Abstract
The primary cilium originates from the mother centriole and participates in critical functions during organogenesis. Defects in cilia biogenesis or function lead to pleiotropic phenotypes. Mutations in centrosome-cilia gene CC2D2A result in Meckel and Joubert syndromes. Here we generate a Cc2d2a-/- mouse that recapitulates features of Meckel syndrome including embryonic lethality and multi-organ defects. Cilia are absent in Cc2d2a-/- embryonic node and other somatic tissues; disruption of cilia-dependent Shh signaling appears to underlie exencephaly in mutant embryos. The Cc2d2a-/- mouse embryonic fibroblasts (MEFs) lack cilia though mother centriole and pericentriolar proteins are detected. Odf2, associated with subdistal appendages, is absent and ninein is reduced in mutant MEFs. In Cc2d2a-/- MEFs, subdistal appendages are lacking or abnormal by transmission-EM. Consistent with this, CC2D2A localizes to subdistal appendages by immuno-EM in wild type cells. We conclude that CC2D2A is essential for the assembly of subdistal appendages, which anchor cytoplasmic microtubules and prime the mother centriole for axoneme biogenesis.
Introduction
The primary cilium is a ubiquitous microtubule-based organelle, projecting from the cell surface to perform specialized sensory and signaling functions during organogenesis and cellular/tissue homeostasis 1-3. In quiescent or post-mitotic cells, the migration of the centrosome towards the plasma membrane sets the stage for biogenesis of the primary cilium, which originates from the mother centriole (MC) acting as the basal body 4. The two centrioles in the centrosome are ultrastructurally distinct; only the mother centriole has distal and subdistal appendages (SDA) 5 and is involved in membrane tethering/docking, whereas the daughter centriole (DC) anchors cytoplasmic microtubule arrays 6. The formation of the ciliary axoneme is initiated at the distal end of mother centriole by the docking of a primary ciliary vesicle followed by coordinated trafficking of hundreds of proteins including signaling molecules and receptors via microtubule motors and intraflagellar transport complexes 7, 8. The efforts to define critical steps in cilia biogenesis have intensified lately because of the association of ciliary function with human diseases.
Given the commonalities in cilia and centrosomes and their nearly universal presence, it is not surprising that aberrant cilia biogenesis and/or function can impact multiple tissues and cell types and manifest as pleiotropic syndromic disorders, collectively termed ciliopathies 9-12. The ciliopathies encompass a spectrum of clinically distinguishable phenotypes that include retinal degeneration, cognitive impairment, neural tube defects, hydrocephalus, polycystic kidney, polydactyly, situs inversus and obesity. The clinical findings could include blindness (as in Leber congenital amaurosis, LCA) and/or cystic kidneys (as in nephronophthesis, NPHP) as the primary manifestation. Alternately, the phenotypes can encompass multiple system defects as in Bardet-Biedl Syndrome (BBS) and Joubert Syndrome (JBTS) or appear as a rare lethal malformation in Meckel Syndrome (MKS). Strikingly, similar syndromic disorders can result from mutations in many different genes, and mutations in one gene can lead to distinct clinical manifestations. The observed phenotypic diversity in ciliopathies might also reflect a cumulative genetic load of variants/mutations and interactions among cilia-associated genes 12-14.
The search for homozygous genomic regions in 10 unrelated MKS fetuses led to the identification of the ciliopathy gene CC2D2A 15, which was independently discovered by homozygosity mapping of consanguineous families with JBTS 16. Concurrently, a third group reported a homozygous splice site mutation in a large Pakistani family with mental retardation and retinitis pigmentosa 17. Follow-up studies suggest that CC2D2A mutations in JBTS patients are less deleterious than those causing MKS 18. CC2D2A encodes a coiled coil and C2-domain containing protein that is required for cilia formation and predicted to be involved in calcium-dependent membrane targeting 15. The CC2D2A protein also includes a catalytically inactive version of the transglutaminase-like (TGL) domain that may provide a peptide-binding interface for microtubules 19. CC2D2A is localized to the basal body and can physically interact with CEP290 16, a cilia-centrosomal protein associated with numerous ciliopathies 20. Interestingly, the multiprotein complex containing Tectonic 1 (Tctn1), associated with regulation of Hedgehog signaling, includes both CC2D2A and CEP290, and these proteins have been localized to the transition zone between the basal body and ciliary axoneme 21.
A fundamental requirement of CC2D2A in organogenesis is implied from embryonic lethality in human MKS 15. The fibroblasts derived from a MKS embryo harboring a CC2D2A mutation are unable to extend ciliary axoneme even though the basal body (i.e., mother centriole) is present, suggesting an essential role of CC2D2A in cilia biogenesis 15. Nevertheless, a nonsense cc2d2a mutation identified in the sentinel zebrafish mutant did not reveal defects in motile cilia number or morphology, though some JBTS-like phenotypes (such as pronephric cysts) were detected 16.
To elucidate the function of CC2D2A in cilia biogenesis and produce a model of MKS, we generated a Cc2d2a null allele in mice. The loss of Cc2d2a (Cc2d2a-/-) results in embryonic lethality with multi-organ defects related to cilia biogenesis. We show that CC2D2A localizes to the SDA in the mother centriole and its loss prevents the assembly of SDA and anchoring of microtubules. Our studies further delineate the fundamental sequence of cilia biogenesis from the mother centriole and uncover ciliary ultrastructural defects associated with the loss of CC2D2A function in MKS.
Results
The loss of Cc2d2a in mouse leads to embryonic lethality
Three protein-coding transcript variants are produced from the Cc2d2a gene. To eliminate all transcripts, we replaced Cc2d2a exons 6 to 8, shared by all variants, with a targeting vector containing a β-gal reporter and a neomycin selection cassette (Fig.1a) through standard homologous recombination in ES cells. Southern blotting of genomic DNA from ES clones using an exon 5-6 probe (Fig. 1a) showed 12.7 and 9.9 kb EcoRI fragments for the wild type and Cc2d2a-/- alleles, respectively (Fig. 1b, Supplementary Fig. 1). We confirmed that the embryos homozygous for the targeted allele (indicated as -/- or Cc2d2a-/-) lacked the Cc2d2a transcript (Fig. 1c). Finally, we demonstrated by immunoblot analysis that Cc2d2a-/- embryos lacked the CC2D2A protein (Fig. 1d, Supplementary Fig. 2).
Figure 1. The loss of Cc2d2a leads to embryonic lethality with pleiotropic defects in organogenesis.
