Abstract
While it has been long known that patients with sepsis often have thrombocytopenia and that septic patients with severe thrombocytopenia have a poor prognosis and higher mortality, the role of platelets in the pathogenesis of sepsis is poorly understood. Here we report a protective role of platelets in septic shock. We show that experimental thrombocytopenia by intraperitoneal injection of an anti-glycoprotein Ibα monoclonal antibody increases mortality and aggravates organ failure whereas transfusion of platelets reduces mortality in Lipopolysaccharide-induced endotoxemia and a bacterial infusion mouse sepsis model. Plasma concentrations of proinflammatory cytokines TNF-α and IL-6 are elevated by thrombocytopenia and decreased by platelet transfusion in septic mice. Furthermore, we identify that platelets protect from septic shock by inhibiting macrophage-dependent inflammation via the COX1/PGE2/EP4 dependent pathway. Thus, these findings demonstrate a previously unappreciated role for platelets in septic shock and suggest that platelet transfusion may be effective in treating severe septic patients.
Platelets play a central role in physiological hemostasis by preventing excess bleeding but are also involvedin pathologic arterial thrombosis. Emerging evidence suggests that platelets are also critical components of immune system1,2. Platelets are activated in patients with systemic inflammation and sepsis, resulting in their sequestration within microcirculation and thrombocytopenia3,4. Severe thrombocytopenia in septic patients is associated with adverse outcome and high mortality5–7. Platelets regulate inflammation and sepsis through multiple mechanisms. Platelets express a lipopolysaccharide (LPS) receptor, TLR4, which contributes to thrombocytopenia through neutrophil-dependent pulmonary sequestration in response to LPS8–10. Platelets also interact with other leukocytes including monocytes11,12. Interaction of activated platelets with monocytes induces nuclear translocation of NF-κB and expression of NF-κB-dependent inflammatory genes13–15. In addition to direct interactions with leukocytes, platelets contribute to inflammation and immune progression by releasing cytokines and mediators stored in alpha and dense granules upon stimulation16,17.
In the present study, we used LPS-induced endotoxemia model and a bacterial infusion sepsis model through intraperitoneally injection of LPS or an E. coli strain ATCC 25922, respectively into mice to investigate the effects of experimental thrombocytopenia and platelet transfusion on septic shock. LPS or endotoxin, a component of the outer membrane of Gram-negative bacteria, plays an essential role in the pathogenesis of sepsis. LPS administration into mice has become a standard inflammation model and is widely used in sepsis research18. Human sepsis is often caused by a single pathogen. The bacterial infusion model introduces a single pathogen into mice in a controlled manner, allowing reproducible infection, which has also been translated to larger animals for the study of systemic and organ-specific hemodynamics. We demonstrate that experimental thrombocytopenia increases mortality and aggravates organ failure whereas transfusion of platelets reduces mortality in LPS-induced endotoxemia and E. coli ATCC 25922-induced sepsis. Our data reveal an important new role for platelets in sepsis and define a mechanism by which platelets protect septic shock.
Results
Thrombocytopenia exacerbates septic shock and organ failure
To establish a role of platelets in sepsis-associated inflammation, we induced thrombocytopenia in mice by intraperitoneal injection of a rat anti-mouse GPIbα monoclonal antibody. Four hours after injection of the antibody, platelet counts were decreased by 90% (Fig. 1a). Platelet counts were not altered by injection of an isotype-matched rat IgG control. We then compared survival rates between the IgG-treated and thrombocytopenic mice after LPS challenge. Unexpectedly, thrombocytopenic mice had a significantly greater mortality rate than the mice administered with control IgG (Fig. 1b). All thrombocytopenic mice died within 36 hours after LPS challenge. In contrast, none of the mice treated with control IgG died within 36 hours after challenge by LPS. Lethality in sepsis is associated with organ failure. Thus, we examined the effects of thrombocytopenia on liver function in mice challenged with LPS. Plasma concentrations of liver enzymes, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) that are released into the circulation upon injury and death of liver cells, were significantly higher in plasma from anti-mouse GPIbα monoclonal antibody-treated mice than those that received control IgG (Fig.1c,d). Lactate dehydrogenase (LDH) is an enzyme found in many tissues, including liver and heart, and may be released into plasma with hepatic and myocardial damage. Accordingly, plasma LDH concentration was higher in the thrombocytopenic mice than that of control mice (Fig. 1e). Creatine kinase (CK), an enzyme expressed by various tissues and cell types, can be elevated in plasma as a consequence of muscle injury or renal failure due to reduced clearance. Plasma CK concentrations were much higher in thrombocytopenic mice than in IgG-treated mice (Fig. 1f). Together, these results demonstrate that thrombocytopenia exacerbates tissue injury associated with sepsis.
Figure 1. Depletion of platelets in mice enhances mortality and worsens organ failure induced by LPS.
(a) C57BL/6 mice were injected with 4 µg/g of body weight of a rat anti-mouse GPIbα monoclonal antibody (n = 7) or control rat IgG (n = 8) by i.p. Platelets counts were measured with a HEMAVET HV950FS multispecies hematology analyzer before and 4 hours after injection of antibody. Values are means ± s.d. (b) Mice were then injected with LPS (10 mg/kg). Survival rate was observed for up to 3 days. P value was calculated by Wald test in a discrete time hazard model using version 9.2 of SAS software. (c–f), 8 hours after LPS treatment, plasma from mice receiving anti-GPIb antibody or IgG control were collected and ALT (c), AST (d), LDH (e), and CK (f) concentrations in plasma were measured. Values are means ± s.d. (n = 4). Differences between two groups were assessed using unpaired two-tailed Student’s t-test. (g,h) Mice were injected intraperitoneally with a rat anti-mouse GPIbα monoclonal antibody or rat IgG control. After 4 hours, the mice were injected intraperitoneally with LPS (10 mg/kg). Fifteen hours after LPS injection, the mice were sacrificed and lungs were collected (g). Sections of lungs were stained with H&E and images were captured. Scale bar, 0.5 mm. (h).
Thrombocytopenia does not cause inflammatory hemorrhage
Previous studies reported that inflammation caused life-threatening hemorrhage during thrombocytopenia19,20. In those studies, the anti-GPIbα monoclonal antibody was injected intravenously or retro-orbitally, which resulted in a lowering of platelet count to less than 2.5% of that of control mice. However, no detectable hemorrhage occurred in lungs (Fig. 1g,h) from mice in which thrombocytopenia was induced by I.P injection of the anti-GPIbα antibody. Additionally, red blood cell counts and hemoglobin concentrations were no significantly different between IgG-treated mice and the anti-GPIbα antibody-treated mice after LPS. Thus, inflammatory hemorrhage did not appear to account for the higher mortality rates with LPS in mice rendered thrombocytopenic by I.P. injection of the anti-GPIbα antibody.
