Abstract
A murine passive transfer model system was employed to ascertain the effects of gestational exposure to a single, intravenous dose of purified, brain-reactive IgG antibodies from individual mothers of children with autism (MAU) or mothers with typically developing children (MTD). Growth and behavioral outcomes in offspring were measured from postnatal days 8 – 65 in each group. Comparisons revealed alterations in early growth trajectories, significantly impaired motor and sensory development, and increased anxiety. This report demonstrates for the first time the effects of a single, low dose gestational exposure of IgG derived from individual MAU on their offspring’s physical and social development.
Keywords: Autism, maternal antibodies, passive transfer, immune, mouse behavior
Introduction
Autism spectrum disorders (ASD) is comprised of a heterogeneous group of behaviorally defined neurodevelopmental disorders that manifest as impairments in three core domains: verbal and non-verbal communication, social reciprocity, and repetitive behaviors. Within ASD, diagnoses of autistic disorder (AU), Asperger syndrome (AS) and pervasive developmental disorder – not otherwise specified (PDD-NOS) are applied based on symptom presentation and severity. Current prevalence estimates of 1 per 88 live births in the United States (CDC, 2012) and a lack of identified causative factors in most cases have fostered much effort towards identifying risk factors for ASD. Although clinical diagnosis is typically made near three years of age, current evidence suggests that the underlying neurodevelopmental changes may occur much earlier, likely in utero. Of the numerous maternal factors that enter fetal circulation during gestation, an etiologic role for brain-reactive IgG antibodies in ASD has now received support in several studies (Braunschweig et al., 2008; Braunschweig et al., 2011; Dalton et al., 2003; Martin et al., 2008; Singer et al., 2008).
Prior to maturation of the mammalian immune system, adaptive immune protection is transferred from mother to fetus via IgG isotype antibodies. In pregnant mice, as well as in humans, specific placental Fc receptors actively transport maternal IgG into the fetal circulation (Kim et al., 2009). These maternal IgG antibodies are known to confer specific immunity on offspring and represent the primary adaptive immunity in the neonate. Antibodies are also transferred postnatally through lactation in both humans and mice (Van de Perre, 2003). Additional non-immune developmental functions of maternal IgG have also been suggested from experimental results in mouse models (Gustafsson et al., 1994), although the precise mechanisms of these remain unclear.
Several deleterious consequences of in utero maternal antibody transfer have been described for autoimmune diseases such as systemic lupus erythematosus (Lee et al., 2009) and arthrogryposis multiplex congenita syndrome arising from myasthenia gravis in the mother (Jacobson et al., 1998). Experimentally, models of passive IgG transfer of human lupus autoantibodies to pregnant mice have replicated significant aspects of the congenital heart block noted in human newborns, supporting a causal role for these antibodies in that disorder (Tran et al., 2002).
Maternal antibodies associated with ASD were first observed over 20 years ago (Warren et al., 1990) and several descriptive behavioral investigations of their effects on offspring development in experimental models have been reported (Martin et al., 2008; Singer et al., 2009). Due to the precise binding specificity of antibodies, as well as their broad tissue access during gestation, several groups have investigated the behavioral sequelae of gestational exposure to IgG antibodies associated with ASD. Whole serum from a mother of two children with ASDs was administered using intraperitoneal injection to pregnant mice, and behavioral changes were observed in the offspring, including altered exploration and motor coordination (Dalton et al., 2003). Singer and coworkers extended these findings with intraperitoneal injection of pooled IgG from 63 mothers of children with ASD to gestating mice (Singer et al., 2009). In their study they identified several changes in juvenile and adult offspring including altered sociability as well as increased immune activation compared with controls. Although a causal role for maternal IgG in ASD etiology has yet to be demonstrated, rodent behavioral models are providing valuable supporting evidence for their pathologic significance.
Previously, our group identified maternal IgG antibodies that specifically bind to human fetal brain proteins of approximately 37kDa and 73kDa exclusively in approximately 12% of mothers of children with autistic disorder (AU), a diagnostic group within ASD manifesting severe impairments in all three clinical domains (Braunschweig et al., 2008). Recently, this work was expanded to include 560 mothers of children with AU, ASD and controls, yielding results that were highly consistent with the original study (Braunschweig et al., 2011). Recently a high prevalence of folate receptor autoantibodies was found in children with ASD (Frye et al., 2012), expanding evidence of dysregulated antibody production in ASD. Although it was the individuals with ASD, and not their mothers who generated the autoantibodies described in that study, the results support the possibility that autoantibodies may play a role in autism risk and may point toward potentially novel clinical intervention.
In the present study, mice were exposed during gestation to purified maternal IgG samples from mothers who possess reactivity to both the 37kDa and 73kDa fetal brain antigens, or to purified IgG from mothers with no history of autism in their families, to model the effects of passive gestational transfer of autism-associated IgG. Developmental and behavioral phenotyping of mice exposed to autism-associated maternal IgG antibodies during gestation identified developmental impairments relevant to ASD.
Methods
Animals and animal care
The UC Davis Institutional Animal Care and Use Committee (IACUC) approved all experiments with mice. Adult female C57Bl/6J mice were purchased from Jackson laboratories and housed in groups of three with adult male breeders of the same strain. All animals were housed and mated at the Mouse Behavioral Assessment Laboratory at the University of California, Davis. Efforts were undertaken to minimize the number of animals used in each experiment. Effects of offspring gender and litter were analyzed independently and grouped where appropriate.
During mating female breeders were examined daily for vaginal plugs. When plugs were detected, females were removed to individual plastic tub type cages with bedding and randomly assigned to injection groups. IgG injections (described below) were performed on gestational day (GD) 12 (plug detection=GD0). On GD 16 nesting materials were provided and pregnant mice were left undisturbed, except for observation for new litters until postnatal day (PND) 8. Pregnant dams were randomly assigned to one of the following treatment groups (Table 1A): injection of IgG isolated from mothers of children with autistic disorder (MAU); injection of IgG isolated from mothers of children with typical development (MTD); or injected with saline vehicle (SAL) as control. At least 5 dams were injected with each of the IgG samples or vehicle control (Table 1B).
Table 1a.
Treatment Groups
| Treatment and Testing Schedule | GD or PND |
|---|---|
|
| |
| Tail vein IgG or Saline injection | GD12 |
| Physical Development | PND 8–40 |
| Neurobehavioral development | PND 8–18 |
| Weanling Test Battery | PND 18–21 |
| Ultrasonic Vocalization | PND 8, 12, 16 |
| Weaning | PND21 |
| Track 1 (1M, 1F from each litter) | Track 2 (1M, 1F from each litter) |
|---|---|
|
| |
| Sociability/social novelty (PND 24) | Social conditioned place preference (PND 31) |
| Social dyadic interaction (PND 26) Spontaneous motor activity (PND 29) |
Olfactory ability (PND 35–45) |
Table 1b.
