Abstract
BACKGROUND
Parkinson's disease (PD) is a complex multi-system age-related neurodegenerative disorder. Targeting the ongoing neuroinflammation in PD patients is one strategy postulated to slow down or halt disease progression. Proof-of-concept studies from our group demonstrated that selective inhibition of soluble Tumor Necrosis Factor (solTNF) by intranigral delivery of dominant negative TNF (DN-TNF) inhibitors reduced neuroinflammation and nigral dopamine (DA) neuron loss in endotoxin and neurotoxin rat models of nigral degeneration.
OBJECTIVE
As a next step toward human clinical trials, we aimed to determine the extent to which peripherally administered DN-TNF inhibitor XPro®1595 could: i) cross the blood-brain-barrier in therapeutically relevant concentrations, ii) attenuate neuroinflammation (microglia and astrocyte), and iii) mitigate loss of nigral DA neurons in rats receiving a unilateral 6-hydroxydopamine (6-OHDA) striatal lesion.
METHODS
Rats received unilateral 6-OHDA (20 μg into the right striatum). Three or 14 days after lesion, rats were dosed with XPro®1595 (10 mg/kg in saline, subcutaneous) every third day for 35 days. Forelimb asymmetry was used to assess motor deficits after the lesion; brains were harvested 35 days after the lesion for analysis of XPro®1595 levels, glial activation, and nigral DA neuron number.
RESULTS
Peripheral subcutaneous dosing of XPro®1595 achieved plasma levels of 1–8 μg/mL and CSF levels of 1–6 ng/mL depending on the time the rats were killed after final XPro®1595 injection. Irrespective of start date, XPro®1595 significantly reduced microglia and astrocyte number in SNpc whereas loss of nigral DA neurons was attenuated when drug was started 3, but not 14 days after the 6-OHDA lesion.
CONCLUSIONS
Our data suggest that systemically administered XPro®1595 may have disease-modifying potential in PD patients where inflammation is part of their pathology.
Keywords: Parkinson's disease, inflammation, tumor necrosis factor, 6-OHDA, microglia, substantia nigra, XPro®1595, astrocytes
Background
Parkinson's disease (PD) is a complex multi-system age-related neurodegenerative disorder. The neuropathologic hallmarks of PD are striatal dopamine (DA) depletion resulting from loss of DA neurons within the substantia nigra pars compacta (SNpc), presence of Lewy bodies, and neuroinflammation. Current therapies attempt to maximize the function of existing neurons but do not target the molecular mechanisms that cause neuronal death. A disease modifying therapy that can protect DA neurons and prevent or delay further loss when given in the pre-motor stages of the disease is sorely needed. Targeting the ongoing neuroinflammation in PD patients is one strategy postulated to slow down or halt disease progression [1, 2]. Inflammation is observed in brains from both living [3] and deceased PD patients (e.g., [4, 5]), and epidemiological data suggests that PD risk can be mitigated with chronic use of nonsteroidal anti-inflammatory drugs [6–8].
Of the inflammatory factors that have been implicated in PD [1] work from our group and others suggests that soluble Tumor Necrosis Factor (solTNF) is required for robust endotoxin- or neurotoxin-induced nigral DA neuron degeneration [9–11]. TNF belongs to a superfamily of ligands and has been implicated in the etiology of several acquired and genetic diseases [12]. TNF is elevated in the cerebrospinal fluid and post-mortem brains of PD patients [5] and correlates with non-motor complications including cognition, depression, sleep, and disability [13–17]. Although other inflammatory cytokines are also elevated (eg: interleukin-1 and -6), solTNF sits at the apex of the inflammatory cascade and is required for the transcription of multiple pro-inflammatory cytokines [18] thereby acting as a master regulator of inflammation [19, 20]. Moreover, microglia imaging studies suggest increases in neuroinflammation occur early in PD [3], targeting solTNF in the earliest stages of PD may be a reasonable disease-modifying therapeutic strategy.
XPro®1595 is a Dominant-Negative TNF (DN-TNF) inhibitor selective for solTNF that capitalizes on the unique chemistry and biology of human solTNF. Human solTNF is a trimer of 3 identical subunits. XPro®1595 is an engineered variant of human solTNF with two amino acid substitutions that disrupt its binding to TNF receptors (TNFRs), but maintains its ability to heterotrimerize with native TNF monomers. The resulting heterotrimers, a mixture of XPro®1595 and human TNF monomers, are likewise devoid of TNFR binding affinity. Therefore, XPro®1595 effectively and selectively neutralizes >99% of solTNF within minutes if the concentration is at least 10 fold higher than the concentration of native solTNF [21]. This specificity differentiates XPro®1595 from FDA-approved anti-TNF biologics that inhibit both solTNF and tmTNF. Because XPro®1595 spares transmembrane TNF (tmTNF) activity, it does not interfere with the role of tmTNF in immunity against infections [22].
Multiple studies in vitro and in vivo demonstrate the selectivity and efficacy of XPro®1595 and related biologics in pre-clinical models of PD. In neuron-glia cultures, XENP345, an earlier version of XPro®1595 that works via the same mechanism of action, decreased microglial activation and improved DA neuron survival in the presence of lipopolysaccharide (LPS). When added up to 72 hours after LPS, XENP345 rescued approximately 50% of the DA neurons from inflammatory stress [9]. In 6-OHDA hemiparkinsonian rats, a direct infusion of XEN345 into the CNS that begins at the time of the lesion prevented DA neuron death and improved locomotor behavior [9]. The ability of DN-TNF to prevent DA neuron death has been confirmed using a lentivirus vector injected directly into the SNpc, allowing constitutive production of an XPro®1595 -like DN-TNF protein to neutralize solTNF. When injected into the SNpc at the time of the 6-OHDA lesion [10] or 2 weeks after 6-OHDA lesion [11], there was at least 50% greater DA neuron survival and improved locomotor behavior [10]. These data represent proof-of-concept that direct administration of XPro®1595 into the brain can significantly reduce nigral DA neuron death when administered prior to significant degeneration.
