Summary
Induction of HIV-1 broad neutralizing antibodies (bnAbs) is a goal of HIV-1 vaccine development but has remained challenging partially due to unusual traits of bnAbs, including high somatic hypermutation (SHM) frequencies and in-frame insertions and deletions (indels). Here we examined the propensity and functional requirement for indels within HIV-1 bnAbs. High-throughput sequencing of the immunoglobulin (Ig) VHDJH genes in HIV-1 infected and uninfected individuals revealed that the indel frequency was elevated among HIV-1-infected subjects, with no unique properties attributable to bnAb-producing individuals. This increased indel occurrence depended only on the frequency of SHM point-mutations. Indel-encoded regions were generally proximal to antigen binding sites. Additionally, reconstruction of a HIV-1 CD4-binding site bnAb clonal lineage revealed that a large compound VHDJH indel was required for bnAb activity. Thus, vaccine development should focus on designing regimens targeted at sustained activation of bnAb lineages to achieve the required SHM and indel events.
Introduction
With the use of antigen-specific memory B cell isolation and antibody rescue techniques, a large number of HIV-1 broad neutralizing antibodies (bnAbs) have been isolated(Haynes et al., 2011). All bnAbs share one or more unusual traits such as autoreactivity, long heavy-chain third complementarity-determining regions (HCDR3s), and high levels of somatic hypermutation(Walker et al., 2011, Walker et al., 2009, Haynes et al., 2005, Haynes et al., 2012, Kwong and Mascola, 2012, Mouquet and Nussenzweig, 2012, Wu et al., 2010, Wu et al., 2011, Zhou et al., 2010, Mascola and Haynes, 2013, Liao et al., 2013) all traits that can limit the induction of such antibodies(Haynes et al., 2005, Haynes et al., 2012, Mascola and Haynes, 2013, Verkoczy et al., 2011).
Some recently isolated HIV-1 bnAbs have been noted to possess another unusual characteristic: multibase in-frame insertions or deletions (indels)(Wu et al., 2010, Walker et al., 2011). Indels are introduced during somatic hypermutation, and are thus found exclusively in germinal center or post-germinal center B cells(Fukita et al., 1998). The proportion of indels among somatic mutations in the normal human B cell repertoire is small. Wilson et al examined IgG memory cells from human tonsil and reported 6 indels in 110,000 bases sequenced from productive genes, comprising both 3- and 6- base insertions and deletions(Wilson et al., 1998). Using high-throughput sequencing, Briney et al(Briney et al., 2012) found slightly higher frequencies of in-frame insertions (1.8% of all sequences) and in-frame deletions (2.0-2.6% of all sequences) in FACS-sorted memory B cells. Smith et al(Smith et al., 1996) studied unselected mutations in the introns between the JH and constant region genes and found that 1-2% of all unselected somatic mutations were single-base insertions or deletions. When indels do occur in normal B cells, they are usually short; their frequency decreases rapidly with length (Wilson et al., 1998). Here we have surveyed bnAb sequences, and found that 40% of reported HIV-1 bnAbs have indels, and that the indel sizes cover the remarkable range from 3 to 33 nucleotides.
Because induction of bnAbs may be central to HIV-1 vaccine development efforts, it is of great interest to determine why indels occur at such great frequency in bnAbs. There are several hypotheses regarding the cause of this striking bias. Perhaps patients who produce bnAbs have an underlying propensity to incorporate indels during affinity maturation. Another possibility is that chronic HIV-1 infection induces the generation of indels, and that indels will be found at greater frequencies in all HIV-1 infected patients. It is also possible that there are genetic polymorphisms that affect the somatic hypermutation mechanism and predispose carriers to produce high levels of somatic hypermutations and indels during the induction of bnAbs. If genetic polymorphins are the cause for elevated SHMs, it would have serious consequences for the development of vaccines to elicit bnAbs, in that only those with such a genetic background will be able to make vaccine-induced bnAbs. In addition it is important to know if indels can be required for bnAb activity. To test these ideas, we examined heavy chain sequences from HIV-1-infected individuals who make bnAbs, as well as from HIV-1 infected individuals who do not. To determine the functional consequences of insertion/deletion events, we made a detailed reconstruction of a VRC01-like CD4 binding site bnAb (CH31) clonal lineage and demonstrated that a large compound indel was essential for both bnAb affinity maturation and HIV-1 neutralization.
Indels are found at high frequency among HIV-1 bnAbs
Many of the recently isolated bnAbs have been observed to have insertions or deletions. To determine whether the occurrence of indels in bnAbs is disproportionally high, we examined the reported 56 HIV-1 bnAb gene-pairs (Supplementary Table 1), that constituted 26 sets of clonally-related heavy and light-chain genes. Among these genes we identified 27 unique in-frame indels. Half of these indels were 3 nt long, but insertions as large as 33 nt and deletions up to 15nt were also observed (Table 1). Overall, 40 of the 108 bnAb genes contained indels. Counting unique indels only, the frequency of indels in this group of genes was 27/108 (25%). This rate is nearly seven times higher than that observed in a collection of ~13,000 human heavy chain variable regions downloaded from NCBI Genbank. Thus, the frequency of indels among bnAbs is elevated.
Table 1.
Insertions and Deletions in broadly neutralizing anti-HIV-1 antibodies.
| Antibody | Indel Type | Length (aa) | Location |
|---|---|---|---|
| CAP206-CH12 | Deletion | 3 | HFR3 |
| NIH45-46 | Deletion | 6 | LCDR1 |
| HJ16 | Deletion | 3 | HCDR2 |
| HJ16 | Insertion | 3 | LFR3 |
| VRC01 | Deletion | 6 | LCDR1 |
| VRC03 | Insertion | 21 | HFR3 |
| CH98 | Deletion | 15 | LFR1 |
| CL103 | Deletion | 9 | LFR1 |
| PGT121-3 | Insertion | 9 | LFR3 |
| PGT125-8 | Insertion | 18 | HCDR2 |
| PGT125-8 | Deletion | 15 | LCDR1 |
| PGT136 | Insertion | 3 | HCDR2 |
| PGT135-7 | Insertion | 15 | HCDR1 |
| PGT135-7 | Insertion | 3 | LFR1 |
| PGT145 | Deletion | 3 | HCDR3 |
| PG9 | Insertion | 3 | HFR1 |
| CH30-34 | Insertion | 27 | HCDR1 |
| 3BNC117, 3BNC60 | Insertion | 12 | HFR3 |
| 3BNC117, 3BNC60 | Deletion | 12 | LCDR1 |
| 8ANC131, 8ANC134 | Deletion | 3 | HCDR2 |
| 8ANC131, 8ANC135 | Deletion | 3 | LCDR1 |
| 8ANC195 | Deletion | 3 | HCDR1 |
| 8ANC195 | Insertion | 15 | HCDR2 |
| 8ANC195 | Insertion | 3 | LCDR1 |
| VRCPG04,04B | Deletion | 9 | LCRD1 |
| VRCPG04,04B | Insertion | 3 | HCDR1 |
| VRCPG04,04B | Insertion | 3 | HFR3 |
To determine whether the high frequency of indels in bnAbs was attributable to an increase in the frequency of indel-bearing antibodies generally in all chronically infected HIV-1 infected individuals, versus selective increase of indels in bnAb-producing individuals, we determined the frequency of indels in unbiased samples of Ig variable-region genes from blood B cells using Roche 454 high-throughput DNA sequencing (HTS) of full-length genomic VHDJH rearrangements. We generated and analyzed HTS data comprising 3,778,439 sequencing reads from 261 samples taken at different times from 75 human subjects. These individuals included 41 HIV-infected individuals (8 bnAb producers and 33 non-bnAb producers), 18 influenza-vaccinated individuals, and 16 HIV-1, uninfected individuals (Supplementary Tables 2-3).
After discarding sequences rearranged out of frame or containing frame-shifting indels, we analyzed a total of 3,352,720 sequences using statistical methods and software developed for this purpose(Kepler, 2013). All alignments between sequencing reads and gene segments used an affine gap scoring function(Gotoh, 1990). Each sequence was scored either as containing or not containing an in-frame insertion and/or deletion.
