Abstract
The genetic basis of 50–60% of prostate cancer is attributable to rearrangements in ETS (ERG, ETV1, ETV4, and ETV5), BRAF and RAF1 genes and overexpression of SPINK1. The development and validation of reliable detection methods are warranted to classify various molecular subtypes of prostate cancer for diagnostic and prognostic purposes. ETS gene rearrangements are typically detected by fluorescence in situ hybridization (FISH) and reverse transcription PCR methods. Recently, monoclonal antibodies against ERG have been developed that detect the truncated ERG protein in immunohistochemical assays where staining levels are strongly correlated with ERG rearrangement status by FISH. However, specific antibodies for ETV1, ETV4 and ETV5 are unavailable, challenging their clinical use. We developed a novel RNA in situ hybridization based assay for the in situ detection of ETV1, ETV4, and ETV5 in formalin fixed paraffin embedded tissues from prostate needle biopsies, prostatectomy, and metastatic prostate cancer specimens using RNA probes. Further, with combined RNA in situ hybridization and immunohistochemistry we identified a rare subset of prostate cancer with dual ETS gene rearrangements in collisions of independent tumor foci. The high specificity and sensitivity of RNA in situ hybridization provides an alternate method enabling bright field in situ detection of ETS gene aberrations in routine clinically available prostate cancer specimens.
Keywords: RNA in situ hybridization, ETS gene fusion, Immunohistochemistry, fluorescence in situ hybridization
Introduction
Prostate cancer (PCa) remains the most commonly diagnosed cancer in American men with an estimated incidence of 238,590 new cases and accounting for 29,720 deaths in 2013. (www.cancer.org). Currently the clinical diagnosis of PCa relies on serum prostate specific antigen (PSA) testing that has well- documented limitations including its unreliability for distinguishing aggressive disease from benign conditions. The identification of PCa specific molecular biomarkers, driving molecular alterations and robust molecular subtypes suggest more accurate diagnosis and prognosis. Using a novel bioinformatics approach, our group reported the first recurrent chromosomal rearrangement in prostate cancer involving fusion of the androgen-regulated TMPRSS2 gene with ERG or ETV1, members of the ETS transcription factor family. Identification of recurrent gene fusions and other molecular aberrations in prostate cancer helped to classify prostate cancer into distinct molecular subtypes [12]. Subsequent studies confirmed the presence of E26 transformation specific (ETS) gene fusions in approximately 50% of prostate specific antigen screened PCa. Among the ETS genes, ERG, ETV1, ETV4 and ETV5 overexpression result from the fusion of these genes with various androgen regulated 5’ partner genes[3–5]. Overexpression of SPINK1 has been identified in 5–10% of cases that do not harbor ETS gene rearrangements [6]. Our group also recently reported the identification of “druggable” RAF kinase gene rearrangements (SLC45A3-BRAF and ESRP1-RAF1) and FGFR gene fusions [7] in 1–2% of PCa patients who are negative for the known ETS family gene rearrangements [8]. The genetic basis of the remaining 30–40% of prostate cancer is yet to be identified.
Considering the emerging therapeutic agents targeting prostate specific markers[9],[10], reliable, efficient methods are warranted for the detection of various markers in both clinical setting for diagnostic or sub classification purposes, as well as in the research setting. Further, the multifocal nature of prostate cancer is well- recognized and several studies have documented the interfocal heterogeneity of ERG rearrangements in PCa harboring multiple tumor foci[11],[12] [13]. Hence genetically distinct tumor foci within the same patient are not unusual.
ETS gene rearrangements were initially detected by fluorescence in situ hybridization (FISH) and reverse transcription polymerase chain reaction (PCR) methods. Recently, monoclonal antibodies against ERG have been developed which are strongly correlated with ERG rearrangement as detected by FISH [14],[15],[6]. We recently developed a novel dual color immunohistochemistry (IHC) assay for the simultaneous detection of ERG-PTEN and ERG-SPINK1 in prostate cancer[16]. ETV1, ETV4 and ETV5 rearrangements, when assessed, are generally detected by FISH and/orreverse transcription PCR due to the lack of specific antibodies for these genes. Since multiple 5’ partner genes are involved in the fusion with ETV1, ETV4 and ETV5 genes, development of PCR based methods require synthesis of multiple primers and reactions. FISH analysis is time consuming, laborious, and can be challenging to identify small foci of interest in biopsies or prostatectomy specimens. In order to overcome the technical limitations associated with PCR and FISH, we have developed a novel RNA based in situ hybridization method (RNA-ISH) for the in situ detection of ETV1, ETV4 and ETV5 in formalin fixed paraffin embedded PCa samples. In this study we validate this novel RNA-ISH method that is comparable to IHC and a viable alternate method for in situ detection of ETS gene rearrangements.
MATERIALS AND METHODS
Study Design
To assess the feasibility of RNA–ISH method for the reliable detection of ETS rearrangement positive cases with high specificity and sensitivity, we initially evaluated RNA-ISH approach by comparing with ERGIHC on previously confirmed ERG-positive and ERG-negative cases from tissue microarray (TMA) samples. We then tested the ETVI, ETV4 and ETV5 RNA-ISH probes on a cohort of previously confirmed positive cases (needle biopsies and TMAs). Lastly we screened a large cohort of localized and metastatic prostate cancer cases from TMAs where the molecular status of these genes was unknown. Each case in the TMA was represented in triplicate cores, 0.6mm in size.
