Abstract
Chronic lymphocytic leukemia (CLL) is characterized by the accumulation of CD5+CD19+ B cells in the peripheral blood, and in primary and secondary lymphoid organs. A major complication associated with CLL is severe recurrent infections, which are often fatal. Vulnerability to infection is due to a wide variety of immunological defects, yet the initiating events of immunodeficiency in CLL are unclear. Using CLL patient samples and a mouse model of CLL, we have discovered that plasmacytoid dendritic cells (pDCs), which underpin the activity of effector immune cells critical for anti-viral immunity and anti-tumor responses, are reduced in number and functionally impaired in progressive CLL. As a result, the levels of interferon alpha (IFNα) production, a cytokine critical for immunity, are markedly reduced. Lower pDC numbers with impaired IFNα production was due to the decreased expression of FMS-like tyrosine kinase 3 receptor (Flt3) and Toll-like receptor 9 (TLR9), respectively. Reduced Flt3 expression was reversed using inhibitors of TGF-β and TNF, an effect correlating with a reduction in tumor load. Defects in pDC numbers and function offer a new insight into mechanisms underpinning the profound immunodeficiency affecting CLL patients and provide a potentially novel avenue for restoring immuno-competency in CLL.
Keywords: Chronic lymphocytic leukemia (CLL), FMS-like tyrosine kinase 3 receptor (Flt3), Interferon alpha (IFNα), plasmacytoid dendritic cells (pDCs), Toll-like receptor 9 (TLR9), Tumour necrosis factor (TNF)
Introduction
Chronic lymphocytic leukemia (CLL) is the most common form of leukemia in the developed world and is characterized by lymphocytosis with accumulation of B-lymphocytes co-expressing CD5, CD19, CD20 and CD23, but with reduced expression of the surface B cell receptor (BCR) (1-3). The majority of CLL patients present with indolent disease, which is often diagnosed via routine blood tests in asymptomatic individuals. As CLL is incurable and often with long progression-free survival (PFS) of 24 years or more, the current treatment strategy is to instigate therapy only upon symptomatic progression (3-5). Although markers indicative of poor prognosis have been identified, such as expression of CD38 (6), unmutated immunoglobulin (Ig) variable heavy chain (IGVH) genes (6), ZAP70 (7) and TCL1 (8), there are still no clinical markers that predict when a patient with indolent CLL might evolve to disease progression requiring treatment.
The EμTCL1-transgenic (Tg) mouse has emerged as a robust model for progressive CLL, sharing many features with the human disease (9). These mice have overexpression of T cell leukemia gene 1 (TCL1) under the Mu (μ) enhancer, confining expression of the transgene to the B cell compartment and resulting in gradual clonal accumulation of circulating CD5+CD19+ B cells. The expansion of these leukemic B cells begins at 3-4 months of age and they accumulate in secondary lymphoid tissues, causing splenomegaly, hepatomegaly and lymphadenopathy over a 12-15 month period (10).
A common complication associated with both indolent and progressive CLL is recurrent viral infection, a major contributor to CLL comorbidity, particularly following chemotherapeutic treatment (11, 12). Progressive immunodeficiency manifests in the early stages of disease and is characterized by hypogammaglobulinemia, poor response to vaccination, poor complement activation and impaired numbers and function of natural killer (NK) cells, neutrophils and macrophages (13, 14). Both CLL patients and EμTCL1-Tg mice display T cell skewing, characterized by decreased T cell activation, increased regulatory T cell (Treg) numbers and attenuated effector function (15). However, the mechanisms underpinning development of immunodeficiency in CLL patients have only been superficially examined and knowledge at this stage is limited to a collection of disparate immune cell defects with little indication as to their possible origin.
Plasmacytoid dendritic cells (pDCs) are highly specialized immune cells which play a major role in promoting innate and adaptive immune responses to viral infection (16), such as herpesvirus, cytomegalovirus (CMV) (17), adenovirus and hepatitis B virus (HBV) (18), which are often responsible for complications in CLL. In addition to some capability for antigen presentation, pDCs are a very important source of type I interferon (IFN-I), which they secrete in large quantities in response to viral infection (16). The IFN-I family includes the closely related IFNα and IFNβ subtypes. All IFN-I cytokines bind to their cognate cell surface IFN-I receptor (IFNAR) composed of the IFNAR1 and IFNAR2 chains. IFNα therapy has previously demonstrated anti-viral efficacy in the treatment of hepatitis C and anti-tumor activity against some cancers (19). However, dose-limiting toxicity of this approach and the potential to precipitate autoimmune disorders has also been reported (19). IFN-I are well-characterized stimulators of the adaptive immune system that enhance antigen-presenting functions by dendritic cells (DC), stimulate effector T cell function and contribute to the differentiation of B cells into antibody-secreting cells (20). IFN-I production stimulates activation of effector T cells, particularly CD8+ T cells, but also NK cells (21), which both play a central role in anti-tumor surveillance. Therefore, this strongly suggests that pDC-derived IFN-I also participates in promoting effective anti-tumor immunity. The role of pDCs in hematological malignancy remains unclear, and no studies have addressed the role of pDCs in CLL progression.
This study provides the first description of pDCs in CLL, and shows that loss of these cells in the peripheral blood of CLL patients differentiates indolent and progressive patient groups. This loss is driven in part by TNF-mediated downregulation of the Flt3 expression. Furthermore, IFNα production by pDCs in CLL is impaired as a consequence of diminished TLR9 expression. Strikingly, these features are recapitulated in the EμTCL1-Tg model of progressive CLL. In view of the documented role of pDCs as essential contributors to anti-viral and anti-tumor immune defenses, our observation provides new insight into mechanisms of immunodeficiency in CLL.
MATERIALS & METHODS
Patients
Patients aged over 18 suffering from CLL fulfilling the National Cancer Institute (NCI) Working Group criteria for diagnosis and staging (3), were prospectively recruited from The Alfred Hospital and Peter MacCallum Cancer Centre. Cryosamples of CLL bone marrow aspirates and lymphocytes were obtained from the Australasian Leukaemia and Lymphoma Group (ALLG) Tissue Bank. Age-matched healthy donor (HD) whole blood samples were collected by the Australian Red Cross Blood Service and provided as per non-clinical supply agreement. All samples from CLL patients were divided into two groups: individuals with stable disease not requiring treatment (indolent) and individuals with advanced disease prior to treatment or who would require treatment in the near future (progressive), according to the NCI Working Group criteria (3). All patients herein gave written informed consent prior to inclusion in this study. Patients receiving therapy for CLL were excluded. All studies were approved by the institutional ethics review boards of the Monash University in accordance with the Declaration of Helsinki principles.
Mice
EμTCL1-Tg mice have previously been described (10) and were kept on a B6C3 background. B6C3 mice were used as wild-type (WT) controls and provided by the Alfred Medical Research and Education Precinct (AMREP) Animal Services (AAS). All experimental mice used were age-matched and housed under high barrier protection and handled with approval from institutional Animal Ethics Committees, in compliance with the Australian code of practice for the care and use of animals for scientific purposes.
Lymphocyte isolation
Lymphocytes from HD and CLL patients were purified from peripheral blood diluted 1:1 in PBS (Invitrogen, Carlsbad, CA, USA) and layered onto Ficoll-Paque (GE Healthcare, Piscataway, NJ, USA) for gradient centrifugation. Mouse splenocytes were isolated following disaggregation of the spleen and 70μm filtration (BD, San Diego, CA, USA) to form a single cell suspension. Red blood cells were lysed with RBC lysis buffer (eBioscience, San Diego, CA, USA). Cells were enumerated using a Z™ Series Coulter Counter (Beckman Coulter Inc., NSW, Australia). Absolute numbers of pDCs were calculated by multiplying the percentage of FACS-gated pDCs by total cell count.
Antibodies, flow cytometry and analysis
For flow cytometric analysis, single-cell suspensions were stained as previously described (22). Intracellular staining was conducted using a Fixation/Permeabilization kit from eBioscience (San Diego, CA, USA) as per manufacturers instructions. Fragment crystallizable (Fc) receptors were blocked using purified CD16/32 monoclonal antibody (mAb) (BD, San Diego, CA, USA). Viable lymphocytes were assessed using Live/Dead Fixable Dead Cell Stain Kit (Invitrogen, Carlsbad, CA, USA). Matching isotype mAbs were used to control for background staining.
