Abstract
We hypothesized that dietary administration of the peroxisomal proliferator-activated receptor α agonist, fenofibrate, to young adult male rats would prevent the fractionated whole-brain irradiation (fWBI)-induced reduction in cognitive function and neurogenesis and prevent the fWBI-induced increase in the total number of activated microglia. Eighty 12–14-week-old young adult male Fischer 344 × Brown Norway rats received either: (1) sham irradiation, (2) 40 Gy of fWBI delivered as two 5 Gy fractions/week for 4 weeks, (3) sham irradiation + dietary fenofibrate (0.2% w/w) starting 7 days prior to irradiation, or (4) fWBI + fenofibrate. Cognitive function was measured 26–29 weeks after irradiation using: (1) the perirhinal cortex (PRh)-dependent novel object recognition task; (2) the hippocampal-dependent standard Morris water maze (MWM) task; (3) the hippocampal-dependent delayed match-to-place version of the MWM task; and (4) a cue strategy preference version of the MWM to distinguish hippocampal from striatal task performance. Neurogenesis was assessed 29 weeks after fWBI in the granular cell layer and subgranular zone of the dentate gyrus using a doublecortin antibody. Microglial activation was assessed using an ED1 antibody in the dentate gyrus and hilus of the hippocampus. A significant impairment in perirhinal cortex-dependent cognitive function was measured after fWBI. In contrast, fWBI failed to alter hippocampal-dependent cognitive function, despite a significant reduction in hippocampal neurogenesis. Continuous administration of fenofibrate prevented the fWBI-induced reduction in perirhinal cortex-dependent cognitive function, but did not prevent the radiation-induced reduction in neurogenesis or the radiation-induced increase in activated microglia. These data suggest that fenofibrate may be a promising therapeutic for the prevention of some modalities of radiation-induced cognitive impairment in brain cancer patients.
INTRODUCTION
Up to 30% of the >1.6 million individuals diagnosed with cancer in 2012 will develop brain metastases (1, 2), and every year ~170,000 patients will receive fractionated partial or whole-brain irradiation (fWBI) (3). Up to 90% of adult patients surviving ≥6 months post-fWBI face the risk of developing radiation-induced cognitive impairments that severely impact their quality of life (QOL) (4, 5). These radiation-induced cognitive impairments encompass several functional domains, including progressive deficits in frontal lobe executive functions, memory, spatial relationships, visual motor processing, quantitative skills and/or attention (5, 6). Short-term interventions have shown temporary efficacy (5), but there are no proven, long-term interventions for preventing radiation-induced cognitive impairment in brain tumor patients.
The mechanisms underlying radiation-induced brain injury and the resulting cognitive impairments remain elusive. Given the central role that the hippocampus plays in learning, consolidation and retrieval of information (7, 8), most rodent brain irradiation studies have focused on the hippocampus. The dentate gyrus (DG) region of the hippocampus is one of two sites of adult neurogenesis in the mammalian brain (9). Neural precursor cells present in the subgranular zone (SGZ) of the DG give rise to new neurons that functionally integrate in the granule cell layer (GCL) of the hippocampus (10). These neural precursor cells are extremely radiosensitive (11, 12); irradiating the rodent brain leads to a significant decrease in the number of newborn mature and immature neurons in the DG. This decrease in the number of newborn and immature neurons has frequently been correlated with hippocampal-dependent cognitive impairment (13, 14). Additionally, previous studies suggest that increased microglial activation may be associated with decreased hippocampal neurogenesis and decreased cognitive function (12, 13, 15).
The peroxisomal proliferator-activated receptor α (PPARα) is a nuclear receptor belonging to the PPAR family of ligand-activated transcription factors (16). PPARα agonists have been shown to confer neuroprotection in a variety of preclinical models, including radiation-induced brain injury (17–19). Administration of dietary fenofibrate to young adult male mice prior to and for 10 weeks after a single WBI dose of 10 Gy of 137Cs γ rays preserved hippocampal neurogenesis and prevented the increase in activated microglia (19). However, behavioral analyses could not be conducted because the mice had a 129Sv genetic background and were not suitable for cognitive function testing due to defects in their corpus callosum (20).
The objective of the current study was to determine if fenofibrate can modulate fWBI-induced cognitive impairment using our well established fWBI rat model (21–26). We employed several established tasks to evaluate cognitive function including the: (1) perirhinal cortex (PRh)-dependent novel object recognition (NOR) task (27, 28); (2) hippocampal-dependent standard Morris water maze (MWM) task (29, 30); (3) hippocampal-dependent delayed match-to-place (DMTP) version of the MWM (31); and (4) cue strategy preference version of the MWM (32, 33) that distinguishes hippocampal from striatal task performance.
As demonstrated previously, fWBI led to a significant reduction in PRh-dependent cognitive function (22, 23, 27). In contrast, fWBI failed to alter hippocampal-dependent function, despite a significant reduction in hippocampal neurogenesis. Continuous administration of fenofibrate prevented the fWBI-induced reduction in PRh-dependent function but did not prevent the radiation-induced decrease in neurogenesis or the radiation-induced increase in microglial activation. These data indicate that fWBI leads to PRh-dependent cognitive impairment in the young adult male rat, without a decrease in hippocampal-dependent cognitive function, despite a large reduction in hippocampal neurogenesis. The ability of fenofibrate to prevent this fWBI-induced PRh-dependent cognitive impairment suggests that fenofibrate may be able to improve the QOL of brain cancer patients receiving fWBI.
MATERIALS AND METHODS
Animals
Eighty 10–12-week-old young adult male Fischer 344 × Brown Norway (F344×BN) rats were obtained from Harlan Laboratories, Inc. (Indianapolis, IN) and housed in pairs on a 12:12 h light-dark schedule with free access to food and water. All animal handling and experiments were performed in strict accordance with the NIH Guide for Care and Use of Laboratory Animals as approved by the Wake Forest School of Medicine Institutional Animal Care and Use Committee. After an acclimation period of 2 weeks, rats were randomized to 4 experimental groups (n = 20 rats/group), Group 1: sham irradiated; Group 2: fWBI; Group 3: sham irradiated + 0.2% w/w of fenofibrate (Sigma-Aldrich, St. Louis, MO) in the diet; and Group 4: fWBI + 0.2% w/w of fenofibrate in the diet. Rats received fenofibrate beginning 3 days before the start of fWBI and continuously until the end of the experiment. All rats were weighed weekly to assess their overall health.
fWBI Procedures
A 40 Gy total dose of fWBI was delivered in 8 fractions of 5 Gy, twice/week, for 4 weeks as described previously (25). Briefly, irradiations were performed on lightly anesthetized [Ketamine (75 mg/kg)/xylazine (7 mg/kg)] rats in a 267 TBq (7,214 Ci) self-shielded 137Cs irradiator using lead and Cerrobend devices to collimate the beam so that the whole brain, including the brain stem, was irradiated. The average dose rate to the midline of the brain was ~4 Gy/min; the eyes and body received ~15% and ~3% of the brain dose, respectively. Doses were delivered to opposite sides of the head on alternate days to ensure that each rat brain received the same midline dose. Sham-irradiated rats were also anesthetized twice weekly for 4 weeks and handled similarly to the irradiated rats.
