SUMMARY
Classical genetic approaches to examine the requirements of genes for T cell differentiation during infection are time-consuming. Here we developed a pooled approach to screen 30–100+ genes individually in separate antigen-specific T cells during infection using short hairpin RNAs in a microRNA context (shRNAmir). Independent screens using T cell receptor (TCR)-transgenic CD4+ and CD8+ T cells responding to lymphocytic choriomeningitis virus (LCMV) identified multiple genes that regulated development of follicular helper (Tfh) and T helper-1 (Th1) cells, and short-lived effector and memory precursor cytotoxic T lymphocytes (CTL). Both screens revealed roles for the positive transcription elongation factor (P-TEFb) component Cyclin T1 (Ccnt1). Inhibiting expression of Cyclin T1, or its catalytic partner Cdk9, impaired development of Th1 cells and protective short-lived effector CTL, and enhanced Tfh and memory precursor CTL formation in vivo. This pooled shRNA screening approach should have utility in numerous immunological studies.
INTRODUCTION
The differentiation of T cells into effector and memory cells is central to adaptive immunity. Transcription factors (TFs) are central regulators of these differentiation processes. Although most current models of T cell differentiation incorporate relatively few regulatory players and rely heavily on the ‘master regulator’ concept, it is abundantly clear that TFs do not act in isolation and that transcription programs that underlie cell differentiation require the concerted actions of multiple factors, including important inducers or repressors of T cell differentiation pathways (Crotty, 2012; Kaech and Cui, 2012; O'Shea and Paul, 2010; Oestreich and Weinmann, 2012; Pipkin and Rao, 2009; Walsh et al., 2002). One large-scale study of the regulation of gene expression in different cell types and tissues found that a given murine cell type could be distinguished from other cell types by a network of approximately 6 TF:TF interactions, and that these TF networks were conserved in human cell types (Ravasi et al., 2010). Thus, the intersecting expression and actions of multiple TFs appear to determine cell fate and function. Recent work on T helper-17 (Th17) cell differentiation suggests that this model also applies to T cells (Ciofani et al., 2012).
The differentiation of naïve CD8+ T cells into CTL is a key process in immunity to viral infections. The differential development of short-lived effector CTL and precursors to long-lived memory CTL are considered alternative cellular ‘fates’ (Chang et al., 2007; Joshi et al., 2007), and understanding this process is critical for prevention and treatment of acute and chronic infections (Doering et al., 2012; Haining and Wherry, 2010; Kaech and Cui, 2012). Activated CD4+ T cells can differentiate into a range of different functional subsets, including Th1, Th2, Th17, peripheral Treg (pTreg), and follicular helper (Tfh) cells, that each have potent capacities to regulate immune responses and eliminate pathogens. Among CD4+ T cells, follicular helper cells (Tfh) are the specialized providers of help to B cells (Crotty, 2011). T-dependent antibody responses are important for protection against a wide range of pathogens. Our understanding of Tfh cells is still in the early stages, and there is much to be learned about the pathways that control Tfh cell differentiation.
A number of excellent studies have characterized the mRNA expression profiles of CD8 and CD4+ T cells isolated ex vivo during the course of antigen-specific responses (Best et al., 2013; Doering et al., 2012; Kaech and Cui, 2012; Kalia et al., 2010; Choi et al., 2013). However, differential mRNA expression studies are likely to overlook a large number of relevant factors responsible for T cell differentiation. For example, of nearly 2,000 predicted conventional DNA-binding transcription factors in the murine genome (Gray et al., 2004), fewer than 15 have validated roles in effector CD8+ T cell differentiation (Kaech and Cui, 2012; Pipkin and Rao, 2009). The same limitations likely hold for Tfh cell differentiation and other CD4+ T cell differentiation pathways (Crotty, 2012; Oestreich and Weinmann, 2012; Vahedi et al., 2013). Thus, a functional genetic approach in which inhibition of a large number of genes individually, in separate cells in parallel, during T cell differentiation has the potential to rapidly identify factors comprising the genetic networks underlying T cell function.
To pursue this objective, we have devised an experimental approach that uses retroviral shRNAmir libraries to diminish the expression of selected gene products one at a time in antigen-specific T cells. Gene function in antiviral responses is then interrogated in pooled screens in mice. We have demonstrated the utility of this approach in two T cell differentiation processes in vivo: CD8+ T cell differentiation into cytotoxic T lymphocytes (CTL), and CD4+ T cell differentiation into Tfh and Th1 cells. Here we show proof of principle that the roles of multiple genes can be interrogated in parallel in T cells during infection, and novel factors were identified that are involved in these differentiation processes. This approach holds promise to substantially accelerate the understanding of T cell differentiation in vivo.
RESULTS
An optimized retroviral vector to express shRNAmir in vivo
Transduction of activated T cells with murine stem cell virus (MSCV) -based retroviral expression vectors (RVs) has previously been used to drive transgene expression, or to deplete expression of endogenous genes by triggering RNA interference (RNAi) using shRNAs upon adoptive transfer in vivo (Araki et al., 2009; Johnston et al., 2009; Joshi et al., 2007; Kao et al., 2011). However, we found that transduction of SMARTA TCR transgenic CD4 T cells (LCMV-specific, gp66–77 IAb restricted) with an MSCV-based (pLMP-derived) RV designed to express shRNAs in the context of miRNA-30 sequences (shRNAmir) resulted in depletion of the transduced cells after an acute LCMV infection (Figure S1A, left panel). This most likely was due to immune rejection of antigens expressed from the pLMP (Figure S1B), as deletion of the puromycin resistance gene from pLMP (LMPd) eliminated this effect (Figure S1A, right panel). We replaced GFP in LMPd with the violet-excitable, yellow-fluorescing GFP variant Ametrine1.1 (LMP-Amt) to expand its utility in FACS (Figures S1B and S1C), and confirmed its functionality for RNAi in vivo by targeting Bcl6. Transferred SMARTA CD4+ T cells transduced with Bcl6-specific shRNAs (LMP-Amt shBcl6-RV, referred to hereafter as shBcl6-RV) displayed a reduced fraction of CXCR5+Bcl6+ cells upon LCMV infection, consistent with a requirement for Bcl6 for differentiation of follicular T helper cells (Tfh) (Figure S1D).