While these data are compelling, central administration (direct infusion or gene therapy) treatment strategies pose significant challenges in the treatment of PD patients. Importantly, recent studies have shown that peripherally administered XPro®1595 successfully decreased neuroinflammation and CNS lesions in EAE models of multiple sclerosis [23, 24]. However, as a protein therapeutic, it has not been established directly whether XPro®1595 can cross the blood-brain-barrier (BBB) to treat central neuroinflammation. Although data in the MOG-EAE model suggested direct central effects of XPro®1595 in the brain, the increased permeability inherent to the MOG-EAE model raised the possibility that disruption of the BBB facilitated entry of XPro®1595 into the CNS. In the current study we used the 6-hydroxydopamine (6-OHDA) hemiparkinsonian rat model. The 6-OHDA model is the gold standard rat model for examining therapeutic strategies (e.g., [25–27]) and does not compromise the BBB, at least to the extent to which non-selective TNF inhibitors can cross into the brain [28]. Herein we report that peripherally administered XPro®1595 can cross into the CNS in therapeutically relevant concentrations to block glial activation and when given three days after the lesion, can attenuate the loss of SNpc DA neurons.
Methods
Animals
Adult male Sprague-Dawley rats (250g) were purchased from Charles River Laboratories International, Inc. (Wilmington, MA) and allowed to acclimate to their new housing quarters for 2 weeks prior to any experimental procedure. Rats were pair-housed in standard transparent Plexiglas cages in a colony room maintained at 22 ± 1°C with a reverse 12-hour light, 12-hour dark cycle (lights on 2100 hrs to 0900 hrs). In all experiments, rats had free access to food and water ad libitum. All manipulations (injections and behavior) were conducted during lights out (rats subjective day) under red light between 1200–1500 hrs with the exception of the stereotaxic surgery, which was performed with lights on between 1100 hrs – 1600 hrs. All studies and animal protocols were approved and guided by the Institutional Animal Care and Use Committee at Emory University.
Surgical procedures
Rats (N=47) were anesthetized with inhalant isoflurane (2–3%; Sigma, St Louis, MO) in oxygen (2.5 L/min), placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA) and received 4 μl (20 μg) of 6-hydroxydopamine (6-OHDA; Sigma, St Louis, MO) solution or sterile saline (mock lesion) in a single unilateral injection at a rate of 0.5 μl/minute [11]. The stereotaxic coordinates were as follows: anteroposterior, 1.0 mm from bregma; mediolateral, −3.0 mm; dorsoventral, −4.5 mm below surface of the dura. After a 5 min waiting period, the needle was slowly retracted. Forty-five minutes prior to surgery and for the following 24 hours, animals received subcutaneous injections of the buprenorphine HCL (0.1 mg/kg; Reckitt Benckiser Pharmaceuticals Inc., Richmond VA) and were monitored closely for signs of pain or discomfort. Soft chow was provided to rats for 5 days following surgery.
XPro®1595 dosing
Beginning 3 or 14 days post 6-OHDA or mock (saline) lesion, rats received subcutaneous (s.c.) injections of XPro®1595 (10 mg/kg in sterile saline) or saline every 3rd day. This dosing schedule is based on a serum half-life for s.c. dose of ~18 hr in rats. Peripherally administered XPro®1595 was assessed for its potential to enter the brain in therapeutic concentrations, reduce microglia and astrocyte numbers, and protect tyrosine hydroxylase neurons (TH/NeuN+) in substantia nigra pars compacta (SNpc).
Determination of XPro®1595 levels in plasma and CSF of rats injected with XPro®1595 peripherally
Rats (n=20) received mock (saline; n=10) or 6-OHDA (n=10) lesion. Rats from both mock and 6-OHDA-lesioned groups received a subcutaneous injection of either saline (n=2) or XPro®1595 treatment beginning on day 3 (n=4) or 14 (n=4). Blood was collected on days 14 (prior to 1st XPro®1595 treatment) and again at the end of the study (day 35) where CSF was also collected.
Plasma and CSF collection for measurement of XPro®1595
Blood was taken by tail vein 14 days and trunk blood 5 weeks after 6-OHDA surgeries. At 5 weeks post surgery, rats were deeply anesthetized with Euthasol (Butler, Dublin, OH) and placed into a stereotaxic frame for CSF collection via the cisterna magna puncture method according to [29] with minor modifications. With the nose positioned downward at approximately 45°, the cisterna magna was exposed by dissecting through the musculature overlying the posterior atlantooccipital membrane. The membrane was punctured using a 27 gauge Hamilton syringe attached to the stereotaxic frame and CSF (~125 μL) was slowly aspirated and immediately flash frozen in liquid nitrogen and stored at −80°C until time of assay. This process took less than 2 min upon which trunk blood was immediately collected. At day 14, tail blood was collected using the tail clip method [30]. Using the tail clip method, blood samples (~200 μL) were collected within 3 min of disturbing the cage by gently stroking the tail. All blood was collected in EDTA coated tubes and immediately placed on ice; samples were on ice < 30 min. Plasma was extracted after a 15 min spin (2000 g) in a pre-cooled (4°C) centrifuge. Plasma was immediately flash frozen in liquid nitrogen and stored at −80°C until time of assay.
Measurement of XPro®1595
Plasma (diluted 1:4) and CSF (undiluted) levels of XPro®1595 (10 mg/kg; s.c.) were measured using the Meso Scale Discovery anti-human TNF ultrasensitive (cat # K151BHC-1) electrochemiluminescent detection immunoassay and quantified using the Meso Scale Discovery SECTOR Imager 2400-A (Meso Scale Diagnostics, LLC, Rockville, MD). To measure XPro®1595, the supplied human TNF protein used to generate a calibrator/standard protein was replaced by the XPro®1595 protein, which increases the sensitivity of the assay substantially. Signals from the linear standard curve range from 3000 (at 0 XPro®1595) to 1 million counts (at 250 ng/mL). All samples were assayed in duplicate by an experimentalist blinded to treatment history. The data were analyzed using the Meso Scale Discovery integrated data analysis software which converts signal to pg/mL values.
Histological and Behavioral analysis of 6-OHDA hemiparkinsonian rats treated with XPro®1595 peripherally
All rats (n=27) received a unilateral lesion of 6-OHDA within the right striatum. Previous studies demonstrate that the contralateral SNpc and striatum remains unaffected by this procedure; thus, each animal also served as its own control [9–11]. 6-OHDA-lesioned rats were then treated with saline or XPro®1595 beginning 3 (saline n=6; XPro®1595 n=6) or 14 (saline n=6; XPro®1595 n=7) days post lesion. Experimental design is as follows with specific details below: Forelimb asymmetry (Cylinder test) was assessed 21 days post lesion. Five weeks after lesion (Day 35), rats were killed and brains were processed for immunohistochemistry followed by stereology.
