, 1 day, 3 days, 1 week, 2 weeks and 4 weeks post-injections, with 4–5 animals per group.
To obtain the spinal cord tissues, animals were anesthetized with an overdose of pentobarbital sodium and euthanized by transcardiac perfusion (250 ml body temperature 0.1M PBS pH 7.4 followed by 200–300ml ice-cold 4% paraformaldehyde/4% sucrose in 0.1M PB pH 7.4). After perfusion, the lumbar spinal cords (L3–5) and a piece of spleen tissue (see below) were removed, postfixed in the same 4% paraformaldehyde fixative for 4 h and then placed in 30% sucrose solution (in 0.1M PB) overnight at 4 °C. Thirty micron thick tissue sections were cut transversely on a cryostat and free-floating immunohistochemical staining for OX-42 (monoclonal mouse anti-rat CD11b, 1:200, Serotec, UK), CD45 (mouse anti-rat CD45 monoclonal antibody, MRC OX-1, 1:20, Serotec), CD11c (mouse anti-rat CD11c, clone 8A2, 1:20, Serotec), MHC class I antigen (mouse anti-rat MHC class I RT1A, MRC OX- 18, 1:2000, Serotec), MHC class II antigen (mouse anti-rat MHC class II RT1B, MRC OX-6, 1:20, Serotec), and Fc receptor (Fc Receptor gamma II rabbit monoclonal antibody, CD32, 1:200, Epitomics Inc., CA). All of the sections were blocked with 5% normal goat serum in 0.3% Triton X-100 for 1 h at room temperature (RT) and incubated over two nights at 4 °C with primary antibody. The sections were then incubated for 90min at RT with a corresponding FITC-conjugated secondary antibody (rat adsorbed, 1:200, Serotec).
In our experiments, replacement of primary antibody by mouse IgG2a, or PBS resulted in no staining, and spleen tissues were positively stained with all above primary antibodies.
For double immunofluorescence, spinal sections were incubated with a mixture of CD45 or MHC class I monoclonal antibody and rabbit anti-Iba1 (microglia marker, 1:2000,Wako), rabbit anti-GFAP (astrocyte marker, 1:1000, Chemicon), rabbit anti-neuron specific enolase (NSE, neuronal marker, 1:1000, Chemicon) over two nights at 4 °C, followed by a mixture of FITC- and Cy3-conjugated secondary antibodies for 90min at RT. The single- or double-stained images were captured with a CCD spot camera and processed using Adobe Photoshop.
To quantitatively analyze the time course of CD45 and MHC class I immunoreactivity, we measured the immunofluorescence intensity as an average percentage of positively stained area, relative to the total outlined interest area [10,20]. The ratio of the injected side to the contralateral side was determined for each animal. Differences in changes of values over time of each group were tested using one-way ANOVA, followed by Bonferroni post hoc analysis.
In both saline-injected (sham injection) and non-treated (naïve) control animals, ramified microglia with OX-42 immunoreactivity were homogeneously distributed throughout the spinal cord gray and white matter and did not express observable CD45 and MHC class I. After peripheral formalin injection, CD45 and MHC class I immunomolecules were induced on the ipsilateral side of the lumbar spinal dorsal horn. The distribution of these immunoreactive cells corresponded to that of the increased OX-42 staining of activated microglia (Fig. 1). Both CD45 and MHC class I expression became evident on day 3, peaked on day 7, and significantly declined on day 14 after the formalin injection (Figs. 2–4). MHC class I expression appeared to be induced earlier than CD45 in that a few faint stained positive cells with MHC class I could be found on day 1 after the formalin injection (Fig. 3). MHC class I was also more strongly expressed than CD45 (Fig. 4).