Thrombocytopenia aggravates inflammatory response to LPS
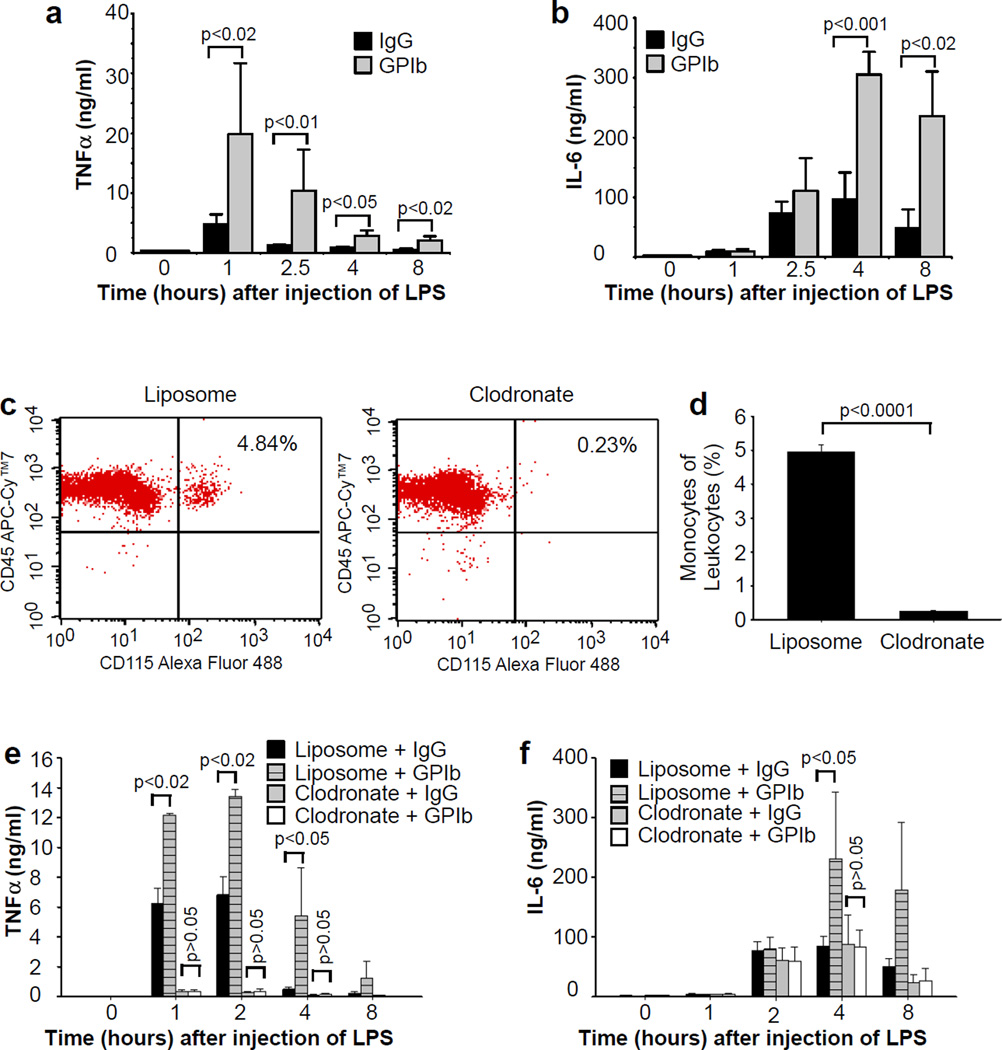
Endotoxemia increases production of endogenous cytokines, including tumor necrosis factor-alpha (TNF-α) and IL-6, which play important roles in development of disseminated intravascular coagulation, acute respiratory distress syndrome, and septic shock21–24. Therefore, we investigated whether platelets protect from endotoxemia by altering expression of these cytokines. Indeed, LPS elevated plasma levels of TNF-α and IL-6, and the effect was amplified in thrombocytopenic mice (Fig. 2a,b). These results suggest that platelets normally inhibit endotoxemia-induced cytokine production. Macrophages are a major source of TNF-α in vivo22,25,26. Therefore, we hypothesize that platelets protect from septic shock by attenuating macrophage-dependent inflammatory response, thereby decreasing concentrations of these proinflammatory cytokines in plasma. We further hypothesize that platelet depletion increases TNF-α and IL-6 production by reducing a macrophage inhibitor factor(s). If these hypotheses are correct, then thrombocytopenia should have no effect on LPS-induced increases in plasma TNF-α and IL-6 levels in the mice lacking macrophages. Macrophages were depleted by retro-orbital injection of liposomal clodronate. Macrophage depletion was confirmed by detecting peripheral monocyte concentration by flow cytometry with an Alexa Fluor 488-labeled anti-CD115 monoclonal antibody. Monocyte concentration was reduced by >95% (Fig. 2c,d). As expected, plasma TNF-α concentrations were significantly decreased in macrophage-depleted mice challenged with LPS, compared with the mice injected with control liposomes (Fig. 2e). The increase in plasma TNF-α observed in thrombocytopenic mice was absent when macrophages were pre-depleted.
Figure 2. The effects of thrombocytopenia on plasma TNF-α and IL-6 concentrations.
(a,b) Mice were injected intraperitoneally with a rat anti-mouse GPIbα monoclonal antibody or rat IgG control. After 4 hours, the mice were injected intraperitoneally with LPS (10 mg/kg). Blood was collected from these mice before orat 1, 2.5, 4, and 8 hours after LPS injection. TNF-α (a) and IL-6(b) concentrations in plasma were measured by an ELISA assay. Values are means ± s.d. (n = 6). Differences between two groups were assessed using unpaired two-tailed Student’s t-test. (c,d) Mice were injected retro-orbitally with clodronate (40 mg/kg) or control liposome. Blood was collected from the mice at 24 hours after injection of clodronate or liposome. Representative flow cytometry plots of monocyte subsets (c) and quantification of monocyte subsets (d) (n = 3) are shown. Error bars indicate s.d. Difference was assessed using unpaired two-tailed Student’s t-test. (e,f) Mice were injected retro-orbitally with clodronate (40 mg/kg) or control liposomes at 24 and 4 hours prior to LPS injection. The mice were also injected intraperitoneally with a rat anti-mouse GPIbα monoclonal antibody or rat IgG control at 4 hours prior to LPS injection. Blood was collected before or at 1, 2, 4, and 8 hours after LPS injection, and plasma TNF-α (e) and IL-6 (f) concentrations were measured. Values are means ± s.d. (n = 5). Differences between two groups were assessed using unpaired two-tailed Student’s t-test.
Macrophages do not appear to be a major source of IL-6 production early after LPS challenge, because depletion of macrophages did not alter plasma IL-6 concentrations until more than 4 hours after injection of LPS (Fig. 2f). By 8 hours after LPS, depletion of macrophages decreased plasma IL-6 concentrations, suggesting that macrophages contribute to plasma IL-6 production at later stages of endotoxemia. Thrombocytopenia increased plasma IL-6 concentrations in control mice administrated liposomes but had no effect in mice pre-depleted with macrophages (Fig. 2f). These results demonstrate that platelets inhibit macrophage-derived IL-6, but have no effect on IL-6 production derived from other cells.
Thrombocytopenia exacerbates bacterial sepsis
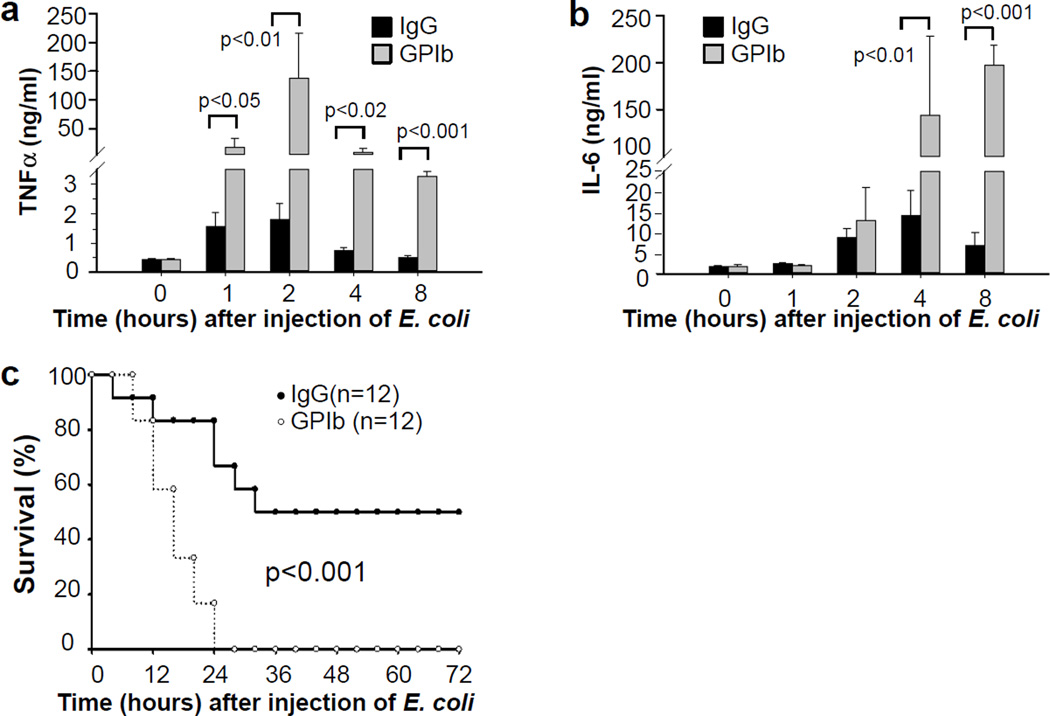
We further investigated the role of platelets in septic shock using a bacterial infusion sepsis model. C57BL/6J mice were injected with the anti-GPIbα monoclonal antibody to deplete platelets. Four hours after injection of the antibody, the mice received intraperitoneally 0.2 mL saline containing 2 × 107 CFU of E. coli ATCC 25922 per mouse. Thrombocytopenic mice had dramatically higher plasma levels of TNF-α and IL-6 (Fig. 3a,b), and a greater mortality rate compared with the mice administered control IgG (Fig.3c).
