Abstract
The function of the CA2 region of the hippocampus is poorly understood. While the CA1 and CA3 regions have been extensively studied, for years the CA2 region has primarily been viewed as a linking area between the two. However, the CA2 region is known to have distinct neurochemical and structural features that are different from the other parts of hippocampus and in recent years it has been suggested that the CA2 region may play a role in the formation and or recall of olfactory-based memories needed for normal social behavior. While this hypothesis has been supported by hippocampal lesion studies that have included the CA2 region, no studies have attempted to specifically lesion the CA2 region of the hippocampus in mice to determine the effects on social recognition memory and olfaction. To fill this knowledge gap, we sought to perform excitotoxic N-methyl-D aspartate (NMDA) lesions of the CA2 region in mice and determine the effects on social recognition memory. We predicted that lesions of the CA2 region would impair social recognition memory. We then went on to test olfaction in CA2 lesioned mice since social memory requires a functional olfactory system. Consistent with our prediction, we found that CA2 lesioned animals have impaired social recognition. These findings are significant because they confirm that the CA2 region of the hippocampus is a part of the neural circuitry that regulates social recognition memory, which may have implications for our understanding of the neural regulation of social behavior across species.
Keywords: social recognition, olfaction, excitotoxic, behavior
Introduction
While the hippocampus is known to play a crucial role in the formation and recall of memories, little is known about the function of the CA2 region. Serving as a link between the CA1 and CA3 regions (Sekino et al., 1997), the CA2 region is structurally and functionally distinct (Lein et al., 2004; Lein et al., 2005). For example, it does not receive rich mossy fiber inputs from the dentate gyrus and lacks the thorny excrescences that are characteristic of mossy fiber synapses (Lorente de No, 1934; Tamamaki et al., 1988). It exclusively expresses a variety of neurochemicals and receptors, such as fibroblast growth factor 2 (Williams et al., 1996), neurotrophin-3 (Vigers et al., 2000), and vasopressin 1b receptors (Avpr1b) (Young et al., 2006). It is also the only part of the hippocampus to receive input from the posterior hypothalamus (Borhegyi & Leranth, 1997; Vertes & McKenna, 2000; Bartesaghi et al., 2006) and the perforant pathway; which connects the entorhinal cortex to the hippocampal formation (Bartesaghi & Gessi, 2004).
A role for the CA2 region in the neural regulation of mammalian social behavior has been previously suggested, since rodents with extensive hippocampal lesions, that include the CA2 region, display reduced aggression and impaired social recognition (Ely et al., 1977a; Maaswinkel et al., 1996; McHugh et al., 2004; Uekita & Okanoya, 2011). However, it is only recently that the importance of the CA2 region in the neural regulation of social behavior has begun to be confirmed. Pagani and colleagues (2014) have shown that the replacement of Avpr1b in the dorsal CA2 region of Avpr1b knockout mice is able to restore attack behaviors and Hitti and Siegelbaum (2014) have shown that genetic inactivation of CA2 pyramidal cells impairs social recognition memory without impacting other forms of hippocampal-dependent memory.
Since social behavior is dependent on the ability to perceive social cues, process them, and elicit the appropriate behavioral response, rodent social interactions largely depend on a functional olfactory system (Ropartz, 1968; Matochik, 1988; Popik et al., 1991). Further, inputs from the olfactory system to the entorhinal cortex, which has projections to the CA1 region of the hippocampus, are important in the coding of olfactory-based memory formation (Petrulis et al., 2005; reviewed in Sanchez-Andrade et al., 2005). Based on these data, we as well as others have hypothesized that the CA2 region of the hippocampus may aid in the formation and/or recall of accessory olfactory-based memories (Young et al., 2006; Caldwell et al., 2008b; Stevenson & Caldwell, 2012).
Unfortunately, previous lesion studies were generally focused on damaging other areas of the hippocampus. For this reason we set out to lesion only the dorsal third of the CA2 region and examine the effects on social recognition and olfaction. We hypothesized that bilateral lesions of CA2 would affect social recognition memory, as measured by the inability to discriminate between a novel and a familiar stimulus animal, but would have no effect on general olfaction.
Methods and Materials
Animals and Housing
Experimental animals were sexually naïve adult male C57BL/6J mice bred in the Kent State University vivarium (Experiment 1) or ordered from The Jackson Laboratory (Bar Harbor, ME) (Experiments 2-4) and stimulus animals were adult female Swiss Webster mice (Jackson Laboratory, Bar Harbor, ME). All animals were kept on a 12:12 light: dark cycle, with lights out at 14:00 hours. C57BL/6J male mice were selected as experimental animals because Avpr1b knockout mice, which were the impetus of this work, have C57BL/6J in their background and much of the work on these mice has been performed in males (Wersinger et al., 2002; Wersinger et al., 2004; Caldwell et al., 2006; Wersinger et al., 2007a; Caldwell & Young, 2009; Caldwell et al., 2010). Swiss Webster female mice were selected as stimulus animals because they are an outbred strain; thus, making the social recognition memory test more rigorous (Macbeth et al., 2009a). At the time of testing, no attempt was made to determine the estrous phase of individual females. This choice was made because some previous work has shown the use of cycling females can result in robust and reliable testing of social recognition memory (Macbeth et al., 2009a) and that when the males are prevented from mounting the females, that the specific day of the estrous cycle does not have much of an effect in terms of investigation time by males (Ingersoll & Weinhold, 1987; Muroi et al., 2006); with sexually naïve males demonstrating no preference for receptive versus non-receptive female odors (Carr et al., 1965). At the time of weaning (postnatal day 18-21), or upon their arrival from Jackson Laboratory, animals were housed in same-sex sibling groups until surgery. Food and water were provided ad libitum, except during testing and prior to the hidden cookie test. All subjects were 2 to 6 months of age at time of testing. Two sets of surgical animals were generated; the first set for Experiment 1 (N=35) and the second set for Experiments 2-4 (N=19). All experiments were conducted in accordance with the National Institutes of Health guidelines on the care and use of animals using an animal study protocol approved by the Kent State University Institutional Animal Care and Use Committee.