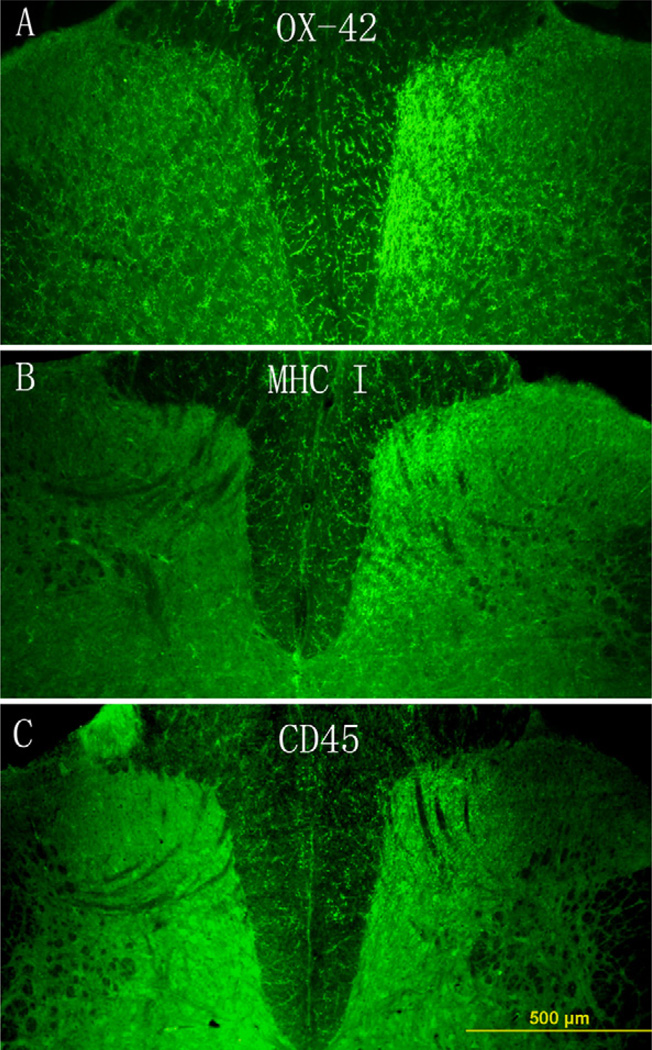
Fig. 1.
The distribution of microglial cells stained by OX-42, MHC class I or CD45 in the lumbar spinal cord on day 7 following peripheral formalin injection. OX-42-positive cells distributed throughout the spinal cord and were significantly increased in the medial portion of the ipsilateral side of the dorsal horn (A). MHC class I and CD45 molecules only expressed in the ipsilateral side of the dorsal horn (B and C), the distribution of these positive