Cylinder test
The cylinder test was performed as described previously [11]. To determine the effect of XPro®1595 on motor deficits induced by a unilateral 6-OHDA lesion, forepaw asymmetry was assessed 21 days following the 6-OHDA (or mock) lesion. Rats were placed into Plexiglas cylinders (15 cm × 45 cm; Diameter × height) and video was recorded for 3 minutes. An investigator blinded to treatment video recorded and later counted the number of ipsilateral and contralateral forepaw touches by each animal. Rats receiving a 6-OHDA lesion in the right striatum have significant motor impairment in the contralateral (left) forepaw. Thus, impaired motor performance in rats with a 6-OHDA lesion in the right striatum will have a greater percentage of right forepaw contacts. The number of right and left forepaw touches were counted. Data presented as percentage of right (non-impaired) forepaw contacts and correlated with loss of TH/NeuN+ cell loss in the SNpc.
Tissue processing
At 5 weeks post 6-OHDA lesion, animals were deeply anesthetized with Euthasol (Butler, Dublin, OH) and transcardially perfused with 250 mL of heparinized (1 ml/l) phosphate-buffered saline; pH 7.4. Rat brains were post-fixed in 4% paraformaldehyde for 24 h at 4°C, and equilibrated in 20–30% (w/v) sucrose in PBS for 24–48 h at 4°C. Brains were cryosectioned into 30 μm-thick coronal sections using a dry-ice cooled SM2010R sliding microtome (Leica, Bannockburn, IL). Coronal sections spaced 120 μm apart throughout the SN (in the range from −4.6 mm to −6.2 mm relative to bregma) were collected.
Stereological estimate of SNpc DA neurons
For the quantification of DA neurons in SN, sections were stained with a polyclonal antibody to the rate-limiting enzyme in DA synthesis tyrosine hydroxylase (anti-TH) (1:1000 dilution; AB152, Millipore, Billerica, MA). Biotinylated anti-rabbit IgG reagent was used as the secondary antibody (1:250 dilution; Vector Laboratories). TH signal was amplified with an ABC Elite kit (Vector Laboratories) and detected with diaminobenzidine (DAB) with nickel (Vector Laboratories). Nuclei were counterstained with anti-NeuN monoclonal antibody (1:1000 dilution, MAB 377, Millipore, Billerica, MA) and biotinylated anti-mouse IgG secondary antibody. NeuN signal was amplified with DAB (brown). Unbiased stereological estimates of dopamine (DA; TH+ cell) and total neuron (NeuN+ cell) numbers were performed using StereoInvestigator analysis software (MicroBrightField, Williston, VT) and the optical fractionator method as previously published [11]. Briefly, boundaries in the substantia nigra pars compacta (SNpc) were outlined according to previously defined anatomical analysis in the rat [31] and cells were counted from 11–13 sections (to ensure coefficient of errors <0.1) by investigators blinded to treatment history under a ×40 oil-immersion objective on a Nikon 80i microscope (Nikon Melville, NY). Stereological parameters: average mounted thickness, 20 μm; optical dissector, 16 μm; and upper and lower guard zones, 2 μm.
Microglia quantification
Coronal sections (240 μm apart) were collected throughout the SNpc (in the range −4.6 mm to −6.2 mm relative to bregma). Every 8th section, a total of 6 sections per brain, were stained with anti-IbaI antibody (1:600 dilution; Abcam, Cambridge, MA) and Alexa Fluro 488 anti-goat IgG (1:1000 dilution; Invitrogen, Carlsbad, CA) secondary antibody. Serial fluorescent microglia pictures were captured. The number of microglia in SN per field was quantified using Nikon NIS-Element software.
Astrocytes quantification
Coronal sections (240 μm apart) were collected throughout the SN (in the range −4.6 mm to −6.2 mm relative to bregma). Every 8th section, a total of 6 sections per brain, were stained with anti-GFAP antibody (1:1000 dilution; Dako, Carpinteria, CA) and Alexa Fluro 594 anti-rabbit IgG (1:1000 dilution; Invitrogen, Carlsbad, CA) secondary antibody. Serial fluorescent astrocyte pictures were captured. The sum intensity of fluorescence in SN per field was quantified by using Nikon NIS-Element software.
Statistical analysis
No differences in any dependent measures were observed between 6-OHDA-lesioned rats treated with saline beginning 3 or 14 days post-lesion. Because of this, these animals were collapsed into one group. This served to increase statistical power and limit the unnecessary use of laboratory animals thereby adhering to the principles of Reduce, Refine, and Reuse (3Rs). In all analyses, data are expressed as a percentage of the intact (contralateral) SNpc and comparisons are made between rats that received a unilateral 6-OHDA lesion and treated with Saline, XPro®1595 given on day 3 (XPro(3)), or XPro®1595 started on day 14 (XPro(14)). A one-way analysis of variance (ANOVA) was used to examine potential treatment differences in DA neurons (TH/NeuN+ cells) in SNpc (Figure 3B), microgliosis (Figure 2B), and astrogliosis (Figure 2C). Pearson's r coefficient was used to examine correlations between percent loss of nigral DA neurons (TH/NeuN+ cells) neurons and the cylinder test (Figure 4D), microgliosis (Figure 4A), and astrogliosis (Figure 4B). Correlations between microgliosis and astrogliosis (Figure 4C), and between plasma and CSF levels of XPro®1595 (Figure 1) were also examined. All parametric tests passed both the normality and equal variance test. Alpha was set at P < 0.05 and data are expressed as mean ± standard error of the mean (SEM). All statistical analyses were performed with GraphPad Prism version 6.0 for mac (GraphPad Software, La Jolla California USA, www.graphpad.com).
Figure 3. Early, but not delayed XPro®1595 attenuates nigral cell death.
(A) Representative images of TH/NeuN+ staining within the SNpc of rats that received a unilateral 6OHDA lesion ± XPro®1595 starting on day 3 [XPro(3)] or 14 [XPro(14)]. (B) Rats injected s.c. with XPro®1595 starting 3 days after 6OHDA lesion displayed reduced dopaminergic (TH/NeuN+) cell loss in the SNpc compared to rats treated with XPro®1595 starting on day 14 or saline-treated rats. Data are expressed as a percentage of the contralateral (intact) SNpc and group differences were analyzed using a one-way ANOVA (F2,24=16.75, p<0.05) and Fisher's LSD post hoc and are expressed as mean ± SEM. Significance is denoted by different letters. (C) Raw stereological counts in the lesioned and non-lesioned SNpc of various treatment groups. Saline, n=12; XPro(3), n=8; XPro(14), n=7.