Anti-mouse CD5-eFluor450 (53-7.3), B220-FITC (RA3-6B2), PDCA1-APC (eBio927), Siglec H-eFluor710 (eBio440c), CD11b-PE (M1/70) and TLR9-FITC (M9.D6) were purchased from eBioscience (San Diego, CA, USA). Gr.1 (Ly-6G)-pacific blue (RB6-8C5) was purchased from Caltag (Buckingham, UK). CD19-PerCP (6D5) was sourced from Biolegend (San Diego, CA, USA). CD11c-PE-Cy7 (HL3) and Flt3-PE (A2F10.1) were purchased from BD (San Diego, CA, USA). Anti-mouse TGF-βR1-PE (Cl141231) was purchased from R&D Systems (Minneapolis, MN, USA).
Human cells were stained with a FITC-conjugated lineage negative (Lin−) cocktail containing antibodies to CD3, CD14, CD16, CD19, CD20, CD56 (NCAM16.2, MφP9, L27, SJ25C1, 3G8, SK7), CD11b-FITC (ICRF44), and with CD123-PE-Cy7 (7G3), CD20-APC-Cy7 (L27), CD5-PE-Cy7 (L17F12) and Flt3-PE (4G8), all purchased from BD (San Diego, CA, USA). CD11c-FITC (3.9) and HLA-DR-eFluor450 (LN3) were purchased from eBioscience (San Diego, CA, USA). BDCA2-APC was sourced from Miltenyi Biotec (Bergisch Gladbach, Germany). BDCA4-PerCP (446921) was purchased from R&D Systems (Minneapolis, MN, USA). TLR9-PE (26C593.2) was purchased from Enzo Life Sciences (Farmingdale, NY, USA). A BD LSRII flow cytometer was used to analyze the flow cytometry samples (San Jose, CA, USA). At least 100,000 events were collected for all analyses. The analysis was conducted using FlowJo software (Treestar, Ashland, OR, USA).
Cell cultures
RPMI and DMEM media (Invitrogen, Carlsbad, CA, USA) were used for all mouse and human cultures, respectively. Media were supplemented with 10% FCS, L-glutamine, 2ME, HEPES and Pen/Strep; all from Invitrogen (Carlsbad, CA, USA). For bone marrow cultures, cells were isolated from the femurs of EμTCL1-Tg and age-matched WT controls. Human bone marrow cryosamples were thawed and washed in sterile PBS. Bone marrow cells were seeded at 105 cells/ml in 48-well cell culture plates (BD, San Diego, CA, USA). For lymphocyte cultures, cells were seeded at 106-107 cells/ml in 24- or 48-well cell culture plates (BD, San Diego, CA, USA). All cultures were incubated at 37°C and 5% CO2. Stimulatory factors were used at the following concentrations: Flt3L, 100-400ng/ml (R&D Systems, Minneapolis, MN, USA); CpG B ODN 7909, 1μM and CpG C ODN 2395, 1μM (Invivogen, San Diego, CA, USA). Optimal CpG stimulations were conducted for 6-12 hrs. In vitro neutralization of TNF and transforming growth factor beta (TGF-β) was achieved using purified anti-mouse TNF (MP6-XT22), anti-mouse TGF-β (TW7-20B9), anti-human TNF (mAb11) and anti-human/mouse TGF-β (19D8) (Biolegend, San Diego, CA, USA), all used at 1μg/ml.
Injections
Mice received one intravenous injection per week for two weeks of either 100μg anti-TNF (MP6-XT22), 100μg anti-TGFβ (TW7-20B9) or 100μg isotype controls (all sourced from Biolegend, San Diego, CA, USA). Sterile PBS (Invitrogen, Carlsbad, CA, USA) was for injection controls.
Serum and secreted cytokine quantification
Serum and secreted IFNα levels were quantified using high-sensitivity ELISA assays from PBL Interferon Source (Piscataway, NJ, USA), as per manufacturer’s protocol. Levels of TNF, TGF-β and Flt3L in serum and supernatant were quantified using ELISA assays purchased from R&D Systems (Minneapolis, MN, USA), as per manufacturer’s protocol. All samples were tested in duplicates.
Statistical analysis
Data are shown as means (± SEM). The student’s t-test was used to determine significant differences between means. All statistical analyses were performed with GraphPad software (Prism Version 6.0d, 2013, San Diego, CA, USA). A P value ≤ 0.05 was considered statistically significant. *P < 0.05; **P < 0.01; ***P < 0.001.
RESULTS
Reduced numbers of splenic pDCs in EμTCL1-Tg mice as disease progresses
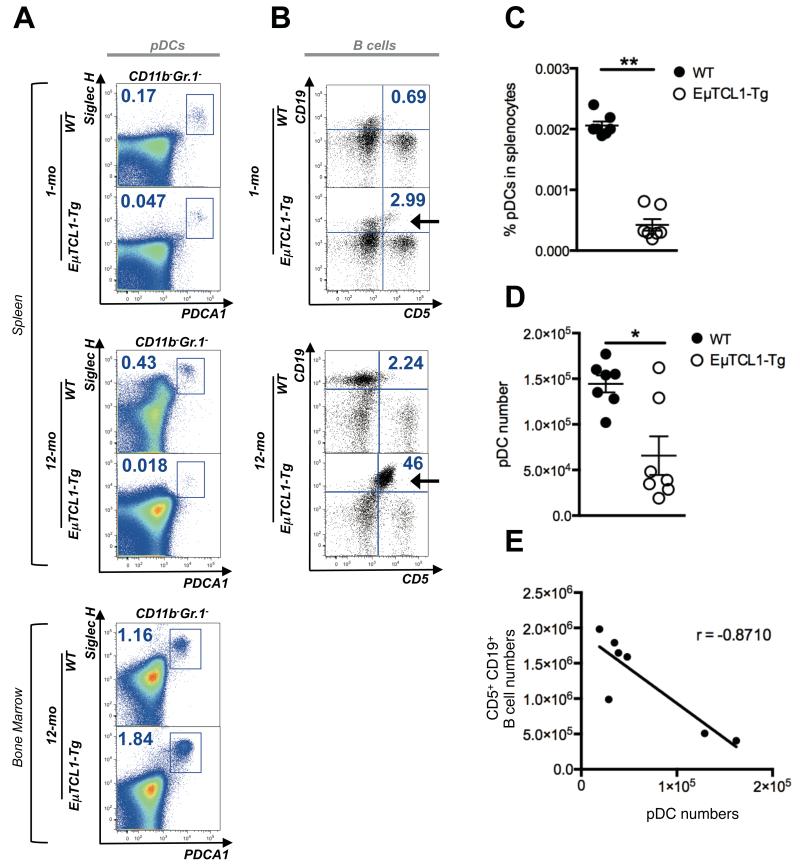
Given the higher incidence of chronic viral infection in most CLL patients either at diagnosis or thereafter (11), we first used the EμTCL1-Tg mouse CLL model to examine possible underlying early immune abnormalities, focusing on pDCs, which are central initiators of effective anti-viral immune responses (reviewed in (20)). Interestingly, proportions of splenic pDCs were significantly reduced in EμTCL1-Tg as they age (Figure 1A, top and middle panels). Significant reduction in pDC numbers was also observed in the peripheral blood of EμTCL1-Tg mice (Supplementary Figure 1). No significant difference was observed in the proportion of pDCs in the bone marrow (BM) of EμTCL1-Tg mice and WT mice (Figure 1A, bottom panel) in keeping with previous work showing that pDC response to growth factors is dependent on the microenvironment and distinct in the BM and the periphery (23). Loss of splenic pDCs paralleled the accumulation of the CD5+CD19+ B cell clone in EμTCL1-Tg mice, which was apparent at 1-mo but striking at 12-mo of age (Figure 1B). Proportions of CD5+CD19+ B cells in WT control mice remained relatively constant over time (Figure 1B) and corresponded to B1 B cells normally present in the spleen (reviewed in (24)). In addition to the proportions (Figure 1C), absolute numbers of splenic pDCs were reduced in EμTCL1-Tg mice (Figure 1D). Numbers of pDCs and tumor cells inversely correlated (Figure 1E). These data suggest that EμTCL1-Tg mice progressively lose peripheral pDCs as disease progresses.
Figure 1. Analysis of splenic and BM pDCs in WT and EμTCL1-Tg mice.
