Abstract
Background
The recently proposed Movement Disorder Society (MDS) Task Force diagnostic criteria for mild cognitive impairment in Parkinson’s disease (PD-MCI) represent a first step towards a uniform definition of PD-MCI across multiple clinical and research settings. Several questions regarding specific criteria, however, remain unanswered including optimal cutoff scores by which to define impairment on neuropsychological tests.
Methods
Seventy-six non-demented PD patients underwent comprehensive neuropsychological assessment and were classified as PD-MCI or PD with normal cognition (PD-NC). Concordance of PD-MCI diagnosis by MDS Task Force Level II criteria (comprehensive assessment), using a range of standard deviation (SD) cutoff scores, was compared to our consensus diagnosis of PD-MCI or PD-NC. Sensitivity, specificity, positive and negative predictive values were examined for each cutoff score. PD-MCI subtype classification and distribution of cognitive domains impaired were evaluated.
Results
Concordance for PD-MCI diagnosis was greatest for defining impairment on neuropsychological tests using a 2 SD cutoff score below appropriate norms. This cutoff also provided the best discriminatory properties for separating PD-MCI from PD-NC, compared to other cutoff scores. With the MDS PD-MCI criteria, multiple domain impairment was more frequent than single domain impairment, with predominant executive function, memory, and visuospatial function deficits.
Conclusions
Application of the MDS Task Force PD-MCI Level II diagnostic criteria demonstrates good sensitivity and specificity at a 2 SD cutoff score. The predominance of multiple domain impairment in PD-MCI with the Level II criteria suggests not only influences of testing abnormality requirements, but also the widespread nature of cognitive deficits within PD-MCI.
Keywords: Executive function, MCI (mild cognitive impairment), Memory, Neuropsychological tests, Parkinson’s disease
Introduction
Mild cognitive impairment in Parkinson’s disease (PD-MCI) has been increasingly recognized as a state of cognitive decline that is beyond that expected with normal aging, but does not meet dementia criteria 1, 2. Although historically described with different criteria, PD-MCI is common, occurring in 20-50% of patients 2, 3 and may reflect a transitional state that precedes dementia in PD 4-6. Recent efforts have focused on developing specific and standardized diagnostic criteria for PD-MCI since prior studies varied greatly in definitions used 2, 3, 7, 8. As such, a Movement Disorder Society (MDS) Task Force proposed diagnostic criteria for PD-MCI in order to enhance clinical and research efforts on identifying clinical characterizations of PD-MCI, predictors of conversion to dementia, and patients who might benefit from early intervention studies, not only in single-site PD cohorts but across multiple clinical and research sites 9. The MDS Task Force criteria delineate inclusionary and exclusionary features for PD-MCI and provide the clinician or researcher with two diagnostic methods: an abbreviated assessment (Level I) or comprehensive assessment (Level II), which can also classify PD-MCI subtypes as single or multiple domains impaired. For Level II criteria, the MDS Task Force recommends formal neuropsychological testing that includes at least two tests for each of the five cognitive domains (i.e., attention/working memory, executive function, language, memory, visuospatial function). Impairment should be present on at least two neuropsychological tests, represented by either two impaired tests in a single cognitive domain or one impaired test in two different cognitive domains and may be demonstrated in one of three ways: performance approximately 1-2 standard deviations (SD) below appropriate norms, significant decline demonstrated on serial cognitive testing, or significant decline from estimated pre-morbid levels.
The proposed MDS PD-MCI diagnostic criteria represent an essential first step towards a uniform definition of PD-MCI across multiple clinical and research settings but await field study of applicability and validation. Several questions for Level II criteria regarding definitions of impairment on neuropsychological tests remain unanswered, including the optimal SD cutoff score below appropriate norms to use. These issues are important to investigate in the validation of the MDS PD-MCI criteria as they can affect the sensitivity of detecting PD-MCI and ultimately, the clinical care and counseling of such patients and their potential eligibility in research studies 10-12. Studies examining the MDS PD-MCI Task Force criteria are emerging but at present, have utilized a 1.5 SD cutoff below appropriate norms in their application of Level II criteria 5, 13, 14. Other PD studies highlight how different cutoff scores, number of tests, and cognitive domain representation can influence the characterization of PD-MCI 11, 12. These studies, however, were published prior to the MDS PD-MCI Task Force criteria and differ in the cognitive domains and tests studied. Accordingly, the aim of our study was to examine one of the unresolved issues raised during the development of the MDS Task Force criteria, namely optimal SD cutoff scores for determining impairment and its effect on PD-MCI characterization and subtype.
Subjects and Methods
Subjects
Seventy-six non-demented PD subjects were recruited from the Rush University Movement Disorders clinic as part of a prospective study of clinical and neuroimaging markers of PD cognitive impairment 15. All PD subjects were examined by a movement disorders neurologist (J.G.G.) and met United Kingdom PD Society Brain Bank criteria 16. Subjects had a disease duration of ≥ 4 years, were on stable medication regimens, and examined in optimal motor state. Exclusionary criteria were: PD dementia by MDS criteria 1, atypical or secondary parkinsonism; severe or unstable depression; anticholinergic medications (e.g., trihexyphenidyl, benztropine, tricyclic antidepressants); other medical or neurological causes of cognitive impairment (e.g., seizures, strokes, head trauma); or contraindications to MRI (e.g., cardiac pacemaker/defibrillator, surgical clips, foreign metallic implants). The study was approved by the Rush University Institutional Review Board, Chicago, IL, and participants provided written informed consent.