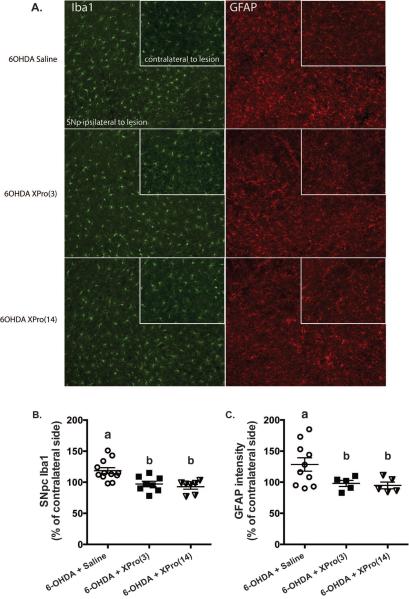
Figure 2. XPro®1595 reduces microglia and astrocyte activation measured at day 35 when started 3 or 14 days after lesion.
(A) Representative immunofluorescence images of Iba1 and GFAP stained SNpc. Inset shows contralateral (intact) SNpc. Rats treated with XPro®1595 beginning 3 or 14 after lesion had reduced Iba1 number (B) and GFAP intensity (C) within the SNpc. Data are expressed as a percentage of the contralateral (intact) SNpc and group differences were analyzed using a one-way ANOVA (Iba1: F2,24=9.72, p<0.05; GFAP: F2,17=3.83, p<0.05) and are expressed as mean ± S.E. Significance is denoted by different letters. For Iba1, Saline, n=12; XPro(3), n=8; XPro(14), n=7. For GFAP, Saline, n=10; XPro(3), n=5; XPro(14), n=5.
Figure 4. Glial activation and motor impairment correlates with loss of nigral DA neurons.
(A) Microglia number denoted by Iba1 (R2=0.24, p<0.05) and (B) astrocyte number denoted by GFAP (R2=0.52, p<0.05) were highly correlated with the percent loss of DA (TH/NeuN+ cell) neurons within the SNpc. (C) Iba1 and GFAP were also highly correlated (R2=0.57, p<0.05) in 6-OHDA-lesioned rats. (D) Forepaw asymmetry 21 days after 6-OHDA lesion correlated with the percentage of SNpc dopaminergic (TH/NeuN+) cell loss on day 35 (R2=0.26, p<0.05).
Figure 1. Plasma levels of peripherally administered XPro®1595 correlate with CSF levels.
Log plasma and CSF levels were highly correlated (R2=0.70, N=20, p<0.05).
Results
Determination of XPro®1595 levels in plasma and CSF of rats injected with XPro®1595 peripherally
XPro®1595 (10 mg/kg in saline, s.c. every third day) was measured in 20 rats (n=4 per group) using a human TNF-specific immunoassay (Meso Scale Discovery) with XPro®1595 substituted as the standard/calibrator. XPro®1595 was detected in the CNS (3 ± 1 ng/mL) and plasma (4787 ± 1931 ng/mL) in rats that received either 6-OHDA or mock lesion. Background signals for XPro®1595 were derived from saline-treated rats. Table 1 illustrates levels of XPro®1595 measured in the various treatment groups. Plasma and CSF XPro®1595 levels were highly correlated (r = 0.84 N=20, p<0.05, one tail; Figure 1). The intra-assay coefficient of variance was less than 5%.
Table 1. Peripherally administered XPro®1595 crosses into the CNS in therapeutically relevant concentrations.
XPro®1595 was measured using a human TNF-specific immunoassay (Meso Scale Discovery) in plasma (Day 14 and 35) and CSF (Day 35). Animals killed two or three days after final injection had plasma levels in the range of 1000–8000 ng/mL and CSF levels of 1.9 – 5.7 ng/mL.
| XPro1595 Levels | N | XPro1595 Plasma Levels Day 13 | XPro1595 Plasma Levels Day 35 | XPro1595 CSF Levels Day 35 | Correlation (Plasma vs CSF) |
|---|---|---|---|---|---|
| Saline/Saline | n=2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | n/a |
| 6OHDA/Saline | n=2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | n/a |
| * Saline/XPro(3) | n=4 | 3427 ± 1105 | 1145 ± 144 | 1.9 ± 0.1 | R2 = 0.87, p<0.05 |
| * 6OHDA/XPro(3) | n=4 | 8968 ± 4252 | 3147 ± 3077 | 2.2 ± 1.4 | R2 = 0.99, p<0.05 |
| ^ ∘ Saline/XPro(14) | n=4 | 0.0 ± 0.0 | 8342 ± 5468 | 5.7 ± 2.7 | R2 = 0.94, p<0.05 |
| ^ ∘ 6OHDA/XPro(14) | n=4 | 0.0 ± 0.0 | 7512 ± 4520 | 3.5 ± 2.2 | R2 = 0.91, p<0.05 |
Animals were killed 3 days after final XPro1595 injection
Animals were killed 2 days after final XPro1595 injection
Blood was taken one day before ihe start of XPro 1595 injections
All values are ng/mL
Histological and behavioral analysis of 6-OHDA-lesioned rats treated with XPro®1595 peripherally
Consistent with a role for solTNF in mediating inflammatory responses induced by 6-OHDA [9–11], XPro®1595 -treated rats displayed reduced microgliosis (F2,24=9.72, p<0.05; Figure 2A) and astrogliosis in SNpc (F2,17=3.83, p<0.05; Figure 2B) compared to saline-treated rats; regardless of whether XPro®1595 treatment began 3 or 14 days post lesion (p<0.05).
Since XPro®1595 entered the brain and reduced inflammation, we sought to determine whether it would also attenuate 6-OHDA-induced nigral DA neuron death. Because the 6-OHDA model employed in the current study has both an early- and late phase of nigral degeneration [11], we investigated whether peripherally administered XPro®1595 could exert neuroprotection when given in either phase. Rats were injected subcutaneously with XPro®1595 every third day beginning 3 or 14 days post lesion. As shown in Figure 3B, 6-OHDA-lesioned rats that started XPro®1595 treatment 3 days following surgery retained a significantly greater number of nigral DA neurons measured at day 35 after the lesion (TH/NeuN+ cells) (one way ANOVA; F2,24=16.75, p<0.05) compared to 6-OHDA-lesioned rats treated with XPro®1595 begining14 days after lesion and saline (p<0.05).
