Abstract
Until the mid-20th century, infectious diseases were the major cause of morbidity and mortality in humans. Massive vaccination campaigns, antibiotics, antivirals and advanced public health measures drastically reduced sickness and death of infections in children and younger adults. Older adults (>65yr of age), however, remain vulnerable to infections, and to date infectious diseases remain amongst the top 5–10 causes of death in this population. The aging of the immune system, often referred to as immune senescence, is the key phenomenon underlying this vulnerability.
This review centers on age-related changes in T cells, which are dramatically and reproducibly altered with aging. I will discuss changes in T cell production, maintenance, function and response to latent persistent infection, particularly against the cytomegalovirus (CMV), that exerts profound influence on the aging T cell pool, concluding with a brief list of measures to improve immune function in older adults.
Introduction: What is aging?
Aging of an organism can be defined as progressive, cumulative and inevitable age-dependent alteration in structure and decline in function of multiple cells, tissues and organs, leading to decreased ability to respond to stress and maintain homeostasis. Given that the ultimate inability to maintain homeostasis is death, this definition also links aging to its final outcome. On the other hand, despite decades of research, the precise molecular mechanism(s) of aging were surprisingly difficult to unambiguously define. There exist more than 40 theories of aging, many of them not mutually exclusive, but few clearly integrated and capable of explaining most of the observations (1). While it is beyond the scope of this review to discuss different theories of aging in detail, a viable unified theory of aging would propose pathway(s) that simultaneously explain molecular, cellular and organismal aging. Moreover, such pathways would operate across different species and within the members of a single species directly proportionally to their life span and chronological age.
What we unambiguously know now comes close to a unified mechanism of aging. Aging is powerfully influenced by alterations in nutrient sensing and metabolism (2). Caloric restriction has been known for over 75 years to extend lifespan in model organisms by 30–40%. Similarly, at least ten individual gene mutations, and at least two pharmacological interventions targeting the mTOR pathway (with rapamycin, (3) and metformin, (4)) have been reported to extend lifespan in model organisms by up to or over 50%. All these mutations/interventions affect cellular growth and nutrient sensing and involve, directly or indirectly, the insulin/insulin growth factor (IGF) pathway. Increased resistance to cellular stress has accompanied these interventions, leading to the “metabolism and cellular stress” theory of aging (5–7), which continues to garner support with time.
Immune system aging and T cell aging
Studying aging of the immune system is mandated by its substantial age-related decline and the concomitant increase in morbidity and mortality from infectious diseases in older adults (8–10). Overall, it is clear that aging of the immune system is a cumulative phenomenon, heterogeneous just as aging itself, and affecting individuals in the community at highly individualized and disparate rates. Given that the immune system is highly integrated and that even within a single cell signaling cascades are precisely spatially and temporally regulated, it is becoming evident that small dysregulations in a series of signaling events and cell-cell communication steps can translate into major deficiencies in the overall immune defense.
With that in mind, distinct differences with aging have been identified in virtually every facet of the immune system examined so far, from the initial contact with a microbial pathogen all the way to its clearance and formation of protective immune memory or to coexistence with a persisting pathogen. Defects in various aspects of innate immune function have been recently discussed (11–13). They include deficiencies in granulocyte, macrophage and NK function (12, 13), diminished or functionally altered function of major innate sensing receptors and soluble systems (including complement)(14) and other age-related changes. However, our understanding of innate immune changes with aging remains incomplete, and some of the above changes lack the consistency and reproducibility between different experimental systems and human subject cohorts.
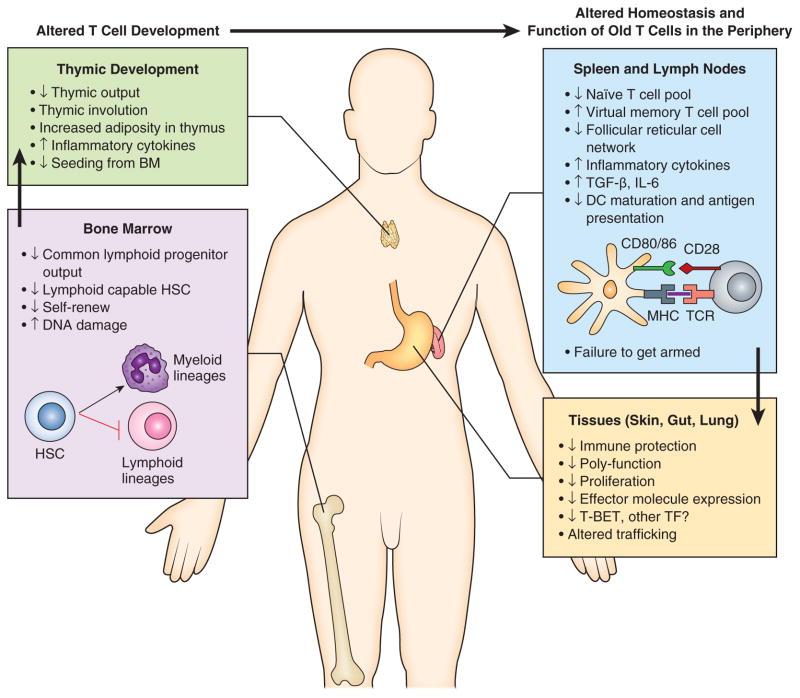
By contrast, changes in adaptive immunity are much better defined and more reproducible. Humoral immunity and B cell alterations with aging have been the subject of an excellent recent review ((15). To that effect, neither innate immune nor B cell changes with aging will be the topic of this text. Rather, I will focus on T cell immunity and maintenance with aging, both of which are amongst the most remarkable and most pronounced changes occurring within an aging immune system. Moreover, fixing T cell defects with aging often leads to restoration of functional and protective immunity in older organisms (16–19). Figure 1 illustrates the multitude of steps necessary for production and function of mature peripheral naïve (N) and memory (M) T cells, most, if not all, of which have been shown to encounter problems in the course of aging.
Figure 1. Multiple defects in the T cell compartment occur during aging.
(Right) T cell development is altered in the bone marrow during aging: the bone marrow stromal changes, as well as cell-intrinsic defects cause hematopoietic stem cells (HSC) and progenitors to shift away from lymphoid and towards myeloid lineages. Thymic T cell development is also altered during aging: there is evidence for decreased seeding from the bone marrow, thymic involution, increased adiposity, and increased expression of proinflammatory cytokines. As a result, the T cell output from the aged thymus is significantly diminished. These developmental defects place stress on peripheral maintenance of T cell pools and T cell function in secondary and tertiary organs (Left). In the peripheral lymphoid organs (spleen and LN) changes in thymic T cell production, along with increased expression of inflammatory cytokines, diminished network of follicular reticular cells and defects in antigen presentation, result in a decrease in the naïve T cell pool, while the virtual memory T cell pool expands (see Fig. 2), hampering responses to new infection. Cell-intrinsic defects as well as impaired Ag processing and presentation synergistically diminish T cell activation, as evident in decreased T cell proliferation and effector molecule expression. Such incompletely or defectively activated effector T cells migrate into tissues (gut, lung, skin, etc.), where decreased effector molecule expression and, potentially, altered trafficking, further contribute to the additive T cell defects with aging, resulting in defective T cell function that limits the response to infection.