Evaluations
Subjects underwent detailed, comprehensive clinical and neuropsychological evaluations including: 1) demographics, disease-related features, and medications, 2) interview with the patient and informant including clinical impressions of the patient’s general cognitive function, decline, and functional abilities 17-19, and 3) neuropsychological testing of cognitive function and mood 20, 21. Cognitive assessments included the MiniMental State Examination (MMSE) 22 and the following individual tests grouped into 5 cognitive domains based on MDS Task Force recommendations and prior studies 6, 9, 13, 14, 23: (a) Attention and working memory (Digit span forwards 24, Letter Number Sequencing 24, Symbol Digit Modalities 25, Trail making Test-A 26), (b) Executive function (Clock Drawing Test 27, Controlled Oral Word Association Test 28, Digits backwards 24, Progressive matrices 29, Trail making Test-B 26, (c) Language (Boston Naming Test 30, Category fluency test of animal naming in 1 minute 30, Similarities 24), (d) Memory (3 trials of word list learning, delayed recall, and recognition from the Consortium to Establish a Registry for AD [CERAD] 30, Logical Memory I and II prose passages 31, total free recall and delayed recall from Free and Cued Selective Reminding Test (FCSRT) 32, figure learning and delayed recall for Figure Memory 33), and (e) Visuospatial function (Clock Copying Test 27, Judgment of Line Orientation 34, pentagons from MMSE 35). Raw scores for cognitive tests were transformed to z-scores based upon normative data from healthy, cognitively normal controls examined at our center 36, 37. Composite scores for each memory test (e.g., CERAD, Logical memory, etc) were computed by averaging individual subcomponent z-scores (e.g., list learning, delayed recall), which avoided over-representing the number of memory test subcomponents comprising this domain. The 5 cognitive domain scores were calculated by averaging z-scores for neuropsychological tests within each of the specific domains.
Cognitive classification
Cognitive classification was determined in a systematic, uniform step-wise process and consensus conference based on methods used at our center 38. Briefly, subjects were classified independently as PD-MCI or cognitively normal (PD-NC) by each of the raters, a neurologist specializing in movement disorders and neuropsychiatry [J.G.G.], senior neuropsychologist administering the tests [B.B], and senior consulting neuropsychologist [G.T.S.]), based on their respective project role (clinical interview/exam, semi-structured interview and neuropsychological testing, and review of neuropsychological testing). Each subject’s information was reviewed in a consensus conference and a final clinical judgment of cognitive status made. This consensus diagnosis was then compared to the MDS Task Force PD-MCI Level II (comprehensive assessment) criteria regarding neuropsychological test impairment 9. Subjects categorized as PD-MCI by the MDS Task Force met the proposed inclusionary and exclusionary criteria for PD-MCI as well as specific guidelines for Level II classification, including impairment present on at least 2 neuropsychological tests, represented by either 2 impaired tests in one cognitive domain or one impaired test in 2 different cognitive domains. For this study, impairment was examined across different SD cutoffs relative to appropriate normative values to determine the influence of the different cutoff scores (i.e., 1 SD, 1.5 SD, 2 SD below appropriate norms as per MDS PD-MCI criteria as well as an exploratory lower limit of 2.5 SD to capture an extended range) on PD-MCI diagnosis. PD-MCI subjects underwent subtyping according to MDS PD-MCI criteria as either PD-MCI single-domain or PD-MCI multiple-domain. Non-demented PD subjects who did not fulfill the MDS PD-MCI criteria were classified as PD-NC.
Statistical analyses
Statistical analyses were performed using SAS 9.1 (Institute Inc., Cary, NC). We compared the clinical features of the PD-NC and PD-MCI subjects using t-tests, Mann-Whitney U, and Chi-square tests, as appropriate. We calculated sensitivity, specificity, positive and negative predictive values, and receiver operating curves (ROC) with area under the curve (AUC) and 95% confidence intervals (CI) and compared diagnostic classification of PD-MCI using MDS PD-MCI Task Force Level II criteria for each SD cutoff score (i.e., 1, 1.5, 2, 2.5 SD) below norms to the classification of PD-MCI by consensus diagnosis criteria. Cohen’s kappa coefficient was calculated to examine the concordance between the 2 PD-MCI classifications at each SD cutoff score. Descriptive statistics were used to characterize PD-MCI subtypes as single and multiple domains impaired and examine clinical characterization. Statistical significance was set at p<0.05.
Results
Clinical characteristics of the PD-MCI cohort
Table 1 depicts the demographic and clinical features of our PD cohort as defined by consensus diagnosis. Compared to the PD-NC subjects, the PD-MCI subjects had worse motor severity as measured by MDS-UPDRS Part III motor examination score (p=0.02) and Hoehn and Yahr stage (p=0.03). The groups were similar regarding age, education, sex, PD duration, medication usage, and mood rating scales. Cognitive performance was worse in PD-MCI subjects compared to PD-NC subjects, consistent with group definitions.
Table 1.