Tissue Selection
Multiple TMAs were used in this study including cases from prostatectomy cases and distant metastases (details of TMAs are provided in Table 1). All tissue samples were collected at the University of Michigan with informed consent and Institutional Review Board approvals. The metastatic prostate carcinoma samples were obtained from patients with hormone-refractory prostate cancer who were part of our posthumous tissue donor program. To date, 60 such autopsies have been performed. Normal and malignant tissues from multiple sites including bone were collected and incorporated in tissue microarrays used in this study.
Table 1.
Types of tissue microarrays and distribution of cases for detection of ETV1, ETV4 and ETV5 rearrangement
| Typeas of TMA | Number of patients |
|---|---|
| Localized PCa | 160 |
| Salvage prostatectomy | 105 |
| Metastatic PCa | 54 |
FISH and IHC validated ERG, and fluorescence in situ hybridization validated ETV1, ETV4 and ETV5 rearrangement positive PCa samples (radical prostatectomy) were used to establish the RNA in situ hybridization method for the reliable detection of ETS rearrangement positive cases in prostate[16–19] cancer.
Immunohistochemistry
ERG IHC was performed using the anti-ERG (EPR3864) rabbit monoclonal primary antibody (1:100) (Cat#790-4576, Ventana Medical Systems, Inc., Tucson, AZ, USA). IHC was performed using an automated protocol developed for the DISCOVERY XT automated slide staining system (Ventana Medical Systems, Inc.,) using Ultramap anti-rabbit HRP (Cat#760-4315,Ventana Medical Systems, Inc.,) as secondary antibody and detected using ChromoMap DAB (Cat#760-159, Ventana Medical Systems Inc.,) for ERG. Hematoxylin (Cat#790-2208 Ventana Medical Systems, Inc.,) was used as the counterstain. ERG immunohistochemistry staining was evaluated by board certified pathologist LPK. Staining of vessels was used as a positive control and slides without staining of vessels were excluded from further analysis. ERG staining in tumor foci was either absent or diffusely strong (2–3+), unless otherwise indicated, and was reported as present/absent. Cases with documented ERG rearrangement by fluorescence in situ hybridization were used as positive control.
ETV1, ETV4, ETV5 RNA ISH probes
RNA scope target probes were designed by Advanced Cell Diagnostics (Hayward, CA) using custom software. The software automatically select from the target sequence a series of oligonucleotides which have an average length of 25 bases and uniform melting temperature and do not cross-hybridize with any off-target sequence in the human transcriptome under the RNA scope assay conditions. The oligonucleotides are synthesized and quantified by measurement of UV absorbance at 260nm. Equi-molar amounts of oligonucleotides were pooled into a single probe set for each target. The human ERG (cat#604021), ETV1 (cat # 311411), ETV4 (cat # 491521), and ETV5 (cat # 590371) probes were used in this study.
RNA in situ Hybridization
RNA-ISH in situ hybridizations were performed essentially as described previously[20]. Briefly, formalin fixed and paraffin embedded sections and tissue microarray slides were baked at 60° C for one hour. RNA-ISH was performed using RNA scope FFPE Reagent Kit 2.0 (Advanced Cell Diagnostics, Hayward, CA) according to manufacturer’s instructions. Briefly, tissues were deparaffinized by immersing in xylene twice for 15 minutes each with periodic agitation. The slides were then immersed in 100% ethanol twice for 3 minutes each with periodic agitation, then air-dried for 5 minutes. Tissues were circled using a pap pen (Vector, #H-4000), allowed to dry, and treated with Pretreatment 1 buffer for 10 minutes. Slides were rinsed in deionized water, and then boiled in 1X Pretreatment 2 for 15 minutes. Slides were rinsed again in deionized water, and then treated with Pretreatment 3 buffer for 30 minutes at 40° C in a humidity chamber. Slides were rinsed twice in deionized water, and then incubated with target probes for either ERG (NM_001136154, region 2933–3913), ETV1 (NM_004956.4, region 998–2031), ETV4 (NM_ 001079675.2, region 431–1891), ETV5 (NM_004454.2, region 2638–3839), (Figure 1) DapB, bacterial gene (as negative control) or POLR2A (as positive control) for 2 hours at 40°C in a humidity chamber. Slides were then washed in 1X Wash Buffer twice for 2 minutes each. Slides were then treated with Amp 1 solution for 30 minutes, Amp 2 solution for 15 minutes, Amp 3 solution for 30 minutes, and Amp 4 solution for 15 minutes, all at 40 C in a humidity chamber with 2 washes in 1X Wash Buffer for 2 minutes each after each step. Slides were then treated with Amp 5 solution for 30 minutes and Amp 6 solution for 15 minutes at room temperature in a humidity chamber with 2 washes in 1X Wash Buffer for 2 minutes each after each step. Color was developed by adding a 1:60 solution of Fast Red B: Fast Red A to each slide and incubating for 10 minutes. Slides were washed twice in deionized water and then immersed in a 50% Hematoxylin (Fisher, #SH26-4D) solution for 2 minutes. Slides were rinsed several times in deionized water, and then immersed in a 0.01% ammonium hydroxide solution. Slides were rinsed in deionized water then dehydrated by dipping 5 times in 70% ethanol twice, dipping 5 times in 95% ethanol twice, immersing twice in 100% ethanol for 5 seconds each, and immersing in xylene for 5 seconds. The slides were mounted in Eco Mount (Fisher, 50–828-32) for viewing under bright-field microscopy.