(A) Murine splenic pDCs were gated by flow cytometry as CD11b− Gr.1− B220+ CD11c+ SiglecH+ PDCA-1+ as indicated. Representative dot plots show splenic pDCs in WT mice and EμTCL1-Tg mice at 1-mo and 12-mo of age as indicated (n=7 per group). Representative dot plots show BM pDCs from WT and EμTCL1-Tg mice at 12-mo of age (n=7 per group; bottom, left panel). Percentages of gated cells are shown. (B) Dot plots showing CD5+CD19+ B cells in splenocytes from 1-mo and 12-mo old WT and EμTCL1-Tg mice as indicated (n=7 per group). Emergence of CD5+CD19+ tumor B cells in EμTCL1-Tg mice is indicated with an arrow. CD19− CD5− non-B cells correspond to CD3+ T cells. Percentages of gated cells are shown. (C) Proportion of splenic pDCs in 12-mo WT and EμTCL1-Tg mice (n=7 per group). (D) Absolute numbers of splenic pDCs in the spleens of 12-mo WT and EμTCL1-Tg mice (n=7 per group). (E) Correlation between absolute numbers of splenic CD5+CD19+ tumor B cells and pDCs in EμTCL1-Tg mice (n=7) with advanced disease. In C and D horizontal bars indicate the mean. In A-E, data are representative of at least three independent experiments.
Impaired pDC numbers in the peripheral blood of CLL patients as disease progresses
We next investigated whether the pDC defect seen in EμTCL1-Tg (Figure 1) could also be detected in blood samples from CLL patients. Indeed, the aberrant pDC phenotype seen in EμTCL1-Tg mice was also seen in CLL patients (Figure 2A). Proportions of pDCs were significantly decreased (5-fold) in the blood of patients with CLL (data not shown). In contrast to the striking absence of pDCs in the peripheral blood, pDCs were detected in the matched BM of patients with progressive CLL (Figure 2A), similar to normal BM sample (Supplementary Figure 2), again confirming different responses of pDCs in distinct microenvironments as described previously(23). pDCs numbers were significantly diminished in patients with progressive CLL but, surprisingly, significantly increased pDC numbers in the blood of patients with indolent CLL relative to healthy donors (HD) (Figure 2B). Overall, pDC numbers in the peripheral blood were found to inversely correlate with the number of CD5+CD19+ B cells (Figure 2C).
Figure 2. Analysis of pDCs from the peripheral blood and BM of HD and CLL patients.
(A) Human pDCs from peripheral blood and BM were gated by flow cytometry as HLA-DR+ CD123+ BDCA2+ BDCA4+ and negative for lineage markers CD3, CD14, CD16, CD19, CD56, CD11c and CD11b. Representative dot plots show pDCs in healthy donor, and patients with indolent and progressive CLL with a representative matched BM sample for the latter patient, as indicated. Percentages of gated cells is shown. (B) Absolute numbers of pDCs per litre in HD (n=12), and patients with indolent (n=14) and progressive (n=12) CLL. (C) Correlation between absolute numbers of CD5+CD19+ B cells and pDCs in progressive CLL patients (n=14). In C, horizontal bars indicate the mean. In A-C, data are representative of at least three independent experiments.
Age-related decline in pDC numbers has been reported previously as a feature of immune senescence in healthy humans over 60 years of age but not to the extent of what we observed in CLL patients compared to age-matched HD (25). Collectively these observations suggest that similar to the EμTCL1-Tg mouse CLL model, progression of CLL is associated with gradual loss of pDCs, which may explain in part the poor anti-viral responses of CLL patients.
Defective pDC function in both EμTCL1-Tg mice and CLL patients
A well-known function of pDCs is to mediate antiviral activity via IFN-I secretion and pDC-derived IFNα accounts for physiological levels of this factor in the serum of healthy individuals (16). Loss of pDCs suggested a possible deficiency in IFNα production in both EμTCL1-Tg mice and CLL patients. Indeed, as they aged, EμTCL1-Tg mice displayed significantly reduced serum IFNα levels relative to WT controls (Figure 3A). Similarly, serum IFNα levels were significantly reduced in patients with indolent CLL relative to HD and further diminished in patients with progressive disease (Figure 3B). Flow cytometry staining for intracellular indoleamine 2,3 dioxygenase (IDO), a well-known immunosuppressive factor abnormally produced by some pDCs in solid tumors (26), was negative in pDCs from EμTCL1-Tg mice and patients with indolent or progressive disease following stimulation (data not shown). This result suggested that pDCs in CLL have not been further subverted beyond an impairment of IFNα production. Moreover, a deficit in pDC-derived IFNα production may also partly explain the vulnerability of CLL patients to infections.
Figure 3. IFNα levels and TLR9 expression in pDCs from EμTCL1-Tg mice and CLL patients.
(A) Serum IFNα levels for WT and Eμ-TCL1-Tg mice at 1-mo (n=7 and n= 6, respectively), 6-mo (n= 6 per group) and 12-mo of age (n=6 per group) were measured by ELISA. (B) Serum IFNα levels from HD (n=17), and patients with indolent CLL (n=13) and progressive CLL (n=9). (C) Intracellular expression of TLR9 was measured by flow cytometry in pDCs of WT (n=6) and EμTCL1-Tg mice (n=5) at 1-mo and 12-mo of age. (D) Intracellular expression of TLR9 in pDCs from HD (n=7), and patients with indolent (n=8) and progressive (n=5) CLL. (E) IFNα production in response to in vitro CpG stimulation of splenic pDCs from WT mice (n=6) and EμTCL1-Tg mice (n=6) assessed by ELISA. (F) IFNα production in response to in vitro CpG stimulation of pDCs from HD (n=6), and patients with indolent (n=6) and progressive CLL (n=6). In A-D, horizontal bars indicate the mean. In A-F, data are representative of at least three independent experiments.
The surprising increase in pDC numbers in patients with indolent CLL despite reduced IFNα levels in the serum of these patients suggested defective IFNα production by their pDCs. pDCs have a restricted TLR profile, primarily expressing TLR7 and TLR9 (16). Ligation of TLR9 by CpG oligonucleotide sequences induces rapid activation and subsequent IFNα production (16). Interestingly, TLR9 expression was significantly reduced in splenic pDCs from 12-mo old EμTCL1-Tg mice with advanced disease (Figure 3C). Likewise, TLR9 expression was significantly reduced in pDCs of patients with indolent CLL and almost completely lost in patients with progressive disease (Figure 3D). As expected, in vitro CpG-stimulated pDCs from WT control mice produced increased levels of IFNα (Figure 3E). In contrast, pDCs from EμTCL1-Tg mice with advanced disease had impaired IFNα production in response to CpG stimulation (Figure 3E). Similarly, blood pDCs from patients with indolent disease had impaired IFNα production in response to CpG stimulation in vitro and negligible levels of IFNα were detected in pDC cultures from patients with progressive disease (Figure 3F). Therefore, the pDC defect in CLL is multifaceted and characterized by both reduced numbers as disease progresses and an inability to produce IFNα upon TLR9 activation. Moreover, increased pDC numbers in the blood of patients with indolent CLL (Figure 2B) may partially offset the reduced production of IFNα by these cells (Figure 3F), hence contributing to a relatively asymptomatic immune state in these patients who do not require therapy.
Elevated serum Flt3L concentration but reduced surface Flt3 expression on pDCs of EμTCL1-Tg mice and CLL patients
Flt3L is a well-known component of pDC differentiation and is essential for their growth and maintenance.(27) In vivo experiments in both mice and humans have demonstrated a significant increase in pDC numbers following administration of Flt3L (27, 28). Surprisingly, serum Flt3L levels were significantly elevated in 12-mo old EμTCL1-Tg mice with advanced disease relative to younger EμTCL1-Tg mice (Figure 4A). Similarly, patients with either indolent or progressive CLL displayed significantly increased serum Flt3L levels compared to HD (Figure 4B). Furthermore, there was no difference in the ability of the BM from either EμTCL1-Tg mice or patients with progressive CLL to generate pDC upon Flt3L treatment ex vivo (Figure 4C and D, respectively). These data suggested that mechanisms underpinning pDC deficiency were confined to the periphery and that increased serum concentrations of Flt3L seen in CLL may be an important compensatory mechanism maintaining high pDC numbers in patient with indolent CLL to offset their reduced IFNα production.
Figure 4. Flt3-Flt3L function in peripheral and BM pDCs of EμTCL1-Tg mice and CLL patients.
