Abstract
Rat skeletal muscle myosin contains small subunits (light chains, here designated LC1, LC2, LC3) of molecular weight 23,000, 17,000 and 15,000, respectively. The synthesis of myosin light chains during differentiation and the accumulation of mRNA which codes for these proteins were investigated in differentiating rat skeletal muscle cultures. When cultures were labeled prior to cell fusion, radioactive light chains which co-migrated with skeletal muscle myosin light chains on gel electrophoresis were absent or only barely detectable. The low molecular weight peptides which were associated with the heavy chain of myosin extracted from pre-fusion cultures differed in their electrophoretic mobility from light chains of skeletal muscle myosin. Following cell fusion, the amount of labeled LC1 and LC2 increased rapidly. The synthesis of LC3 was barely detectable during cell fusion and never exceeded one-fifth of the amounts of LC1 and LC2 synthesized.
Polyadenylated RNA extracted at different times during differentiation was translated in the wheat germ cell-free system. The products were analyzed on isoelectric focusing-SDS two-dimensional gel electrophoresis, and the radioactivity of the polypeptides co-migrating with myosin light chains was measured. Small amounts of radioactive products co-migrating with LC1 and LC2 became detectable among products of RNA preparations extracted several hours prior to cell fusion. However, the cell-free system directed by post-fusion RNA synthesized much larger amounts of LC1- and LC2-like polypeptides. Rapid accumulation of translatable mRNA for LC1 and LC2 was closely correlated with cell fusion. Radioactive polypeptides co-migrating with LC3 were synthesized in significant amounts in a cell-free system directed by pre-fusion RNA and increased only moderately when RNA extracted after fusion was used.
Introduction
During the differentiation of skeletal muscle cells, multinucleated fibers are formed by the fusion of mononucleated cells. This is associated with distinctive changes in the pattern of protein synthesis such as great increase in myosin synthesis, increases in activity of several enzymes, and transition in isoenzymes (e.g. [1–5]). The myosin molecule is composed of two subunits of molecular weight 200,000 and several small polypeptides ranging in molecular weight from 15,000 to 25,000 daltons [6]. The small subunits show high tissue specificity. Distinctive differences in the size of light chains have been reported for myosin extracted from various kinds of muscle from the same organism [7–9]. Rat skeletal (thigh) muscle myosin contains light chains with molecular weights of 23,000, 17,000 and 15,000 (LC1, LC2 and LC3, respectively). We have shown that polyadenylated RNA extracted from differentiated rat skeletal muscle cell cultures directs in the wheat germ cell-free system (CFS) the synthesis of polypeptides co-migrating with light chains of rat skeletal muscle myosin in isoelectric focusing and in SDS gels. The polypeptides synthesized in the CFS also become specifically associated with myosin heavy chain during a treatment which causes the dissociation and reassociation of myosin subunits [10, 11].
In the present study, we investigated the relation between muscle cell fusion, the synthesis of myosin light chains and the appearance in the cells of mRNA coding for these proteins. The experiments showed a temporal correlation between cell fusion, rapid accumulation of translatable mRNA which directs the synthesis of polypeptides with the properties of skeletal muscle myosin light chains 1 and 2, and the synthesis of these light chains in the intact cells1.
Methods
Materials
Fresh commercial wheat germ was supplied by the “Bar-Rav” mill, Tel Aviv, Israel and was stored under reduced pressure at 4°C. [35S]methionine (5.6 mCi/ml, 360 Ci/mmole) was obtained from the Radiochemical Centre, Amersham, U.K. Oligo(dT)-cellulose was from Collaborative Research, Waltham, Mass. NP-40 was a gift from Paz Oil Co., Israel. Rats used were of the Wistar non-inbred strain.
Cell Cultures
Primary cultures of rat skeletal muscle cells were prepared and grown as described previously [13]. Cultures were grown first in fetal calf serum and embryo extract-enriched medium (FE medium) which promotes cell proliferation without cell fusion. About 40 h after plating, the medium was changed to the fusion-permissive S medium. A phase of very intensive cell fusion started 18 h after the change to S medium [14].
Fibroblasts were isolated from well-fused muscle cultures and passaged in growth medium used for primary muscle cultures. Tertiary cultures were used for myosin extraction.
All cultures were grown in 100-mm Falcon tissue culture plates coated with 0.1% gelatin.
Measurement of Cell Fusion
Cultures were rinsed three times with phosphate buffered saline solution, fixed in absolute methanol and stained with Giemsa stain. The number of nuclei within fibers was counted in 10 randomly selected fields. The values given in the Results are the average per microscope field (×400).
Isolation of Unlabeled Myosin
Myosin was purified from the hind legs of adult rats, from primary muscle cultures, and from fibroblast cultures, according to Naus, Kitagawa and Gergely [15], except that the extraction buffer was 0.5 M KCl, 10 mM Tris pH 7.4, 1 mM EDTA, from the beginning, and the highspeed centrifugation was performed in the presence of 10 mM ATP. This was followed by ammonium sulfate fractionation as described in [15].