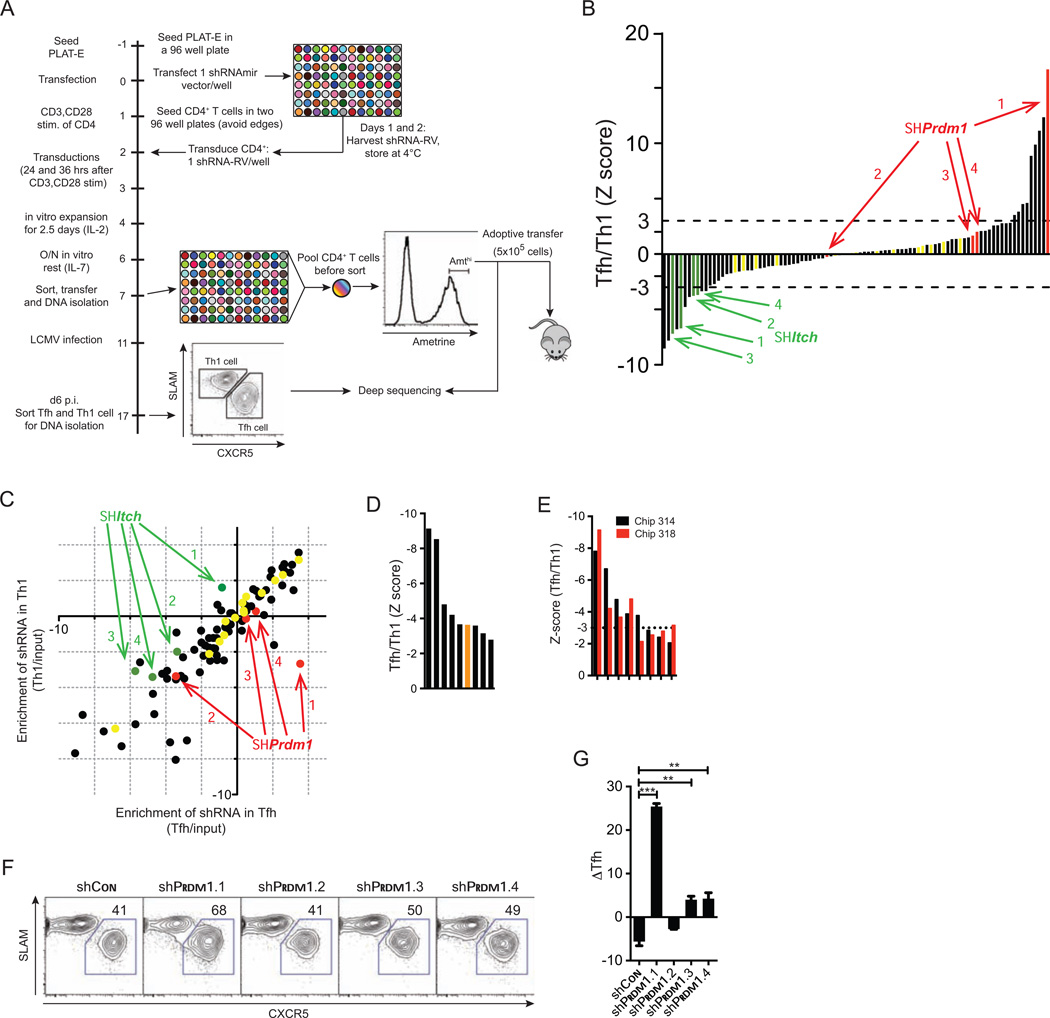
A pooled screening system using shRNAs in CD8+ T cells during LCMV infection
We parallelized the shRNAmir-RV approach in order to interrogate the functions of numerous genes simultaneously. The experimental strategy was to introduce a pool of TCR transgenic T cells carrying individual shRNAs into host mice, and assay alterations in the responding T cells during a viral infection (Figure 1A). In effect, each T cell is barcoded by the integrated shRNA-RV, and the fate of individual cells carrying each shRNA can be monitored in T cell populations of interest by deep sequencing DNA libraries derived from the integrated provirus (Beronja et al., 2013; Zuber et al., 2011) (Figures 1B and 1C). We optimized conditions in 96-well format to produce arrays of high titer RV supernatants without concentration, sufficient to transduce ≥ 70% of LCMV-specific P14 TCR transgenic CD8+ T cells 18 hr after TCR stimulation (Figure 1B, Figures S2A and S2B). The day after transduction, cells from each well were pooled (Figure 1B, day 0) and immediately transferred to recipient mice without cell sorting (sorting reduced P14 accumulation in vivo, Figure S2C), and the recipients were infected with LCMV 1 hour later. In addition, an aliquot of Ametrine high cells was FACS-purified and saved as the “input”.
Figure 1. Optimization of conditions for a pooled screening approach using shRNAmirs in CD8+ T cells in vivo to identify genes that regulate CTL differentiation during infection.
(A) A conceptual representation depicting the principle of the pooled screening strategy.
(B) Scheme for the shRNAmir screen using P14 cells and LCMV infection.
(C) Scheme for quantifying shRNAmirs. DNA libraries generated by PCR of the integrated shRNAmir provirus are analyzed by deep sequencing to quantify shRNA representation in the cell subsets.
(D) Total P14 cell numbers recovered in the spleen in the presence or absence of LCMV infection. Error bars indicate standard deviations.
(E) Blimp1-YFPhi, CD25hi KLRG-1hi and IL-7Rαhi cell frequencies at the indicated time points after infection. Symbols represent values from individual mice. Red = LCMV infected mice. Black = uninfected mice.
(F) Representative flow cytometry plots of KLRG-1 and IL-7Rα staining on P14 cells under conditions used for screening.
Genomic DNA was prepared from the input and samples of P14 cells isolated by flow cytometry on day 7 after LCMV infection. Deep sequencing was used to quantify shRNA representation (Figure 1A and Figure S2D–H), after a single step PCR of the shRNAmir from genomic DNA template to generate the sequencing libraries (Figure 1B and 1C). Multiple PCR conditions were interrogated (Figure S2D–G). Independent libraries generated from different DNA template amounts at low PCR cycles (22 or 26 cycles, Figure S2F and S2G) exhibited high correlations in shRNA representation, with both 314 (medium density) and 318 (high density) PGM sequencing chips (Figure S2H). Thus, the sequencing approach was robust.
To establish conditions for screening pools of shRNAmir-RV+ P14 CD8+ T cells in the context of infection, numerous factors were optimized and standardized (Figure S3). Naïve Thy1.1+ Blimp1-YFP transgenic P14 cells were activated in vitro and transfered to B6 hosts subjected to LCMV infection and the P14 cells were examined as a function of (i) cell transfer number (Figure S3A), (ii) the timing of the infection relative to cell transfer (data not shown), (iii) LCMV dose (Figure S3B), and (iv) LCMV strain (Figure S3C). Transfer of 500,000 activated P14 cells followed by intraperitoneal (IP) infection with 1.5 × 105 of LCMV-clone 13 (LCMV-cl13) resulted in a robust infection that induced accumulation of 106 P14 cells in the spleen by day 7, ~50-fold more than in uninfected recipients (Figure 1D and Figure S3D). Under these conditions virus replication was strongly inhibited (see below), and the responding P14 cells exhibited CD8+ T cell phenotypes typical of acute infection, based on interleukin-2 receptor α (IL-2Rα) (CD25), KLRG-1, IL-7Rα (CD127) and Blimp1-YFP reporter expression (Figures 1E, 1F and Figure S4A). LCMV-cl13 is more virulent than LCMV Armstrong (Wherry et al., 2003) but was controlled due to the P14 cell transfers. In addition, we confirmed that short-lived effector (KLRG-1hi IL-7Rαlo) and memory precursor (KLRG-1lo IL-7Rαhi) P14 populations exhibited different potentials for memory cell formation and “recall” capacity (Figures S4B–S5E). Altogether, these results demonstrate robust in vivo conditions for effector and memory CTL development.
The number of distinct shRNAmirs that could be tested in parallel was constrained by the number of adoptively transferred T cells. In order to ensure library complexity, we aimed to represent each shRNA with ~500 cells per mouse upon engraftment. This depth of representation is similar to, or exceeds recent in vivo shRNA-based screens (Beronja et al., 2013; Zhou et al., 2014; Zuber et al., 2011). Based on data from adoptively transferred naïve CD8+ T cells (Badovinac et al., 2007) and activated CD8+ T cells (Pipkin et al., 2010), we assumed that ~10% of transferred cells would engraft. Thus, we initially analyzed a pool of 500,000 cells representing ~100 unique shRNAs in a single experiment. We also considered the recovery of effector and memory precursor populations. Based on P14 accumulation data at day 7 post infection (Figure 1D), we expected to recover ~ 300,000 KLRG-1hi IL-7Rαlo and ~ 30,000 KLRG-1lo IL-7Rαhi cells per mouse. To assure cell numbers would not limit library complexity at the end of the experiment, and reduce potential founder effects from variation in individual mice (Zuber et al., 2011), sorted cells were pooled from 5 or more infected mice in each experiment.
Identification of genes underlying CTL differentiation using an shRNA screen in vivo
We selected 34 genes to screen to test the approach. These included genes differentially expressed in Tfh cells and CTLs (Choi et al., 2013), broadly expressed chromatin and transcriptional regulators with unknown roles in effector T cells, and multiple positive and negative control genes. Each gene was targeted by 1–5 shRNAmirs, depending on availability in the original library (see Experimental Procedures). The use of multiple shRNAs per gene is important, as not all shRNAs are functional. 110 unique shRNAs that target these genes were subcloned as a pool into pLMPd-Amt (Table S1 and Figure S5A and S5B). Individual colonies from this transformation were picked, sequence verified, and re-arrayed into 96 well plates.