cells corresponded to that of the increased OX-42 staining of activated microglia. Scale bar, 500 µm.
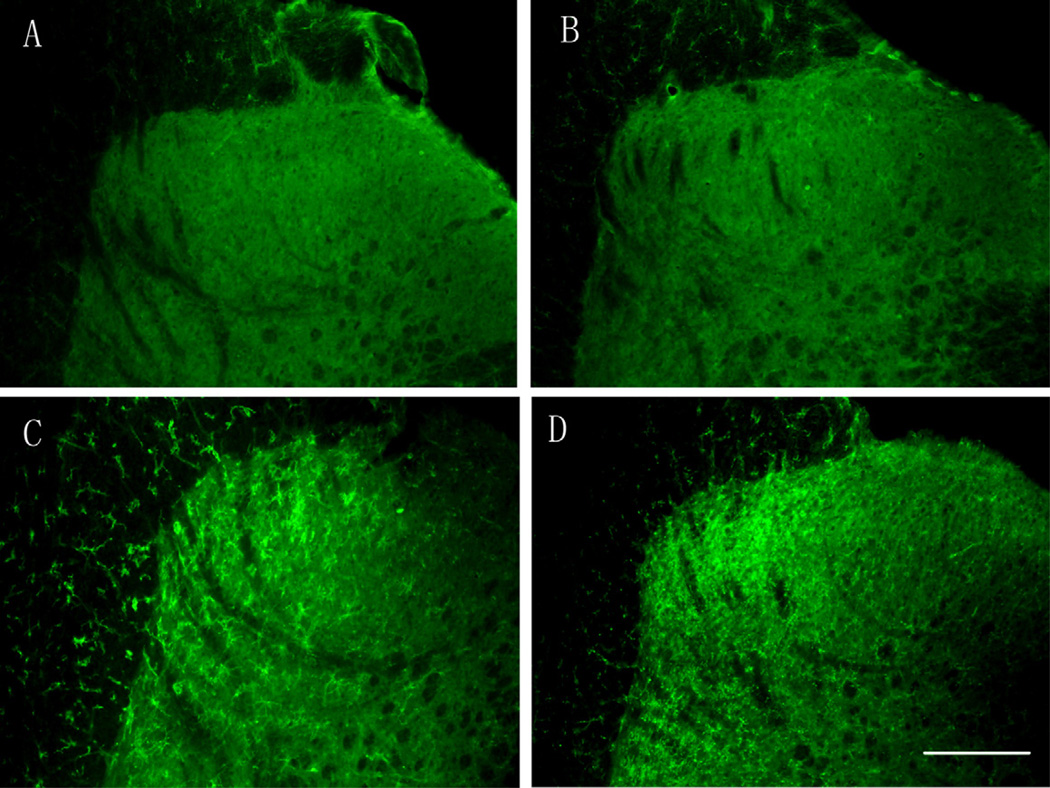
Fig. 2.
Time-course of CD45 expression in the ipsilateral side of the spinal cord following peripheral formalin injection. CD45 was not expressed at 2 h (A) and 1 day after the formalin injection (B); the CD45 expression was evident on day 3 (C) with the peak expression on day 7 (D). Scale bar, 200 µm.
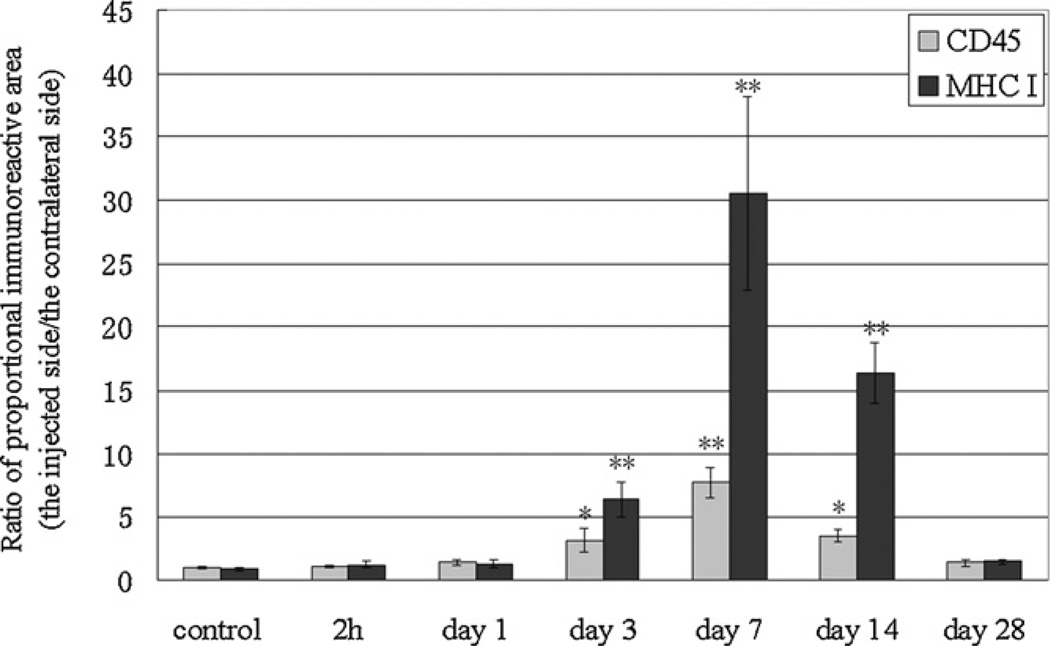
Fig. 4.