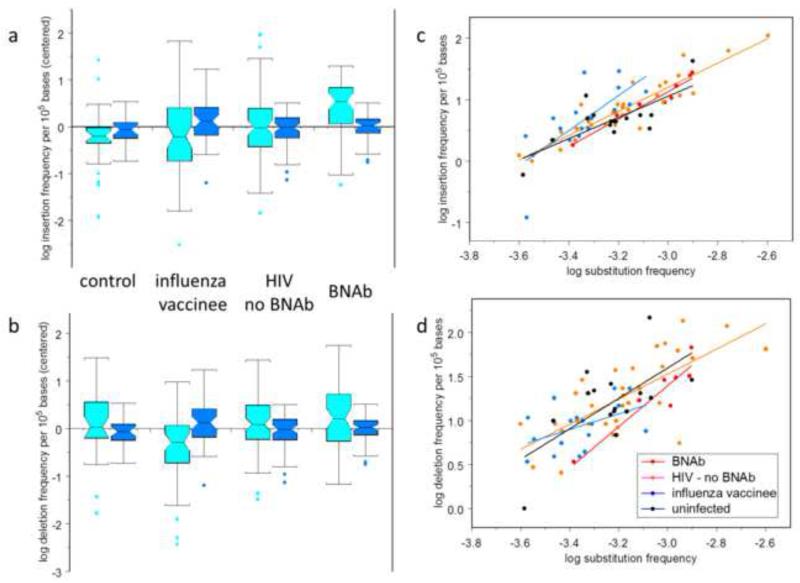
We found that VHDJH genes from HIV-1-infected bnAb and non-bnAb subjects have higher levels of indels than do those from healthy influenza-vaccinated and non-HIV-1-infected groups (Fig. 1a,b). Compared to non-HIV-1 infected subjects, the geometric mean insertion frequency was 27% higher in HIV-1 infected individuals (p = 0.03; ANOVA); the geometric mean deletion frequency was similarly 23% higher in HIV-1 infected individuals (p = 0.03; ANOVA). Importantly, with HIV-1 infected individuals, indel frequencies did not significantly differ between bnAb-producing and - non-producing subjects (p > 0.1 in both cases; Tukey multiple-comparisons test).
Figure 1. Comparison of VH mutation frequencies in HIV-1 bnAbs with those in other antibodies.
Box plots showing centered log frequencies (cyan) for in-frame insertions (a) and deletions (b) per 105 VH bases in potentially functional heavy chain genes; and insertion and deletion log frequency residuals (blue) after subtracting log frequency predicted from substitution frequency alone. Subject classifications: Subjects known to produce broadly neutralizing antibodies against HIV-1 (bnAb), subjects who had been vaccinated against influenza one week prior to sampling (influenza vaccinee), HIV-1 infected subjects who had not produced bnAbs by the time of this study (HIV no bnAb), and uninfected controls (control). Boxes cover from the first to the third quartiles; whiskers extend to the standard span—1.5 times the interquartile range. Cyan boxes were centered by subtracting the mean frequency over all subjects; blue boxes were not centered. c: Log of the insertion frequency per 105 bases (c) and deletion frequency (d) as a function of the log of the substitution frequency per base. The colored line segments show the linear regression for each of the subject classifications; the gray line segment shows the linear regression for all subjects at once.
Mean indel frequency is predicted by mean mutation frequency
The frequency of point mutations is similarly elevated in HIV-1 infected individuals (ANOVA p < 0.01 for differences attributable to subject classification; Tukey multiple comparisons, p < 0.05 for differences specific to HIV-1 status). This result raises the possibility that both point-mutation and indel frequencies are increased together by the same underlying cause. We hypothesized that since indels are induced by the same mechanism as point-substitutions, a higher overall mutation level could account for the higher indel frequency. To evaluate this possibility, we performed linear regressions of log average insertion or deletion frequency against the log of the substitution frequency. The regressions were performed with separate regression coefficients for each subject classification group as well as with a common regression coefficient for all subjects. The regression coefficients were not statistically different among subjects for either insertions or deletions (p > 0.1, ANOVA; Figs. 1c, d); the estimate (± standard error) for the common regression coefficient was 1.95±0.13 for insertions and 1.44±0.22 for deletions. Figure 1a and b also show the residuals remaining after subtracting the insertion and deletion frequencies predicted using the common-coefficient regression models, illustrating that most of the variability among classifications was attributable to differences in point-substitution frequency.
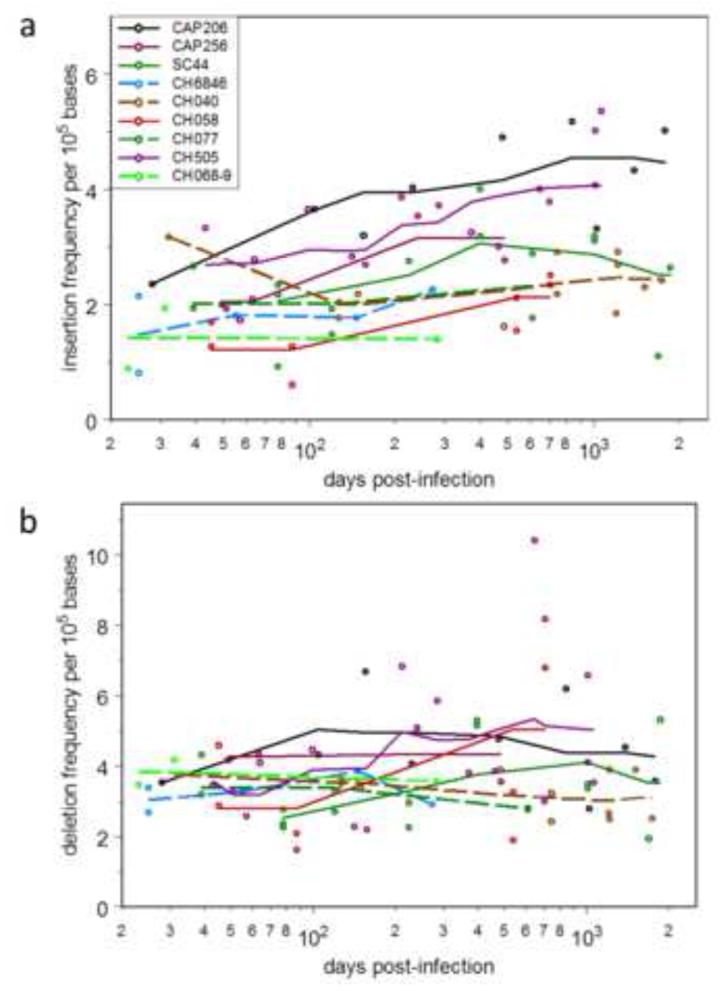
We were able to study nine HIV-1 infected individuals (five among the bnAb-producers and four among the non-bnAb producers) with VHDJH sequencing data from three or more sampling times. The frequency of indel-containing sequences was variable over time (Fig. 2). Simple linear regression of insertion or deletion frequencies against days post-infection found regression coefficients for insertion significantly greater than zero in 2/9 cases (p < 0.05) while8/9 regression coefficient estimates were positive, significantly more than expected if there were no temporal trend (p = 0.004, two-sided sign test). No such trend was evident for deletions—no correlation coefficients differed statistically from zero, and 4/9 were positive. Although indel frequency is volatile, there is a weak but detectable tendency for insertions, but not deletions, to increase over time with HIV infection.
Figure 2.
Time course of in-frame insertion (a) and deletion (b) frequencies in days post-infection for HIV-infected subjects. The five subjects known to produce bnAbs are indicated with solid lines; the four who have not made bnAbs are indicated with dashed lines. Only subjects with samples taken at three or more times were included.