NOR Test Procedures
The cognitive function of each rat was assessed 26 weeks after the completion of fWBI using a PRh-dependent version of the NOR task as previously described (27, 28). The NOR task is comprised of three phases: sample, delay and test phases. Before testing, each animal was habituated to the testing arena (an opaque rectangular container: 24 in × 18 in × 20 in) by allowing exploration of the empty arena for 5 min a day for 5 days. After habituation, the following trials were divided into a sample phase and a test phase, with a delay occurring between the two phases. During the sample phase, the rat was allowed to explore and become familiar with two identical objects (A1 and A2), placed in two adjacent corners of the testing arena, for 3 min. Following the sample phase, a 1 min delay was introduced during which the animal was removed from the arena and returned to its home cage. A test phase followed in which two objects (A3 and B1) were placed in the arena, and the rat was allowed to explore the arena for 3 min. Object A3 was identical to A1 and A2, while B1 was a novel object; activity of the rat was recorded using an automated tracking system (Ethovision, Nodulus, Leesburg, VA). The objects were secured to the arena floor with Velcro patches so that the subject could not displace them during exploration. Recognition memory was measured as the time spent exploring the novel object compared to the time spent exploring the familiar object. The position (left or right) of the novel object in the test phase was balanced between sessions to avoid any spatial preference.
The basic measurement was the time the rat spent exploring an object, defined as placing his nose within ≤2 cm of the object and actively exploring it. The following parameters were obtained: E1, the total time spent exploring the identical objects A1 and A2 in the sample phase (3 min); E2, the total time spent exploring object A3 and the novel object B1 in the test phase (3 min); and D1, the index of discrimination defined as the difference in time spent exploring objects A3 and B1 in the test phase (i.e., B1 – A3). The discrimination ratio (D1/E2), a measure of the rat’s recognition memory, was calculated and the average discrimination ratio for each treatment group compared.
MWM Test Procedures
Spatial learning, reference memory and spatial reversal learning were evaluated 27–29 weeks after completion of fWBI using the MWM. As previously described (34), rats were placed in a circular white plastic tank filled with opaque water and surrounded by dark geometric cues affixed to white curtains. The tank was divided into 4 imaginary quadrants with an escape platform 2 cm under the water surface in the middle of one quadrant.
During the standard version of the MWM, spatial learning and reference memory were measured. The escape platform was placed in quadrant 4, the rats were introduced into the quadrants in a systematically random pattern, and each rat’s performance was recorded using an automated tracking system (Ethovision). For each block (Supplementary Table S1; http://dx.doi.org/10.1667/RR13202.1.S3) the first two days consisted of two training trials each day and the third day consisted of one training trial and one probe trial. During each training trial, the rat was allowed to search for 90 s to locate the platform and the total distance to the platform, the path length to the platform and the escape latency were measured. During the probe trial, the platform was lowered beyond reach, the rat was allowed to search for 30 s, and the mean distance and latency to the previous platform site was measured. This schedule was repeated for 4 blocks (Supplementary Table S1; http://dx.doi.org/10.1667/RR13202.1.S3). In the reversal version of the MWM, the platform location was moved to the opposite quadrant with the visual cues remaining in the same positions. This task required the rats to inhibit their previously learned response and remember a new location to find the escape platform.
Spatial working memory was also assessed using a DMTP procedure that started on the last day of the standard MWM (Supplementary Table S1; http://dx.doi.org/10.1667/RR13202.1.S3). The reversal day of the standard MWM was the first day of the DMTP procedure that lasted for a total of 4 days. Each DMTP testing day consisted of 4 trials, a sample trial (trial 1) and three match trials (trial 2–4). The escape platform in the sample phase each day was located 2 cm under the water surface, in a unique location (Supplementary Fig. S2; http://dx.doi.org/10.1667/RR13202.1.S2) and remained in the same location for the remaining match trials for that day. The delays between daily trials were 20 min, 15 s and 15 s.
Strategy preference was assessed 24 h after the last day of DMTP testing. A visible platform was placed in the opposite quadrant to where the hidden platform was previously located. The rats were given two 60 s strategy probe trials. Start locations were on either side of the tank, equidistant from the visible cue platform and the prior hidden platform location. A “place strategy” was recorded if the rat crossed within 5 cm of the prior hidden platform location before escaping to the visible platform. A “cue strategy” was recorded if the rat did not cross the prior hidden platform before swimming to the visible platform. Data were scored by a blinded observer.
Visual acuity was assessed after the cue strategy task was performed. The task consisted of 4 consecutive trials for each rat. A visible platform was placed in each quadrant for each trial excluding the quadrant where the rat entered the maze. The rats were given 60 s to locate the platform. Locomotor behavior was assessed using swim velocity during the visual acuity trials in order to reduce confounding factors of trial acquisition on motor behavior. Data were scored by an automated tracking system (Ethovision, Nodulus, Leesburg, VA).
Tissue Processing
Following completion of the cognitive function testing at 29 weeks post-fWBI, rats were euthanized by decapitation. The brains were removed rapidly and hemisected. The right hemisphere was flash frozen in liquid nitrogen, the left hemisphere was immersion fixed in phosphate-buffered 4% paraformaldehyde for 24 h. The left hemispheres were then cryoprotected for 24 h in 10, 20 and 30% sucrose, frozen in embedding medium, cryosectioned at a thickness of 40 μm in the coronal plane, placed in an antifreeze solution (1:1:2 ethylene glycol, glycerol and 0.1 M of phosphate-buffered saline) and stored at −20°C.
Immunohistochemistry
Based on previously published studies, immunohistochemistry was performed on the left hemispheres of 4 brains selected at random from each experimental group (19, 26). For each brain, the first section was randomly selected from the first 12 sections through the DG (bregma −1.8 to bregma −6.8). Subsequently, every 12th section was then washed in tris-buffered saline (TBS; pH 7.4) to remove the cryoprotectant. For immunohistochemical staining, endogenous per-oxidase activity was reduced by 30 min incubation in 0.6% hydrogen peroxide in TBS. To label immature neurons, sections were incubated in blocking solution (5% normal serum + 0.2% Triton X-100 in TBS) and then overnight at 4°C with primary antibody. The primary antibodies used were a goat polyclonal anti-rat doublecortin (DCX+) antibody [sc-8066 (C18), Santa Cruz Biotechnology, Inc., Santa Cruz, CA; 1 μg/mL; 1:200] and mouse monoclonal anti-rat CD68 (ED1 clone), which labels activated macrophages/microglia (MCA341R, AbD Serotec, Raleigh, NC; 1:400). The primary antibody was detected using biotinylated secondary antibodies [Dcx: horse anti-goat, ED1: horse anti-mouse (Santa Cruz Biotechnology, Inc.; 1:300)] and visualized using peroxidase-conjugated avidin-biotin complex (ABC Elite kit) with nickel enhanced diaminobenzidine (DAB) as substrate (Vector Laboratories, Burlingame, CA). Sections were counterstained with the nuclear binding dye, 4′, 6-diamidino-2-phenylindole dihydrochloride (DAPI, Sigma Aldrich, St. Louis, MO), to facilitate recognition of anatomical landmarks.
Stereological Analyses of Tissue Sections
The estimated total number of Dcx+ cells in the GCL and SGZ of the DG and ED1+ cells in the DG/hilus were estimated by the optical fractionator method using Stereo Investigator software (Microbright-field, Inc., Colchester, VT). The counting parameters for Dcx+ cells in the GCL/SGZ were: sampling grid size (100 × 100 μm), counting frame size (100 × 100 μm), disector height (10 μm) and guard-zone thickness (2 μm). The counting parameters for ED1+ cells in the DG/hilus were: sampling grid size (150 × 150 μm), counting frame size (75 × 75 μm), disector height (10 μm) and guard-zone thickness (5 μm). The precision of the stereological counts was measured using the coefficient of error (CE) by the Gundersen-Jensen CE estimator. The variance introduced by the stereological analysis was never more than 50% of the observed group variance (33, 34).
Statistical Analysis
All analyses were performed using GraphPad Prism (La Jolla, CA), SPSS v. 21 (Chicago, IL) and/or SAS (Cary, NC) software. P < 0.05 were considered significant.