We also examined the extent to which inflammation correlated with the loss of nigral DA neurons and locomotor deficits. Both microgliosis (r = 0.49, N=26, p<0.05, one tail; Figure 4A) and astrogliosis (r = 0.72, N=15, p<0.05, one tail; Figure 4B) correlated with the percent loss of nigral DA neurons within the SNpc. Rats that had high levels of microgliosis also had high levels of astrogliosis (r = 0.76, N=16, p<0.05, one tail; Figure 4C). Finally, rats with the greatest amount of nigral DA neuron loss were the same rats displaying paw preference (asymmetry) on the cylinder test (r = 0.51, N=22, p<0.05, on tail; Figure 4D).
Discussion
Targeting the ongoing neuroinflammation in PD patients is one strategy postulated to slow down or halt disease progression. Our initial findings that central administration of DN-TNF peptide or lentiviral-derived inhibitors attenuated nigral DA neuron death and neuroinflammation in rat models of PD represented the first proof-of-concept for the validity of the solTNF targeting approach. In an effort to translate this therapeutic strategy to patients with PD, in the current study we sought to determine whether peripheral administration of XPro®1595 could block neuroinflammation and nigral DA neuron death induced by intrastriatal 6-OHDA.
Our first objective was to determine whether peripheral administration of XPro®1595 could gain access to the brain in therapeutically relevant concentrations. XPro®1595 was dosed at 10 mg/kg in saline every third day. This systemic dosing achieved terminal plasma levels of 1–8 μg/mL and CSF levels of 1–6 ng/mL depending on whether the rats were killed two or three days after final XPro®1595 injection. These levels did not depend on a compromised blood-brain-barrier as mock-lesioned rats injected with XPro®1595 had comparable CSF levels of drug. These levels of XPro®1595 within the CSF (1–6 ng/mL) are sufficient to neutralize >99.9% of soluble TNF at the pathological levels found in most chronic inflammatory neurological disorders, such as the 30–90 pg/mL reported for Parkinson's patients CSF [5, 32].
Our second objective was to investigate whether once XPro®1595 got into the brain, it could attenuate markers of neuroinflammation. We examined the extent to which early versus delayed XPro®1595 treatment could attenuate microglia and astrocyte activation in rats that received a unilateral 6-OHDA intrastriatal lesion. Consistent with previous studies in which DNTNF was administered centrally (e.g., [9–11]), peripherally administered XPro®1595 attenuated both microglia and astrocyte activation regardless of treatment start date. These data provide further support that brain levels of 1–6 ng/mL of XPro®1595 are sufficient to attenuate neuroinflammation.
As a final test of efficacy for peripherally administered XPro®1595 , we sought to determine whether early versus delayed administration attenuated nigral DA neuron death and motor impairment in a well-characterized hemiparkinsonian rat model where direct intranigral delivery of this biologic had already shown good efficacy. Our findings demonstrate that blocking solTNF shortly after 6-OHDA lesion is key to achieving robust neuroprotection of nigral dopaminergic neurons. We found that when XPro®1595 was given beginning 3 days after lesion, 15% of nigral DA neurons were lost. Rats that began XPro®1595 treatment 14 days after lesion had comparable nigral DA neuron loss (44%) to saline-treated rats (55%). Because these analyses were done in the same animals, we were able to show that those rats with increased nigral DA neuron death also had increased (correlated highly) microglia and astrocyte number and motor dysfunction. In summary, where XPro®1595 attenuated nigral cell death, it also attenuated neuroinflammation and motor deficits in those same animals.
In hemiparkinsonian rats, the bulk of DA neuron loss occurs within the first 7 days [33–37]. Our early intervention began 3 days after lesion, suggesting that XPro®1595 can attenuate an ongoing degenerative process in which inflammation is a necessary component. Recent clinical data suggests that in humans with PD this window is approximately 4 years from time of diagnosis to maximum nigral DA neuron loss [38]. These data lead us to conclude that a disease-modifying window may exist even after a patient has received a clinical diagnosis of PD and that XPro®1595 will be effective in those PD patients where inflammation may be accelerating neuropathology. Given the emergent data that inflammation may also underlie some of the non-motor symptoms of PD [13], there may be a beneficial and protective role for XPro®1595 at several different stages of the disease. Together, these data demonstrate that peripheral administration of XPro®1595 can robustly attenuate nigral DA neuron loss even when given some time after the beginning of an insult that triggers oxidative stress.
While timing appears to be critical to attenuate nigral DA neuron death, XPro®1595 was able to block neuroinflammation regardless of when it was administered in relation to the lesion, as both microglia and astrocyte markers were reduced in rats given XPro®1595 beginning on day 3 or at day 14. The significance of being able to block inflammation at any point during the disease might have benefits for non-motor symptoms [13, 39]. Subsequently, others have shown that TNF [16] and c-reactive protein (CRP), classic companion biomarkers of inflammation, were correlated with fatigue [15], depression [40], cognition [41], and hallucination [42] in PD patients and plasma solTNF receptors were correlated with cognitive impairment in PD patients [17]. Elevated CRP has also been observed to correlate with motor dysfunction in PD patients [43–46]. If these specific inflammatory factors are tied to the mechanism of non-motor (depression or cognition) symptoms, a single therapy may treat both motor and non-motor symptoms of PD by suppressing central and peripheral inflammation. Consistent with this idea, TNF antagonism with the TNF antibody infliximab was recently evaluated in patients with refractory depression. The authors found that peripheral anti-TNF therapy could ameliorate depressive symptoms in a subset of patients, and that these patients were defined by elevated levels of plasma CRP [47]. The authors proposed CRP as a biomarker for treatment response to anti-TNF therapy. These findings may have major implications for PD; specifically for identifying PD patients with active inflammation who should derive CNS benefit from systemic XPro®1595 therapy. Regardless, the ability of peripherally administered XPro®1595 to attenuate the neuroinflammatory response suggests its utility may reach beyond the motor symptoms of PD and should be investigated in other models of disease that are characterized by chronic activation of microglia and astrocytes.