Treatment Groups
MAU – Mother of a child with autism (plasma derived IgG)
MTD – Mother of a child with typical development (plasma derived IgG)
SAL – Saline control injection
Selection of serum samples and purification of human IgG
IgG was purified from blood plasma from three MAU (MAU 1–3), as well as three MTD (MTD1–3). MAU and MTD subjects were matched for maternal age, child age and sex (Table 2). AU diagnosis or typical development was determined by expert examination at the UC Davis MIND Institute (Hertz-Picciotto et al., 2006). All children were assessed for cognitive function with the Mullen’s Scales of Early Learning (MSEL) (Mullen, 1995), and adaptive function using the Vineland Adaptive Behavior Scales (VABS)(Sparrow, 1984). Both measures yielded scores in the typical range for the TD children, and scores indicating significant impairment for the AU children. The MAU samples were from a group of mothers identified in a previous report (Braunschweig et al. 2008) who possess IgG antibody reactivity recognizing the 37 kDa and 73 kDa fetal brain proteins, and have at least one child with a confirmed AU diagnosis. No fetal brain IgG reactivity was observed for any of the MTD samples. No history of clinical autoimmunity or immune dysfunction was noted for any of the mothers enrolled in the study. All subjects provided informed consent, and the Institutional Review Board at UC Davis approved the study.
Table 2.
Demographics
| Sample | Maternal Age | Child Age | Parity | MSEL | VABS |
|---|---|---|---|---|---|
|
| |||||
| MTD1 | 25.8 | 3 | 1 | 110 | 110 |
| MTD2 | 27.8 | 4.5 | 2 | 109 | 108 |
| MTD3 | 34.4 | 3.2 | 2 | 100 | 90 |
|
| |||||
| Mean (SD) | 29.3 (4.5) | 3.6 (0.8) | 1.7 (0.6) | 106.3 (5.5) | 102.7 (11) |
|
| |||||
| MAU1 | 24.8 | 4.6 | 1 | 49 | 45 |
| MAU2 | 21.1 | 3.4 | 1 | 49 | 56 |
| MAU3 | 37.2 | 2.8 | 1 | 47 | 32 |
|
| |||||
| Mean (SD) | 27.7 (8.4) | 3.6 (0.9) | 1 (0) | 48.3 (1.2) | 44.3 (12) |
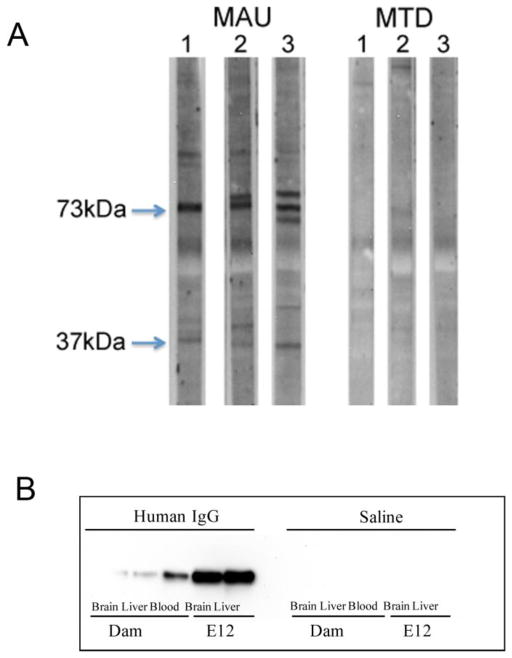
One yellow-top acid-citrate dextrose tube of blood was collected from each subject, yielding 5 ml of plasma that was used for IgG purification. Plasma samples were diluted (1:1) with binding buffer (Pierce, Rockford, IL) and centrifuged at 10,000 x g. Samples were filtered through 0.22μm syringe filter onto a chromatography column containing 1ml protein A/G plus agarose beads (Pierce). Columns were then washed 5 times with binding buffer. IgG was eluted with an acid elution buffer (Pierce) and immediately neutralized with 1M phosphate. Purified IgG was then desalted and adjusted to a concentration of 5mg/ml in sterile phosphate buffered saline (PBS). Reactivity of purified MAU IgG towards mouse fetal brain proteins at approximately 37 and 73 kDa was confirmed by western blot, as well as the absence of similar reactivity among MTD samples (Figure 1A). Purified IgG was stored at −20°C until use. Absence of bacterial contamination was confirmed using the Limulus Amebocyte Lysate Pyrotell test (Associates of Cape Cod, East Falmouth, MA).
Figure 1.
A) Western blot demonstrating MAU and MTD reactivity towards E12 fetal brain protein from C57Bl6/J mice. B) Human IgG in sterile saline, or saline alone, was administered IV to E12 gestating dams at a dose of 1.5μg per gram body weight. 4 hours later, tissues were harvested and 15μg protein from each tissue was assayed for the presence of human IgG by Western blot.
Western Blot
Protein samples were prepared by homogenizing and sonicating fetal brain tissue from GD12 C57Bl/6J mice in a 10-fold greater volume of 20 mM HEPES-OH, pH 7.5 buffer supplemented with (in mM) 1 EDTA, 5 DTT, 1 PMSF, 0.2 Na2VO3, 1 NaF and one tablet Complete protease inhibitor cocktail (Roche, Indianapolis, IN; per 50 ml buffer). The protein sample concentration was then adjusted to 3.5 mg/ml, as determined by BCA assay (Pierce). Western blots were then performed as described previously (Braunschweig et al., 2011), using 1:400 diluted purified IgG (from above) to verify maternal antibody reactivity to mouse fetal brain protein (Figure 1A). Additional western blots were performed to assess tissue distribution of maternal IgG 4 hours after injection and are described below.
IgG injection
Purified human IgG (1.5 μg/g body weight) from an individual MAU or MTD mother, or SAL control was injected by tail vein on GD 12 in all dams whose offspring were used for behavioral characterization.
IgG tissue distribution
In order to evaluate the distribution of human maternal IgG in the mouse fetal brain and liver as well as the dam’s blood, brain and liver, tissue was harvested four hours after tail vein injection from one dam who received human IgG pooled from the MAU and MTD sources and one dam who received SAL (Figure 1B). Tissues were weighed and purified protein was prepared for each sample as described elsewhere (Braunschweig et al., 2011). This protein was subjected to western blotting and visualized using a goat anti-human secondary antibody that was pre-adsorbed against mouse IgG (Invitrogen, Carlsbad, CA) in order to determine the presence of human IgG in each tissue protein sample.
Study design
The study design included three treatment groups: the autism group consisting of three subgroups (MAU1–3), each receiving IgG from a different mother of a child with AU and possessing fetal-brain reactive IgG; a typically developing antibody control group consisting of three subgroups, each receiving IgG from a different mother of a typically developing child (MTD1–3); and a SAL control group (Table 1B). The SAL and MTD antibody control groups were compared to determine whether nonspecific human IgG antibody injections affected the endpoints under study. The MAU group was compared to both controls to determine the specific effects of maternal IgG administration during mouse gestation. Hypotheses based on the differential effects of maternal antibody sources, which may be relevant to autism symptoms, were tested by comparing the three MAU subgroups when an MAU-MTD treatment group difference was found for a given endpoint.
All pups in each litter were tested on a neurobehavioral development battery as well as separation-induced ultrasonic vocalizations prior to weaning (PND21) (Table 1A). To control for litter effects and provide for litter-based statistical analysis, dam was included as a random variable in the analyses on all preweaning tests. At weaning, 1 male and 1 female from each litter were assigned to Track 1 tests (sociability/social novelty, social dyadic interaction, spontaneous motor activity) or to Track 2 tests (social conditioned place preference, olfactory ability). This process also provided a litter-based statistical analysis. Each individual mouse received a maximum of three post-weaning tests in Track 1 or Track 2.