Lesion Surgeries
Prior to surgery animals were randomly assigned to one of the following surgical groups: 1) bilateral excitotoxic lesion of the CA2 region 2) sham surgery of the CA2 region (surgical control) or 3) bilateral excitotoxic lesion of the striatum (lesion control for Experiment 1 only). At the time of surgery animals were anesthetized using a 2% isoflurane/oxygen mixture. Using an Ultraprecise stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) and a 2μl Hamilton syringe (Hamilton Company, Reno, NV), groups of C57BL/6J male mice either underwent bilateral, site-specific microinjections of 10mg/ml NMDA solution or a bilateral sham operation in which the needle was inserted but no injection administered. For CA2 injections, a total of 6 separate injections (20nl of NMDA per injection) were performed, bilaterally, in order to localize the lesion to the dorsal third of the CA2 region. This area of CA2 was selected because the Avpr1b is prominently expressed here and is known to be important for normal social recognition memory (Wersinger et al., 2002; Young et al., 2006; Caldwell et al., 2008a; Caldwell et al., 2008b). The coordinates used were: from Bregma (a) ML = ±1.3, AP = −1.3, DV = −1.45 (b) ML = ± 1.5, AP = −1.5, DV = −1.5 (c) ML = ± 1.9, AP = −1.8, DV = −1.6 (from top of the brain). For striatum injections 40nl of NMDA was injected bilaterally at the following coordinates: from Bregma ML = ±1.9, AP = +0.7, DV = −2.5 (from the top of the brain). Burr holes were made using a hand-held drill (Dremel®, Racine, WI) with an engraving cutter bit (model #105, Dremel®, Racine, WI). Once the burr hole was produced, the needle tip was placed at the appropriate depth and allowed to sit for 2 minutes prior to injection to allow the brain to reposition. Each injection took place over approximately 30 seconds and then the needle remained in place for another 2 minutes; allowing the NMDA time to diffuse away from the needle tip. The needle was then slowly removed, the skin brought back together over the skull and closed with a wound clip. Following surgery, animals were administered 0.3ml of warm saline (0.9%) intraperitoneally (i.p.) to aid in recovery and returned to their home cage. To prevent seizures, lesioned animals were administered chlordiazepoxide, i.p., at a dose of 10mg/kg immediately after surgery. All animals were allowed to recover for at least 1 week before behavioral testing and wound clips removed at the end of that week.
Experiment 1: 2-Trial Social Discrimination Test
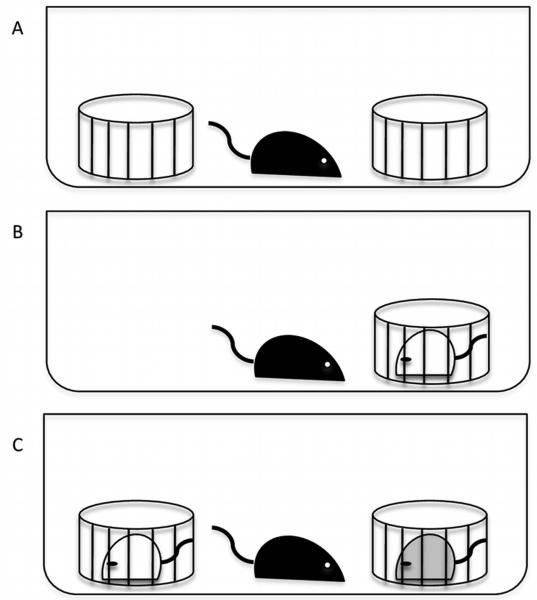
One week before testing, the stimulus animals (gonadally-intact female Swiss Webster mice ages 2-4 months) were single-housed while the 35 surgical animals remained group housed. On the day of testing, all animals were moved to the testing room at time of lights out. Experimental animals were then separated into individual clean cages and allowed to habituate for 30 minutes. After the 30-minute habituation, 2 clean wire corrals were placed inside the cage on opposite ends and the animals were then allowed to habituate for another 30 minutes (Figure 1A). Testing commenced 1 hour after lights out under dim red light illumination during the dark phase of the light: dark cycle. At the start of Trial 1, one of the corrals was removed and a stimulus animal was placed in the remaining corral for 5 minutes (Figure 1B). During this time the experimental animal was videotaped. The stimulus animal was then designated as the “familiar” animal and removed for 30 minutes, returned to its home cage and the second corral placed back into the experimental animal’s test cage (Figure 1A). During Trial 2, the “familiar” female from Trial 1 was placed back into its original corral, which had been moved to the opposite side of the cage, and a second “novel” female was placed into the remaining corral (Figure 1C); this test was also videotaped for 5 minutes. The videotapes were later scored using Noldus Observer software (Leesburg, VA) by an observer blind to the surgical treatments. The amount of time spent investigating each stimulus female, i.e. any time the experimental animal would insert their nose and/or forepaw through the bars of the corral or sniff any portion of the stimulus animal that was outside of the corral, duration of non-social behavior (defined as the time the resident spent investigating the cage and self-grooming (as previously described in Dhakar et al., 2012)), and the frequency of tail rattles were scored.
Figure 1.
The 2-Trial social discrimination test used in Experiment 1. A) An illustration of the placement of the corrals in the test cage during the 30-minute habituation period. B) An illustration of Trial 1 in which the experimental animal is exposed to a single corralled female for 5 minutes. C) An illustration of Trial 2 in which the experimental animal is exposed to both the “familiar” female (white) from Trial 1 and the “novel” female (grey) for 5 minutes, following a 30-minute inter-trial interval.
Experiment 2: 11-Trial Habituation/Dishabituation Social Recognition Test
One week prior to testing, the stimulus animals (gonadally-intact female Swiss Webster mice ages 2-4 months) were single-housed while the 20 surgical animals were kept group housed. On the day of testing, all experimental and stimulus animals were moved to the testing room at the time of lights out. The experimental animals were then individually housed in clean cages with a clean wire corral placed in the center of the cage. All animals were allowed to habituate to their new environment for 1 hour. At the time of testing, a randomly assigned stimulus female was placed into the corral for 1 minute (Trial 1) and the experimental animal was observed for olfactory investigation of the female. After 1 minute the stimulus animal was removed and placed back into its home cage for 5 minutes. This series of exposures occurred 9 more times with the same “familiar” stimulus female for a total of 10 1-minute trials with an inter-trial interval of 5 minutes (habituation). On the eleventh trial, a “novel” stimulus female was placed into the wire corral for 1 minute (dishabituation). All 11 trials were videotaped and scored with the Noldus Observer system (Leesburg, VA) by an observer blind to the surgical treatment. Time spent investigating the stimulus animal (as described above), time spent in non-social behavior (as described above), and tail rattles were scored.