To address the question of whether chronic infections other than HIV are associated with increased rates of insertions and deletions, we examined HTS heavy-chain Ig repertoire data from subjects chronically infected with cytomegalovirus (CMV), Epstein-Barr virus (EBV), both viruses, or neither(Wang et al., 2014). There were no detectable differences among these groups in the rates of in-frame insertions or deletions (p > 0.1, ANOVA; Fig. S2).
Indel placement and antigen recognition
To determine whether indels in bnAbs were preferentially found in antigen-interacting regions or were more randomly distributed, we analyzed a set of 11 antigen-bound bnAb structures containing a total of 18 indels (Supplementary Methods). Fifteen of 18 (83%) indels were found within 10 Å of the respective antigen binding site (Fig. 3). In comparison, analysis of loop regions (assumed to be able to more easily accommodate indels due to fewer structural constraints compared to beta strand regions) revealed that only ~1/3 of loop residues were found within 10 Å of the respective antigen, even when removing potential skewness due to over-representation of specific antibody classes (Fig. S1). The distributions of the indel and loop residue locations were significantly different (p < 0.0001, Mann-Whitney test). These results indicated that while there was little constraint on the location where indels can be accommodated, their presence related directly to antigen recognition. One potential caveat of this analysis is that proximity to antigen was measured based on the Env subunit antigen constructs in the available crystal structures, rather than a functional HIV-1 spike, thus possibly underestimating the proximity to antigen for some indels. Overall, indels were preferentially found in close proximity to antigen binding sites, and thus likely improved, interactions with antigen.
Figure 3. Structural analysis of antibody indels.
a and b, Distribution of distances between antigen and: indels (a) or loop residues (b). These distributions are statistically distinguishable (p < 0.0001, Mann-Whitney test). Distances are shown as bins in 5 Å increments. (c-e) Location of insertions and deletions in antigen-bound structures of antibodies VRC-CH31 (c), VRC01 (d), 3BNC117 (e), and PGT128 (f). Structures are shown in ribbon representation with antibody heavy chains in green, light chains in blue, and the respective antigens in grey. For PGT128, antibody-interacting glycans on antigen are shown as grey sticks. The regions where insertions and deletions were observed are highlighted in yellow and red, respectively. See also Figure S1.
The indel in VRC01-like CD4 binding site CH30-CH34 clonal lineage was required for bnAb affinity maturation
We have recently reported the occurrence of two different sets of clonally-related bnAbs in the same HIV-1 infected individual that together accounted for all of the plasma bnAb activity(Wu et al., 2011, Bonsignori et al., 2012). This individual (CH0129) had both VRC01-like CD4 binding site and V1V2 quaternary bnAb activity(Bonsignori et al., 2012),(Bonsignori et al., 2011). The VRC01-like CD4 binding-site clone CH30-CH34 experienced indel formation, while the V1V2 PG9-like bnAb clonal lineage CH01-04 did not. We reconstructed the corresponding CH31 clonal lineage using statistical inference on the originally isolated genes, supplemented with targeted HTS reads to determine the affinity maturation history and the role of indel in affinity maturation and acquisition of neutralization breadth in the observed CH31 lineage CD4bs antibodies (Fig. 4, Table 2).
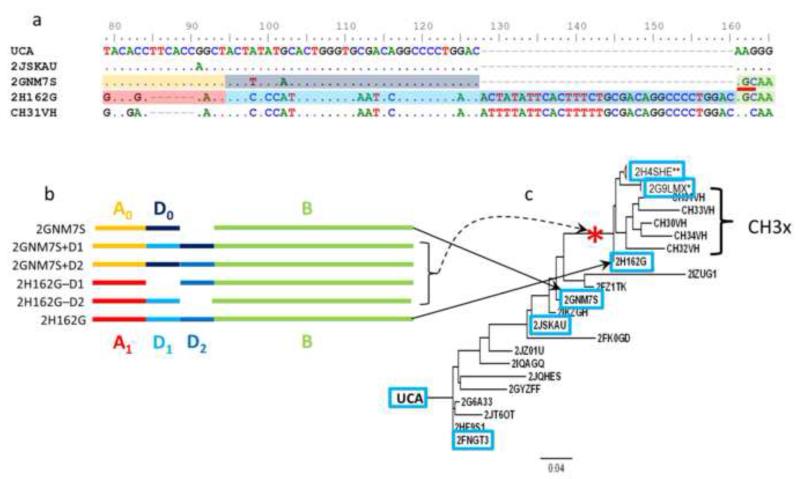
Figure 4. Maturation pathway of CD4BS bnAb.
CH31 clonal lineage. a, Alignment of the observed CD4BS bnAb CH31, and observed CH31 clonally related VHDJH sequences from HTS shows the likely duplication and deletion events leading to the mature antibody. An AID hot spot “AGC” at alignment position 162 (red line segment) appears as a result of nucleotide substitution in 2GNM7S. Dots indicate identity with the nucleotides in the UCA; dashes represent gaps. Shading of the sequences corresponds to the schematic deconstruction in part b. b, A schematic showing the structures of the observed heavy chains (2GNM7S, 2H162G) in terms of the sequence elements A, A’; D0, D1, D2, and B, and the artificial constructs made by inserting and deleting the duplicons. c, A maximum-likelihood tree incorporating all antibody members (CH30-34) of the CH31 clonal lineage VHDJH_ and the VHDJH isolated by HTS. The VHDJH genes indicated in blue boxes have been synthesized and paired with intermediate-4 light chain for production of recombinant monoclonal antibodies for testing the binding and neutralizing activity. The compound insertion-deletion event occurred on the branch indicated by the red asterisk. The asterisks on the sequence titles 2H4SHE and 2G9LMX indicate that each of these sequences has hundreds (**) or tens (*) of closely-related sequences not shown on the tree.
Table 2.
Neutralization of HIV-1 tier 1 and 2 isolates by members of CD4BS bnAb CH31 clonal lineage.
| TCID50, ug/ml |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| HIV-1 isolates |
Control | ||||||||
| Antibody | B.MN.3 | C.MW965 | B.SF162 | B.W61D | C.C3347 | C.DU172 | A.Q23 | A.TRO.11 | MuLV |
| UCA | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| 2FNGT3 | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 | >50 |
| 2JSKAU | >50 | >50 | >50 | >50 | >50 | >50 | 1.51 | >50 | >50 |
| 2GNM7S | 30.1 | >50 | >50 | >50 | >50 | >50 | 6.57 | >50 | >50 |
|
| |||||||||
| 2H162G | 0.03 | 6.78 | 1.07 | 2.62 | 10.4 | 5.61 | 0.08 | 0.94 | >50 |
| 2H4SHE | <0.023 | 2.45 | 0.402 | 0.023 | 3.86 | 1.63 | 0.085 | 0.75 | >50 |
| 2G9LMX | <0.023 | 1.43 | 0.443 | 0.326 | 6.48 | 7.7 | 0.07 | 0.95 | >50 |
| I1 | 3.86 | 10.2 | 1.34 | >50 | 0.98 | 0.91 | <0.23 | 0.46 | >50 |
| I2 | 7.19 | 15.8 | 1.68 | >50 | 0.77 | 1.03 | <0.23 | 0.54 | >50 |
| I3 | 5.55 | 9.09 | 1.12 | >50 | 0.46 | 0.71 | <0.23 | 0.47 | >50 |
| I4 | 2.26 | 5.38 | 0.77 | >50 | 0.34 | 0.61 | <0.23 | 0.33 | >50 |
| CH31 | 0.0225 | 0.993 | 0.121 | 8.34 | 0.17 | 1.15 | <023 | 0.15 | >50 |
|
| |||||||||
| 2H162G − D1 | >50 | >50 | >50 | >50 | >50 | >50 | 3.19 | >50 | >50 |
|
| |||||||||
| 2GNM7S+D.1 | 14.3 | >50 | 49.7 | 38.3 | >50 | >50 | 4.21 | >50 | >50 |
| 2GNM7S+D2 | 16.2 | >50 | 27.4 | 5.8 | >50 | >50 | 25.0 | >50 | >50 |
The members of the CH31 clonal lineage are similar to other VRC01-like bnAbs clone in their use of the VH1-2*02 gene segment(Wu et al., 2011, Bonsignori et al., 2012). Moreover, the crystal structure of CH31 complexed to gp120 is superimposable on that of VRC01(Zhou et al., 2013). Most strikingly, perhaps, is the observation that both VRC03 (a member of the VRC01 clonal lineage) and all the members of the CH31 clonal lineage contain large indels. VRC03 has a 21 nt insertion in framework region 3. The CH31 clonal lineage genes have a net 27 nt insertion within CDR1 that resolves into a 6 nt deletion and a 33 nt tandem duplication (Fig. 4a). Using HTS targeted at VHDJH genes rearranging to the VH and JH gene segments used by CH31 lineage antibodies, we were able to isolate genes situated close to the inferred unmutated common ancestor (UCA) on the clonal tree and prior to the indels. Importantly, we isolated 14 unique VHDJH genes that were clearly members of the CH31 clonal lineage, but did not have the indel, as well as isolated several hundred more mutated VHDJH genes that did have the indel (Figure 4c).