Survival and growth
Dams were weighed at the time of injection and left undisturbed until 8 days after birth of their litters when behavioral evaluations began. Pups were weighed daily from PND 8 to PND 21 at the time of testing. Additional assessments of growth conducted on PND 14, 18, 21, 24, 28 and 40 were body length (crown-rump length) and biparietal diameter (head width measured at top base of the pinna with a micrometer). Death of dams or pups was recorded in the study log along with any potentially related observations.
Developmental behavioral phenotyping
Behavioral tests
Each test was conducted at the same time of day within a 3.5 h range for all mice during the light cycle. Postnatal testing day (PND) for each measure is described below. The test battery emphasized testing of social behavior in juvenile (prepubertal) offspring. All surfaces were cleaned with disinfectant (Nolvasan, Fort Dodge Animal Health, Fort Dodge, IA) between testing sessions. Litters tested on a given day underwent testing with order balanced for treatment group. Testing was conducted blind for treatment group.
Developmental test battery (PND 8–18)
The condition of the nest was rated before the pups were removed from the cage for behavioral testing. Pups were then randomly removed from their individual cages and placed in individual holding cups on heating pad for testing. Testing was carried out for individual pups on a Plexiglas board maintained at 36° C. Maturation of reflexes and simple behaviors were evaluated as described previously (Golub et al., 2004; Ta et al., 2011). These include: righting, cliff aversion, forelimb and hindlimb grasping, vibrissa placing, ear opening, ear twitch, screen grasp, pull and climb, dowel foot placement, eye opening, palpebral reflex, visual placement, auditory startle and popcorn (jumping) behavior. Endpoints were scored from 0–3 with 3 being a fully mature response. Composite scores were obtained by adding daily scores on tests that reflected motor (fore and hind limb grasp, fore and hind limb dowel placement, fore and hind limb wide stick placement, wire cling, wire climb, wire pull resistance) or sensory (vibrissa placing, eye opening, visual placing, auditory startle) development. Such scores have been shown to correlate with brain morphometric measures of development (Wahlsten, 1974).
Weanling test battery (PND 19–21)
Litters were also observed daily from PND 19–21 for a 3-min period on the Plexiglas testing board (without heat support) for attainment of age-appropriate postures and movement (Golub et al., 2004). A grid (6.6 cm squares) was placed under the testing board for locomotor activity scoring. In each session, the number of rearing, pivoting, sitting, and grooming movements as well as grid lines crossed was recorded. Gait characterization categories and abnormalities were also tallied. Additionally, measures of hind limb grasp and visual placement, which mature late in the pre-weaning period, were continued from the developmental test battery.
Ultrasonic vocalizations (PND 8, 12, 16)
This test of separation-induced vocalizations has been described previously (Jaubert et al., 2007; Ta et al., 2011). Mouse pups were removed from the nest and placed within 15 sec in a 7.5 cm diameter holding cup in a sound attenuated chamber. Over the next 2 min, ultrasound vocalizations were recorded using a duration cutoff of 10 msec and a frequency range of 30 to 50 kHz with commercial software (Ultravox, Noldus, Wagingen, The Netherlands). An amplitude filter was used to eliminate extraneous peripheral noise. All pups in the litter were tested successively in a random order. This test was conducted prior to the Developmental Test Battery on postnatal days when both tests were scheduled.
Sociability/social novelty preference (Track 1, PND 24)
Sociability and preference for social novelty were tested using previously developed techniques (Moy et al., 2004). Mice were habituated for 10 min to a 58 × 40 × 22 cm chamber divided into 3 compartments (left/center/right) accessible through a 2 cm wide doorway. The next day, two 10-min sessions were conducted. In the first session, a small wire cage was placed in one side chamber and an identical cage containing a mouse in the opposite side chamber. For the second session, the now-familiar mouse was placed in the cage on one side and a “stranger” mouse was placed in the cage on the other side. Groups were balanced for side to equalize side preference, and side was recorded and used as a covariate for statistical analysis and determined to have no effect. Movement of the test mouse was tracked using TopScan (Cleversys, Reston, VA) software, which recorded the amount of time spent in each of the three compartments, the amount of time spent in the area adjacent to the cages (within 7.5 cm), and the amount of time spent sniffing the cages (body oriented with head toward the cage). The two object mice (novel and familiar) were selected from a group of experienced adult males maintained for this purpose.
Social dyadic interaction (Track 1, PND 26)
Mice were videotaped during a 10 min interaction with an unfamiliar mouse of the same sex and age (PND 24–26) in a 10 × 10 × 10 cm Plexiglas chamber under red light. Mice were first marked on the tail with dry mark pens for identification during video scoring. The session was conducted after a 5 min adaptation of both mice in identical chambers located on the two sides of the test chamber. Chambers were thoroughly cleaned with Nolvasan between mice. Social interaction was scored using Observer 4.1 software (Noldus Information Tech., Tacoma, WA) with an ethogram previously developed for juvenile mice (Jaubert et al., 2007).
Spontaneous motor activity (Track 1, PND 29)
Activity was recorded for 60 min in a 27.5 × 27.5 × 37.5 cm Plexiglas chamber (Truscan Coulbourn Instruments, Allentown, PA) using infrared photo beam interruption. The software provided measures of horizontal (floor plane) and vertical activity (i.e., rearing) as well as time in various areas of the arena. (i.e., margin time). Specific measures included total number of floor plane moves, duration of each floor plane move, floor plane move distance and time spent around the margins of the arena.
Social conditioned place preference (Track 2, PND 31)
This test of social reward is based on the conditioned place preference paradigm using cues associated with social interaction or solitary activity (Panksepp and Lahvis, 2007). To establish the cue association, mice were placed in cages with different bedding (paper pellets or wood shavings) and different enrichment objects (tubing with or without internal threads) on alternate days (all day) from PND 21 to PND 30. One of the caging environments (randomly assigned) was also a social environment and housed the mouse’s littermates. On PND 31, the mouse individually was placed in the center compartment of a 3-compartment chamber for 30 min and given the opportunity to access both the caging environment (bedding and enrichment object) associated with littermate housing, or that caging environment that was not associated with littermate housing.
Olfactory ability: buried food (Track 2, PND 35–45)
For this test of olfactory competence (Nathan et al., 2004), mice were placed in a cage with a 1-g piece of their standard diet (bait) hidden 0.5 cm below the surface of the bedding and allowed 5 min to find the food. One trial per day was conducted on 5 successive days. Mice were food deprived prior to testing (0.2 g food/day in addition to the 1 g obtained during testing). If the bait was not found, it was fed to the mouse in its home cage. Latency to retrieve the bait was recorded. Olfaction is a major mediator of social interaction in mice (Arakawa et al., 2008).
Stereotypical Behavior: home cage observation (Track 2, PND 60–65)
Adult mice were videotaped during the first two hours of the dark cycle from 7–9 pm. Videos were scored for bar mouthing, jumping, looping, route tracing, twirling and wiping. The amount of time spent performing a stereotypical behavior was divided by total observation time to yield a “time budget” for the particular behavior.
Brain histology and stereology
Once behavioral testing was complete mice were sacrificed (100mg/kg body weight I.P. Euthasol injection) for stereological examination. Mice were then perfused with 20 ml of 0.1M phosphate buffer (PB) (pH 7.4) followed by a 20 min gravitational drip of 4% paraformaldehyde (PFA) in 0.1M sodium phosphate buffer. After perfusion brains were removed and post-fixed for 1 hour in 4% PFA, followed by cryoprotection in a solution of 10% sucrose in 0.1M PB for 1 hour and then 30% sucrose in 0.1M PB for 24 hours. After cryoprotection brains were flash frozen in dry ice and stored at −80° C until sectioning.