Experiment 3: Hidden Cookie Test
Approximately 12 hours prior to testing, experimental animals were weighed and food-restricted, but not water-restricted, overnight. On the day of testing, all experimental animals were moved to the testing room at the time of lights out and allowed to acclimate to their new environment for 1 hour. Just prior to testing, the mice were again weighed, to ensure that they did not lose more than 10% of their body weight. A small piece of Nabisco Nutter Butter™ cookie (5mm cube) was then randomly placed on either the left or right side of an empty mouse cage and covered with approximately 4mm of woodchip bedding. The subject was then placed into the center of the cage and allowed to investigate for up to 5 minutes, i.e., 300 seconds, or until the cookie was found. Finding the cookie was defined as having both front paws on the cookie piece. The latency to find the hidden cookie was recorded with a stopwatch. If the mouse failed to find the cookie a latency score of 300 seconds was assigned.
Experiment 4: Habituation/Dishabituation Olfactory Test
On the day of testing, experimental animals were moved to the testing room at the time of lights out, housed individually in clean cages, and allowed to acclimate to their new environment for 1 hour. Immediately before use, a cotton-tipped applicator was soaked in the appropriate odorant. Each cotton swab was presented to the subject by suspension from the cage lid, approximately 4cm above the bedding. The odorants (in order: water, almond extract (Market Pantry®, Target Brands, Inc., Minneapolis, MN), vanilla extract (Market Pantry®, Target Brands, Inc., Minneapolis, MN), male urine, and female urine were all presented at a 1:100 dilution) were thawed immediately before use and kept on ice. The urine had previously been collected from male and female mice that were a mixture of C57BL/J6 and 129/SvJ strains by placing 4-5 mice of each sex (at separate times) in an open field for 15 minutes. The floor of the open field was covered with aluminum foil and urine was pipetted off of the floor into a microcentrifuge tube. The urine was then diluted to 1:100 with sterile water, aliquoted, and frozen at −20°C until used for testing. For each animal tested a fresh microcentrifuge tube of each odorant was used. Each odorant was presented for 2 minutes with an intertrial-interval of 1 minute for 3 trials apiece (habituation) before switching to the next novel odorant (dishabituation). So, there were 15 trials in total, which consisted of three trials each of the five odorants. The trials were videotaped and an observer blind to the surgical treatment scored the amount of time spent in olfactory investigation (<1cm proximity) of the cotton swab using the Noldus Observer system (Leesburg, VA).
Immunohistochemistry for NeuN
At the conclusion of behavioral testing all experimental animals were euthanized via cervical dislocation, their brains removed and fixed in 4% paraformaldehyde. The brains remained in fixative at least 24 hours before being cut into two series of 50μm sections using a vibratome (Pelco® 102 vibratome sectioning system, Ted Pella, Inc., Redding, CA) and placed into scintillation vials containing 1X phosphate buffered saline (PBS). The brains were then stained using immunocytochemistry for NeuN so that the extent of the lesions could be determined. Briefly, sections were washed in 1XPBS 2 times for 5 minutes each before blocking with 1X Power Block™ Universal Blocking Reagent (BioGenex, San Ramon, CA) at room temperature (RT) for 30 minutes and washing again in 1XPBS for 5 minutes. The sections were then incubated overnight at 4°C in primary antibody (Millipore™, Temecula, CA, mouse anti-NeuN, A60) diluted to 1:500 with antisera diluent (PBS + 1% normal goat serum + 0.3% Triton X-100). Following incubation in primary antibody the sections were washed in 1XPBS for 5 minutes and then incubated for 1 hour in secondary antibody (biotinylated goat anti-mouse 1:500) from a Mouse Vectastain ABC kit from Vector Laboratories (Burlingame, CA, USA, PK-4002). Following incubation, the tissue was washed in 1XPBS for 5 minutes, incubated in 3% hydrogen peroxide for 20 minutes, and washed again in 1XPBS 2 times for 5 minutes. During this time the ABC complex was prepared according to kit instructions and allowed to sit for 30 minutes. The sections were then incubated in the ABC complex for 1 hour at RT, washed in 1XPBS for 5 minutes, and incubated with diaminobenzidine (DAB) solution for 2-10 minutes (Vector Laboratories, Burlingame, CA, USA, DAB substrate kit, SK-4100). Lastly, sections were washed in 1XPBS 2 times for 5 minutes, mounted onto gel-subbed slides, air dried, and coverslipped.
Lesion Quantification
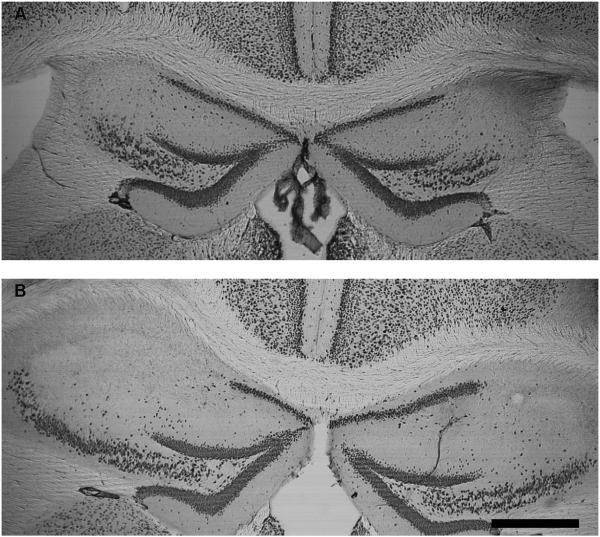
Once the sections were stained for NeuN, the number of sections containing bilateral lesions of the CA2 region were counted from Bregma −0.94 to −2.30 (the dorsal third) and the percentage of CA2 lesioned sections determined (number of lesioned sections/12 * 100 = percent lesioned). Lesions were calculated this way because there were 12 sections from Bregma −0.94 to −2.30 available for histological analysis for each brain. There was an average hit rate of 80% for bilateral CA2 lesions. Representative lesion photomicrographs can be found in Figure 2. For all experiments, only animals with no detectable bilateral lesions were excluded from the statistical analyses. This resulted in one animal being excluded from statistical analysis in Experiment 1 and one animals being excluded from statistical analysis in Experiments 2-4.