Thymic involution: the when, how and why?
One of the most remarkable age-related changes in any tissue or organ in the body is the involution of the thymus, the central organ orchestrating production of new T cells (age-related changes affecting the thymus were recently reviewed in depth in three expert reviews,(20–22)). This organ involutes relatively early in life in humans, with changes being evident even before puberty, and by some accounts even soon after birth (23, 24). In mice, involution appears to be more gradual, but it is debatable whether the organ has any significant output after 15 months of age in C57BL/6 laboratory mice, which are certainly the best studied animal model. Current data suggests that early thymic involution involves a primary thymic stromal defect, whereby one or more epithelial cell components, possibly the epithelial cell precursor/stem cells, are affected by the process of aging. Expression of key differentiation and growth factors for thymic epithelial cells, such as the master transcriptional regulator FoxN1 and the keratinocyte growth factor (KGF) is altered with aging. Consistent with that, histological changes in the involuting thymus include decreased volumes of both cortical and medullary regions, disorganization of epithelial cell architecture and of the corticomedularry junction and replacement of the stroma by adipose tissue. It remains unclear whether the last observation is due to true epithelial-to-mesenchymal transdifferentiation or to death/lack of production of epithelial cells and expansion of adipose tissue (21). Adipose cells have the potential to produce a number of proinflammatory cytokines that can affect thymopoiesis. Subsequently, one finds an inverse correlation between thymic adiposity and thymic function, that are modulated in opposite directions by caloric restriction and obesity. Therefore, an increase in thymic adipocytes has the potential to accelerate or aggravate the loss of thymic function with aging, regardless of the exact origin of the fat cell increase with aging.
These early changes are compounded later in life with hematopoietic cell defects, which exhibit diminished differentiation towards the lymphoid lineage, with reduced generation of common lymphoid precursors (CLP) and increased propensity towards myeloid differentiation (rev. in (20). It is not clear whether, once differentiated towards lymphoid lineage, aged precursors may also face difficulties in seeding the old thymus, either because of cell-intrinsic defects or because of further age-related changes in stromal niches, that may make them less receptive for seeding. But those old precursors that make it into an old thymus have decreased differentiation potential, suggestive of true cell-intrinsic defects with aging. Thus, ETP isolated from young and old mice were used to seed the fetal thymic organ cultures, and the number of ETP-derived cells from the old mouse was 10 fold lower than the young (25). Regardless of the number and exact nature of above defects, they all lead to a single outcome: a reduced thymocyte-stroma cross-talk, resulting in diminished export of new naïve T cells into the periphery. Of interest, even once they are produced, recent thymic CD4+ emigrants from old mice may not be able to seed the peripheral pool as well as their younger counterparts (26).
Peripheral T cell maintenance with aging: who’s naïve now?
Regardless of the exact mechanism(s) of involution, the result of thymic involution is diminished, and ultimately negligible, production of new naïve T cells. A key question, then, is: How long is the thymus functional in different species? A recent manuscript compared rates of naïve T cell production in mice and humans (27), concluding that, relative to lifespan, the thymus in the mouse is a much more prominent contributor to the dynamics of the peripheral T cell pool, whereas in humans, it is peripheral maintenance that does most of the heavy lifting. This is consistent with the anecdotal observations on stress resistance of T cells from longer-lived species (e.g. human T cells undergo much less cell death than the mouse T cells following isolation, and can be rested overnight in vitro or frozen and thawed without significant loss of function(28)), and also with data that the peripheral CD8 T cell compartment could not be regenerated following antibody-mediated depletion in middle-aged (10–16 year old) cynomolgus macaques (Macaca fascicularis) regardless of whether the animals were thymectomized or not (29).
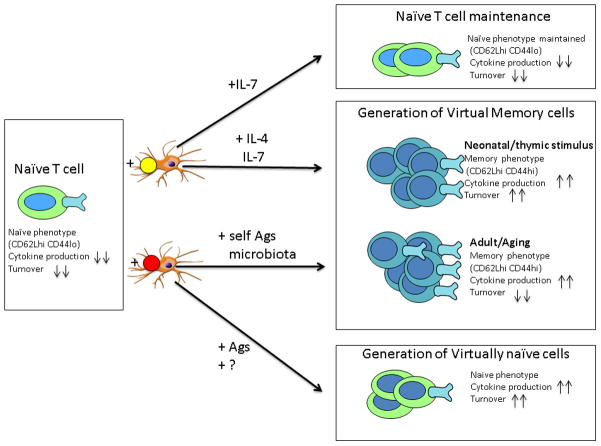
The lack of thymic production places significant stress upon peripheral maintenance of the remaining peripheral naïve T cells, which need to protect the organism against new infections for the remainder of the lifetime with no “cavalry” coming from the thymus to the rescue. Peripheral T cell maintenance relies on low-intensity, subthreshold stimulation via the T cell receptor (essential for naïve T cell maintenance) and/or stimulation with the homeostatic cytokines, IL-7 (necessary for survival of naïve T cells but can be used by memory cells as well) and IL-15 (essential for maintenance of memory cells) (rev. in (30). In the process of this maintenance, which is normally composed of low-grade tonic signals that only very infrequently lead to cell division, cells maintain the phenotype and function consistent with their prior differentiation state. Importantly, relative (e.g. lymphopenia) or absolute excess of trophic cytokines leads to intensification of homeostatic proliferation (HP) and often results in a change of phenotypic and functional status of the cells, so that naïve cells assume a pseudo-memory phenotype. Several observations over the past 10 years have concordantly indicated that the process of naïve T cell homeostasis is profoundly affected by aging, with the changes aimed to maintain naïve T cell pool eventually leading to its further depletion and demise. For example, both human naïve CD4 T cells (31) and non-human primate naïve CD8 T cells (32) exhibit increased turnover with aging, indicative of increased homeostatic proliferation that has the potential to convert naïve T cells into virtual memory cells. Formal proof that this is occurring on a large scale was subsequently found in mice, where we showed that naïve aging T cell precursors frequently assume virtual memory (VM) phenotype and function in the absence of immunization (33), Figure 2). These findings were subsequently corroborated by other investigators (34, 35), and it was shown that in wild-type mice, these virtual memory cells dominate in the central memory compartment (35). Of interest, an accumulating body of evidence suggests that virtual memory cells accumulating in old mice arise by mechanisms distinct from those that produce VM cells in young adult mice and also exhibit differences in function. VM cells in adult mice exhibit superior immediate effector function and proliferation compared to their true naïve (TNa) counterparts (36). They likely arise due to neonatal lymphopenia, driven to proliferate and become VM in response to the excess of IL-7 in an absolutely or relatively “empty” mouse neonatal peripheral compartment (36), although in some mouse strains a portion of these cells can also arise in the developing thymus in response to IL-4 stimulation by NK-T cells (37), Figure 2). By contrast, VM cells in old wild type mice exhibit signs of increased TCR avidity, as judged by elevated CD5 levels and decreased pMHC dissociation rates (33). Moreover, in TCR transgenic mice, these cells do not accumulate if additional TCR rearrangements are blocked by the absence of Rag genes (38), suggesting the role of TCR ligands in driving accumulation of these cells (Fig. 2). Consistent with this, VM CD8 T cells from both TCR transgenic and wild type old mice show signs of functional alterations: they competently secrete T1 type cytokines, but exhibit inferior proliferation relative to their TNa counterparts (38). Experiments are in progress to elucidate which antigens drive this TCR-dependent conversion and/or accumulation from naïve to memory phenotype with aging.