Figure 3. Platelets inhibit inflammation and protect against septic shock in a bacterial infusion sepsis model.
(a,b) Mice were injected intraperitoneally with a rat anti-mouse GPIbα monoclonal antibody or rat IgG control. After 4 hours, the mice were injected intraperitoneally with 0.2 mL saline containing 2 × 107 CFU of E. coli ATCC 25922 per mouse. Blood was collected from these mice before or at 1, 2, 4, and 8 hours after injection of bacteria. TNF-α (a) and IL-6 (b) concentrations in plasma were measured by ELISA assays. Values are means ± s.d. (n = 6). Differences between two groups were assessed using unpaired two-tailed Student’s t-test. (c) C57BL/6 mice were injected intraperitoneally with a rat anti-mouse GPIbα monoclonal antibody or control rat IgG (4 µg/g of body weight). After 4 hours, the mice were injected with 0.2 mL saline containing 2 × 107 CFU of E. coli ATCC 25922. Survival rate was observed for up to 3 days. P value was calculated by Wald test in a discrete time hazard model using version 9.2 of SAS software.
Platelet transfusion protects against septic shock
Because platelets inhibited macrophage-derived TNF-α and IL-6 production, platelet transfusion may reduce in vivo inflammatory response during sepsis, thereby protect from septic shock. C57BL/6J mice were injected retro-orbitally with washed platelets from the same background (1 × 109 per mouse) immediately after injection of LPS (i.p). Injection of 1 × 109 platelet per mouse increased platelet counts by ~25% (Fig. 4a). Plasma TNF-α and IL-6 concentrations following LPS were significantly lower in mice injected with platelets versus saline (Fig. 4b,c). Platelet transfusion also attenuated thrombocytopenia during endotoxemia (Fig. 4a). Platelet transfusion increased survival rate from 16.0% to 41.7% (Fig. 4d) and 6.7% to 40.0% (Fig. 4e), respectively in LPS-induced endotoxemia model and bacterial infusion sepsis model.
Figure 4. The effects of platelet transfusion on plasma TNF-α and IL-6 concentrations and mortality in septic animals.
(a–c) Mice were injected with LPS i.p. (10 mg/kg) followed by retro-orbital injection of 1 × 109 platelets in 0.2 ml saline or saline. Blood were collected before or at 1, 2.5, 4, and 8 hours after LPS injection. Platelets counts were measured before or at 1 and 8 hours after LPS injection and expressed as relative to the counts of saline-treated mice before injection of LPS (a). Plasma TNF-α (b) and IL-6 (c) concentrations were measured. Values are means ± s.d. (n = 5). Differences between two groups were assessed using unpaired two-tailed Student’s t-test. (d) Mice were injected with LPS (10 mg/kg) immediately followed by retro-orbital injection of 1 × 109 platelets in 0.2 ml saline or saline. Survival rate was observed for 3 days. P value was calculated by Wald test in a discrete time hazard model using version 9.2 of SAS software. (e) C57BL/6 mice were injected intraperitoneally with 0.2 mL saline containing 2.5 × 107 CFU of E. coli ATCC 25922 immediately followed by retro-orbital injection of 1 × 109 platelets in 0.2 ml saline or saline. Survival rate was observed for up to 3 days. P value was calculated by Wald test in a discrete time hazard model using version 9.2 of SAS software. (f) Mice were injected retro-orbitally with clodronate or control liposomes at 24 and 4 hours prior to LPS injection (5 mg/kg of body weight), immediately followed by retro-orbital injection of 1 × 109 platelets in 0.2 ml saline or saline. Survival rate was observed for up to 3 days. p<0.001 between liposome group and clodronate group; p>0.2 between clodronate group injected with saline and clodronate group injected with platelets. P value was calculated by Wald test in a discrete time hazard model using version 9.2 of SAS software.
Protection against sepsis by platelet depends on macrophages
To determine whether the protective effect of platelet transfusion on sepsis depends on inhibition of macrophage function, macrophages were depleted by retro-orbital injection of liposomal clodronate. Since injection of control liposome increased mortality rate in the LPS-challenged mice, a lower dose of LPS was applied. Depletion of macrophage increased survival rate from 8.33% to 50.0% in response to LPS challenge (Fig. 4f). Transfusion of platelets did not significantly increase the survival rate in the macrophage-depleted mice. These results suggest that the protective effect of platelet transfusion on endotoxemia depends on macrophages.
Platelets inhibit macrophage-derived TNF-α and IL-6
Next, in vitro experiments were performed to address whether platelets directly inhibit macrophage-derived cytokines in response to LPS. Mouse bone marrow-derived macrophages (BMDMs) were stimulated with LPS in the presence or absence of washed mouse platelets. Although addition of platelets increased TNF-α production from macrophages in response to a low-dose LPS (Fig. 5a), TNF-α production elicited by higher concentrations of LPS was dramatically reduced by addition of platelets (Fig. 5a). Similarly, IL-6 production induced by LPS was reduced by addition of platelets (Fig. 5a). Because LPS at 100 ng/ml induced maximal TNF-α and IL-6 production from macrophages, this concentration of LPS was chosen to investigate the mechanism whereby platelets inhibit macrophage-derived TNF-α in the following experiments.
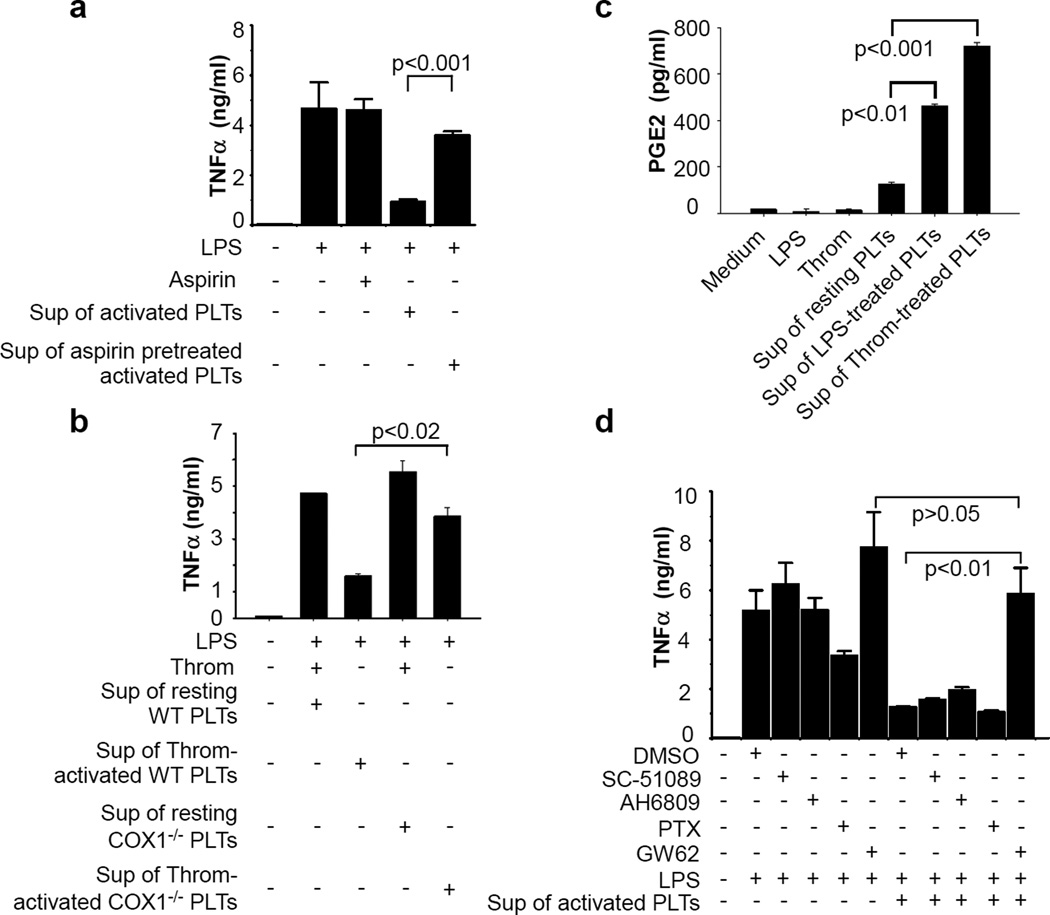
Figure 5. Platelets or platelet releasates inhibit TNF-α and IL-6 production induced by LPS in bone marrow-derived macrophages (BMDMs).