Time course of the increased level of CD45 and MHC class I immunoreactivity, the ratio of the percentage of immunoreactive area on the injected side to the contralateral side, following the formalin injection. *p < 0.05; **p < 0.01 vs. no treatment control group.
Fig. 3.
Time-course of MHC class I expression in the ipsilateral side of the spinal cord following peripheral formalin injection. MHC class I was not expressed at 2h after the formalin injection (A); a few faint-stained positive cells were found on day 1 (B), which were significantly increased on day 3 with morphological appearances of activated microglia (C). The peak expression was observed on day 7 (D). Scale bar, 200 µm.
To identify the cell types, we performed double immunostaining with several cell-specific markers: NSE (neurons), GFAP (astrocytes), and Iba1 (microglia). CD45 and MHC class I did not colocalize with either NSE or GFAP, but completely colocalized with Iba1 (Fig. 5). The double stained microglia demonstrated morphological activation (Fig. 5).
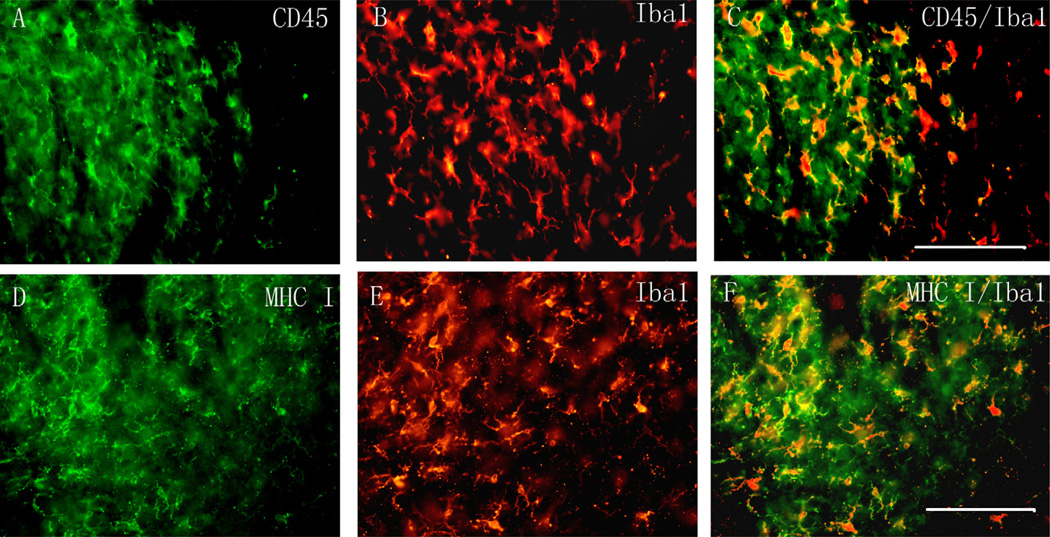
Fig. 5.
Double immunofluorescence on the ipsilateral side of the spinal cord dorsal horn for CD45 (green; A) or MHC class I (green; D) and Iba1 (a microglial marker, red; B and E). Images were merged using Adobe Photoshop, both CD45 and MHC class I were colocalized with Iba1(C and F). Scale bar, 100 µm.
There was a very low basal expression of MHC class II, CD11c, and the Fc receptor. This expression did not change following the formalin injection.
In the present study, we reported that the activated microglia increased expression of CD45 and MHC class I antigen. These antigens were not expressed in the ramified microglia in the spinal cord from normal rats and did not increase within 1 day following the peripheral formalin injection.
Microglia can be rapidly activated without morphological changes following peripheral inflammation induced by carrageenan as indicated by the increased expression of p38 MAP kinase in the spinal microglia [17]. The increases in p38 MAP kinase were blocked by intrathecal administration of minocycline (a functional microglia inhibitor), which also blocked hyperalgesia [17]. Other reports found that peripheral formalin injection could also rapidly increase phosphorylation of p38 in microglia within minutes after the formalin injection before morphological changes, and pretreatment with p38 MAPK inhibitors could suppress formalin-induced paw flinching behaviors and spinal neuronal c-fos expression [30]. This activation of p38 MAP kinase in microglia within minutes contrasts with the increase in CD45, MHC class I, and morphological changes which take more than 24 h.