Retroviral supernatants prepared from cells transfected with the array of 110 shRNAmir DNAs were used to transduce P14 cells. Transduction of each construct was confirmed by Ametrine fluorescence, and P14 cells were pooled. An aliquot was removed for the input sample, and 500,000 cells were transferred into multiple recipient mice that were then infected with LCMV. On day 7 post infection, short-lived effector (KLRG-1hiIL-7Rαlo) and memory precursor (KLRG-1loIL-7Rαhi) P14 cells were sorted, genomic DNA was extracted from each population, and shRNAmir sequencing libraries were prepared. After sequencing, 642,718, 233,902, and 487,865 reads aligned to the reference shRNA sequences, derived from the input, effector, and memory precursor cell populations, respectively. All intended shRNAs introduced during T cell transduction were recovered from input samples, but two were not detected in either the effector or memory-precursor cell populations. The memory precursor: short-lived effector cell ratio for each shRNA was calculated. Values from negative control shRNAs targeting genes not expressed in CD8+ T cells (Cd4, Cd14, Cd19, Ms4a1 (CD20)) based on RNA-seq analysis (MEP unpublished data) were used to calculate Z-scores for each shRNA (Figure 2A and Table S1), and the ratios of shRNAs in effector and memory precursor subsets relative to the input cells (Figure 2B). Several shRNAs were substantially reduced in both short-lived effector and memory precursor P14 cell subsets relative to input, suggesting the genes were required for the accumulation of P14 T cells during infection (Figure 2B).
Figure 2. A pooled RNAi screen in CD8+ T cells in vivo identifies potential regulators of effector and memory precursor CTL formation.
(A) Relative enrichment of shRNAs in memory precursor and short-lived effector P14 cell populations is reported as Z-scores for each shRNA in the library. Each bar represents a single shRNA. Negative control shRNAs are colored yellow.
(B) Scatter plot shows the log2 ratio of normalized reads of all shRNAs in each sorted CD8+ T cell subset versus the input sample. Each dot represents a unique shRNA and is color-coded as in (A).
(C) Tbx21 mRNA expression in shTbx21+ P14 CD8+ T cells , after 6 days of culture (10 U/mL IL-2). shCon = Control shRNAmir.
(D) Intracellular IFN-γ staining in P14 CD8+ T cells, gated on shTbx21+ cells. Cells were cultured for 6 days (10 U/mL IL-2) and restimulated with PMA and Ionomycin for 4 hours before staining.
(E) T-bet expression in shTbx21+ P14 CD8+ T cells from spleens at 8 days post LCMV infection (normalized geometric MFI). T-bet staining is shown for representative mice (right).
(F) Contour plots show KLRG-1 and IL-7Rα staining on shTbx21+ P14 CD8+ T cells from representative mice at 8 days after LCMV infection.
(G-I) Quantitation of CD8+ T cell subsets resulting from shTbx21+ P14 cells in vivo. (G) Shortlived effector cells (KLRG-1hi IL-7Rαlo). (H) Memory precursor cells (KLRG-1lo IL-7Rαhi). (I) Ratio of memory precursor to short-lived effector phenotype P14 cells, per mouse.
(J) Prdm1 mRNA expression was determined by qRT-PCR in transduced P14 CD8+ T cells after sorting from spleens 7 days post LCMV infection. Blimp1 protein expression was determined by Western blot analysis after 4 days of culture with IL-12 (5ng/mL) and IL-2 (100 U/mL).
(K) Map of Prdm1 with shRNA targeted regions indicated.
(L) Contour plots of KLRG-1 and IL-7Rα staining on shPrdm1+ P14 CD8+ T cells from representative mice at 7 days after LCMV infection.
(M) Ratios of memory precursor to effector P14 CD8+ T cells . Each symbol represents T cells from an individual mouse.
Data are pooled from 3 (H, I) and 2 (J, L) independent experiments. *P<0.05, **P<0.01, ****P<0.0001. Error bars indicate standard deviations.
To focus on factors with differential effects on short-lived effector versus memory-precursor CD8+ T cell subsets, we identified genes for which 2 or more cognate shRNAs were enriched with Z-score values of ≥ |3.0|, and classified these as hits. None of the negative control genes exhibited this pattern (Figure 2A and Table S1). Genes that met these criteria were identified in both effector and memory precursor subsets (Figure 2A and Table S1). As expected from studies with gene-deficient mice (Cannarile et al., 2006; Intlekofer et al., 2005; Joshi et al., 2007; Yang et al., 2011), Tbx21 (T-bet) and Id2 specific shRNAs were enriched in memory precursor cells, as these genes are necessary for effector CTL generation (Figure 2A and Table S1). Conversely, all three shRNAs targeting Id3 were enriched in effector CTL (Figure 2A and Table S1), but were just below the criteria to be designated a hit, consistent with a mild early defect in memory precursor formation in Id3-deficient mice (Ji et al., 2011; Yang et al., 2011).
Tbx21 and Prdm1 shRNAs impair effector CTL development during LCMV infection
We validated results of the screen by examining the impact of shRNAs individually. Both shTbx21.2 and shTbx21.3, but not shTbx21.1, strongly depleted Tbx21 mRNA and T-bet protein (Figure 2C, E), inhibited interferon-γ (IFN-γ) expression (Figure 2D), and limited development of short-lived effector cells in vivo (P <0.01, Figure 2F–I). These results correlated directly with the enrichment of these shRNAs in the screen (Figure 2A and Table S1), and confirm the role of T-bet in the generation of effector CD8+ T cells .
Prdm1 (encoding Blimp-1) has known roles in effector CD8+ and CD4+ T cell differentiation (Rutishauser et al., 2009; Shin et al., 2009)(Johnston et al., 2009). Consistent with this, Prdm1 shRNAs were preferentially enriched in memory precursor CD8+ T cells, but the magnitude of their effects differed in replicates of the in vivo screen (Figure S5C and Table S1). Analysis of each Prdm1 shRNA individually showed that three of four shRNAs impaired expression of both Blimp-1 mRNA and protein (Figure 2J). The fourth shRNA impaired Blimp-1 protein expression but did not reduce its mRNA (Figure 2J), perhaps because it targeted the Prdm1 3’ UTR (Figure 2K). All four Prdm1 shRNAs impaired effector CD8+ T cell frequencies and increased the ratio of memory precursor cells to short-lived effector cells in vivo (P < 0.01 – 0.05, Figures 2L and M). These data indicate that shRNAs can have variable effects but confirmed that Prdm1 expression is required for short-lived effector CD8+ T cell differentiation.
Identification of genes underlying Tfh cell differentiation using an shRNA screen in vivo
In parallel, we developed a pooled screen in CD4+ T cells to discover genes important for Tfh and Th1 cell differentiation in vivo (Figure 3A). 5×105 shRNAmir+ Amthi SMARTA cells were transferred into B6 hosts, and mice were infected 3–4 days later with LCMV Armstrong (Figure 3A). DNA was also isolated from an aliquot of cells before the transfer (input). Six days after LCMV infection, virus-specific Tfh cells (CXCR5+SLAMlo) and Th1 cells (CXCR5-SLAMhi) (Choi et al., 2013; Johnston et al., 2009) were isolated by flow cytometry and deep sequenced for differential representation of the shRNAs. The Tfh/Th1 ratios for each shRNA were calculated and their Z-scores were plotted (Figures 3B and 3C). 14 control shRNAs expected not to affect Tfh or Th1 differentiation (not known to be expressed in CD4+ T cells : Cd14, Cd19, Cd22, Ms4a1, Cd8, Smarca1) were equally distributed in both populations (Figure 3B), and their effects on cell accumulation were also assessed (Figure 3C). Based on a Z-score cutoff of ≥ |3.0| for each shRNA, factors encoded by Prdm1, Chd4, Id2, and Ccnt1 were identified as candidate positive regulators of Th1 cells or inhibitors of Tfh cell differentiation (Figures 3B and 3C, Table S1). Prdm1 is a positive control, as an inhibitor of Tfh cell differentiation (Johnston et al., 2009), and is discussed further below. Genes Fosb, Plagl1, Mta3, and Runx3 were identified as potential positive regulators of Tfh cells or inhibitors of Th1 cell differentiation (Table S1). Plagl1 is highly expressed in Tfh cells (Hale et al., 2013; Yusuf et al., 2010), and MTA3 is known to interact with Bcl6 in B cells (Fujita et al., 2004). Itch was selected as a known positive regulator of Tfh cell differentiation, based on a profound loss of Tfh in Itchfl/fl Cd4-cre+ mice (Xiao et al., 2014). Notably, all four Itch shRNAs were severely depleted from Tfh cells and highly enriched in the Th1 cell population (Figures 3B and 3C). In a second replicate of the CD4+ T cell screen, the validated Bcl6 shRNA (shBcl6.2, Figure S1D) was depleted from the Tfh cell population, as expected (Figure 3D). Comparisons of the two independent screens indicated that the in vivo screens generated reproducible results (Figure 3E), and also showed that shRNA representation was similar even when the libraries were sequenced with increased coverage using the higher density PGM 318 chip (Figure 3E). These results suggest that the CD4+ T cell shRNAmir-RV screening approach in vivo was also robust.