(a) LPS (1 ng/ml~10 µg/ml) was added with buffer or washed platelets (3 × 108/ml) to BMDMs and incubated for 6 hours. TNF-α and IL-6 concentrations in the supernatant were measured. (b) Washed platelets or medium were incubated with 3H-LPS (250 ng/ml) at 37°C for 2 hours and centrifuged. DPMI in medium, platelet pellet or supernatant was measured. (c) LPS (100 ng/ml) was added to BMDMs in the presence or absence of platelets (3 × 108/ml), and incubated for 6 hours. Washed platelets (3 × 108/ml) were also incubated with LPS (100 ng/ml) for 30 min or thrombin (Enzyme Research Laboratories, South Bend, IN, 0.1 U/ml) in aggregometer at 37°C for 5 min and centrifuged. The resulting supernatants with LPS were added to BMDMs and incubated for 6 hours. LPS plus thrombin (Throm) were added to BMDMs and incubated for 6 hours as a control. TNF-α and IL-6 concentrations in the supernatant were measured. (d) Washed platelets from Gq knockout mice or wild-type littermates were incubated with thrombin in aggregometer for at 37°C 5 min and centrifuged. Supernatants plus LPS were added to BMDMs and incubated for 6 hours. (e) Platelets (3 × 108/ml) were preincubated with thrombin in aggregometer at 37°C for 5 min and centrifuged. SAA (1 µg/ml) together with buffer or supernatant from thrombin-stimulated platelets were added to BMDMs and incubated for 6 hours. Values are means ± s.d. (n = 3 for all). Differences between two groups were assessed using unpaired two-tailed Student’s t-test.
Platelets express the LPS receptor, TLR4. To exclude the possibility that inhibition of LPS-induced TNF-α production is due to platelet sequestration of LPS, platelets were incubated with H3-labled LPS for two hours, and the amount of radioactivity associated with platelets was measured. Despite of the presence of LPS receptor on platelets, radioactivity remained in the supernatant rather than with platelets (Fig. 5b).
Platelet releasates inhibit TNF-α and IL-6 production
To determine whether platelets inhibit macrophage-derived cytokines through direct interaction between platelets and macrophages or by releasing inhibitory factors, platelets were incubated with LPS for 30 minutes, and then centrifuged. The resulting supernatant containing LPS was added to macrophages. LPS in the presence of platelet releasate elicited less TNF-α and IL-6 production than did LPS alone (Fig. 5c).
The coagulation system is activated during sepsis, resulting in thrombin generation27,28, a physiological platelet agonist. Therefore, during sepsis, platelets are activated not only by LPS but also by physiological agonists such as thrombin. To determine whether platelet activation induced by a physiological agonist has an effect on macrophages, platelets from C57BL/6 mice were stimulated with thrombin and stirred in aggregometer at 37°C for 5 min. Supernatant from the thrombin-stimulated platelets markedly inhibited LPS-induced TNF-α and IL-6 production by macrophages (Fig. 5c). Thrombin-induced platelet activation and secretion requires Gq signaling29. As expected, supernatant of thrombin-stimulated wild-type platelets, but not Gq deficient platelets, inhibited LPS-induced TNF-α production (Fig. 5d). Taken together, these results demonstrate an inhibitory role of platelet activation in LPS-induced TNF-α production by macrophages.
We next examined the effects of platelet releasate on TNF-α production from macrophages induced by serum amyloid A (SAA) to determine whether platelet activation plays a common role in regulation of macrophage response to inflammatory challenge. Addition of supernatant of thrombin-stimulated platelets reduced SAA-stimulated TNF-α production by macrophages (Fig. 5e).
The effect of platelet secretion on TNF-α production
Platelets contain two major types of granules, α granules containing soluble and membrane-associated proteins and dense granules containing small molecules such as nucleotides30. In response to agonist activation, including LPS, platelets secrete granule contents31–33. We therefore investigated whether granule releasate contributes to the inhibitory effects of platelets on macrophages. Most components secreted fromα granules are proteins16 that are sensitive to heating or proteinase K treatment. However, neither heating (Supplementary Fig. S1a) nor proteinase K (Supplementary Fig. S1b) treatment blunted the inhibitor effects of activated platelet supernatant on TNF-α production by macrophages.
ADP secreted from dense granules plays an essential role in platelet activation through its receptor P2Y12 and P2Y134. The addition of the ADP scavenger a pyrase did not block the inhibitory effect of platelet releasate on LPS-induced TNF-α production by macrophages (Supplementary Fig. S2a). Furthermore, platelet releasate inhibited LPS-induced TNF-α from macrophages lacking the ADP receptor P2Y12 (Supplementary Fig. S2b), indicating that ADP is not required for the inhibition of macrophages by platelets. Munc13-4 is critical for cargo release from both α and dense granules of platelets35. Platelet releasats from Unc13dJinx mice that lack Munc13-4 inhibited LPS-induced TNF-α by macrophages (Supplementary Fig. 2c). Taken together, these results suggest that inhibition of macrophage-derived TNF-α by platelets is independent of granule cargo release from platelets.
Platelets inhibit TNF-α through the COX1-dependent pathway
Synthesis of TXA2 from cyclooxygenase1 (COX1) signaling is an important positive feedback mechanism for platelet activation. To determine whether COX1 is involved in platelet inhibition of macrophages, we examined whether aspirin, a COX1 inhibitor, could reverse the inhibitory effect of platelets on macrophages. Pre-treatment of platelets with aspirin markedly reversed inhibition of LPS-induced TNF-α production from macrophages by platelet releasates (Fig. 6a). Accordingly, supernatant from thrombin-activated COX1 deficient platelets had less effect than that of wild-type platelets on inhibiting macrophage-derived TNF-α (Fig. 6b). Considering the importance of TXA2 in regulating platelet activation, we asked whether TXA2 synthesized from COX1 signaling is responsible for platelet inhibition on macrophages. Addition of a stable TXA2 analog, U46619, failed to inhibit LPS-induced TNF-α production by macrophages (Supplementary Fig. S3a). Supernatant from thrombin-stimulated wild-type platelets inhibited LPS-induced TNF-α production by macrophages lacking the TXA2 receptor, TP (Supplementary Fig. S3b). Thus, platelet inhibition of macrophage function is likely independent of TXA2.
Figure 6. Platelets inhibit TNF-α production by macrophages through the COX1/PGE2/EP4 pathway.
(a) Washed platelets were pre-incubated with aspirin (1 mM) or buffer for 5 min and then incubated with thrombin in aggregometer at 37°C for 5 min and centrifuged. LPS plus the supernatants of platelets were added to BMDMs and incubated for 6 hours. Thrombin or thrombin plus aspirin were added to the wells with LPS in the absence of platelet supernatant as controls. (b) Platelets from COX1 knockout mice (COX1−/−) or wild-type littermates (COX1+/+) were incubated with buffer (resting) or thrombin in aggregometer at 37°C for 5 min. LPS plus the supernatants of platelets were added to BMDMs and incubated for 6 hours. (c) Washed platelets from C57BL/6J mice were incubated with LPS for 30 min or thrombin in aggregometer at 37°C for 5 min. PGE2 in the supernatant were measured by an ELISA assay (Cayman Chemical, Ann Arbor, MI). (d) BMDMs were pre-incubated with buffer, SC -51089 10 µM, AH 6809 10 µM, pertusis toxin (PTX) 0.5 µg/ml, or GW627368X (GW62) at 37°C for 15 min. BMDMs were then added with LPS or LPS plus supernatant from thrombin-activated platelets and incubated at 37°C for 6 hours. Values are means ± s.d. (n = 3 for all). Differences between two groups were assessed using unpaired two-tailed Student’s t-test.