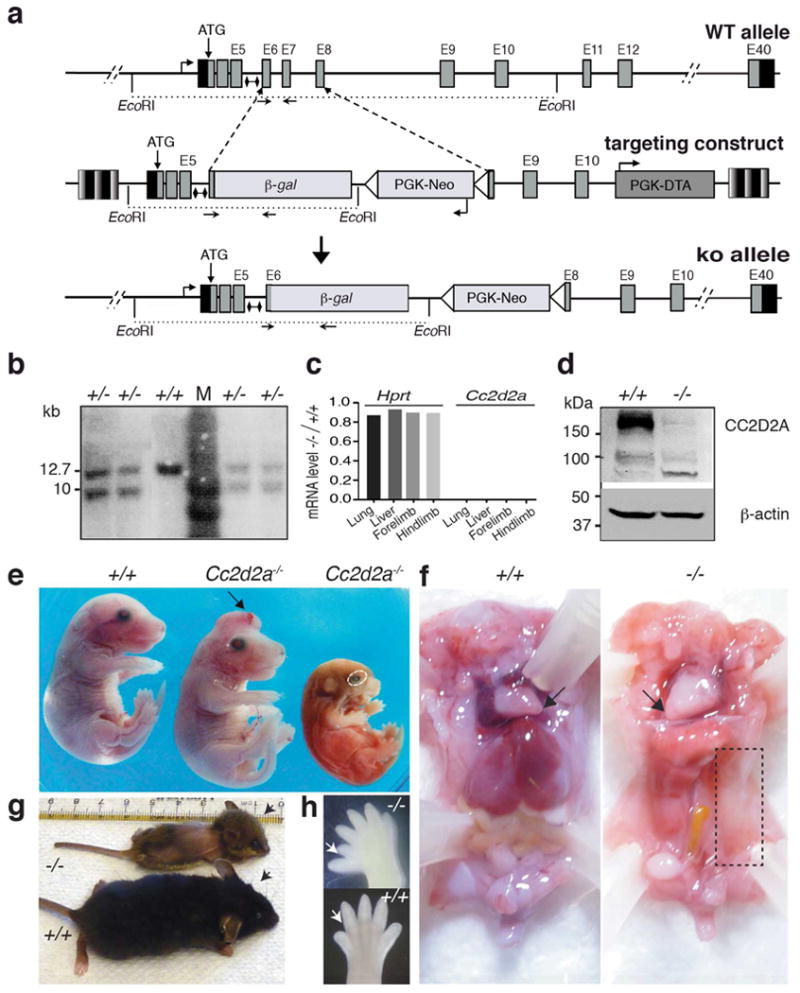
(a) The design for generating Cc2d2a knockout (ko) allele in mouse. The wild type Cc2d2a locus at mouse chromosome 5 (top), the targeting vector (middle) and the knockout allele (bottom) are shown. Small lines with diamond caps indicate the DNA fragment used as a probe for Southern blot analysis. Mice were genotyped using the forward and reverse primers indicated by arrows. (b) Validation of Cc2d2a ko allele in ES cells by Southern blot analysis after digestion with EcoRI, as shown in (a). Successful recombination with the targeting vector would yield both wild type (12.7 kb) and targeted (9.9 kb) fragments. M, DNA ladder; +, wild type allele; -, knockout allele. (c). RT-PCR analysis of Cc2d2a transcripts. The ratio of Hprt (control) or Cc2d2a transcripts in -/- vs. +/+ tissues of newborn (P0) mouse is shown. Cc2d2a transcripts were undetectable in the KO embryos. (d) Immunoblot analysis with anti-CC2D2A antibody on protein extract from the wild type and Cc2d2a ko embryos aged E14. A 187 kDa CC2D2A protein was detected in the wild type (+/+), whereas in the -/- embryos CC2D2A was undetectable. β-actin was used as a loading control. (e) Gross phenotype of E16 Cc2d2a-/- embryos compared to wild type (+/+) littermates. Mutant embryos display a range of developmental defects, including exencephaly or open rostral neural tube (arrow; middle), small size (right) and microphthalmia (dotted oval). (f) Visceral phenotype of Cc2d2a-/- embryos. The -/- embryos display dextrocardia (arrow) and this one lacks kidney, spleen on the left side, in addition to large part of intestine (dotted rectangle). (g) The phenotype of a single rare -/- survivor at P27, along with its littermate control. The -/- (top) animal has a domed head representing profound hydrocephalus compared to its +/+ littermate (lower). See also Supplementary Fig. 3. (h) Hind limbs from wild type and -/- embryos. Note the pre-axial polydactyly on the hind limb of -/- embryo (arrow).
The analysis of F2 litters produced by crossing heterozygous Cc2d2a+/-mice identified live pups with only Cc2d2a+/+ and Cc2d2a+/- genotypes, suggesting lethality of Cc2d2a-/- homozygous null embryos. We then assessed at what age Cc2d2a-/- embryos are lost. We were able to identify Cc2d2a-/- embryos at close to predicted ratios (25%) on embryonic day (E) 14 and E16. However, the ratio of Cc2d2a-/- embryos declined sharply at E18 (to 4%), and null mutants rarely survived beyond that age (Table 1). At E16-18, Cc2d2a-/- embryos displayed pleiotropic phenotypes resembling MKS, with many showing severe degeneration and resorption during early embryogenesis (Fig. 1e). In addition to hemorrhage, open neural tube, microphthalmia, and anophthalmia, Cc2d2a-/- embryos often revealed situs inversus and dextrocardia, and on occasion lacked abdominal organs (Fig. 1f, boxed area). Polydactyly was observed frequently in Cc2d2a-/- embryos (Fig. 1h, arrows). Thus, Cc2d2a is broadly required for organogenesis in mice. Among hundreds of Cc2d2a-/- mutants generated in our laboratory, only one mouse survived for 27 days and showed gross underdevelopment with marked lethargy, loss of hair on its dorsal surface, hydrocephalus (Fig. 1g, compare arrows), and retinal dystrophy (Supplementary Fig. 3).
Table 1. Genotypes obtained from heterozygous (+/-) interbreeding.
| Age | Cc2d2a genotype (%) | Total (N) | ||
|---|---|---|---|---|
| +/+ | +/- | -/- | ||
| Predicted % | 25 | 50 | 25 | |
| E14 | 29 | 54 | 17 | 41 |
| E16 | 24 | 52 | 24 | 25 |
| E18 | 32 | 64 | 4 | 25 |
| P0 | 34 | 66 | 0.2 | 1022 |
First column shows the age of the embryos/animals analysed, indicated as either embryonic (E) day or as P0 for live-born pups. The last column shows total number of animals genotyped at each age. Predicted percentages are italicized.
Cc2d2a-/- embryos have defects in motile and sensory cilia
The situs inversus phenotype suggested defects in the embryonic node and establishment of left-right asymmetry. Scanning electron microscopy of the E8 Cc2d2a-/- embryos revealed flattening of the node with only a few cilia-like structures (Fig. 2a-c). Immunostaining of the embryonic node using anti-acetylated α–tubulin antibody validated these findings (Fig. 2d). The analysis of other ciliated tissues also showed profound defects in cilia biogenesis. The kinocilia in the cochlea were frequently absent or abnormal (green signal in Fig. 2e, right) and stereociliary bundles deformed (red signal in Fig. 2e, right) in Cc2d2a-/- embryos. The cilia were also absent or abnormal in Cc2d2a-/- embryonic liver (green signal in Fig. 2f, right). Perinatal kidney tubules and tracheal epithelium revealed few cilia in Cc2d2a-/- embryos (green signal in Fig. 2g and h, right panels). By comparison, kidney tubules in wild type embryos had prominent, primary cilia projecting into the lumen (Fig. 2g, left), and tracheal epithelia presented tufts of motile cilia (Fig. 2h, left).
Figure 2. Motile and sensory cilia biogenesis is defective in Cc2d2a-/- embryos.

(a to c) Scanning electron micrographs of E8 embryonic node in increasing order of magnification. The wild type node is comprised of ciliated cells, whereas the -/- embryo had only rare cilia (arrowheads in c). (d) Immunostaining for cilia markers (anti-α-acetylated tubulin, green) on E8 embryonic node (within the dashed circled area). The wild type embryo (left panel) had many cilia compared to the -/- embryo (right panel) that had almost none. (e) E18 cochlea showing kinocilia (anti-α-acetylated tubulin, green) and stereocilia (phalloidin, red) in wild type (+/+) and Cc2d2a-/- embryos. In the wild type, kinocilia were evenly spaced and aligned, whereas those in the -/- were missing or misoriented (arrowhead). (f) E13.5 liver from wild type (+/+) and Cc2d2a-/- embryos showing cilia staining (anti-α-acetylated tubulin, green). Cilia were underdeveloped in -/- liver (arrowheads). (g) Kidney tubule and (h) trachea from P0 wild type (+/+) and Cc2d2a-/- mice showing cilia staining (anti-α-acetylated tubulin, green). The wild type kidney had many long luminal cilia that are not detected in the -/- mice (arrowheads). Similarly, the -/- trachea had no cilia (arrowheads) compared to tufts of multiple motile cilia on each epithelial cell in the wild type. The scale bars in all panels are 5 μm except in ‘a’, which is 50 μm. In d-h, anti-α-acetylated tubulin is green and nuclear DAPI counterstain is blue.