Figure 1.

Schematic diagram showing the genomic organization of A) ERG, B) ETV1, C) ETV4 and D) ETV5 genes. Horizontal black bar indicate the location of the probe in the gene and the numbers in the bar indicate the probe coverage region.
RNA-ISH evaluation criteria
RNA-ISH scoring guidelines were established to classify tumor foci as described previously[20]. In positive cases, RNA–ISH staining appeared as distinct punctate dots within the tumor cells ranging from 0- >10 in each cell. All tumor foci were evaluated and scanned at 20X magnification. Scoring for a tumor focus was based on the highest intensity observed. Based on the number of dots/cell we established five grading levels ranging from 0to 4:tumor foci with no staining or less than 1 dot/cell under 20X magnification are scored as 0 or negative; tumor foci with 1–3 dots/cell in > 5% of the tumor were scored as 1;tumor foci with 4–10 dots/cell with no or very few dot clusters (fused overlapping dots) in >5% of tumor were scored as 2;tumor foci with more than 10 dots/cell with <10% of positive cells having dot clusters were scored as 3;tumor foci with > 10 dots/cell with >10% of positive cells having dot clusters were scored as 4. For the purposes of this study, tumor foci showing scores of 2–4 were classified as positive whereas tumor foci with scores 0–1 were considered negative. All slides were reviewed by a study pathologist (LPK).
Fluorescence in situ hybridization
BAC clones were used to generate the dual color break-apart FISH probes for ERG (RP11-476D17- 3’ probe, RP11-95I21-5’ probe), ETV1 (RP11-79G16-3’probe, RP11-661L15-5’probe), ETV4 (CTD-3215I16-3’ probe; RP11-147C10-5’probe) and ETV5 (RP11-480B15-3’probe; RP11-822O23-5’probe). All clones were tested on normal human metaphase chromosomes to validate map position and these clones have been used extensively in various studies from our laboratory and others [17],[19].
BAC DNA Preparation
200 ml overnight cultures for each BAC clone were grown in LB medium containing 12.5g/ml of chloramphenicol at 37°C for 14–16 hours with constant shaking. DNA was prepared using Qiagen- midiprep kit using Qiatip-100 according to the protocol provided by the manufacturer (Qiagen, USA).
Probe labeling
All FISH probes were prepared by nick translation labeling using modified nucleotides conjugated with biotin or digoxigenin utilizing biotin nick translation mix (11745824910, Roche, USA) for 3’ ERG and PTEN locus probes; digoxigenin nick translation mix (11745816910, Roche, USA) for 5’ ERG and chromosome 10 control probes. Probe DNA was precipitated and dissolved in hybridization mixture containing 50% formamide, 2XSSC, 10% dextran sulphate, and 1% Denhardt’s solution. Approximately 200ng of each labeled probe was used for hybridization. Fluorescent signals were detected with Streptavidin Alexa fluor 594 (S-32356, Invitrogen, USA) and anti-digoxigenin fluorescein Fab fragments (11207741910, Roche, USA) for red and green colors, respectively.
Image capture and FISH signal analysis
FISH analysis was performed by experienced cytogeneticist (NP) and pathologist (LPK). ERG rearrangement by translocation and/or deletion was recorded when the corresponding abnormal signal pattern was observed in more than 10–15% of cells. Fluorescent images were captured using a high resolution CCD camera controlled by ISIS image processing software (Metasystems, Germany).
Sequential Hybridization: RNA-ISH and ERG IHC
We developed standardized protocols to sequentially perform RNA-ISH and ERG IHC on radical prostatectomy formalin fixed paraffin embedded whole sections and/or TMA cores with multifocal tumor foci. The criteria for scoring the RNA-ISH and ERGIHC remained identical to that described above.
RESULTS
Comparison of ERG expression at RNA and Protein level by RNA-ISH and IHC
RNA-ISH in situ hybridization was positive in all (100%) of the 12 cases with ERG rearrangement confirmed by IHC and FISH. Additionally, RNA-ISH was negative in all the 70 ERG-negative cases as determined by IHC, indicating a 100% concordance between IHC and RNA-ISH (Figure 2) with a 0% false positive rate. For the 12 ERG-positive cases, RNA-ISH results were scored as follows: 2 (n=2), 3 (n=3) and 4 (n=7).
Figure 2.
Comparison of ERG RNA ISH and immunohistochemistry. Left panel showing the images of ERG RNA ISH and right panel showing images of ERG immunohistochemistry in benign prostate tissue (A&B); strong positive ERG by RNA ISH with score 4 (C) and positive ERG by immunohistochemistry (D) in a localized prostate carcinoma; weak staining of ERG by RNA ISH with score 1and weak ERG by immunohistochemistry in a metastatic prostate carcinoma (E and F); ERG negative both by RNA ISH (G) and immunohistochemistry (H) in a metastatic prostate carcinoma.
Validation of ETV1, ETV4 and ETV5 expression by RNA-ISH in situ hybridization
Based on results from the RNA-ISH and FISH/IHC comparison for ERG, we subsequently tested ETV1, ETV4, and ETV5 RNA-ISH probes on known positive cases and screened a large cohort of cases where the rearrangement status was unknown. We selected 11 positive cases, (5 ETV1, 4 ETV4and 2 ETV5) identified by FISH and performed RNA-ISH separately with ETV1, ETV4 and ETV5 probes. Positive RNA in situ staining was observed in cases with corresponding probes only in the fluorescence in situ hybridization confirmed positive cases. No cross reactivity or nonspecific hybridization of any of the probes other than the designated positive cases (Figure 3) was observed in either localized or metastatic PCa samples. The RNA-ISH intensity scores for positive cases were as follows: ETV1 3 (n=2), 4 (n=3); ETV4 3 (n=1) and 4 (n=3) and ETV5 2 (n=1) and 4 (n=1).