Cognitive Studies
A two-way ANOVA was used to analyze the NOR. For all MWM data, the escape latency, distance to platform and path length, over the course of training and probe trials were analyzed using repeated measures ANOVA; variables were corrected for sphericity using Greenhouse-Geisser correction. Additionally, probe trial data were analyzed using a mixed effects model to determine if the rats exhibited a preference for the target quadrant (defined as percentage time (%) in the target quadrant > 25%), and to determine if there were any drug, radiation, probe or interaction effects. MWM visual acuity and swim velocity were analyzed using a two-way ANOVA with Bonferroni corrected pair-wise post-test comparisons. Strategy selection was assessed using χ2 analysis.
Immunohistochemical Studies
Data are presented as the mean ± SEM and include CE calculations. A two-way ANOVA was used to determine the effect of fenofibrate, fWBI or any interaction effects. Bonferroni post-tests were used for pair-wise comparisons.
RESULTS
NOR Testing Results
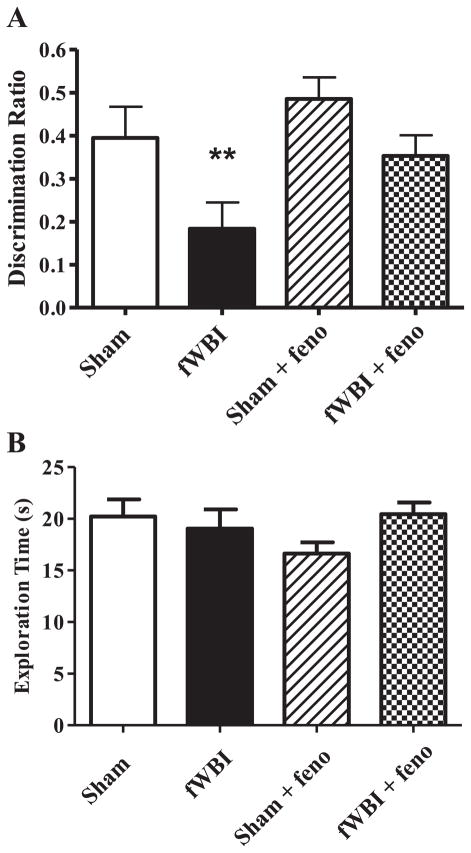
Consistent with previously published results using the PRh-dependent NOR task with this rat model (10, 20), fWBI produced a significant radiation effect at 26 weeks post-fWBI [Fig. 1A; fWBI effect (F1.68 = 8.64, P < 0.005)]. There was also a significant dietary fenofibrate effect (F1.68 = 4.92, P < 0.030), but no significant interaction between dietary fenofibrate and fWBI (F1.68 = 0.46, P > 0.5). Administration of dietary fenofibrate before, during and after fWBI prevented this PRh-dependent cognitive impairment without any significant effect on the cognitive function of the sham-irradiated controls (Fig. 1A). Data analysis using two-way ANOVA showed that there was no significant difference in the total time spent exploring both objects in the test phase (E2) between any of the groups (Fig. 1B).
FIG. 1.
PRh-dependent cognitive behavior was assessed by the NOR. Administering fenofibrate to young adult male F344×BN rats prevents the fractionated whole-brain irradiation (fWBI) induced reduction in PRh-dependent cognitive function (panel A). Total exploration (E2) is not different between any of the treatment groups (panel B). Rats received either sham irradiation (Sham), 40 Gy of fWBI, sham irradiation and dietary 0.2% w/w fenofibrate (sham + feno) or 40 Gy of fWBI + fenofibrate (fWBI + feno). Fenofibrate was continuously administered by diet to the rats starting 7 days before the beginning of fWBI. PRh-dependent cognitive function was assessed 26 weeks after completion of fWBI. Data represent the mean ± SEM; n = 17–20/group; **P < 0.005.
Standard MWM Testing Results
No overall deficits in spatial learning, reference memory or spatial reversal learning were measured 27–29 weeks post-fWBI using the standard MWM (Figs. 2–4).
FIG. 2.
Hippocampal-dependent behavior was assessed by the standard Morris water maze (MWM). There were no significant main effects of radiation or drug on MWM performance. No deficits in spatial learning were detected in escape latency post-fWBI using the standard MWM (panel A). There was a significant radiation by block by radiation effect (P < 0.001) on the distance to the platform, with sham-irradiated groups tending to have a longer distance compared to fWBI groups (panel B). Path length also had a significant block by radiation effect (P < 0.01; panel C). There was no significant difference between any of the groups in the time to platform annulus in the probe trials (panel D). Cognitive function was assessed 27–29 weeks after completion of fWBI. Data represent the mean ± SEM; n = 17–20/group.
FIG. 4.
Neither fWBI nor dietary fenofibrate had an effect on reversal in the MWM. During the reversal trial, the hidden platform was moved to a new location from that of the previous training trials. There were no group differences in escape latency (panel A) or in the total distance to the platform (panel B). Cognitive function was assessed 27–29 weeks after completion of fWBI. Data represent the mean ± SEM; n = 17–20/group.
Training Trials
Escape Latency
There were no main effects of radiation, drug or interaction on escape latency (drug: F1,67 < 1, P < 0.5; radiation: F1,67 < 1, P < 0.4; radiation × drug: F1,67 < 1, P < 0.3). All groups had a similar decrease in escape latency over successive trials and blocks (Fig. 2A). There was a significant effect of block, trial and trial by block interaction (block: F2.5,165.8 = 227.6, P < 0.001; trial: F3.6,240.7 = 22.0, P < 0.001, block × trial: F8.2,550.9 = 7.0, P < 0.001). All other interactions were not significant.
Distance to Platform
There were no significant main radiation, drug or interaction effects on total distance to platform during the standard MWM (radiation: F1,65 < 1, P < 0.8; drug: F1,65 = 1.5, P < 0.3; radiation × drug: F1,65 < 1, P < 0.4; Fig. 2B). There were significant block, trial, block by radiation and block by trial effects as well as a marginally significant block by trial by radiation effect (block: F2.3,164.2 = 211.2, P < 0.001; block × radiation: F2.5,164.2 = 10.5, P < 0.001; trial: F3.8,256.9 = 19.9, P < 0.001; block × trial: F7.2,481.6 = 5.9, P < 0.001; block × trial × radiation: F7.2,481.6 = 2.0, P < 0.053; Fig. 2B). The block by radiation effect was driven by performance in block 1 where sham-irradiated animals swam longer distances than irradiated counterparts, and in turn the tertiary interaction between block, trial and radiation was driven by differences in trial 1 more than later trials. The initial trials in block 1 had the highest number of animals that failed to reach the platform within the time allotted (65% in trial 1 block 1); however, failure to reach the platform was equally distributed between the treatment groups and cannot explain this difference in swim distance.
Path Length
There were no main effects of radiation, drug or interaction on path length during the standard MWM (radiation: F1,67 = 1.6, P < 0.3; drug: F1,67 < 1, P < 0.9; radiation × drug: F1,67 < 1, P < 0.6; Fig. 2C). There were significant block, trial, block by radiation and block by trial effects (block: F2.5,168.3 = 228.3, P < 0.001; block × radiation: F2.5,168.3 = 4.7, P < 0.01; trial: F3.8,252.6 = 13.1, P < 0.001; block × trial: F7.8,519.3 = 4.2, P < 0.001; Fig. 2C). The animals that received fWBI had a greater total path length; as with the distance to platform, this effect was driven by performance in block 1.
Probe Trials
Latency to platform annulus
There was a significant effect of probe day (3, 6, 9, 12) indicating improved performance at later time points, but no significant drug or radiation or interaction effects (day: F3,71 = 17.0, P < 0.0001; drug: F1,71 < 1, p > 0.6; radiation: F1,71 < 1, p > 0.6; drug x radiation: F1,71 = 0.44, p > 0.5; day x drug: F3,71 = 0.34, p > 0.7; day x radiation: F3,71 = 1.03, p > 0.3; Fig. 2D).