Microglial activation has been demonstrated in almost all pain models using OX-42 immunohistochemical marker, which stained both resting and activated microglia. In the present study, the upregulation of both CD45 and MHC class I antigen in microglia was found, beginning at 1–3 days after the formalin injection, and peaking on day 7. The time course of CD45 and MHC class I upregulation is exactly the same as the time course of morphologically microglial activation detected by OX-42 immunohistochemistry [10,20]. It seems that microglia could respond to peripheral tissue injury, but only in the later stages did microglia acquire the morphological and immunological phenotypic changes. Thus, microglia may undergo at least two distinct stages of activation on the basis of their morphological and phenotypic changes. (1) Early-activated microglia display a “resting” ramified morphology with a relatively small cell body and weakly express molecules normally present in other haematopoietic lineages, such as CD45, MHC class I antigen, and other immunomolecules. (2) Late-activated microglia show upregulation of CD45 and MHC class I and a morphology characterized by the hypertrophic cell body and the shortening of cellular processes. Given that MHC class I antigen can be easily and strongly stained in the late-activated microglia as compared with CD45, it may be used as a marker to differentiate this late activation state from the early-activated microglia.
Under normal conditions, in the CNS, microglia exist in a state characterized by a distinct ramified morphology, a slow rate of turnover, and a CD45low expression pattern [28]. Microglia have the capacity to become activated during the CNS inflammation, acquiring phenotypic and morphologic features of peripheral macrophages [1,11,27,28].Microglia also have been shown to differentiate into cells that resemble dendritic cells and express CD11c in animal models of multiple sclerosis [7,27]. Microglial activation in the spinal cord has been extensively investigated in animal pain models, but few reported their phenotypic changes [16]. Upregulation of MHC class I and class II on glia has been observed following nerve injury including facial nerve transection and sciatic nerve injury [14,16,29]. In a rodent model of nerve root injury, methotrexate was reported to attenuate tactile sensitivity at doses that inhibited the expression of MHC class II on microglia in the lumbar spinal cord [14]. The present immunophenotypic analyK.- sis shows that the peripheral formalin injection induces microglia to express CD45 and MHC class I antigen, but not MHC class II antigen, the Fc receptor, and CD11c. We previously reported that CFA induced much more severe inflammatory reactions in the peripheral hindpaw tissues than the formalin injection, but did not produce significant microglial morphological changes and increases of OX-42 immunostaining in the ipsilateral lumbar spinal cord [20]. Moreover, the injection of a low concentration (1%) formalin evoked the typical two phases of pain behavior, but no long-term morphological activation of microglia was found [9]. In the present study, we also did not find a significant upregulation of CD45 and MHC class I antigen in the spinal microglia in CFA and 1% formalin injected animals (data not shown). The resulting microglial phenotypic changes might depend on the identity of the activating agents and/or the stimulus intensity (i.e., the intensity of peripheral stimulation). Although microglial activation has been commonly observed in animal pain models, the present and previous data indicate that the time course and stage-dependent microglial activation (both number and phenotypic change) may well be different in different types of animal pain models.
Various immunomolecules such as CD45 and MHC class I antigen are key players in the role of immune responses and in the regulation of inflammation associated with a variety of neurological disorders, but very little is known with regard to their roles in pain modulation [14]. Subcutaneous formalin injection has long been used as a model of acute inflammatory pain, but the injection produces tissue injury and pain responses to both mechanical and thermal stimuli that is applied to the injected area are lost [8,20]. However, the injury causes hyperalgesic responses on the opposite surface of the injected hindpaw 3 days after the injection, which lasts 2–3 weeks [8]. The pain behavior parallels the time course of the microglial phenotypic changes. Our data suggest distinct roles for the upregulated CD45 and MHC class I molecules of the spinal microglia in pain modulation that warrants further study.