Figure 3. A pooled RNAi screen in CD4+ T cells identifies potential regulators of Tfh and Th1 cell differentiation in vivo.
(A) Scheme for the shRNAmir screening approach using SMARTA CD4+ T cells .
(B) Relative enrichment of shRNAs in the Tfh or Th1 cell populations in vivo, reported as Z-score values for each shRNA in the library. Z-scores of |3| and |2| are indicated by a dotted line, and a tick, respectively.
(C) Scatter plot shows the log2 of normalized reads of all shRNA in Tfh and Th1 cell populations vs. the input sample. This reveals effects on cell survival or proliferation. shRNA are color-coded as in (B).
(D) Z-scores are shown for the shRNAs most depleted from the Tfh cell population in the Ion 318 Chip experiment. shBcl6 is highlighted in orange.
(E) Z-scores of shRNAs depleted from Tfh in two independent deep sequencing reactions: Ion 314 Chip (black bars), Ion 318 Chip (red bars). The dotted line is a Z-score of −3.
(F) SMARTA CD4+ T cells were transduced with the indicated shRNAs, transferred into B6 mice, and analyzed 6 days after LCMV infection. shCon = a control shRNAmir. Representative flow cytometry plots are shown of shRNA and shRNA+ SMARTA CD4+ T cells with Tfh cell (CXCR5+SLAMlo) gate drawn.
(G) The differences in percentages of Tfh (%Tfh of Amt+ - %Tfh of Amt−) for each shRNAmir in SMARTA CD4+ T cells are shown. **P<0.01, ***P<0.001. Error bars indicate standard deviations.
To confirm results from the primary screen (Figure 3B), the effects of Prdm1 shRNAs were examined individually. SMARTA CD4+ T cells transduced with shPrdm1.1-RV exhibited the strongest Tfh cell bias in vivo (P < 0.001, Figures 3F and 3G), consistent with results from the screen. A modest but significant Tfh cells bias was observed in shPrdm1.3+ and shPrdm1.4+ SMARTA CD4+ T cells when compared to untransduced CD4+ T cells (P < 0.01, Figure 3F and 3G). ShPrdm1.2 had no effect on Tfh cell differentiation (Figures 3F and 3G), consistent with it having the weakest effect on Prdm1 mRNA expression in CD4+ T cells (data not shown). These results correlated with the observed distribution of the 4 shRNA in the primary screen in CD4+ T cells (Figure 3B), and also indicate that the activity of individual shRNAs might depend on the specific cellular context (e.g., CD4 versus CD8+ T cells ; Figures 3B and 2G). Thus, results of the pooled shRNA screen were consistent with experiments using individual constructs.
Ccnt1 is required for both Th1 cell and effector CD8+ T cell differentiation in vivo
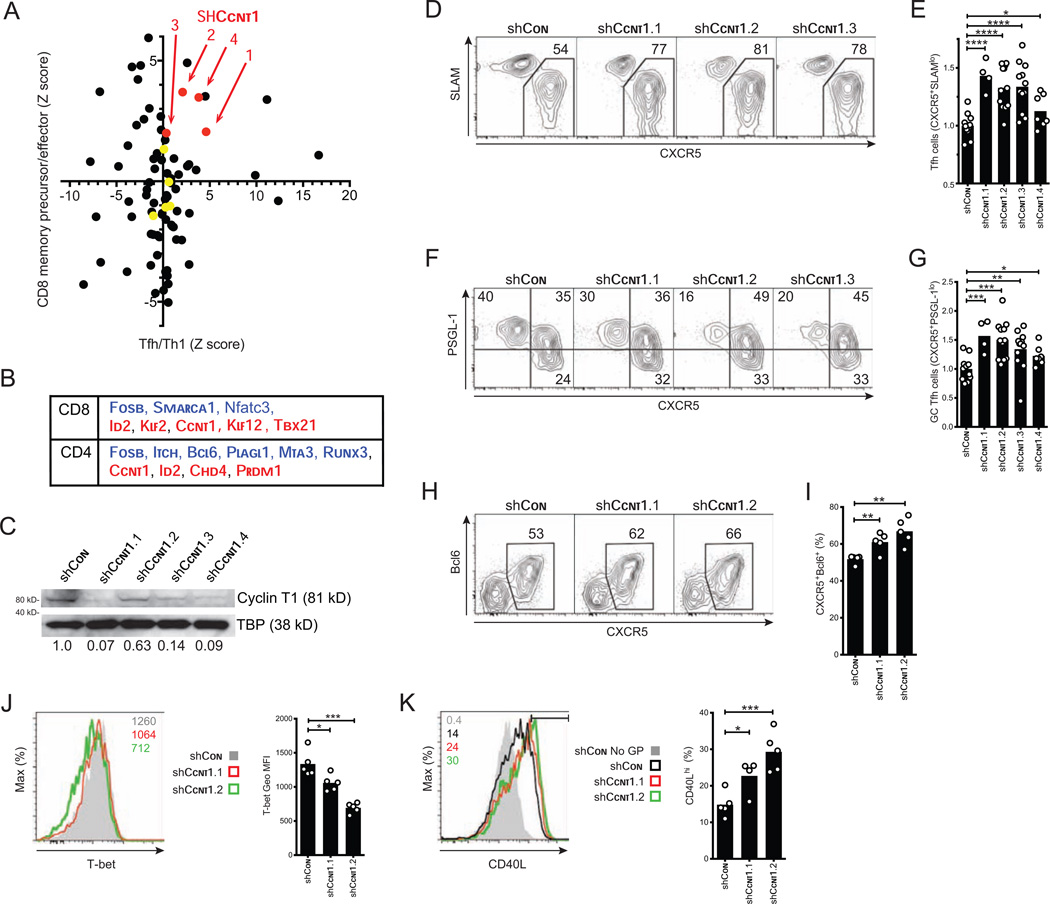
We compared the full datasets from the CD8 and CD4+ T cells screens and found that inhibition of several different genes affected differentiation of both effector CD4 and CD8+ T cells (Figures 4A and 4B). Ccnt1, encoding Cyclin T1, is a non-canonical cyclin that is a regulatory subunit of the RNA polymerase II positive transcription elongation factor (P-TEFb). All four shRNAs targeting Ccnt1 were depleted from KLRG-1hiIL-7Rαlo terminal effector CD8+ T cells and from Th1 cells in the screens (Figure 4A). Based on the notion that functional parallels might exist between differentiation of CD4+ and CD8+ T cells during infection (Choi et al 2013; Yang et al., 2011), we further explored the roles of Cyclin T1 in both subsets.
Figure 4. Ccnt1 depletion promotes development of Tfh cells during viral infection.