Path length
During the probe trials, there was a significant effect of probe day (3, 6, 9, 12) with shorter path lengths at later time points, but no significant drug or radiation effects (day: F3,71 = 15.1, P < 0.0001; drug: F1,71 = 1.4, p > 0.2; radiation: F1,71 = 1.6, p > 0.2). There was a significant interaction effect between radiation and drug (F1,71 = 6.11, P < 0.02).
Target quadrant preference
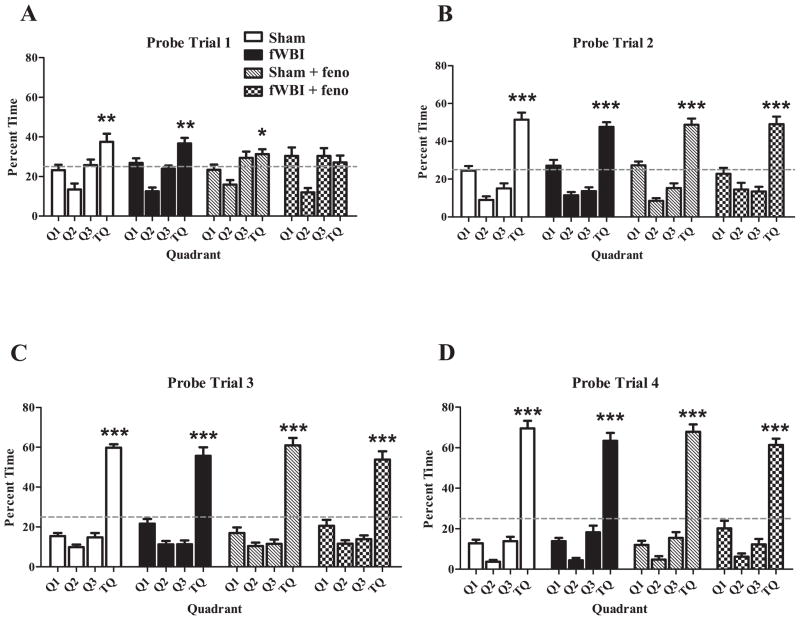
For the first probe trial (Day 3), the fWBI + fenofibrate group failed to show a significant preference for the target quadrant, defined as the percentage time in target quadrant being greater than 25% (T9,68 < 1, P < 0.5; Fig. 3A). All other groups, (sham, fWBI and sham + fenofibrate) showed a significant preference for the target quadrant in probe one (T9,68 = 3.8, P < 0.001; T9,68 = 3.4, P < 0.001; T9,68 = 2.2, P < 0.04, respectively; Fig. 3A). All groups showed a significant preference for the target quadrant in probes 2–4 (P < 0.0001, all groups). There were no significant differences or interactions between the groups in target quadrant preference in the probe trials (drug: F1,68 = 2.8, P < 0.1; radiation: F1,68 = 2.9, P < 0.1; drug × probe: F3,68 = 0.54, P < 0.70; drug × radiation: F1,68 = 0.02, P < 0.9; radiation × probe: F3,68 = 0.59, P < 0.63); probe day was significant (F3,68 = 66.3, P < 0.0001), indicating increased preference over time for all groups (Fig. 3A–D).
FIG. 3.
Fractionated WBI had no effect on Morris water maze (MWM) probe trial results. There were no group differences during any of the probe trials (panels A, B, C, D) regarding the percentage of time spent in the target quadrant (TQ) where the platform was previously located. The sham-irradiated, fWBI and sham + fenofibrate groups showed a significant preference for the TQ (time spent in TQ was greater than 25%) during the MWM probe trial 1. During the probe trials 2–4, all groups showed an increased preference for the TQ (panels B–D). The dashed line indicates platform choice equivalent to random chance (25%). Cognitive function was assessed 27–29 weeks after completion of fWBI. Data represent the mean ± SEM; n = 17–20/group. *P < 0.04; **P < 0.001; ***P < 0.0001.
Reversal Trials
During the reversal trial of the MWM, there were no group differences in escape latency (Fig. 4A) or total distance to the platform (Fig. 4B).
DMTP Testing Results
There were no significant differences in spatial working memory between the groups using the DMTP version of the MWM (Figs. 5 and 6). Escape latency decreased over the successive training days and trials (Fig. 5A; day effect (F3,213 = 11.0, P < 0.0001) and trial effect (F3,213 = 100.1, P < 0.001), indicating that all the rats were able to learn the task. There were no group differences in escape latency (Fig. 5A) or total distance to the platform (Fig. 5B). The percentage of time the rat spent where the platform was the previous day is an indication of how much the previous MWM training interfered with learning the new platform location; there was no significant difference among the groups (Fig. 6A). Additionally, the rats exhibited no difference in savings, (i.e., the time difference between escape latency of trial 1–trial 2), indicating that all groups were equally able to learn the new platform location regardless of fWBI or fenofibrate treatment (Fig. 6B).
FIG. 5.
Hippocampal-dependent behavior assessed using the DMTP task is unaffected by fWBI or dietary fenofibrate. All groups had decreasing escape latencies (panel A) over the trials each day, indicating that all rats were equally able to learn the new platform locations. There were also no differences in the distance to the platform (panel B) between the groups. Cognitive function was assessed 27–29 weeks after completion of fWBI. Data represent the mean ± SEM; n = 17–20/group.
FIG. 6.
Neither fWBI nor fenofibrate had an effect on relearning aspects of the DMTP task. There was no difference in the amount of time that the rats spent in the previous platform location or the relearning of the new platform location between the groups in the DMTP task. There were no group differences in the percentage of time the rats spent in the previous platform location (panel A). In addition, rats were equally able to relearn the new platform location, indicated as the percentage of time “saved” (trial 2–trial 1) between the first two trials (panel B). Cognitive function was assessed 27–29 weeks after completion of fWBI. Data represent the mean ± SEM; n = 17–20/group.
Cue vs. Place Strategy Testing Results
There was no significant difference among the groups in the cue strategy task for test 1 (χ2; P < 0.91) or test 2 (χ2; P < 0.701, Table 1). More rats (73%) tended to use a spatial, hippocampal-based strategy rather than a cue, striatal-based strategy during test 1. Significant preference for the spatial strategy was seen in the sham irradiated (P < 0.02) and fWBI + fenofibrate groups (P < 0.03). A marginal preference for the spatial strategy was seen in the fWBI group (P < 0.09), but no preference was observed in the sham irradiated + fenofibrate group (P < 0.2). For test 2, 44% of rats performed using a spatial strategy however no significant preference was observed in any group (sham: P < 0.7, sham + fenofibrate: P < 0.2, fWBI: P < 0.9, fWBI + fenofibrate: P < 0.9).
TABLE 1.
The Number of Rats Selecting a Place Strategy or a Cue Strategy
| Sham irradiated | fWBI | Sham irradiated + fenofibrate | fWBI + fenofibrate | |
|---|---|---|---|---|
| Part A | ||||
| Competition trial 1 | ||||
| Place strategy | 14* | 12 | 12 | 13 * |
| Cue strategy | 4 | 5 | 6 | 4 |
| Part B | ||||
| Competition trial 2 | ||||
| Place strategy | 8 | 9 | 6 | 8 |
| Cue strategy | 10 | 8 | 12 | 9 |
Notes. Strategy preference was assessed using the cue strategy task which uses a visible (cue) platform in a new location verses a hidden (place) platform that is in the same location as the prior training trials. In trial 1 more rats (73%) tended to use a spatial, hippocampal-based strategy rather than a cue, striatal-based strategy (part A). For trial 2, 44% of rats performed using a spatial strategy however no significant preference was observed in any group (part B). Cognitive function was assessed 27–29 weeks after completion of fWBI. Data represent the mean the number of rats choosing a particular strategy (place or cue). n = 17–20/group,
P < 0.05.