The CH31 clonal lineage history demonstrated a striking accumulation of mutations in the vicinity of the eventual indel, including the formation of an activation-induced cytidine deaminase (AID) AGC hotspot that likely contributed to the genetic instability responsible for the indel (Fig 4a). Indeed, a feature common to all of the bnAbs that have experienced indels is an accumulation of point mutations on either side of the insertion or deletion (Fig. S1).
We next expressed antibody members of the CH31 clonal lineage representative of key stages along the maturation pathway as well as four artificial heavy chain constructs (Figs. 4b) that were intended to probe the specific contributions of the indel to the affinity, kinetic parameters, and neutralization capacity of CH31 bnAb lineage antibodies generated before and after indel occurrence.
Consider the line of descent leading from the UCA to the mature antibodies CH30-CH34 (Fig. 4b). GNM7S is an observed heavy-chain sequence lying just off the line of descent. It arose just before the indel was acquired, and is the indel-free sequence farthest from the UCA. Among post-indel sequences, H162G is closest to the UCA along the line of descent. GNM7S and H162G differ by 16 nucleotide substitutions in addition to the indel. The differences between the two in terms of their biophysical properties are likely due both to the indel and the nucleotide substitutions. To isolate the influence of the indel from the influence of the simple substitutions, we designed and synthesized four heavy chains that represent hybrids of antibodies H162G and GNM7S involving inclusion or removal of the tandem duplication.
The relationships between the observed and synthetic sequences are shown schematically in Fig. 4b. Each heavy chain sequence can be decomposed into three elements: the duplicated element, or duplicon(Eichler, 1998), and the elements 5′ and 3′ respectively, of the duplicon. The 5′ element is denoted A, the duplicon D, and the 3′ element B. The structure of GNM7S is given by A0 D0 B and that of H162G is A1 D1 D2 B. The duplicons D0, D1, and D2 are homologous 33-nucleotide tracts that differ from each other by 5-13 nucleotides. A0 and A1 differ from each other by 17 nucleotide substitutions and a 6 nucleotide deletion. GNM7S and H162G share an identical B element. The following four antibody heavy chains were synthesized by adding a duplicon to GNM7S (A1 D1 D0 B and A1 D0 D2 B) or deleting a duplicon from H162G (A1 D1 B and A1 D2 B). Each artificial heavy chain was paired with the light chain of a statistically-inferred post-insertion antibody for full antibody expression.
We estimated antibody binding kinetic parameters affected by the indel by surface plasmon resonance (SPR) analysis of CH31 lineage members binding to HIV-1 AE.A244 gp120(Alam et al., 2013). Figure 5 shows that, in the natural CH31 lineage, the association rate of lineage antibodies for AE.A244 gp120 binding increased and the dissociation rate decreased with evolutionary distance (in expected number of mutations per base) from the inferred UCA. The artificial antibody constructs in which an indel duplicon was inserted but no other changes made showed that this single change brought about an 8-fold increase in the association rate (Fig. 5a). When the indel duplicon was deleted from the post-indel heavy chain, the association rate of the antibody for binding to AE.A244 gp120 decreased by an order of magnitude (2H162G-D1, Fig. 5a). The dissociation rate was slightly decreased by insertion of the duplicon from 2GNM7S to both 2GNM7S+D1 and 2GNM7S+D2. Upon its deletion, however, the dissociation rate was increased 3-fold (2H162G-D1, Fig.5b). No antibodies less diverged than 2JSKAU from the UCA showed binding of any measurable degree to AE.A244 gp120 (not shown).
Figure 5. SPR-estimated kinetic rate constants.
a, association rate, ka. b, dissociation rate, kd for the tested antibodies as a function of evolutionary distance (expected nucleotide changes per base) from the inferred unmutated common ancestor (UCA). Solid line segments indicate clonal descent. Dashed line segments indicate artificial descent by insertion or deletion of the respective duplicon. c, The estimated dissociation, Kd (the ratio of the on-rate to the off-rate, Kd = kd/ka) vs. the evolutionary distance from the UCA.
Broad Neutralization by CH31 antibody is acquired after indel formation
Finally, we assayed members of the CH31 clonal lineage for their ability to neutralize heterologous HIV-1 strains in the TZBbl neutralization assay (Table 2). We found that neutralization breadth did not arise until after the occurrence of the indel. The only tier 2 (difficult to neutralize) strain neutralized before the indel was A.Q23, with neutralization of other strains only acquired after the indel with antibodies 2H162G and 2H4HE representing the point in the CH31 lineage with neutralization breadth.
Discussion
In this study, we have demonstrated the high frequency of indels among HIV-1 broad neutralizing antibodies, showed that these indels occur predominantly near bnAb antigen contact sites, and demonstrated an example of the requirement for an indel for affinity maturation and bnAb activity in the VRC01-like CH31 bnAb lineage.
Not all HIV-1 infected individuals make bnAbs, although it has recently been shown that the ability to make BnAbs is not dichotomous. That is, serum neutralization breadth is graded among infected individuals(Hraber et al., 2014). What is not yet known is whether there are host factors such as a genetic predisposition that are responsible for the differences there are. Alternatively, there may be as-yet undetermined differences in the circumstances of the infection itself, such as total antigenic variability or viral load that drives bnAb production. On the other hand, there may be no host or pathogen factors that determine bnAb status. Instead, bnAb status may be stochastic, driven essentially by the stochastic nature of B cell affinity maturation itself. In this study we demonstrate that insertion and deletions occur at unusually high frequencies among bnAbs and that the occurrence of indels in HIV-1 bnAbs is determined by the degree of SHM that occurs in HIV-1 infection, rather than by the action of factors specific to those patients that have detectable plasma bnAbs. By examining a total of 3,352,720 genomic DNA sequencing reads from individuals with and without HIV-1 infection, we found that the rate of indels could be accounted for by a single mutation frequency driving both point-substitution and indels in all groups, with only this single mutation frequency varying among groups. Furthermore, the frequencies of both indels and substitutions were only slightly higher in HIV-1 infected patients, and there was no statistically significant difference between HIV-1 infected patients who have detectable plasma bnAbs and those who did not. Moreover, we found that the indel frequency depends on the substitution frequency raised to a power greater than one. Mathematically, the relationship between the frequency of insertions or deletions f and the frequency of substitutions μ is given by
| (0.0) |
where β > 1. The interpretation of this finding is that the excess SHM frequency of bnAbs is sufficient by itself to account for the increase in indel frequency. Thus, high indel frequency does not represent a separate class of bnAb anomaly and does not implicate unknown host factors or genetic predisposition to generate bnAbs. In this regard, we have recently performed full exon-sequencing on 50 bnAbs and 50 non-bnAbs HIV-1 infected individuals and demonstrated no genome-wide gene maturation associated with the ability to make bnAbs (Shea, P, Haynes, B, Goldstein, unpublished).