Figure 2. Self renewal and differentiation on Naïve CD8+ T cells into phenotypically and functionally distinct CD8 T cell subsets.
Their phenotypic attributes, proliferative potential and the functional properties (cytokine production) are illustrated as increased (up arrows ) or decreased (down arrows). Naïve CD8 T cells are maintained by low avidity TCR signaling following pMHC contact and IL-7 (top right). Lymphopenia-driven homeostatic proliferation gives rise to neonatal virtual memory (VM) CD8 T cells (middle right). These VM CD8 T cells can arise early (during the neonatal period) in the thymus (in response to weak TCR signals and IL-4, (37) or in the periphery from the first wave of thymic emigrants, in response to IL-7 (36, 103). Alternatively, over the lifespan, a different type of VM cells can arise in the periphery in response to near-threshold TCR signals that are likely stronger than TCR interactions which maintain naïve T cells in the naïve state (33–35, 38). Ligands promoting such VM cells could originate from self antigens or microbiota; these cells accumulate with aging, unlike the neonatal-origin VM cells. Finally, in humans, we recently found a virtually naïve (VN) subset (functionally differentiated yet lacking effector markers) (Pulko, V. et al., in preparation) although it is not yet clear whether such cells are representative of populations generated in the course of antimicrobial responses to foreign antigens or are driven by homeostatic cues.
By contrast to the clear numerical decline of naïve CD8 T cells in aging mice (39) and humans (40), the CD4 T cell compartment appears to be numerically better conserved, because it shows no appreciable decrease across ages, at least in CMV-negative humans (40). However, the above VM conversion at least in part applies to CD4 T cells in old TCR transgenic mice (38) and initial results suggest that wild-type CD4 T cells may show similar conversion with aging (Deshpande, N. et al., in preparation). Human counterparts of the murine age-related VM CD8 T cells have not yet been conclusively identified. However, interesting results have been reported by Su et al. (41), who found that humans across different ages exhibited memory-phenotype CD4 T cells that were specific for a variety of viral antigens, some of whom (e.g. tetanus, smallpox etc.) were unlikely to have infected the subjects in question. While conclusive evidence that such cells accumulate in human aging is lacking at the present, these results have been interpreted to suggest that the memory compartment in humans may be exquisitely crossreactive as a consequence of diverse antigenic experiences (41). Whether such T cell crossreactivity produces protective heterologous immunity (42) and whether aging makes that crossreactivity more pronounced is unclear at the moment, although such a mechanism has been proposed as an adaptive feature of the aging immune system (43).
T cell repertoire in the last third of life: intrinsic and extrinsic modifiers
As the numbers of naïve CD8 T cells decline with aging, one would expect that the diversity of the TCR repertoire follows suite. Indeed, there are notable examples of TCR diversity reduction with aging in mice and humans (Rev. in. (44–46), initially detected based on anti-TCRVβ antibody staining (47, 48) and/or TR-BV CDR3 length (49) analysis (50, 51). Subsequent analysis included single-cell PCR (52) and high-throughput next-generation sequencing (53).
These studies, while informative, all come with limitations. The mouse and most of the human studies have analyzed the mobilized T cell repertoire, activated in response to immunization/infection. That carries the problem of age-related differences that may be related to antigen uptake, processing, presentation, costimulation, and other issues not primarily related to the maintenance of the naïve T cell repertoire per se. A second limitation is that numerous studies simply analyzed total CD4 or CD8 (or even worse, total T) cell pools, using relatively low definition techniques (usually CDR3 length polymorphism), providing some information on total TCR diversity, but where the changes in population representation with aging (relative dominance of memory over naïve T cells) can drastically impair data interpretation. Third, most, if not all, of the above studies, have been limited to TCRVβ or TR-BV analysis. Finally, to this date, the field has not benefitted from a definitive, well controlled, high-throughput next-generation sequencing approach to inform us about diversity reduction with aging in individual subsets of T cells. In humans, the most stringent and well-controlled results in that regard were recently published on unseparated T cells and total CD4+ T cells (53), showing that TCRβ diversity decreases roughly linearly with age, and is noticeable already by the age 40, paralleling the decline in N cells and consistent with earlier data of Naylor et al. (31). Using inter-individual analysis, these authors found that there were fewer than 10,000 representative and shared TCR clonotypes, and that their abundance within the top 100,000 clones correlated with overall TCR diversity and was reduced with aging (53). Moreover, in octogenerians, this group found both an increase in percentage of naïve CD4 cells and an increase in TCR diversity relative to individuals around the mean age of 62. This strongly suggests selection/survival effects. The important point of this study is the detection of reduced diversity in total CD4 T cells, which are much less prone than the CD8 T cells to numerically decline in human peripheral blood with aging (40). That further suggests a genuine loss of diversity, rather than a simple replacement of diverse N CD4 cells with less diverse CM and EM CD4 T cells with aging. Unfortunately, the above study (53) did not directly analyze purified major T cell subsets (N, CM, EM) and also did not account for the dramatic effects of CMV infection, which is known to massively alter T cell pools with aging. A single mouse study published so far on a similar topic (54) suffers from similar limitations inasmuch total CD4 T cells were analyzed from spleen and bone marrow. Of interest, these authors concluded that spleen, but not bone marrow, exhibits reduced CD4+ T lymphocyte TCRβ diversity with aging; they further conclude that this is due to concomitant expansion of a large number of clones
Memory/effector memory TCRβ repertoire changes with aging were investigated by single-cell PCR in total mouse T cells in one mouse longitudinally, following LCMV infection at 23 days, 15 and 26 months post infection, and in two additional animals at 26 months p.i. (52), with drastic reduction of diversity, which, in one mouse, was complete (i.e. one clone was found in 100% of sequences). While the scope of that study was not broad, the reduction of diversity was striking.