Isolation of Labeled Myosin from Cultures
When myosin from cultures is purified by ammonium sulfate fractionation as described above, a considerable amount of myosin is lost. This method is thus not suitable for quantitative measurements. In addition, in the method described above, the nuclei are broken and the partially purified myosin preparation (before the step of high speed centrifugation) contains a large amount of actin, as well as low molecular weight proteins apparently originating in the nuclei. This makes it impossible to load on the gels amounts of myosin large enough to enable accurate determination of the changes in light chain synthesis during the early stages of differentiation. Also, the background of proteins in the myosin light chain area was high. Therefore, the following procedure was adopted. Cultures were labeled for 1 h in 5 ml of complete medium containing 30 μCi of [34S]methionine per ml (Amersham; specific activity 400 Ci/mmole). At the end of the labeling period, myosin was extracted in the cold as described in Table 1.
Table 1.
Purification of labeled myosin extracted from cultures


|
NaCl was used instead of KCl to make it possible to directly analyze the total extract on SDS gels (without obtaining a precipitate of KDS)
The content of myosin heavy chain in the discarded fraction was negligible. No myosin was detected in the supernatant after the salt concentration was lowered to precipitate myosin. Actin appeared in large amounts in all the discarded fractions
The peptide comigrates with myosin heavy chain on SDS polyacrylamide gel
Preparation of Messenger RNA
Total cytoplasmic polyadenylated RNA was isolated from 11 to 20 cultures by a modification of the method described by Singer and Penman [16]. TNM buffer (0.01 M Tris, pH 7.4, 0.25 M NaCl, 0.01 M MgCl2) was used to rinse the cultures and TNM buffer containing 1% NP-40 was used to lyse the cells.
Translation of RNA in Cell-Free System Prepared from Wheat Germ
Preincubated extracts were prepared and protein synthesis assays were carried out as described by Roberts and Paterson [17] and Paterson et al. [18], except that no tRNA was added and KCl in the reaction mixture was replaced by K acetate. 10 to 15 μCi [35S] methionine were added to 50 μl reaction mixture. Polyadenylated RNA was added to final concentrations as indicated. The reaction mixture was incubated at 22° C for 90 min.
SDS-polyacrylamide Gel Electrophoresis
Protein extracts and cell-free products were analyzed on SDS-polyacrylamide gels, as described by Laemmli [19], using a 10 to 20% polyacrylamide gradient slab gel (0.65 × 140 × 80 mm) prepared according to Maizel [20].
Two-Dimensional Gel Electrophoresis
Isoelectric focusing in the first dimension, followed by SDS electrophoresis in the second dimension, were performed according to O’Farrell [21], except that the ampholines used were of pH ranging between 3.5 and 10 and the second dimension gel was built from a gradient of 10 to 20% polyacrylamide.
Measurements of Radioactivity Incorporated into Myosin and into Specific CFS Products
Gels were stained and fixed with 50% methanol, 7.5% acetic acid and 0.1% Coomassie Brilliant Blue and destained with 5% methanol, 7.5% acetic acid. Gels were dried and exposed to an X-ray film (Kodak RP-54X-Omat). After radioautography, specific bands were cut out from the dried gels, swollen in a mixture of 90% NCS in water at 50° C for 3 h and their radioactivity was measured in toluene scintillant [22].
Determination of Molecular Weight of Myosin Light Chains
This was done on SDS-polyacrylamide gels prepared as described by Laemmli [19], using a 12.5% polyacrylamide slab gel. Molecular weights were determined with the help of a calibration curve obtained by plotting the electrophoretic mobilities of several proteins against the logarithm of the molecular weights of their polypeptide chains [23].
Results
Synthesis of Myosin Subunits in Cell Culture
Myosin extracted from differentiated primary skeletal muscle cell cultures contains light chains which co-migrate with rat thigh muscle myosin LC1 and LC2 (mw 23,000 and 17,000, respectively). LC3 (mw 15,000) is absent or barely detectable. Myosin extracted from fibroblast cultures contains three major low molecular weight proteins, none of which co-migrates on SDS gels with light chains of skeletal muscle myosin (Fig. 1).
Fig. 1.