Brains were blocked and sectioned at 50 μm on a sliding microtome (AO model 860). Slices were preserved in 0.1% sodium azide in 0.1M PB until mounting. Every 5th section was mounted starting approximately at Bregma −1.46 and ending at approximately Bregma −2.92 and cresyl violet stained. A total of 7 mounted sections for each subject were used for unbiased stereological analysis of total neuron number in the CA1 subregion of one hemisphere of the hippocampus using the optical fractionator probe (StereoInvestigator, Microbrightfield, Williston, VT). In all cases the Gundersen coefficient of error (CE) was less than 0.06 with m=1 ensuring precision of the cell sampling design.
Statistical analysis
All data analyses were conducted using SAS version 9.2 (SAS Institute, Cary, NC, USA). For repeated measures (i.e. the measures taken successively on the same animal), linear mixed effects models were used to analyze the data with each pup as the analysis unit. Treatment, sex and time, as well as significant interactions (e.g. treatment*age, treatment*sex) were treated as fixed effects, and dam was treated as a random effect to control for litter effects as appropriate (Holson et al., 2008). Repeated measurements over time for each pup were analyzed using the compound symmetry covariance structure or the autoregressive-1 (AR(1)) covariance structure, whichever was appropriate. The Kenward-Rogers method was used to calculate adjusted degrees of freedom, which is recommended when analyzing repeated measures data (Littell et al., 2006). Significant treatment*age or treatment*sex interactions were further analyzed using simple-effect slice ANOVAs for each age or sex. For cross-sectional measures, a linear mixed effects model was used to analyze the data, with each pup as the analysis unit, treatment and sex (and treatment*sex if significant) as fixed effects, and dam as a random effect to control for litter effects when appropriate.
Analysis was done either using both females and males together, or using female and male mice separately as appropriate for the test being analyzed (see Methods for individual tests). Similar analyses were also repeated using antibody source as an independent variable to investigate whether the antibody source was significantly associated with outcome variables within the MAU group. These tests were conducted if results showed significant differences between the MAU and the MTD antibody control groups on the outcome variables.
Comparisons between treatment groups and antibody sources were planned analyses, and no corrections for multiple testing were needed. Planned comparisons subsequent to significant treatment effects compared the MTD antibody control group to the SAL control group in order to identify the appropriate control for the MAU treatment. These comparisons (MTD vs. SAL) demonstrated overall differences between the two control groups on many behavioral and non-behavioral endpoints. Consequently the MTD antibody control group was selected as the more appropriate control for examining the effects of the MAU treatment. Thus, in addition to planned comparison between MTD and SAL groups, a planned comparison between MTD and MAU groups was conducted subsequent to a significant treatment effect. Planned comparisons of antibody source (subgroups within treatment) were carried out only subsequent to significant MTD vs. MAU findings. The antibody sources within the MAU group (MAU1, MAU2 and MAU3) were evaluated with planned pairwise tests. This stratified approach to statistical analysis was used to avoid inflating false positive results by conducting all possible comparisons of treatment groups and antibody source groups.
Results
Detection of injected antibody in maternal and fetal tissues
A test of distribution of human IgG after tail vein injection in GD12 mice was carried out to confirm that the route of administration would be effective in delivering human IgG to fetal tissue. As shown in Figure 1B, human IgG was detected at the highest relative concentration in the E12 fetal brain and liver. Human IgG was also detected in blood from the dam, and was observed albeit at much lower levels in protein derived from dam brain and liver. No detectable reactivity to human IgG was observed in tissues of the SAL group (Figure 1B).
Human maternal IgG administration was positively associated with growth parameters versus SAL, but was attenuated in MAU versus MTD
There were no statistically significant differences between treatment groups in dam and pup survival. Of 61 dams successfully injected, all completed pregnancy. Five dams died prior to completing lactation, three in the MAU2 injection group, one in the MAU3 group and one in the MTD1 group. It is interesting to note that 4 of the 5 dams that died during lactation received MAU IgG, although this finding did not reach statistical significance due to the overall high survival rate. Pup survival between birth and weaning was 78% in the SAL control group, 88% in the MTD antibody injected control group and 89% in the MAU antibody injected group (excluding pups in litters where the dam died).
Analysis of postnatal/pre-weaning pup body weights showed a significant effect of treatment on postnatal pup body weights by RMANOVA (F(2,49)=4.89, p=0.011), with the body weights of the MAU and MTD antibody groups being greater than those of the SAL control group, although only the difference between the MTD group and the SAL control group reached significance (p=0.008)(Figure 2A). However, there was a significant treatment*age interaction (F(20,2867)=4.13, p<0.0001), and post hoc comparisons showed that the MAU group weighed significantly less than the MTD antibody control group on PND 12 (p=0.048).
Figure 2.

Growth curves showing MAU vs. MTD vs. SAL: weight and biparietal diameter. A) Body weight of offspring of MAU IgG treated dams indicated a generally slower growth trajectory than MTD IgG offspring, reaching significance at PND12 (*p=0.048). While both MAU and MTD offspring were somewhat heavier than SAL across days, this difference only reached statistical significance for the MTD offspring (p=0.008). B) The rapid inflection of biparietal diameter increase typically observed around PND24 was significantly delayed among offspring of MAU IgG treated dams when compared to offspring of MTD treated dams (p=0.016).
After weaning, pup weights at PND 21, PND 24, PND 28 and PND 40 continued to show a similar pattern (data not shown). There was a significant treatment effect (F(2,76)=4.22, p=0.018, and a significant treatment*age interaction (F(6,316)=3.29, p=0.0037). In post hoc tests, the MAU group weighed less than the MTD antibody control on PND 21 (p=0.026) and PND 40 (p=0.025). Additionally, offspring from the MTD antibody groups were heavier than the SAL control groups at all ages (p=0.005). No pairwise comparisons of individual antibody sources within the MAU group were significant at the ages when the MAU and MTD groups differed.
Growth was also evaluated with measurements of body length (crown-rump) and head width (biparietal diameter) at intervals prior to puberty. For body length, RMANOVA showed a significant treatment*age interaction (F(10,1185)=2.45, p=0.007). Treatment effects were seen for body length on PNDs 24 (p=0.01) and 40 (p=0.04). Post hoc tests showed that the MAU group body length was shorter than the MTD antibody control group on PND 24 (p=0.006) and PND 40 (p=0.041). The MTD antibody control group body length was longer than the SAL control group at these two ages (PND 24, p=0.03, PND 40, p=0.02).
Biparietal diameter showed a treatment *age interaction (F(10,1185)=2.52, p=0.005) in RMANOVA (Figure 2B). The treatment effect was significant on PND 28 (F(2,122)=3.39, p=0.037) with the MAU biparietal diameter smaller than the MTD offspring on PND 28 (p=0.016). There were no differences between the two control groups (SAL, MTD) on PND 28.
Comparison of the three antibody sources (MAU1, MAU2 and MAU3) at ages when MAU and MTD groups differed in body length and biparietal diameter showed that the MAU2 group had shorter body length than the MAU1 across all time points (p=0.02) and shorter body length than MAU3 on PND 14 (p=0.05), and PND 28 (p=0.02). However, on PND 28 the biparietal diameter of the MAU3 subgroup was smaller than that of the MAU1 (p=0.002) or MAU2 (p=0.02) groups.