Shh signaling is perturbed in Cc2d2a-/- embryos
As Cc2d2a-/- embryos often exhibited exencephaly (see Fig. 1e), we hypothesized that the open neural tube phenotype is due to abnormal cilia biogenesis and perturbed Shh signaling 22-24. Our analysis of embryonic day (E) 12 embryos revealed the presence of cilia in the neural tube in the wild type (Fig. 3a, upper middle panel) but not in the Cc2d2a-/- mutants (Fig. 3a, lower middle panel). We noted the presence of basal bodies in the neural tube of both genotypes (Fig. 3a, left panels).
Figure 3. Cilia-mediated Shh signaling is perturbed in the developing neural tube of Cc2d2a-/- embryos.
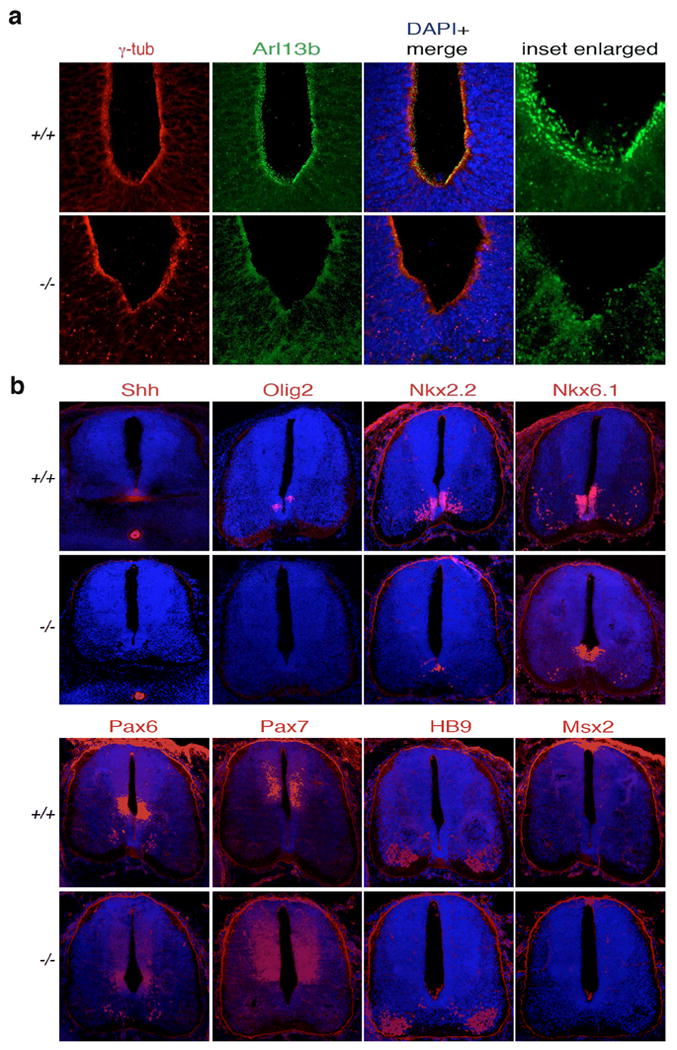
(a) Immunostaining for anti-γ tubulin (marker of basal bodies; red) and anti-Arl13b (highlighting cilia; green) in embryonic (E12) neural tube. In wild type embryo (+/+) neural tube showed presence of both basal bodies (red) and cilia (green). In KO embryo (-/-), the cilia are missing and the basal bodies are mislocalised. The inset region is enlarged and shown at the right. (b) Immunostaining for indicated markers (red) in embryonic E12 neural tube. In the wild type (+/+), the Shh signal was clearly seen in the ventral floor plate and notochord whereas in the KO (-/-) the Shh signal was absent in the floor plate. Olig2 and Nkx2.2 were both evident in ventral domains near the floor plate in the wild type, but in the mutant Olig2 was absent and Nkx2.2 was seen ectopically at the floor plate. Compared to the wild type, Nkx6.1 signal was shifted ventrally, and Pax6 domain also showed a slight expansion ventrally. Pax7, HB9 and the roof plate maker Msx2 did not show any apparent difference in signal intensity and pattern in the wild type and the KO embryos. The nuclear staining is done with DAPI (blue). The images are taken in rostrocaudal axis approximately in the hind limb level. The scale bars are 50 μm.
We then investigated a potential defect in patterning of neural tube domain markers due to lack of cilia-mediated Shh signaling. In wild type embryos, Shh signal localizes to the floor plate of neural tube and at the notochord (Fig. 3b). In contrast, Cc2d2a-/- embryos lack Shh signal at the floor plate (Fig. 3b). In contrast to the wild type embryos showing Nkx2.2 and Nkx6.1 expression (Fig. 3b) at the ventral neural tube, Nkx2.2 signal was found at the floor plate in the Cc2d2a-/- embryos and Nkx6.1 expressing domain shifted toward the ventral midline (Fig. 3b). Furthermore, the Pax6 domain also expanded ventrally. These data indicate the loss of floor plate cell fate and a reduction in V3 progenitors, which require high levels of Shh for induction. Olig2 signal, marking progenitors of the oligodendrocyte lineage that also depend on Shh signaling for induction, was absent in the Cc2d2a-/- embryos (Fig. 3b). We note that Pax7, HB9 and Msx2 showed no discernable difference in Cc2d2a-/- embryos compared to the wild type (Fig. 3b). Thus, the neural tube patterning defect of Cc2d2a-/- embryos mostly affects ventral cell fates, consistent with a perturbation of Shh signaling. The neural tube patterning defect in this mutant is similar to that of the Mks1 mutant 25.
Cc2d2a-/- fibroblasts have basal body but not ciliary axoneme
To delineate the underlying defect in cilia biogenesis, we isolated and cultured embryonic fibroblasts (MEFs) from Cc2d2a-/- mutants. Unlike MEFs from wild type littermates (Fig. 4a, upper panel), Cc2d2a-/- MEFs did not grow cilia upon serum starvation (Fig. 4a, lower panel). We however noted that ∼10% of Cc2d2a-/- MEFs escaped this phenotype. Both Cc2d2a-/- and wild type MEFs showed similar mother centriole staining with γ-tubulin, yet Arl13b labeling was compromised in Cc2d2a-/- MEFs (Fig. 4a). In addition, microtubule (MT) staining with acetylated α–tubulin showed presence of axoneme in the wild type but not in Cc2d2a-/- MEFs; nonetheless, cytoplasmic MTs were visible in Cc2d2a-/- MEFs (Fig. 4c-d). These data suggest that ciliary axoneme biogenesis was not initiated from the mother centriole in the absence of Cc2d2a. The mouse Cc2d2a transgene could rescue the axoneme assembly defect in Cc2d2a-/- MEFs (Fig. 4b; note that the non-transfected cells in the same field do not have cilia), demonstrating a direct role of CC2D2A in the genesis of cilia from the mother centriole.
Figure 4. Lack of cilia and defects in mother centriole of Cc2d2a-/- MEFs can be rescued by Cc2d2a transgene.

(a) Cc2d2a-/- MEFs have mother centriole but no cilia. Anti-Arl13b is used to highlight cilia. MC (basal body) is stained with γ-tubulin antibody. (b) Cc2d2a transgene rescues cilia biogenesis in Cc2d2a-/- MEFs. Anti-Arl13b staining is shown in red circle. GFP (green) represents the transfected cells. Non-transfected cells in the same field do not have any cilia. Nuclei are stained with DAPI (blue). In c-d cilia are marked by acetylated α– tubulin (green). (c) Pericentrin (a proximal end marker of mother centriole) staining (in red) is unaltered in Cc2d2a-/- MEFs. See also Supplementary Fig. 4. (d) Immunostaining of ninein that marks both SDA and the mother centriole proximal end is significantly reduced in Cc2d2a-/- MEFs. (e) Anti-Odf2 immunostaining which is detected at SDA in wild type is barely visible in Cc2d2a-/- MEFs. (f) Trichoplein immunostaining is reduced in Cc2d2a-/- MEFs. All scale bars are 5 μm. Antibody is indicated above each panel. In merged images, blue nuclear stain is DAPI. The number of cells used for quantitation is indicated in all graphs. The graph in a compares percentage of cells with cilia (y-axis) between wild type (+/+) and Cc2d2a-/- genotypes (x-axis). The graphs in remaining panels compare the level of antibody-generated fluorescence (red signal in each panel) between the two genotypes, where x- and y-axes are genotypes and corrected fluorescence intensity (arbitrary units, (a. u.)), respectively. Bars indicate s.e.m. The sample size (n) is indicated on the bar diagram, and the experiment was repeated three times. P-value was derived from unpaired two tailed t-test. Statistical significance is marked with asterisks (* and **** indicate P≤0.05 and 0.0001, respectively).