Subunits of rat myosin. Unlabeled myosin was extracted from (a) muscle fibroblast culture; (b) adult rat thigh muscle; (c) highly differentiated primary muscle culture (multinucleated fibers), and analyzed on SDS-polyacrylamide gradient slab gels stained with Coomassie Brilliant Blue. MHC: myosin heavy chain; A: actin; LC1, LC2, LC3: light chains of molecular weight 23,000, 17,000 and 15,000, respectively
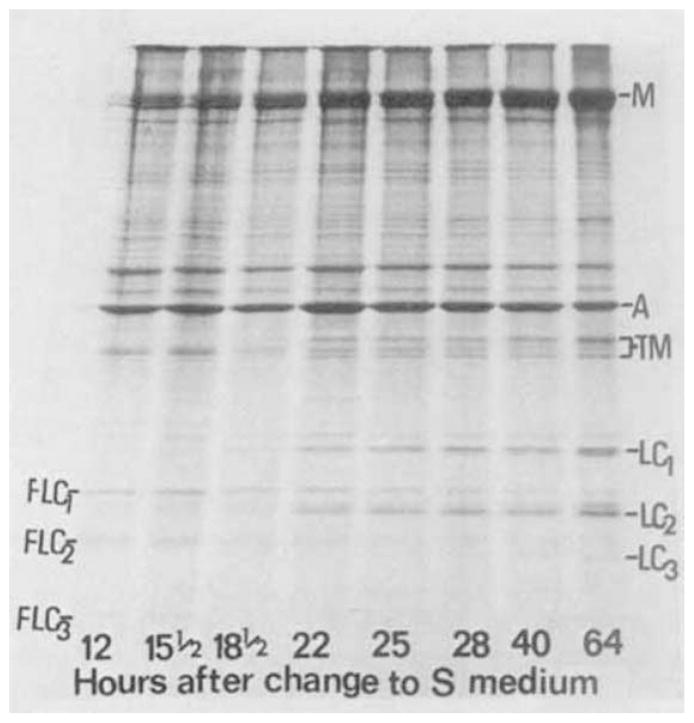
As described in Methods, primary rat muscle cultures grown at the appropriate cell density in FE medium proliferate without cell fusion. Changing the medium to the fusion-permissive S medium results in the onset of a phase of rapid cell fusion after about 18 h. In order to study the changes in synthesis of light chains in relation to the differentiation of such cultures, cultures were exposed for 1 h to [35S]methionine at different times following the change from FE to S medium. After labeling, the cells were harvested and myosin was extracted as described in Table 1. SDS gel analysis of myosin and associated proteins after step A of purification (designated Total Myosin) are shown in Figure 2. For all time points, the same amount of protein was applied on the gels. However, it can be seen that radioactive bands co-migrating with LC1 and LC2 are distinguishable only in samples extracted after the onset of fusion (18 h in S medium). The changes in the synthesis of LC1 and LC2, relative to the synthesis of myosin heavy chain, are given in Table 2.
Fig. 2.
Radioautograph of total myosin extracted from primary muscle culture at various times during differentiation, after step A of purification. Cultures grown in FE medium were changed to S medium. Cultures were labeled at various time points with [35S]methionine for 1 h. Myosin was extracted at the end of the labeling period, as described in Table 1, Step A, and analyzed in SDS-polyacrylamide gels. The numbers below the gels indicate the time in S medium when the 1-h labeling period ended. The phase of rapid cell fusion started 18 h after the change from FE to S medium (see Fig. 4). The number of cultures used to extract myosin at 12, 15½, 18½, 22, 25, 28, 40 and 64 h was 6, 8, 5, 5, 4, 3, 2 and 2, respectively. The samples put on the gels were the equivalents of 0.43, 0.16, 0.18, 0.16, 0.16, 0.12, 0.08, 0.02 cultures, respectively. Amounts of radioactivity put on the gels were 54,980; 58,690; 42,360; 51,485; 51,610; 41,815; 40,995; 41,310 cpm, respectively. The gels were exposed to X-ray film for 3 days. The migrations of unlabeled markers are indicated alongside the radioautograph: M: Myosin; A: actin; TM: tropomyosin; LC1, LC2, LC3: light chains of rat skeletal muscle myosin; FLC1, FLC2, FLC3: low molecular weight polypeptides associated with myosin extracted from fibroblasts
Table 2.
Change in ratio of radioactive myosin heavy chain to light chain, during differentiation of primary muscle cultures.
Myosin extracted at various times following the change from FE medium to S medium was subjected after step A of purification (Table 1) to SDS-polyacrylamide gel electrophoresis and the radioactivity of the bands co-migrating with myosin heavy and light chains was measured as described in Methods
| Time in S medium | Ratio of myosin light to heavy chain (%)
|
||
|---|---|---|---|
| LC1 | LC2 | ||
| 12 | 0.54a | 0.59a | |
|
|
0.31a | 0.55a | |
|
|
1.20 | 1.89 | |
| 22 | 2.45 | 3.01 | |
| 25 | 4.61 | 4.4 | |
| 28 | 7.50 | 7.84 | |
| 40 | 7.38 | 6.80 | |
| 64 | 7.45 | 6.94 | |
Not accurate due to low radioactivity
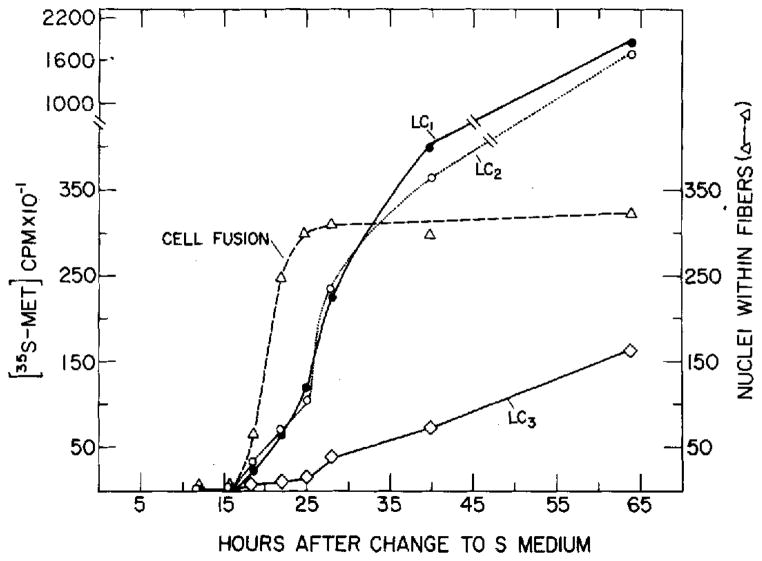
As can be seen from Figure 2, after step A of purification a considerable amount of other proteins is still left in the myosin preparation. Some of these proteins migrate to the light chain region. Therefore, in order to obtain myosin as free as possible from other proteins, step B of purification was performed. SDS analysis of myosin after this step (Myosin I, Table 1) is shown in Figure 3. This purification resulted in the efficient removal of most extraneous polypeptides. LC1 appeared on SDS gel as a split band and remained split after step B of purification. The 2 bands migrated to the same position in isoelectric focusing (data not shown). It has been reported that myosin extracted from rabbit muscle shows a split band on SDS gel in both LC1 and LC2 positions [7, 8, 24]. The split band was taken as one unit for measurement of synthesis of LC1. The changes in radioactivity of the peptides co-migrating with the muscle myosin light chain markers, calculated per culture, are shown in Figure 4. A very similar curve was obtained when the radioactivity of the bands co-migrating with LC1 and LC2 after step A of purification (Fig. 2) was measured (LC3 could not be accurately measured