The results in the current work are selective for solTNF, and therefore XPro®1595 . Commercially available anti-TNF inhibitors are non-selective and therefore block the activities of both solTNF and tmTNF. The positive effects of TNF antagonism (i.e. inhibiting inflammation) occur primarily via solTNF, whereas inhibition of tmTNF signaling can compromise host defense, inhibit myelination and impair reparative functions [22, 23, 48–50]. In addition to safety liabilities, there is no compelling data that any of the commercially available FDA-approved TNF-antagonists can access the brain in the absence of significant CNS pathology and significant disruption of the BBB. Because of their non-selectivity against soluble and tmTNF, TNF antagonists currently on the market (including infliximab) have warning labels against use in patients with neurologic diseases due to significant liabilities in terms of both immune suppression and inhibition of myelination, leading to susceptibility to infection and precipitation or worsening of demyelinating disorders [51–53]. XPro®1595 is the only anti-TNF therapy demonstrated to selectively antagonize solTNF, access the brain, and attenuate neuroinflammation and nigral DA neuron death when administered peripherally.
In summary, the data presented herein demonstrate that peripheral administration of XPro®1595 can enter the brain and significantly attenuate neuroinflammation. To attenuate nigral DA neuron death, it was important that XPro®1595 be given early during the course of disease when inflammation is playing a central role in neuronal demise. That inflammation can be attenuated by XPro®1595 early or later in the disease process demonstrates the potential utility of this drug in treating PD-associated ailments that may have an inflammatory component. In fact, XPro®1595 has shown to be effective in other disease models where inflammation is part of the disease process, including multiple sclerosis (e.g.,[23, 50]). Thus, where inflammation is part of pathology, XPro®1595 can be therapeutically efficacious when administered peripherally. We have reproducibly attenuated nigral DA neuron death using various pre-clinical models of PD, different species, and different routes of administration. This capstone study has clear clinical implications and provides compelling rationale for moving XPro®1595 toward a clinical trial in pre-motor or newly diagnosed PD patients.
Acknowledgments including sources of support
These studies were conducted with financial support from The Michael J. Fox Foundation Therapeutic Development Initiative awarded to FPRT Bio, Inc. (RJT) and Emory under subcontract. We thank D.E. Szymkowski at Xencor Inc. for providing XPro®1595 . Additional funding support for this work came from the NIEHS T32 (5T32ES012870-10; CJB).
Footnotes
Conflict of Interest R.J Tesi is CEO of FPRT Bio, Inc., which retains commercial rights to XPro®1595 . M. G. Tansey is a co-inventor on patents describing the DN-TNF technology and an ex-employee of Xencor Inc., the biotherapeutic company that developed the DN-TNF technology and XPro®1595 but holds no significant financial stake in the company. The remaining authors have no conflict of interest to report.
References
- [1].Tansey MG, Goldberg MS. Neuroinflammation in Parkinson's disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010;37:510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Luo L, Rodriguez E, Jerbi K, Lachaux JP, Martinerie J, Corbetta M, Shulman GL, Piomelli D, Turrigiano GG, Nelson SB, Joels M, de Kloet ER, Holsboer F, Amodio DM, Frith CD, Block ML, Zecca L, Hong JS, Dantzer R, Kelley KW, Craig AD. Ten years of Nature Reviews Neuroscience: insights from the highly cited. Nat Rev Neurosci. 2010;11:718–726. doi: 10.1038/nrn2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ. In vivo imaging of microglial activation with [11C](R)- PK11195 PET in idiopathic Parkinson's disease. Neurobiol Dis. 2006;21:404–412. doi: 10.1016/j.nbd.2005.08.002. [DOI] [PubMed] [Google Scholar]
- [4].McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- [5].Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. Tumor necrosis factor-alpha (TNF-alpha) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994;165:208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- [6].Chen H, Jacobs E, Schwarzschild MA, McCullough ML, Calle EE, Thun MJ, Ascherio A. Nonsteroidal antiinflammatory drug use and the risk for Parkinson's disease. Ann Neurol. 2005;58:963–967. doi: 10.1002/ana.20682. [DOI] [PubMed] [Google Scholar]
- [7].Chen H, Zhang SM, Hernan MA, Schwarzschild MA, Willett WC, Colditz GA, Speizer FE, Ascherio A. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol. 2003;60:1059–1064. doi: 10.1001/archneur.60.8.1059. [DOI] [PubMed] [Google Scholar]
- [8].Samii A, Etminan M, Wiens MO, Jafari S. NSAID use and the risk of Parkinson's disease: systematic review and meta-analysis of observational studies. Drugs Aging. 2009;26:769–779. doi: 10.2165/11316780-000000000-00000. [DOI] [PubMed] [Google Scholar]
- [9].McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, Botterman BR, Tansey KE, Tansey MG. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson's disease. J Neurosci. 2006;26:9365–9375. doi: 10.1523/JNEUROSCI.1504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].McCoy MK, Ruhn KA, Martinez TN, McAlpine FE, Blesch A, Tansey MG. Intranigral lentiviral delivery of dominant-negative TNF attenuates neurodegeneration and behavioral deficits in hemiparkinsonian rats. Mol Ther. 2008;16:1572–1579. doi: 10.1038/mt.2008.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Harms AS, Barnum CJ, Ruhn KA, Varghese S, Trevino I, Blesch A, Tansey MG. Delayed dominant-negative TNF gene therapy halts progressive loss of nigral dopaminergic neurons in a rat model of Parkinson's disease. Mol Ther. 2011;19:46–52. doi: 10.1038/mt.2010.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tansey MG, Frank-Cannon TC, McCoy MK, Lee JK, Martinez TN, McAlpine FE, Ruhn KA, Tran TA. Neuroinflammation in Parkinson's disease: is there sufficient evidence for mechanism-based interventional therapy? Front Biosci. 2008;13:709–717. doi: 10.2741/2713. [DOI] [PubMed] [Google Scholar]