The existence of a mother centriole but lack of axoneme suggested that Cc2d2a is needed in early ciliogenic processes. After polarity-guided centriolar migration, the mother centriole docks to the membrane with distal appendages, whereas the anchoring of MT arrays requires SDA 6. Even though MT nucleation starts with aster formation at both centrioles, only the mother centriole is able to sustain a stable MT array, a process requiring ninein 26, 27. Immunolabeling with anti-ninein antibody revealed a significant reduction of ninein signal at the mother centriole in Cc2d2a-/- MEFs (Fig. 4d). It should be noted that ninein is present predominantly at the distal end of the mother centriole, on SDA, while it is also detectable at the proximal ends of both centrioles. Our results thus indicate a basic structural defect in mother centriole.
Distal end components are abnormal in MC of Cc2d2a-/- MEFs
Immunolabeling of wild type and Cc2d2a-/- MEFs using rootletin and pericentrin antibodies revealed no significant differences (Supplementary Fig. 4a and Fig. 4c), implying structural integrity of pre-proximal and proximal ends of the two centrioles. Absence of the ciliary axoneme and reduced ninein staining prompted us to examine Odf2, an established marker of SDA 28, which is needed for cilia biogenesis 29. The anti-Odf2 signal at the mother centriole was dramatically reduced in Cc2d2a-/- MEFs compared to the wild type (Fig. 4e). We then performed immunolabeling against trichoplein, which controls MT anchoring at the mother centriole by binding to Odf2 and ninein 30. Interestingly, trichoplein signal was also significantly reduced at the mother centriole in Cc2d2a-/- MEFs (Fig. 4f). Our results suggest that recruitment of Odf2 and trichoplein to the SDA is compromised when CC2D2A is not present, and that CC2D2A is needed for SDA assembly.
CC2D2A is required to form subdistal appendages
The loss of cc2d2a in zebrafish photoreceptors resulted in mislocalisation of Rab8 31, which interacts with Odf2 and is needed for cilia biogenesis 32. In Cc2d2a-/- MEFs, Rab8 staining is significantly reduced compared to the wild type (Fig. 5a). Furthermore, transfection of Rab8a-mCherry in wild type MEFs showed sharp localization at the base of the cilium, whereas the signal was diffuse and cytoplasmic in Cc2d2a-/- MEFs (Fig. 5b). Notably, immunoblotting demonstrated similar levels of Rab8a protein in the wild type and Cc2d2a-/- MEFs (Supplementary Fig. 5). These results thus indicate defective vesicle docking or membrane tethering at mother centriole in the absence of CC2D2A.
Figure 5. Accumulation of transport vesicles and lack of or abnormal subdistal appendages (SDA) in Cc2d2a-/- MEFs.
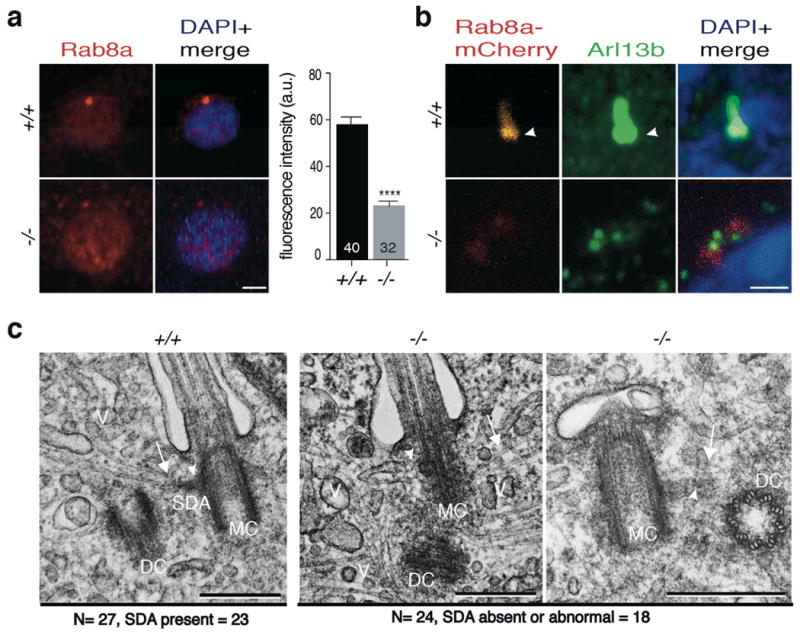
(a) Immunostaining of Rab8a (red) that marks transport vesicles in wild type and Cc2d2a-/- MEFs. Rab8a localization is diffuse in cytoplasm in Cc2d2a-/- MEFs compared to the wild type. The x- and y-axes are genotypes and corrected fluorescence intensity (arbitrary units, (a.u.)), respectively. Bars indicate s.e.m. Signal intensity quantification shows a highly significant difference (P-value was derived from unpaired two tailed t-test; **** indicates P-value ≤0.0001). The sample size (n) is indicated on the bar diagram, and the experiment was repeated three times. (b) Immunostaining for anti-Arl13b (green) and Rab8a-mCherry (red) transfected in wild type and Cc2d2a-/- MEFs. Rab8a-mCherry signal is sharp at the base of the cilium (arrowhead) in wild type cells but diffuse in -/- MEFs. Nuclei are stained with DAPI (blue). (c) Transmission electron micrographs of cilia from wild type and Cc2d2a-/- MEFs. In wild type, the mother centriole has well-defined SDA (arrow head), to which microtubules (arrow) are anchored for trafficking of vesicles (V). In Cc2d2a-/- MEFs, the SDA is not visible at a comparable location (middle panel, arrowhead) or an abnormal SDA with compromised transition zone (right panel, arrowhead) and the microtubules (arrow) are not anchored. The vesicles accumulate in the cytoplasm around the mother centriole. The total number (N) of MEFs analysed for the presence of SDA or abnormal SDA is indicated. MC and DC are mother and daughter centriole, respectively. Scale bars in a and b are 5 μm, and in c is 500 nm.
To further investigate cellular ultrastructure at and near the mother centriole, we took advantage of transmission electron microscopy (TEM). In wild type MEFs, normal ciliary structures were evident with microtubules anchored at SDA and oriented in a regular array from the cytoplasm toward the basal body (Fig. 5c; left, arrow). However, in Cc2d2a-/- MEFs, mother centriole lacked or had abnormal SDA, had disorganized microtubules and showed accumulation of vesicle-like structures nearby (Fig. 5c, middle panel). Notably, in a fraction of Cc2d2a-/- MEFs, the mother centriole docked to ciliary vesicles but apparently no transition zone developed (Fig. 5c, right panel). This finding was not obtained in the wild type MEFs. We therefore conclude that CC2D2A is needed for the assembly of SDA at mother centriole, for stable MT anchoring and docking of transport vesicles, and for further elaboration of the transition zone.
MT regrowth assays using α- and γ-tubulin 33 were then performed to evaluate whether MT anchoring at the mother centriole requires CC2D2A. In wild type and Cc2d2a-/- MEFs, MT regrowth did not initiate immediately after removing nocodazole (Fig. 6, 0 min). At 5 min, the MT started reappearing at the MC in the wild type, but not seen in Cc2d2a-/- MEFs. At 30 min of recovery from nocodazole MT aster anchoring at the mother centriole was visible in wild type cells (Fig. 6, 30 min). At 30 min, though MTs were visible in Cc2d2a-/- MEFs, these did not form an aster and anchor at the basal body. These data demonstrate a critical role of CC2D2A in MT anchoring at the basal body.