In infected humans, and mice, memory T cell repertoire with aging is under an extremely strong influence of the CMV virus (55), which infects 60–70% humans in the Western hemisphere, with numbers being even higher in older adults. CMV induces strong (56, 57) and absolute inflation of both the EM CD8 and EM CD4 subsets (40, 58, 59), likely via direct stimulation of a wide repertoire of responding T cells with its complex peptides (60). It follows that humans (and other naturally or experimentally infected animals) will experience drastically different aging patterns in the presence and the absence of CMV infection, and that was shown for total CD8 TCR repertoire (61–63). In one study, this repertoire shrinkage was even linked with decreased residual life span (61), but the mechanistic underpinnings remain missing.
A single-cell approach, but with simultaneous sequencing of TR-AV and BV genes from human CMV-specific effector memory CD8 T cells (64) showed that individuals with high anti-CMV Ab titers (usually used as a correlate of high CMV reactivation/activity) had lower CD8 T cell diversity in their anti-CMV response. That study did not evaluate aging as an independent variable, a critical and important question. Finally, several studies evaluated the impact of deliberate, controlled life-long mouse CMV infection upon immune responsiveness to third-party infection in old mice. All three studies found diminished CD8 responses to third-party infectious challenge in CMV-infected, but not control or mice infected with acute microbial pathogens (39, 65, 66), and one of the studies found that CMV-infected mice exhibited a thoroughly different and nonoverlapping TCR utilization in these responses (39). While at the present it is unclear at what level CMV manipulates TCR utilization in old mice following lifelong infection, this result represents a stark example of external influences upon TCR diversity with aging.
Functional changes in old T cells
The above alterations in TCR repertoire could, by themselves, alter TCR avidity and thereby functional output of old T cells. However, data on TCR avidity in aging is controversial. In the mouse, we have described an increase in TCR avidity for pMHC in old mice both by direct pMHC dissociation assays and by CD5 levels (33), Renkema, K.R. et al., in preparation). Consistent with that, we did not see a decline in functional avidity of old CD8 T cells responding to infection in mice ((67) or to lifelong systemic HSV in mice (68) or rhesus CMV in monkeys (69). These measurements have been made at the peak of primary or in the course of ongoing effector-memory responses, and therefore may not reflect the responsiveness of naïve T cells. Indeed, a list of functional and signaling defects in old T cells has been accumulated over the past 40 years of research (rev. in (70–72)), including data on reduced T cell proliferation, effector function and synapse formation, impaired generation of early signaling intermediates, blunted induction of key fate-determining transcription factors, incomplete effector differentiation and effector molecule expression and altered trafficking. As this topic was recently extensively reviewed for both CD4 and CD8 T cells (71–73), I will not review it in exhaustive details.
Rather, I will focus on the key question: to what extent are the above defects intrinsic to old T cells and to what extent can they be ascribed to defective antigen uptake, processing and presentation, suboptimal dendritic cell maturation/migration and/or problems in cytokine/inflammatory environment, all of which have been documented to a greater or lesser extent? Certainly, in vivo whole animal testing and testing of unseparated T cell populations cannot resolve this question. Rather, most informative are the in vitro studies of minimally manipulated purified T cell subsets and in vivo transfers of the same purified subsets from old or adult mice into adult, T cell deficient hosts. Focusing on these types of studies, one can discern clear patterns of cell-intrinsic defects in naïve T cells. Thus, Miller, Garcia and colleagues have shown that in vitro stimulated naïve old CD4 T cells exhibit impaired cytoskeletion signaling, polarization, LAT and ZAP-70 recruitment and CD3ξ phosphorylation all the way to the induction of NF-AT (rev. in (74). Similar studies have not been performed in naïve CD8 T cells. Haynes, Swain and their colleagues have performed transfers of CD4 recent thymic emigrants between old and adult hosts and found out that both the environment and the intrinsic T cell defects play a role in that setting (75). The same group also documented that inflammatory cytokines can overcome some of the defects in T cell helper function (16, 17), but the exact target for these cytokines has not been defined. In a mouse model of the West Nile virus, we have shown that transferred total CD8 or CD4 T cells from an old donor do not confer protection upon a young Rag-KO recipient, whereas adult donor T cell subsets provide substantial protection (76). This experiment did not purify naïve T cells, and we have subsequently shown that numbers of WNV-specific (and all other) precursors decline with aging by 60–90% (33, 38), so a true test of intrinsic defects on a per-cell basis still remains to be done. However, at a minimum, this result strongly suggests that whatever T cells with increased CD8 TCR avidity (33) or CD4 crossreactivity (41), Deshpande et al., in preparation) may accumulate/ selectively survive with aging, they cannot provide sufficient heterologous immunity against viral infection. Finally, benign CD8 T cell clonal expansions (TCE), which arise due to unknown causes with aging in laboratory mice (47, 51) and whose emergence is enhanced by increased peripheral T cell turnover (77), were shown to adversely affect new primary responses if they were occupying the TCRVβ family needed for the primary response (78). If such TCE are of known specificity for an infectious microorganism (e.g. a virus), their ability to respond to the virus in the late memory phase is often functionally compromised (79).
Memory T cell responses are also affected with aging, but in an asymmetrical manner. Specifically, memory responses generated in youth or adulthood appear to be much better preserved compared to those generated in the old age from a primary response that is already ridden with different age-specific deficits (rev. in (43, 80). Incomplete expansion and function during recall responses in old mice have been observed in CD8 T cells responding to LCMV (81), Listeria (67) and influenza (82) and in CD4 T cell response to model antigens (83). By contrast, immunization of young adult individuals with vaccinia or infection with smallpox was shown to preserve robust CD8 T cell immune responses for decades, and perhaps for life (84), and to provide strong protection against other poxvirus infections (85).
T cell trafficking with aging has been investigated in the human model and it was shown that mobilization of cutaneous CD4 T cell immunity was defective in older adults, despite intact systemic immunity detected in blood (86), however, due to the limitations of the human model, it remained unanswered whether that defect is cell-intrinsic. Much more research is needed on age-related T cell trafficking defects.
Effector differentiation has been conclusively shown to be defective in CD8 and Th1 CD4 lineage in response to infection, with reduced expression of important effector cytokines such as IFNγ, TNF-α, granzyme B (76, 87) or IL-2 (88–90). Less is known about differentiation into other lineages, although it appears that Th2 lineage is similarly impaired with aging (rev. in (91, 92) with reduced production of GATA-3 (93), whereas Th17 function could be exacerbated (94) or decreased (95, 96). Regulatory T cells were found to be increased in old mice (97) and humans (98). Some studies suggested that aging increased the Th17/Treg ratio in humans (99), with an increased Th17 proportion, however, absolute numbers were not available and functional differences were not confirmed in culture supernatants(99). By contrast, another study found a decreased proportion of Th17 cells under nonpolarizing, but higher proportion under polarizing, conditions(100). Overall, it remains unclear whether aging can lead to selective favoring of one of the functional T cell fates at the expense of others, and there is a dearth of studies on the function in aging of Th9 and Tfh cells.
Conclusions: What is wrong with old T cells and how to fix them?