(A) Serum Flt3L levels for WT and Eμ-TCL1-Tg mice at 1-mo (n=5 and n=3, respectively) and 12-mo of age (n=4 and n=6, respectively) measured by ELISA. (B) Serum Flt3L levels for HD (n=11), and patients with indolent CLL (n=5) and progressive CLL (n=6). (C) BM cells from WT mice and EμTCL1-Tg mice (n=6 per group) at 1-mo and 12-mo of age was cultured for 9-d with or without Flt3L and assessed for pDC generation by flow cytometry as in Figure 1. (D) BM tissue from CLL patients with progressive disease (n=3) was cultured for 9-d with or without Flt3L and assessed for pDC generation by flow cytometry as in Figure 2. (E) Flt3 expression was measured by flow cytometry on freshly isolated pDCs from BM and spleens of WT and EμTCL1-Tg mice (n=6 per group). (F) Flt3 expression on pDCs from peripheral blood of HD (n=3), and patients with indolent (n=7) and progressive (n=5) CLL. In A-F, horizontal bars indicate the mean. In A-F, data are representative of at least two independent experiments.
The disparity between decreased pDC numbers and elevation of peripheral Flt3L levels suggested a dampened response to growth signals mediated by the receptor Flt3. EμTCL1-Tg mice with advanced disease (9-mo old) displayed diminished Flt3 expression on splenic pDCs compared to WT mice (Figure 4E). Flt3 expression was significantly higher on BM pDCs from EμTCL1-Tg compared to splenic pDCs from the same mice (Figure 4E). This higher Flt3 expression may explain the maintenance of BM pDC numbers in EμTCL1-Tg mice in contrast to Flt3lo splenic counterparts.
Likewise, pDCs from CLL patients with indolent disease expressed significantly increased levels of Flt3 compared to HD (Figure 4F). Conversely, significant downregulation of Flt3 expression was observed on pDCs from patients with progressive CLL (Figure 4F). Higher Flt3 expression on pDCs from patients with indolent CLL may account for the increased numbers of circulating pDCs in these individuals (Figure 2B). These observations suggest that loss of pDCs may be linked to gradual loss of Flt3 expression as disease progresses and subsequent impaired maintenance of these cells.
Increased pDC numbers and IFNα secretion following combined Flt3L and CpG stimulation of pDCs from EμTCL1-Tg mice or CLL patients
Exogenous Flt3L increased pDC numbers in 5-d cultures of splenocytes from WT mice (Figure 5A). In contrast, pDCs from EμTCL1-Tg mice with advanced disease failed to expand in response to Flt3L in culture (Figure 5A). A similar pattern was observed in human PBMC cultures. Modest increases in pDC numbers were observed in 4-d Flt3L cultures of PBMCs from patients with indolent CLL, in contrast to Flt3-supplemented cultures of PBMCs from HD in which significantly higher numbers of pDCs were generated (Figure 5B). Strikingly, Flt3-supplemented cultures with PBMCs from patients with progressive CLL failed to increase pDC numbers (Figure 5B), mirroring the effect seen in EμTCL1-Tg splenocyte cultures. This may reflect the lower sensitivity of these pDCs to Flt3L due to reduced Flt3 expression (Figure 4E and F). Furthermore, ex-vivo amplification of pDC numbers from WT mice or HD with exogenous Flt3L alone was enough to modestly elevate IFNα levels (Figure 5C and D). Interestingly, pDCs from patients with indolent CLL also responded in vitro to Flt3L stimulation by producing IFNα in similar amounts to that of control cultures. Importantly, cultures supplemented with Flt3L and CpG resulted in visibly increased IFNα secretion by pDCs from EμTCL1-Tg mice (Figure 5C) and patients with indolent and progressive CLL (Figure 5D). Of note, detected IFNα levels were lower in unstimulated cell culture conditions than in serum (Figure 3B). This is expected in prolonged in vitro culture without exogenous stimuli, and IFNα levels mirrored those of serum when stimulation was provided. We observed no effect of CpG on Flt3 expression on pDCs (data not shown). These observations suggest that combining these immunostimulatory agents could restore some pDC activity (Figure 5C and D).
Figure 5. In vitro stimulation of pDCs from EμTCL1-Tg and CLL patients with Flt3L and CpG.
(A) Numbers of pDCs from 5-d cultures of splenocytes from WT and EμTCL1-Tg mice (n=4 per group) were determined by flow cytometry as in Figure 1A. (B) Numbers of pDCs from 4-d cultures of PBMCs from HD, and patients with indolent CLL and progressive CLL (n=6 per group) were determined by flow cytometry as in Figure 2A. (C) pDCs isolated from WT and EμTCL1-Tg mice (n=4 per group) were cultured with or without Flt3L and/or CpG and supernatants assessed for IFNα levels by ELISA (D) IFNa levels in supernatants of pDCs from HD, and patients with indolent CLL and progressive CLL (n=6 per group) cultured with or without Flt3L and/or CpG. In A-D, horizontal bars indicate the mean. In A-D, data are representative of at least two independent experiments.
TNF and TGF-β-dependent downregulation of Flt3 expression in pDCs from CLL patients and EμTCL1-Tg mice
TNF and TGF-β are well-established negative regulators of pDCs via modulation of Flt3 expression during haematopoiesis (29, 30). Interestingly, TLR9 downregulation in pDCs has also been linked to increased TNF production.(31) TNF is highly produced by activated macrophages, and to a lesser extent, activated T cells, B cells and NK cells (reviewed in (32)). TNF has also been described as an autocrine growth factor produced by malignant CLL B cells and is a significant contributor to tumor persistence (33). We detected elevated serum TNF levels in EμTCL1-Tg mice with advanced disease relative to younger EμTCL1-Tg mice, and age-matched WT controls which did not show detectable levels of TNF (Figure 6A). Likewise, we observed a significant increase in TNF levels in the serum of patients with progressive CLL compared to that of both HD and patients with indolent disease (Figure 6B). Furthermore, in vitro TNF stimulation of pDCs from EμTCL1-Tg mice and CLL patients resulted in significantly reduced Flt3 and TLR9 mRNA levels, respectively (Figure 6C and D). This suggested that TNF modulated Flt3 and TLR9 expression at the transcriptional level. Strikingly, multiple IFN-responsive genes and Flt3-inducing genes were also significantly downregulated in TNF treated pDCs from EμTCL1-Tg mice and CLL patients (Supplementary Figure 3). pDCs purified from HD and co-cultured in transwell assays with CLL B cells also displayed reduced TLR9 and Flt3 protein level expression confirming a role for tumor-derived soluble factors in driving TLR9 and Flt3 downregulation (Supplementary Figure 4). These observations strongly suggest that TNF signalling in CLL mediates broad transcriptional deregulation in pDCs to subvert their numbers and function.
Figure 6. Increased pDC numbers following TNF or TGF-β inhibition in EμTCL1-Tg mice and CLL patients.
(A) Serum TNF levels in WT and Eμ-TCL1-Tg mice at 1-mo and 12-mo of age (n=3 per group), measured by ELISA. (B) Serum TNF levels from HD (n=7), and patients with indolent CLL (n=4) and progressive CLL (n=5), measured by ELISA. (C) Flt3 and TLR9 mRNA levels in TNF-treated pDCs from EμTCL1-Tg mice and (D) CLL patients. (E) TGF-βR1 expression on BM and splenic pDCs from WT and EμTCL1-Tg mice (n=5 per group, p = 0.0079), measured by flow cytometry as in Figure 1A. (F) pDC numbers and (G) Flt3 expression from 12-h cultures of splenocytes from WT and EμTCL1-Tg mice (n=4 and n=5 per group, p = 0.0075) stimulated with or without anti-TNF in vitro, measured by flow cytometry as in Figure 1A. (H) Enumeration of pDCs by flow cytometry as in Figure 2A, from 12-h cultures of PBMCs from HD (n=5), and patients with indolent CLL (n=6) and progressive CLL (n=4, p = 0.0357). (I) Flt3 expression on cultured pDCs from HD (n=3), and patients with indolent CLL (n=7) and progressive CLL (n=7, p = 0.038) stimulated with or without anti-TNF in vitro, measured by flow cytometry (J) Enumeration of splenic CD5+CD19+ B cells and (K) pDCs by flow cytometry as in Figure 1A and B from EμTCL1-Tg mice with advanced disease, injected twice with 100μg anti-TNF (n=7) or anti-TGF-β (n=6) neutralizing mAbs or isotype control (n=6). (L) Serum IFNα levels for Eμ-TCL1-Tg mice injected twice with 100μg anti-TNF (n=7) or anti-TGF-β (n=6) neutralizing mAbs or isotype control (n=6), measured by ELISA. In A-K, horizontal bars indicate the mean. Data are representative of at least two independent experiments.