Demographic and clinical features of the PD cohort
| PD-NC, n=28 | PD-MCI, n=48 | p value | |
|---|---|---|---|
| Demographics | |||
| Age, y | 72 ± 6.5 | 73.2 ± 5.8 | 0.41 |
| Male, n (%) | 20 (71.4) | 39 (81.3) | 0.32 |
| Education, y | 15.5 ± 2.8 | 15.1 ± 3.4 | 0.55 |
| PD duration, y | 8.7 ± 2.9 | 9.7 ± 4.4 | 0.24 |
| Motor features | |||
| MDS-UPDRS Part III Motor score | 31 ± 8.9 | 36.8 ± 11.3 | 0.02 |
| Hoehn and Yahr stage, median (range) | 2.0 (2-3) | 2.0 (2-5) | 0.03 |
| Medications | |||
| LEDD, mg/d | 821.6 ± 492.1 | 736.8 ± 395.5 | 0.41 |
| Dopamine agonist, n (%) | 16 (57.1) | 18 (37.5) | 0.10 |
| Sleep medication, n (%) | 5 (17.9) | 15 (31.2) | 0.20 |
| Antidepressant, n (%) | 5 (17.9) | 10 (20.8) | 0.75 |
| Cognitive enhancing medications, n (%) | 2 (7.1) | 4 (8.3) | 1 |
| Antipsychotic, n (%) | 1 (3.6) | 2 (4.2) | 1 |
| Cognitive and neuropsychological features | |||
| Cognitive decline by patient, informant, or clinician, n (%) | 21 (75) | 48 (100) | 0.001 |
| CDR Global score, median (range) | 0 (0-0.5) | 0.5 (0-0.5) | <0.0001 |
| Functional Assessment Questionnaire, median (range) | 0 (0-5) | 2 (0-11) | 0.001 |
| MMSE scores | 28.6 ± 1.1 | 27.7 ± 1.7 | 0.005 |
| Attention/working memory domain | -0.32 ± 0.58 | -1.3 ± 0.85 | <0.0001 |
| Executive function domain | -0.33 ± 0.62 | -1.52 ± 0.97 | <0.0001 |
| Language domain | -0.15 ± 0.72 | -0.81 ± 0.65 | <0.0001 |
| Memory domain | -0.23 ± 0.51 | -1.33 ± 0.70 | <0.0001 |
| Visuospatial domain | -0.21 ± 0.77 | -1.48 ± 1.77 | <0.0001 |
| Hamilton depression rating scale | 5.1 ± 3.1 | 6.1 ± 3.9 | 0.27 |
| Beck anxiety inventory | 8.4 ± 6.3 | 7.3 ± 7.8 | 0.56 |
Results are expressed as mean (SD), unless otherwise noted. Abbreviations: CDR = Clinical Dementia Rating Scale, LEDD = levodopa equivalent daily doses, MDS-UPDRS = Movement Disorder Society-Unified Parkinson’s Disease Rating Scale, MMSE = Mini-Mental State Examination
Classification function and concordance
The sensitivity and specificity of the MDS PD-MCI Level II criteria for accurate classification of PD-MCI vs. PD-NC relative to the consensus diagnosis varied across the different SD cutoff scores used to define impairment on neuropsychological tests (Table 2). The best sensitivity and specificity measures were achieved using the cutoff of 2 SD below norms, with 85.4% and 78.6%, respectively. The other cutoff scores compromised either specificity, such as with 1 and 1.5 SD below norms (21.4% and 60.7%, respectively), or sensitivity, such as with our exploratory analyses of 2.5 SD below norms (58.3%). Positive and negative predictive values were optimal at the cutoff of 2 SD below norms with 87.2% and 75.9%, respectively and lower for the other cutoff scores. ROC analyses revealed an AUC value of 0.9 for the 2 SD cutoff score (Figure 1). Concordance between the two PD-MCI classification methods was highest for the 2 SD cutoff score, demonstrating good agreement (kappa = 0.64 [0.46-0.81]), but for all other cutoff scores, only fair to moderate. At the MDS PD-MCI Level II 2 SD cutoff score, 47/76 (61.8%) of subjects were diagnosed as PD-MCI, whereas 48/76 (63.2%) of subjects were classified as PD-MCI by consensus diagnosis.
Table 2.
Diagnosis of PD-MCI by MDS Task Force criteria using different cutoff scores below norms for neuropsychological impairment
| PD-MCI by MDS Level II criteria, n | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) | Kappa, (95% CI) | |
|---|---|---|---|---|---|---|
| 1 SD | 69 | 97.9 (88.9-99.7) | 21.4 (8.3-41.0) | 68.1 (55.8-78.8) | 85.7 (42.2-97.6) | 0.23 (0.05-0.41) |
| 1.5 SD | 56 | 93.8 (82.8-98.6) | 60.7 (40.6-78.5) | 80.4 (67.6-89.8) | 85 (62.1-96.6) | 0.58 (0.39-0.77) |
| 2 SD | 47 | 85.4 (72.2-93.9) | 78.6 (59.0-91.7) | 87.2 (74.3-95.1) | 75.9 (56.5-89.7) | 0.64 (0.46-0.81) |
| 2.5 SD | 29 | 58.3 (43.2-72.4) | 96.4 (81.6-99.4) | 96.6 (82.2-99.4) | 57.5 (42.2-71.7) | 0.48 (0.31-0.65) |
SD = standard deviation
FIG. 1.