Figure 2.
Representative photomicrograph demonstrating the size of lesions caused by injection of 10mg/ml NMDA into the CA2 region of hippocampus and visualized using with immunocytochemistry for NeuN. These are coronal sections of the hippocampus at 50x magnification. A) Illustrates a smaller lesion approximately −1.46mm to Bregma. B) Illustrates a larger lesion approximately −1.70 to Bregma. The scale bar = 0.5mm. (Franklin & Paxinos, 2008)
Statistical Analysis
For Experiment 1, tail rattles, the duration of non-social behavior, and Trial 1 of the 2-Trial social discrimination, were analyzed using a one-way analysis of variance (ANOVA) (SPSS 16.0 for Mac, IBM, Armonk, NY). If a significant effect was found a Fisher’s least significant difference (LSD) post hoc test was used. For Trial 2 of the 2-Trial social discrimination test, a 1-tailed paired-sample Student’s t-test was run within each group to directly compare time spent during Trial 2 with “familiar” versus “novel” females and the changes between trials (as previously used in Macbeth et al., 2009a). For Experiment 2, surgical groups were compared within individual trials using a one-way ANOVA and a repeated measures ANOVA was used to determine if there were trial × surgery differences between trials 10 and 11. For Experiment 3, the latency to find the cookie was analyzed using a one-way ANOVA. For Experiment 4, the time spent in olfactory investigation were compared between surgical groups using a repeated measures ANOVA (trial × surgery). For all statistical tests a result was considered statistically significant if p ≤ 0.05.
Results
Experiments 1 and 2: Social Memory Tests
CA2 sham surgery controls (n = 10), animals with lesions to the CA2 region (n = 15), and animals with lesions to the striatum (n = 10) were tested in a 2-Trial social discrimination test, while a second set of animals (control animals n = 11 and lesioned animals n = 8), were tested in an 11-Trial habituation/dishabituation social recognition test. In the 2-Trial social discrimination test, following site verification and data analysis, one animal was removed from the CA2 sham surgery group because it was a statistical outlier (>2.3 SD from the mean) and one animal was removed from the striatum lesion group because no lesion was observed (mentioned in the above section). In the 11-Trial test, one animal was removed due to an absence of a lesion (mentioned in the section above) and two more were removed due to experimenter error, resulting in n = 11 control animals and n = 6 lesioned animals.
In both tests we found that social recognition was impaired in animals with bilateral lesions to the CA2 region compared to controls. Lesioned animals did not show the same pattern of chemoinvestigation as controls in either test for social memory (Figure 3A-3C). In the first trial of the 2-Trial social discrimination test CA2 sham animals spent significantly more time investigating stimulus females (reported as mean ± standard error of the mean: 86.94±6.86 secs) than CA2 lesioned animals (58.11±5.51 secs) or those with striatum lesions (65.49±5.33 secs, F2, 32 = 6.036 *p = 0.006) (Figure 3A). In the second trial, in which experimental animals were presented with a familiar female from Trial 1, as well as a novel female, the CA2 sham and striatum lesioned animals spent significantly more time with the novel female than the familiar (CA2 sham: 109.90±16.77 secs for the novel female versus 61.74±5.65 secs for the familiar female, t(8) = −2.459, *p = 0.020; Striatum lesion: 79.79± 6.40 secs for the novel female versus 62.91 ± 5.90 secs for the familiar female, t(8) = −1.979, *p = 0.041) indicating the ability to socially discriminate. Conversely, CA2 lesioned animals spent a similar amount of time investigating both stimulus females (66.54±6.77 secs for the novel female versus 75.87±7.44 secs for the familiar female, t(14) = −0.892, p = 0.19) (Figure 3B).
Figure 3.
Social recognition memory in CA2 lesioned and control mice. A) Time spent in olfactory investigation of the familiar female in Trial 1 of the 2-trial social discrimination test. The CA2 sham group spent significantly more time in chemoinvestigation than the lesion groups (*p = 0.006), as determined by a one-way analysis of variance (ANOVA). B) The time spent in olfactory investigation of the stimulus females in Trial 2 of the 2-trial social discrimination test. The CA2 sham and striatum lesion groups demonstrated normal social recognition memory by spending significantly more time with the novel vs. familiar female (*p = 0.02 for CA2 sham and *p = 0.041 for striatum lesion) while the CA2 lesion group showed impaired social recognition memory (p = 0.19), as determined by a 1-tailed paired-sample Student’s t-test. C) The time spent in olfactory investigation of the stimulus female in each trial of an 11-trial habituation/dishabituation social recognition test. In the control group there was a significant decrease in the time spent in olfactory investigation between the surgical groups in Trial 10 and an increase in Trial 11 (*p = 0.019 and 0.042 respectively), as determined by a one-way ANOVA. There was also a trial by surgical group effect between trials 10 and 11 (*p = 0.008), with CA2 lesioned animals not displaying the expected increase in olfactory investigation, as determined by a repeated measures ANOVA.
When the mice were presented with the same stimulus female over a series of ten trials, the CA2 lesioned animals spent significantly more time with the stimulus female than control animals only in the 10th trial (14.62±2.29 secs for CA2 lesions, 7.43±1.60 secs for controls, F1,16 = 6.885, *p = 0.019) and significantly less time investigating the novel female in the 11th trial (12.20±1.57 secs for CA2 lesions, 18.87±2.02 secs for controls, F1,16 = 4.940, *p = 0.042) (Figure 3C). For the comparison between Trials 10 and 11, there was a trial by surgery effect (F1,15 = 9.495, *p=0.008) with control animals having normal social recognition as demonstrated by significantly increased investigation times between the 10th and 11th trial (7.43±1.60 secs with familiar female vs. 18.87±2.02 secs with novel female) and CA2 lesioned males showing no increased investigation time between the 10th and 11th trial, as would be indicative of normal social recognition memory (14.62±2.29 secs with familiar female vs. 12.20±1.57 secs with novel female). In neither experiment were there any statistically significant differences between surgical groups in the number of tail rattles or the duration of non-social behaviors.