from these gels, due to the high background). It can be seen that prior to cell fusion, radioactive polypeptides co-migrating with skeletal muscle light chains are barely detectable. After cell fusion, the amount of labeled LC1 and LC2 increases rapidly. The two curves describing the rate of incorporation of [35S]methionine into the two peptides are very similar. The rate of incorporation of amino acids into LC3 during the phase of rapid cell fusion is only slightly above background. A moderate increase occurs much later and never exceeds 1/10–⅕ of LC1 and LC2. A similar difference in incorporation is observed also when [14C]leucine is used instead of labeled methionine. LC3 is not present or barely detectable when the gels are stained and the amount of accumulated protein is measured by optical density (Fig. 1). These results indicate that the difference in radioactivity between LC3 and the other light chains is not due to a difference in amino acid composition.
Fig. 3.

Radioautograph of myosin extracted from primary muscle culture during differentiation, after step B of purification. Cultures were labeled with [35S]methionine for 1 h. Myosin was extracted as described in Table 1 and analyzed on SDS-polyacrylamide gels. The numbers below the gels indicate the time in S medium (hours) when the 1-h labeling ended. The number of cultures used to extract myosin at each time point is listed in Figure 2. The samples loaded on the gels at 12, 15½, 18½, 22, 25, 28, 40 and 64 h were the equivalents of 1.8, 1.47, 1.39, 1.49, 1.59, 0.44, 0.31 and 0.08 cultures, respectively. Counts per min on gels at each time point were 4,740; 7,810; 10,160; 14,375; 32,410; 17,240; 17,860; and 26,721, respectively. The gel was exposed to the X-ray film for 3 days
Fig. 4.
Synthesis of myosin light chains during differentiation of primary muscle culture. The gel used for the radioautograph shown in Figure 3 was used to measure the radioactivity (per culture) of the bands comigrating with LC1, LC2 and LC3, as described in Methods. Measurement of radioactivity incorporated into myosin light chains was performed by solubilizing the protein bands cut out of the dried gels after radioautography. Starting 12 h after the change from FE to S medium and up until the end of this experiment, the amount of DNA did not significantly increase. Thus, a very similar curve was obtained when the results were calculated relative to the amount of DNA. The number of nuclei within fibers was determined as described in Methods
As can be seen from Figure 2, small amounts of a protein with mobility of myosin heavy chain are synthesized prior to cell fusion. However, a great part of it does not precipitate in the presence of ATP and remains in the low ionic strength supernatant after step B of purification (Myosin II, Table 1). This protein was precipitated with NH4SO4 (90% saturation) and run on an SDS gel. As can be seen from Figure 5, the low molecular weight peptides associated with this fraction differ from the light chains of skeletal muscle myosin. They co-migrate with the low molecular weight protein associated with myosin extracted from fibroblasts2.
Fig. 5.

Radioautography of “Myosin II” extracted from primary skeletal muscle culture. Cultures grown for 14.5 h in S medium were labeled with [35S]methionine for 1 h and “Myosin II” was isolated as described in Table 1, Step B, and analyzed on SDS gels. The amount of radioactivity put on gel was 27,864 cpm.
Radioautography was made for 3 days. (No peptides comigrating with LC1, LC2 and LC3 were found after 17 days of exposure.)
Differences in sizes of light chains between myosin extracted from chick skeletal muscle cell cultures prior to cell fusion and differentiated cultures have been reported by Chi et al. [25, 26].
Accumulation of Translatable mRNA for Light Chains during Differentiation
In order to investigate the relation between differentiation and appearance of mRNA coding for light chains, cytoplasmic polyadenylated mRNA was extracted from cultures taken at various times following the change from FE to S medium and its ability to direct protein synthesis in the wheat germ cell-free system was tested. Since inactive mRNA may not be associated with polysomes, total cytoplasmic polyadenylated RNA was used for translation in the CFS. At concentrations below 1.3 μg RNA/50 μl of reaction mix, preparations of RNA extracted at various times during differentiation showed the same linear relation between amount of RNA added to CFS and stimulation of incorporation of [35S]methionine into polypeptides. The concentration of polyadenylated RNA in the CFS did not affect the pattern of radioactive bands produced on SDS gels. In all experiments reported below, RNA added to the CFS was in rate-limiting concentrations.