The receiver operating characteristic curve is illustrated for a classification of mild cognitive impairment in Parkinson’s disease (PD-MCI) using Movement Disorder Society PD-MCI Level II criteria at different standard deviation (SD) cutoff scores.
PD-MCI subtype classification
Using the MDS PD-MCI Level II criteria, multiple domain impairment was more frequent than single domain impairment for all SD cutoff scores. With the 2 SD cutoff score, multiple domain impairment occurred in 43/47 (91.5%) subjects and single domain impairment, in 4/47 (8.5%) subjects. The number of multiple domains impaired ranged from 2-5 with 34.9% subjects having 3 domains and 32.5% subjects having 4 domains impaired (Figure 2). When single domains were impaired, they included: executive function (n=1), memory (n=1), and visuospatial function subtypes (n=2). Regardless of the SD cutoff score, executive function was the most frequently impaired cognitive domain. For the 2 SD cutoff score, the cognitive profile was represented by deficits in executive function (78.7%), followed by memory (70.2%) and visuospatial function (70.2%), attention/working memory (59.6%), and language (48.9%) (Table 3).
FIG. 2.
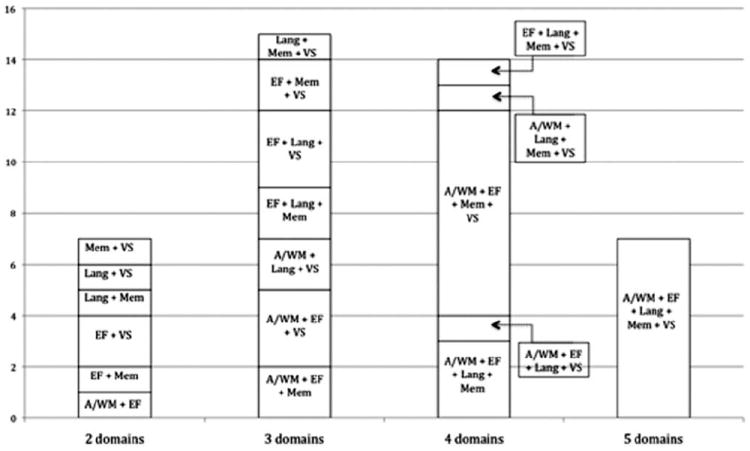
Mild cognitive impairment in Parkinson’s disease (PD-MCI) multiple domain impairment subtypes are illustrated according to Movement Disorder Society PD-MCI Level II criteria at a cutoff of 2 standard deviations (SD). Frequency counts of PD-MCI subtypes and specific cognitive domains affected are illustrated. Lang indicates language; Mem, memory; VS, visuospatial function; EF, executive function; A/WM, attention/working memory.
Table 3.
Cognitive domain profile of PD-MCI subjects by MDS Task Force criteria using different cutoff scores
| Attention/working memory, % | Executive function, % | Language, % | Memory, % | Visuospatial function, % | |
|---|---|---|---|---|---|
| 1 SD (n=69) | 75.4 | 91.3 | 76.8 | 82.6 | 81.2 |
| 1.5 SD (n=56) | 80.4 | 85.7 | 60.7 | 80.4 | 67.9 |
| 2 SD (n=47) | 59.6 | 78.7 | 48.9 | 70.2 | 70.2 |
| 2.5 SD (n=29) | 65.5 | 89.7 | 10.3 | 58.6 | 58.6 |
SD = standard deviation
Discussion
Our study demonstrates several findings. First, the MDS PD-MCI Task Force Level II diagnostic criteria can be applied in clinical research settings and provide a reliable diagnosis of PD-MCI, with good concordance with PD-MCI classification by consensus diagnosis criteria. Second, PD-MCI impairment was best defined using a cutoff of 2 SD below norms in our cohort. Third, multiple domain impairment was more common than single domain PD-MCI, using all SD cutoff scores, with predominantly executive dysfunction.
This study represents a first step in the application and validation of the MDS PD-MCI Level II criteria and in addressing one of the criteria’s unresolved issues, specifically SD cutoff scores used to define neuropsychological test impairment. Compared to other SD cutoff scores, the 2 SD cutoff score below norms provided the best discriminative properties for classifying subjects as PD-MCI with good concordance with our consensus PD-MCI classification. While most studies of MCI, either in PD or non-PD populations 10, 39-41, have utilized 1 SD, 1.5 SD, 1.96 SD, or 2 SD cutoff scores, we extended our score analyses to a lower limit of 2.5 SD below norms, and thus, on the other extreme end, confirm that the 2 SD cutoff score below norms provides the best PD-MCI diagnostic classification. Moreover, a normative cutoff score of 1 SD may identify 16% of healthy individuals as impaired, assuming that measures are normally distributed in healthy populations, and thus, has been considered too liberal to permit meaningful specificity in the assessment of cognitive decline 10. While the MDS PD-MCI Task Force Level II diagnostic criteria recommend a broad definition of impairment ranging between 1-2 SD below norms, to avoid missing the diagnosis of PD-MCI in high-functioning people, our study suggests that the 1 or 1.5 SD cutoff scores are too inclusive and sacrifice specificity. Therefore, at present, we would recommend a more conservative 2 SD cutoff below norms for defining PD-MCI by MDS PD-MCI Level II criteria. As our PD cohort had a mean age of 72.7 (6.0) years and mean education of 15.3 (3.2) years, examination of this cutoff in PD-MCI cohorts with different levels of education and diverse backgrounds may provide additional insights and further refinement of the criteria.