Neurobehavioral Indices
Motor and sensory development was examined from PND 8–18. A composite motor development score was calculated for each of the 0–3 scores of individual motor tests across testing days (i.e., forelimb and hindlimb grasping, forelimb and hind limb dowel placement, forelimb and hindlimb wide stick placement, wire cling, wire climb, and wire pull resistance). Likewise, sensory development was quantified as a composite developmental score across testing days from each of the 0–3 scores of sensory function (i.e., vibrissa placing, eye opening, visual placing and auditory startle).
Pups exposed in utero to MAU IgG showed slower motor and sensory development during pre-weaning periods when these abilities typically develop most rapidly. There was a significant effect of age on both motor and sensory scores as anticipated, but no significant effect of sex.
There were no overall treatment effects for the RMANOVA analysis of motor development scores, but a significant treatment*age interaction was seen (F(20,2854)=4.32, p<0.0001). Treatment effects were significant on PND 12 (p=0.018) and 13 (p=0.036). Individual group comparisons showed that on PND 12 the MAU group had lower motor developmental scores than the MTD antibody control group (p=0.0006) (Figure 3A).
Figure 3.
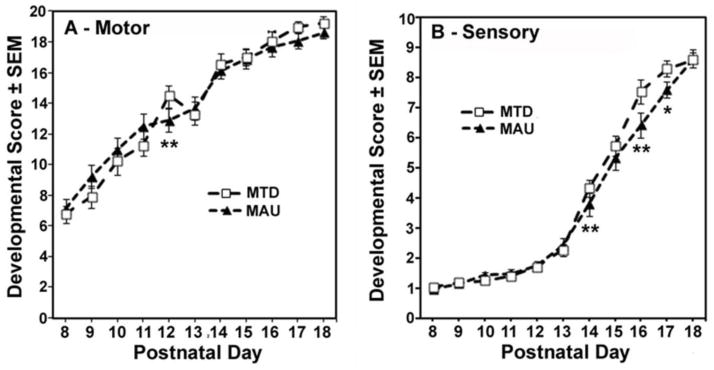
Offspring of MAU injected dams exhibit significantly delayed motor and sensory development. A) Motor development was lower in MAU treated animals at PND 12 (p=0.0006). B) Sensory development was delayed in the MAU treatment group versus the MTD antibody control group on PND 14 (p=0.005), and PND 16 (p<0.0001) and PND 17 (p=0.02).
Sensory scores also demonstrated a significant treatment*age interaction (F(20,28)=7.52, p<0.0001) with no overall treatment effect. For sensory scores, treatment effects were significant on PND 9 (p=0.009), PND 14 (p<0.0001) PND 16 (p<0.001) and PND 17 (p=0.035). The MAU group had lower scores than the MTD antibody control group on PND 14 (p=0.005), and PND 16 (p<0.0001) and PND 17 (p=0.02) (Figure 3B).
These results indicate that MAU antibody administration delayed motor development of offspring on PND 12 and sensory development between PNDs 14–17, periods of the most rapid development of these functions. Examination of the individual tests comprising the motor and sensory scores suggested that delayed maturation was reflected in multiple test items on these days.
Comparison of the motor and sensory scores of MAU antibody source subgroups at the ages when MAU and MTD groups differed showed that the MAU1 subgroup was least affected. For motor scores the order of mean scores was MAU2<MAU3<MAU1 on PND 12 and 13. For sensory scores, the order of mean scores was MAU2<MAU3<MAU1 on PND 14 and MAU3<MAU2<MAU1 on PND 17. Thus, MAU1 IgG produced the least severe motor and sensory alterations and characterization of the antigenic epitopes may reveal the cause of greater delay in the MAU2 and MAU3 subgroups.
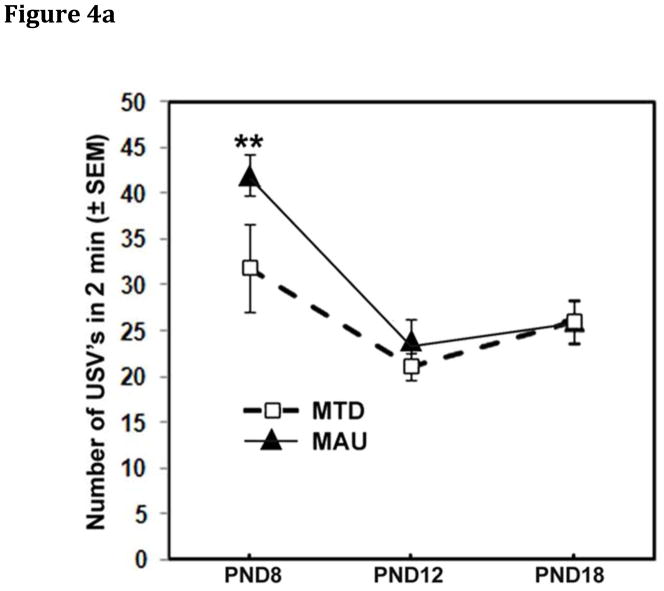
Ultrasound vocalizations: Pups exposed in-utero to MAU antibody show anxiety at PND 8
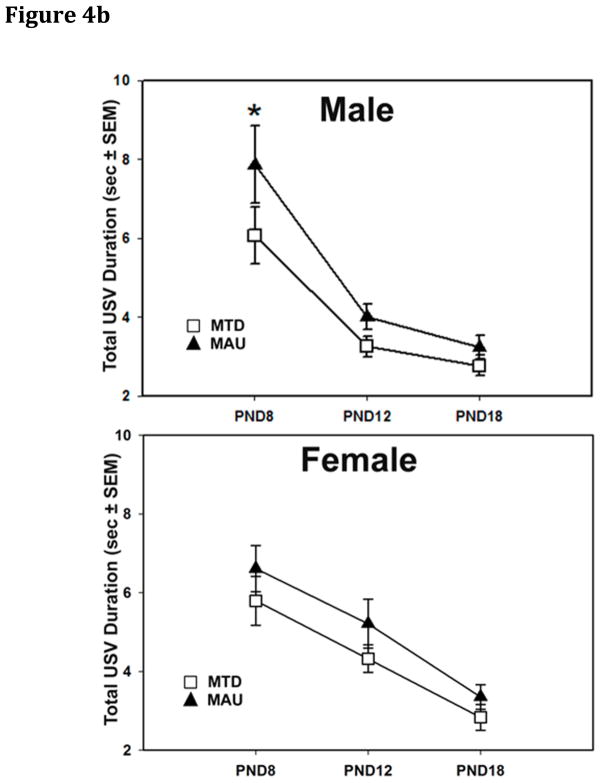
Three endpoints for ultrasonic vocalizations (USV) were analyzed: the number of vocalizations, the mean duration of individual vocalizations and the total duration of vocalizing during the 2 min recording period. All three endpoints showed a significant effect of age (p<0.0001), declining across the three ages, but no significant effect of sex. No effects of treatment were seen in RMANOVA over all three ages (PND 8, 12, 16). However, a significant age*treatment interaction was seen for the number of USVs over the 2 min test session (F(4,521)=2.44, p=0.046). Examination of treatment effects on individual days yielded a significant difference in the number of vocalizations on PND 8 only (p=0.009). The MAU group had a higher number of vocalizations (p=0.002) on PND 8 compared to the MTD antibody control group (Figure 4A). Mean total duration of vocalization demonstrated a significant sex*age*treatment interaction (F(8, 620)=2.00, p=0.045) with MAU males displaying a longer total duration of vocalization compared to MTD males on PND 8 (p=0.002)(Figure 4B), but not on PNDs 12 or 18. There were no significant differences seen for female mice in this test. Comparison of MAU antibody sources on PND 8 demonstrated that MAU2 had a higher frequency of vocalizations than MAU1 (p=0.0001) or MAU3 (p=0.004).