As the reader could conclude from the above deliberations, numerous steps involved in generation, development, activation, effector differentiation, homeostasis and trafficking are altered in T cells with aging (Fig. 1). Memory differentiation is less affected, but memory generated in the old age nonetheless ends up functionally inferior; it is only T cell memory generated in youth that remains relatively unscathed by the process of aging. From the standpoint of immune defense, it is important to reiterate that the above alterations need not be quantitatively remarkable in order to impact microbial clearance: small defects readily synergize along the same pathway to produce a multiplied and leveraged effect.
A practical question then arises as to what we can do to improve T cell function with aging. A detailed discussion of that topic deserves a review by itself. It is warranted to say, however, that our potential to intervene has never been greater. New adjuvants targeting specific innate sensors are being discovered and tested at an increasing rate (101), and several past publications have shown that TLR agonists(101), adjuvants(92), cytokines (17) and live attenuated vaccines (19) can all improve T cell responses, and often also protective immunity, in old animals and humans. Transduction with specific transcription factors is also being attempted, but is practically less likely to be deployable. Finally, once naïve T cells decline below a certain point, only T cell rejuvenation will be able to replenish the lost reserve. Good news is that T cell rejuvenation is by no means impossible, as several treatments can impressively regrow an old thymus (102). Less good news is that thymic involution and loss of function with age is a complex and composite process, and it is now clear that single treatments are unlikely to restore the totality of function, including robust export of functional T cells to the periphery, to allow for improved immune defense (22, 102)}. The journey to improving T cell function with aging, and in particular to fully rejuvenating thymic production and peripheral T cell performance, promises to be at the same time difficult, exciting and highly rewarding.
Acknowledgments
Supported in part by the USPHS awards AG020719, AG035309 and AI81680 and contracts HHSN 272201100017C and HHSN272200900059C from the National Institutes of Health and the Elizabeth Bowman Endowed Professorship in Medical Science to J.N-Ž.
I would like to thank the past and present members of the Nikolich lab for their experimental and intellectual contributions to this work, and specifically to Drs. Megan J. Smithey, Emily L. Goldberg and Heather L. Thompson for critical perusing and editing of this manuscript and helpful discussion. I further fully realize that due to the brevity of the format, I did not have the space to extensively quote primary literature and thereby adequately acknowledge the work of many exceptionally deserving colleagues in the field. I hope they will graciously excuse these involuntary omissions.
Abbreviations
- CMV
cytomegalovirus
- M
memory (T cells)
- mTOR
mechanistic (mammalian) target of rapamycin
- N
naïve (T cells)
- TNa
true naïve T cells
- VM
virtual memory T cells
References
- 1.Behl C, Ziegler C. Theories and Mechanisms of Aging. 2014:21–97. [Google Scholar]
- 2.Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 6.Miller RA. Cell stress and aging: new emphasis on multiplex resistance mechanisms. J Gerontol A Biol Sci Med Sci. 2009;64:179–182. doi: 10.1093/gerona/gln072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardner P, Pabbatireddy S. Vaccines for women age 50 and older. Emerg Infect Dis. 2004;10:1990–1995. doi: 10.3201/eid1011.040469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC. West Nie Virus and Other Arboviral Diseases - United States, 2012. Morbidity and Mortality Weekly Report. 2013;62:513–517. [PMC free article] [PubMed] [Google Scholar]
- 10.Control, C. f. D. Multistate Outbreak of Listeriosis Linked to Whole Cantaloupes from Jensen Farms, Colorado. CDC; 2012. [Google Scholar]
- 11.Kovacs EJ, Palmer JL, Fortin CF, Fulop T, Jr, Goldstein DR, Linton PJ. Aging and innate immunity in the mouse: impact of intrinsic and extrinsic factors. Trends Immunol. 2009;30:319–324. doi: 10.1016/j.it.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol. 2012;24:331–341. doi: 10.1016/j.smim.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13:875–887. doi: 10.1038/nri3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Panda A, Arjona A, Sapey E, Bai F, Fikrig E, Montgomery RR, Lord JM, Shaw AC. Human innate immunosenescence: causes and consequences for immunity in old age. Trends Immunol. 2009;30:325–333. doi: 10.1016/j.it.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kogut I, Scholz JL, Cancro MP, Cambier JC. B cell maintenance and function in aging. Semin Immunol. 2012;24:342–349. doi: 10.1016/j.smim.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Haynes L, Eaton SM, Swain SL. The defects in effector generation associated with aging can be reversed by addition of IL-2 but not other related gamma(c)-receptor binding cytokines. Vaccine. 2000;18:1649–1653. doi: 10.1016/s0264-410x(99)00501-0. [DOI] [PubMed] [Google Scholar]
- 17.Haynes L, Eaton SM, Burns EM, Rincon M, Swain SL. Inflammatory cytokines overcome age-related defects in CD4 T cell responses in vivo. J Immunol. 2004;172:5194–5199. doi: 10.4049/jimmunol.172.9.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Messaoudi I, Warner J, Fischer M, Park B, Hill B, Mattison J, Lane MA, Roth GS, Ingram DK, Picker LJ, Douek DC, Mori M, Nikolich-Zugich J. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc Natl Acad Sci U S A. 2006;103:19448–19453. doi: 10.1073/pnas.0606661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uhrlaub JL, Brien JD, Widman DG, Mason PW, Nikolich-Zugich J. Repeated In Vivo Stimulation of T and B Cell Responses in Old Mice Generates Protective Immunity against Lethal West Nile Virus Encephalitis. J Immunol. 2011;186:3882–3891. doi: 10.4049/jimmunol.1002799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berent-Maoz B, Montecino-Rodriguez E, Dorshkind K. Genetic regulation of thymocyte progenitor aging. Semin Immunol. 2012;24:303–308. doi: 10.1016/j.smim.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dixit VD. Impact of immune-metabolic interactions on age-related thymic demise and T cell senescence. Semin Immunol. 2012;24:321–330. doi: 10.1016/j.smim.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Chinn IK, Blackburn CC, Manley NR, Sempowski GD. Changes in primary lymphoid organs with aging. Semin Immunol. 2012;24:309–320. doi: 10.1016/j.smim.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinmann GG. Changes in the human thymus during aging. Curr Top Pathol. 1986;75:43–88. doi: 10.1007/978-3-642-82480-7_2. [DOI] [PubMed] [Google Scholar]