To the best of our knowledge, our study is the first to examine this issue with the MDS PD-MCI Level II criteria. Few studies have examined the recently published MDS PD-MCI criteria, but those in the literature thus far have applied a 1.5 SD cutoff score below norms. In addition, these studies explored different issues from our study, namely the demonstration of a decline from pre-morbid levels, requirement of cognitive complaints, inclusion of 2 tests abnormal per domain 14 or the discriminatory power of individual neuropsychological tests 13. Other recent studies, prior to the MDS PD-MCI criteria, affirm that the issue of defining impairment cutoff scores is an important and influential one regarding the frequency and characterization of PD-MCI, with PD-MCI estimates varying from approximately 10-90% using cutoffs of 1 SD, 1.5 SD, or 2 SD below norms 2, 3, 11, 12, 42-44. These studies, however, also varied in other features including number of cognitive domains assessed (range 3-5), number of tests per domain (range 2-7), number of abnormal tests per domain required (i.e., 1 test abnormal per 1 or 2 cognitive domains or 2 tests abnormal per 1 or 2 cognitive domains), educational level of participants (mean 11-16 years), and definition of PD dementia 1, 45. Moreover, application of more liberal or conservative cutoff scores may have additional clinical and research significance, beyond cross-sectional frequency estimates. In studies of non-PD populations, MCI diagnostic approaches influenced estimates of MCI stability or progression to dementia, in general and by subtype categorization 39, 40. This may have particular relevance in advancing our understanding of PD-MCI subtype differences and selecting PD-MCI patients for trials of therapies to halt or slow conversion to PD dementia.
Classification of PD-MCI into subtypes is important for exploring whether impairments in different cognitive domains have distinct underlying neurobiological substrates and clinical courses. In our cohort, PD-MCI multiple domain impairment was more frequent than single domain impairment, which is similar to a recent report of PD-MCI subtype distributions using the MDS PD-MCI Task Force Level II diagnostic criteria 14. This PD-MCI subtype distribution of greater multiple domain impairment persisted despite use of different SD cutoff scores in our study, though the 2 SD cutoff score classified the greatest number of subjects as having single domain impairment. These findings differ from older studies, including our prior work 36, which reported greater single domain impairment, either nonamnestic 4, 42-44, 46, 47 or amnestic subtypes 3. Potential reasons for the greater frequency of PD-MCI overall and in multiple domain impairment include differences in PD-MCI diagnostic criteria, number of tests, number and type of cognitive domains, PD duration (prevalent vs. incident cases), and populations (clinic-based vs. community).
Of the cognitive domains impaired in our current study, executive function was most frequently affected, followed by memory and visuospatial function, at the 2 SD cutoff score. Multiple combinations of cognitive deficits occurred in PD-MCI multiple domain subtype. Distributions and combinations of domains impaired did not appear to be driven by the number of individual cognitive tests within each domain. In most PD-MCI studies, to date, cognitive domains have been variably weighted with 2-7 tests per cognitive domain 11-13. Excessive or imbalanced number of tests per domain may bias PD-MCI classification 9, and fewer than 2 tests per domain also may be inadequate for diagnosis and subtyping 10. The MDS PD-MCI criteria recommend at least 2 tests per domain with impairment demonstrated on at least 2 neuropsychological tests as either 2 impaired tests in 1 cognitive domain or 1 impaired test in 2 different cognitive domains, but the optimal number of tests per domain and number of tests impaired per domain remain areas that may merit additional study. The optimal grouping of tests in domains also has been debated; some tests have potentially overlapping features (e.g., executive components of visuospatial tests), and others may be sensitive to deficits in more than one area (e.g., category fluency and frontal or temporal/posterior cortical dysfunction 6, 23).
Compared to PD-NC subjects, our PD-MCI subjects had worse motor function but did not differ regarding age, education, PD duration, and medications used for motor and non-motor features (e.g., hallucinations, sleep, mood). Several studies demonstrate an association between older age and lower education levels and PD cognitive impairment 3, 6, 48, but this association was not found in other studies 14, 42, 43, 47, 49. Although our PD-MCI subjects were slightly older and had slightly fewer years of education than PD-NC subjects, this was not statistically significant and may relate to demographic patterns or sample size. The association of worse cognitive function and greater motor severity is in keeping with other studies, which also link these two features to shared neurobiological substrates, increased dementia risk, and earlier onset of dementia in PD 4, 6, 50-52. Longitudinal follow-up studies of our PD cohort will permit examination of their cognitive and motor progression.