Previously, Krause et al(Krause et al., 2011) investigated the role of an insertion in a human antibody against influenza HA by deleting the insert while leaving all other somatic mutations intact and comparing binding affinities between the two forms of the antibody. They found that the removal of the insertion increased the equilibrium dissociation constant 35-fold, largely through increasing the kinetic dissociation rate. Pejchal et al(Pejchal et al., 2011) deleted the inserts from two gp-120-binding anti-HIV-1 antibodies, while leaving the rest of the molecules intact. They found that the binding affinity for the antigen, and their ability to neutralize the virus, were diminished by the removal of the insert. Our goal and our findings were different from these studies in an important way. While the previous groups of investigators sought to determine the role of the insert in the function of the extant antibodies, we sought to elucidate the role of the insertion event in the affinity maturation of the bnAb clonal lineage. Thus, we isolated lineage members from before and after the compound insertion/deletion event and produced four constructs that represent possible intermediate forms. We found that after an insertion or deletion event, point-mutations continue to accumulate in the context of the indel. These post-indel mutations may have very different effects in the presence of the indel than they would in its absence. Removing the insert from the mature antibody did not return the antibody to its pre-indel state because the post-indel point mutations were still intact. Our studies more closely approximate the history of the lineage at the time of the indel’s occurrence.
Our analyses demonstrate that insertions and deletions are prevalent at very high frequency in bnAbs, that indels can be critical for bnAb activity, and that indel frequency increases with and is predicted by the frequency of SHM-mediated substitution. The last point is important because it suggests that the continued accumulation of point mutations in HIV-infected individuals naturally leads to more substantial sequence alterations, such as insertions and deletions, without additional genetic predispositions. It follows that a vaccine strategy specifically designed to target and persistently activate bnAb precursors and their descendants(Haynes et al., 2012) can induce the full spectrum of antibody somatic hypermutations seen in bnAbs and may induce bnAbs as well.
Experimental Procedures
Human Samples
DNA samples prepared from a total of 261 peripheral blood mononuclear cell (PBMC) samples from 75 human subjects for analysis of VHDJH by HTS. These individuals included 41 HIV-infected individuals (8 bnAb producers and 33 non-bnAb producers)(Shen et al., 2009, Tomaras et al., 2011, Morris et al., 2011, Moore et al., 2011), 16 healthy, uninfected individuals, and 18 healthy individuals vaccinated with the 2007-2008 or 2008-2009 seasonal inactivated influenza vaccines as described(Moody et al., 2011). Those characterized as bnAb producers either had broad neutralizing plasma antibodies by previously described criteria from the CHAVI chronic HIV-1 infection cohort(Tomaras et al., 2011) and/or had bnAbs isolated from blood B cells as described(Shen et al., 2009, Morris et al., 2011). PBMC samples were used for DNA isolation for HTS from the influenza vaccinated individuals taken before, 7 days and 21 days after vaccination(Moody et al., 2011) using the methods as described previously(Wrammert et al., 2008, Liao et al., 2009, Moody et al., 2011). All work related to human subjects was in compliance with Institutional Review Board protocols approved by the Duke University Health System Institutional Review Board.
Inference of unmutated common ancestor (UCA) and identification of clone members
The inference of the VHDJH and VLJL of the UCA and intermediate antibodies of CD4BS bnAb VRC-CH30-34 clonal lineage is described in detail elsewhere(Kepler, 2013, Kepler et al., 2014). Briefly, we parameterize the VDJ rearrangement process in terms of its gene segments, recombination points, and n-regions. Given any multiple sequence alignment A for the set of clonally related genes and any tree T describing a purported history, we compute the likelihood for all parameter values, and subsequently the posterior probabilities on the rearrangement parameters conditional on A and T. We can then find the UA with the greatest posterior probability and compute the maximum likelihood alignment A* and tree T* given this UA, and then recompute the posterior probabilities on rearrangement parameters conditional on A* and T*. We iterate the alternating conditional optimizations until convergence is reached.
To infer likely clonal relatedness, we use the following statistical procedure. Two sequences are regarded as potential relatives if they are inferred to have used the same IGHV and IGHJ genes (without regard to allelic differences) and if the number of differences between the two sequences in their CDR3 is not so large that the following hypothesis is rejected. The hypothesis is that the two CDR3 evolved from a common precursor and that along each of the two branches, the mutation frequency is as estimated from the count of point-mutations in IGHV alone. The test itself is a z-test based on the Gaussian approximation to the binomial.
Isolation of VHDJH and VL genes and expression of VHDJH and VLJL genes as full-length IgG1 recombinant monoclonal antibodies (mAbs)
The VHDJH and VLJL gene segment pairs were isolated by RT/PCR from sorted single plasma cells from PBMC collected from healthy blood donors and from vaccinees 7 days after vaccination with either the 2007-2008 or 2008-2009 inactivated influenza vaccine according to a protocol approved by the Duke University Health System Institutional ReviewBoard(Moody et al., 2011). The VHDJH and VLJL gene segment pairs of the observed VRC-CH30-34 were obtained as described previously(Wu et al., 2011). Additional VHDJH were identified by HTS(Wu et al., 2011, Boyd et al., 2009). Clonally related sequences derived from the observed CH31-34 and from the cDNA HTS were combined and used to generate maximum-likelihood phylogenetic trees (Fig 3c). The VHDJH_gene sequences identified by HTS indicated in blue boxes in Figure 1c have been synthesized and paired with intermediate-4 light chain for production of recombinant mAbs for testing the binding and neutralizing activity using the method as described previously(Liao et al., 2011, Liao et al., 2013).
Structural analysis
A set of 11 antigen-bound structures for the following indel-containing bnAbs were included in the analysis: VRC01, VRC03, VRC-CH31, VRC-PG04, NIH45-46, 3BNC117, VRC-PG20, CH103, PG9, PGT128, and PGT135(Wu et al., 2010, Bonsignori et al., 2012, Falkowska et al., 2012, Scheid et al., 2011, Chuang et al., 2013, Walker et al., 2009, Liao et al., 2013, Walker et al., 2011). A total of 18 indels were found in the 11 antibodies. For a given antibody, the distance between an insertion and the corresponding antigen was computed as the minimum heavy-atom (C, N, O, and S) distance between any residue part of the insertion and any residue/glycan part of the antigen. Similarly, the distance between an antibody deletion and the corresponding antigen was computed as the minimum heavy-atom distance between either residue flanking the deletion and any residue/glycan part of the antigen. For a given antibody, loop residues were defined by the loop, bend, and turn categories in DSSP(Kabsch and Sander, 1983), with distances computed as the minimum heavy-atom distance between each loop residue and any residue/glycan in the corresponding antigen. In the above analysis, missing residues in the input structure were ignored.
Surface plasmon resonance (SPR) affinity and kinetics measurements
Binding Kd and rate constant (association rate ka, dissociation rate kd) measurements of recombinant mAbs of VRC-CH30-34 clonal lineage to A244 Δ11 gp120(Liao et al., 2013, Alam et al., 2013) were carried out on BIAcore 3000 instruments as described previously(Alam et al., 2011, Alam et al., 2007, Alam et al., 2008). All data analysis was performed using the BIAevaluation 4.1 analysis software (GE Healthcare).
Neutralization assays
Neutralizing antibody assays in TZM-bl cells were performed as described previously(Montefiori, 2005). Neutralizing activity of plasma samples in 8 serial 3-fold dilutions starting at 1:20 dilution and for recombinant mAbs in 8 serial 3-fold dilutions starting at 50ug/ml were tested against autologous and heterologous HIV-1 Env-pseudotyped viruses in TZM-bl-based neutralization assays using the methods as described(Montefiori, 2005),(Seaman et al., 2010). The data were calculated as a reduction in luminescence units compared with control wells and reported as IC50 in μg/ml for mAbs.
Supplementary Material
Highlights.
Indels are frequent among HIV-1 broad neutralizing antibodies (bnAbs)
Indel occurrence depends solely on the frequency of somatic hypermutation.
Indels in HIV-1 bnAbs are proximal to antigen binding sites.
A compound indel in a CD4-binding site bnAb is required for activity.