Figure 4.
Ultrasonic Vocalizations (USV) among offspring of IgG treated dams. A) The total number of vocalizations was significantly increased in MAU pups on PND8 (p=0.002) compared to MTD pups, but returned to normal levels by PND 12. B) A significant sex*age*treatment interaction in total USV duration was observed, reflecting a longer total duration of USVs in MAU males, but not females, compared with MTD offspring on PND 8 (p=0.002).
Sociability and social novelty
Sociability was examined in a three-chamber testing apparatus, where time spent with an unfamiliar mouse located in one of the side chambers versus time in the empty chamber was measured. Preference for social novelty was tested by placing a second “new” mouse in the opposite chamber and measuring time spent with the now familiar versus new mouse. There were no significant effects of IgG treatment on sociability (mouse versus empty chamber) or preference for social novelty (familiar mouse versus novel mouse) (data not shown).
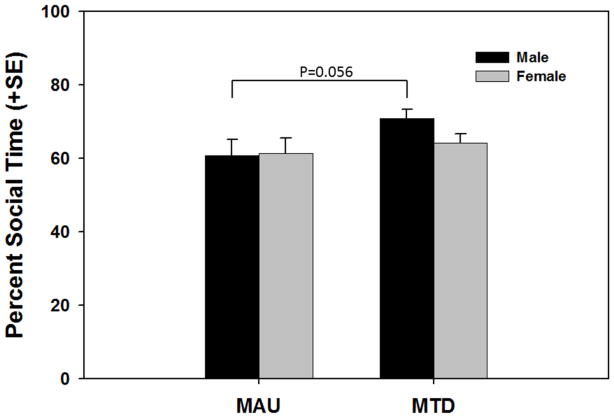
Social dyadic interaction: Juvenile mice exposed to MAU antibody show marginally shorter social interactions
In the social dyadic interaction test the percent time spend in one of the following behavioral states over a 10 min session was measured: social interaction, social withdrawal, inactivity, self-groom, freezing, stereotypy. A treatment effect was detected for the duration of social interaction (F(2,69)=6.17, p=0.003). The difference between the MAU group and the MTD antibody group approached but did not reach statistical significance (p=0.064). To further explore this, MAU and MTD groups were compared within sex (Figure 5). The treatment effect was significant in males (F(2,37)=5.04, p=0.012), and post hoc tests showed that male MAU mice had marginally lower social interaction durations than the MTD antibody group (p=0.056). The MTD antibody control group also showed more social interactions than the SAL control group (p=0.0008) and, specifically, male MTD antibody offspring had higher duration of social activity than male SAL controls (p=0.004) (data not shown).
Figure 5.
Duration of social interaction as a percentage of the 10-minute testing period. Male offspring of MAU injected dams displayed a marginally lower percentage of the testing period engaged in social interaction.
Social conditioned place preference: There were no significant treatment effects in this test of juvenile mice
Across all groups, there were more entries into the social side (side with cues associated with social interaction) (RMANOVA F(1,63)=8.92, p=0.004), longer duration of individual visits in the social side (F(1,63)=11.50, p=0.001) and a larger percent of total time spent in the social side (50% versus 34%, F=42.04, p<0.001) as compared to the isolation side. No treatment effects were detected for these variables.
Spontaneous motor activity: Male juvenile mice exposed in-utero to MAU IgG exhibit fewer bouts of movement and more anxiety
No treatment effects were noted on distance traveled, total time spent moving or vertical rearing. There were sex*treatment interactions for two variables, floor plane moves, (F(2,79)=6.63, p=0.002), a variable reflecting horizontal movement, and margin time score, defined as margin time minus center time (F(2,79)=3.87, p=0.025). Margin time score was used as a measure of thigmotaxis, an index of anxiety in mice (Simon et al., 1994). Treatment effects were seen in males only (floor plane moves, p=0.002; margin time score p=0.018). For floor plane moves, the MAU group had fewer moves than the MTD control (p=0.0005) (Figure 6A). For margin time score, the MAU group spent more time in the margin than the MTD antibody control (p=0.041) (Figure 6B). Comparison of MAU sources for floor plane moves revealed MAU1>MAU3 (p= 0.044) and MAU1>MAU2 (p=0.003). For margin time score, the antibody source effect was not significant and no post hoc comparisons were made. The lack of significant correlations between margin time score and ambulatory distance (r=−0.02) and move time (r=−0.06), the two major indices of movement, confirmed that the increased time in the margin was not secondary to changes in activity.
Figure 6.
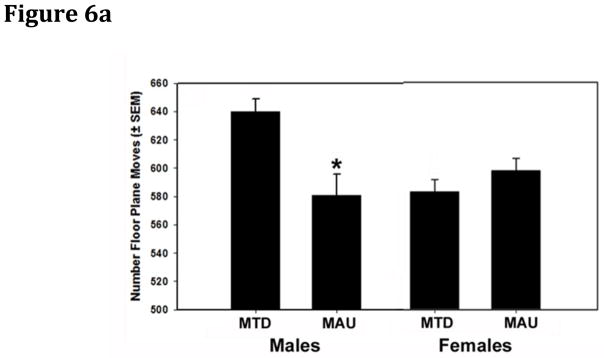
Floor plane moves and margin time score among offspring of treated dams. A) Total numbers of floor plane moves were lower among male (p=0.002), but not female offspring of MAU IgG treated dams. B) Margin time score was increased among male (p=0.011), but not female offspring of MAU IgG treated dams compared to MTD controls.
Olfactory ability: No significant treatment effects were found in pubertal mice
There were no treatment effects, treatment*sex interactions, or treatment*test day interactions for time required to retrieve the buried food item. Retrieval times decreased over the five days of testing (F(4,58)=211.52, p<0.0001), averaging 20 sec on the last day, with no significant interaction between treatment*test day or treatment*sex.
Home cage stereotypy
Home-cage stereotypical behaviors were coded from videotapes using The Noldus Observer software. Bar mouthing was the only stereotypic behavior observed. There were no treatment effects or treatment*sex interactions for this measure of stereotypy.
Stereologic Brain Measurements
There were no significant differences in the total number of neurons in the CA1 subregion of the hippocampus between any of the treatment groups. The mean number of neurons, as determined by weighted section thickness for each treatment group is shown in Table 3.
Table 3.