Our study has several notable strengths including our well-defined PD cohort, diagnoses by experienced specialists in movement disorders and neuropsychology, comprehensive clinical and neuropsychological assessments, as well as the application of the recently published MDS PD-MCI Task Force Level II criteria. Limitations include our university setting, sample size, and high educational levels, which may affect generalizability. While our cohort size is smaller than several large, community-based or multi-center PD-MCI cohorts 3, 14, 42, 44, it is comparable to other PD-MCI studies in clinic-based populations 13, 43, 47, 53. Educational levels of PD cohorts vary in the literature, depending on the country and setting, ranging from mean ~ 9-11 years 13, 42, 48 to ≥ 15 years 14, 43, 54, the latter, comparable to our cohort. Future studies, however, with larger PD-MCI cohorts representing different educational levels, disease durations, and PD-MCI subtypes will be needed to further characterize PD-MCI. At present, there is no “gold standard” to validate the diagnosis of PD-MCI, and studies of this are in beginning phases. As such, in order to examine the issue of optimal SD cutoff scores, we compared the MDS PD-MCI Level II criteria to our consensus diagnosis, using a systematized approach similar to those applied in aging and dementia studies 38, 55. Future studies with longitudinal follow up of PD-MCI subjects will permit the examination of stability or progression of PD-MCI and the use of the MDS PD-MCI criteria to predict PDD. These studies will help establish the sensitivity/specificity and optimal SD cutoff scores for the MDS PD-MCI criteria in predicting PDD.
We conclude that the MDS PD-MCI Task Force Level II criteria can be readily applied in the clinical research setting and that consideration should be given for using a 2 SD cutoff score below norms in the diagnosis of PD-MCI. The development of these criteria mark a first step in creating a uniform definition of PD-MCI that can be used in multiple centers and for interventional trials, and further validation of our observations in larger and diverse cohorts will be important steps in the testing and application of the MDS PD-MCI criteria.
Acknowledgments
Dr. Goldman has received grant/research support from NIH K23NS060949 and the Parkinson’s Disease Foundation.
Full Financial Disclosures of all Authors for the Past Year: Information concerning all sources of financial support and funding for the preceding twelve months, regardless of relationship to current manuscript. List sources or “none”:
Jennifer G. Goldman, MD, MS
|
| |
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
|
| |
| Consultancies: Merz, Pfizer | Expert Testimony: none |
|
| |
| Advisory Boards: none | Employment: Rush University Medical Center |
|
| |
| Partnerships: none | Contracts: none |
|
| |
| Honoraria: Movement Disorders Society, American Academy of Neurology, Teva, Johns Hopkins Dystonia and Spasticity Practicum | Royalties: none |
|
| |
| Grants: NIH K23NS060949, Michael J. Fox Foundation (BioFIND, site-PI), Parkinson’s Disease Foundation, Rush University, Teva (Moderato study, site-PI) | Other: none |
|
| |
Samantha Holden, MD:
|
| |
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
|
| |
| Consultancies: none | Expert Testimony: none |
|
| |
| Advisory Boards: none | Employment: Rush University Medical Center |
|
| |
| Partnerships: none | Contracts: none |
|
| |
| Honoraria: none | Royalties: none |
|
| |
| Grants: none | Other: none |
|
| |
Bryan Bernard, PhD:
|
| |
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
|
| |
| Consultancies: none | Expert Testimony: none |
|
| |
| Advisory Boards: none | Employment: Rush University Medical Center |
|
| |
| Partnerships: none | Contracts: none |
|
| |
| Honoraria: none | Royalties: none |
|
| |
| Grants: none | Other: none |
|
| |
Bichun Ouyang, PhD:
|
| |
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
|
| |
| Consultancies: none | Expert Testimony: none |
|
| |
| Advisory Boards: none | Employment: Rush University Medical Center |
|
| |
| Partnerships: none | Contracts: none |
|
| |
| Honoraria: none | Royalties: none |
|
| |
| Grants: none | Other: none |
|
| |
Christopher G. Goetz, MD:
|
| |
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
|
| |
| Consultancies: AOP Orphan, Addex Pharma, Advanced Studies of Medicine, Boston Scientific, CHDI, Health Advances, ICON Clinical Research, Ingenix (i3 Research), National Institutes of Health, Neurocrine, Oxford Biomedica, Synthonics | Expert Testimony: none |
|
| |
| Advisory Boards: AOP Orphan, Addex Pharma, Advanced Studies of Medicine, Boston Scientific, CHDI, Health Advances, ICON Clinical Research, Ingenix (i3 Research), National Institutes of Health, Neurocrine, Oxford Biomedica, Synthonics | Employment: Rush University Medical Center |
|
| |
| Partnerships | Contracts: None |
|
| |
| Honoraria: Movement Disorder Society, American Academy of Neurology, Movement Disorder Society, University of Pennsylvania, University of Chicago, University of Luxembourg | Royalties: Oxford University Press, Elsevier Publishers, Wolters Kluwer Health-Lippincott, Wilkins and Williams |
|
| |
Glenn Stebbins, PhD:
|
| |
| Stock Ownership in medically-related fields: none | Intellectual Property Rights: none |
|
| |