COX1 activation results in the production of many types of prostaglandins. Prostaglandin E2 (PGE2) modulates macrophage function and decreases TNF-α and IL-6 production via its receptors EP1–436,37. LPS and thrombin stimulated PGE2 production in platelets (Fig. 6c), and PGE2 or heated PGE2 inhibited LPS-induced TNF-α production in macrophages (Supplementary Fig. S3c). To determine whether PGE2 is required for platelet inhibition of LPS-induced TNF-α production by macrophages, macrophages were pre-incubated with antagonists of EP1–4. EP4 antagonist, GW62, but not the antagonists for EP1–3, reversed the inhibitory effect of platelet releasates on LPS-induced TNF-α production from macrophages (Fig. 6d), indicating that platelet inhibition of macrophage function involves the PGE2/EP4 pathway.
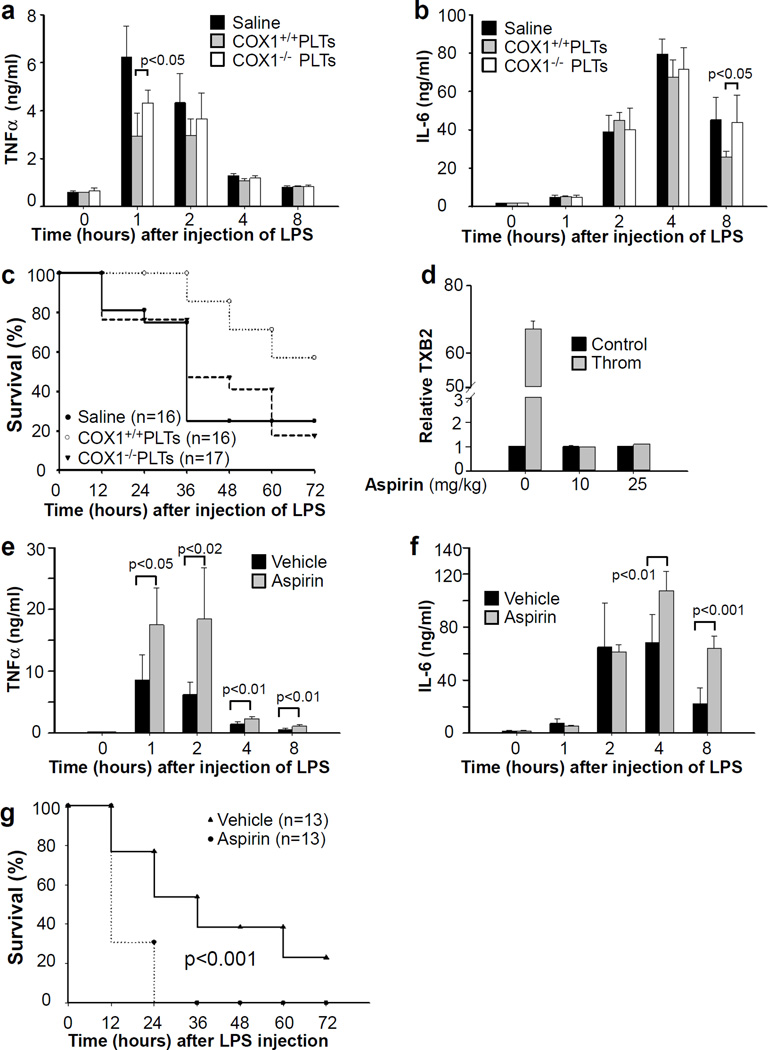
In agreement with the in vitro observations, plasma TNF-α and IL-6 levels were higher in LPS-treated C57BL/6 mice injected with COX1 deficient platelets than with platelets from wild-type littermates (Fig. 7a,b). Injection of COX1 deficient platelets failed to protect from LPS-induced septic shock (Fig. 7c). Next, we investigated the role of aspirin on sepsis. We first determined the effective dose of aspirin in inhibiting platelet COX1 activity in vivo. Thrombin-induced TXA2 production was completely abolished in the platelets isolated from mice administrated with at 10 or 25 mg/kg of aspirin (Fig. 7d). Aspirin (25 mg/kg) pre-treated mice had significantly higher plasma TNF-α and IL-6 concentrations than control mice (Fig. 7e,f). Aspirin pre-treatment also reduced survival rate in the LPS-induced sepsis model (Fig. 7g). Taken together, these results demonstrate that platelet inhibition of inflammatory responses and LPS-induced TNF-α production from macrophages is dependent on platelet COX-1 activity.
Figure 7. The effects of COX1 knockout and aspirin on platelet-dependent inhibition of inflammation and protection against sepsis.
(a,b) C57BL/6 mice received intraperitoneal injection of LPS (10 mg/kg) immediately followed by retro-orbital injection of saline or 1 × 109 platelets from COX1 deficient mice (COX1−/−) or wild-type littermates (COX1+/+) in 0.2 ml saline. Blood was collected before or at 1, 2, 4, and 8 hours after LPS injection. TNF-α (a) and IL-6 (b) concentrations in plasma were measured. Values are means ± s.d. (n = 5). Differences between two groups were assessed using unpaired two-tailed Student’s t-test. (c) C57BL/6 mice received intraperitoneal injection of LPS (10 mg/kg) immediately followed by retro-orbital injection of 1 × 109 platelets from either COX1 knockout mice or wild type littermates in 0.2 ml saline or saline. Survival rate was observed for 3 days. P<0.001 between control group and the group injected with wild type platelets; p>0.2 between control group and the group injected with COX1−/− platelets by Wald test.(d) C57BL/6 mice received intraperitoneal injection of aspirin (10 or 25 mg/kg). Blood was collected at 30 min after aspirin injection. Washed platelets were incubated with thrombin (0.1 U/ml) for 5 min, and TXB2 in supernatant was measured by ELISA kit and expressed as relative to platelets from control mice (without aspirin injection) in the absence of thrombin. Values are means ± s.d. (n = 3). Differences between two groups were assessed using unpaired two-tailed Student’s t-test. (e,f) C57BL/6 mice received intraperitoneal injection of aspirin (25 mg/kg) 30 min prior to intraperitoneal injection of LPS (10 mg/kg). Blood was collected before or at 1, 2, 4, and 8 hours after LPS injection. TNF-α (e) and IL-6 (f) concentrations in plasma were measured. Values are means ± s.d. (n = 6). Differences between two groups were assessed using unpaired two-tailed Student’s t-test. (g) C57BL/6 mice received intraperitoneal injection of aspirin (25 mg/kg) at 30 min prior to intraperitoneal injection of LPS (10 mg/kg). Survival rate was observed for 3 days. P value was calculated by Wald test in a discrete time hazard model using version 9.2 of SAS software.
Discussion
Growing evidence indicates that platelets are key effectors in many inflammatory diseases. In sepsis, thrombocytopenia is a frequent complication and closely associates with increased mortality6,7. Whether thrombocytopenia is a marker for more disseminated disease or a contributor to poor outcomes is not known. In the present study, we demonstrate that antibody-induced thrombocytopenia in mice increases mortality in a LPS-induced endotoxemia and in a bacterial infusion sepsis model. Depletion of platelets worsened organ injury during septic shock, whereas transfusion of platelets significantly reduced mortality. These findings imply that platelets play a beneficial role in sepsis and protect against septic shock.