(A) Comparison of shRNAmir screening results in both CD4 and CD8+ T cells. Tfh and Th1 CD4+ T cells differentiation results, plotted against memory precursor and effector CD8+ T cell differentiation results. Z-score values are shown. Common negative control shRNAs (yellow), and those targeting Ccnt1 are highlighted (red).
(B) Table of top hits for genes required for memory precursor CD8+ T cell or Tfh CD4+ T cells differentiation (blue), and short-lived effector CD8+ T cell or Th1 CD4+ T cells differentiation (red).
(C) Cyclin T1 protein expression in MCC T cells after transduction with the indicated shRNAs and 4 days of culture. The ratios of Cyclin T1 to TBP relative to the control shRNA are indicated.
(D-E) Flow cytometry plots (D) and quantitation (normalized) (E) of Tfh cell differentiation (CXCR5+SLAMlo) by shCcnt1+ SMARTA CD4+ T cells at 6 days after LCMV infection.
(F-G) Flow cytometry plots (F) and quantitation (normalized) (G) of GC Tfh cell differentiation (CXCR5+PSGL1lo) by shCcnt1+ SMARTA CD4+ T cells at 6 days after LCMV infection.
(H-I) Flow cytometry plots (H) and quantitation (I) of CXCR5 and Bcl6 expression by shCcnt1+ SMARTA CD4+ T cells at 4 days after LCMV infection.
(J) T-bet expression in shCcnt1+ SMARTA CD4+ T cells in vivo, 3 days after LCMV infection. T-bet geometric MFIs are graphed (right panel).
(K) CD40L expression by shCcnt1+ SMARTA CD4+ T cells at 4 days after LCMV infection, after 2 hour restimulation with GP61–80 peptide. The percentages of CD40Lhi cells are indicated.
Each symbol represents T cells from an individual mouse.
Data are pooled from 3 (F, G) or representative of 2 (K) or 3 independent experiments (H-J).
*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
In CD4+ T cells, all four Ccnt1-shRNAs inhibited Cyclin T1 protein expression to varying degrees; three of four caused robust inhibition (Figure 4C and Figure S6A). Each Ccnt1 shRNA was examined individually in SMARTA CD4+ T cells 6 days after LCMV infection (Figures 4D-4G). Neither CD4+ T cell proliferation (Figure S6B, S6C), nor CD4 or CD44 expression was affected by Ccnt1 shRNAs (data not shown). Ccnt1 shRNAs both increased Tfh cell development (CXCR5+SLAMlo), and decreased Th1 cell formation (P < 0.0001 – 0.05; Figures 4D and 4E). Germinal center Tfh (GC Tfh) cells are a fully polarized subset of Tfh cells (CXCR5+PSGL1lo, ref Crotty, 2011; Poholek et al., 2010) and their frequencies were increased by Ccnt1 shRNAs (P < 0.001 – 0.05; Figures 4F and 4G).
We also examined T cell differentiation at earlier times points and found that Ccnt1 shRNAs substantially increased the proportion of early CXCR5+Bcl6+ Tfh cells (P < 0.01; Figures 4H and 4I, and P < 0.01 – 0.05; Figure S6D). The increased Tfh cell differentiation of shCcnt1+ CD4+ T cells after LCMV infection could be a reflection of a decreased potential of these cells to differentiate into Th1 cells. Consistent with this hypothesis, Ccnt1 shRNAs resulted in decreased expression of T-bet in vivo (P < 0.001 – 0.05; Figure 4J). Reciprocally, the expression of CD40L, an essential component of T cell help to B cells, was also increased in shCcnt1+ cells (P < 0.001 – 0.05; Figure 4K). These results suggest that Cyclin T1 promotes Th1 cell differentiation at the expense of Tfh differentiation in vivo.
To test the requirement for Cyclin T1 in T cell differentiation in vitro, we cultured shCon+ and shCcnt1+ CD4+ T cells in Th1 cell-biasing conditions. Ccnt1 shRNAs impaired T-bet expression under these conditions (P < 0.0001; Figure 5A), resulting in a substantial loss of IFN-γ production upon restimulation (P < 0.0001-0.001; Figures 5B and 5C). The defect was cell intrinsic, as no defect in IFN-γ production was observed in untransduced CD4+ T cells in the same wells (Figure S6E). These results support a model in which reduced Cyclin T1 expression impairs Th1 cell development and favors Tfh cell development.
Figure 5. Cyclin T1 and Cdk9 depletion impairs Th1 cell differentiation in vitro and in vivo.
(A-E) CD4+ T cells were transduced with Ccnt1 shRNAs and cultured under Th1-biasing conditions for 4 days before restimulation with PMA and ionomycin for 1 (D-E) or 4 hours (A-C).
(A) T-bet expression by shCcnt1+ CD4+ T cells . Quantitation and an example histogram are shown.
(B) Flow cytometry plots of IFN-γ expression by shCcnt1+ CD4+ T cells upon restimulation.
(C) Quantitation of B, for all samples.
(D) Cdk9 protein expression in shCdk9+ MCC T cells.
(E) Flow cytometry plots of IFN-γ expression by shCdk9+ CD4+ T cells upon restimulation.
(F) Quantitation of E, for all samples.
(G-H) Flow cytometry plots (G) and quantitation (H) of Tfh cell differentiation (CXCR5+SLAMlo) by shCdk9+ SMARTA CD4+ T cells at 6 days after LCMV infection.
(I-J) Flow cytometry plots (I) and quantitation (J) of GC Tfh differentiation (CXCR5+PSGL1lo) by shCdk9+ SMARTA CD4+ T cells at 6 days after LCMV infection.
(K-L) Flow cytometry plots (K) and quantitation (L) of CXCR5 and Bcl6 expression by shCdk9+ SMARTA CD4+ T cells, 4 days after LCMV infection.
(M) Histograms of CD40L expression on shCdk9+ SMARTA CD4+ T cells , after isolation from spleens 4 days after LCMV infection and restimulation with GP61-80 peptide for 4 hours. The percentages of CD40Lhi SMARTA are shown, and summarized (right). Data are representative of 2 independent experiments. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
The P-TEFb subunit Cdk9 is necessary for Th1 cell differentiation
Canonical P-TEFb comprises Cdk9 (catalytic subunit) and a regulatory subunit (e.g., Cyclin T1 or T2). To test whether Cyclin T1 was likely acting via P-TEFb, we examined two Cdk9 shRNAs in SMARTA CD4+ T cells for their effects on Tfh vs Th1 cell differentiation. Both shRNAs inhibited Cdk9 expression in vitro (Figure 5D). CD4+ T cells transduced with Cdk9 shRNAs and cultured under Th1 cell-biasing conditions showed impaired production of IFN-γ, similar to the effect of Ccnt1 shRNAs (P < 0.001 – 0.01; Figures 5E and 5F), suggesting that both Cyclin T1 and Cdk9 promote Th1 cell differentiation in vitro.
Cdk9 depletion in vivo favored Tfh (CXCR5+SLAMlo) cell development while reducing Th1 cell differentiation (shCdk9.1, P < 0.0001; Figures 5G and 5H), without impairing T cell expansion (Figure S7A) or normal expression of CD4 and CD44. Furthermore, Cdk9 depletion increased the frequency of GC Tfh cells, measured as CXCR5+PSGL1lo cells (shCdk9.1, P < 0.001; Figures 5I and 5J), or CXCR5+Bcl6hi cells (Figures S7B–C). Additionally, based on CXCR5 and Bcl6 expression, early Tfh cell differentiation in shCdk9+ CD4+ T cells was enhanced 4 days after LCMV infection (P < 0.01 – 0.05; Figures 5K and 5L), with normal cell expansion (Figure S7D). Intriguingly, similar to what was observed in the absence of Cyclin T1, Cdk9 deficiency also increased CD40L expression by CD4+ T cells (P < 0.001 – 0.01; Figure 5M). Altogether, these data recapitulated those obtained with shCcnt1+ CD4+ T cells and suggest that P-TEFb might preferentially promote Th1 cell differentiation of activated CD4 + T cells .