Acknowledgements
This study was supported by the National Institutes of Allergy and Infectious Diseases and by intramural NIH support for the NIAID Vaccine Research Center, by grants from the NIH, NIAID, AI068501 (the Center for HIV/AIDS Vaccine Immunology),AI100645 (the Center for Vaccine Immunology-Immunogen Discovery) and HHSN272201000053C (Multiscale Systems Immunology). The authors acknowledge the contributions of the Center for HIV/AIDS Vaccine Discovery (CHAVI) Clinical Core Team at Chapel Hill, North Carolina (Joe Eron); Blantyre, Malawi (Johnstone Kumwenda, Taha Taha); Lilongwe, Malawi (Irving Hoffman, Gift Kaminga); Johannesburg, South Africa (Helen Rees); Moshi, Tanzania (Sam Noel, Saidi Kapiga, John Crump); London, UK (Sarah Fidler). The authors thank Haiyan Chen, Melissa Cooper, Shi-Mao Xia and Julie Blinn for expert technical assistance.
T.B.K. designed the indel studies, performed antibody gene sequence analyses and other statistical analyses, wrote and edited the paper. H.X.L. produced recombinant antibodies and HIV-1 Envs, designed assays, analyzed data, wrote and edited the paper; S.M.L. performed SPR analysis; R.B. assisted with antibody gene sequence analysis; R.R.Z. isolated and produced antibodies; S.S. and K.A. performed SPR assays; R.P., K.E.L., C.S. performed immunoassays; J.P., E.S., E.F performed antibody and HIV-1 Env production; L.M., S.S.A.K. and M.S.C. provided PBMC samples of HIV-1 infected bnAb individuals; G.K. contributed to the design of the experiments; E.W. carried out influenza vaccination and sample collection from influenza vaccinees; M.A.M. performed single plasma cell sorting for isolation of VHDJH and VLJL genes from healthy blood donors and influenza vaccinees; X.W. isolated VRC-CH30-34 bnAbs, H.R.A-T., I.S.G., and P.D.K., perform the structure analysis, S.D.B. and A.Z.F performed HTS; J.R.M. isolated antibodies, designed assays, analyzed data, and edited the paper; B.F.H. designed and directed the immunologic and clinical studies, directed the overall study, analyzed data, and wrote and edited the paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ALAM SM, LIAO HX, DENNISON SM, JAEGER F, PARKS R, ANASTI K, FOULGER A, DONATHAN M, LUCAS J, VERKOCZY L, NICELY N, TOMARAS GD, KELSOE G, CHEN B, KEPLER TB, HAYNES BF. Differential reactivity of germ line allelic variants of a broadly neutralizing HIV-1 antibody to a gp41 fusion intermediate conformation. J Virol. 2011;85:11725–31. doi: 10.1128/JVI.05680-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALAM SM, LIAO HX, TOMARAS GD, BONSIGNORI M, TSAO CY, HWANG KK, CHEN H, LLOYD KE, BOWMAN C, SUTHERLAND L, JEFFRIES TL, JR., KOZINK DM, STEWART S, ANASTI K, JAEGER FH, PARKS R, YATES NL, OVERMAN RG, SINANGIL F, BERMAN PW, PITISUTTITHUM P, KAEWKUNGWAL J, NITAYAPHAN S, KARASAVVA N, RERKS-NGARM S, KIM JH, MICHAEL NL, ZOLLA-PAZNER S, SANTRA S, LETVIN NL, HARRISON SC, HAYNES BF. Antigenicity and immunogenicity of RV144 vaccine AIDSVAX clade E envelope immunogen is enhanced by a gp120 N-terminal deletion. J Virol. 2013;87:1554–68. doi: 10.1128/JVI.00718-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALAM SM, MCADAMS M, BOREN D, RAK M, SCEARCE RM, GAO F, CAMACHO ZT, GEWIRTH D, KELSOE G, CHEN P, HAYNES BF. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J Immunol. 2007;178:4424–35. doi: 10.4049/jimmunol.178.7.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALAM SM, SCEARCE RM, PARKS RJ, PLONK K, PLONK SG, SUTHERLAND LL, GORNY MK, ZOLLA-PAZNER S, VANLEEUWEN S, MOODY MA, XIA SM, MONTEFIORI DC, TOMARAS GD, WEINHOLD KJ, KARIM SA, HICKS CB, LIAO HX, ROBINSON J, SHAW GM, HAYNES BF. Human immunodeficiency virus type 1 gp41 antibodies that mask membrane proximal region epitopes: antibody binding kinetics, induction, and potential for regulation in acute infection. J Virol. 2008;82:115–25. doi: 10.1128/JVI.00927-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONSIGNORI M, HWANG KK, CHEN X, TSAO CY, MORRIS L, GRAY E, MARSHALL DJ, CRUMP JA, KAPIGA SH, SAM NE, SINANGIL F, PANCERA M, YONGPING Y, ZHANG B, ZHU J, KWONG PD, O’DELL S, MASCOLA JR, WU L, NABEL GJ, PHOGAT S, SEAMAN MS, WHITESIDES JF, MOODY MA, KELSOE G, YANG X, SODROSKI J, SHAW GM, MONTEFIORI DC, KEPLER TB, TOMARAS GD, ALAM SM, LIAO HX, HAYNES BF. Analysis of a clonal lineage of HIV-1 envelope V2/V3 conformational epitope-specific broadly neutralizing antibodies and their inferred unmutated common ancestors. J Virol. 2011;85:9998–10009. doi: 10.1128/JVI.05045-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONSIGNORI M, MONTEFIORI DC, WU X, CHEN X, HWANG KK, TSAO CY, KOZINK DM, PARKS RJ, TOMARAS GD, CRUMP JA, KAPIGA SH, SAM NE, KWONG PD, KEPLER TB, LIAO HX, MASCOLA JR, HAYNES BF. Two distinct broadly neutralizing antibody specificities of different clonal lineages in a single HIV-1-infected donor: implications for vaccine design. J Virol. 2012;86:4688–92. doi: 10.1128/JVI.07163-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD SD, MARSHALL EL, MERKER JD, MANIAR JM, ZHANG LN, SAHAF B, JONES CD, SIMEN BB, HANCZARUK B, NGUYEN KD, NADEAU KC, EGHOLM M, MIKLOS DB, ZEHNDER JL, FIRE AZ. Measurement and clinical monitoring of human lymphocyte clonality by massively parallel VDJ pyrosequencing. Sci Transl Med. 2009;1:12ra23. doi: 10.1126/scitranslmed.3000540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRINEY BS, WILLIS JR, CROWE JE., JR. Location and length distribution of somatic hypermutation-associated DNA insertions and deletions reveals regions of antibody structural plasticity. Genes and Immunity. 2012;13:523. doi: 10.1038/gene.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUANG GY, ACHARYA P, SCHMIDT SD, YANG Y, LOUDER MK, ZHOU T, KWON YD, PANCERA M, BAILER RT, DORIA-ROSE NA, NUSSENZWEIG MC, MASCOLA JR, KWONG PD, GEORGIEV IS. Residue-Level Prediction of HIV-1 Antibody Epitopes Based on Neutralization of Diverse Viral Strains. J Virol. 2013 doi: 10.1128/JVI.00984-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EICHLER EE. Masquerading Repeats: Paralogous Pitfalls of the Human Genome. Genome Research. 1998;8:758–762. doi: 10.1101/gr.8.8.758. [DOI] [PubMed] [Google Scholar]