- 24.Haynes BF, Sempowski GD, Wells AF, Hale LP. The human thymus during aging. Immunol Res. 2000;22:253–261. doi: 10.1385/IR:22:2-3:253. [DOI] [PubMed] [Google Scholar]
- 25.Min H, Montecino-Rodriguez E, Dorshkind K. Reduction in the developmental potential of intrathymic T cell progenitors with age. J Immunol. 2004;173:245–250. doi: 10.4049/jimmunol.173.1.245. [DOI] [PubMed] [Google Scholar]
- 26.Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic output in aged mice. Proc Natl Acad Sci U S A. 2006;103:8447–8452. doi: 10.1073/pnas.0601040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mogling R, Bregje de Boer A, Willems N, Schrijver EH, Spierenburg G, Gaiser K, Mul E, Otto SA, Ruiter AF, Ackermans MT, Miedema F, Borghans JA, de Boer RJ, Tesselaar K. Maintenance of peripheral naive T cells is sustained by thymus output in mice but not humans. Immunity. 2012;36:288–297. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Kreher CR, Dittrich MT, Guerkov R, Boehm BO, Tary-Lehmann M. CD4+ and CD8+ cells in cryopreserved human PBMC maintain full functionality in cytokine ELISPOT assays. J Immunol Methods. 2003;278:79–93. doi: 10.1016/s0022-1759(03)00226-6. [DOI] [PubMed] [Google Scholar]
- 29.Wertheimer AM, Uhrlaub JL, Hirsch A, Medigeshi G, Sprague J, Legasse A, Wilk J, Wiley CA, Didier P, Tesh RB, Murray KO, Axthelm MK, Wong SW, Nikolich-Zugich J. Immune response to the West Nile virus in aged non-human primates. PLoS One. 2010;5:e15514. doi: 10.1371/journal.pone.0015514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol. 2011;12:478–484. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naylor K, Li G, Vallejo AN, Lee WW, Koetz K, Bryl E, Witkowski J, Fulbright J, Weyand CM, Goronzy JJ. The influence of age on T cell generation and TCR diversity. J Immunol. 2005;174:7446–7452. doi: 10.4049/jimmunol.174.11.7446. [DOI] [PubMed] [Google Scholar]
- 32.Cicin-Sain L, Messaoudi I, Park B, Currier N, Planer S, Fischer M, Tackitt S, Nikolich-Zugich D, Legasse A, Axthelm MK, Picker LJ, Mori M, Nikolich-Zugich J. Dramatic increase in naive T cell turnover is linked to loss of naive T cells from old primates. Proc Natl Acad Sci U S A. 2007;104:19960–19965. doi: 10.1073/pnas.0705905104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudd BD, Venturi V, Li G, Samadder P, Ertelt JM, Way SS, Davenport MP, Nikolich-Zugich J. Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor:pMHC interactions. Proc Natl Acad Sci U S A. 2011;108:13694–13699. doi: 10.1073/pnas.1107594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Decman V, Laidlaw BJ, Doering TA, Leng J, Ertl HC, Goldstein DR, Wherry EJ. Defective CD8 T cell responses in aged mice are due to quantitative and qualitative changes in virus-specific precursors. J Immunol. 2012;188:1933–1941. doi: 10.4049/jimmunol.1101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiu BC, Martin BE, Stolberg VR, Chensue SW. Cutting edge: Central memory CD8 T cells in aged mice are virtual memory cells. J Immunol. 2013;191:5793–5796. doi: 10.4049/jimmunol.1302509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinreich MA, Odumade OA, Jameson SC, Hogquist KA. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat Immunol. 2010;11:709–716. doi: 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Renkema KR, Li G, Wu A, Smithey MJ, Nikolich-Zugich J. Two separate defects affecting true naive or virtual memory T cell precursors combine to reduce naive T cell responses with aging. J Immunol. 2014;192:151–159. doi: 10.4049/jimmunol.1301453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smithey MJ, Li G, Venturi V, Davenport MP, Nikolich-Zugich J. Lifelong persistent viral infection alters the naive T cell pool, impairing CD8 T cell immunity in late life. J Immunol. 2012;189:5356–5366. doi: 10.4049/jimmunol.1201867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wertheimer AM, Bennett MS, Park B, Uhrlaub JL, Martinez C, Pulko V, Currier NL, Nikolich-Zugich D, Kaye J, Nikolich-Zugich J. Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans. J Immunol. 2014;192:2143–2155. doi: 10.4049/jimmunol.1301721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity. 2013;38:373–383. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Welsh RM, Che JW, Brehm MA, Selin LK. Heterologous immunity between viruses. Immunol Rev. 2010;235:244–266. doi: 10.1111/j.0105-2896.2010.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woodland DL, Blackman MA. Immunity and age: living in the past? Trends Immunol. 2006;27:303–307. doi: 10.1016/j.it.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nikolich-Zugich J, Slifka MK, Messaoudi I. The many important facets of T-cell repertoire diversity. Nat Rev Immunol. 2004;4:123–132. doi: 10.1038/nri1292. [DOI] [PubMed] [Google Scholar]
- 45.Nikolich-Zugich J. Ageing and life-long maintenance of T-cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8:512–522. doi: 10.1038/nri2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blackman MA, Woodland DL. The narrowing of the CD8 T cell repertoire in old age. Curr Opin Immunol. 2011;23:537–542. doi: 10.1016/j.coi.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Callahan JE, Kappler JW, Marrack P. Unexpected expansions of CD8-bearing cells in old mice. J Immunol. 1993;151:6657–6669. [PubMed] [Google Scholar]
- 48.Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammapathy”. J Exp Med. 1994;179:609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pannetier C, Even J, Kourilsky P. T-cell repertoire diversity and clonal expansions in normal and clinical samples. Immunol Today. 1995;16:176–181. doi: 10.1016/0167-5699(95)80117-0. [DOI] [PubMed] [Google Scholar]
- 50.Hingorani R, I, Choi H, Akolkar P, Gulwani-Akolkar B, Pergolizzi R, Silver J, Gregersen PK. Clonal predominance of T cell receptors within the CD8+ CD45RO+ subset in normal human subjects. J Immunol. 1993;151:5762–5769. [PubMed] [Google Scholar]
- 51.LeMaoult J, Messaoudi I, Manavalan JS, Potvin H, Nikolich-Zugich D, Dyall R, Szabo P, Weksler ME, Nikolich-Zugich J. Age-related dysregulation in CD8 T cell homeostasis: kinetics of a diversity loss. J Immunol. 2000;165:2367–2373. doi: 10.4049/jimmunol.165.5.2367. [DOI] [PubMed] [Google Scholar]