Ccnt1 and Cdk9 are required for development of protective effector CTL in vivo
Next, we explored the requirements of Ccnt1 in CD8+ T cells . Three of the four Ccnt1-specific shRNAs resulted in near complete inhibition of Cyclin T1 protein expression in cultured CD8+ T cells; one shRNA exhibited strong but incomplete inhibition (Figure 6A). The accumulation of P14 CD8+ T cells transduced with Ccnt1 shRNAs was not impaired during culture (Figure 6B), or in vivo (Figure 6C). However, Ccnt1 shRNAs strongly impaired generation of short-lived effector P14 T cells (Figures 6D-6E). There was a concomitant increase in the fraction of memory precursor phenotype P14 cells (Figures 6D, 6F), which increased the ratio of memory precursor to short-lived effector P14 cells (Figure 6G).
Figure 6. Cyclin T1 and Cdk9 depletion impairs generation of effector CD8+ T cells during LCMV infection.
(A) Western blot analysis of Cyclin T1 in FACS-sorted shCcnt1+ P14 CD8+ T cells . Cells were cultured 6 days in low IL-2 (10 U/ml).
(B) Expansion of FACS-sorted shCcnt1+ P14 CD8 T in culture. Low IL-2 (10 U/ml), high IL-2 (100 U/ml).
(C-L) Adoptively transferred P14 CD8+ T cells transduced with the indicated shRNAs were analyzed on day 7 (C-G) or day 8 (H-L) after LCMV infection.
(C) The numbers and percentages of shCcnt1+ P14 cells in the spleen.
(D) Contour plots show KLRG-1 and IL-7Rα staining on shCcnt1+ P14 CD8+ T cells from representative mice at 8 days after LCMV infection.
(E-G) Quantitation of CD8+ T cell subsets from shCcnt1+ P14 cells in vivo. (E) Short-lived effector cells (KLRG-1hi IL-7Rαlo). (F) Memory precursor cells (KLRG-1lo IL-7Rαhi). (G) Ratio of memory precursor to short-lived effector phenotype P14 cells, per mouse.
(H) Contour plots show KLRG-1 and IL-7Rα staining by shCdk9+ P14 CD8+ T cells from representative mice at 8 days after LCMV infection.
(I-L) Quantitation of CD8+ T cell subsets from shCdk9+ P14 cells in vivo. (I) Short-lived effector cells (KLRG-1hi IL-7Rαlo). (J) Memory precursor cells (KLRG-1lo IL-7Rαhi). (K) Ratio of memory precursor to short-lived effector phenotype P14 cells, per mouse. (L) Summarized T-bet expression based on intracellular staining and flow cytometry.
Each symbol represents T cells from separate mice. Data are pooled from 2 independent experiments. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Error bars indicate standard deviations.
To examine whether the effects of Ccnt1 shRNAs in CD8+ T cells were related to Cyclin T1 function as a subunit of P-TEFb, we examined the effects of Cdk9-specific shRNAs (Figure 6H). Similar to shCcnt1, shCdk9+ P14 cells exhibited reduced short-lived effector cell and increased memory precursor cell formation in vivo (Figures 6I-6K). This correlated with decreased T-bet expression in vivo (Figure 6L). Notably, T-bet expression in shCcnt1+ or shCdk9+ P14 cells was impaired in KLRG-1lo IL-7Rαlo cells, a stage that presumably precedes development of either short-lived effector or memory precursor CD8+ T cells (data not shown). These data show that wild type amounts of Cyclin T1 and Cdk9 are necessary for efficient development of short-lived effector CTL, and that they normally limit generation of memory precursor CD8+ T cells .
The defect in shCcnt1+ and shCdk9+ short-lived effector CD8+ T cell formation brought into question whether these cells were effective at viral control. Thus, we examined LCMV burden on day 8 of infection. LCMV titers in the spleen of host mice with shCcnt1+ or shCdk9+ P14 cells were at least 2–5 fold higher than controls (Figure 7A). Correlating with this finding, shCcnt1+ or shCdk9+ P14 cells from infected mice expressed less granzyme B (Figure 7B). Finally, we examined shCcnt1+ or shCdk9+ P14 cells under culture conditions that strongly induce CTL differentiation (Pipkin et al, 2010). Under these conditions, all shRNAs targeting either Ccnt1 or Cdk9 also specifically inhibited perforin protein expression (Figure 7C). Thus, normal amounts of Cyclin T1 and Cdk9 are required for the upregulation of genes encoding cytotoxic effector functions, and for effective CTL-mediated protection from viral infection.
Figure 7. Cyclin T1 and Cdk9 are required for antiviral CTL functions.
(A) LCMV titers the in spleen were determined 8 days after LCMV infection.
(B) Granzyme B expression in P14 cells at day 8 post-infection. Histogram (left) and quantitation (geometric MFI; right).
(C) Western blot analysis of Cyclin T1, Cdk9, Perforin and p-actin expression in whole cell lysates from flow cytometry-sorted shCcnt1+ and shCdk9+ P14 CD8+ T cells after 6 days in culture (100 U/ml).
DISCUSSION
We have demonstrated the applicability of a pooled approach using shRNAmirs in T cells to screen for factors that regulate CD4+ and CD8+ T cell differentiation in response to viral infection. Using an improved shRNAmir vector that enhances suitability of shRNA-mediated RNAi in studies that depend upon cell transfers, more than 100 unique shRNAs were screened simultaneously in vivo. In separate proof of principle screens during LCMV infection using virus-specific TCR transgenic SMARTA CD4+ T cells or P14 CD8+ T cells, we identified multiple candidate genes with potential roles in the development of Th1 and Tfh CD4+ T cells, as well as short-lived effector and memory precursor CD8+ T cells. Detailed follow-up analyses of one factor identified in both screens revealed specific roles for Cyclin T1 and Cdk9, components of P-TEFb, for promoting Th1 cell development of CD4+ T cells and protective effector CTL development of CD8+ T cells during infection, while limiting differentiation of Tfh cells and memory precursor CD8+ T cells. These data suggest that regulation of transcription elongation by P-TEFb might be an important mechanism underpinning differentiation of T cells during immune responses.
Several important aspects distinguish the screen presented here from other recently reported pooled in vivo shRNA-screens (Beronja et al., 2013; Zhou et al., 2014; Zuber et al., 2011). The screening approach here used an array strategy to ensure independent transductions and equal representation of shRNAmirs in the pools. In addition, in contrast to other screens based on shRNA-dependent cell accumulation or depletion as the main readout to identify primary hits (Beronja et al., 2013; Zhou et al., 2014; Zuber et al., 2011), the approach presented here was a phenotypic screen of cell differentiation during an infection. As such, it was complicated by the nature of T cell responses during infection, which are constrained by factors such as the frequencies of antigen-specific T cells (Badovinac et al., 2007; Obar et al., 2008), and thus, is distinct from a T cell adoptive immunotherapy setting, which affords transferring much larger T cell numbers (Zhou et al., 2014).
The ability to conduct large-scale pooled screens using shRNAs has advanced, although interpreting the results of shRNA-based assays remains complicated. As our data on the Prdm1, Tbx21 and Ccnt1 genes emphasize, interrogating the effects of multiple shRNAs targeting the same gene in several assays tends to clarify the role of each gene, as each shRNA can result in non-identical phenotypes attributable to differential effects on particular target RNA isoforms, unintended off-target effects, or partial attenuation of target-specific gene expression. In our experience, ~ 60% of shRNA sequences derived from the GIPZ library (the source of most shRNAs for this study), impaired target gene expression or caused a measurable biological phenotype (data not shown). However, newer algorithms trained on functional data have improved predicting shRNAmirs that trigger RNAi more potently and specifically than previous designs (Fellmann et al., 2011). Our study employed some of theses designs and they are likely to enhance the fidelity of future large-scale screens in vivo.