- FALKOWSKA E, RAMOS A, FENG Y, ZHOU T, MOQUIN S, WALKER LM, WU X, SEAMAN MS, WRIN T, KWONG PD, WYATT RT, MASCOLA JR, POIGNARD P, BURTON DR. PGV04, an HIV-1 gp120 CD4 binding site antibody, is broad and potent in neutralization but does not induce conformational changes characteristic of CD4. Journal of virology. 2012;86:4394–4403. doi: 10.1128/JVI.06973-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKITA Y, JACOBS H, RAJEWSKY K. Somatic Hypermutation in the Heavy Chain Locus Correlates with Transcription. Immunity. 1998;9:105–114. doi: 10.1016/s1074-7613(00)80592-0. [DOI] [PubMed] [Google Scholar]
- GOTOH O. Optimal sequence alignment allowing for long gaps. Bulletin of Mathematical Biology. 1990;52:359–373. doi: 10.1007/BF02458577. [DOI] [PubMed] [Google Scholar]
- HAYNES BF, FLEMING J, ST CLAIR EW, KATINGER H, STIEGLER G, KUNERT R, ROBINSON J, SCEARCE RM, PLONK K, STAATS HF, ORTEL TL, LIAO HX, ALAM SM. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–8. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- HAYNES BF, KELSOE G, HARRISON SC, KEPLER TB. B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nat Biotechnol. 2012;30:423–33. doi: 10.1038/nbt.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYNES BF, MOODY MA, LIAO HX, VERKOCZY L, TOMARAS GD. B cell responses to HIV-1 infection and vaccination: pathways to preventing infection. Trends Mol Med. 2011;17:108–16. doi: 10.1016/j.molmed.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HRABER P, SEAMAN MS, BAILER RT, MASCOLA JR, MONTEFIORI DC, KORBER BT. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. AIDS. 2014;28:163–169. doi: 10.1097/QAD.0000000000000106. 10.1097/QAD.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KABSCH W, SANDER C. Dictionary of protein secondary structure: Pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983;22:2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- KEPLER TB. Reconstructing a B-cell clonal lineage. I. Statistical inference of unobserved ancestors. F1000Res. 2013;2:103. doi: 10.12688/f1000research.2-103.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEPLER TB, MUNSHAW S, WIEHE K, ZHANG R, YU JS, WOODS CW, DENNY TN, TOMARAS GD, ALAM SM, MOODY MA, KELSOE G, LIAO HX, HAYNES BF. Reconstructing a B-Cell Clonal Lineage. II. Mutation, Selection, and Affinity Maturation. Front Immunol. 2014;5:170. doi: 10.3389/fimmu.2014.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAUSE JC, EKIERT DC, TUMPEY TM, SMITH PB, WILSON IA, CROWE JE. An Insertion Mutation That Distorts Antibody Binding Site Architecture Enhances Function of a Human Antibody. mBio. 2011:2. doi: 10.1128/mBio.00345-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KWONG PD, MASCOLA JR. Human antibodies that neutralize HIV-1: identification, structures, and B cell ontogenies. Immunity. 2012;37:412–25. doi: 10.1016/j.immuni.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIAO HX, BONSIGNORI M, ALAM SM, MCLELLAN JS, TOMARAS GD, MOODY MA, KOZINK DM, HWANG KK, CHEN X, TSAO CY, LIU P, LU X, PARKS RJ, MONTEFIORI DC, FERRARI G, POLLARA J, RAO M, PEACHMAN KK, SANTRA S, LETVIN NL, KARASAVVAS N, YANG ZY, DAI K, PANCERA M, GORMAN J, WIEHE K, NICELY NI, RERKS-NGARM S, NITAYAPHAN S, KAEWKUNGWAL J, PITISUTTITHUM P, TARTAGLIA J, SINANGIL F, KIM JH, MICHAEL NL, KEPLER TB, KWONG PD, MASCOLA JR, NABEL GJ, PINTER A, ZOLLA-PAZNER S, HAYNES BF. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity. 2013;38:176–86. doi: 10.1016/j.immuni.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIAO HX, CHEN X, MUNSHAW S, ZHANG R, MARSHALL DJ, VANDERGRIFT N, WHITESIDES JF, LU X, YU JS, HWANG KK, GAO F, MARKOWITZ M, HEATH SL, BAR KJ, GOEPFERT PA, MONTEFIORI DC, SHAW GC, ALAM SM, MARGOLIS DM, DENNY TN, BOYD SD, MARSHAL E, EGHOLM M, SIMEN BB, HANCZARUK B, FIRE AZ, VOSS G, KELSOE G, TOMARAS GD, MOODY MA, KEPLER TB, HAYNES BF. Initial antibodies binding to HIV-1 gp41 in acutely infected subjects are polyreactive and highly mutated. J Exp Med. 2011;208:2237–49. doi: 10.1084/jem.20110363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIAO HX, LEVESQUE MC, NAGEL A, DIXON A, ZHANG R, WALTER E, PARKS R, WHITESIDES J, MARSHALL DJ, HWANG KK, YANG Y, CHEN X, GAO F, MUNSHAW S, KEPLER TB, DENNY T, MOODY MA, HAYNES BF. High-throughput isolation of immunoglobulin genes from single human B cells and expression as monoclonal antibodies. J Virol Methods. 2009;158:171–9. doi: 10.1016/j.jviromet.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASCOLA JR, HAYNES BF. HIV-1 neutralizing antibodies: understanding nature’s pathways. Immunological Reviews. 2013;254:225–244. doi: 10.1111/imr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONTEFIORI DC. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr Protoc Immunol. 2005 doi: 10.1002/0471142735.im1211s64. Chapter 12. Unit 12 11. [DOI] [PubMed] [Google Scholar]
- MOODY MA, ZHANG R, WALTER EB, WOODS CW, GINSBURG GS, MCCLAIN MT, DENNY TN, CHEN X, MUNSHAW S, MARSHALL DJ, WHITESIDES JF, DRINKER MS, AMOS JD, GURLEY TC, EUDAILEY JA, FOULGER A, DEROSA KR, PARKS R, MEYERHOFF RR, YU JS, KOZINK DM, BAREFOOT BE, RAMSBURG EA, KHURANA S, GOLDING H, VANDERGRIFT NA, ALAM SM, TOMARAS GD, KEPLER TB, KELSOE G, LIAO HX, HAYNES BF. H3N2 influenza infection elicits more cross-reactive and less clonally expanded anti-hemagglutinin antibodies than influenza vaccination. PLoS One. 2011;6:e25797. doi: 10.1371/journal.pone.0025797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE PL, GRAY ES, SHEWARD D, MADIGA M, RANCHOBE N, LAI Z, HONNEN WJ, NONYANE M, TUMBA N, HERMANUS T, SIBEKO S, MLISANA K, ABDOOL KARIM SS, WILLIAMSON C, PINTER A, MORRIS L, STUDY ATC. Potent and Broad Neutralization of HIV-1 Subtype C by Plasma Antibodies Targeting a Quaternary Epitope Including Residues in the V2 Loop. Journal of Virology. 2011;85:3128–3141. doi: 10.1128/JVI.02658-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRIS L, CHEN X, ALAM M, TOMARAS G, ZHANG R, MARSHALL DJ, CHEN B, PARKS R, FOULGER A, JAEGER F, DONATHAN M, BILSKA M, GRAY ES, ABDOOL KARIM SS, KEPLER TB, WHITESIDES J, MONTEFIORI D, MOODY MA, LIAO H-X, HAYNES BF. Isolation of a Human Anti-HIV gp41 Membrane Proximal Region Neutralizing Antibody by Antigen-Specific Single B Cell Sorting. PLoS ONE. 2011;6:e23532. doi: 10.1371/journal.pone.0023532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOUQUET H, NUSSENZWEIG MC. Polyreactive antibodies in adaptive immune responses to viruses. Cell Mol Life Sci. 2012;69:1435–45. doi: 10.1007/s00018-011-0872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEJCHAL R, DOORES KJ, WALKER LM, KHAYAT R, HUANG P-S, WANG S-K, STANFIELD RL, JULIEN J-P, RAMOS A, CRISPIN M, DEPETRIS R, KATPALLY U, MAROZSAN A, CUPO A, MALOVESTE S, LIU Y, MCBRIDE R, ITO Y, SANDERS RW, OGOHARA C, PAULSON JC, FEIZI T, SCANLAN CN, WONG C-H, MOORE JP, OLSON WC, WARD AB, POIGNARD P, SCHIEF WR, BURTON DR, WILSON IA. A Potent and Broad Neutralizing Antibody Recognizes and Penetrates the HIV Glycan Shield. Science. 