In contrast to TNF, no difference was detected in the serum TGF-β levels between WT and EμTCL1-Tg mice. In fact, TGF-β levels were slightly decreased in both WT and EμTCL1-Tg with age (data not shown). In contrast, expression of TGF-βR1 was significantly higher on splenic pDCs from EμTCL1-Tg mice compared to WT (Figure 6E). BM pDCs from EμTCL1-Tg mice showed no difference in TGF-βR1 expression compared to WT mice (Figure 6E). These data suggest that splenic pDCs in EμTCL1-Tg mice might have increased sensitivity to circulating TGF-β.
We next examined the restorative potential of TNF inhibition. In vitro blockade of TNF significantly increased pDC numbers and this effect correlated with restored Flt3 expression on pDCs from EμTCL1-Tg mice with advanced disease (Figure 6F and G). Similar results were obtained using these culture conditions with pDCs from patients with indolent and in particular, the progressive CLL cohort (Figure 6H and I). We next investigated whether this approach would have beneficial effects in vivo. EμTCL1-Tg mice with advanced disease were injected twice, over 14 days, with antibodies blocking either TNF or TGF-β. Mice responded to both TGF-β and TNF blockade with a significant reduction in splenic tumor burden that correlated with a significant increase in splenic pDC numbers relative to control mice (Figure 6J and K). Interestingly, serum IFNα levels were significantly increased in mice treated with either anti-TNF or anti-TGF-β, and this correlated with restoration of pDC numbers (Figure 6L). We also observed increased numbers of both CD8+ T cells in anti-TNF-treated EμTCL1-Tg mice (Supplementary Figure 5). Collectively, these observations suggest that TNF production and TGF-βR1 overexpression on peripheral pDCs compromises Flt3-dependent pDC maintenance during CLL progression. Normal TNF levels in the serum of patients with indolent CLL compared to HD may also explain unaffected Flt3 expression on the pDCs from these patients (Figure 6C). It remains to be seen whether the reduction in splenic tumor burden is solely due to neutralization of autocrine TNF signals that usually support tumor persistence or whether increased pDC numbers also restores some immuno-competence. Encouragingly, similar results obtained with TGF-β inhibition, which does not directly affect CLL cells(34), suggest that increased pDC numbers may indeed play an important role.
DISCUSSION
Infection is the leading cause of death in CLL (13, 35); strongly indicating that immunodeficiency in CLL patients is a significant underlying problem. Despite this, current treatments emphasize tumor debulking and are immunosuppressive, limiting their long-term effectiveness (36). The immune system is the best natural protection against infections and cancers, and a strategy aimed at restoring adequate immune defenses in CLL patients is likely to improve patients’ survival and quality of life. However, it has been difficult to pinpoint the mechanism of immunodeficiency in CLL, as almost all immune players appear to be affected. Our work describes, an early and progressive deficiency in pDC numbers and function. pDC activity underpins the effector function of most downstream immune cells including B cells, CD4+ and CD8+ T cells, and NK cells, which are critical to fight infections and cancers (37). We have also identified possible mechanisms explaining loss of pDCs, in particular pathways that are currently exploited for the treatment of CLL. Moreover, the pDC defects we described are all recapitulated in a mouse model of CLL, suggesting a conserved and important mechanism.
Decreased IFNα concentrations resulted from reduced TLR9 expression in pDCs. Defects in pDC function are known to disable downstream NK and CD8 T cell effector functions during infection (20). Importantly, these NK and CD8 T cell abnormalities are also described in CLL (13, 14). Given the central role of pDCs in immunity, it is likely that progressive loss of pDC function in CLL underlies the major immunodeficiency affecting CLL patients. The role of pDCs in cancer appears to be complex (38), however, pDC production of IFN-I has a well-described anti-tumor activity (21, 39). IFNAR−/− mice display significantly enhanced mortality when injected with melanoma and carcinoma cell lines (40) and, inhibition of IFN-I with polyclonal blocking antibodies significantly exacerbated grafting of tumors (41).
The efficacy of IFN-I therapy in the CLL arena was first described over 20 years ago (42). IFN-I has since been extensively used in the treatment of several malignancies including hairy cell leukemia, B cell lymphoma and T cell lymphoma as well as solid tumors such as melanoma, renal carcinoma and Kaposi’s sarcoma (43,44).
The prolific secretion of IFNα by pDCs is essential for mitigating infectious complications (16), and enhancing antigen cross-presentation by DCs to prime CD8+ CTLs. (45, 46) Thus, our observation that pDC numbers are drastically decreased or nearly absent in progressive CLL explains the attenuation of IFNα production and may contribute to chronic infection in these patients. Enumeration of blood pDCs appears to constitute a novel and straightforward clinical tool to distinguish indolent and progressive groups. Longitudinal studies will be needed to determine whether increased pDC numbers contribute to disease stability in patients with indolent CLL and if those patients with lower pDC numbers are at increased risk of evolving to progressive disease. The loss of pDCs specifically in the periphery but not in the BM correlated with Flt3 expression, which was specifically reduced in the periphery. This observation further illustrates the fact that maintenance of splenic pDCs is different than that of BM pDCs and requires IFNα in addition to Flt3 signalling (47) both of which are specifically impaired in splenic pDCs of EμTCL1-Tg mice.
TNF-mediated reduction in TLR9 expression in pDCs of CLL patients suggested that IFNα deficiency arose from concomitant reduction in pDC number and reduced activation, at least in progressive CLL. Increased pDC numbers in patients with indolent disease, which is most likely supported by greater Flt3 expression on these cells, may offset in part reduced IFNα production in response to CpG stimulation. Maintenance of basal serum IFNα production may account for a relatively asymptomatic immune profile in some patients. Downregulation of TLR9 in pDCs within the tumor environment has been previously described, as has their resulting insensitivity to CpG stimulation at the tumor site (48). However, TLR9 down modulation in pDCs has not been previously reported in CLL. The anti-tumor activity of TLR9 agonists has been demonstrated in a number of tumor models including glioma (49) and neuroblastoma (50). Injection of CpG has shown efficacy in the clearance of solid tumors such as HNSCC (48) but effective treatment of hematological malignancies like CLL with CpG is still being investigated. The class B ODN, CpG 7909 is currently in Phase I/II clinical trials for progressive CLL (51) where it may act to trigger apoptosis through an autocrine interleukin-10 (IL-10) secretion by the B cell tumor (52). Our data suggests class C CpG stimulation, targeting both B cells and pDCs, may induce increased levels of IFNα whilst simultaneously aiding IL-10 production. The effect of this agonist on the remnant pDCs of CLL patients is of particular interest in the context of the immune phenotype we observed. IFNα is an important pDC survival and maturation factor, at least in vitro (53) and future work will need to ascertain the importance of IFNα restoration in CpG-treated patients and its implications for infection-related mortality.
Interestingly, increasing pDC numbers with exogenous Flt3L in vitro was enough to increase basal IFNα secretion but this was more potently induced with concomitant CpG stimulation. The use of Flt3L therapy alone or in combination with CpG 7909 to restore pDC numbers in the periphery and increase IFNα production may be a possible therapeutic avenue for improving immunity.
TNF, along with TGF-β, are two well-known negative regulators of Flt3 expression (30). We observed increased serum levels of TNF but not TGF-β in EμTCL1-Tg mice relative to WT. Furthermore, increased pDC numbers and Flt3 expression following in vitro TNF neutralization supported a TNF-mediated decrease in Flt3 expression and subsequent reduction of pDC numbers. While TNF is a well-known autocrine growth factor for CLL B cells (54), our findings suggest its pathogenic role extends beyond tumor survival to the disruption of pDC maintenance and induction of an immunodeficient state.
Based on the rationale that overcoming TNF-induced tumor proliferation would increase the potency of rituximab, anti-TNF therapy in the form of soluble TNF receptor, etanercept has been trialed in combination with rituximab to treat relapsed CLL (55). This study showed a 29% response rate with all these patients achieving durable, greater than 12 months, remissions (55). In addition, the anti-TNF monoclonal antibody, infliximab, which is currently approved for the treatment of rheumatoid arthritis, has been associated with increased total IFNα in these patients, potentially due to reactivation of pDC activity (56). Although the responses in the etanercept-rituximab trial was attributed solely to direct inhibition of tumor growth, four patient responders had remained treatment-free ranging from 32 to 56 months (53). This raises an interesting question as to whether this durability of remission may have been assisted by restoration of pDC numbers and immune competency, and it would be worthwhile to investigate further anti-TNF therapy alone or in other combinations with for example, chlorambucil or fludaribine in CLL patients.