Experiments 3 and 4: Olfaction Tests
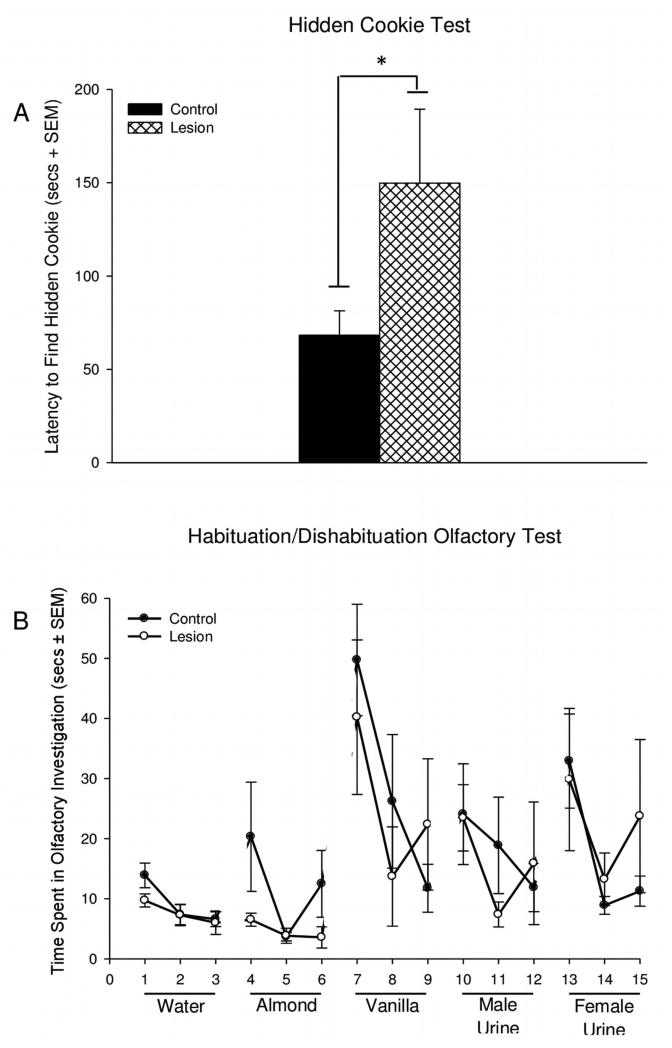
The animals from the 11-trial social recognition test were then tested in a hidden cookie test and a habituation/dishabituation olfactory test. There was a significant difference between treatment groups in the hidden cookie test (F1, 18 = 4.930, *p = 0.040) with CA2 lesioned animals having a longer latency to find the hidden cookie than controls (68.28±13.05 secs for controls and 149.82±39.52 secs for CA2 lesions) (Figure 4A). Two of the CA2 lesion animals did not find the hidden cookie and were assigned the maximum time of 300 seconds. There were no significant differences observed between treatment groups in the amount of olfactory investigation of the 5 odorants in the habituation/dishabituation olfactory test (Figure 4B). The only odorant that the mice did not habituate to was the almond extract.
Figure 4.
Olfaction in CA2 lesioned and control mice. A) The latency to find a hidden piece of cookie. Mice with lesions of the CA2 region took significantly more time to find the hidden cookie than control animals (*p = 0.040), as determined by a one-way analysis of variance B) The time spent in olfactory investigation of 5 different odorants in a habituation/dishabituation test of olfaction. In this test, animals with lesions to the CA2 region showed no significant differences in olfaction compared to controls. For the measurements in the habituation/dishabituation olfactory experiment a repeated measures ANOVA was used for each odorant (trial × surgical group).
Discussion
In the current study we examined the effects of excitotoxic lesions of the CA2 region of the hippocampus on social recognition memory and olfaction. We found that CA2 lesions resulted in impairments in social recognition memory. Unlike surgical and lesion controls, CA2 lesioned animals were unable to discriminate between a novel and a familiar stimulus animal in both tests for social memory. These data are consistent with previous studies in rodents, which have reported impaired social recognition in animals with lesions of the hippocampus that included the CA2 region (Maaswinkel et al., 1996; Uekita & Okanoya, 2011) and are also consistent with a recently published study that found that genetically targeted inactivation of CA2 pyramidal cells results in a loss of social memory, while having no effect on anxiety-like behavior or hippocampal-dependent forms of memory, i.e. spatial and contextual memory (Hitti & Siegelbaum, 2014). Taken together with previous work, the specificity of our lesions and their effects on social behavior, the data suggest that the CA2 region of the hippocampus is indeed important for normal social memory.
CA2 Lesions and Social Memory
We also observed in Trial 1 of Experiment 1 that CA2 lesioned animals showed significantly reduced investigation times compared to controls. These data suggest that lesions to the CA2 region may result in a reduction in an animal’s motivation to interact with social stimuli; though, as animals with striatum lesions also appeared to have lower investigation times in Trial 1 and there being no apparent differences in lesions versus control animals in Trial 1 of the habituation-dishabituation test, it is difficult to know if this observation is meaningful. Reduced social motivation in CA2 lesioned mice could potentially help to explain the unusual up and down patterns in the habituation/dishabituation social recognition test. Specifically, lesioned animals initially were interested in the stimulus female, but this interest did not show the typical decline with repeated exposures, but rather had a high level of variability. As social motivation is essential to normal displays of social behavior, a test specifically focused on this behavior would be needed to determine whether or not CA2 contributes to more global changes in the perception and/or response to social stimuli. It is also possible that the decreased amount of social investigation of the stimulus female in Trial 1 by the CA2 lesioned animals was due to insufficient learning about the stimulus that resulted in the impairment in social discrimination seen in Trial 2. An argument could also be made that increased anxiety was responsible for the sporadic behavior observed in CA2 lesioned animals, but since there were no observable differences in self-grooming or tail rattles between the treatment groups, this seems unlikely. Further, as past studies of hippocampal lesioned animals report a reduction in anxiety-like behavior rather than an increase (Deacon et al., 2002) and selective inactivation of dorsal CA2 pyramidal neurons has no effect on anxiety-like behavior (Hitti & Siegelbaum, 2014), it is doubtful that lesions of the CA2 region would result in increased anxiety-like behavior.