TNF-α plays a key role in sepsis pathogenesis21,22,25. Platelets potentiate macrophage-derived TNF-α production in response to low-dose LPS (≤100 pg/ml) under conditions where TNF-α increased only several fold over the baseline level38,39. Platelets also potentiate TNF-α production and inflammation in the mice injected with low-dose LPS40,41. While we observed that platelets enhanced low-dose LPS (1 ng/ml)-elicited TNF-α production, our findings demonstrate that platelets dramatically inhibit high-dose LPS (≥10 ng/ml)-induced TNF-α and IL-6 production by macrophages. Platelets also inhibited human macrophage-derived TNF-α production (Supplementary Fig. S4). Therefore, platelets appear to serve as a switch for the inflammatory response during sepsis in that they may promote inflammation at early stage of infection, but inhibit macrophage-dependent inflammation when plasma concentrations of LPS are high. Plasma LPS concentration, which can reach several ng/ml to over 100 µg/ml, in severe sepsis carries prognostic significance. High LPS concentrations are associated with increased adverse outcome and higher mortality rate42,43.
Plasma IL-6 also predicts mortality in sepsis24,44. Although antibiotics decrease overall mortality of septic mice, all animals with IL-6 levels in plasma greater than 14.0 ng/mL die, regardless of whether they receive antibiotics24, suggesting that uncontrolled inflammation is an important cause of mortality. Interestingly, in the bacterial infusion sepsis model, we observed 100% mortality in control mice (injected with IgG) that had plasma IL-6 concentrations > 14.0 ng/mL, whereas all of the mice with plasma IL-6 concentrations < 14.0 ng/mL survived. Plasma IL-6 levels in thrombocytopenic mice increased 10-and 29-fold over control animals (> 63 ng/mL and 176 ng/mL, respectively) at 4 and 8 hours after injection of bacteria. TNF-α concentrations in thrombocytopenic mice increased 77-fold over controls at two hours after injection of bacteria. These data indicate that platelets play an essential role in modulating inflammation during sepsis, and that thrombocytopenia, which often occurs in severe septic patients, may be an important cause for the uncontrolled inflammation and death.
A previous study reported lethal lung hemorrhage in thrombocytopenic mice challenged with LPS19. In that study, the anti-GPIbα monoclonal antibody was injected intravenously, which resulted in a lowering of platelet count to less than 2.5% of that of control mice. They then performed platelet transfusion studies to determine the minimal concentration of platelets that was required for preventing inflammatory bleeding. Platelets from IL4Rα/GPIbα transgenic mice, which were resistant to the anti-GPIbα antibody, were injected into the inflamed mice. It was found that 10–15% of normal platelet counts was sufficient to prevent inflammatory bleeding19. In our study, thrombocytopenia was elicited by intraperitoneal injection of an anti-GPIbα monoclonal antibody, which lowered platelet counts to ~10% of control. Consistent with previous studies, which reported that platelet counts of 10% – 15% normal were sufficient to prevent inflammatory bleeding19,45, no significant hemorrhage was observed in lungs and brains from the thrombocytopenic mice. Therefore, in the present study, the increased mortality from sepsis in thrombocytopenic mice does not appear to be due to inflammatory hemorrhage, but rather to an exacerbated inflammatory response. In order reconcile previous findings19,20 with our data, we induced thrombocytopenia in mice by retro-orbital injection of the anti-GPIbα antibody. In agreement with the previous studies, retro-orbital injection of the anti-GPIbα antibody induced much severe thrombocytopenia (platelet counts were reduced to < 5% of that of control mice), and injection of LPS caused severe lung hemorrhage (Supplementary Fig. S5).
Our in vitro and in vivo data suggest that the beneficial effect of platelets is likely due to COX1-dependent generation of PGE2 that acts on macrophage EP4 receptors. PGE2 is a key macrophage modulator36,46–48. LPS stimulates COX2 expression and PGE2 production in many cells by up-regulating COX2 transcription through NF-κB-dependent pathways37,49,50. However, COX1 is the dominant isoform in platelets. Previous studies reported that pretreatment with nonsteroidal anti-inflammatory drugs (NSAIDs) enhances endotoxin-induced production of TNFα, IL-6, and IL-8 concentrations in blood of human volunteers51,52. Consistent with these findings, aspirin pre-treated mice had significantly higher plasma TNF-α and IL-6 concentrations and reduced survival rate than that of control mice in the LPS-induced sepsis model. COX metabolizes arachidonic acid to many biologically active eicosanoids such as prostaglandins, prostacyclins and thromboxane, and both COX1 and COX2 are activated during sepsis. However, they appear to play distinct roles in septic shock. While aspirin reduced survival rate in LPS-challenged mice, a COX2 specific inhibitor NS-398 increased survival rate in LPS-induced endotoxemia53, suggesting that in contrast to COX1, COX2-derived products aggravate sepsis. Platelet-specific deletion ofCOX1 or the PGE2 synthase may be helpful in further evaluating the protective role of platelets in septic shock. Our data do suggest that other pathways may also be involved in platelet inhibition of macrophage function, because aspirin or COX1 deficiency only partially reversed platelet inhibition of LPS-induced TNF-α production by macrophages (Fig. 6a,b). In fact, following injection of COX1 deficient platelets, plasma TNF-α concentrations were lower, although to a less degree than observed after injection of wild-type platelets (Fig. 7a). In this regard, a previous study showed that platelet-derived microparticles reduced the release of TNF-α by macrophages activated with LPS54.
Platelet transfusion has been recommended in septic patients with severe thrombocytopenia, in particular, in patients with bleeding or in patients at risk for bleeding55. Historically, the purpose of platelet transfusion in sepsis is to halt or prevent bleeding. Our results suggest a potential beneficial effect of platelet transfusion in modulating immune response in sepsis, especially in the severe septic patients. Further studies of platelet transfusion in high-risk sepsis patients may be warranted.
Methods
Mice
Mice deficient in Gαq29, P2Y1256, TP57, Munc 13-4 (Unc13dJinx mice)35, COX158 and wild-type littermates from heterozygous breeding were used in this study. C57BL/6J mice were purchased from Jackson Laboratories. Mice were bred and maintained in the University of Kentucky Animal Care Facility following institutional and National Institutes of Health guidelines after approval by the Institutional Animal Care and Use Committee. Eight to twelve week-old males with a variety of genetic manipulations were used in most experiments.
Preparation of mouse platelets
Blood was collected from abdominal aortas of isofluorane-anesthetized mice (8–10 weeks) using 1/7 volume of ACD (85 mM trisodium citrate, 83 mM dextrose, and 21 mM citric acid) as anticoagulant59. Platelets were then washed once with CGS (0.12 M sodium chloride, 0.0129 M trisodium citrate, 0.03 M D-glucose, pH 6.5), resuspended in saline at 5 × 109/ml for in vivo experiments or in RPMI 1640 medium containing 10 mM HEPES at 3 × 108/ml for in vitro experiments, and incubated for 1 h at 22°C before use.
Survival studies
LPS endotoxemia model: Male mice (8–10 weeks old) were injected intraperitoneally with 10 mg/kg LPS (0111:B4, purchased from Sigma) in a volume of 200 µl that was diluted in saline. Survival after LPS challenge was assessed every 12 h for 3 days. All survived mice were euthanized at the end of the third day.
Bacterial infusion sepsis model: E. coli ATCC 25922 was grown in LB medium overnight. After washed once with sterile saline, the bacteria were resuspended and diluted in sterile saline. Male mice (8–10 weeks old) were injected intraperitoneally with bacteria in a volume of 200 µl saline. Survival after injection of bacteria was assessed every 4 h for 3 days. All survived mice were euthanized at the end of the third day.
Platelet depletion and transfusion
Mice were injected intraperitoneally with a commercially available rat anti-mouse GPIbα monoclonal antibody (Emfret Analytics, Wurzburg, Germany, 4 µg/g body weight) for platelet depletion. Mice were injected with same amount of isotype-matched rat IgG as controls. For platelet counts, mice were bled from the retro-orbital plexus under isoflurane anesthesia (IsoFlo; Abbott Laboratories, Abbott Park, IL). Blood was collected into a heparin-containing tube, and added with 10 mM (final concentration) EDTA as anticoagulant. Platelet counts in whole blood were analyzed with a HEMAVET HV950FS multispecies hematology analyzer. For platelet transfusion, 1 × 109 washed platelets resuspended in 0.2 ml saline were injected into the retro-orbital plexus for each recipient mouse.