Interestingly, TGF-β inhibition demonstrated similar effects to anti-TNF treatment in EμTCL1-Tg mice and the effect appeared to be linked to higher TGF-βR1 expression on pDCs, suggesting greater sensitivity to TGF-β. This result is of particular interest as CLL B cells are normally resistant to TGF-β signalling(34) and inhibition of TGF-β offered a way to study this effect away from any direct effect on CLL cells (34). Remarkably, like TNF inhibition, anti-TGF-β restores Flt3 expression and pDC numbers, and these effects correlated with reduced tumor burden. Collectively, our study indicates a beneficial effect of restoring pDCs and potentially activating anti-tumor immunity.
Of interest, previous work has already shown that increased TGF-βR1 expression, rather than TGF-β levels, is the key determinant in modulating pDC sensitivity to TGF-β (57, 58). Indeed, anti-TGF-β therapies are currently being tested in patients with solid tumors (reviewed in (59)) and our study suggests that TGF-β inhibition may also be a potential strategy for immune reactivation in CLL patients.
Our study represents an important turning point in our understanding of the mechanisms leading to immunodeficiency in CLL and offers a promising avenue for innovative therapeutic interventions aimed at restoring vital natural immune protection in CLL patients and reducing infection-related mortalities.
Supplementary Material
Supplementary Figure 1. Analysis of pDCs from the peripheral blood of WT and EμTCL1-Tg mice.
Murine blood pDCs were gated by flow cytometry as CD11b− Gr.1− B220+ CD11c+ SiglecH+ PDCA-1+ as in Figure 1A. Dot plots show proportion of blood pDCs in 12-mo WT and EμTCL1-Tg mice (n=7 per group).
Supplementary Figure 2. Analysis of human pDCs from normal BM.
BM from a patient with non-diffuse lymphoma and no BM infiltrate was used as a normal BM control. The representative dot plot shows pDCs were gated by flow cytometry as HLA-DR+ CD123+ BDCA2+ and BDCA4+ and negative for lineage markers CD3, CD14, CD16, CD19, CD56, CD11c and CD11b. Percentages of pDCs are shown.
Supplementary Figure 3. Aberrant gene expression in TNF-stimulated pDCs from EμTCL1-Tg mice and CLL patients.
irf1, irf7, irf8, Bcl11a and E2-2 mRNA levels were measured in TNF-treated pDCs from (A) EμTCL1-Tg mice and (B) CLL patients. Data are expressed as fold difference and are representative of at least two independent experiments.
Supplementary Figure 4. Downregulation of TLR9 and Flt3 expression on healthy pDCs in the presence of CLL B cells.
(A) Expression of intracellular TLR9 and (B) surface Flt3 on purified pDCs from of HD was measured by flow cytometry following 12-h transwell co-culture with CLL B cells placed in the upper chamber and pDCs in the bottom chamber (n=4 per group).
Supplementary Figure 5. Numbers of CD8+ T cells from anti-TNF injected Eμ TCL1-Tg mice.
Numbers of murine splenic CD8+ T cells, defined as B220− CD4− CD3+ CD8+, isolated from anti-TNF injected EμTCL1-Tg mice with advanced disease.
Key Points.
pDC numbers and IFNα production decrease with disease progression in CLL in a TNF-dependent manner.
TNF or TGF-β inhibition restores pDC numbers in experimental CLL, offering a new strategy to improve immuno-competency in CLL.
Acknowledgments
We thank Professor Stephen Jane for critical appraisal of the manuscript, the AMREP flow cytometry team for technical assistance, Dr. Meredith O’Keefe for technical suggestions, Dr. Pohan Lukito for control BM samples, the AMREP Animal Service staff for colony health management, and the ALLG Tissue Bank for provision of BM and PBL samples. We especially thank all patients involved in this study. SBT is supported by an NHMRC RD Wright Career Development Fellowship. This study was funded by the Association for International Cancer Research (AICR) UK, and the National Health and Medical Research Council (NHMRC) of Australia.
ABBREVIATIONS
- ALL
acute lymphocytic leukemia
- ALLG
Australasian Leukaemia and Lymphoma Group
- AMREP
Alfred Medical Research and education Precinct
- BAFF
B cell activating factor from the TNF family
- BCR
B cell receptor
- BM
bone marrow
- CLL
Chronic lymphocytic leukemia
- CMV
cytomegalovirus
- DCs
dendritic cells
- Fc
Fragment crystallizable
- Flt3
FMS-like tyrosine kinase 3 receptor
- Flt3L
FMS-like tyrosine kinase 3 ligand
- HBV
Hepatitis B virus
- HD
healthy donors
- IDO
indoleamine 2,3 dioxygenase
- Ig
immunoglobulin
- IFNAR
type I interferon receptor
- IFNα
interferon alpha
- IFN-I
type I interferon
- IGVH
immunoglobulin variable heavy chain
- Lin−
lineage negative
- mAb
monoclonal antibody
- NK
natural killer
- PBMC
peripheral blood mononuclear cells
- pDC
plasmacytoid dendritic cells
- PFS
progression-free survival
- TCL1
T cell leukemia gene 1
- Tg
transgenic
- TGF-β
transforming growth factor beta
- TLR
toll-like receptor
- TNF
tumour necrosis factor
- Treg
regulatory T cells
- WT
wild-type
Footnotes
Supplementary information is available at Leukemia’s website.
Conflict of Interest Disclosures
The authors have no conflict of interest to declare.
REFERENCES
- 1.Ternynck T, Dighiero G, Follezou J, Binet JL. Comparison of normal and CLL lymphocyte surface Ig determinants using peroxidase-labeled antibodies. I. Detection and quantitation of light chain determinants. Blood. 1974;43(6):789–95. Epub 1974/06/01. [PubMed] [Google Scholar]
- 2.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. The World Health Organization classification of hematological malignancies report of the Clinical Advisory Committee Meeting, Airlie House, Virginia, November 1997. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc. 2000;13(2):193–207. doi: 10.1038/modpathol.3880035. Epub 2000/03/04. [DOI] [PubMed] [Google Scholar]
- 3.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–56. doi: 10.1182/blood-2007-06-093906. Epub 2008/01/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrisqueta P, Pereira A, Rozman C, Aymerich M, Gine E, Moreno C, et al. Improving survival in patients with chronic lymphocytic leukemia (1980-2008): the Hospital Clinic of Barcelona experience. Blood. 2009;114(10):2044–50. doi: 10.1182/blood-2009-04-214346. Epub 2009/06/26. [DOI] [PubMed] [Google Scholar]
- 5.Orchard JA, Ibbotson RE, Davis Z, Wiestner A, Rosenwald A, Thomas PW, et al. ZAP-70 expression and prognosis in chronic lymphocytic leukaemia. Lancet. 2004;363(9403):105–11. doi: 10.1016/S0140-6736(03)15260-9. Epub 2004/01/17. [DOI] [PubMed] [Google Scholar]
- 6.Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94(6):1840–7. Epub 1999/09/09. [PubMed] [Google Scholar]
- 7.Rassenti LZ, Jain S, Keating MJ, Wierda WG, Grever MR, Byrd JC, et al. Relative value of ZAP-70, CD38, and immunoglobulin mutation status in predicting aggressive disease in chronic lymphocytic leukemia. Blood. 2008;112(5):1923–30. doi: 10.1182/blood-2007-05-092882. Epub 2008/06/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herling M, Patel KA, Weit N, Lilienthal N, Hallek M, Keating MJ, et al. High TCL1 levels are a marker of B-cell receptor pathway responsiveness and adverse outcome in chronic lymphocytic leukemia. Blood. 2009;114(21):4675–86. doi: 10.1182/blood-2009-03-208256. Epub 2009/09/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan XJ, Albesiano E, Zanesi N, Yancopoulos S, Sawyer A, Romano E, et al. B cell receptors in TCL1 transgenic mice resemble those of aggressive, treatment-resistant human chronic lymphocytic leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(31):11713–8. doi: 10.1073/pnas.0604564103. Epub 2006/07/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bichi R, Shinton SA, Martin ES, Koval A, Calin GA, Cesari R, et al. Human chronic lymphocytic leukemia modeled in mouse by targeted TCL1 expression. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(10):6955–60. doi: 10.1073/pnas.102181599. Epub 2002/05/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perkins JG, Flynn JM, Howard RS, Byrd JC. Frequency and type of serious infections in fludarabine-refractory B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma: implications for clinical trials in this patient population. Cancer. 2002;94(7):2033–9. Epub 2002/04/05. [PubMed] [Google Scholar]