- [13].Barnum CJ, Tansey MG. Neuroinflammation and non-motor symptoms: the dark passenger of Parkinson's disease? Curr Neurol Neurosci Rep. 2012;12:350–358. doi: 10.1007/s11910-012-0283-6. [DOI] [PubMed] [Google Scholar]
- [14].Doty RL. Olfaction in Parkinson's disease and related disorders. Neurobiol Dis. 2012;46:527–552. doi: 10.1016/j.nbd.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lindqvist D, Hall S, Surova Y, Nielsen HM, Janelidze S, Brundin L, Hansson O. Cerebrospinal fluid inflammatory markers in Parkinson's disease--associations with depression, fatigue, and cognitive impairment. Brain Behav Immun. 2013;33:183–189. doi: 10.1016/j.bbi.2013.07.007. [DOI] [PubMed] [Google Scholar]
- [16].Lindqvist D, Kaufman E, Brundin L, Hall S, Surova Y, Hansson O. Non-motor symptoms in patients with Parkinson's disease - correlations with inflammatory cytokines in serum. PLoS One. 2012;7:e47387. doi: 10.1371/journal.pone.0047387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Rocha NP, Teixeira AL, Scalzo PL, Barbosa IG, de Sousa MS, Morato IB, Vieira EL, Christo PP, Palotas A, Reis HJ. Plasma levels of soluble tumor necrosis factor receptors are associated with cognitive performance in Parkinson's disease. Mov Disord. 2014;29:527–531. doi: 10.1002/mds.25752. [DOI] [PubMed] [Google Scholar]
- [18].Harms AS, Lee JK, Nguyen TA, Chang J, Ruhn KM, Trevino I, Tansey MG. Regulation of microglia effector functions by tumor necrosis factor signaling. Glia. 2012;60:189–202. doi: 10.1002/glia.21254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Frankola KA, Greig NH, Luo W, Tweedie D. Targeting TNF-alpha to elucidate and ameliorate neuroinflammation in neurodegenerative diseases. CNS Neurol Disord Drug Targets. 2011;10:391–403. doi: 10.2174/187152711794653751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Steed PM, Tansey MG, Zalevsky J, Zhukovsky EA, Desjarlais JR, Szymkowski DE, Abbott C, Carmichael D, Chan C, Cherry L, Cheung P, Chirino AJ, Chung HH, Doberstein SK, Eivazi A, Filikov AV, Gao SX, Hubert RS, Hwang M, Hyun L, Kashi S, Kim A, Kim E, Kung J, Martinez SP, Muchhal US, Nguyen DH, O'Brien C, O'Keefe D, Singer K, Vafa O, Vielmetter J, Yoder SC, Dahiyat BI. Inactivation of TNF signaling by rationally designed dominant-negative TNF variants. Science. 2003;301:1895–1898. doi: 10.1126/science.1081297. [DOI] [PubMed] [Google Scholar]
- [22].Zalevsky J, Secher T, Ezhevsky SA, Janot L, Steed PM, O'Brien C, Eivazi A, Kung J, Nguyen DH, Doberstein SK, Erard F, Ryffel B, Szymkowski DE. Dominant-negative inhibitors of soluble TNF attenuate experimental arthritis without suppressing innate immunity to infection. J Immunol. 2007;179:1872–1883. doi: 10.4049/jimmunol.179.3.1872. [DOI] [PubMed] [Google Scholar]
- [23].Brambilla R, Ashbaugh JJ, Magliozzi R, Dellarole A, Karmally S, Szymkowski DE, Bethea JR. Inhibition of soluble tumour necrosis factor is therapeutic in experimental autoimmune encephalomyelitis and promotes axon preservation and remyelination. Brain. 2011;134:2736–2754. doi: 10.1093/brain/awr199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Evangelidou M, Karamita M, Vamvakas SS, Szymkowski DE, Probert L. Altered expression of oligodendrocyte and neuronal marker genes predicts the clinical onset of autoimmune encephalomyelitis and indicates the effectiveness of multiple sclerosis-directed therapeutics. J Immunol. 2014;192:4122–4133. doi: 10.4049/jimmunol.1300633. [DOI] [PubMed] [Google Scholar]
- [25].Blandini F, Armentero MT. Animal models of Parkinson's disease. FEBS J. 2012;279:1156–1166. doi: 10.1111/j.1742-4658.2012.08491.x. [DOI] [PubMed] [Google Scholar]
- [26].Gombash SE, Lipton JW, Collier TJ, Madhavan L, Steece-Collier K, Cole-Strauss A, Terpstra BT, Spieles-Engemann AL, Daley BF, Wohlgenant SL, Thompson VB, Manfredsson FP, Mandel RJ, Sortwell CE. Striatal pleiotrophin overexpression provides functional and morphological neuroprotection in the 6-hydroxydopamine model. Mol Ther. 2012;20:544–554. doi: 10.1038/mt.2011.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gombash SE, Manfredsson FP, Mandel RJ, Collier TJ, Fischer DL, Kemp CJ, Kuhn NM, Wohlgenant SL, Fleming SM, Sortwell CE. Neuroprotective potential of pleiotrophin overexpression in the striatonigral pathway compared with overexpression in both the striatonigral and nigrostriatal pathways. Gene Ther. 2014 doi: 10.1038/gt.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhou QH, Sumbria R, Hui EK, Lu JZ, Boado RJ, Pardridge WM. Neuroprotection with a brain-penetrating biologic tumor necrosis factor inhibitor. J Pharmacol Exp Ther. 2011;339:618–623. doi: 10.1124/jpet.111.185876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nirogi R, Kandikere V, Mudigonda K, Bhyrapuneni G, Muddana N, Saralaya R, Benade V. A simple and rapid method to collect the cerebrospinal fluid of rats and its application for the assessment of drug penetration into the central nervous system. J Neurosci Methods. 2009;178:116–119. doi: 10.1016/j.jneumeth.2008.12.001. [DOI] [PubMed] [Google Scholar]
- [30].Barnum CJ, Blandino P, Jr, Deak T. Adaptation in the corticosterone and hyperthermic responses to stress following repeated stressor exposure. J Neuroendocrinol. 2007;19:632–642. doi: 10.1111/j.1365-2826.2007.01571.x. [DOI] [PubMed] [Google Scholar]
- [31].German DC, Manaye KF. Midbrain dopaminergic neurons (nuclei A8, A9, and A10): three-dimensional reconstruction in the rat. J Comp Neurol. 1993;331:297–309. doi: 10.1002/cne.903310302. [DOI] [PubMed] [Google Scholar]
- [32].Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, Hirsch EC. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson's disease. Neurosci Lett. 1994;172:151–154. doi: 10.1016/0304-3940(94)90684-x. [DOI] [PubMed] [Google Scholar]