Macrophage depletion
Mice were injected retro-orbitally with 40 mg/kg of liposomal clodronate (L-α-Phosphatidyl-choline/cholesterol clodronate; Encapsula NanoSciences, Nashville, TN) at 24 and 4 hours, respectively before challenged with LPS. Same amount of plain liposomes were injected as control. This method is specific for depletion of monocytes and macrophages, which undergo apoptosis following phagocytosis of the liposomal clodronate. Depletion of monocytes/macrophages was confirmed by flow cytometry asnalysis. EDTA anticoagulated blood was subjected to red blood cell lysis. White blood cells were resuspended in flow buffer (Hank's Balanced Salt Solution with 0.1% BSA and 5 mM EDTA), and incubated with Alexa Fluor 488-labeled anti-CD115 and APC-Cy™ 7-labeled anti-CD45 antibodies.
Isolation of Macrophages
Macrophages were isolated from mouse bone marrow and plated in a plastic flask60. Bone marrow macrophages were cultured for 7 days in the presence of 15%conditional medium from L929 cells.
Blood chemistry and cytokine measurements
AST, ALT, CK, and LDH were measured by Comparative Clinical Pathology Services (Columbia, MO). TNF-α and IL-6were measured by ELISA (eBioscience, San Diego, CA). To observe the effects of platelets on TNF-α and IL-6 production in macrophages, washed platelets or medium were incubated with LPS (100 ng/ml) for 30 min and added to 2 × 105 macrophages pre-coated in 96-well plates. To observe the effects of platelet releasates on TNF-α and IL-6 production in macrophages, washed platelets were incubated with LPS (100 ng/ml) at room temperature for 30 minor thrombin (0.1 U/ml) in aggregometer with stirring at 1000 rpm at 37°C for 5 min, and then centrifuge at 2,500 rpm at 22°C for 3 min. Supernatants from LPS- or thrombin-stimulated platelets were added with LPS to 96-well plates pre-coated with 2 × 105 macrophages/well, and incubated at 37°C for6 hours.
Histologic examination
Mice were injected intraperitoneally with an anti-mouse GPIbα monoclonal antibody or control IgG. After 4 hours, LPS (10 mg/kg) was injected i.p. Mice were anesthetized and lungs and brains were isolated at 15 hours after injection of LPS. Lungs and brains were fixed with 4% paraformaldehyde in 0.1 m phosphate buffer (pH 7.4) for 2 days at 4°C and then processed to paraffin embedding. Sections were cut at 5 µm and stained by hematoxylin and eosin (H&E). Images were captured with a Leica DM IRB microscope using 20 × objective (Leica Microsystems, Wetzlar, Germany).
Statistical Analyses
The survival assay was analyzed by Wald test in a discrete time hazard model using version 9.2 of SAS software. Significance in experiments comparing two groups was examined by unpaired two-tailed Student's t test. Values are reported as the mean ± S.D. A value of p < 0.05 was considered significant.
Supplementary Material
Acknowledgments
This work is supported by American Heart Association Midwest Affiliate Grant-in-Aid 0855698G (to Z.L.), and in part supported by the American Society of Hematology (ASH) bridge Grant Award and the NIH/National Center for Research Resources Centers of Biomedical Research Excellence in Obesity and Cardiovascular Disease Grant P20 RR021954. B. Xiang is a recipient of the American Heart Association Postdoctoral Fellowship Award. This work was support in part by resources provided by the Lexington VA Medical Center. We would like to thank Dr. Richard Charnigo for his assistance with statistical analysis and Dr. Wendy S. Katz for her technical support for the histologic analysis. We would like to thank Dr. Prabhakara R. Nagareddy and Judy F. Glass for their assistance with detection of monocyte depletion by flow cytometry.
Footnotes
Contributions
B.X. and Z.L. designed the research; Z. L. supervised the study; B.X. and G.Z. carried out most of the experiments; L.G. and X.L. carried out some experiments of in vivo sepsis model; S. W. W. provided Jinx mice and helped with analysis of secretion data; A. J. M. helped with PGE2 measurement; A. J. M., A.D., S.S.S. provided extra technical assistance; S.S.S. provided valuable suggestions; B.X., A.D., S.S.S, and Z.L. wrote the manuscript. All authors commented on the manuscript.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 2.Li Z, Yang F, Dunn S, Gross AK, Smyth SS. Platelets as immune mediators: their role in host defense responses and sepsis. Thromb Res. 2011;127:184–188. doi: 10.1016/j.thromres.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russwurm S, et al. Platelet and leukocyte activation correlate with the severity of septic organ dysfunction. Shock. 2002;17:263–268. doi: 10.1097/00024382-200204000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Levi M. Platelets in sepsis. Hematology. 2005;10(Suppl 1):129–131. doi: 10.1080/10245330512331390177. [DOI] [PubMed] [Google Scholar]
- 5.Akca S, et al. Time course of platelet counts in critically ill patients. Crit Care Med. 2002;30:753–756. doi: 10.1097/00003246-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Sharma B, et al. Thrombocytopenia in septic shock patients--a prospective observational study of incidence, risk factors and correlation with clinical outcome. Anaesth Intensive Care. 2007;35:874–880. doi: 10.1177/0310057X0703500604. [DOI] [PubMed] [Google Scholar]
- 7.Moreau D, et al. Platelet count decline: an early prognostic marker in critically ill patients with prolonged ICU stays. Chest. 2007;131:1735–1741. doi: 10.1378/chest.06-2233. [DOI] [PubMed] [Google Scholar]
- 8.Andonegui G, et al. Platelets express functional Toll-like receptor-4. Blood. 2005;106:2417–2423. doi: 10.1182/blood-2005-03-0916. [DOI] [PubMed] [Google Scholar]
- 9.Clark SR, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 10.Ma AC, Kubes P. Platelets, neutrophils, and neutrophil extracellular traps (NETs) in sepsis. J Thromb Haemost. 2008;6:415–420. doi: 10.1111/j.1538-7836.2007.02865.x. [DOI] [PubMed] [Google Scholar]
- 11.Evangelista V, et al. Src family kinases mediate neutrophil adhesion to adherent platelets. Blood. 2007;109:2461–2469. doi: 10.1182/blood-2006-06-029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vieira-de-Abreu A, Campbell RA, Weyrich AS, Zimmerman GA. Platelets: versatile effector cells in hemostasis, inflammation, and the immune continuum. Semin Immunopathol. 2012;34:5–30. doi: 10.1007/s00281-011-0286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weyrich AS, McIntyre TM, McEver RP, Prescott SM, Zimmerman GA. Monocyte tethering by P-selectin regulates monocyte chemotactic protein-1 and tumor necrosis factor-alpha secretion. Signal integration and NF-kappa B translocation. J Clin Invest. 1995;95:2297–2303. doi: 10.1172/JCI117921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weyrich AS, et al. Activated platelets signal chemokine synthesis by human monocytes. J Clin Invest. 1996;97:1525–1534. doi: 10.1172/JCI118575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dixon DA, et al. Expression of COX-2 in platelet-monocyte interactions occurs via combinatorial regulation involving adhesion and cytokine signaling. J Clin Invest. 2006;116:2727–2738. doi: 10.1172/JCI27209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev. 2009;23:177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cloutier N, et al. Platelets can enhance vascular permeability. Blood. 2012;120:1334–1343. doi: 10.1182/blood-2012-02-413047. [DOI] [PubMed] [Google Scholar]
- 18.Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest. 2009;119:2868–2878. doi: 10.1172/JCI39421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goerge T, et al. Inflammation induces hemorrhage in thrombocytopenia. Blood. 2008;111:4958–4964. doi: 10.1182/blood-2007-11-123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boulaftali Y, et al. Platelet ITAM signaling is critical for vascular integrity in inflammation. J Clin Invest. 2013;123:908–916. doi: 10.1172/JCI65154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tracey KJ, Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]