Using a conservative approach, we showed that more than 100 unique shRNAmirs represented by 500,000 adoptively transferred P14 cells could be assayed in fewer than 10 mice in approximately 1 week. Under these conditions, we estimated that each shRNA was represented ~500 times per mouse after T cell engraftment, which is ~10-fold higher representation of each shRNA than a recent pooled screen that examined skin cell development in vivo (Beronja et al., 2013; Zuber et al., 2011), and involved transferring 10-fold fewer T cells than a recent immunotherapy screen in T cells (Zhou et al., 2014). Taking these studies into account with our results, we anticipate that by using our system and applying deeper sequencing it is likely to be feasible to perform phenotypic screens on pools of 1000 or more shRNAs in parallel in T cells.
The screen discovered unanticipated roles for Cyclin T1 and Cdk9 in the regulation of T cell differentiation during antiviral immune responses, and emphasizes the specificity that ubiquitously expressed factors can have. Cyclin T1 and Cdk9 are two widely expressed components of P-TEFb (Oven et al., 2007; Peterlin and Price, 2006), which stimulates the transition of paused RNA Polymerase II complexes into productive elongation (Peterlin and Price, 2006). The regulation of transcription elongation may govern a substantial fraction of differential expression of transcriptionally active genes (Min et al., 2011; Peterlin and Price, 2006; Rahl et al., 2010), and it is notable that this process is also critical in the regulation of HIV-transcription in CD4+ T cells . The fact that depletion of Cyclin T1 or Cdk9 in activated T cells results in specific alterations in their differentiation in vitro and in vivo indicates these factors are utilized in context-specific regulation of gene expression in T cells, despite their ubiquitous expression. Indeed, ChIP-seq analysis showed that Cyclin T1 is specifically recruited to subsets of genes, including Tbx21, Prf1, Ifng, Il2ra, that are activated in response to TCR-like stimulation of CD8+ T cells (Pipkin ME, et al. manuscript submitted).
Given the known functions of Cyclin T1 and Cdk9 in P-TEFb, one interpretation of our results is that T cell differentiation is regulated via transcriptional elongation by P-TEFb. However, the phenotypes upon Cyclin T1 and Cdk9 depletion were not identical, although they were similar. One simple explanation of this outcome is that shRNA-mediated depletion of Cdk9 was less efficient than for Cyclin T1, resulting in different amounts of functional P-TEFb in each case.
Another interpretation is alternative factors that “compensate” for reductions in wild type Cyclin T1 or Cdk9 amounts, and which possess distinct activities or targeting, caused the observed phenotypes. Indeed, other Cyclins and Cyclin dependent kinases can phosphorylate the C-terminal domain of RNA Pol II at Serine 2 and regulate transcription, and could be cell type-specific (Blazek et al., 2011). Finally, Cyclin T1 and Cdk9 could have roles in T cells apart from their established roles in P-TEFb. Future studies to elucidate the specific roles of Cyclin T1 and Cdk9 and how they integrate with the external signals that govern CD4 and CD8+ T cell differentiation are likely to open previously unappreciated insights into T cell function. In summary, the functional genetic approach described here is likely to facilitate the identification of many previously unknown players.
EXPERIMENTAL PROCEDURES
Animals and viruses
C57BL/6 (B6) were purchased from the Jackson Laboratory. CD45.1+ SMARTA (SM; lymphocytic choriomeningitis virus [LCMV] gp66-77-IAb specific)(Oxenius et al., 1998) and Blimp1-YFP mice were bred in-house. LCMV gp33-41 specific P14 Thy1.1+ mice used for in vivo analysis were a gift from Dr. Rafi Ahmed (Emory University); P14 Tcra−/− mice were used for in vitro studies (Taconic). All mice were maintained in specific pathogen-free facilities and used according to protocols approved by the animal care and use committees of the LIAI and TSRI-FL. Virus stocks were made as described (Johnston et al., 2009), and LCMV titers in tissues were assessed by plaque assay.
Pooled Screening Approaches
Please refer to Supplemental Material for detailed Experimental Procedures and Protocols.
Flow cytometry
Single cell suspensions of spleens were prepared by mechanical disruption. Surface staining for flow cytometry was performed using standard techniques (Johnston et al., 2009) and the following clones: CD4 (RM4-5), CD45.1 (A20), CD44 (IM7) and CD62L (MEL-14) (eBiosciences); CD8 (53-6.7) and B220 (RA3-6B2) (BD Biosciences); as well as CD8 (53-6.7), CD127 (A7R34), KLRG-1 (2F1), CD90.1 (OX-7), SLAM (TC15-12F12.2) (BioLegend). CXCR5 staining was performed as described (Choi et al., 2013). Intracellular staining after surface stains was performed using the “Foxp3 staining buffer” set (eBiosciences), using anti-Bcl6 monoclonal antibody (K112-91, BD Biosciences), anti-Tbet (4B10) or anti-Granzyme B (GB11) (Biolegend).
Adoptive transfer analysis of individual shRNAmirs in CD8 or CD4+ T cells
For in vivo confirmation of “hits” SMARTA CD4 or P14 CD8+ T cells were transduced with viral supernatants generated from individual shRNAmir-RV constructs (Supplementary Experimental Procedures). 5×105 P14 cells were transferred into 6-week-old B6 mice 1 or 2 days after activation and analyzed on day 7 or 8 after infection. Note, transfer of P14 cells on day 1 rather than day 2 after activation was found to recapitulate differentiation more physiologically (data not shown). For CD4+ T cells experiments 25,000 SMARTA cells were transferred.
RNA and protein analysis
Total RNA was isolated from transduced (Ametrine+) CD4+ or CD8+ T cells and used for cDNA synthesis as previously described (Johnston et al., 2009). qPCR reactions were performed in triplicate using the SYBR Select Master Mix (Life Technologies) on a Roche Lightcycler 480, using primers specific to Prdm1 (F-5’-TTCTCTTGGAAAAACGTGTGGG-3’; R-5’-GGAGCCGGAGCTAGACTTG-3’) and Tbx21 (F-5’ -ACCAACAACAAGGGGGCTTC-3’; R-5’ -CTCTGGCTCTCCATCATTCACC −3’). For western blot analysis, whole cell lysates were obtained from CD8+ T cells on day 6 after activation, CD4+ T cells 5 days after activation, or from MCC-T cells by sorting transduced (Ametrine+) cells and lysis in 150 mM NaCl, 25 mM Tris pH 7.5, 1 % Triton X-100, 0.1% SDS, 0.5% Deoxycholate, and complete protease inhibitors (Roche). 25 µg of protein was resolved by 8% SDS PAGE, transferred to nitrocellulose membranes and probed with anti-Cyclin T1 (sc-10750), anti-Blimp1 (sc-47732), anti-Cdk9 (sc-484) (Santa Cruz Biotechnology), anti-Perforin (ab16074) and anti-beta Actin (Abcam ab8227).
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NIH grant RC4 AI092763 for MEP, RC, SS, BP, and AR; NIH R01 AI095634 to MEP; NIH R01 CA42471 to AR; NIH R01 072543 for SC; and NIH U19 AI109976 for SC and MEP; and Frenchmen's Creek Women for Cancer Research to RC. We thank Gustavo Martinez for advice and assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
RC established the pooled screen methods, performed the CD8+ T cell screen and assisted writing the paper. SB designed, performed and analyzed the CD4+ T cell screen and experiments, and assisted writing the paper. MAF performed CD8+ T cell follow-up analysis. BL designed the sequencing bioinformatic pipeline. RJ designed vectors and did preliminary experiments. SS, NX and YL provided reagents and advice. AR assisted with experimental design, provided resources, and assisted writing the paper. BP designed the sequencing analysis pipeline and supervised statistical analyses. MEP and SC conceived of the studies, designed experiments, analyzed data, supervised the projects and wrote the paper.
We have no conflict of interest.