Earlier analysis of CFS products on SDS and urea gels showed that polypeptides co-migrating with LC1 and LC2 become detectable as early as 5 h after the change from FE to S medium [10]. However, products of CFS directed by RNA extracted from primary cultures produce on one-dimensional SDS gels a high background of radioactive bands in the LC1 and LC2 region. Therefore, isoelectric focusing-SDS two-dimensional gels were used in the present study in order to quantitate the changes in radioactivity of light chains. On these gels, the peptides co-migrating with the three light chains are relatively clean of background and can be easily isolated for quantitative measurement of radioactivity. Products of CFS directed by RNA extracted at various times after the transition from FE medium to S medium were subjected to gel electrophoresis, and the gels were radioautographed (Fig. 6). The radioactivity of peptides co-migrating with LC1, LC2 and LC3 markers was measured by cutting out the spots of the markers and counting the radioactivity. The results calculated as activity per microgram of RNA in the CFS and per plate are shown in Figures 7a and 7b.
Fig. 6a–c.
Radioautograph of [35S]methionine-labeled cell-free products analyzed by two-dimensional gel electrophoresis. CFS products directed by RNA extracted from primary muscle cultures 12, 19 and 29 h after the change to S medium were separated by isoelectric focusing in the first dimension and according to molecular weight by SDS electrophoresis in the second dimension. A, 1, 2, 3 denote the place where unlabeled actin and the three light chain markers migrated (the tops of the letters). Of the 2 spots on top of the figure 1, only the lower migrates with LC1. The position of tropomyosin is indicated by two dashes (TM). In addition to the changes in radioactivity of myosin light chain, several other radioactive spots appear during cell fusion (e.g., a cluster of 3 spots to the right of LC3). Amount of radioactivity put on the gels at 12, 19 and 29 h: 248,315, 257,235 and 260,880 cpm, respectively. Radioautography lasted 4 days
Three radioactive spots which co-migrated with LC1, LC2 and LC3 became detectable at least 12 h following the change from FE to S medium. However, while the radioactive peptide which co-migrates with LC3 was quite prominent at all time points, the intensity of spots migrating with LC1 and LC2 in CFS products directed by RNA extracted prior to cell fusion was relatively low. There was a very large increase coinciding with the phase of rapid cell fusion. The radioactivity of the LC2 peptide produced in CFS directed by RNA extracted at the end of the phase of rapid cell fusion (29 h) is 20 times as great as radioactivity of LC2 produced in CFS directed by RNA extracted just prior to the onset of cell fusion (16 h). It can also be seen from Figure 6 that the synthesis in the CFS of several other polypeptides increases very markedly during cell fusion. Two of them were identified as α and β tropomyosin subunits (Carmon and Yaffe, to be published).
Investigations with sea urchin eggs have shown that following fertilization there is polyadenylation of preexisting RNA [29, 30], In order to test the possibility that nonpolyadenylated mRNA coding for myosin light chains is present prior to cell fusion and is lost during the purification of the RNA on oligo(dT)cellulose, we also analyzed in two-dimensional gels the products of CFS directed by total cytoplasmic RNA extracts prior to purification on oligo(dT)cellulose. 10 μg RNA per 50 μl reaction mixture (rate-limiting concentration) gave ca. 15-fold stimulation above background, of incorporation of amino acids into acid-insoluble products. The results with regard to the time of appearance of mRNA coding for polypeptides co-migrating with light chains were similar to those obtained with oligo(dT)-bound RNA.
Discussion
During cell fusion the synthesis of myosin increases severalfold [28, 4]. Small amounts of a protein which co-migrates in SDS gels with myosin heavy chain are synthesized in the cells prior to cell fusion. However, the analyses on SDS gels showed that the low molecular weight polypeptides associated with this protein differ from skeletal muscle light chains. Significant amounts of light chains with the properties of skeletal muscle myosin LC1 and LC2 become associated with myosin only after cell fusion. The curve describing the increase in amount of labeled LC1 and LC2 in the myosin preparation indicates that the synthesis of these polypeptides follows cell fusion. The amount of LC1 and LC2 which was found prior to cell fusion was so small that it could well have been synthesized by the small numbers of multinucleated cells present in the cultures prior to the main phase of cell fusion. Similar results were obtained also when whole cytoplasmic fractions (not subjected to extraction of myosin) were analyzed on two-dimensional gels and radioactivity of polypeptides co-migrating with light chains was measured (data not shown). The synthesis of a peptide which co-migrates with LC3 is barely detectable during the phase of rapid cell fusion and increases only many hours later. Embryonic muscle myosin of chick [31, 32], rabbit [24] and rat [Yablonka and Yaffe, unpublished] contains predominantly the two heavier light chains, whereas the third light chain appears later during development. In this respect, the differentiation of fibers in muscle cell cultures resembles that in embryonic muscle. Experiments with chick cultures suggested that the fast-migrating light chain (LC3) is produced by the fibroblasts present in primary muscle cultures and not in the myotubes [26]. However, the experiments with rat skeletal muscle cells do not support such a conclusion: none of the detectable polypeptides associated with myosin extracted from pre-fusion cultures or fibroblasts co-migrated with LC3 (Figs. 1, 2, 5).