Figure 6. CC2D2A is required for microtubule anchoring at the basal body.

The microtubule array (α-tubulin, green) formation at the basal body (γ-tubulin, red) is not visible at 0 min in the wild type (+/+) and the KO cells (-/-). At 5 min, the microtubule array is apparent at the basal body in the wild type cells but not in the KO cells. At 30 min, a profuse array of microtubule forming an aster is observed at the basal body in the wild type but not in the KO cells even though cytoplasmic microtubules are detected. The area in the inset is shown at the higher magnification on the right side of each image. The scale bar is 5 μm.
CC2D2A localizes to subdistal appendages
Immunolabeling in ciliated IMCD3 cells revealed the localization of CC2D2A protein to the mother centriole. The CC2D2A staining partially overlapped with RPGR, a cilia transition zone marker 34, and anti-GT335 (Fig. 7a, b), which labels polyglutamylated tubulin and marks both the basal body and the proximal axoneme 35, 36. Interestingly, CC2D2A immunostaining appeared similar to that of centriolin 35 and Odf2 28. To further refine the localization of CC2D2A, we took advantage of immuno-EM. Immunogold labeling for CC2D2A was clearly visible on the SDA both in IMCD3 cells and in monkey retina (Fig. 7c, d). Our data are concordant with preceding MEF studies and strengthen the proposition of a role for CC2D2A in SDA assembly.
Figure 7. CC2D2A localizes to SDA where ciliary transition zone begins.

(a) Immunostaining for anti-GT335 (green) and anti-CC2D2A (red) in IMCD3 cells that were induced to generate cilia. Anti-GT335 signal marks mother centriole and the proximal part of the axoneme, whereas anti-CC2D2A staining is restricted to the mother centriole (third panel, circled). Inset shows magnified view of the circled cilium. (b) Immunostaining for anti-α-acetylated tubulin (magenta), anti-RPGR (green) and anti-CC2D2A (red) in IMCD3 cells. RPGR is a cilia transition zone marker. The CC2D2A immunostaining partially overlaps with anti-RPGR signal (circled, enlarged in inset) at the base of axoneme marked by anti-α-acetylated tubulin. Nuclei are stained with DAPI (blue) in a and b. (c) Immuno-EM of mother centrioles labeled with anti-CC2D2A from ciliated IMCD3 cells. Gold particles (10 nm) are localized to SDA on mother centriole in both micrographs. The upper micrograph shows a ciliary vesicle of an extending cilium axoneme (arrow). (d) Immuno-EM of a monkey retina, using CC2D2A antibody in c. The gold particles align on the SDA. Mother centriole is supported by a rootlet. Scale bars in a and b are 5 μm, and c and d are 500 nm.
No cell cycle defect in Cc2d2a-/- MEFs
We detected similar anti-GT335 immunostaining in wild type and Cc2d2a-/- MEFs (Supplementary Fig. 4b, lower panels), consistent with the hypothesis that CC2D2A includes a catalytically inactive transglutaminase like domain 19. To test whether CC2D2A might be required for sensing Ca2+ level changes during cell cycle 37, we analysed the cell cycle profile in MEFs. No significant difference was identified between the wild type and Cc2d2a-/- MEFs (Supplementary Fig. 6).
Discussion
Embryonic lethality observed in MKS represents the most severe of all phenotypes in ciliopathies. CC2D2A is one of the ten centrosome-cilia genes associated with MKS. Mutations in CC2D2A also cause JBTS, another relatively severe disease with a plethora of clinical findings. Previous studies implicate a fundamental role of CC2D2A in cilia biogenesis that when interrupted would lead to MKS or JBTS. However, the precise disease mechanism and CC2D2A function have been elusive. Here, we demonstrate that CC2D2A is required for the assembly of SDA that are critical for anchoring of microtubules, vesicle docking and initiation of ciliary axoneme. Loss of CC2D2A, as in the Cc2d2a-/- embryos reported here, appears to prevent cilia-mediated functions of motile or sensory cilia, thereby leading to defects in organogenesis in MKS. We suggest that the less severe JBTS ciliopathy phenotype likely results from residual function of certain mutant CC2D2A alleles or from partial compensation by a modifier gene 14, 38, 39.
The Cc2d2a-/- mouse model recapitulates the phenotype reported in human MKS fetuses that inherit a nonsense mutation in CC2D2A 15. The lethality observed in cc2d2a-null mutants of zebrafish 40 reflects the evolutionary conservation of Cc2d2a function in cilia biogenesis. Interestingly, though a Cc2d2a gene-trap mouse exhibits embryonic lethality, the MEFs derived from this mutant could extend a ciliary axoneme 21, suggesting that the gene-trap allele may not be a complete null. Different mouse mutants therefore offer an opportunity to better elucidate underlying cilia biogenesis defects and pathologies in distinct syndromes caused by mutations in CC2D2A.
We note that the situs inversus phenotype in Cc2d2a-/- embryos can be explained by the lack of nodal cilia, as in IFT-defective kif3B mice 41. However, the cilia defects are remarkably different in Cc2d2a-/- mutants compared to the IFT mutants. Generally, in IFT mutants cilia are formed with different degrees of abnormality depending on the mutation: shorter, rounder, or of normal length but with bulges near the tip 42. By contrast, the cilia are not formed in the Cc2d2a mutant, suggesting that CC2D2A is required at an early step of ciliogenesis. IFTs are utilized as transport modules at a later stage to build and maintain the cilia.
The Shh pathway is initiated in primary cilia for both neural tube and limb bud patterning 22, 23. Notably, Shh signaling is perturbed in the developing Cc2d2a-/- neural tube (see Fig. 3b). Ventral progenitor domains marked by Shh and Olig2 are absent in Cc2d2a-/- embryos. Nkx2.2 is shifted and confined to the floor plate, and both Nkx6.1 and Pax6 show a slight shift toward the ventral midline. These data indicate lost floor plate and diminished V3 progenitor domain, both of which require high levels of Shh signaling for induction. On the other hand, dorsal and lateral fates are largely unaffected in Cc2d2a-/- embryos. The neural tube patterning defect is consistent with reduced Shh signaling and is similar to those reported for Mks1 mutant 25 and some other ciliogenesis or trafficking mutants 23, 43. Polydactyly is a common finding in MKS and JBTS 44, and is caused by aberrant Shh signaling 45 via its effector Gli proteins localized on the ciliary membrane 22, 46, 47. We conclude that both the neural tube defect and polydactyly in Cc2d2a-/- embryos result from a disruption in cilia-based Shh signaling.
The two centrioles in the centrosome constitute the primary center for cytoplasmic microtubule nucleation, anchoring and elongation in animal cells. Distinct ultrastructural characteristics, specifically the presence of distal appendages and SDA, distinguish the mother centriole from daughter centriole and provide cellular architecture for building the ciliary axoneme using molecular motors and intraflagellar transport 6, 48, 49. Initiation of cilia biogenesis requires the assembly of microtubule bundles, aster formation and their stabilization at the mother centriole; the absence of CC2D2A will compromise this process. While aberrant immunostaining of ninein, Odf2 and trichoplein indicate a SDA defect, TEM of Cc2d2a-/- MEFs clearly shows the absence or abnormality of SDA. Thus, SDA assembly at the mother centriole requires CC2D2A along with ninein, Odf2 and trichoplein. Odf2 appears to be a core component since SDA are missing in the Odf2-/- model 28. Ninein and ε-tubulin are also essential because their depletion halts MT aster formation 50, 51. In addition, centriolin and Cep170 are associated with SDA 35, 52, though whether they play a direct role is less clear. Additional investigations are required to determine whether distinct proteins are assembled directly at the mother centriole, or if a pre-assembled complex is attached to the mother centriole to generate SDA. The catalytically inactive TGL domain in CC2D2A is a good candidate for providing the microtubule interaction surface for SDA assembly (see Figure 8 for a schematic).