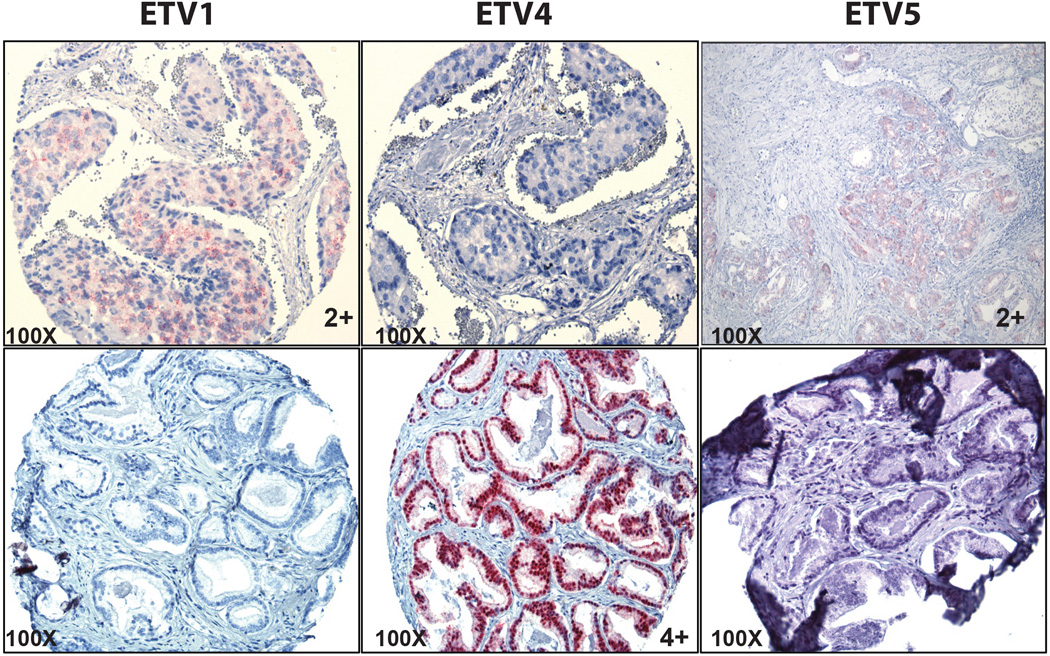
Figure 3.
Unique expression of ETV1, ETV4 and ETV5 genes detected by RNA ISH in corresponding positive cases (ETVI RNA-ISH score 2, ETV4 RNA-ISH score 4 and ETV5 RNA ISH score 2 with no cross reactivity or nonspecific hybridization of the probes in the negative cases.
We further tested ETV1, ETV4 and ETV5 probes on 319 cases represented on different TMAs comprising 265 localized PCa and 54 metastatic PCa samples with unknown ETS rearrangement status (details of TMA listed in Table 1).We identified 10 ETV1 (5localized and 5 metastatic PCa)(3.4%) and 3 ETV4 (localized PCa) (1%) and no ETV5 positive cases. The RNA-ISH intensity scores for ETV1 positive cases were,2 (n=2), 3 (n=5) and 4 (n=3); RNA-ISH intensity scores for ETV4 were as follows: 3 (n=1) and 4 (n=2). No non-specific background signals were observed for any of these three probes in any of the negative cases indicating the high specificity and sensitivity of the RNA probes. All RNA-ISH in positive cases were independently validated by respective break-apart FISH.
Identification of dual ETS gene rearrangements by Immunohistochemistry and RNA in situ hybridization
In the majority of the positive cases, the RNA-ISH was homogeneous within the tumor focus; a small subset of cases (three ETV1-positive cases and in one ETV4-positive case) of localized PCa from a large cohort (n=319) showed heterogeneous staining pattern within the triplicate cores of the TMA tissue microarray i.e 1–2 cores of a case were positive while the remaining cores were negative.