The discrepancy between the low rate of synthesis of LC3 in the intact cells and the comparatively large synthesis of polypeptides co-migrating with LC3 in CFS has been discussed elsewhere [11].
Several proteins, formerly considered homogeneous, were found to exist in more than one isozymic form (e.g., actin; [33, 34, 35]). It is impossible therefore to exclude that different separation methods or amino acid sequencing might reveal that LC1 and/or LC2 contain more than one species of light chain. However, the kinetics of their synthesis during differentiation permits ignoring this consideration in the present study. The finding that radioactive LC1 and LC2 which are found associated with myosin heavy chain after cell fusion differ from the polypeptides found prior to cell fusion, and the possibility of synthesizing light chains in a cell-free system makes these polypeptides useful markers with which to follow the mode of control of the time of appearance of specific proteins during differentiation. It was shown earlier that application of actinomycin D to rat muscle cultures approaching the phase of cell fusion does not prevent the formation of multinucleated fibers and the associated changes in enzymatic activity for several hours [2, 28]. Furthermore, examination of the changes in isozymic pattern of creatine kinase in actinomycin D-treated cultures has shown that like in untreated cultures, prior to cell fusion, the active creatine kinase is predominantiy of the brain isozyme; the big increase during cell fusion is mainly of the muscle type [10; Dym et al., to be published]. These results suggested the presence of mRNA for the muscle-specific proteins prior to cell fusion. The suggestion that post-transcriptional control plays a role in the regulation of protein synthesis in these cells gained further support from the observation that in bovine muscle cultures a 26S polyadenylated RNA is synthesized prior to cell fusion. This RNA, which is assumed to be the mRNA for myosin heavy chain, is found prior to cell fusion in a 100S particle and becomes associated with polysomes during cell fusion, when myosin synthesis increases [36]. The existence of factors which regulate the synthesis of specific muscle proteins has also been indicated [37].
It was, therefore, assumed in the present study that if control of the onset of synthesis of muscle-specific proteins were made on the translational level, then the pre-existence of mRNA for these proteins would be detectable by translation of purified RNA extracts in a suitable cell-free system. Small amounts of radioactive polypeptides co-migrating with LC1 and LC2 were detectable on the two-dimensional gels among the products of CFS directed by RNA extracted before the stage of fusion. However, during the phase of rapid cell fusion there was a very large increase in the capacity of the extracted RNA to direct the synthesis of LC1 and LC2. Assuming that the changes in the relative amount of a radioactive polypeptide produced by the different RNA preparations under the same CFS conditions reflect the changes in the relative availability of the corresponding mRNA in a translatable form, the present study shows a very close temporal correlation between cell fusion and the rapid accumulation of translatable mRNA for LC1- and LC2-like polypeptides. These results thus suggest that mRNA coding for myosin light chains appears in the cells following cell fusion or very shortly prior to fusion. Comparison of the curves describing the changes in translation of LC1 and LC2 in CFS and synthesis in the intact cells shows that while the capacity to direct the synthesis in CFS builds up rapidly and almost coincides with the phase of rapid cell fusion, the rate of synthesis of LC1 and LC2 in the cells appears to increase more slowly. This may suggest a lag in the order of 3 to 4 h between appearance of mRNA and its utilization. It is, however, possible that the observed lag is due to differences in the sensitivity of the methods of assaying the synthesis of light chains in the cultures and in the CFS.
It should be noted, however, that while the results of the present study do not provide evidence for the suggestion that mRNAs involved in terminal differentiation are transcribed during the period preceding cell fusion, they do not exclude such a possibility, for the following reasons:
The CFS assays only translatable mRNA. Thus, the CFS would detect inactive mRNA if control of activation of mRNA were based on factors other than changes in the structure of the mRNA (initiation factors, etc.; [37, 38]). However, transcribed mRNA not fully processed [39–41, 29, 30] might not be translatable in this CFS and would remain undetected.
The CFS assays the changes in the relative amounts of mRNA, whereas by pulse-labeling of RNA the rate of RNA synthesis is measured. Thus if mRNAs for muscle-specific proteins are also produced prior to cell fusion but the rate of degradation of these mRNAs decreases during cell fusion [42, 36], one would expect to observe their accumulation following cell fusion.
More sensitive methods are needed to resolve these questions.
Fig. 7a, b.

Changes in synthesis of light chains in CFS directed by RNA extracted at various times during differentiation of primary muscle cultures. Polyadenylated mRNA was extracted from primary muscle cultures at various times after the change to S medium and translated in the wheat germ cell-free system at a concentration of 1 μg RNA per 50 μl of reaction mix. After incubation, 15 μl of the reaction mix were analyzed by two-dimensional gel electrophoresis, as described in Figure 6. After radioautography, the light chain marker spots were cut out of the gel and their radioactivity was measured. The background radioactivity was measured in neighboring pieces of gel (which did not produce visible spots on the X-ray film) and was substracted. The corrected values (ordinate) are given in cpm per 1 μg RNA (50 μl reaction mix) in a. These values were normalized for the amount of polyadenylated mRNA extracted from each time group and are presented in cpm per culture in b. The amount of polyadenylated RNA extracted per culture at time points 0, 12, 16, 19, 29, 43 were 2.65, 3.0 3.37, 3.0 4.27 and 4.00 μg per plate, respectively
Acknowledgments
The skillful technical assistance of Ms. R. Meller and Z. Levi is gratefully acknowledged. Thanks are due to Mrs. M. Baer for helping to prepare the manuscript. This work was supported by the Muscular Dystrophy Association, Inc., USA, by a grant from the NIH (#R01 GM 22767) and by the US-Israel Binational Science Foundation, Jerusalem, Israel.