Figure 8. A schematic of the base of primary cilium showing the mother centriole (basal body) and the proposed function of CC2D2A at subdistal appendages.

Mother centriole includes triplet microtubules (purple cylinders) that continue as the doublet microtubules of the axoneme (pink cylinders). The plus and minus ends of microtubules are marked. The proteins localised to the cilium and mother centriole are indicated. Nine extensions of each microtubule bundle at the border of mother centriole and transition zone represent the SDA (blue-green-yellow pyramids) and distal appendages (blue rods). The proteins localized to SDA are shown in an order from center to periphery, based on the current experimental evidence. The SDA anchors MT arrays and helps in the docking of transport vesicles at the base of cilium. The distal appendages help in tethering the mother centriole to the plasma membrane, separating the cilium compartment from the cytoplasm. The green circle at the far end of the distal appendage, on the right side, represents a Rab8a tagged vesicle. Rab8a may help tether plasma membrane to distal appendages with support from Odf2 and CC2D2A. Inset shows the top view of mother centriole at the level of SDA, which are shown anchored to MT. The electron micrographs on the right show a cross-sectional view at the level indicated: doublet axoneme (top), at the level of appendages (middle), and triplet mother centriole (basal body) (bottom).
A transition zone membrane complex required to build cilia contains CC2D2A, in addition to other MKS-JBTS associated proteins such as CEP290, Tectonic1 and B9D1 21. It appears that the SDA region is at the base of the ciliary transition zone where triplet microtubules of the mother centriole terminate and doublet microtubules of the axoneme begin 4, 53, 54. The overlap in RPGR and CC2D2A immunostaining, reported here (see Fig. 7b), is consistent with this model (see Fig. 8) and with their interaction with CEP290 16, 55; however, these proteins might exist in distinct protein complexes in SDA or transition zone 12, 14, 56. A substantial fraction of mutant MEFs revealed the docking of mother centriole with the ciliary vesicle but no transition zone was apparent, suggesting a requirement of CC2D2A in transition zone formation (Fig. 5c, right panel). We hypothesize that CC2D2A interacts with Odf2, which in turn facilitates the delivery of Rab8a vesicles to tether the membrane at the SDA 32. Accumulation of vesicles observed in Cc2d2a-/- MEFs (see Fig. 5) and mislocalisation of Rab8 in zebrafish mutant photoreceptors 31 further support the role of CC2D2A and other SDA proteins in membrane loading and/or tethering during cilia biogenesis.
In summary, we demonstrate an essential role of CC2D2A in the formation or stabilization of sub-distal appendages to initiate the process of cilia biogenesis from the basal body. Defects in nodal and primary cilia, and consequently Shh signaling, can explain severe pleiotropic phenotypes. Our studies thus provide molecular insights into the MKS disease caused by CC2D2A mutations.
Methods
Generation of Cc2d2a-/- mice and MEFs for phenotyping
All animal experiments were performed after obtaining approval of the animal care and use committee of the National Eye Institute (ASP-NEI 650).
The mouse Cc2d2a gene on chromosome 5 spans almost 80 kb and includes 40 exons (Gene ID: 231214) with three protein-coding splice variants. Following standard homologous recombination 41, 57, Exons 6 to 8 were replaced with a gene-targeting cassette containing homology arms and a β-gal reporter. Southern blotting and PCR validated the correct integration of the targeting cassette. qRT-PCR analysis of different tissues was performed using a TaqMan assay (Applied Biosystems; Mm01211431_m1). The method for RNA-seq analysis of MEFs has been reported 58. The procedures for electroretinography (ERG), histology and immunohistochemistry are reported elsewhere 59. Analysis of the neural tube for cilia and Shh signaling associated proteins was largely performed as detailed 43.
MEFs were prepared from E12.5-E13.5 mouse embryos, as instructed (Millipore Tech. Publications). For immunostaining, 70,000 MEFs were plated in each well of a chamber slide (ibidi, WI) with 300 μl of medium. After overnight growth, cilia biogenesis was induced with serum free medium. The cells were grown to confluence for 48 h, washed with PBS, fixed with 4% paraformaldehyde at room temperature for 15 min, and subjected to standard immunostaining protocol. MEFs were imaged on LSM 700 (Zeiss, Germany) confocal microscope, and the images were edited with Adobe Photoshop CS5 and assembled on Adobe Illustrator CS5. The fluorescence intensity in the images was analyzed with ImageJ64 (NIH) as performed earlier 60. Briefly, fluorescence signal (pixel area) in an image was selected with a tool (circle) and integrated density (mean gray value) of the area was measured. Similarly, from the same field, an area with no fluorescence signal (next to a cell) was measured for background intensity. The corrected fluorescence (CF) was calculated by the formula, CF= integrated density – (area of pixel with signal X mean fluorescence of background readings). Statistical analysis of difference in fluorescence intensity was performed by t-test (2 tailed, type 2) in Excel (Microsoft).
Antibodies
The following primary antibodies were used: anti-CC2D2A (Rb, 1:300, custom made), anti-acetylated α-tubulin (Ms, 1:500; Sigma, T6793), anti-α-tubulin (Ms, 1:2000; Sigma, T6199 (DM1A)), anti-γ–tubulin (Ms, 1:500; Sigma, T6557), anti-GT335 (Ms, 1:500; Adipogen, AG-20B-0020), anti-Arl13b (Rb, 1:500; Proteintech, 17711-1-AP;), anti-trichoplein (Rb, 1:100; Masaki Inagaki), anti-human cenexin (ODF2 Rb, 1:50; Kyung Lee), anti-ninein-L77 and –L79 (Rb, 1:2500; Michel Bornens), anti-Rab8a (Rb, 1:250; Proteintech, 55296-1-AP), anti-rootletin6 (Rb, 1:500; Tiansen Li) and anti-RPGR-S3 (Chk, 1:300; Tiansen Li), anti-pericentrin (Rb, 1:500; Abcam, ab448), Anti-Olig2 (Rb, 1:200; R&D, AF2418). The following monoclonal antibodies (Ms, 1:200, DSHB): Shh, Nkx2.2, Nkx6.1 (clone F55A10), HB9, Msx2, Islet-1 (clone 40.2.D6) Pax6 and Pax7. The secondary antibodies for immune-fluorescence analysis were coupled to Alexa Fluor 488 or 568 dyes (Molecular Probes).
Microtubule regrowth assay
MEFs were plated at a high confluence onto the chamber slide (70-80%) and the following day the cells were treated with 5 uM nocodazole (Sigma) in complete media (DMEM) and incubated for 1.5 h at 37°C. Subsequently, the media was aspirated and the cells were washed once with PBS and replaced with complete media. After treatment, the cells were fixed at time points of interest with 100% methanol 3 min on ice and then processed for immunofluorescence staining, with anti-γ–tubulin and anti-α-tubulin, sequentially. The images were captured on LSM 700 confocal microscope (Zeiss, Germany).
Scanning Electron Microscopy
Embryos (E8) were fixed in paraformaldehyde (4%) and glutaraldehyde (2%) in cacodylate buffer (0.1 M) for 2 h at room temperature and then washed in cacodylate buffer thrice (10 min each). The embryos were treated for 1 h in osmium tetroxide (1%), washed thrice (10 min each) in cacodylate buffer, and dehydrated in graded ethanol (35%, 50%, 70%, 95%, 2 times for 10 min each, and then 3 times with 100%). After treating with tetramethylsilane solution (3 times for 10 min each), the embryos were mounted on a SEM stub and sputter coated with gold/palladium using an EMITECH K575 high-resolution coater. Imaging was performed on S-3000N scanning electron microscope (Hitachi, Japan).
Transmission Electron Microscopy (TEM) and Immuno EM
The serum-starved MEFs were fixed and processed for TEM as described 61. Sections of cells in Epoxy blocks (80 nm) were prepared with Leica UCT Ultramicrotome, mounted on 200 mesh copper grids and coated with carbon. The sections were imaged using H-7600 transmission electron microscope (Hitachi).