It should also be noted that there is a lack of consensus in the literature about whether or not using cycling females as stimulus animals in a social recognition task is best, as there may be a potential confound due to sexual motivation. In our previous work we have used ovariectomized females, in which we extinguished sexual behavior (Wersinger et al., 2007b; Lee et al., 2008), but found that a number of animals always had to be excluded due to displays of mounting behavior during testing. Thus, based on some recent publications on social recognition in mice we decided to use corraled cycling females (Macbeth et al., 2009a; Macbeth et al., 2009b), as they consitute a robust stimulus. There is a report that gonadally-intact males do not display estrous cycle-dependent differences in the sniffing of female volatile odors. With the only major difference in sniffing by males being between cycling females and ovariectomized females; with males sniffing cycling females, at any state of the estrous cycle, more than ovariectomized females (Muroi et al., 2006). These data do however contrast with work by Achiraman and collegues (2010) in mice as well as the work by others in Syrian hamsters (eg., Huck et al., 1989; delBarco-Trillo et al., 2009), which suggests that gonadally-intact males show an odor preference for urine or vaginal secretions from estrus females. Thus, we cannot definatively rule out the possibility that the stage of the estous cycle of the stimulus females in our social recognition tests (Experiments 1 and 2) did not influence our results.
CA2 Lesions and Olfaction
Since social behaviors in rodents rely heavily on the ability to discern olfactory cues (Bronson, 1971; Schultz & Tapp, 1973) we chose to investigate olfaction following bilateral CA2 lesions. Even though there have been no reports of disrupted olfaction in rodents with extensive hippocampal lesions (Bunsey & Eichenbaum, 1995; Burton et al., 2000), the possibility of the CA2 region being important to olfactory processing could not be ruled out since it receives inputs from the entorhinal cortex (Petrulis et al., 2005; reviewed in Sanchez-Andrade et al., 2005). Thus, lesions to the CA2 region could hypothetically cause a disconnection between olfactory cues and the formation and/or recall of accessory olfactory-based memories. In reality, the mild impairments we observed were test-specific and may not represent an olfactory deficit. The hidden cookie test is not a very rigorous test of olfaction and it is possible that lesions of the CA2 region may have compromised some aspect of the motivation to eat rather than olfaction itself. It is also plausible that our observations are the result of food neophobia rather than an olfactory deficit. In the recent paper by Hitti and colleagues (2014), which genetically lesioned CA2 pyramidal cells, no CA2 dependent-deficits in the hidden cookie test were observed, but the animals were preexposed to the food reward a day prior to testing, thus reducing the chances of food neophobia. Further, the fact that we do not see any differences in the habituation/dishabituation olfactory test leads us to believe that olfaction has not been compromised in CA2 lesioned animals.
Conclusion
In summary, our hypothesis that the CA2 region of the hippocampus is important to normal displays of social recognition was supported. Interestingly, the deficits that were observed in social recognition are similar to the deficits observed in mice lacking the Avpr1b (for review see Caldwell et al., 2008a; Caldwell et al., 2008b; Stevenson & Caldwell, 2012). In fact, the impetus for the current work was the impaired social recognition memory that is observed in Avpr1b knockout mice as well as the prominent expression of the Avpr1b in the dorsal third of the CA2 region of the hippocampus (Wersinger et al., 2002; Young et al., 2006). We and others have suggested that Avpr1b within the CA2 region may be important to displays of specific social behaviors, specifically to the coupling of the olfactory cue with the “what” and “when” components of the odor context (Caldwell et al., 2008b; DeVito et al., 2009). This hypothesis is supported by a recent paper, which found that the restoration of Avpr1b into the CA2 region of Avpr1b knockout mice increases socially motivated attack behaviors (Pagani et al., 2014).
The role of the CA2 region in social recognition memory will drive future work in our laboratory, which will focus on whether lesions of the CA2 region have other effects on social behavior and the determination of whether vasopressin acting through the Avpr1b in this region that is important to social memory. Further, it is becoming ever more clear that the CA2 region of hippocampus is important for normal social behaviors, including social memory, in animal models (Ely et al., 1977b; Maaswinkel et al., 1996; McHugh et al., 2004; Uekita & Okanoya, 2011; Hitti & Siegelbaum, 2014; Pagani et al., 2014), and may be important across species, including humans. In humans, for instance, there is a report of a decrease in the volume the CA2-3 subfield that is associated with impaired learning and memory in Parkinson’s disease (Pereira et al., 2014), as well as report of a reduction in the number of nonpyramidal cells in the CA2 region in schizophrenic and manic depressive patients (Benes et al., 1998). Thus, understanding the role of the CA2 region of the hippocampus may have important implications for our understanding of the neural regulation behaviors across species.
Acknowledgements
This work was supported by NIH R21 MH083963-05 to H.K.C. We thank Amy Gilkerson for her assistance with the olfactory studies, as well as, Megan Rich and Shannah Witchey for their expertise in animal husbandry. We also thank W. Scott Young, III for comments on this manuscript as well as the excellent care provided to our animals by the members of the vivarium staff at KSU.