Footnotes
Part of this study was presented at the Fifth International Conference on Muscular Dytrophy in Durango, Colorado (USA) in 1976 [12]
References
- 1.Reporter MC, Königsberg IR, Strehler BL. Kinetics of accumulation of creatine phosphokinase activity in developing embryonic skeletal muscle in vivo and in monolayer culture. Exp Cell Res. 1963;30:410. doi: 10.1016/0014-4827(63)90313-6. [DOI] [PubMed] [Google Scholar]
- 2.Shainberg A, Yagil G, Yaffe D. Alterations of enzymatic activities during muscle differentiation in vitro. Dev Biol. 1971;25:1. doi: 10.1016/0012-1606(71)90017-0. [DOI] [PubMed] [Google Scholar]
- 3.Coleman JR, Coleman AW. Muscle differentiation and macromolecular synthesis. J Cell Physiol. 1968;72(Suppl 1):19. doi: 10.1002/jcp.1040720404. [DOI] [PubMed] [Google Scholar]
- 4.Paterson BM, Strohman RC. Myosin synthesis in cultures of differentiating chicken embryo skeletal muscle. Dev Biol. 1972;29:113. doi: 10.1016/0012-1606(72)90050-4. [DOI] [PubMed] [Google Scholar]
- 5.Turner DC, Maier V, Eppenberger HM. Creatine kinase and aldolase isoenzyme transitions in cultures of chick skeletal muscle cells. Dev Biol. 1974;37:63. doi: 10.1016/0012-1606(74)90170-5. [DOI] [PubMed] [Google Scholar]
- 6.Tonomura Y. Muscle Protein, Muscle Contraction and Cation Transport. University of Tokyo Press; 1972. The structure of the myosin molecule; p. 27. [Google Scholar]
- 7.Lowey S, Risby D. Light chains from fast and slow muscle myosin. Nature. 1971;234:81. doi: 10.1038/234081a0. [DOI] [PubMed] [Google Scholar]
- 8.Sarkar S, Sréter FA, Gergely J. Light chains of myosins from white, red, and cardiac muscles. Proc Natl Acad Sci (US) 1971;68:946. doi: 10.1073/pnas.68.5.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weeds AG, Trentham PR, Kean CJC, Baller AJ. Myosin from cross reinnervated cat muscle. Nature. 1974;247:135. doi: 10.1038/247135a0. [DOI] [PubMed] [Google Scholar]
- 10.Yaffe D, Yablonka Z, Kessler G, Dym H. mRNA and protein synthesis in differentiating muscle cultures. Proc. Xth FEBS Meeting; Paris. 1975. [Google Scholar]
- 11.Yablonka Z, Yaffe D. Synthesis of polypeptides with the properties of myosin light chains, directed by RNA extracted from muscle cultures. Proc Nat Acad Sci (US) 1976;73:4599. doi: 10.1073/pnas.73.12.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaffe D, Yablonka Z, Kessler G. Studies on the synthesis of myosin light chain. Proc. Muscular Dystrophy Association International Scientific Conference; Durango, Colorado. June 1976. (in press) [Google Scholar]
- 13.Yaffe D. Rat skeletal muscle cells. In: Kruse PF Jr, Patterson MK Jr, editors. Tissue Culture, Methods and Applications. New York: Academic Press; 1973. p. 106. [Google Scholar]
- 14.Yaffe D. Developmental changes preceding cell fusion during muscle differentiation in vitro. Exp Cell Res. 1971;66:33. doi: 10.1016/s0014-4827(71)80008-3. [DOI] [PubMed] [Google Scholar]
- 15.Naus KM, Kitagawa S, Gergely J. Pyrophosphate binding to the adenosine triphosphatase activity of myosin and its proteolytic fragments. J Biol Chem. 1969;244:755. [PubMed] [Google Scholar]
- 16.Singer R, Penman S. mRNA in HeLa cells: Kinetics of formation and decay. J Mol Biol. 1973;78:321. doi: 10.1016/0022-2836(73)90119-8. [DOI] [PubMed] [Google Scholar]
- 17.Roberts BE, Paterson BM. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in cell-free system from commercial wheat germ. Proc Nat Acad Sci (US) 1973;70:2330. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paterson BM, Roberts BW, Yaffe D. Determination of actin messenger RNA in cultures of differentiating embryonic chick skeletal muscle. Proc Nat Acad Sci (US) 1974;71:4467. doi: 10.1073/pnas.71.11.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laemmli VK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Maizel JV., Jr . Polyacrylamide gel electrophoresis of viral proteins. In: Maramarosch K, Koprowski H, editors. Methods in Virology. V. New York: Academic Press; 1971. p. 176. [Google Scholar]
- 21.O’Farrell PH. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007. [PMC free article] [PubMed] [Google Scholar]
- 22.Basch RS. An improved method for counting tritium and carbon-14 in acrylamide gels. Anal Biochem. 1968;26:184. doi: 10.1016/0003-2697(68)90044-4. [DOI] [PubMed] [Google Scholar]
- 23.Weber K, Osborn M. The reliability of molecular weight determination by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969;244:4406. [PubMed] [Google Scholar]