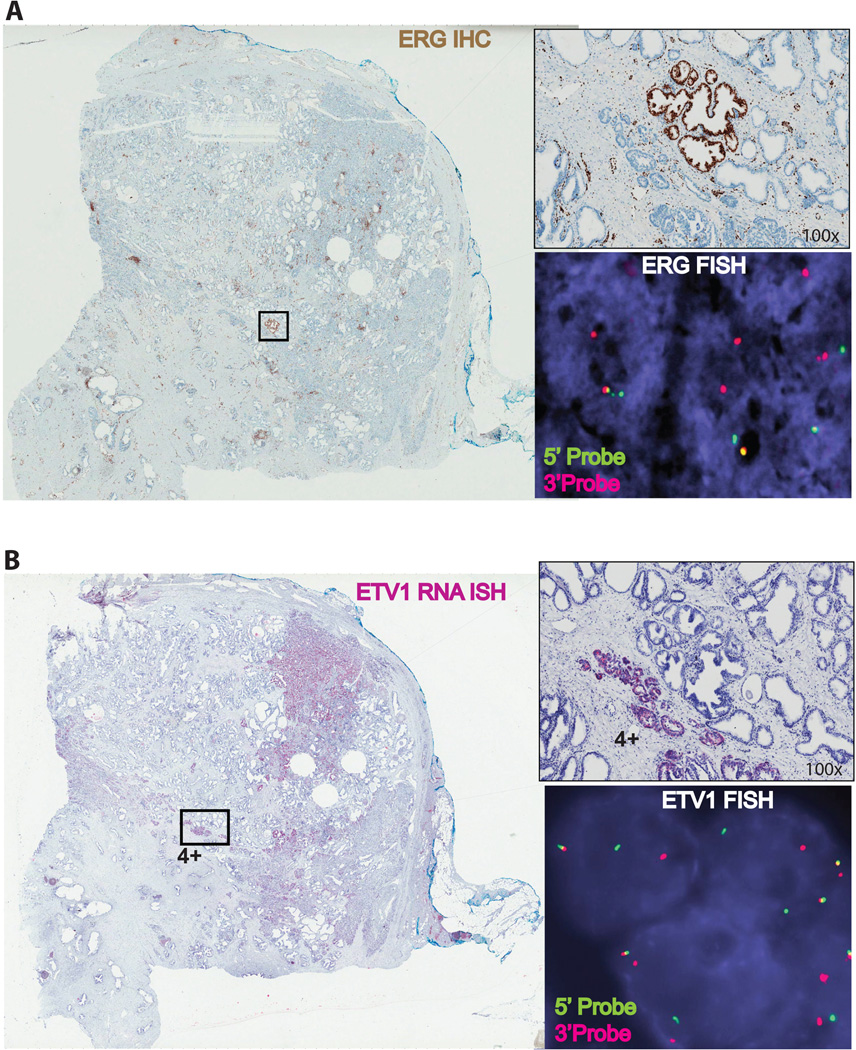
Sequential RNA-ISH and ERGIHC were performed on these 4 cases on representative whole block sections from radical prostatectomy specimens (3 cases) and TMA (1 case). RNA-ISH for ETV1 was repeated and small foci of tumor were negative on the whole sections. ERGIHC sequentially performed on the same blocks found that small ETVI negative tumor foci were positive with ERG staining (See Figure 4 and 5).
Figure 4.
Whole section view of a prostate carcinoma tissue showing tumor molecular heterogeneity with a small foci showing ERG expression by immunohistochemistry (A) and a large tumor foci showing ETV1 expression by RNA ISH (Score 4).Confirmation of ERG and ETV1 rearrangements by FISH (Insets) in the corresponding tumor foci. Green and red color signals indicate 5’ and 3’ probes, respectively.
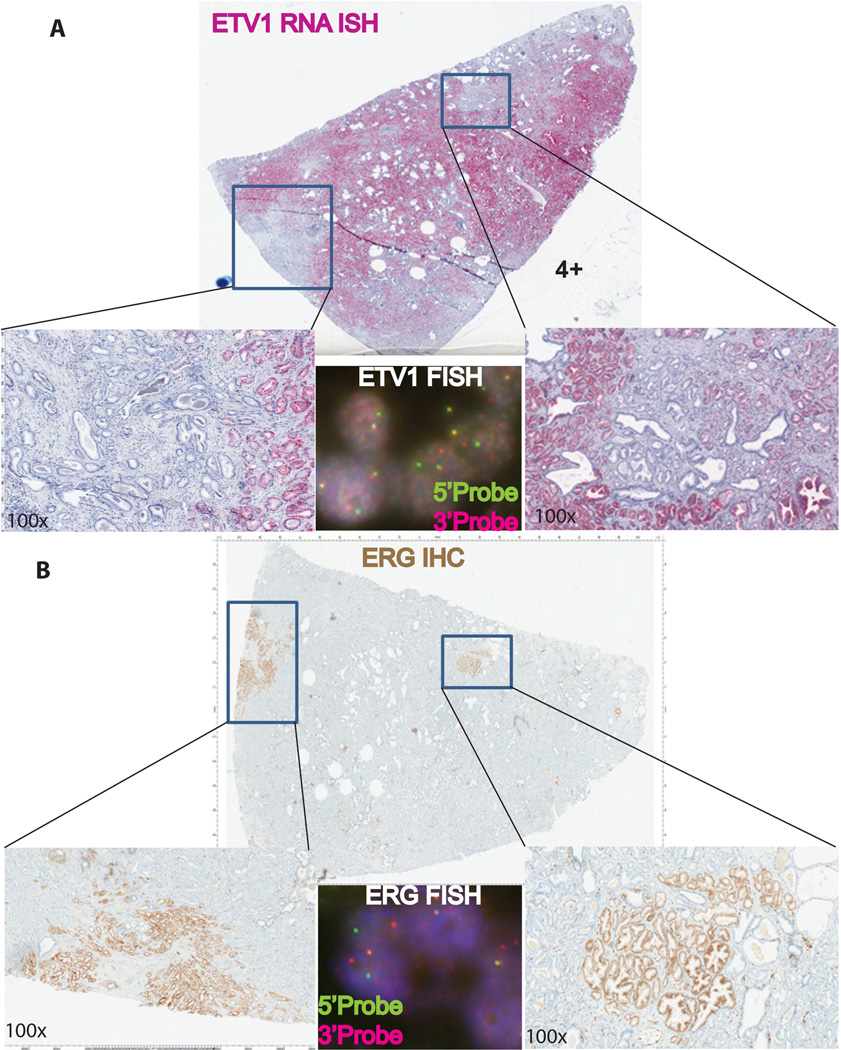
Figure 5.