Immunohistochemistry Results
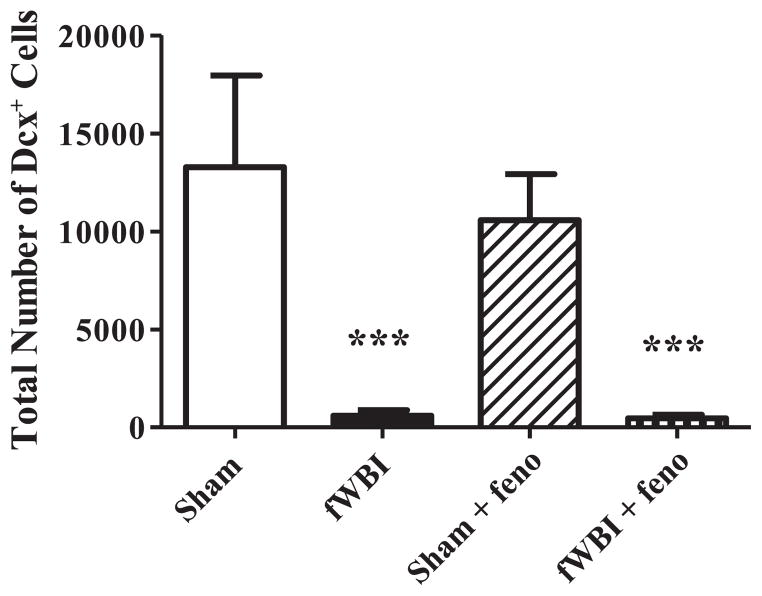
Immature neurons (DCX+ cells) were quantified within the GCL and SGZ of the DG as an indirect marker of neurogenesis, since not all newborn neurons are incorporated into functional networks (36). There was a highly significant reduction in the total number of DCX+ cells at 29 weeks after fWBI (F1.12 = 18.8, P < 0.001; Fig. 7). Continuous administration of dietary fenofibrate did not prevent this fWBI-induced reduction in the total number of DCX+ cells. CE values were as follows: sham =0.05, fWBI = 0.27, sham + fenofibrate = 0.06, fWBI + fenofibrate = 0.19.
FIG. 7.
Dietary fenofibrate did not prevent the fWBI-mediated reduction in neurogenesis in rats. Following the completion of the cognitive function testing at 29 weeks post-fWBI/sham-fWBI, tissues were collected, processed and stained using a marker for immature neurons (DCX+) in the granular and subgranular zones of the DG in the hippocampus. All analyses were performed using stereological techniques. There was a marked reduction in the estimated total number of DCX+ cells in the DG after fWBI, which was not modulated by continuous administration of fenofibrate. Data represent the mean ± SEM; n = 4 rats/group; ***P < 0.001.
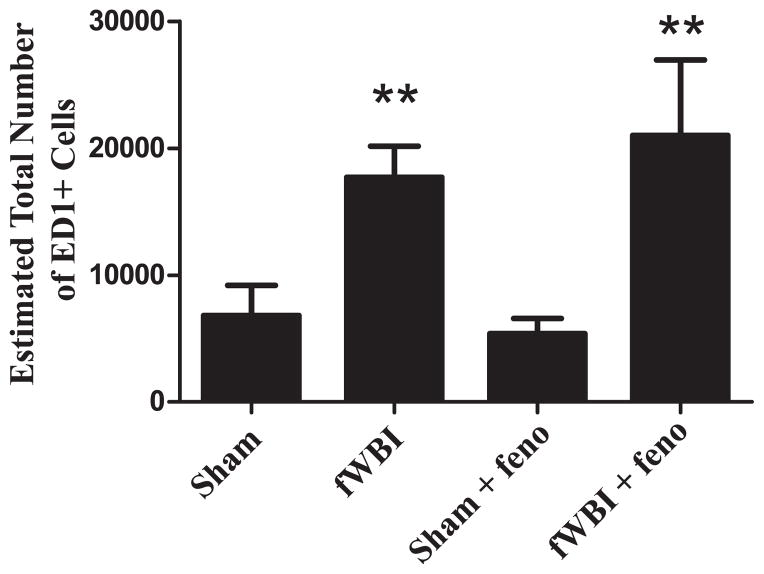
The total number of activated microglia (ED1+ cells) were quantified within the DG/hilus as an indicator of neuro-inflammation. There was a significant increase in the total number of ED1+ cells at 29 weeks after fWBI (F1.12 = 14.5, P < 0.003; Fig. 8). Continuous administration of dietary fenofibrate did not prevent this fWBI-induced increase. CE values were as follows: sham =0.15, fWBI = 0.09, sham + fenofibrate = 0.15, fWBI + fenofibrate = 0.07.
FIG. 8.
Dietary fenofibrate did not prevent the fWBI-mediated increase in the total number of activated microglia in rats. Following the completion of the cognitive function testing at 29 weeks post-fWBI/sham-fWBI, tissues were collected, processed and stained using a marker for activated microglia (ED1+) in the DG/hilus in the hippocampus. All analyses were performed using stereological techniques. There was a significant increase in the estimated total number of ED1+ cells in the DG/hilus after fWBI, which was not modulated by continuous administration of fenofibrate. Data represent the mean ± SEM; n = 4 rats/group; **P < 0.003.
Body Weight
There was a significant decrease in body weight with radiation (F1,69 = 22.2, P < 0.001), drug (F1,69 = 82.8, P < 0.001) and interaction between radiation and drug (F1,69 = 8.7, P < 0.01). Bonferroni corrected comparisons to explore these main effects indicated that sham-irradiated animals were significantly heavier than all other treatment groups (P < 0.001), while fWBI were significantly heavier than fWBI + fenofibrate rats (P < 0.01). Sham + fenofibrate rats did not significantly differ in weight from either fWBI (P < 0.2) or fWBI + fenofibrate rats (P < 0.5). There were significant effects of week (F2.7,189.5 = 2272.0, P < 0.001), week by radiation (F2.7,189.5 = 25.9, P < 0.001), week by drug (F2.7,189.5 = 97.3, P < 0.001) and week by radiation by drug (F2.7,189.5 = 13.5, P < 0.001; Supplementary Fig. S1; http://dx.doi.org/10.1667/RR13202.1.S1).
Visual Acuity and Locomotor Behavior
The average swimming distance to the visible platform over 4 trials was unaffected by fWBI or fenofibrate treatment (Supplementary Fig. S2A; http://dx.doi.org/10.1667/RR13202.1.S2). Swim velocity during visual acuity trials was assessed as an indicator of locomotor behavior. Swim velocity was significantly slower with fenofibrate (drug: F1,68 = 11.2, P < 0.01) though not irradiation (F1,68 = 3.0, P < 0.1). The drug by radiation interaction was significant (F1,68 = 6.3, P < 0.02) with post-hoc testing revealing that the fWBI + fenofibrate group was significantly slower than the sham-irradiated group (Bonferroni, P < 0.01; Supplementary Fig. S2B; http://dx.doi.org/10.1667/RR13202.1.S2). All other post-hoc comparisons were not significant.