Post embedding Immuno EM
The procedure of post-embedding immuno EM was previously described 62. Briefly, cultured IMCD3 cells (CRL-2123, ATCC) were fixed in phosphate-buffered saline containing formaldehyde (4% v/v) and glutaraldehyde (0.05% v/v) for 2 h, then dehydrated in a series of cold ethanol (35%, 50%, 70%, 95%, and 100%). The cells were infiltrated in 1:1 and 1:2 mixtures of 100% ethanol and LR White resin for 1hr each, then in LR White resin overnight. The cells were embedded in LR White resin and cured in a 55°C oven for 24 h. Thin sections (90-100 nm) were then mounted on 300-meshed nickel grids that were first blocked by a commercial blocking buffer for goat antibodies and then incubated in a serial dilution of primary antibody, followed by immunogold-conjugated secondary antibody (10 nm particles). The grids were washed in Tris-buffer (pH 7.4) containing BSA (0.1% w/v), NaCl (250 mM), and Tween-20 (0.01% v/v). The grids were stained in uranyl acetate and lead citrate and examined by electron microscope.
Post embedding immunolabeling of monkey photoreceptors
Monkey eyes (Macaca mulatta) were obtained from the Washington National Primate Research Center at the University of Washington. Eyes were fixed in 2% paraformaldehyde and 0.5% glutaraldehyde in phosphate buffer for 1 h, then en bloc stained in uranyl acetate, dehydrated and infiltrated in LR White as previously described 63. Thin sections were collected on nickel grids, blocked in normal goat serum and incubated in anti-CC2D2A antibody (1:100) for 2 h, washed and incubated in goat anti-rabbit 10 nm gold secondary for 1 h. Sections were stained with uranyl acetate and lead citrate and examined on a JEOL 1010 transmission electron microscope. Negative controls (no primary) were run in parallel.
Flow cytometry
DNA content was assayed by flow cytometry using propidium iodide. Samples were acquired using a FACSCalibur (BD Bioscience, CA) and the data analysed with FlowJo V9.5 (TreeStar Inc, OR) using Watson Pragmatic Modeling.
Supplementary Material
Acknowledgments
We are grateful to Michel Bornens and James Sillibourne for anti-Ninein, Masaki Inagaki and Akhito Inoko for anti-Trichoplein, Kyung Lee for anti-hOdf2 antibodies, James Goldenring for Rab8a-mCherry plasmid, and Andrew Kodani and Jeremy Reiter for microtubule regrowth assay protocol. We thank Megan Kopera and Seid Ali with mouse matings, Rafael Villasmil with flow sorting, Adam Harned with EM, and Ethan Tyler for an illustration. We thank Jacob Nellissery, Sharda Yadav, and Haiyan Guo for technical assistance. The following monoclonal antibodies - Shh, Nkx2.2, HB9, Msx2, Islet-1 (clone 40.2.D6) (developed by T.M. Jessell and S. Brenner-Morton); Nkx6.1 (clone F55A10) (developed by O.D. Madsen); Pax6, and Pax7 (developed by A. Kawakami) - were obtained from the Developmental Studies Hybridoma Bank created under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. Our studies are supported by the Intramural Research Program of the National Eye Institute and by a contract to SAIC from National Cancer Institute.
Footnotes
Author contributions: S.V., T.L. and A.S. conceived the study, designed the experiments, analysed the data and wrote the manuscript. R.A.R. performed some of the data analysis and was involved in manuscript revision. S.V., S.H.M., H.M., M.B., T.A.F. and T.A.L. performed the experiments. P.L., S.V., and L.D. generated the knockout mice. S.V., C.G., K.N. and R.N.F. performed confocal or electron microscopy.
Competing financial interests: The authors declare no competing financial interests.
References
- 1.Singla V, Reiter JF. The primary cilium as the cell's antenna: signaling at a sensory organelle. Science. 2006;313:629–633. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 2.Gerdes JM, Davis EE, Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell. 2009;137:32–45. doi: 10.1016/j.cell.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eggenschwiler JT, Anderson KV. Cilia and developmental signaling. Annu Rev Cell Dev Biol. 2007;23:345–373. doi: 10.1146/annurev.cellbio.23.090506.123249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ishikawa H, Marshall WF. Ciliogenesis: building the cell's antenna. Nat Rev Mol Cell Biol. 2011;12:222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- 5.Paintrand M, Moudjou M, Delacroix H, Bornens M. Centrosome organization and centriole architecture: their sensitivity to divalent cations. J Struc Biol. 1992;108:107–128. doi: 10.1016/1047-8477(92)90011-x. [DOI] [PubMed] [Google Scholar]
- 6.Bornens M. Centrosome composition and microtubule anchoring mechanisms. Curr Opin Cell Biol. 2002;14:25–34. doi: 10.1016/s0955-0674(01)00290-3. [DOI] [PubMed] [Google Scholar]
- 7.Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170:103–113. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haimo LT, Rosenbaum JL. Cilia, flagella, and microtubules. J Cell Biol. 1981;91:125s–130s. doi: 10.1083/jcb.91.3.125s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badano JL, Mitsuma N, Beales PL, Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 10.Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011;364:1533–1543. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nigg EA, Raff JW. Centrioles, centrosomes, and cilia in health and disease. Cell. 2009;139:663–678. doi: 10.1016/j.cell.2009.10.036. [DOI] [PubMed] [Google Scholar]
- 12.Novarino G, Akizu N, Gleeson JG. Modeling human disease in humans: the ciliopathies. Cell. 2011;147:70–79. doi: 10.1016/j.cell.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaghloul NA, Katsanis N. Functional modules, mutational load and human genetic disease. Trends Genet. 2010;26:168–176. doi: 10.1016/j.tig.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sang L, et al. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell. 2011;145:513–528. doi: 10.1016/j.cell.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tallila J, Jakkula E, Peltonen L, Salonen R, Kestila M. Identification of CC2D2A as a Meckel syndrome gene adds an important piece to the ciliopathy puzzle. Am J Hum Genet. 2008;82:1361–1367. doi: 10.1016/j.ajhg.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorden NT, et al. CC2D2A is mutated in Joubert syndrome and interacts with the ciliopathy-associated basal body protein CEP290. Am J Hum Genet. 2008;83:559–571. doi: 10.1016/j.ajhg.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noor A, et al. CC2D2A, encoding a coiled-coil and C2 domain protein, causes autosomal-recessive mental retardation with retinitis pigmentosa. Am J Hum Genet. 2008;82:1011–1018. doi: 10.1016/j.ajhg.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bachmann-Gagescu R, et al. Genotype-phenotype correlation in CC2D2A-related Joubert syndrome reveals an association with ventriculomegaly and seizures. J Med Genet. 2012;49:126–137. doi: 10.1136/jmedgenet-2011-100552. [DOI] [PubMed] [Google Scholar]
- 19.Zhang D, Aravind L. Novel transglutaminase-like peptidase and C2 domains elucidate the structure, biogenesis and evolution of the ciliary compartment. Cell Cycle. 2012;11:3861–3875. doi: 10.4161/cc.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coppieters F, Lefever S, Leroy BP, De Baere E. CEP290, a gene with many faces: mutation overview and presentation of CEP290base. Hum Mut. 2010;31:1097–1108. doi: 10.1002/humu.21337. [DOI] [PubMed] [Google Scholar]
- 21.Garcia-Gonzalo FR, et al. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet. 2011;43:776–784. doi: 10.1038/ng.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haycraft CJ, et al. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet. 2005;1:e53. doi: 10.1371/journal.pgen.0010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huangfu D, Anderson KV. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci U S A. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huangfu D, Anderson KV. Signaling from Smo to Ci/Gli: conservation and divergence of Hedgehog pathways from Drosophila to vertebrates. Development. 2006;133:3–14. doi: 10.1242/dev.02169. [DOI] [PubMed] [Google Scholar]