| Consultancies: IMPAX Laboratories, Inc.; Ceregene, Inc.; Biovail Technologies, LTD; Santhera Pharmaceuticals; i3 | Expert Testimony: none |
|
| |
| Advisory Boards: none | Employment: Rush University Medical Center |
|
| |
| Partnerships: none | Contracts: none |
|
| |
| Honoraria: none | Royalties: none |
|
| |
| Grants: NIH, Michael J. Fox Foundation for Parkinson’s Research, American Cancer Society, Fragile × Foundation | Other: Editorial Board, Journal of Clinical and Experimental Neuropsychology |
|
| |
Footnotes
Jennifer G. Goldman, MD, MS: 1) Research project: A. Conception, B. Organization, C. Execution; 2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3) Manuscript: A. Writing of the first draft, B. Review and Critique
Samantha Holden, MD: 1) Research project: C. Execution; 3) Manuscript: A. Writing of the first draft, B. Review and Critique
Bryan Bernard, PhD: 1) Research project: C. Execution; 3) Manuscript: B. Review and Critique
Bichun Ouyang, PhD: 1) Research project: C. Execution; 2) Statistical Analysis: C. Review and Critique; 3) Manuscript: B. Review and Critique
Christopher G. Goetz, MD: 1) Research project: B. Organization; 3) Manuscript: B. Review and Critique
Glenn Stebbins, PhD: 1) Research project: A. Conception, B. Organization, C. Execution; 2) Statistical Analysis: C. Review and Critique; 3) Manuscript: B. Review and Critique
References
- 1.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. quiz 1837. [DOI] [PubMed] [Google Scholar]
- 2.Litvan I, Aarsland D, Adler CH, et al. MDS task force on mild cognitive impairment in Parkinson’s disease: Critical review of PD-MCI. Mov Disord. 2011;26(10):1814–1824. doi: 10.1002/mds.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aarsland D, Bronnick K, Williams-Gray C, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010;75(12):1062–1069. doi: 10.1212/WNL.0b013e3181f39d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson’s disease: progression to dementia. Mov Disord. 2006;21(9):1343–1349. doi: 10.1002/mds.20974. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen KF, Larsen JP, Tysnes OB, Alves G. Prognosis of Mild Cognitive Impairment in Early Parkinson Disease: The Norwegian ParkWest Study. JAMA Neurol. 2013:1–7. doi: 10.1001/jamaneurol.2013.2110. [DOI] [PubMed] [Google Scholar]
- 6.Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130(Pt 7):1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 7.Goldman JG, Litvan I. Mild cognitive impairment in Parkinson’s disease. Minerva Med. 2011;102(6):441–459. [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 9.Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov Disord. 2012;27(3):349–356. doi: 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schinka JA, Loewenstein DA, Raj A, et al. Defining mild cognitive impairment: impact of varying decision criteria on neuropsychological diagnostic frequencies and correlates. Am J Geriatr Psychiatry. 2010;18(8):684–691. doi: 10.1097/JGP.0b013e3181e56d5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalrymple-Alford JC, Livingston L, MacAskill MR, et al. Characterizing mild cognitive impairment in Parkinson’s disease. Mov Disord. 2011;26(4):629–636. doi: 10.1002/mds.23592. [DOI] [PubMed] [Google Scholar]
- 12.Liepelt I, V A, Baysal G, et al. Influence of different classification criteria on the interpretation of group differences in PD-MCI. Movement Disorders. 2009;24(Suppl 1):205. [Google Scholar]
- 13.Biundo R, Weis L, Pilleri M, et al. Diagnostic and screening power of neuropsychological testing in detecting mild cognitive impairment in Parkinson’s disease. J Neural Transm. 2013;120(4):627–633. doi: 10.1007/s00702-013-1004-2. [DOI] [PubMed] [Google Scholar]
- 14.Marras C, Armstrong MJ, Meaney CA, et al. Measuring mild cognitive impairment in patients with Parkinson’s disease. Mov Disord. 2013 doi: 10.1002/mds.25426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman JG, Ghode RA, Ouyang B, Bernard B, Goetz CG, Stebbins GT. Dissociations among daytime sleepiness, nighttime sleep, and cognitive status in Parkinson’s disease. Parkinsonism Relat Disord. 2013 doi: 10.1016/j.parkreldis.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes AJ, Daniel SE, Blankson S, Lees AJ. A clinicopathologic study of 100 cases of Parkinson’s disease. Arch Neurol. 1993;50(2):140–148. doi: 10.1001/archneur.1993.00540020018011. [DOI] [PubMed] [Google Scholar]
- 17.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 18.Morris JC. Clinical Dementia Rating: Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 19.Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer’s Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21(3):249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton M. Development of a rating scale for primary depressive illness. Brit Jrl Soc Clin Psych. 1967;6:278–297. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 21.Beck AT, Steer RA. Beck Anxiety Inventory Manual. San Antonio, TX: Harcourt Brance and Company; 1993. [Google Scholar]
- 22.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Pagonabarraga J, Kulisevsky J, Llebaria G, Garcia-Sanchez C, Pascual-Sedano B, Gironell A. Parkinson’s disease-cognitive rating scale: a new cognitive scale specific for Parkinson’s disease. Mov Disord. 2008;23(7):998–1005. doi: 10.1002/mds.22007. [DOI] [PubMed] [Google Scholar]
- 24.Weschler D. Weschler Adult Intelligence Scale-III. New York: The Psychological Corporation; 1991. [Google Scholar]