DISCUSSION
We employed several well established tasks to evaluate the impact of fWBI on cognitive function, including the PRh-dependent NOR task, hippocampal-dependent standard and DMTP versions of the MWM task, and a strategy preference version of the MWM task. As demonstrated previously, fWBI led to a significant PRh-dependent cognitive impairment in young adult male rats at 26 weeks postirradiation (Fig. 1A) (22, 23, 27). In contrast, there was no measurable hippocampal-dependent cognitive impairment after fWBI (Figs. 2–6; Table 1A), despite a large reduction in the number of immature neurons in the DG of the hippocampus (Fig. 7). Administering the PPARα agonist, fenofibrate, starting 1 week before, during and for up to 29 weeks after fWBI prevented the radiation-induced PRh-dependent cognitive impairment (Fig. 1A); however, fenofibrate treatment did not prevent the fWBI-induced reduction in the number of immature neurons (Fig. 7) or the fWBI-induced increase in activated microglia (Fig. 8). These data suggest that long-term administration of the PPARα agonist, fenofibrate, appears to prevent fWBI-induced PRh-dependent cognitive impairment.
Chronic administration of fenofibrate was well tolerated. However, there was decreased locomotor behavior in the fWBI + fenofibrate group in the visual acuity trials (Supplementary Fig. S2B; http://dx.doi.org/10.1667/RR13202.1.S2). Despite the apparent decrease in locomotor behavior in the fWBI + fenofibrate group, these rats had the same escape latency as all other groups in the standard MWM trials. Additionally there appears to be a temporary detrimental effect of fenofibrate treatment on hippocampal-dependent spatial memory during the first MWM probe trial (probe 1, Fig. 3A), as only the fWBI + fenofibrate group failed to show a statistically significant preference for the target quadrant. However, the fWBI + fenofibrate group initially reached the platform annulus in the same amount of time as all the other groups (Fig. 2D), and the percentage of time spent in the target quadrant during the first probe trial was not significantly different from any of the treatment groups. Further, the fWBI + fenofibrate group significantly preferred a hippocampal mediated place strategy that supports a functioning hippocampal circuitry. These additional observations are inconsistent with a strong conclusion for a defect in the fWBI + fenofibrate group in spatial memory. Taken together, these results indicate that fenofibrate, even in the presence of fWBI, has limited if any impact on spatial memory. Confirming previous observations (22, 35), fWBI was associated with a significant reduction in body weight (Supplementary Fig. S1; http://dx.doi.org/10.1667/RR13202.1.S1). Fenofibrate administration was also associated with a reduced body weight compared to sham controls (Supplementary Fig. S1; http://dx.doi.org/10.1667/RR13202.1.S1). Previous studies have reported fenofibrate-mediated reductions in body weight in fatty Zucker (36) and diet-induced obese Wistar rats (37), due to reduced adiposity independent of changes in food intake. In contrast, fenofibrate has been shown not to affect body weight in Sprague Dawley (SD) rats (38). This inconsistency in a fenofibrate-mediated effect on body weight may reflect strain differences (F334×BN, Zucker and Wistar vs. SD rats) and/or differences in length of the fenofibrate administration. Despite the differences in weight, it is important to note that we did not observe any apparent health issues in any of the animals.
Using our well characterized rat model, we demonstrate for the first time that fenofibrate can prevent fWBI-induced PRh-dependent cognitive impairment (Fig. 1A). We have previously reported that fWBI leads to a chronic, progressive decline in PRh-dependent cognitive function assessed using the NOR task, such that by 52 weeks after fWBI, rats no longer exhibit a preference for either object (27). Whether or not the prevention of fWBI-induced PRh-dependent cognitive impairment seen at 26 weeks reflects permanent or only temporary protection requires additional studies. However, previous observations using the PPARγ agonist, pioglitazone, suggest that administering the PPAR agonist prior to, during and for only 4 weeks after fWBI was sufficient to prevent PRh-dependent cognitive impairment determined 1 year after irradiation (24). This suggests long-lasting effects of PPAR agonists that may even be sustained after drug treatment is stopped.
We also used several well validated tasks to determine if fWBI impaired hippocampal-dependent cognitive impairment. Given that irradiation has been shown to impair hippocampal-dependent cognitive function (13, 14, 29), and fenofibrate has been shown to prevent the decline in hippocampal-dependent cognitive function in a rat model of Huntington’s disease (39), we hypothesized that fenofibrate would prevent fWBI-induced impairment of hippocampal-dependent cognitive function. Although there were transient effects of fWBI on distance to platform and path length in Block 1 of the standard MWM (Fig. 2B and C), we were unable to detect consistent impairment in hippocampal-dependent cognitive function assessed using several robust variants of the hippocampal-dependent MWM task up to 29 weeks post-fWBI (Figs. 2–6). Cumulatively, our observations do not support a conclusion of fWBI-induced defects in hippocampal function for this study. The effects of fWBI on the hippocampal-dependent MWM task are still unclear; however these results confirm previous published observations of other laboratories. Chen et al. (40) delivered 30 Gy in 10 fractions over a 2 week time frame to 45-day-old Wistar rats and observed no hippocampal-dependent learning deficits 10–13 months after fWBI. Yoneoka et al. (41) delivered 40 Gy in 8 fractions over a 4 week time frame to 6-month-old F344 male rats and failed to observe any deficit in cognitive performance using the MWM at 6, 9 and 12 months postirradiation. Of interest, they did observe cognitive impairment using the hippocampal-dependent passive avoidance task at 12 months postirradiation. Similar changes have been reported in aged (16–27 months) rats that received 30 Gy in 10 fractions delivered over a 2 week time frame. MWM performance was unchanged 7 months postirradiation, while avoidance tasks revealed cognitive deficits 6–7 months postirradiation (42). However, radiation-induced impairments in MWM performance were observed 1 year after fWBI in F344×BN that were 12 months old at the time of irradiation (29). Thus, the effect of fWBI on hippocampal-dependent cognitive function in the adult rat remains controversial and is the subject of ongoing investigations.
Despite the lack of any measurable hippocampal-dependent cognitive impairment, fWBI resulted in a dramatic reduction in the number of immature neurons present in the DG at 29 weeks after fWBI (Fig. 7), confirming previous observations in this rat model (21). We have previously reported that administering fenofibrate to young adult mice starting 2 weeks prior to and for 8 weeks after a single dose of 10 Gy WBI preserved hippocampal neurogenesis by promoting the survival of newborn cells in the DG (19). In contrast to previous findings in the mouse study (19), chronic administration of fenofibrate failed to prevent this radiation-induced decrease in neurogenesis in our fWBI rat model (Fig. 7). This finding was unexpected, given that: (1) the same dose of fenofibrate was administered to young adult mice and our rats; and (2) previous studies have shown that PPARα agonists are protective in rodent models of CNS injury (19). However, it should be noted that the mouse study (19) analyzed Ki67 as a marker of actively proliferating cells and analyzed the number of surviving newborn neurons (NeuN+ cells in a cohort of BrdU labeled cells) as a marker of “neurogenesis”; the number of immature neurons (DCX+) was not determined. This significantly complicates direct comparison between the studies.
Previous studies have shown that PPARα agonists inhibit inflammatory responses of a variety of cell types, including microglia and astrocytes (43, 44). Previously, we demonstrated that fenofibrate administration to young adult male mice receiving WBI (10 Gy single dose) prevented the radiation-induced increase in microglial activation at 1 week postirradiation (28). In the current study, fenofibrate treatment did not prevent the radiation induced increase in microglial activation in the DG/hilus (Fig. 8). Perhaps delaying assessment in the present study till 6 months post-fWBI, rather than at 1 week and 2 months after irradiation in the mouse study is responsible for this difference. Additionally, at the 2 month time frame in the mouse study, microglial activation was not significantly different between any of the treatment groups. In contrast, at 6 months post-fWBI the rats had a significant increase in the total number of activated microglia in the DG/hilus. Differences between activated microglia counts could also be due to species-specific responses to irradiation and/or reflect differences in the irradiation response of the brain to single and fractionated doses of WBI.