REFERENCES
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beronja S, Janki P, Heller E, Lien WH, Keyes BE, Oshimori N, Fuchs E. RNAi screens in mice identify physiological regulators of oncogenic growth. Nature. 2013;501:185–190. doi: 10.1038/nature12464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JA, Blair DA, Knell J, Yang E, Mayya V, Doedens A, Dustin ML, Goldrath AW, Monach P, Shinton SA, et al. Transcriptional insights into the CD8(+) T cell response to infection and memory T cell formation. Nat Immunol. 2013;14:404–412. doi: 10.1038/ni.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazek D, Kohoutek J, Bartholomeeusen K, Johansen E, Hulinkova P, Luo Z, Cimermancic P, Ule J, Peterlin BM. The Cyclin K/Cdk12 complex maintains genomic stability via regulation of expression of DNA damage response genes. Genes Dev. 2011;25:2158–2172. doi: 10.1101/gad.16962311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannarile MA, Lind NA, Rivera R, Sheridan AD, Camfield KA, Wu BB, Cheung KP, Ding Z, Goldrath AW. Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nat Immunol. 2006;7:1317–1325. doi: 10.1038/ni1403. [DOI] [PubMed] [Google Scholar]
- Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- Choi YS, Yang JA, Yusuf I, Johnston RJ, Greenbaum J, Peters B, Crotty S. Bcl6 Expressing Follicular Helper CD4 T Cells Are Fate Committed Early and Have the Capacity To Form Memory. J Immunol. 2013 doi: 10.4049/jimmunol.1202963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciofani M, Madar A, Galan C, Sellars M, Mace K, Pauli F, Agarwal A, Huang W, Parkurst CN, Muratet M, et al. A validated regulatory network for Th17 cell specification. Cell. 2012;151:289–303. doi: 10.1016/j.cell.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S. Follicular helper CD4 T cells (TFH) Annu Rev Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- Crotty S. The 1-1-1 fallacy. Immunol Rev. 2012;247:133–142. doi: 10.1111/j.1600-065X.2012.01117.x. [DOI] [PubMed] [Google Scholar]
- Doering TA, Crawford A, Angelosanto JM, Paley MA, Ziegler CG, Wherry EJ. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity. 2012;37:1130–1144. doi: 10.1016/j.immuni.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellmann C, Zuber J, McJunkin K, Chang K, Malone CD, Dickins RA, Xu Q, Hengartner MO, Elledge SJ, Hannon GJ, Lowe SW. Functional identification of optimized RNAi triggers using a massively parallel sensor assay. Mol Cell. 2011;41:733–746. doi: 10.1016/j.molcel.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita N, Jaye DL, Geigerman C, Akyildiz A, Mooney MR, Boss JM, Wade PA. MTA3 and the Mi-2/NuRD complex regulate cell fate during B lymphocyte differentiation. Cell. 2004;119:75–86. doi: 10.1016/j.cell.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Gray PA, Fu H, Luo P, Zhao Q, Yu J, Ferrari A, Tenzen T, Yuk DI, Tsung EF, Cai Z, et al. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science. 2004;306:2255–2257. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- Haining WN, Wherry EJ. Integrating genomic signatures for immunologic discovery. Immunity. 2010;32:152–161. doi: 10.1016/j.immuni.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Hale JS, Youngblood B, Latner DR, Mohammed AU, Ye L, Akondy RS, Wu T, Iyer SS, Ahmed R. Distinct memory CD4+ T cells with commitment to T follicular helper- and T helper 1-cell lineages are generated after acute viral infection. Immunity. 2013;38:805–817. doi: 10.1016/j.immuni.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- Ji Y, Pos Z, Rao M, Klebanoff CA, Yu Z, Sukumar M, Reger RN, Palmer DC, Borman ZA, Muranski P, et al. Repression of the DNA-binding inhibitor Id3 by Blimp-1 limits the formation of memory CD8+ T cells. Nat Immunol. 2011;12:1230–1237. doi: 10.1038/ni.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B, Dent AL, Craft J, Crotty S. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Kao C, Oestreich KJ, Paley MA, Crawford A, Angelosanto JM, Ali MA, Intlekofer AM, Boss JM, Reiner SL, Weinmann AS, Wherry EJ. Transcription factor T-bet represses expression of the inhibitory receptor PD-1 and sustains virus-specific CD8+ T cell responses during chronic infection. Nat Immunol. 2011;12:663–671. doi: 10.1038/ni.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min IM, Waterfall JJ, Core LJ, Munroe RJ, Schimenti J, Lis JT. Regulating RNA polymerase pausing and transcription elongation in embryonic stem cells. Genes Dev. 2011;25:742–754. doi: 10.1101/gad.2005511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327:1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreich KJ, Weinmann AS. Master regulators or lineage-specifying? Changing views on CD4+ T cell transcription factors. Nat Rev Immunol. 2012;12:799–804. doi: 10.1038/nri3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oven I, Brdickova N, Kohoutek J, Vaupotic T, Narat M, Peterlin BM. AIRE recruits P-TEFb for transcriptional elongation of target genes in medullary thymic epithelial cells. Mol Cell Biol. 2007;27:8815–8823. doi: 10.1128/MCB.01085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur J Immunol. 1998;28:390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Peterlin BM, Price DH. Controlling the elongation phase of transcription with P-TEFb. Mol Cell. 2006;23:297–305. doi: 10.1016/j.molcel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Pipkin ME, Rao A. SnapShot: effector and memory T cell differentiation. Cell. 2009;138:606. doi: 10.1016/j.cell.2009.07.020. e601–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poholek AC, Hansen K, Hernandez SG, Eto D, Chandele A, Weinstein JS, Dong X, Odegard JM, Kaech SM, Dent AL, et al. In vivo regulation of Bcl6 and T follicular helper cell development. J Immunol. 2010;185:313–326. doi: 10.4049/jimmunol.0904023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahl PB, Lin CY, Seila AC, Flynn RA, McCuine S, Burge CB, Sharp PA, Young RA. c-Myc regulates transcriptional pause release. Cell. 2010;141:432–445. doi: 10.1016/j.cell.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravasi T, Suzuki H, Cannistraci CV, Katayama S, Bajic VB, Tan K, Akalin A, Schmeier S, Kanamori-Katayama M, Bertin N, et al. An atlas of combinatorial transcriptional regulation in mouse and man. Cell. 2010;140:744–752. doi: 10.1016/j.cell.2010.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Blackburn SD, Intlekofer AM, Kao C, Angelosanto JM, Reiner SL, Wherry EJ. A role for the transcriptional repressor Blimp-1 in CD8(+) T cell exhaustion during chronic viral infection. Immunity. 2009;31:309–320. doi: 10.1016/j.immuni.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahedi G, Kanno Y, Sartorelli V, O’Shea JJ. Transcription factors and CD4 T cells seeking identity: masters, minions, setters and spikers. Immunology. 2013;139:294–298. doi: 10.1111/imm.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh JC, DeKoter RP, Lee HJ, Smith ED, Lancki DW, Gurish MF, Friend DS, Stevens RL, Anastasi J, Singh H. Cooperative and antagonistic interplay between PU.1 and GATA-2 in the specification of myeloid cell fates. Immunity. 2002;17:665–676. doi: 10.1016/s1074-7613(02)00452-1. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao N, Eto D, Elly C, Peng G, Crotty S, Liu YC. The E3 ubiquitin ligase Itch is required for the differentiation of follicular helper T cells. Nat Immunol. 2014;15:657–666. doi: 10.1038/ni.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CY, Best JA, Knell J, Yang E, Sheridan AD, Jesionek AK, Li HS, Rivera RR, Lind KC, D’Cruz LM, et al. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nat Immunol. 2011;12:1221–1229. doi: 10.1038/ni.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf I, Kageyama R, Monticelli L, Johnston RJ, Ditoro D, Hansen K, Barnett B, Crotty S. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150) J Immunol. 2010;185:190–202. doi: 10.4049/jimmunol.0903505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Shaffer DR, Alvarez Arias DA, Nakazaki Y, Pos W, Torres AJ, Cremasco V, Dougan SK, Cowley GS, Elpek K, et al. In vivo discovery of immunotherapy targets in the tumour microenvironment. Nature. 2014;506:52–57. doi: 10.1038/nature12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, Magoon D, Qi J, Blatt K, Wunderlich M, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478:524–528. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.