Stereology
| IgG source | Mean Number of CA1 Pyramidal Neurons +/− SEM | # Animals |
|---|---|---|
|
| ||
| MAU1 | 77506 +/− 4866 | 4 |
| MAU2 | 66874 +/− 3145 | 6 |
| MAU3 | 62534 +/− 5736 | 6 |
| MTD (combined) | 70541 +/− 2279 | 11 |
| Saline | 74375 +/− 3802 | 5 |
Discussion
Building upon previous reports of behavioral changes in offspring following gestational exposure to autism-associated maternal IgG, this study describes for the first time, the developmental and behavioral effects on offspring following a single-dose IV administration of such IgG in a pregnant mouse model. The goal of this administration paradigm was to create an ecologically valid model of gestational transfer of human IgG in a murine system while minimizing the effects from foreign IgG exposure, verify that the IgG could be detected in the fetal brain, and independently assess behavioral and developmental endpoints among several autism and control antibody sources. GD 12 was chosen as the injection date based on the timing of IgG transfer across the inverted yolk-sac placenta of the mouse (Morphis and Gitlin, 1970), as well as earlier studies showing that activation of the mouse maternal immune system at this gestational day by poly I:C injections results in behavioral changes in their offspring (Shi et al., 2009). Our exposure protocol clearly produces passive transfer of human IgG across the placenta to the developing fetal brain. Furthermore, since the neonatal Fc receptor (FcRn) is known to mediate transplacental IgG passage as well as delay IgG catabolism, the half-life of this administered IgG should be expected to be approximately 6–8 days in this model (Roopenian and Akilesh, 2007). The consequent window of fetal exposure to administered human IgG, from mid-gestation through parturition, supports the potential of a direct interaction between brain-specific IgG and its target molecules. This type of autoantibody-target antigen interaction forms the basis for numerous autoimmune disorders, and may underlie some cases of autism.
Our findings of delayed physical and sensory-motor development along with an increased response to stressors, as indicated both by the USV results as well as increased anxiety in the open field (i.e., increased margin time), among pups of MAU treated dams are consistent with reports from previous animal studies using intra-peritoneal, mid-gestation serum and IgG administration and support a role for maternal IgG in some cases of ASD. Indeed, motor delays are observed in at least 50% of individuals with ASD (Ming et al., 2007) and may precede characteristic behavioral features of the disorder. Furthermore, symptoms of anxiety co-occur with core features of ASD (Weisbrot et al., 2005), and have been hypothesized to represent coping mechanisms in individuals with ASD (Wink et al., 2010). The anxiety noted by increased time spent in the margin of the open field test by MAU mice was confirmed to be unrelated to amount of locomotor activity or time spent moving, supporting this measure as an indicator of anxious behavior. Additionally, increased numbers of vocalizations in the USV test among MAU animals indicates a heightened response to the stress of being removed from the home environment.
Taken together, these outcomes, along with the nearly significant effects on social interaction in the males, using only a single dose of autism-associated maternal IgG demonstrate a robust model of ASD-associated behaviors in a murine system, and add to the growing list of mouse models that recapitulate features of ASD (Silverman et al., 2010). Additionally, it is interesting to note that the altered sociability noted by Singer et al. (Singer et al., 2009) was observed only among adult mice, beyond the ages studied herein. This delayed symptom onset may reflect differences between human and rodent development.
Differences in antibody titer or epitope specificity may underlie developmental and behavioral differences noted between MAU1, MAU2 and MAU3 offspring. Significant differences at specific time points in MAU injection groups were noted in measurements of body length (MAU2 < MAU3 < MAU1), biparietal diameter (MAU3 < MAU2 < MAU1), mean motor and sensory scores (MAU2 < MAU3 < MAU1 on PND 14 and MAU3< MAU2< MAU1 on PND 17) and number of ultrasonic vocalizations (MAU2 > MAU3 > MAU1). The general consistency in these findings is surprising considering low dosage and selection criteria of the administered IgG, and may represent an opportunity for further behavioral categorization of titer effects of brain reactive antibodies on development. These rankings do not appear to relate to antibody titer, but may stem from the precise epitopes that the antibodies recognize, adding urgency to future studies on this topic.
Previous work with intra-peritoneal administrations spanning a wide gestational window produced significant behavioral and physiologic alterations (Singer et al., 2009). In designing the current study, we reasoned that exposure to a single low-dose of IgG IV should limit confounding influences of exposure. Because most IgG is found in circulation, we also rationalized that a single IV injection would be less likely to cause immune reactivity in the dams towards the human IgG. Furthermore, the observations noted in this study resulted from an IgG dosage that was at least 200-fold lower than other reports in the literature, substantially decreasing the amount of maternal IgG necessary to produce developmental alterations. A potential limitation of this minimal dosing strategy is that the full effects of gestational (and post-natal in the murine model) exposure to fetal brain reactive maternal IgG may not have been realized, leading to several results that approached significance. Future experiments will address the dose-response of gestational exposure to autism associated maternal IgG, while identification of the targets of this IgG, currently under investigation, will allow development of model systems with endogenous expression of brain-reactive IgG. Additionally, as our hypothesis is that antigen specificity underlies the effect of gestational transfer of autism-associated maternal antibodies, we would suspect that autism in individuals whose mothers did not harbor such antibodies during gestation would have alternate etiologies, although investigating the effects of IgG from antibody-negative mothers might provide additional insight.
Supporting a direct mechanism of interaction between MAU IgG and the developing fetal brain, human IgG was detected in fetal brain tissue after a single dose administered on GD12. Furthermore, differential growth and behavior findings between MAU and MTD IgG offspring indicate that the activity of brain-reactive IgG, which differentiates the MAU and MTD groups, is retained at this low dosing level. Although stereologic counting of CA1 hippocampal neurons did not reveal significant differences between injection groups, future studies will examine other brain regions for evidence of effects stemming from maternal antibody exposure. It remains to be determined whether the observed behavioral manifestations arise from direct interaction between maternal antibodies and their targets in the developing brain, or are the consequence of immune activation and inflammation that may be triggered.
Further development of the murine maternal antibody related autism (MAR) model, including modifications to dosage, timing of IgG treatment, age at evaluation of social behavior and mouse strain, will allow us to pinpoint the critical developmental windows that are perturbed in maternal antibody associated autism. The MAU and MTD individuals used in these experiments were chosen to be representative of the population of mothers of children with autism who possess fetal-brain reactive antibodies, and mothers of typically developing children who do not, but follow up studies with larger samples are urgently needed. Additionally, it is important to note that by controlling for litter, our conclusions are not compromised by potential litter effects.
Unexpectedly, nonspecific effects of human IgG administration (unrelated to immune protection) were identified through comparison of mice in the SAL control group with mice in the MTD group. These antibody-injected controls were larger in body weight and length during the pre-weaning and juvenile period, showed advanced sensory development, and were more socially active in two tests of direct interactions with conspecifics (social dyad tests). The physiological effects of IgG injection unrelated to passive immunity have not been previously studied to our knowledge.
In conclusion, we report the effects of a single gestational IV-administered dose of purified IgG from mothers of children with autism, with autoantibodies to very specific fetal brain proteins, on development and behavior. Clearly, a more carefully developed model is needed to fully understand the pathologic significance of these MAR antibodies. Future studies utilizing the specific autoantigens recognized by these antibodies are the next logical step in understanding what role, if any, these antibodies play in the etiology of autism.