The beneficial effect of fenofibrate on fWBI-induced deficits in some cognitive tasks suggests that it may be beneficial clinically, particularly since PPARα agonists, in addition to being neuroprotective, are increasingly recognized as potent antitumor agents. PPARα expression and activity have been reported in a variety of cancer cell lines including glioblastoma, colon, breast and prostate cancer (45). Treatment of melanoma cells with fenofibrate inhibited cell migration and colony formation by downregulation of Akt phosphorylation in vitro (46), fenofibrate also prevented the establishment of lung metastases in vivo (47). In addition, fenofibrate potently inhibited primary tumor formation in mice by regulating proangiogenic and proinflammatory pathways (45). Therefore it is reasonable to suggest that fenofibrate is likely to enhance radiation-induced tumor cell kill in primary/metastatic brain cancer patients, as well as prevent some cognitive impairment.
In summary, we have shown that fenofibrate prevents fWBI-induced PRh-dependent cognitive impairment. Fenofibrate is an FDA approved drug for the treatment of hypercholesterolemia and hypertriglyceridemia (48), can cross the blood–brain barrier (49) and is well tolerated in humans. Thus, fenofibrate may be a promising therapeutic for the prevention of radiation-induced cognitive impairment and improve the QOL of brain cancer patients receiving fWBI.
Supplementary Material
http://dx.doi.org/10.1667/RR13202.1.S1; Body weight was reduced by fWBI and/or dietary fenofibrate. The body weight of the rats during the study indicated that there was a significant effect of fWBI, with sham-irradiated rats sustaining a higher body weight compared to all other groups. In addition, dietary fenofibrate also significantly reduced body weight; both sham-irradiated + fenofibrate rats and fWBI + fenofibrate rats had lower body weights than sham-irradiated and fWBI rats. There was no significant difference between the body weights of rats in the fWBI and fWBI + fenofibrate groups. Data represent the mean ± SEM; n = 17–20/group.
http://dx.doi.org/10.1667/RR13202.1.S2; Visual acuity and locomotor behavior in the MWM. Visual acuity was unaffected by fWBI or dietary fenofibrate (panel A). The average distance to the visible platform swam over the four trials was not different between any of the treatment groups (panel A). There was a significant reduction in swim velocity in the fWBI + fenofibrate group compared to the sham-irradiated group over the four trials (panel B). Visual acuity and locomotor behavior was assessed ~28 weeks after completion of fWBI. Data represent the mean ± SEM; n = 17–20/group. **P < 0.01,***P < 0.001.
http://dx.doi.org/10.1667/RR13202.1.S3; The timing and order of the Morris Water Maze tasks performed.
Acknowledgments
This work was supported by NIH grants CA112593, (MER and LMB) and CA113267 (MER). We would like to dedicate this article to the memory of the senior author, Mike Robbins, PhD (1954–2012).
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003;29(6):533–540. doi: 10.1016/s0305-7372(03)00105-1. [DOI] [PubMed] [Google Scholar]
- 3.Khuntia D, Brown P, Li J, Mehta MP. Whole-brain radiotherapy in the management of brain metastasis. J Clin Oncol. 2006;24(8):1295–1304. doi: 10.1200/JCO.2005.04.6185. [DOI] [PubMed] [Google Scholar]
- 4.Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12(3):627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- 5.Shaw EG, Rosdhal R, D’Agostino RB, Jr, Lovato J, Naughton MJ, Robbins ME, et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. J Clin Oncol. 2006;24(9):1415–1420. doi: 10.1200/JCO.2005.03.3001. [DOI] [PubMed] [Google Scholar]
- 6.Meyers CA, Smith JA, Bezjak A, Mehta MP, Liebmann J, Illidge T, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22(1):157–165. doi: 10.1200/JCO.2004.05.128. [DOI] [PubMed] [Google Scholar]
- 7.Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44(1):109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Eichenbaum H. The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behavioural Brain Research. 2001;127:199–207. doi: 10.1016/s0166-4328(01)00365-5. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 10.Taupin P, Gage FH. Adult neurogenesis and neural stem cells of the central nervous system in mammals. J Neurosci Res. 2002;69(6):745–749. doi: 10.1002/jnr.10378. [DOI] [PubMed] [Google Scholar]
- 11.Mizumatsu S, Monje ML, Morhardt DR, Rola R, Palmer TD, Fike JR. Extreme sensitivity of adult neurogenesis to low doses of x-irradiation. Cancer Res. 2003;63(14):4021–4027. [PubMed] [Google Scholar]
- 12.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8(9):955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 13.Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162(1):39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 14.Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188(2):316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Monje ML, Vogel H, Masek M, Ligon KL, Fisher PG, Palmer TD. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol. 2007;62:515–520. doi: 10.1002/ana.21214. [DOI] [PubMed] [Google Scholar]
- 16.Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: From orphan receptors to drug discovery. J Med Chem. 2000;43(4):527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- 17.Schintu N, Frau L, Ibba M, Caboni P, Garau A, Carboni E, et al. PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson’s disease. Eur J Neurosci. 2009;29(5):954–963. doi: 10.1111/j.1460-9568.2009.06657.x. [DOI] [PubMed] [Google Scholar]
- 18.Bordet R, Ouk T, Petrault O, Gele P, Gautier S, Laprais M, et al. PPAR: a new pharmacological target for neuroprotection in stroke and neurodegenerative diseases. Biochem Soc Trans. 2006;34(Pt 6):1341–1346. doi: 10.1042/BST0341341. [DOI] [PubMed] [Google Scholar]
- 19.Ramanan S, Kooshki M, Zhao W, Hsu FC, Riddle DR, Robbins ME. The PPARalpha agonist fenofibrate preserves hippocampal neurogenesis and inhibits microglial activation after whole-brain irradiation. Int J Radiat Oncol Biol Phys. 2009;75(3):870–877. doi: 10.1016/j.ijrobp.2009.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, et al. Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology. 1997;132(2):107–124. doi: 10.1007/s002130050327. [DOI] [PubMed] [Google Scholar]
- 21.Conner KR, Payne VS, Forbes ME, Robbins ME, Riddle DR. Effects of the AT1 receptor antagonist L-158,809 on microglia and neurogenesis after fractionated whole-brain irradiation. Radiat Res. 2010;173(1):49–61. doi: 10.1667/RR1821.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robbins ME, Payne V, Tommasi E, Diz DI, Hsu FC, Brown WR, et al. The AT1 receptor antagonist, L-158,809, prevents or ameliorates fractionated whole-brain irradiation-induced cognitive impairment. Int J Radiat Oncol Biol Phys. 2009;73(2):499–505. doi: 10.1016/j.ijrobp.2008.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown WR, Blair RM, Moody DM, Thore CR, Ahmed S, Robbins ME, et al. Capillary loss precedes the cognitive impairment induced by fractionated whole-brain irradiation: a potential rat model of vascular dementia. J Neurol Sci. 2007;257(1–2):67–71. doi: 10.1016/j.jns.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Zhao W, Payne V, Tommasi E, Diz DI, Hsu F-C, Robbins ME. Administration of the peroxisomal proliferator-activated receptor (PPAR)g agonist pioglitazone during fractionated brain irradiation prevents radiation-induced cognitive impairment. Int J Radiat Oncol Biol Phys. 2007;67:6–9. doi: 10.1016/j.ijrobp.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 25.Brown WR, Thore CR, Moody DM, Robbins ME, Wheeler KT. Vascular damage after fractionated whole-brain irradiation in rats. Radiat Res. 2005;164(5):662–668. doi: 10.1667/rr3453.1. [DOI] [PubMed] [Google Scholar]