- 12.Anaissie EJ, Kontoyiannis DP, O’Brien S, Kantarjian H, Robertson L, Lerner S, et al. Infections in patients with chronic lymphocytic leukemia treated with fludarabine. Annals of internal medicine. 1998;129(7):559–66. doi: 10.7326/0003-4819-129-7-199810010-00010. Epub 1998/10/03. [DOI] [PubMed] [Google Scholar]
- 13.Tsiodras S, Samonis G, Keating MJ, Kontoyiannis DP. Infection and immunity in chronic lymphocytic leukemia. Mayo Clinic proceedings Mayo Clinic. 2000;75(10):1039–54. doi: 10.4065/75.10.1039. Epub 2000/10/21. [DOI] [PubMed] [Google Scholar]
- 14.Freeman JA, Crassini KR, Best OG, Forsyth CJ, Mackinlay NJ, Han P, et al. Immunoglobulin G subclass deficiency and infection risk in 150 patients with chronic lymphocytic leukemia. Leukemia & lymphoma. 2013;54(1):99–104. doi: 10.3109/10428194.2012.706285. Epub 2012/06/29. [DOI] [PubMed] [Google Scholar]
- 15.Gorgun G, Ramsay AG, Holderried TA, Zahrieh D, Le Dieu R, Liu F, et al. E(mu)-TCL1 mice represent a model for immunotherapeutic reversal of chronic lymphocytic leukemia-induced T-cell dysfunction. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(15):6250–5. doi: 10.1073/pnas.0901166106. Epub 2009/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reizis B, Bunin A, Ghosh HS, Lewis KL, Sisirak V. Plasmacytoid dendritic cells: recent progress and open questions. Annual review of immunology. 2011;29:163–83. doi: 10.1146/annurev-immunol-031210-101345. Epub 2011/01/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akbar AN. The silent war against CMV in CLL. Blood. 2010;116(16):2869–70. doi: 10.1182/blood-2010-07-293431. Epub 2010/10/23. [DOI] [PubMed] [Google Scholar]
- 18.Yagci M, Ozkurt ZN, Yegin ZA, Aki Z, Sucak GT, Haznedar R. Hepatitus B virus reactivation in HBV-DNA negative and positive patients with hematological malignancies. Hematology. 2010;15(4):240–4. doi: 10.1179/102453309X12583347114059. Epub 2010/07/31. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Navajas JM, Lee J, David M, Raz E. Immunomodulatory functions of type I interferons. Nature reviews Immunology. 2012;12(2):125–35. doi: 10.1038/nri3133. Epub 2012/01/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunological reviews. 2010;234(1):142–62. doi: 10.1111/j.0105-2896.2009.00881.x. Epub 2010/03/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33(6):955–66. doi: 10.1016/j.immuni.2010.11.020. Epub 2010/12/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou LJ, Smith HM, Waldschmidt TJ, Schwarting R, Daley J, Tedder TF. Tissue-specific expression of the human CD19 gene in transgenic mice inhibits antigen-independent B-lymphocyte development. Molecular and cellular biology. 1994;14(6):3884–94. doi: 10.1128/mcb.14.6.3884. Epub 1994/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swiecki M, Wang Y, Vermi W, Gilfillan S, Schreiber RD, Colonna M. Type I interferon negatively controls plasmacytoid dendritic cell numbers in vivo. The Journal of experimental medicine. 2011;208(12):2367–74. doi: 10.1084/jem.20110654. Epub 2011/11/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nature reviews Immunology. 2011;11(1):34–46. doi: 10.1038/nri2901. Epub 2010/12/15. [DOI] [PubMed] [Google Scholar]
- 25.Shodell M, Siegal FP. Circulating, interferon-producing plasmacytoid dendritic cells decline during human ageing. Scandinavian journal of immunology. 2002;56(5):518–21. doi: 10.1046/j.1365-3083.2002.01148.x. Epub 2002/11/02. [DOI] [PubMed] [Google Scholar]
- 26.Munn DH, Mellor AL, Rossi M, Young JW. Dendritic cells have the option to express IDO-mediated suppression or not. Blood. 2005;105(6):2618. doi: 10.1182/blood-2005-01-0122. Epub 2005/03/05. [DOI] [PubMed] [Google Scholar]
- 27.Maraskovsky E, Pulendran B, Brasel K, Teepe M, Roux ER, Shortman K, et al. Dramatic numerical increase of functionally mature dendritic cells in FLT3 ligand-treated mice. Advances in experimental medicine and biology. 1997;417:33–40. doi: 10.1007/978-1-4757-9966-8_6. Epub 1997/01/01. [DOI] [PubMed] [Google Scholar]
- 28.Maraskovsky E, Daro E, Roux E, Teepe M, Maliszewski CR, Hoek J, et al. In vivo generation of human dendritic cell subsets by Flt3 ligand. Blood. 2000;96(3):878–84. Epub 2000/07/27. [PubMed] [Google Scholar]
- 29.Veiby OP, Jacobsen FW, Cui L, Lyman SD, Jacobsen SE. The flt3 ligand promotes the survival of primitive hemopoietic progenitor cells with myeloid as well as B lymphoid potential. Suppression of apoptosis and counteraction by TNF-alpha and TGF-beta. J Immunol. 1996;157(7):2953–60. Epub 1996/10/01. [PubMed] [Google Scholar]
- 30.Ramsfjell V, Borge OJ, Cui L, Jacobsen SE. Thrombopoietin directly and potently stimulates multilineage growth and progenitor cell expansion from primitive (CD34+ CD38-) human bone marrow progenitor cells: distinct and key interactions with the ligands for c-kit and flt3, and inhibitory effects of TGF-beta and TNF-alpha. J Immunol. 1997;158(11):5169–77. Epub 1997/06/01. [PubMed] [Google Scholar]
- 31.Schroeder JT, Chichester KL, Bieneman AP. Toll-like receptor 9 suppression in plasmacytoid dendritic cells after IgE-dependent activation is mediated by autocrine TNF-alpha. The Journal of allergy and clinical immunology. 2008;121(2):486–91. doi: 10.1016/j.jaci.2007.09.049. Epub 2007/11/27. [DOI] [PubMed] [Google Scholar]
- 32.Vincent FB, Morand EF, Murphy K, Mackay F, Mariette X, Marcelli C. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Annals of the rheumatic diseases. 2013;72(2):165–78. doi: 10.1136/annrheumdis-2012-202545. Epub 2012/11/28. [DOI] [PubMed] [Google Scholar]
- 33.Ferrajoli A, Keating MJ, Manshouri T, Giles FJ, Dey A, Estrov Z, et al. The clinical significance of tumor necrosis factor-alpha plasma level in patients having chronic lymphocytic leukemia. Blood. 2002;100(4):1215–9. Epub 2002/08/01. [PubMed] [Google Scholar]
- 34.Schiemann WP, Rotzer D, Pfeifer WM, Levi E, Rai KR, Knaus P, et al. Transforming growth factor-beta (TGF-beta)-resistant B cells from chronic lymphocytic leukemia patients contain recurrent mutations in the signal sequence of the type I TGF-beta receptor. Cancer detection and prevention. 2004;28(1):57–64. doi: 10.1016/j.cdp.2003.11.001. Epub 2004/03/26. [DOI] [PubMed] [Google Scholar]
- 35.Morrison VA. Infectious complications in patients with chronic lymphocytic leukemia: pathogenesis, spectrum of infection, and approaches to prophylaxis. Clinical lymphoma & myeloma. 2009;9(5):365–70. doi: 10.3816/CLM.2009.n.071. Epub 2009/10/28. [DOI] [PubMed] [Google Scholar]
- 36.Morrison VA, Rai KR, Peterson BL, Kolitz JE, Elias L, Appelbaum FR, et al. Impact of therapy With chlorambucil, fludarabine, or fludarabine plus chlorambucil on infections in patients with chronic lymphocytic leukemia: Intergroup Study Cancer and Leukemia Group B 9011. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2001;19(16):3611–21. doi: 10.1200/JCO.2001.19.16.3611. Epub 2001/08/16. [DOI] [PubMed] [Google Scholar]
- 37.Guven H, Gilljam M, Chambers BJ, Ljunggren HG, Christensson B, Kimby E, et al. Expansion of natural killer (NK) and natural killer-like T (NKT)-cell populations derived from patients with B-chronic lymphocytic leukemia (B-CLL): a potential source for cellular immunotherapy. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2003;17(10):1973–80. doi: 10.1038/sj.leu.2403083. Epub 2003/09/27. [DOI] [PubMed] [Google Scholar]