Whole section view of a second prostate carcinoma tissue showing tumor molecular heterogeneity with a large tumor foci showing ETV1 expression by RNA ISH (Score 4) and two independent small foci showing ERG expression by immunohistochemistry (B). Confirmation of ERG and ETV1 rearrangements by FISH (Insets) in the corresponding tumor foci. Green and red color signals indicate 5’ and 3’ probes, respectively.
Sequential ERGIHC performed on the TMA sample with one core negative with ETV4 RNA-ISH was confirmed to be ERG-positive (Figure 6) and thus dual ETS rearrangement (ERG rearrangement and ETV1/ETV4 rearrangement in separate tumor foci/ areas were confirmed in 4 cases. The three ERG/ETV1and one ERG /ETV4 rearrangement cases were observed in localized, multifocal, and organ confined PCa with Gleason Score 3+4=7 (n=2), 4+3=7 (n=1) and 3+3=6 (n=1) respectively.
Figure 6.
A prostate adenocarcinoma sample showing ERG and ETV4 rearrangement in two independent tumor foci. A) ERG IHC; B) ETV1 RNA ISH; C) ETV4 RNA ISH with score 4; D) ETV5 RNA ISH.
DISCUSSION
In the present study, we demonstrate the utility of a novel RNA-ISH in-situ hybridization technique to detect ETV1, ETV4 and ETV5 rearrangements in formalin-fixed paraffin embedded PCa samples with high sensitivity and specificity. We systematically validated this technique initially by comparing the sensitivity and specificity of ERG RNA-ISH in situ hybridization with previously validated techniques such as FISH and IHC. We subsequently screened a large cohort of localized and metastatic PCa samples to identify the rare ETV1, ETV4 and ETV5 rearrangements using this novel technique.
Since the initial discovery of recurrent gene fusions in PCa, other prostate-specific molecular aberrations have been identified that stratify prostate cancer into distinct molecular subtypes, many of which are associated with altered clinical behavior[2]. Recent studies demonstrated that in addition to protein coding genes, non-coding RNA[21 22] and lineage- and cancer-specific pseudogenes[23 24] may serve as potential new biomarkers for disease classification and patient stratification in PCa.
RNA-based in situ detection methods are a viable approach to evaluate tissue level expression of non-coding RNAs and genes for which specific antibodies are not available. ERG, ETV1, ETV4 and ETV5 are highly expressed only in the presence of ETS gene fusions in PCa while benign prostate and ETS fusion-negative PCa show low or complete absence of ETS gene expression. This unique expression pattern is optimal for the identification of ETS rearrangement by RNA-ISH without any ambiguity based on expression. The utility of RNA-ISH is particularly evident for the detection of ETV1, ETV4 and ETV5 gene fusion at the tissue level as no specific antibodies exist unlike for ERG fusions.
Importantly, we identified the existence of rare molecular subsets of prostate cancer with dual ETS gene rearrangements in different tumor focus of the same tumor. Given the multifocal nature of prostate cancer, it has been shown that prostate tumors showed inter tumoral molecular heterogeneity with ERG rearrangement by deletion in one focus and translocation in other focus and intra tumoral heterogeneity with more than one 5’ ERG fusion partner gene (NDRG1. SLC45A3, TMPRSS2) rearrangement based on FISH evaluation [25–27]. Here, we observed heterogeneous expression pattern of ETV1 and ETV4 in a small subset of positive cases (3/11 ETV1 and 1/3 ETV4 cases) from the large cohort (n=319 cases) screened for these rearrangements. In samples with heterogeneous expression of ETV1 and ETV4, of the triplicate cores in tissue microarray, 1–2 were positive while the remaining cores were negative. We were intrigued by this observation and hypothesized that such small foci may have rearrangement with other ETS family gene or unknown gene (s). Since ERG accounts for the vast majority of ETS rearrangements, we performed ERG immunohistochemistry for these cases on whole sections and found ERG positivity in the small tumor foci that were negative for ETV1 or ETV4. Using RNA-ISH and ERGIHC sequentially, we demonstrated small ERG-positive foci admixed with large ETVI rearranged prostate cancer tumor focus (Figure 4 and 5). We also found one case where of the triplicate TMA cores; one core was ERG-positive by IHC and 1 core strongly positive for ETV4 by RNA-ISH (Figure 6). No tumor foci had concomitant staining with ERG and ETV1 or ETV4. While inter-focal heterogeneity of ERG rearrangements within multifocal prostate cancer is well described[28], to the best of our knowledge this is the first time dual ETS rearrangement in prostate cancer has been demonstrated in formalin fixed and paraffin embedded samples by sequential RNA in situ hybridization and ERG immunohistochemistry methods (Supplemental Figure 1). On morphologic evaluation these tumor foci appeared to be a single focus. Hence our results demonstrate that in such rare instances, what appears to be a single tumor focus by routine histologic evaluation are actually collisions of two or more distinct tumor foci.
Gene expression based microarray studies have shown high level expression of ERG, ETV1, ETV4 and ETV5 almost exclusively in ETS positive prostate cancers while benign prostate and ETS negative prostate cancers show very low or complete absence of ETS gene expression. This unique expression pattern is optimal for the identification of ETS rearrangement without any ambiguity based on RNA-ISH. Its utility is specifically critical in the recognition of ETV1, ETV4 and ETV5 genes which lack specific antibodies at the tissue level by a methodology analogous to immunoperoxidase detection.