- 26.Lee TC, Greene-Schloesser D, Payne V, Diz DI, Hsu FC, Kooshki M, et al. Chronic administration of the Angiotensin-converting enzyme inhibitor, ramipril, prevents fractionated whole-brain irradiation-induced perirhinal cortex-dependent cognitive impairment. Radiat Res. 2012;178(1):46–56. doi: 10.1667/rr2731.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atwood T, Payne VS, Zhao W, Brown WR, Wheeler KT, Zhu JM, et al. Quantitative magnetic resonance spectroscopy reveals a potential relationship between radiation-induced changes in rat brain metabolites and cognitive impairment. Radiat Res. 2007;168(5):574–581. doi: 10.1667/RR0735.1. [DOI] [PubMed] [Google Scholar]
- 28.Winters BD, Saksida LM, Bussey TJ. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev. 2008;32(5):1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Shi L, Adams MM, Long A, Carter CC, Bennett C, Sonntag WE, et al. Spatial learning and memory deficits after whole-brain irradiation are associated with changes in NMDA receptor subunits in the hippocampus. Radiat Res. 2006;166(6):892–899. doi: 10.1667/RR0588.1. [DOI] [PubMed] [Google Scholar]
- 30.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 31.Steele RJ, Morris RG. Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus. 1999;9(2):118–136. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 32.Da Cunha C, Wietzikoski S, Wietzikoski EC, Silva MH, Chandler J, Ferro MM, et al. Pre-training to find a hidden platform in the Morris water maze can compensate for a deficit to find a cued platform in a rat model of Parkinson’s disease. Neurobiol Learn Mem. 2007;87(4):451–463. doi: 10.1016/j.nlm.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 33.McDonald RJ, White NM. Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol. 1994;61(3):260–270. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- 34.Shi L, Adams MM, Long A, Carter CC, Bennett C, Sonntag WE, et al. Spatial learning and memory deficits after whole-brain irradiation are associated with changes in NMDA receptor subunits in the hippocampus. Radiat Res. 2006;166(6):892–899. doi: 10.1667/RR0588.1. [DOI] [PubMed] [Google Scholar]
- 35.Lee TC, Greene-Schloesser DM, Payne V, Diz DI, Hsu F-C, Kooshki M, et al. Chronic administration of the ACE inhibitor, ramipril, prevents fractionated whole-brain irradiation-induced cognitive impairment. Radiat Res. 2012 Jul;178(1):46–56. doi: 10.1667/rr2731.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaput E, Saladin R, Silvestre M, Edgar AD. Fenofibrate and rosiglitazone lower serum triglycerides with opposing effects on body weight. Biochem Biophys Res Commun. 2000;271(2):445–450. doi: 10.1006/bbrc.2000.2647. [DOI] [PubMed] [Google Scholar]
- 37.Mancini FP, Lanni A, Sabatino L, Moreno M, Giannino A, Contaldo F, et al. Fenofibrate prevents and reduces body weight gain and adiposity in diet-induced obese rats. FEBS Lett. 2001;491(1–2):154–158. doi: 10.1016/s0014-5793(01)02146-9. [DOI] [PubMed] [Google Scholar]
- 38.De Vos P, Lefebvre AM, Miller SG, Guerre-Millo M, Wong K, Saladin R, et al. Thiazolidinediones repress ob gene expression in rodents via activation of peroxisome proliferator-activated receptor gamma. J Clin Invest. 1996;98(4):1004–1009. doi: 10.1172/JCI118860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhateja DK, Dhull DK, Gill A, Sidhu A, Sharma S, Reddy BV, et al. Peroxisome proliferator-activated receptor-α activation attenuates 3-nitropropionic acid induced behavioral and biochemical alterations in rats: possible neuroprotective mechanisms. Eur J Pharmacol. 2012;674(1):33–43. doi: 10.1016/j.ejphar.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 40.Chen QM, Lamproglou I, Poisson M, Le Poncin M, Delattre JY. Long-term effects of cranial irradiation in the rat: A behavioural study. J Neurol Sci. 1992;239 (Suppl 2):116. [Google Scholar]
- 41.Yoneoka Y, Satoh M, Akiyama K, Sano K, Fujii Y, Tanaka R. An experimental study of radiation-induced cognitive dysfunction in an adult rat model. Br J Radiol. 1999;72:1196–1201. doi: 10.1259/bjr.72.864.10703477. [DOI] [PubMed] [Google Scholar]
- 42.Lamproglou I, Chen QM, Boisserie G, Mazeron JJ, Poisson M, Baillet F, et al. Radiation-induced cognitive dysfunction: an experimental model in the old rat. Int J Radiat Oncol Biol Phys. 1995;31:65–70. doi: 10.1016/0360-3016(94)00332-F. [DOI] [PubMed] [Google Scholar]
- 43.Drew PD, Xu J, Storer PD, Chavis JA, Racke MK. Peroxisome proliferator-activated receptor agonist regulation of glial activation: relevance to CNS inflammatory disorders. Neurochem Int. 2006;49(2):183–189. doi: 10.1016/j.neuint.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Ramanan S, Kooshki M, Zhao W, Hsu FC, Robbins ME. PPARalpha ligands inhibit radiation-induced microglial inflammatory responses by negatively regulating NF-kappaB and AP-1 pathways. Free Radic Biol Med. 2008;45(12):1695–1704. doi: 10.1016/j.freeradbiomed.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Panigrahy D, Kaipainen A, Huang S, Butterfield CE, Barnes CM, Fannon M, et al. PPAR{alpha} agonist fenofibrate suppresses tumor growth through direct and indirect angiogenesis inhibition. PNAS. 2008;105(3):985–990. doi: 10.1073/pnas.0711281105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grabacka M, Plonka PM, Urbanska K, Reiss K. Peroxisome proliferator-activated receptor {alpha} activation decreases meta-static potential of melanoma cells in vitro via down-regulation of Akt. Clin Cancer Res. 2006;12(10):3028–3036. doi: 10.1158/1078-0432.CCR-05-2556. [DOI] [PubMed] [Google Scholar]
- 47.Grabacka M, Placha W, Plonka PM, Pajak S, Urbanska K, Laidler P, et al. Inhibition of melanoma metastases by fenofibrate. Arch Dermatol Res. 2004;296(2):54–58. doi: 10.1007/s00403-004-0479-y. [DOI] [PubMed] [Google Scholar]
- 48.McKeage K, Keating GM. Fenofibrate: a review of its use in dyslipidaemia. Drugs. 2011;71:1917–1946. doi: 10.2165/11208090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 49.Deplanque D, Gele P, Petrault O, Six I, Furman C, Bouly M, et al. Peroxisome proliferator-activated receptor-{alpha} activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J Neurosci. 2003;23(15):6264–6271. doi: 10.1523/JNEUROSCI.23-15-06264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
http://dx.doi.org/10.1667/RR13202.1.S1; Body weight was reduced by fWBI and/or dietary fenofibrate. The body weight of the rats during the study indicated that there was a significant effect of fWBI, with sham-irradiated rats sustaining a higher body weight compared to all other groups. In addition, dietary fenofibrate also significantly reduced body weight; both sham-irradiated + fenofibrate rats and fWBI + fenofibrate rats had lower body weights than sham-irradiated and fWBI rats. There was no significant difference between the body weights of rats in the fWBI and fWBI + fenofibrate groups. Data represent the mean ± SEM; n = 17–20/group.
http://dx.doi.org/10.1667/RR13202.1.S2; Visual acuity and locomotor behavior in the MWM. Visual acuity was unaffected by fWBI or dietary fenofibrate (panel A). The average distance to the visible platform swam over the four trials was not different between any of the treatment groups (panel A). There was a significant reduction in swim velocity in the fWBI + fenofibrate group compared to the sham-irradiated group over the four trials (panel B). Visual acuity and locomotor behavior was assessed ~28 weeks after completion of fWBI. Data represent the mean ± SEM; n = 17–20/group. **P < 0.01,***P < 0.001.
http://dx.doi.org/10.1667/RR13202.1.S3; The timing and order of the Morris Water Maze tasks performed.