- 38.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nature immunology. 2004;5(12):1219–26. doi: 10.1038/ni1141. Epub 2004/11/19. [DOI] [PubMed] [Google Scholar]
- 39.Hartmann G, Battiany J, Poeck H, Wagner M, Kerkmann M, Lubenow N, et al. Rational design of new CpG oligonucleotides that combine B cell activation with high IFN-alpha induction in plasmacytoid dendritic cells. European journal of immunology. 2003;33(6):1633–41. doi: 10.1002/eji.200323813. Epub 2003/06/05. [DOI] [PubMed] [Google Scholar]
- 40.Picaud S, Bardot B, De Maeyer E, Seif I. Enhanced tumor development in mice lacking a functional type I interferon receptor. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2002;22(4):457–62. doi: 10.1089/10799900252952244. Epub 2002/05/30. [DOI] [PubMed] [Google Scholar]
- 41.Oberg K, Alm G, Magnusson A, Lundqvist G, Theodorsson E, Wide L, et al. Treatment of malignant carcinoid tumors with recombinant interferon alfa-2b: development of neutralizing interferon antibodies and possible loss of antitumor activity. Journal of the National Cancer Institute. 1989;81(7):531–5. doi: 10.1093/jnci/81.7.531. Epub 1989/04/05. [DOI] [PubMed] [Google Scholar]
- 42.Ziegler-Heitbrock HW, Schlag R, Flieger D, Thiel E. Favorable response of early stage B CLL patients to treatment with IFN-alpha 2. Blood. 1989;73(6):1426–30. Epub 1989/05/01. [PubMed] [Google Scholar]
- 43.Peggs KS, Quezada SA, Korman AJ, Allison JP. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Current opinion in immunology. 2006;18(2):206–13. doi: 10.1016/j.coi.2006.01.011. Epub 2006/02/09. [DOI] [PubMed] [Google Scholar]
- 44.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. The Journal of experimental medicine. 2009;206(8):1717–25. doi: 10.1084/jem.20082492. Epub 2009/07/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lapenta C, Santini SM, Spada M, Donati S, Urbani F, Accapezzato D, et al. IFN-alpha-conditioned dendritic cells are highly efficient in inducing cross-priming CD8(+) T cells against exogenous viral antigens. European journal of immunology. 2006;36(8):2046–60. doi: 10.1002/eji.200535579. Epub 2006/07/21. [DOI] [PubMed] [Google Scholar]
- 46.Kranzer K, Bauer M, Lipford GB, Heeg K, Wagner H, Lang R. CpG-oligodeoxynucleotides enhance T-cell receptor-triggered interferon-gamma production and up-regulation of CD69 via induction of antigen-presenting cell-derived interferon type I and interleukin-12. Immunology. 2000;99(2):170–8. doi: 10.1046/j.1365-2567.2000.00964.x. Epub 2000/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen YL, Chen TT, Pai LM, Wesoly J, Bluyssen HA, Lee CK. A type I IFN-Flt3 ligand axis augments plasmacytoid dendritic cell development from common lymphoid progenitors. The Journal of experimental medicine. 2013 doi: 10.1084/jem.20130536. Epub 2013/10/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hartmann E, Wollenberg B, Rothenfusser S, Wagner M, Wellisch D, Mack B, et al. Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer research. 2003;63(19):6478–87. Epub 2003/10/16. [PubMed] [Google Scholar]
- 49.Carpentier AF, Xie J, Mokhtari K, Delattre JY. Successful treatment of intracranial gliomas in rat by oligodeoxynucleotides containing CpG motifs. Clinical cancer research: an official journal of the American Association for Cancer Research. 2000;6(6):2469–73. Epub 2000/06/29. [PubMed] [Google Scholar]
- 50.Carpentier AF, Chen L, Maltonti F, Delattre JY. Oligodeoxynucleotides containing CpG motifs can induce rejection of a neuroblastoma in mice. Cancer research. 1999;59(21):5429–32. Epub 1999/12/20. [PubMed] [Google Scholar]
- 51.Zent CS, Smith BJ, Ballas ZK, Wooldridge JE, Link BK, Call TG, et al. Phase I clinical trial of CpG oligonucleotide 7909 (PF-03512676) in patients with previously treated chronic lymphocytic leukemia. Leukemia & lymphoma. 2012;53(2):211–7. doi: 10.3109/10428194.2011.608451. Epub 2011/08/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang X, Moseman EA, Farrar MA, Bachanova V, Weisdorf DJ, Blazar BR, et al. Toll-like receptor 9 signaling by CpG-B oligodeoxynucleotides induces an apoptotic pathway in human chronic lymphocytic leukemia B cells. Blood. 2010;115(24):5041–52. doi: 10.1182/blood-2009-03-213363. Epub 2010/03/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ito T, Amakawa R, Inaba M, Ikehara S, Inaba K, Fukuhara S. Differential regulation of human blood dendritic cell subsets by IFNs. J Immunol. 2001;166(5):2961–9. doi: 10.4049/jimmunol.166.5.2961. Epub 2001/02/24. [DOI] [PubMed] [Google Scholar]
- 54.Cordingley FT, Bianchi A, Hoffbrand AV, Reittie JE, Heslop HE, Vyakarnam A, et al. Tumour necrosis factor as an autocrine tumour growth factor for chronic B-cell malignancies. Lancet. 1988;1(8592):969–71. doi: 10.1016/s0140-6736(88)91782-5. Epub 1988/04/30. [DOI] [PubMed] [Google Scholar]
- 55.Woyach JA, Lin TS, Lucas MS, Heerema N, Moran ME, Cheney C, et al. A phase I/II study of rituximab and etanercept in patients with chronic lymphocytic leukemia and small lymphocytic lymphoma. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2009;23(5):912–8. doi: 10.1038/leu.2008.385. Epub 2009/02/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palucka AK, Blanck JP, Bennett L, Pascual V, Banchereau J. Cross-regulation of TNF and IFN-alpha in autoimmune diseases. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(9):3372–7. doi: 10.1073/pnas.0408506102. Epub 2005/02/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bonnefoy F, Couturier M, Clauzon A, Remy-Martin JP, Gaugler B, Tiberghien P, et al. TGF-beta-exposed plasmacytoid dendritic cells participate in Th17 commitment. J Immunol. 2011;186(11):6157–64. doi: 10.4049/jimmunol.1002497. Epub 2011/04/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li L, Liu S, Zhang T, Pan W, Yang X, Cao X. Splenic stromal microenvironment negatively regulates virus-activated plasmacytoid dendritic cells through TGF-beta. J Immunol. 2008;180(5):2951–6. doi: 10.4049/jimmunol.180.5.2951. Epub 2008/02/23. [DOI] [PubMed] [Google Scholar]
- 59.Seoane J. The TGFBeta pathway as a therapeutic target in cancer. Clinical & translational oncology: official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2008;10(1):14–9. doi: 10.1007/s12094-008-0148-2. Epub 2008/01/23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Analysis of pDCs from the peripheral blood of WT and EμTCL1-Tg mice.
Murine blood pDCs were gated by flow cytometry as CD11b− Gr.1− B220+ CD11c+ SiglecH+ PDCA-1+ as in Figure 1A. Dot plots show proportion of blood pDCs in 12-mo WT and EμTCL1-Tg mice (n=7 per group).
Supplementary Figure 2. Analysis of human pDCs from normal BM.
BM from a patient with non-diffuse lymphoma and no BM infiltrate was used as a normal BM control. The representative dot plot shows pDCs were gated by flow cytometry as HLA-DR+ CD123+ BDCA2+ and BDCA4+ and negative for lineage markers CD3, CD14, CD16, CD19, CD56, CD11c and CD11b. Percentages of pDCs are shown.
Supplementary Figure 3. Aberrant gene expression in TNF-stimulated pDCs from EμTCL1-Tg mice and CLL patients.
irf1, irf7, irf8, Bcl11a and E2-2 mRNA levels were measured in TNF-treated pDCs from (A) EμTCL1-Tg mice and (B) CLL patients. Data are expressed as fold difference and are representative of at least two independent experiments.
Supplementary Figure 4. Downregulation of TLR9 and Flt3 expression on healthy pDCs in the presence of CLL B cells.
(A) Expression of intracellular TLR9 and (B) surface Flt3 on purified pDCs from of HD was measured by flow cytometry following 12-h transwell co-culture with CLL B cells placed in the upper chamber and pDCs in the bottom chamber (n=4 per group).
Supplementary Figure 5. Numbers of CD8+ T cells from anti-TNF injected Eμ TCL1-Tg mice.
Numbers of murine splenic CD8+ T cells, defined as B220− CD4− CD3+ CD8+, isolated from anti-TNF injected EμTCL1-Tg mice with advanced disease.