Abstract
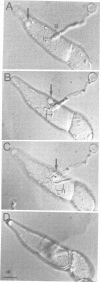
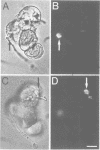
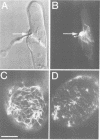
We describe a novel system of reduced complexity for analysing molecular plant-fungus interactions. The system consists of suspension-cultured parsley (Petroselinum crispum) cells infected with a phytopathogenic fungus (Phytophthora infestans) which adheres to a coated glass plate and thus immobilizes the plant cells for live microscopy. Conventional light and electron microscopy as well as time-lapse video microscopy confirmed the virtual identity of fungal infection structures and of several characteristic early plant defence reactions in the cultured cells and whole-plant tissue. Using this new system to approach previously unresolved questions, we made four major discoveries: (i) rapid translocation of plant cell cytoplasm and nucleus to the fungal penetration site was associated with local depolymerization of the microtubular network; (ii) the directed translocation was dependent on intact actin filaments; (iii) a typical plant defence-related gene was activated in the fungus-invaded cell; and (iv) simultaneous activation of this gene in adjacent, non-invaded cells did not require hypersensitive death of the directly affected cell.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper J. A. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987 Oct;105(4):1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzemeier K. H., Cretin C., Kombrink E., Rohwer F., Taylor J., Scheel D., Hahlbrock K. Transient Induction of Phenylalanine Ammonia-Lyase and 4-Coumarate: CoA Ligase mRNAs in Potato Leaves Infected with Virulent or Avirulent Races of Phytophthora infestans. Plant Physiol. 1987 Sep;85(1):34–41. doi: 10.1104/pp.85.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horoyan M., Benoliel A. M., Capo C., Bongrand P. Localization of calcium and microfilament changes in mechanically stressed cells. Cell Biophys. 1990 Dec;17(3):243–256. doi: 10.1007/BF02990720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kombrink E., Hahlbrock K. Responses of cultured parsley cells to elicitors from phytopathogenic fungi : timing and dose dependency of elicitor-induced reactions. Plant Physiol. 1986 May;81(1):216–221. doi: 10.1104/pp.81.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreimer G., Melkonian M. Reflection confocal laser scanning microscopy of eyespots in flagellated green algae. Eur J Cell Biol. 1990 Oct;53(1):101–111. [PubMed] [Google Scholar]
- Köhle H., Jeblick W., Poten F., Blaschek W., Kauss H. Chitosan-elicited callose synthesis in soybean cells as a ca-dependent process. Plant Physiol. 1985 Mar;77(3):544–551. doi: 10.1104/pp.77.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C. J., Lawton M. A., Dron M., Dixon R. A. Signals and transduction mechanisms for activation of plant defenses against microbial attack. Cell. 1989 Jan 27;56(2):215–224. doi: 10.1016/0092-8674(89)90894-5. [DOI] [PubMed] [Google Scholar]
- Northen H. T. STUDIES ON THE PROTOPLASMIC NATURE OF STIMULATION AND ANESTHESIA. II. Plant Physiol. 1940 Oct;15(4):645–660. doi: 10.1104/pp.15.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quader H., Robinson D. G. Structure, synthesis and orientation of microfibrils. VI. The role of ions in microfibril deposition in Oocystis solitaria. Eur J Cell Biol. 1979 Oct;20(1):51–56. [PubMed] [Google Scholar]
- Ragg H., Kuhn D. N., Hahlbrock K. Coordinated regulation of 4-coumarate:CoA ligase and phenylalanine ammonia-lyase mRNAs in cultured plant cells. J Biol Chem. 1981 Oct 10;256(19):10061–10065. [PubMed] [Google Scholar]
- Scheel D., Parker J. E. Elicitor recognition and signal transduction in plant defense gene activation. Z Naturforsch C. 1990 Jun;45(6):569–575. doi: 10.1515/znc-1990-0601. [DOI] [PubMed] [Google Scholar]
- Schmelzer E., Jahnen W., Hahlbrock K. In situ localization of light-induced chalcone synthase mRNA, chalcone synthase, and flavonoid end products in epidermal cells of parsley leaves. Proc Natl Acad Sci U S A. 1988 May;85(9):2989–2993. doi: 10.1073/pnas.85.9.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer E., Kruger-Lebus S., Hahlbrock K. Temporal and Spatial Patterns of Gene Expression around Sites of Attempted Fungal Infection in Parsley Leaves. Plant Cell. 1989 Oct;1(10):993–1001. doi: 10.1105/tpc.1.10.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somssich I. E., Schmelzer E., Kawalleck P., Hahlbrock K. Gene structure and in situ transcript localization of pathogenesis-related protein 1 in parsley. Mol Gen Genet. 1988 Jul;213(1):93–98. doi: 10.1007/BF00333403. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Kron S. J., Sheetz M. P. Movement of myosin-coated beads on oriented filaments reconstituted from purified actin. Nature. 1985 Jun 13;315(6020):584–586. doi: 10.1038/315584a0. [DOI] [PubMed] [Google Scholar]
- Stäb M. R., Ebel J. Effects of Ca2+ on phytoalexin induction by fungal elicitor in soybean cells. Arch Biochem Biophys. 1987 Sep;257(2):416–423. doi: 10.1016/0003-9861(87)90585-6. [DOI] [PubMed] [Google Scholar]
- Traas J. A., Doonan J. H., Rawlins D. J., Shaw P. J., Watts J., Lloyd C. W. An actin network is present in the cytoplasm throughout the cell cycle of carrot cells and associates with the dividing nucleus. J Cell Biol. 1987 Jul;105(1):387–395. doi: 10.1083/jcb.105.1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeda T. Q., Furuya M. Evidence for active interactions between microfilaments and microtubules in myxomycete flagellates. J Cell Biol. 1989 May;108(5):1727–1735. doi: 10.1083/jcb.108.5.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson R. E., Ashley C. C. Free Ca2+ and cytoplasmic streaming in the alga Chara. Nature. 1982 Apr 15;296(5858):647–650. doi: 10.1038/296647a0. [DOI] [PubMed] [Google Scholar]
- Wolniak S. M., Hepler P. K., Jackson W. T. Detection of the membrane-calcium distribution during mitosis in Haemanthus endosperm with chlorotetracycline. J Cell Biol. 1980 Oct;87(1):23–32. doi: 10.1083/jcb.87.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolniak S. M., Hepler P. K., Jackson W. T. Ionic changes in the mitotic apparatus at the metaphase/anaphase transition. J Cell Biol. 1983 Mar;96(3):598–605. doi: 10.1083/jcb.96.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]