Acknowledgments
The authors gratefully acknowledge the Mouse Behavioral Assessment Laboratory at UC Davis. We acknowledge Dr. Sam Goth for performing tail vein injections. This work was supported in part by NIEHS 1P01ES11269-0 and U.S. EPA Grant R829388, a Targeted Research Grant from Autism Speaks and an unrestricted research grant from the JB Johnson Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arakawa H, Blanchard DC, Arakawa K, Dunlap C, Blanchard RJ. Scent marking behavior as an odorant communication in mice. Neurosci Biobehav Rev. 2008;32:1236–1248. doi: 10.1016/j.neubiorev.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig D, Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, Pessah IN, Van de Water J. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2008;29:226–231. doi: 10.1016/j.neuro.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig D, Duncanson P, Boyce R, Hansen R, Ashwood P, Pessah IN, Hertz-Picciotto I, Van de Water J. Behavioral Correlates of Maternal Antibody Status Among Children with Autism. Journal of Autism and Developmental Disorders. 2011 doi: 10.1007/s10803-011-1378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Prevalence of autism spectrum disorders - autism and developmental disabilities monitoring network, 14 sites, United States, 2008. MMWR Surveill Summ. 2012;61:1–19. [PubMed] [Google Scholar]
- Dalton P, Deacon R, Blamire A, Pike M, McKinlay I, Stein J, Styles P, Vincent A. Maternal neuronal antibodies associated with autism and a language disorder. Ann Neurol. 2003;53:533–537. doi: 10.1002/ana.10557. [DOI] [PubMed] [Google Scholar]
- Frye RE, Sequeira JM, Quadros EV, James SJ, Rossignol DA. Cerebral folate receptor autoantibodies in autism spectrum disorder. Mol Psychiatry. 2012 doi: 10.1038/mp.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Germann SL, Lloyd KC. Behavioral characteristics of a nervous system-specific erbB4 knock-out mouse. Behav Brain Res. 2004;153:159–170. doi: 10.1016/j.bbr.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Gustafsson E, Mattsson A, Holmdahl R, Mattsson R. Pregnancy in B-cell-deficient mice: postpartum transfer of immunoglobulins prevents neonatal runting and death. Biol Reprod. 1994;51:1173–1180. doi: 10.1095/biolreprod51.6.1173. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect. 2006;114:1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holson RR, Freshwater L, Maurissen JP, Moser VC, Phang W. Statistical issues and techniques appropriate for developmental neurotoxicity testing: a report from the ILSI Research Foundation/Risk Science Institute expert working group on neurodevelopmental endpoints. Neurotoxicol Teratol. 2008;30:326–348. doi: 10.1016/j.ntt.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Polizzi A, Vincent A. An animal model of maternal antibody-mediated arthrogryposis multiplex congenita (AMC) Ann N Y Acad Sci. 1998;841:565–567. doi: 10.1111/j.1749-6632.1998.tb10984.x. [DOI] [PubMed] [Google Scholar]
- Jaubert PJ, Golub MS, Lo YY, Germann SL, Dehoff MH, Worley PF, Kang SH, Schwarz MK, Seeburg PH, Berman RF. Complex, multimodal behavioral profile of the Homer1 knockout mouse. Genes Brain Behav. 2007;6:141–154. doi: 10.1111/j.1601-183X.2006.00240.x. [DOI] [PubMed] [Google Scholar]
- Kim J, Mohanty S, Ganesan LP, Hua K, Jarjoura D, Hayton WL, Robinson JM, Anderson CL. FcRn in the yolk sac endoderm of mouse is required for IgG transport to fetus. J Immunol. 2009;182:2583–2589. doi: 10.4049/jimmunol.0803247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Huerta PT, Zhang J, Kowal C, Bertini E, Volpe BT, Diamond B. Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nat Med. 2009;15:91–96. doi: 10.1038/nm.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell R, Milliken G, Stroup W, Wolfinger R, Schabenberber O. SAS for Mixed Models. Sas Publishing; Carey, NC: 2006. [Google Scholar]
- Martin LA, Ashwood P, Braunschweig D, Cabanlit M, Van de Water J, Amaral DG. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immun. 2008;22:806–816. doi: 10.1016/j.bbi.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming X, Brimacombe M, Wagner GC. Prevalence of motor impairment in autism spectrum disorders. Brain Dev. 2007;29:565–570. doi: 10.1016/j.braindev.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Morphis LG, Gitlin D. Maturation of the maternofoetal transport system for human gamma-globulin in the mouse. Nature. 1970;228:573. doi: 10.1038/228573a0. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Mullen E. Mullen Scale of Early Learning. American Guidance Service; Circle Pines, MN: 1995. [Google Scholar]
- Nathan BP, Yost J, Litherland MT, Struble RG, Switzer PV. Olfactory function in apoE knockout mice. Behav Brain Res. 2004;150:1–7. doi: 10.1016/S0166-4328(03)00219-5. [DOI] [PubMed] [Google Scholar]
- Panksepp JB, Lahvis GP. Social reward among juvenile mice. Genes Brain Behav. 2007;6:661–671. doi: 10.1111/j.1601-183X.2006.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- Shi L, Smith SE, Malkova N, Tse D, Su Y, Patterson PH. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav Immun. 2009;23:116–123. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon P, Dupuis R, Costentin J. Thigmotaxis as an index of anxiety in mice. Influence of dopaminergic transmissions. Behav Brain Res. 1994;61:59–64. doi: 10.1016/0166-4328(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Singer HS, Morris C, Gause C, Pollard M, Zimmerman AW, Pletnikov M. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: A pregnant dam mouse model. J Neuroimmunol. 2009;211:39–48. doi: 10.1016/j.jneuroim.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Singer HS, Morris CM, Gause CD, Gillin PK, Crawford S, Zimmerman AW. Antibodies against fetal brain in sera of mothers with autistic children. J Neuroimmunol. 2008;194:165–172. doi: 10.1016/j.jneuroim.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Sparrow SBDA, Cicchetti DV. Vineland Adaptive Behavior Scales Survey Form Manual. American Guidance Service; Circle Pines, MN: 1984. [Google Scholar]
- Ta TA, Koenig CM, Golub MS, Pessah IN, Qi L, Aronov PA, Berman RF. Bioaccumulation and behavioral effects of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) in perinatally exposed mice. Neurotoxicol Teratol. 2011;33:393–404. doi: 10.1016/j.ntt.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran HB, Macardle PJ, Hiscock J, Cavill D, Bradley J, Buyon JP, Gordon TP. Anti-La/SSB antibodies transported across the placenta bind apoptotic cells in fetal organs targeted in neonatal lupus. Arthritis Rheum. 2002;46:1572–1579. doi: 10.1002/art.10316. [DOI] [PubMed] [Google Scholar]
- Van de Perre P. Transfer of antibody via mother’s milk. Vaccine. 2003;21:3374–3376. doi: 10.1016/s0264-410x(03)00336-0. [DOI] [PubMed] [Google Scholar]
- Wahlsten D. A developmental time scale for postnatal changes in brain and behavior of B6D2F2 mice. Brain Res. 1974;72:251–264. doi: 10.1016/0006-8993(74)90863-4. [DOI] [PubMed] [Google Scholar]
- Warren RP, Cole P, Odell JD, Pingree CB, Warren WL, White E, Yonk J, Singh VK. Detection of maternal antibodies in infantile autism. J Am Acad Child Adolesc Psychiatry. 1990;29:873–877. doi: 10.1097/00004583-199011000-00005. [DOI] [PubMed] [Google Scholar]
- Weisbrot DM, Gadow KD, DeVincent CJ, Pomeroy J. The presentation of anxiety in children with pervasive developmental disorders. J Child Adolesc Psychopharmacol. 2005;15:477–496. doi: 10.1089/cap.2005.15.477. [DOI] [PubMed] [Google Scholar]
- Wink LK, Erickson CA, McDougle CJ. Pharmacologic treatment of behavioral symptoms associated with autism and other pervasive developmental disorders. Curr Treat Options Neurol. 2010;12:529–538. doi: 10.1007/s11940-010-0091-8. [DOI] [PubMed] [Google Scholar]