- [33].Blandini F, Levandis G, Bazzini E, Nappi G, Armentero MT. Time-course of nigrostriatal damage, basal ganglia metabolic changes and behavioural alterations following intrastriatal injection of 6-hydroxydopamine in the rat: new clues from an old model. Eur J Neurosci. 2007;25:397–405. doi: 10.1111/j.1460-9568.2006.05285.x. [DOI] [PubMed] [Google Scholar]
- [34].Przedborski S, Levivier M, Jiang H, Ferreira M, Jackson-Lewis V, Donaldson D, Togasaki DM. Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by intrastriatal injection of 6-hydroxydopamine. Neuroscience. 1995;67:631–647. doi: 10.1016/0306-4522(95)00066-r. [DOI] [PubMed] [Google Scholar]
- [35].Armentero MT, Levandis G, Nappi G, Bazzini E, Blandini F. Peripheral inflammation and neuroprotection: systemic pretreatment with complete Freund's adjuvant reduces 6-hydroxydopamine toxicity in a rodent model of Parkinson's disease. Neurobiol Dis. 2006;24:492–505. doi: 10.1016/j.nbd.2006.08.016. [DOI] [PubMed] [Google Scholar]
- [36].Walsh S, Finn DP, Dowd E. Time-course of nigrostriatal neurodegeneration and neuroinflammation in the 6-hydroxydopamine-induced axonal and terminal lesion models of Parkinson's disease in the rat. Neuroscience. 2011;175:251–261. doi: 10.1016/j.neuroscience.2010.12.005. [DOI] [PubMed] [Google Scholar]
- [37].Maia S, Arlicot N, Vierron E, Bodard S, Vergote J, Guilloteau D, Chalon S. Longitudinal and parallel monitoring of neuroinflammation and neurodegeneration in a 6-hydroxydopamine rat model of Parkinson's disease. Synapse. 2012;66:573–583. doi: 10.1002/syn.21543. [DOI] [PubMed] [Google Scholar]
- [38].Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, Bartus RT. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain. 2013;136:2419–2431. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Pessoa Rocha N, Reis HJ, Vanden Berghe P, Cirillo C. Depression and cognitive impairment in Parkinson's disease: a role for inflammation and immunomodulation? Neuroimmunomodulation. 2014;21:88–94. doi: 10.1159/000356531. [DOI] [PubMed] [Google Scholar]
- [40].Hassin-Baer S, Cohen OS, Vakil E, Molshazki N, Sela BA, Nitsan Z, Chapman J, Tanne D. Is C-reactive protein level a marker of advanced motor and neuropsychiatric complications in Parkinson's disease? J Neural Transm. 2011;118:539–543. doi: 10.1007/s00702-010-0535-z. [DOI] [PubMed] [Google Scholar]
- [41].Zhang L, Yan J, Xu Y, Long L, Zhu C, Chen X, Jiang Y, Yang L, Bian L, Wang Q. The combination of homocysteine and C-reactive protein predicts the outcomes of Chinese patients with Parkinson's disease and vascular parkinsonism. PLoS One. 2011;6:e19333. doi: 10.1371/journal.pone.0019333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sawada H, Oeda T, Umemura A, Tomita S, Hayashi R, Kohsaka M, Yamamoto K, Sudoh S, Sugiyama H. Subclinical elevation of plasma C-reactive protein and illusions/hallucinations in subjects with Parkinson's disease: case-control study. PLoS One. 2014;9:e85886. doi: 10.1371/journal.pone.0085886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Song IU, Chung SW, Kim JS, Lee KS. Association between high-sensitivity C-reactive protein and risk of early idiopathic Parkinson's disease. Neurol Sci. 2011;32:31–34. doi: 10.1007/s10072-010-0335-0. [DOI] [PubMed] [Google Scholar]
- [44].Song IU, Kim JS, Chung SW, Lee KS. Is there an association between the level of high-sensitivity C-reactive protein and idiopathic Parkinson's disease? A comparison of Parkinson's disease patients, disease controls and healthy individuals. Eur Neurol. 2009;62:99–104. doi: 10.1159/000222780. [DOI] [PubMed] [Google Scholar]
- [45].Song IU, Kim YD, Cho HJ, Chung SW. Is neuroinflammation involved in the development of dementia in patients with Parkinson's disease? Intern Med. 2013;52:1787–1792. doi: 10.2169/internalmedicine.52.0474. [DOI] [PubMed] [Google Scholar]
- [46].Andican G, Konukoglu D, Bozluolcay M, Bayulkem K, Firtiina S, Burcak G. Plasma oxidative and inflammatory markers in patients with idiopathic Parkinson's disease. Acta Neurol Belg. 2012;112:155–159. doi: 10.1007/s13760-012-0015-3. [DOI] [PubMed] [Google Scholar]
- [47].Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Haroon E, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Olleros ML, Vesin D, Bisig R, Santiago-Raber ML, Schuepbach-Mallepell S, Kollias G, Gaide O, Garcia I. Membrane-bound TNF induces protective immune responses to M. bovis BCG infection: regulation of memTNF and TNF receptors comparing two memTNF molecules. PLoS One. 2012;7:e31469. doi: 10.1371/journal.pone.0031469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Olleros ML, Vesin D, Lambou AF, Janssens JP, Ryffel B, Rose S, Fremond C, Quesniaux VF, Szymkowski DE, Garcia I. Dominant-negative tumor necrosis factor protects from Mycobacterium bovis Bacillus Calmette Guerin (BCG) and endotoxin-induced liver injury without compromising host immunity to BCG and Mycobacterium tuberculosis. J Infect Dis. 2009;199:1053–1063. doi: 10.1086/597204. [DOI] [PubMed] [Google Scholar]
- [50].Taoufik E, Tseveleki V, Chu SY, Tselios T, Karin M, Lassmann H, Szymkowski DE, Probert L. Transmembrane tumour necrosis factor is neuroprotective and regulates experimental autoimmune encephalomyelitis via neuronal nuclear factor-kappaB. Brain. 2011;134:2722–2735. doi: 10.1093/brain/awr203. [DOI] [PubMed] [Google Scholar]
- [51].Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, Paulus HE, Mudano A, Pisu M, Elkins-Melton M, Outman R, Allison JJ, Suarez Almazor M, Bridges SL, Jr, Chatham WW, Hochberg M, MacLean C, Mikuls T, Moreland LW, O'Dell J, Turkiewicz AM, Furst DE. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–784. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- [52].Galloway JB, Hyrich KL, Mercer LK, Dixon WG, Fu B, Ustianowski AP, Watson KD, Lunt M, Symmons DP. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford) 2011;50:124–131. doi: 10.1093/rheumatology/keq242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Singh S, Kumar N, Loftus EV, Jr, Kane SV. Neurologic complications in patients with inflammatory bowel disease: Increasing relevance in the era of biologics. Inflamm Bowel Dis. 2012 doi: 10.1002/ibd.23011. [DOI] [PubMed] [Google Scholar]