- 52.Bunztman A, Vincent BG, Krovi H, Steele S, Frelinger JA. The LCMV gp33-specific memory T cell repertoire narrows with age. Immun Ageing. 2012;9:17. doi: 10.1186/1742-4933-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Britanova OV, Putintseva EV, Shugay M, Merzlyak EM, Turchaninova MA, Staroverov DB, Bolotin DA, Lukyanov S, Bogdanova EA, Mamedov IZ, Lebedev YB, Chudakov DM. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. J Immunol. 2014;192:2689–2698. doi: 10.4049/jimmunol.1302064. [DOI] [PubMed] [Google Scholar]
- 54.Shifrut E, Baruch K, Gal H, Ndifon W, Deczkowska A, Schwartz M, Friedman N. CD4(+) T Cell-Receptor Repertoire Diversity is Compromised in the Spleen but Not in the Bone Marrow of Aged Mice Due to Private and Sporadic Clonal Expansions. Front Immunol. 2013;4:379. doi: 10.3389/fimmu.2013.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Looney RJ, Falsey A, Campbell D, Torres A, Kolassa J, Brower C, McCann R, Menegus M, McCormick K, Frampton M, Hall W, Abraham GN. Role of cytomegalovirus in the T cell changes seen in elderly individuals. Clin Immunol. 1999;90:213–219. doi: 10.1006/clim.1998.4638. [DOI] [PubMed] [Google Scholar]
- 56.Solana R, Tarazona R, Aiello AE, Akbar AN, Appay V, Beswick M, Bosch JA, Campos C, Cantisan S, Cicin-Sain L, Derhovanessian E, Ferrando-Martinez S, Frasca D, Fulop T, Govind S, Grubeck-Loebenstein B, Hill A, Hurme M, Kern F, Larbi A, Lopez-Botet M, Maier AB, McElhaney JE, Moss P, Naumova E, Nikolich-Zugich J, Pera A, Rector JL, Riddell N, Sanchez-Correa B, Sansoni P, Sauce D, van Lier R, Wang GC, Wills MR, Zielinski M, Pawelec G. CMV and Immunosenescence: from basics to clinics. Immun Ageing. 2012;9:23. doi: 10.1186/1742-4933-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Derhovanessian E, Maier AB, Hahnel K, Beck R, de Craen AJ, Slagboom EP, Westendorp RG, Pawelec G. Infection with cytomegalovirus but not herpes simplex virus induces the accumulation of late-differentiated CD4+ and CD8+ T-cells in humans. J Gen Virol. 2011;92:2746–2756. doi: 10.1099/vir.0.036004-0. [DOI] [PubMed] [Google Scholar]
- 58.Chidrawar S, Khan N, Wei W, McLarnon A, Smith N, Nayak L, Moss P. Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin Exp Immunol. 2009;155:423–432. doi: 10.1111/j.1365-2249.2008.03785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Litjens NH, de Wit EA, Betjes MG. Differential effects of age, cytomegalovirus-seropositivity and end-stage renal disease (ESRD) on circulating T lymphocyte subsets. Immun Ageing. 2011;8:2. doi: 10.1186/1742-4933-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sylwester AW, Mitchell BL, Edgar JB, Taormina C, Pelte C, Ruchti F, Sleath PR, Grabstein KH, Hosken NA, Kern F, Nelson JA, Picker LJ. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J Exp Med. 2005;202:673–685. doi: 10.1084/jem.20050882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hadrup SR, Strindhall J, Kollgaard T, Seremet T, Johansson B, Pawelec G, thor Straten P, Wikby A. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J Immunol. 2006;176:2645–2653. doi: 10.4049/jimmunol.176.4.2645. [DOI] [PubMed] [Google Scholar]
- 62.Pourgheysari B, Khan N, Best D, Bruton R, Nayak L, Moss PA. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J Virol. 2007;81:7759–7765. doi: 10.1128/JVI.01262-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Khan N, Shariff N, Cobbold M, Bruton R, Ainsworth JA, Sinclair AJ, Nayak L, Moss PA. Cytomegalovirus seropositivity drives the CD8 T cell repertoire toward greater clonality in healthy elderly individuals. Journal of Immunology. 2002;169:1984–1992. doi: 10.4049/jimmunol.169.4.1984. [DOI] [PubMed] [Google Scholar]
- 64.Wang GC, Dash P, McCullers JA, Doherty PC, Thomas PG. T cell receptor alphabeta diversity inversely correlates with pathogen-specific antibody levels in human cytomegalovirus infection. Sci Transl Med. 2012;4:128ra142. doi: 10.1126/scitranslmed.3003647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cicin-Sain L, Brien JD, Uhrlaub JL, Drabig A, Marandu TF, Nikolich-Zugich J. Cytomegalovirus infection impairs immune responses and accentuates T-cell pool changes observed in mice with aging. PLoS Pathog. 2012;8:e1002849. doi: 10.1371/journal.ppat.1002849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mekker A, V, Tchang S, Haeberli L, Oxenius A, Trkola A, Karrer U. Immune senescence: relative contributions of age and cytomegalovirus infection. PLoS Pathog. 2012;8:e1002850. doi: 10.1371/journal.ppat.1002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smithey MJ, Renkema KR, Rudd BD, Nikolich-Zugich J. Increased apoptosis, curtailed expansion and incomplete differentiation of CD8(+) T-cells combine to decrease clearance of L. monocytogenes in old mice. Eur J Immunol. 2011 doi: 10.1002/eji.201041141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lang A, Nikolich-Zugich J. Functional CD8 T cell memory responding to persistent latent infection is maintained for life. J Immunol. 2011;187:3759–3768. doi: 10.4049/jimmunol.1100666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cicin-Sain L, Sylwester AW, Hagen SI, Siess DC, Currier N, Legasse AW, Fischer MB, Koudelka CW, Axthelm MK, Nikolich-Zugich J, Picker LJ. Cytomegalovirus-specific T cell immunity is maintained in immunosenescent rhesus macaques. J Immunol. 2011;187:1722–1732. doi: 10.4049/jimmunol.1100560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miller RA. Effect of aging on T lymphocyte activation. Vaccine. 2000;18:1654–1660. doi: 10.1016/s0264-410x(99)00502-2. [DOI] [PubMed] [Google Scholar]
- 71.Nikolich-Zugich J, Li G, Uhrlaub JL, Renkema KR, Smithey MJ. Age-related changes in CD8 T cell homeostasis and immunity to infection. Semin Immunol. 2012;24:356–364. doi: 10.1016/j.smim.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haynes L, Swain SL. Aged-related shifts in T cell homeostasis lead to intrinsic T cell defects. Semin Immunol. 2012;24:350–355. doi: 10.1016/j.smim.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goronzy JJ, Li G, Yu M, Weyand CM. Signaling pathways in aged T cells - A reflection of T cell differentiation, cell senescence and host environment. Semin Immunol. 2012 doi: 10.1016/j.smim.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller RA, Berger SB, Burke DT, Galecki A, Garcia GG, Harper JM, Sadighi Akha AA. T cells in aging mice: genetic, developmental, and biochemical analyses. Immunol Rev. 2005;205:94–103. doi: 10.1111/j.0105-2896.2005.00254.x. [DOI] [PubMed] [Google Scholar]
- 75.Clise-Dwyer K, Huston GE, Buck AL, Duso DK, Swain SL. Environmental and intrinsic factors lead to antigen unresponsiveness in CD4(+) recent thymic emigrants from aged mice. J Immunol. 2007;178:1321–1331. doi: 10.4049/jimmunol.178.3.1321. [DOI] [PubMed] [Google Scholar]