Abbreviations
- ANOVA
analysis of variance
- Avpr1b
vasopressin 1b receptor
- DAB
diaminobenzidine
- i.p
intraperitoneal
- NMDA
N-methyl-D aspartate
- PBS
phosphate buffered saline
References
- Achiraman S, Ponmanickam P, Ganesh DS, Archunan G. Detection of estrus by male mice: synergistic role of olfactory-vomeronasal system. Neurosci Lett. 2010;477:144–148. doi: 10.1016/j.neulet.2010.04.051. [DOI] [PubMed] [Google Scholar]
- Bartesaghi R, Gessi T. Parallel activation of field CA2 and dentate gyrus by synaptically elicited perforant path volleys. Hippocampus. 2004;14:948–963. doi: 10.1002/hipo.20011. [DOI] [PubMed] [Google Scholar]
- Bartesaghi R, Migliore M, Gessi T. Input-output relations in the entorhinal cortex-dentate-hippocampal system: evidence for a non-linear transfer of signals. Neuroscience. 2006;142:247–265. doi: 10.1016/j.neuroscience.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44:88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- Borhegyi Z, Leranth C. Distinct substance P- and calretinin-containing projections from the supramammillary area to the hippocampus in rats; a species difference between rats and monkeys. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1997;115:369–374. doi: 10.1007/pl00005706. [DOI] [PubMed] [Google Scholar]
- Bronson FH. Rodent pheromones. Biol Reprod. 1971;4:344–357. [PubMed] [Google Scholar]
- Bunsey M, Eichenbaum H. Selective damage to the hippocampal region blocks long-term retention of a natural and nonspatial stimulus-stimulus association. Hippocampus. 1995;5:546–556. doi: 10.1002/hipo.450050606. [DOI] [PubMed] [Google Scholar]
- Burton S, Murphy D, Qureshi U, Sutton P, O'Keefe J. Combined lesions of hippocampus and subiculum Do not produce deficits in a nonspatial social olfactory memory task. J Neurosci. 2000;20:5468–5475. doi: 10.1523/JNEUROSCI.20-14-05468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, Dike OE, Stevenson EL, Storck K, Young WS., 3rd Social dominance in male vasopressin 1b receptor knockout mice. Horm Behav. 2010;58:257–263. doi: 10.1016/j.yhbeh.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, Lee HJ, Macbeth AH, Young WS., 3rd Vasopressin: Behavioral roles of an "original" neuropeptide. Prog Neurobiol. 2008a;84:1–24. doi: 10.1016/j.pneurobio.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell HK, Stewart J, Wiedholz LM, Millstein RA, Iacangelo A, Holmes A, Young WS, 3rd, Wersinger SR. The acute intoxicating effects of ethanol are not dependent on the vasopressin 1a or 1b receptors. Neuropeptides. 2006;40:325–337. doi: 10.1016/j.npep.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Wersinger SR, Young WS., 3rd The role of the vasopressin 1b receptor in aggression and other social behaviours. Prog Brain Res. 2008b;170:65–72. doi: 10.1016/S0079-6123(08)00406-8. [DOI] [PubMed] [Google Scholar]
- Caldwell HK, Young WS., 3rd Persistence of reduced aggression in vasopressin 1b receptor knockout mice on a more "wild" background. Physiol Behav. 2009;97:131–134. doi: 10.1016/j.physbeh.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr WJ, Loeb LS, Dissinger ML. Responses of Rats to Sex Odors. Journal of comparative and physiological psychology. 1965;59:370–377. doi: 10.1037/h0022036. [DOI] [PubMed] [Google Scholar]
- Deacon RM, Bannerman DM, Rawlins JN. Anxiolytic effects of cytotoxic hippocampal lesions in rats. Behav Neurosci. 2002;116:494–497. doi: 10.1037//0735-7044.116.3.494. [DOI] [PubMed] [Google Scholar]
- delBarco-Trillo J, LaVenture AB, Johnston RE. Male hamsters discriminate estrous state from vaginal secretions and individuals from flank marks. Behavioural processes. 2009;82:18–24. doi: 10.1016/j.beproc.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVito LM, Konigsberg R, Lykken C, Sauvage M, Young WS, 3rd, Eichenbaum H. Vasopressin 1b receptor knock-out impairs memory for temporal order. J Neurosci. 2009;29:2676–2683. doi: 10.1523/JNEUROSCI.5488-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakar MB, Rich ME, Reno EL, Lee HJ, Caldwell HK. Heightened aggressive behavior in mice with lifelong versus postweaning knockout of the oxytocin receptor. Horm Behav. 2012;62:86–92. doi: 10.1016/j.yhbeh.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Ely DL, Greene EG, Henry JP. Effects of hippocampal lesion on cardiovascular, adrenocortical and behavioral response patterns in mice. Physiol Behav. 1977a;18:1075–1083. doi: 10.1016/0031-9384(77)90014-2. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Elsevier/Academic Press, Amsterdam; Boston: 2008. [Google Scholar]
- Hitti FL, Siegelbaum SA. The hippocampal CA2 region is essential for social memory. Nature. 2014;508:88–92. doi: 10.1038/nature13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck UW, Lisk RD, Kim S, Evans AB. Olfactory discrimination of estrous condition by the male golden hamster (Mesocricetus auratus) Behavioral and neural biology. 1989;51:1–10. doi: 10.1016/s0163-1047(89)90608-0. [DOI] [PubMed] [Google Scholar]
- Ingersoll DW, Weinhold LL. Modulation of male mouse sniff, attack, and mount behaviors by estrous cycle-dependent urinary cues. Behavioral and neural biology. 1987;48:24–42. doi: 10.1016/s0163-1047(87)90544-9. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, Young WS., 3rd A conditional knockout mouse line of the oxytocin receptor. Endocrinology. 2008;149:3256–3263. doi: 10.1210/en.2007-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Callaway EM, Albright TD, Gage FH. Redefining the boundaries of the hippocampal CA2 subfield in the mouse using gene expression and 3-dimensional reconstruction. J Comp Neurol. 2005;485:1–10. doi: 10.1002/cne.20426. [DOI] [PubMed] [Google Scholar]
- Lein ES, Zhao X, Gage FH. Defining a molecular atlas of the hippocampus using DNA microarrays and high-throughput in situ hybridization. J Neurosci. 2004;24:3879–3889. doi: 10.1523/JNEUROSCI.4710-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente de No R. Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. J Psychol Neurol. 1934;46:113–177. [Google Scholar]