Although ETS gene fusions are common in PCa, and are known to be an early event in prostate carcinogenesis, the role of these genes in the prostate tumor development is not known. ERG, ETV1 and ETV4 belong to the ETS gene family and in the unusual cases with dual ETS rearrangement in a given prostate cancer specimen, the overall clinical course of the disease may be driven by the ETS rearrangement that is dominant by tumor grade and/or volume. For example in the case depicted in Figures 4 and 5 the larger tumor harboring ETVI rearrangement may drive disease progression in contrast to the smaller ERG-positive tumor foci with similar or lower grade Recent data suggests that ETV1 rearrangement may be associated with a more adverse prognosis. Baena et al.,[29] showed that ETV1 under PTEN deletion background in mice developed a more invasive carcinoma compared to ERG with PTEN deletion background implicating differential oncogenic potential of ERG and ETV1 in prostate cancer development.
The cases we observed herewith dual ETV1 and ERG rearrangement, a majority of the tumor focus was ETV1 positive whereas smaller foci were ERG-positive suggesting independent clonal origin of the tumor harboring distinct molecular aberration. It is however not clear whether such cases have altered clinical behavior. Given that the majority of the tumor foci are ETV1 positive, such cases can be easily misdiagnosed as only ETV1-positive which underscores the importance of in situ detection methods for the simultaneous identification of multiple molecular subtypes of prostate cancer in a single specimen. Our preliminary studies here revealed the hitherto unidentified molecular subset of prostate cancer that warrants further in depth investigations to understand the molecular heterogeneity and associated clinical behavior.
In this study, we evaluated tissue microarrays containing predominantly specimens selected from major tumor foci and identified 4 cases with dual ETS rearrangement. Although, our small cohort of dual ETS rearranged tumors failed to reveal any specific histomorphologic features, further studies of larger cohorts of these unusual carcinomas are required to fully elucidate their clinical behavior and implications for prognosis. In addition to IHC and FISH, RNA-ISH in situ would be a valuable addition to the repertoire of tools used for the molecular sub classification of PCa. We are currently successfully using this RNA-ISH technique for determining the ETV1 status in an ongoing multi-institutional clinical trial that is testing the role of ETS fusions status from metastatic tumor biopsy as predictive biomarkers for response to abirater one (ABI) alone or with ABT-888 (a PARP1 inhibitor) in metastatic castrate resistant prostate cancer patients (NCI 9012, a randomized ETS fusion –stratified Phase II trial).
In summary, this study shows that RNA-ISH is a viable alternative for the in situ detection of genes for which no specific antibodies are available or for non-coding RNAs. Further, RNA-ISH assays can be easily performed on formalin fixed and paraffin embedded tissues from needle biopsies, radical prostatectomies and tissue microarrays that can be standardized and scaled for high-throughput screening of large number of samples.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported in part by the US National Institutes of Health Early Detection Research Network (U01 CA111275 and U01 CA113913), NIH S.P.O.R.E. (P50 CA69568), and R01 CA132874. N.P. is supported by a University of Michigan Prostate SPORE Career Development Award. A.M.C. is supported by the Howard Hughes Medical Institute, the Doris Duke Foundation and the Prostate Cancer Foundation and is an American Cancer Research Professor and a Taubman Scholar.
We thank Jyoti Athanikar for critical reading of this manuscript.
The University of Michigan has been issued a patent on ETS gene fusions in prostate cancer, on which S.A.T. and A.M.C. are co-inventors. The diagnostic field of use has been licensed to Gen-Probe Inc. /Hologic, who has sublicensed some rights to Ventana Medical Systems Inc. /Roche. A.M.C. serves on the advisory board of Ventana/Roche. S.A.T. has received honoraria from and consults for Ventana/Roche. Ventana/Roche did not play a role in the design and conduct of this study, in the collection, analysis, or interpretation of the data, or in the preparation, review, or approval of the article. N.P. receives research funding from Ventana/Roche but this funding did not play a part in development of the assays.
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
The remaining authors declare no conflicts of interest.