- 76.Brien JD, Uhrlaub JL, Hirsch A, Wiley CA, Nikolich-Zugich J. Key role of T cell defects in age-related vulnerability to West Nile virus. J Exp Med. 2009;206:2735–2745. doi: 10.1084/jem.20090222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Messaoudi I, Warner J, Nikolich-Zugich D, Fischer M, Nikolich-Zugich J. Molecular, cellular, and antigen requirements for development of age-associated T cell clonal expansions in vivo. J Immunol. 2006;176:301–308. doi: 10.4049/jimmunol.176.1.301. [DOI] [PubMed] [Google Scholar]
- 78.Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Zugich J. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J Exp Med. 2004;200:1347–1358. doi: 10.1084/jem.20040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kohlmeier JE, Connor LM, Roberts AD, Cookenham T, Martin K, Woodland DL. Nonmalignant clonal expansions of memory CD8+ T cells that arise with age vary in their capacity to mount recall responses to infection. J Immunol. 185:3456–3462. doi: 10.4049/jimmunol.1001745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nikolich-Zugich J, Rudd BD. Immune memory and aging: an infinite or finite resource? Curr Opin Immunol. 2010;22:535–540. doi: 10.1016/j.coi.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kapasi Z, Murali-Krishna K, McRae M, Ahmed R. Defective generation but normal maintenance of memory T cells in old mice. European Journal of Immunology. 2002;32:1567–1573. doi: 10.1002/1521-4141(200206)32:6<1567::AID-IMMU1567>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 82.Valkenburg SA, Venturi V, Dang TH, Bird NL, Doherty PC, Turner SJ, Davenport MP, Kedzierska K. Early priming minimizes the age-related immune compromise of CD8(+) T cell diversity and function. PLoS Pathog. 2012;8:e1002544. doi: 10.1371/journal.ppat.1002544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc Natl Acad Sci U S A. 2003;100:15053–15058. doi: 10.1073/pnas.2433717100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 85.Hammarlund E, Lewis MW, Carter SV, Amanna I, Hansen SG, Strelow LI, Wong SW, Yoshihara P, Hanifin JM, Slifka MK. Multiple diagnostic techniques identify previously vaccinated individuals with protective immunity against monkeypox. Nat Med. 2005;11:1005–1011. doi: 10.1038/nm1273. [DOI] [PubMed] [Google Scholar]
- 86.Agius E, Lacy KE, Vukmanovic-Stejic M, Jagger AL, Papageorgiou AP, Hall S, Reed JR, Curnow SJ, Fuentes-Duculan J, Buckley CD, Salmon M, Taams LS, Krueger J, Greenwood J, Klein N, Rustin MH, Akbar AN. Decreased TNF-alpha synthesis by macrophages restricts cutaneous immunosurveillance by memory CD4+ T cells during aging. J Exp Med. 2009;206:1929–1940. doi: 10.1084/jem.20090896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Po JL, Gardner EM, Anaraki F, Katsikis PD, Murasko DM. Age-associated decrease in virus-specific CD8+ T lymphocytes during primary influenza infection. Mech Ageing Dev. 2002;123:1167–1181. doi: 10.1016/s0047-6374(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 88.Miller RA, Stutman O. Limiting dilution analysis of IL-2 production: studies of age, genotype, and regulatory interactions. Lymphokine Res. 1982;1:79–86. [PubMed] [Google Scholar]
- 89.Effros RB, Walford RL. The immune response of aged mice to influenza: diminished T-cell proliferation, interleukin 2 production and cytotoxicity. Cell Immunol. 1983;81:298–305. doi: 10.1016/0008-8749(83)90237-x. [DOI] [PubMed] [Google Scholar]
- 90.Haynes L, Linton PJ, Eaton SM, Tonkonogy SL, Swain SL. Interleukin 2, but not other common gamma chain-binding cytokines, can reverse the defect in generation of CD4 effector T cells from naive T cells of aged mice. J Exp Med. 1999;190:1013–1024. doi: 10.1084/jem.190.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haynes L, Maue AC. Effects of aging on T cell function. Curr Opin Immunol. 2009;21:414–417. doi: 10.1016/j.coi.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang W, Brahmakshatriya V, Swain SL. CD4 T cell defects in the aged: Causes, consequences and strategies to circumvent. Exp Gerontol. 2014;54C:67–70. doi: 10.1016/j.exger.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hasegawa A, Miki T, Hosokawa H, Hossain MB, Shimizu C, Hashimoto K, Kimura MY, Yamashita M, Nakayama T. Impaired GATA3-dependent chromatin remodeling and Th2 cell differentiation leading to attenuated allergic airway inflammation in aging mice. J Immunol. 2006;176:2546–2554. doi: 10.4049/jimmunol.176.4.2546. [DOI] [PubMed] [Google Scholar]
- 94.Tesar BM, Du W, Shirali AC, Walker WE, Shen H, Goldstein DR. Aging augments IL-17 T-cell alloimmune responses. Am J Transplant. 2009;9:54–63. doi: 10.1111/j.1600-6143.2008.02458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang MC, Liao JJ, Bonasera S, Longo DL, Goetzl EJ. Nuclear factor-kappaB-dependent reversal of aging-induced alterations in T cell cytokines. FASEB J. 2008;22:2142–2150. doi: 10.1096/fj.07-103721. [DOI] [PubMed] [Google Scholar]
- 96.Lim MA, Lee J, Park JS, Jhun JY, Moon YM, Cho ML, Kim HY. Increased Th17 differentiation in aged mice is significantly associated with high IL-1beta level and low IL-2 expression. Exp Gerontol. 2014;49:55–62. doi: 10.1016/j.exger.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 97.Raynor J, Lages CS, Shehata H, Hildeman DA, Chougnet CA. Homeostasis and function of regulatory T cells in aging. Curr Opin Immunol. 2012;24:482–487. doi: 10.1016/j.coi.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vukmanovic-Stejic M, Rustin MH, Nikolich-Zugich J, Akbar AN. Immune responses in the skin in old age. Curr Opin Immunol. 2011;23:525–531. doi: 10.1016/j.coi.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 99.Schmitt V, Rink L, Uciechowski P. The Th17/Treg balance is disturbed during aging. Exp Gerontol. 2013;48:1379–1386. doi: 10.1016/j.exger.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 100.Lee JS, Lee WW, Kim SH, Kang Y, Lee N, Shin MS, Kang SW, Kang I. Age-associated alteration in naive and memory Th17 cell response in humans. Clin Immunol. 2011;140:84–91. doi: 10.1016/j.clim.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Behzad H, Huckriede AL, Haynes L, Gentleman B, Coyle K, Wilschut JC, Kollmann TR, Reed SG, McElhaney JE. GLA-SE, a synthetic toll-like receptor 4 agonist, enhances T-cell responses to influenza vaccine in older adults. J Infect Dis. 2012;205:466–473. doi: 10.1093/infdis/jir769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ventevogel MS, Sempowski GD. Thymic rejuvenation and aging. Curr Opin Immunol. 2013;25:516–522. doi: 10.1016/j.coi.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Akue AD, Lee JY, Jameson SC. Derivation and maintenance of virtual memory CD8 T cells. J Immunol. 2012;188:2516–2523. doi: 10.4049/jimmunol.1102213. [DOI] [PMC free article] [PubMed] [Google Scholar]