- Maaswinkel H, Baars AM, Gispen WH, Spruijt BM. Roles of the basolateral amygdala and hippocampus in social recognition in rats. Physiol Behav. 1996;60:55–63. doi: 10.1016/0031-9384(95)02233-3. [DOI] [PubMed] [Google Scholar]
- Macbeth AH, Edds JS, Young WS., 3rd Housing conditions and stimulus females: a robust social discrimination task for studying male rodent social recognition. Nature protocols. 2009a;4:1574–1581. doi: 10.1038/nprot.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macbeth AH, Lee HJ, Edds J, Young WS., 3rd Oxytocin and the oxytocin receptor underlie intrastrain, but not interstrain, social recognition. Genes Brain Behav. 2009b;8:558–567. doi: 10.1111/j.1601-183X.2009.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matochik JA. Role of the main olfactory system in recognition between individual spiny mice. Physiol Behav. 1988;42:217–222. doi: 10.1016/0031-9384(88)90073-x. [DOI] [PubMed] [Google Scholar]
- McHugh SB, Deacon RM, Rawlins JN, Bannerman DM. Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav Neurosci. 2004;118:63–78. doi: 10.1037/0735-7044.118.1.63. [DOI] [PubMed] [Google Scholar]
- Muroi Y, Ishii T, Komori S, Nishimura M. A competitive effect of androgen signaling on male mouse attraction to volatile female mouse odors. Physiol Behav. 2006;87:199–205. doi: 10.1016/j.physbeh.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Pagani JH, Zhao M, Cui Z, Williams Avram SK, Caruana DA, Dudek SM, Young WS. Role of the vasopressin 1b receptor in rodent aggressive behavior and synaptic plasticity in hippocampal area CA2. Mol Psychiatry. 2014 doi: 10.1038/mp.2014.47. epub ahead of print: May 27, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JB, Valls-Pedret C, Ros E, Palacios E, Falcon C, Bargallo N, Bartres-Faz D, Wahlund LO, Westman E, Junque C. Regional vulnerability of hippocampal subfields to aging measured by structural and diffusion MRI. Hippocampus. 2014;24:403–414. doi: 10.1002/hipo.22234. [DOI] [PubMed] [Google Scholar]
- Petrulis A, Alvarez P, Eichenbaum H. Neural correlates of social odor recognition and the representation of individual distinctive social odors within entorhinal cortex and ventral subiculum. Neuroscience. 2005;130:259–274. doi: 10.1016/j.neuroscience.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Popik P, Vetulani J, Bisaga A, van Ree JM. Recognition cue in the rat's social memory paradigm. J Basic Clin Physiol Pharmacol. 1991;2:315–327. doi: 10.1515/jbcpp.1991.2.4.315. [DOI] [PubMed] [Google Scholar]
- Ropartz P. The relation between olfactory stimulation and aggressive behaviour in mice. Animal behaviour. 1968;16:97–100. doi: 10.1016/0003-3472(68)90117-6. [DOI] [PubMed] [Google Scholar]
- Sanchez-Andrade G, James BM, Kendrick KM. Neural encoding of olfactory recognition memory. The Journal of reproduction and development. 2005;51:547–558. doi: 10.1262/jrd.17031. [DOI] [PubMed] [Google Scholar]
- Schultz EF, Tapp JT. Olfactory control of behavior in rodents. Psychological bulletin. 1973;79:21–44. doi: 10.1037/h0033817. [DOI] [PubMed] [Google Scholar]
- Sekino Y, Obata K, Tanifuji M, Mizuno M, Murayama J. Delayed signal propagation via CA2 in rat hippocampal slices revealed by optical recording. J Neurophysiol. 1997;78:1662–1668. doi: 10.1152/jn.1997.78.3.1662. [DOI] [PubMed] [Google Scholar]
- Stevenson EL, Caldwell HK. The vasopressin 1b receptor and the neural regulation of social behavior. Horm Behav. 2012;61:277–282. doi: 10.1016/j.yhbeh.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Abe K, Nojyo Y. Three-dimensional analysis of the whole axonal arbors originating from single CA2 pyramidal neurons in the rat hippocampus with the aid of a computer graphic technique. Brain Res. 1988;452:255–272. doi: 10.1016/0006-8993(88)90030-3. [DOI] [PubMed] [Google Scholar]
- Uekita T, Okanoya K. Hippocampus lesions induced deficits in social and spatial recognition in Octodon degus. Behav Brain Res. 2011;219:302–309. doi: 10.1016/j.bbr.2011.01.042. [DOI] [PubMed] [Google Scholar]
- Vertes RP, McKenna JT. Collateral projections from the supramammillary nucleus to the medial septum and hippocampus. Synapse. 2000;38:281–293. doi: 10.1002/1098-2396(20001201)38:3<281::AID-SYN7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Vigers AJ, Baquet ZC, Jones KR. Expression of neurotrophin-3 in the mouse forebrain: insights from a targeted LacZ reporter. J Comp Neurol. 2000;416:398–415. doi: 10.1002/(sici)1096-9861(20000117)416:3<398::aid-cne10>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Caldwell HK, Christiansen M, Young WS., 3rd Disruption of the vasopressin 1b receptor gene impairs the attack component of aggressive behavior in mice. Genes Brain Behav. 2007a;6:653–660. doi: 10.1111/j.1601-183X.2006.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersinger SR, Caldwell HK, Martinez L, Gold P, Hu SB, Young WS., 3rd Vasopressin 1a receptor knockout mice have a subtle olfactory deficit but normal aggression. Genes Brain Behav. 2007b;6:540–551. doi: 10.1111/j.1601-183X.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Ginns EI, O'Carroll AM, Lolait SJ, Young WS., III Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Kelliher KR, Zufall F, Lolait SJ, O'Carroll A, Young WS., Iii Social motivation is reduced in vasopressin 1b receptor null mice despite normal performance in an olfactory discrimination task. Horm Behav. 2004:638–645. doi: 10.1016/j.yhbeh.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Williams TE, Meshul CK, Cherry NJ, Tiffany NM, Eckenstein FP, Woodward WR. Characterization and distribution of basic fibroblast growth factor-containing cells in the rat hippocampus. J Comp Neurol. 1996;370:147–158. doi: 10.1002/(SICI)1096-9861(19960624)370:2<147::AID-CNE2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Young WS, Li J, Wersinger SR, Palkovits M. The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience. 2006;143:1031–1039. doi: 10.1016/j.neuroscience.2006.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]