REFERENCES
- 1.Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer. Nat Rev Cancer. 2008;8(7):497–511. doi: 10.1038/nrc2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubin MA, Maher CA, Chinnaiyan AM. Common gene rearrangements in prostate cancer. J Clin Oncol. 2011;29(27):3659–68. doi: 10.1200/JCO.2011.35.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310(5748):644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 4.Helgeson BE, Tomlins SA, Shah N, et al. Characterization of TMPRSS2:ETV5 and SLC45A3:ETV5 gene fusions in prostate cancer. Cancer Res. 2008;68(1):73–80. doi: 10.1158/0008-5472.CAN-07-5352. [DOI] [PubMed] [Google Scholar]
- 5.Tomlins SA, Mehra R, Rhodes DR, et al. TMPRSS2:ETV4 gene fusions define a third molecular subtype of prostate cancer. Cancer Res. 2006;66(7):3396–400. doi: 10.1158/0008-5472.CAN-06-0168. [DOI] [PubMed] [Google Scholar]
- 6.Tomlins SA, Rhodes DR, Yu J, et al. The role of SPINK1 in ETS rearrangement-negative prostate cancers. Cancer Cell. 2008;13(6):519–528. doi: 10.1016/j.ccr.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu YM, Su F, Kalyana-Sundaram S, et al. Identification of Targetable FGFR Gene Fusions in Diverse Cancers. Cancer Discov. 2013;3(6):636–647. doi: 10.1158/2159-8290.CD-13-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palanisamy N, Ateeq B, Kalyana-Sundaram S, et al. Rearrangements of the RAF kinase pathway in prostate cancer, gastric cancer and melanoma. Nat Med. 2010;16(7):793–798. doi: 10.1038/nm.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner JC, Ateeq B, Li Y, et al. Mechanistic rationale for inhibition of poly (ADP-ribose) polymerase in ETS gene fusion-positive prostate cancer. Cancer Cell. 2011;19(5):664–678. doi: 10.1016/j.ccr.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ateeq B, Tomlins SA, Laxman B, et al. Therapeutic targeting of SPINK1-positive prostate cancer. Sci Transl Med. 2011;3(72):72ra17. doi: 10.1126/scitranslmed.3001498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young A, Palanisamy N, Siddiqui J, et al. Correlation of urine TMPRSS2:ERG and PCA3 to ERG+ and total prostate cancer burden. Am J Clin Pathol. 2012;138(5):685–696. doi: 10.1309/AJCPU7PPWUPYG8OH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barry M, Perner S, Demichelis F, et al. TMPRSS2-ERG fusion heterogeneity in multifocal prostate cancer: clinical and biologic implications. Urology. 2007;70(4):630–633. doi: 10.1016/j.urology.2007.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagle RB, Algotar AM, Cortez CC, et al. ERG overexpression and PTEN status predict capsular penetration in prostate carcinoma. Prostate. 2013;73(11):1233–1240. doi: 10.1002/pros.22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park K, Tomlins SA, Mudaliar KM, et al. Antibody-based detection of ERG rearrangement-positive prostate cancer. Neoplasia. 2010;12(7):590–598. doi: 10.1593/neo.10726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomlins SA, Palanisamy N, Siddiqui J, et al. Antibody-based detection of ERG rearrangements in prostate core biopsies, including diagnostically challenging cases: ERG staining in prostate core biopsies. Arch Pathol Lab Med. 2012;136(8):935–946. doi: 10.5858/arpa.2011-0424-OA. [published Online First: Epub Date]|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhalla R, Kunju LP, Tomlins SA, et al. Novel dual-color immunohistochemical methods for detecting ERG-PTEN and ERG-SPINK1 status in prostate carcinoma. Mod Pathol. 2013;26(6):835–848. doi: 10.1038/modpathol.2012.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han B, Mehra R, Dhanasekaran SM, et al. A fluorescence in situ hybridization screen for E26 transformation-specific aberrations: identification of DDX5-ETV4 fusion protein in prostate cancer. Cancer Res. 2008;68(18):7629–7637. doi: 10.1158/0008-5472.CAN-08-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han B, Mehra R, Lonigro RJ, et al. Fluorescence in situ hybridization study shows association of PTEN deletion with ERG rearrangement during prostate cancer progression. Mod Pathol. 2009;22(8):1083–1093. doi: 10.1038/modpathol.2009.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han B, Mehra R, Suleman K, et al. Characterization of ETS gene aberrations in select histologic variants of prostate carcinoma. Mod Pathol. 2009;22(9):1176–1185. doi: 10.1038/modpathol.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warrick JI, Tomlins SA, Carskadon SL, et al. Evaluation of tissue PCA3 expression in prostate cancer by RNA in situ hybridization-a correlative study with urine PCA3 and TMPRSS2-ERG. Mod Pathol. 2013 doi: 10.1038/modpathol.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prensner JR, Iyer MK, Balbin OA, et al. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol. 2011;29(8):742–749. doi: 10.1038/nbt.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prensner JR, Iyer MK, Sahu A, et al. The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nat Genet. 2013 doi: 10.1038/ng.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalyana-Sundaram S, Kumar-Sinha C, Shankar S, et al. Expressed pseudogenes in the transcriptional landscape of human cancers. Cell. 2012;149(7):1622–1634. doi: 10.1016/j.cell.2012.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tay Y, Kats L, Salmena L, et al. Coding-independent regulation of the tumor suppressor PTEN by competing endogenous mRNAs. Cell. 2011;147(2):344–357. doi: 10.1016/j.cell.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehra R, Han B, Tomlins SA, et al. Heterogeneity of TMPRSS2 gene rearrangements in multifocal prostate adenocarcinoma: molecular evidence for an independent group of diseases. Cancer Res. 2007;67(17):7991–7995. doi: 10.1158/0008-5472.CAN-07-2043. [DOI] [PubMed] [Google Scholar]
- 26.Svensson MA, LaFargue CJ, MacDonald TY, et al. Testing mutual exclusivity of ETS rearranged prostate cancer. Lab Invest. 2011;91(3):404–412. doi: 10.1038/labinvest.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clark J, Attard G, Jhavar S, et al. Complex patterns of ETS gene alteration arise during cancer development in the human prostate. Oncogene. 2008;27(14):1993–2003. doi: 10.1038/sj.onc.1210843. [DOI] [PubMed] [Google Scholar]
- 28.Mehra R, Tomlins SA, Yu J, et al. Characterization of TMPRSS2-ETS gene aberrations in androgen-independent metastatic prostate cancer. Cancer Res. 2008;68(10):3584–3590. doi: 10.1158/0008-5472.CAN-07-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baena E, Shao Z, Linn DE, et al. ETV1 directs androgen metabolism and confers aggressive prostate cancer in targeted mice and patients. Genes Dev. 2013;27(6):683–698. doi: 10.1101/gad.211011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.