2011;334:1097–1103. doi: 10.1126/science.1213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEID JF, MOUQUET H, UEBERHEIDE B, DISKIN R, KLEIN F, OLIVERA TY, PIETZSCH J, FENYO D, ABADIR A, VELINZON K, HURLEY A, MYUNG S, BOULAD F, POIGNARD P, BURTON D, PEREYRA F, HO DD, WALKER BD, SEAMAN MS, BJORKMAN PJ, CHAIT BT, NUSSENZWEIG MC. Sequence and Structural Convergence of Broad and Potent HIV Antibodies That Mimic CD4 Binding. Science. 2011;333:633–7. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEAMAN MS, JANES H, HAWKINS N, GRANDPRE LE, DEVOY C, GIRI A, COFFEY RT, HARRIS L, WOOD B, DANIELS MG, BHATTACHARYA T, LAPEDES A, POLONIS VR, MCCUTCHAN FE, GILBERT PB, SELF SG, KORBER BT, MONTEFIORI DC, MASCOLA JR. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol. 2010;84:1439–52. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN X, PARKS RJ, MONTEFIORI DC, KIRCHHERR JL, KEELE BF, DECKER JM, BLATTNER WA, GAO F, WEINHOLD KJ, HICKS CB, GREENBERG ML, HAHN BH, SHAW GM, HAYNES BF, TOMARAS GD. In Vivo gp41 Antibodies Targeting the 2F5 Monoclonal Antibody Epitope Mediate Human Immunodeficiency Virus Type 1 Neutralization Breadth. Journal of Virology. 2009;83:3617–3625. doi: 10.1128/JVI.02631-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH D, CREADON G, JENA P, PORTANOVA J, KOTZIN B, WYSOCKI L. Di- and trinucleotide target preferences of somatic mutagenesis in normal and autoreactive B cells. The Journal of Immunology. 1996;156:2642–2652. [PubMed] [Google Scholar]
- TOMARAS GD, BINLEY JM, GRAY ES, CROOKS ET, OSAWA K, MOORE PL, TUMBA N, TONG T, SHEN X, YATES NL, DECKER J, WIBMER CK, GAO F, ALAM SM, EASTERBROOK P, ABDOOL KARIM S, KAMANGA G, CRUMP JA, COHEN M, SHAW GM, MASCOLA JR, HAYNES BF, MONTEFIORI DC, MORRIS L. Polyclonal B Cell Responses to Conserved Neutralization Epitopes in a Subset of HIV-1-Infected Individuals. Journal of Virology. 2011;85:11502–11519. doi: 10.1128/JVI.05363-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERKOCZY L, KELSOE G, MOODY MA, HAYNES BF. Role of immune mechanisms in induction of HIV-1 broadly neutralizing antibodies. Curr Opin Immunol. 2011;23:383–90. doi: 10.1016/j.coi.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER LM, HUBER M, DOORES KJ, FALKOWSKA E, PEJCHAL R, JULIEN J-P, WANG S-K, RAMOS A, CHAN-HUI PY, MOYLE M, MITCHAM JL, HAMMOND PW, OLSEN OA, PHUNG P, FLING S, WONG C-H, PHOGAT S, WRIN T, SIMEK M, INVESTIGATOR P, KOFF WC, WILSON MJ, BURTON D, POIGNARD P, PHOGAT SK, CHAN-HUI PY, WAGNER D, PHUNG P, GOSS JL, WRIN T, SIMEK MD, FLING S, MITCHAM JL, LEHRMAN JK, PRIDDY FH, OLSEN OA, FREY SM, HAMMOND PW, KAMINSKY S, ZAMB T, MOYLE M, KOFF WC, POIGNARD P, BURTON DR. Broad neutralization covarage of HIV by multiple highly potent antibodies. Nature. 2011;477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER LM, PHOGAT SK, CHAN-HUI P-Y, WAGNER D, PHUNG P, GOSS JL, WRIN T, SIMEK MD, FLING S, MITCHAM JL, LEHRMAN JK, PRIDDY FH, OLSEN OA, FREY SM, HAMMOND PW, INVESTIGATORS PGP, KAMINSKY S, ZAMB T, MOYLE M, KOFF WC, POIGNARD P, BURTON DR. Broad and Potent Neutralizing Antibodies from an African Donor Reveal a New HIV-1 Vaccine Target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG C, LIU Y, XU LT, JACKSON KJL, ROSKIN KM, PHAM TD, LASERSON J, MARSHALL EL, SEO K, LEE J-Y, FURMAN D, KOLLER D, DEKKER CL, DAVIS MM, FIRE AZ, BOYD SD. Effects of Aging, Cytomegalovirus Infection, and EBV Infection on Human B Cell Repertoires. The Journal of Immunology. 2014;192:603–611. doi: 10.4049/jimmunol.1301384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON PC, BOUTEILLER OD, LIU Y-J, POTTER K, BANCHEREAU J, CAPRA JD, PASCUAL V. Somatic Hypermutation Introduces Insertions and Deletions into Immunoglobulin V Genes. The Journal of Experimental Medicine. 1998;187:59–70. doi: 10.1084/jem.187.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WRAMMERT J, SMITH K, MILLER J, LANGLEY WA, KOKKO K, LARSEN C, ZHENG NY, MAYS I, GARMAN L, HELMS C, JAMES J, AIR GM, CAPRA JD, AHMED R, WILSON PC. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–71. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU X, YANG ZY, LI Y, HOGERKORP CM, SCHIEF WR, SEAMAN MS, ZHOU T, SCHMIDT SD, WU L, XU L, LONGO NS, MCKEE K, O’DELL S, LOUDER MK, WYCUFF DL, FENG Y, NASON M, DORIA-ROSE N, CONNORS M, KWONG PD, ROEDERER M, WYATT RT, NABEL GJ, MASCOLA JR. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–61. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU X, ZHOU T, ZHU J, ZHANG B, GEORGIEV I, WANG C, CHEN X, LONGO NS, LOUDER M, MCKEE K, O’DELL S, PERFETTO S, SCHMIDT SD, SHI W, WU L, YANG Y, YANG Z-Y, YANG Z, ZHANG Z, BONSIGNORI M, CRUMP JA, KAPIGA SH, SAM NE, HAYNES BF, SIMEK M, BURTON DR, KOFF WC, DORIA-ROSE NA, CONNORS M, PROGRAM NCS, MULLIKIN JC, NABEL GJ, ROEDERER M, SHAPIRO L, KWONG PD, MASCOLA JR. Focused Evolution of HIV-1 Neutralizing Antibodies Revealed by Structures and Deep Sequencing. Science. 2011;333:1593–1602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU T, GEORGIEV I, WU X, YANG Z-Y, DAI K, FINZI A, DO KWON Y, SCHEID JF, SHI W, XU L, YANG Y, ZHU J, NUSSENZWEIG MC, SODROSKI J, SHAPIRO L, NABEL GJ, MASCOLA JR, KWONG PD. Structural Basis for Broad and Potent Neutralization of HIV-1 by Antibody VRC01. Science. 2010;329:811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOU T, ZHU J, WU X, MOQUIN S, ZHANG B, ACHARYA P, GEORGIEV IVELINS, ALTAE-TRAN HANR, CHUANG G-Y, JOYCE MG, DO KWON Y, LONGO NANCYS, LOUDER MARKK, LUONGO T, MCKEE K, SCHRAMM CHAIMA, SKINNER J, YANG Y, YANG Z, ZHANG Z, ZHENG A, BONSIGNORI M, HAYNES BARTONF, SCHEID JOHANNESF, NUSSENZWEIG MICHELC, SIMEK M, BURTON DENNISR, KOFF WAYNEC, MULLIKIN JAMESC, CONNORS M, SHAPIRO L, NABEL GARYJ, MASCOLA JOHNR, KWONG PETERD. Multidonor Analysis Reveals Structural Elements, Genetic Determinants, and Maturation Pathway for HIV-1 Neutralization by VRC01-Class Antibodies. Immunity. 2013 doi: 10.1016/j.immuni.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.