- 24.Sréter F, Balint M, Gergely J. Structural and functional changes of myosin during development. Comparison with adult fast, slow and cardiac myosin. Dev Biol. 1975;46:317. doi: 10.1016/0012-1606(75)90108-6. [DOI] [PubMed] [Google Scholar]
- 25.Chi JC, Fellini SA, Holtzer H. Differences among myosins synthesized in nonmyogenic cells, presumptive myoblasts, and myoblasts. Proc Nat Acad Sci (US) 1975;72:4999. doi: 10.1073/pnas.72.12.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chi JCH, Rubinstein N, Strahs K, Holtzer H. Synthesis of myosin heavy and light chains in muscle cultures. J Cell Biol. 1975;67:523. doi: 10.1083/jcb.67.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baril EF, Love DS, Hermann H. Investigation of myosin heterogeneity observed during chromatography on diethylaminoethyl cellulose. J Biol Chem. 1966;241:822. [PubMed] [Google Scholar]
- 28.Yaffe D, Dym H. Gene expression during differentiation of contractile muscle fibers. Cold Spr Harb Symp Quant Biol. 1972;37:543. [Google Scholar]
- 29.Slater PW, Slater I, Gillespie DH, Gillespie S. Postfertilization polyadenylation during transcriptive and translational inhibition. Biochem Biophys Res Commun. 1974;60:1222. doi: 10.1016/0006-291x(74)90329-5. [DOI] [PubMed] [Google Scholar]
- 30.Wilt FH. Polyadenylation of maternal RNA of sea urchin eggs after fertilization. Proc Nat Acad Sci (US) 1973;70:2345. doi: 10.1073/pnas.70.8.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dow J, Stracher A. Identification of the essential light chains of myosin. Proc Nat Acad Sci (US) 1971;68:1107. doi: 10.1073/pnas.68.6.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sréter F, Holtzer S, Gergely J, Holtzer H. Some properties of embryonic myosin. J Cell Biol. 1972;55:586. doi: 10.1083/jcb.55.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whalen RG, Butler-Browne GS, Gros F. Protein synthesis and actin heterogeneity in calf muscle cells in culture. Proc Nat Acad Sci (US) 1976;73:2018. doi: 10.1073/pnas.73.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Storti RB, Cohen DM, Rich A. Tissue-specific forms of actin in the developing chick. Cell. 1976;8:521. doi: 10.1016/0092-8674(76)90220-8. [DOI] [PubMed] [Google Scholar]
- 35.Garrels JI, Gibson W. Identification and characterization of multiple forms of actin. Cell. 1976;9:793. doi: 10.1016/0092-8674(76)90142-2. [DOI] [PubMed] [Google Scholar]
- 36.Buckingham ME, Cohen A, Gros F. Cytoplasmic distribution of pulse-labeled poly A-containing RNA, particularly 26S RNA, during myoblast growth and differentiation. J Mol Biol. 1976;103:611. doi: 10.1016/0022-2836(76)90220-5. [DOI] [PubMed] [Google Scholar]
- 37.Heywood SM, Kennedy DS, Bester AJ. Separation of specific initiation factors involved in the translation of myosin. Proc Nat Acad Sci (US) 1974;71:2428. doi: 10.1073/pnas.71.6.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Revel M, Groner Y, Pollack Y, Cnaani D, Zeller H, Nudel U. Biochemical mechanisms to control protein synthesis: mRNA specific initiation factors. Acta Endocrinol Suppl; Proc Karolinska Symp on Research Methods in Reproductive Endocrinology; 1973. p. 54. [DOI] [PubMed] [Google Scholar]
- 39.Johnson LF, Williams JG, Abelson HT, Green H, Penman S. Changes in RNA in relation to growth of the fibroblasts. III Posttranscriptional regulation of mRNA formation investigated in growing cells. Cell. 1975;4:69. doi: 10.1016/0092-8674(75)90135-x. [DOI] [PubMed] [Google Scholar]
- 40.Both GW, Banerjee AK, Shatkin AJ. Methylation-dependent translation of viral mRNA in vitro. Proc Nat Acad Sci (US) 1975;72:1189. doi: 10.1073/pnas.72.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prives C, Aviv H, Gilboa E, Revel M, Winocour E. The cell-free translation of SV40 messenger RNA. Cold Spr Harb Symp Quant Biol. 1975;39:309. doi: 10.1101/sqb.1974.039.01.041. [DOI] [PubMed] [Google Scholar]
- 42.Yaffe D, Feldman M. The effect of actinomycin D on heart and thigh muscle cells grown in vitro. Dev Biol. 1964;9:347. doi: 10.1016/0012-1606(64)90030-2. [DOI] [PubMed] [Google Scholar]
- 43.Young RB, Goll DE, Stromer MH. Isolation of myosin synthesizing polysomes from cultures of embryonic chicken myoblasts before fusion. Dev Biol. 1975;47:123. doi: 10.1016/0012-1606(75)90268-7. [DOI] [PubMed] [Google Scholar]