Acknowledgements
The research was supported by the program for New Century Excellent Talents in University (NCET) and Project 30371544 by National Natural Science Foundation of China (K.Y.F.) as well as by the US PHS RO1 grants DE18214 and DE18538 (J.M.).
References
- 1.Carson MJ, Reilly CR, Sutcliffe JG, Lo D. Maturemicroglia resemble immature antigen-presenting cells. Glia. 1998;22:72–85. doi: 10.1002/(sici)1098-1136(199801)22:1<72::aid-glia7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 2.Clark AK, Gentry C, Bradbury EJ, McMahon SB, Malcangio M. Role of spinal microglia in rat models of peripheral nerve injury and inflammation. Eur. J. Pain. 2007;11:223–230. doi: 10.1016/j.ejpain.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Coyle DE. Partial peripheral nerve injury leads to activation of astroglia and microglia which parallels the development of allodynic behavior. Glia. 1998;23:75–83. [PubMed] [Google Scholar]
- 4.Denker SP, Ji S, Dingman A, Lee SY, Derugin N, Wendland MF, Vexler ZS. Macrophages are comprised of resident brain microglia not infiltrating peripheral monocytes acutely after neonatal stroke. J. Neurochem. 2007;100:893–904. doi: 10.1111/j.1471-4159.2006.04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Echeverry S, Shi XQ, Zhang J. Characterization of cell proliferation in rat spinal cord following peripheral nerve injury and the relationship with neuropathic pain. Pain. 2008;135:37–47. doi: 10.1016/j.pain.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Finsen BR, Jorgensen MB, Diemer NH, Zimmer J. Microglial, MHC antigen expression after ischemic and kainic acid lesions of the adult rat hippocampus. Glia. 1993;7:41–49. doi: 10.1002/glia.440070109. [DOI] [PubMed] [Google Scholar]
- 7.Fischer HG, Reichmann G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J. Immunol. 2001;166:2717–2726. doi: 10.4049/jimmunol.166.4.2717. [DOI] [PubMed] [Google Scholar]
- 8.Fu KY, Light AR, Maixner W. Long-lasting inflammation and long-term hyperalgesia after subcutaneous formalin injection into the rat hindpaw. J. Pain. 2001;2:2–11. doi: 10.1054/jpai.2001.9804. [DOI] [PubMed] [Google Scholar]
- 9.Fu KY, Light AR, Maixner W. Relationship between nociceptor activity, peripheral edema, spinal microglial activation and long-term hyperalgesia induced by formalin. Neuroscience. 2000;101:1127–1135. doi: 10.1016/s0306-4522(00)00376-6. [DOI] [PubMed] [Google Scholar]
- 10.Fu KY, Light AR, Matsushima GK, Maixner W. Microglial reactions after subcutaneous formalin injection into the rat hind paw. Brain Res. 1999;825:59–67. doi: 10.1016/s0006-8993(99)01186-5. [DOI] [PubMed] [Google Scholar]
- 11.Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28:12–18. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Graeber MB, Streit WJ, Kreutzberg GW. Axotomy of the rat facial nerve leads to increased CR3 complement receptor expression by activated microglial cells. J. Neurosci. Res. 1988;21:18–24. doi: 10.1002/jnr.490210104. [DOI] [PubMed] [Google Scholar]
- 13.Hashizume H, DeLeo JA, Colburn RW, Weinstein JN. Spinal glial activation and cytokine expression after lumbar root injury in the rat. Spine. 2000;25:1206–1217. doi: 10.1097/00007632-200005150-00003. [DOI] [PubMed] [Google Scholar]
- 14.Hashizume H, Rutkowski MD, Weinstein JN, DeLeo JA. Central administration of methotrexate reduces mechanical allodynia in an animal model of radiculopathy/sciatica. Pain. 2000;87:159–169. doi: 10.1016/S0304-3959(00)00281-5. [DOI] [PubMed] [Google Scholar]