- 25.Weatherbee SD, Niswander LA, Anderson KV. A mouse model for Meckel syndrome reveals Mks1 is required for ciliogenesis and Hedgehog signaling. Hum Mol Genet. 2009;18:4565–4575. doi: 10.1093/hmg/ddp422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mogensen MM, Malik A, Piel M, Bouckson-Castaing V, Bornens M. Microtubule minus-end anchorage at centrosomal and non-centrosomal sites: the role of ninein. J Cell Sci. 2000;113(Pt 17):3013–3023. doi: 10.1242/jcs.113.17.3013. [DOI] [PubMed] [Google Scholar]
- 27.Piel M, Meyer P, Khodjakov A, Rieder CL, Bornens M. The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J Cell Biol. 2000;149:317–330. doi: 10.1083/jcb.149.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishikawa H, Kubo A, Tsukita S. Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nat Cell Biol. 2005;7:517–524. doi: 10.1038/ncb1251. [DOI] [PubMed] [Google Scholar]
- 29.Hehnly H, Hung HF, Doxsey S. One among many: ODF2 isoform 9, a.k.a. Cenexin-1, is required for ciliogenesis. Cell Cycle. 2013;12:1021. doi: 10.4161/cc.24330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibi M, et al. Trichoplein controls microtubule anchoring at the centrosome by binding to Odf2 and ninein. J Cell Sci. 2011;124:857–864. doi: 10.1242/jcs.075705. [DOI] [PubMed] [Google Scholar]
- 31.Bachmann-Gagescu R, et al. The ciliopathy gene cc2d2a controls zebrafish photoreceptor outer segment development through a role in Rab8-dependent vesicle trafficking. Hum Mol Genet. 2011;20:4041–4055. doi: 10.1093/hmg/ddr332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshimura S, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol. 2007;178:363–369. doi: 10.1083/jcb.200703047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kodani A, Salome Sirerol-Piquer M, Seol A, Garcia-Verdugo JM, Reiter JF. Kif3a interacts with Dynactin subunit p150 Glued to organize centriole subdistal appendages. EMBO J. 2013;32:597–607. doi: 10.1038/emboj.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong DH, et al. RPGR isoforms in photoreceptor connecting cilia and the transitional zone of motile cilia. Invest Ophthalmol Vis Sci. 2003;44:2413–2421. doi: 10.1167/iovs.02-1206. [DOI] [PubMed] [Google Scholar]
- 35.Gromley A, et al. A novel human protein of the maternal centriole is required for the final stages of cytokinesis and entry into S phase. J Cell Biol. 2003;161:535–545. doi: 10.1083/jcb.200301105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bobinnec Y, et al. Centriole disassembly in vivo and its effect on centrosome structure and function in vertebrate cells. J Cell Biol. 1998;143:1575–1589. doi: 10.1083/jcb.143.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takuwa N, Zhou W, Takuwa Y. Calcium, calmodulin and cell cycle progression. Cell Signalling. 1995;7:93–104. doi: 10.1016/0898-6568(94)00074-l. [DOI] [PubMed] [Google Scholar]
- 38.Rachel RA, et al. Combining Cep290 and Mkks ciliopathy alleles in mice rescues sensory defects and restores ciliogenesis. J Clin Invest. 2012;122:1233–1245. doi: 10.1172/JCI60981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ocbina PJ, Eggenschwiler JT, Moskowitz I, Anderson KV. Complex interactions between genes controlling trafficking in primary cilia. Nat Genet. 2011;43:547–553. doi: 10.1038/ng.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Owens KN, et al. Identification of genetic and chemical modulators of zebrafish mechanosensory hair cell death. PLoS Genet. 2008;4:e1000020. doi: 10.1371/journal.pgen.1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nonaka S, et al. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 42.Liem KF, Jr, et al. The IFT-A complex regulates Shh signaling through cilia structure and membrane protein trafficking. J Cell Biol. 2012;197:789–800. doi: 10.1083/jcb.201110049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ko HW, Liu A, Eggenschwiler JT. Analysis of hedgehog signaling in mouse intraflagellar transport mutants. Methods Cell Biol. 2009;93:347–369. doi: 10.1016/S0091-679X(08)93017-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaghloul NA, Katsanis N. Mechanistic insights into Bardet-Biedl syndrome, a model ciliopathy. J Clin Invest. 2009;119:428–437. doi: 10.1172/JCI37041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeller R, Lopez-Rios J, Zuniga A. Vertebrate limb bud development: moving towards integrative analysis of organogenesis. Nat Rev Genet. 2009;10:845–858. doi: 10.1038/nrg2681. [DOI] [PubMed] [Google Scholar]
- 46.Litingtung Y, Dahn RD, Li Y, Fallon JF, Chiang C. Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. Nature. 2002;418:979–983. doi: 10.1038/nature01033. [DOI] [PubMed] [Google Scholar]
- 47.Caspary T, Larkins CE, Anderson KV. The graded response to Sonic Hedgehog depends on cilia architecture. Dev Cell. 2007;12:767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol. 2010;26:59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baldari CT, Rosenbaum J. Intraflagellar transport: it's not just for cilia anymore. Curr Opin Cell Biol. 2010;22:75–80. doi: 10.1016/j.ceb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dammermann A, Merdes A. Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J Cell Biol. 2002;159:255–266. doi: 10.1083/jcb.200204023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang P, Giddings TH, Jr, Winey M, Stearns T. Epsilon-tubulin is required for centriole duplication and microtubule organization. Nat Cell Biol. 2003;5:71–76. doi: 10.1038/ncb900. [DOI] [PubMed] [Google Scholar]
- 52.Guarguaglini G, et al. The forkhead-associated domain protein Cep170 interacts with Polo-like kinase 1 and serves as a marker for mature centrioles. Mol Biol Cell. 2005;16:1095–1107. doi: 10.1091/mbc.E04-10-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Craige B, et al. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J Cell Biol. 2010;190:927–940. doi: 10.1083/jcb.201006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garcia-Gonzalo FR, Reiter JF. Scoring a backstage pass: Mechanisms of ciliogenesis and ciliary access. J Cell Biol. 2012;197:697–709. doi: 10.1083/jcb.201111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang B, et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Hum Mol Genet. 2006;15:1847–1857. doi: 10.1093/hmg/ddl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rachel RA, Li T, Swaroop A. Photoreceptor sensory cilia and ciliopathies: focus on CEP290, RPGR and their interacting proteins. Cilia. 2012;1:22. doi: 10.1186/2046-2530-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas KR, Capecchi MR. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987;51:503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- 58.Brooks MJ, Rajasimha HK, Roger JE, Swaroop A. Next-generation sequencing facilitates quantitative analysis of wild-type and Nrl(-/-) retinal transcriptomes. Mol Vis. 2011;17:3034–3054. [PMC free article] [PubMed] [Google Scholar]
- 59.Roger JE, et al. Preservation of cone photoreceptors after a rapid yet transient degeneration and remodeling in cone-only Nrl-/- mouse retina. J Neurosci. 2012;32:528–541. doi: 10.1523/JNEUROSCI.3591-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gavet O, Pines J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev Cell. 2010;18:533–543. doi: 10.1016/j.devcel.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonda MA, Aaronson SA, Ellmore N, Zeve VH, Nagashima K. Ultrastructural studies of surface features of human normal and tumor cells in tissue culture by scanning and transmission electron microscopy. J Natl Cancer Inst. 1976;56:245–263. doi: 10.1093/jnci/56.2.245. [DOI] [PubMed] [Google Scholar]
- 62.Chang J, et al. Essential role of Cenexin1, but not Odf2, in ciliogenesis. Cell Cycle. 2013;12:655–662. doi: 10.4161/cc.23585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Erickson PA, Lewis GP, Fisher SK. Postembedding immunocytochemical techniques for light and electron microscopy. Methods Cell Biol. 1993;37:283–310. doi: 10.1016/s0091-679x(08)60255-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