- 25.Smith A. Symbol Digits Modality Test. Los Angeles: Western Psychological Services; 1973. [Google Scholar]
- 26.Reitan RM, Wolfson D. The Trail-making Test. 2. Tucson, AZ: Neuropsychology Press; 1993. [Google Scholar]
- 27.Goodglass H, Kaplan E. Assessment of aphasia and related disorders. 2. Philadelphia: Lea and Febiger; 1983. Clock Drawing Test. [Google Scholar]
- 28.Benton AL, Hamsher Kd. Controlled Oral Word Association (FAS): Multilingual aphasia examination. Iowa City: AJA Associates; 1989. [Google Scholar]
- 29.Raven J, Raven JC, Court JH. Manual for Raven’s Progressive Matrices and Vocabulary Scales. San Antonio, TX: Harcourt Assessment; 2003. [Google Scholar]
- 30.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 31.Weschler D. Weschler Memory Scale - III manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 32.Grober E, Buschke H. Genuine memory deficits in dementia. Dev Neuropsychol. 1987;3:13–36. [Google Scholar]
- 33.Wilson RS, Gilley DW, Bennett DA, Beckett LA, Evans DA. Person-specific paths of cognitive decline in Alzheimer’s disease and their relation to age. Psychol Aging. 2000;15(1):18–28. doi: 10.1037//0882-7974.15.1.18. [DOI] [PubMed] [Google Scholar]
- 34.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging. 2002;17(2):179–193. [PubMed] [Google Scholar]
- 35.Bourke J, Castleden CM, Stephen B, Dennis M. A comparison of clock and pentagon drawing in Alzheimer’s disease. Int J Geriatr Psychiatry. 1995;10:703–705. [Google Scholar]
- 36.Goldman JG, Weis H, Stebbins G, Bernard B, Goetz CG. Clinical differences among mild cognitive impairment subtypes in Parkinson’s disease. Mov Disord. 2012;27(9):1129–1136. doi: 10.1002/mds.25062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25(4):163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 38.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59(2):198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 39.Busse A, Hensel A, Guhne U, Angermeyer MC, Riedel-Heller SG. Mild cognitive impairment: long-term course of four clinical subtypes. Neurology. 2006;67(12):2176–2185. doi: 10.1212/01.wnl.0000249117.23318.e1. [DOI] [PubMed] [Google Scholar]
- 40.Jak AJ, Bondi MW, Delano-Wood L, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry. 2009;17(5):368–375. doi: 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol. 2005;62(7):1160–1163. doi: 10.1001/archneur.62.7.1160. discussion 1167. [DOI] [PubMed] [Google Scholar]
- 42.Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. 2009;72(13):1121–1126. doi: 10.1212/01.wnl.0000338632.00552.cb. [DOI] [PubMed] [Google Scholar]
- 43.Caviness JN, Driver-Dunckley E, Connor DJ, et al. Defining mild cognitive impairment in Parkinson’s disease. Mov Disord. 2007;22(9):1272–1277. doi: 10.1002/mds.21453. [DOI] [PubMed] [Google Scholar]
- 44.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65(8):1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 45.Association AP. Diagnostic and statistical manual of mental disorders, text revision. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 46.Foltynie T, Brayne CE, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson’s patients in the UK. The CamPaIGN study. Brain. 2004;127(Pt 3):550–560. doi: 10.1093/brain/awh067. [DOI] [PubMed] [Google Scholar]
- 47.Sollinger AB, Goldstein FC, Lah JJ, Levey AI, Factor SA. Mild cognitive impairment in Parkinson’s disease: subtypes and motor characteristics. Parkinsonism Relat Disord. 2010;16(3):177–180. doi: 10.1016/j.parkreldis.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim JW, Cheon SM, Park MJ, Kim SY, Jo HY. Cognitive impairment in Parkinson’s disease without dementia: subtypes and influences of age. J Clin Neurol. 2009;5(3):133–138. doi: 10.3988/jcn.2009.5.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elgh E, Domellof M, Linder J, Edstrom M, Stenlund H, Forsgren L. Cognitive function in early Parkinson’s disease: a population-based study. Eur J Neurol. 2009;16(12):1278–1284. doi: 10.1111/j.1468-1331.2009.02707.x. [DOI] [PubMed] [Google Scholar]
- 50.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60(3):387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 51.Bohnen NI, Muller ML, Koeppe RA, et al. History of falls in Parkinson disease is associated with reduced cholinergic activity. Neurology. 2009;73(20):1670–1676. doi: 10.1212/WNL.0b013e3181c1ded6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yarnall A, Rochester L, Burn DJ. The interplay of cholinergic function, attention, and falls in Parkinson’s disease. Mov Disord. 2011;26(14):2496–2503. doi: 10.1002/mds.23932. [DOI] [PubMed] [Google Scholar]
- 53.Janvin C, Aarsland D, Larsen JP, Hugdahl K. Neuropsychological profile of patients with Parkinson’s disease without dementia. Dement Geriatr Cogn Disord. 2003;15(3):126–131. doi: 10.1159/000068483. [DOI] [PubMed] [Google Scholar]
- 54.Mamikonyan E, Moberg PJ, Siderowf A, et al. Mild cognitive impairment is common in Parkinson’s disease patients with normal Mini-Mental State Examination (MMSE) scores. Parkinsonism Relat Disord. 2009;15(3):226–231. doi: 10.1016/j.parkreldis.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gabel MJ, Foster NL, Heidebrink JL, et al. Validation of consensus panel diagnosis in dementia. Arch Neurol. 2010;67(12):1506–1512. doi: 10.1001/archneurol.2010.301. [DOI] [PMC free article] [PubMed] [Google Scholar]