- 15.Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, Clohisy DR, Mantyh PW. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–598. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- 16.Hu P, Bembrick AL, Keay KA, McLachlan EM. Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain. Behav. Immun. 2007;21:599–616. doi: 10.1016/j.bbi.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Hua XY, Svensson CI, Matsui T, Fitzsimmons B, Yaksh TL, Webb M. Intrathecal minocycline attenuates peripheral inflammation-induced hyperalgesia by inhibiting p38 MAPK in spinal microglia. Eur. J. Neurosci. 2005;22:2431–2440. doi: 10.1111/j.1460-9568.2005.04451.x. [DOI] [PubMed] [Google Scholar]
- 18.Inoue K. The function of microglia through purinergic receptors: neuropathic pain and cytokine release. Pharmacol. Ther. 2006;109:210–226. doi: 10.1016/j.pharmthera.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Jensen MB, Finsen B, Zimmer J. Morphological and immunophenotypic microglial changes in the denervated fascia dentata of adult rats: correlation with blood-brain barrier damage and astroglial reactions. Exp. Neurol. 1997;143:103–116. doi: 10.1006/exnr.1996.6337. [DOI] [PubMed] [Google Scholar]
- 20.Lin T, Li K, Zhang FY, Zhang ZK, Light AR, Fu KY. Dissociation of spinal microglia morphological activation and peripheral inflammation in inflammatory pain models. J. Neuroimmunol. 2007;192:40–48. doi: 10.1016/j.jneuroim.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matyszak MK, Denis-Donini S, Citterio S, Longhi R, Granucci F, Ricciardi-Castagnoli P. Microglia induce myelin basic protein-specific T cell energy or T cell activation, according to their state of activation. Eur. J. Immunol. 1999;29:3063–3076. doi: 10.1002/(SICI)1521-4141(199910)29:10<3063::AID-IMMU3063>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 22.McMahon SB, Cafferty WB, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp. Neurol. 2005;192:444–462. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Molander C, Hongpaisan J, Svensson M, Aldskogius H. Glial cell reactions in the spinal cord after sensory nerve stimulation are associated with axonal injury. Brain Res. 1997;747:122–129. doi: 10.1016/s0006-8993(96)01230-9. [DOI] [PubMed] [Google Scholar]
- 24.Morioka T, Kalehua AN, Streit WJ. Progressive expression of immunomolecules on microglial cells in rat dorsal hippocampus following transient forebrain ischemia. Acta Neuropathol. 1992;83:149–157. doi: 10.1007/BF00308474. [DOI] [PubMed] [Google Scholar]
- 25.Muzio L, Martino G, Furlan R. Multifaceted aspects of inflammation in multiple sclerosis: the role of microglia. J. Neuroimmunol. 2007;191:39–44. doi: 10.1016/j.jneuroim.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 26.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 27.Ponomarev ED, Shriver LP, Maresz K, Dittel BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J. Neurosci. Res. 2005;81:374–389. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- 28.Sedgwick JD, Schwender S, Imrich H, Dorries R, Butcher GW, ter Meulen V. Isolation and direct characterization of resident microglial cells from the normal and inflamed central nervous system. Proc. Natl. Acad. Sci. U.S.A. 1991;88:7438–7442. doi: 10.1073/pnas.88.16.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Streit WJ, Graeber MB, Kreutzberg GW. Peripheral nerve lesion produces increased levels of major histocompatibility complex antigens in the central nervous system. J. Neuroimmunol. 1989;21:117–123. doi: 10.1016/0165-5728(89)90167-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J. Neurochem. 2003;86:1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- 31.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 32.Watkins LR, Milligan ED, Maier SF. Spinal cord glia: newplayers in pain. Pain. 2001;93:201–205. doi: 10.1016/S0304-3959(01)00359-1. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J, Hoffert C, Vu HK, Groblewski T, Ahmad S, O’Donnell D. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur. J. Neurosci. 2003;17:2750–2754. doi: 10.1046/j.1460-9568.2003.02704.x. [DOI] [PubMed] [Google Scholar]