- 22.Parameswaran N, Patial S. Tumor necrosis factor-alpha signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20:87–103. doi: 10.1615/critreveukargeneexpr.v20.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kruttgen A, Rose-John S. Interleukin-6 in sepsis and capillary leakage syndrome. J Interferon Cytokine Res. 2012;32:60–65. doi: 10.1089/jir.2011.0062. [DOI] [PubMed] [Google Scholar]
- 24.Turnbull IR, et al. Antibiotics improve survival in sepsis independent of injury severity but do not change mortality in mice with markedly elevated interleukin 6 levels. Shock. 2004;21:121–125. doi: 10.1097/01.shk.0000108399.56565.e7. [DOI] [PubMed] [Google Scholar]
- 25.Pfeffer K, et al. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 26.Flynn JL, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 27.Esmon CT, et al. Inflammation, sepsis, and coagulation. Haematologica. 1999;84:254–259. [PubMed] [Google Scholar]
- 28.Levi M, van der Poll T, Schultz M. Systemic versus localized coagulation activation contributing to organ failure in critically ill patients. Semin Immunopathol. 2012;34:167–179. doi: 10.1007/s00281-011-0283-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Offermanns S, Toombs CF, Hu YH, Simon MI. Defective platelet activation in G alpha(q)-deficient mice. Nature. 1997;389:183–186. doi: 10.1038/38284. [DOI] [PubMed] [Google Scholar]
- 30.Ren Q, Ye S, Whiteheart SW. The platelet release reaction: just when you thought platelet secretion was simple. Curr Opin Hematol. 2008;15:537–541. doi: 10.1097/MOH.0b013e328309ec74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shashkin PN, Brown GT, Ghosh A, Marathe GK, McIntyre TM. Lipopolysaccharide is a direct agonist for platelet RNA splicing. J Immunol. 2008;181:3495–3502. doi: 10.4049/jimmunol.181.5.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cognasse F, et al. Toll-like receptor 4 ligand can differentially modulate the release of cytokines by human platelets. Br J Haematol. 2008;141:84–91. doi: 10.1111/j.1365-2141.2008.06999.x. [DOI] [PubMed] [Google Scholar]
- 33.Zhang G, et al. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J Immunol. 2009;182:7997–8004. doi: 10.4049/jimmunol.0802884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahner BN, Shankar H, Murugappan S, Prasad GL, Kunapuli SP. Nucleotide receptor signaling in platelets. J Thromb Haemost. 2006;4:2317–2326. doi: 10.1111/j.1538-7836.2006.02192.x. [DOI] [PubMed] [Google Scholar]
- 35.Ren Q, et al. Munc13-4 is a limiting factor in the pathway required for platelet granule release and hemostasis. Blood. 2010;116:869–877. doi: 10.1182/blood-2010-02-270934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Strassmann G, Patil-Koota V, Finkelman F, Fong M, Kambayashi T. Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J Exp Med. 1994;180:2365–2370. doi: 10.1084/jem.180.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu D, Meydani SN. Mechanism of age-associated up-regulation in macrophage PGE2 synthesis. Brain Behav Immun. 2004;18:487–494. doi: 10.1016/j.bbi.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 38.Aiura K, et al. Interaction with autologous platelets multiplies interleukin-1 and tumor necrosis factor production in mononuclear cells. J Infect Dis. 1997;175:123–129. doi: 10.1093/infdis/175.1.123. [DOI] [PubMed] [Google Scholar]
- 39.Scull CM, Hays WD, Fischer TH. Macrophage pro-inflammatory cytokine secretion is enhanced following interaction with autologous platelets. J Inflamm (Lond) 2010;7:53. doi: 10.1186/1476-9255-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aslam R, et al. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood. 2006;107:637–641. doi: 10.1182/blood-2005-06-2202. [DOI] [PubMed] [Google Scholar]
- 41.Looney MR, et al. Platelet depletion and aspirin treatment protect mice in a two-event model of transfusion-related acute lung injury. J Clin Invest. 2009;119:3450–3461. doi: 10.1172/JCI38432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Opal SM, et al. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J Infect Dis. 1999;180:1584–1589. doi: 10.1086/315093. [DOI] [PubMed] [Google Scholar]
- 43.Behre G, et al. Endotoxin concentration in neutropenic patients with suspected gram-negative sepsis: correlation with clinical outcome and determination of anti-endotoxin core antibodies during therapy with polyclonal immunoglobulin M-enriched immunoglobulins. Antimicrob Agents Chemother. 1992;36:2139–2146. doi: 10.1128/aac.36.10.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17:463–467. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Loria GD, Romagnoli PA, Moseley NB, Rucavado A, Altman JD. Platelets support a protective immune response to LCMV by preventing splenic necrosis. Blood. 2013;121:940–950. doi: 10.1182/blood-2011-08-376822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu XJ, Reichner JS, Mastrofrancesco B, Henry WL, Jr, Albina JE. Prostaglandin E2 suppresses lipopolysaccharide-stimulated IFN-beta production. J Immunol. 2008;180:2125–2131. doi: 10.4049/jimmunol.180.4.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakamoto A, Matsumura J, Mii S, Gotoh Y, Ogawa R. A prostaglandin E2 receptor subtype EP4 agonist attenuates cardiovascular depression in endotoxin shock by inhibiting inflammatory cytokines and nitric oxide production. Shock. 2004;22:76–81. doi: 10.1097/01.shk.0000129338.99410.5d. [DOI] [PubMed] [Google Scholar]
- 48.Nemeth K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D'Acquisto F, et al. Involvement of NF-kappaB in the regulation of cyclooxygenase-2 protein expression in LPS-stimulated J774 macrophages. FEBS Lett. 1997;418:175–178. doi: 10.1016/s0014-5793(97)01377-x. [DOI] [PubMed] [Google Scholar]
- 50.Abate A, Oberle S, Schroder H. Lipopolysaccharide-induced expression of cyclooxygenase-2 in mouse macrophages is inhibited by chloromethylketones and a direct inhibitor of NF-kappa B translocation. Prostaglandins Other Lipid Mediat. 1998;56:277–290. doi: 10.1016/s0090-6980(98)00061-6. [DOI] [PubMed] [Google Scholar]
- 51.Martich GD, Danner RL, Ceska M, Suffredini AF. Detection of interleukin 8 and tumor necrosis factor in normal humans after intravenous endotoxin: the effect of antiinflammatory agents. J Exp Med. 1991;173:1021–1024. doi: 10.1084/jem.173.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spinas GA, Bloesch D, Keller U, Zimmerli W, Cammisuli S. Pretreatment with ibuprofen augments circulating tumor necrosis factor-alpha, interleukin-6, and elastase during acute endotoxinemia. J Infect Dis. 1991;163:89–95. doi: 10.1093/infdis/163.1.89. [DOI] [PubMed] [Google Scholar]
- 53.Reddy RC, et al. Selective inhibition of COX-2 improves early survival in murine endotoxemia but not in bacterial peritonitis. Am J Physiol Lung Cell Mol Physiol. 2001;281:L537–L543. doi: 10.1152/ajplung.2001.281.3.L537. [DOI] [PubMed] [Google Scholar]
- 54.Sadallah S, Eken C, Martin PJ, Schifferli JA. Microparticles (ectosomes) shed by stored human platelets downregulate macrophages and modify the development of dendritic cells. J Immunol. 2011;186:6543–6552. doi: 10.4049/jimmunol.1002788. [DOI] [PubMed] [Google Scholar]
- 55.Zimmerman JL. Use of blood products in sepsis: an evidence-based review. Crit Care Med. 2004;32:S542–S547. doi: 10.1097/01.ccm.0000145906.63859.1a. [DOI] [PubMed] [Google Scholar]
- 56.Foster CJ, et al. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001;107:1591–1598. doi: 10.1172/JCI12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas DW, et al. Coagulation defects and altered hemodynamic responses in mice lacking receptors for thromboxane A2. J Clin Invest. 1998;102:1994–2001. doi: 10.1172/JCI5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Langenbach R, et al. Prostaglandin synthase 1 gene disruption in mice reduces arachidonic acid-induced inflammation and indomethacin-induced gastric ulceration. Cell. 1995;83:483–492. doi: 10.1016/0092-8674(95)90126-4. [DOI] [PubMed] [Google Scholar]
- 59.Zhang G, et al. Distinct roles for Rap1b protein in platelet secretion and integrin alphaIIbbeta3 outside-in signaling. J Biol Chem. 2011;286:39466–39477. doi: 10.1074/jbc.M111.239608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davies JQ, Gordon S. Isolation and culture of murine macrophages. Methods Mol Biol. 2005;290:91–103. doi: 10.1385/1-59259-838-2:091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.