Abstract
Following an acute T cell response, most activated effector cells die, while some survive and become memory cells. The pro-apoptotic Bcl-2 family member, Bcl-2 interacting mediator of death (Bim) is critical for eliminating most effector T cells, while expression of CD127 (IL-7Rα) has been proposed to mark effector cells destined to become memory cells. Here, we examined the effects of Bim on the death of effector T cells in relationship to CD127 expression and on development of T cell memory following lymphocytic choriomeningitis virus (LCMV) infection. We found that large numbers of CD127lo LCMV-specific CD4+ and CD8+ T cells were lost in wild-type mice, but were spared in Bim−/− mice. Further, while the numbers of CD127hi T cells declined only slightly during contraction of the response in wild-type mice, they increased significantly in Bim−/− mice due to re-expression of CD127 on CD127lo T cells that had avoided apoptosis. Functional memory T cells were significantly increased in Bim−/− mice; however, they underwent a slow attrition due to decreased proliferative renewal. Taken together, these data suggest that the absence of Bim-mediated death of LCMV-specific CD4+ and CD8+ T cells in vivo can increase T cell memory, but other homeostatic mechanisms control the long-term maintenance of memory cells.
Keywords: Apoptosis, Knockout mice, Memory T cells, Transgenic, Viral infection
Introduction
Antigen-driven activation of naïve T cells during the course of an immune response results in massive expansion of antigen (Ag)-specific T cells [1, 2]. In general, following clearance of the stimulus, the majority of activated T cells die by apoptosis, while a fraction become memory T cells. This decision between death and survival is likely crucial for avoiding autoimmunity and for promoting the development of immunological memory and protective immunity. The molecular mechanism(s) that underlie this cell fate decision are poorly understood.
The two major pathways of cell death are the so-called “extrinsic” or death receptor pathway and “intrinsic” or mitochondrial pathway. The death receptor pathway is triggered when extracellular ligands oligomerize cell surface “death receptors” and activate an apoptotic cascade via recruitment of caspases [3–5]. Previous studies regarding Fas/FasL and/or TNF-α on controlling contraction of T cell responses are controversial [6–13]. In the lymphocytic choriomeningitis virus (LCMV) model, Fas/FasL interactions as well as TNF-R signaling can play a role in controlling contraction of CD8+ T cell responses during persistent LCMV infection [7, 14]. Although recent data suggest a minor role for TNF-R signaling on T cell contraction following acute LCMV infection [6], previous results showed little, if any, role for either TNF-R signaling or Fas/FasL in acute infection [11]. Further, contraction of herpes simplex virus (HSV)-specific CD8+ T cell responses does not require Fas/FasL interactions [12]. Thus, death receptor signaling does not appear to play a major role in the contraction of Ag-specific T cell responses during acute infections.
In contrast, the mitochondrial pathway involves pro- and anti-apoptotic members of the Bcl-2 family. Antiapoptotic Bcl-2 family members appear to function, at least in part, by sequestering and inactivating pro-apoptotic BH-3 only proteins [15, 16]. Regulated decreases in the levels of anti-apoptotic molecules can be key to initiation of mitochondrial apoptosis [10]. In T cells, Bcl-2 levels are significantly down-regulated after activation [10, 17, 18], an event that we showed sensitizes T cells to the pro-apoptotic effects of Bcl-2 interacting mediator of death (Bim) following super-antigen injection [10]. Similar phenomena occur during acute HSV infection [10, 12]. However, the long-term survival of effector T cells and their development into memory in the absence of Bim was not examined in either of these studies. Thus, while Bim plays a critical role in controlling contraction of T cell responses in vivo, it remains unclear whether or not avoidance of Bim-mediated apoptosis affects T cell memory.
Recent data have also suggested that expression of CD127 (IL-7Rα) is an early marker for CD8+ effector T cells that possess memory potential [19, 20]. At the peak of the T cell response, very few CD8+ T cells express high levels of CD127 and during contraction, the percentage CD8+ T cells expressing high levels of CD127 is increased [19]. CD127lo effector CD8+ T cells express lower levels of Bcl-2 and Bcl-xL compared to their CD127hi counterparts, suggesting that CD127lo T cells are destined for death [19, 21]. However, the mechanism(s) that drive the death of CD127lo T cells remains unclear. Further, whether or not similar phenomena also occur in Ag-specific CD4+ T cells is unclear. Here, we investigated the role of Bim in the death of LCMV-specific CD4+ and CD8+ T cells, their expression of CD127, and their development into memory cells following LCMV infection.
In this study, we found that during acute LCMV infection large numbers of LCMV-specific CD4+ and CD8+ CD127lo T cells were lost in Bim+/+ mice, but were largely spared in Bim−/− mice. LCMV-specific CD4+ and CD8+ T cells that avoided apoptosis in Bim−/− mice appeared functional, re-expressed CD127 and initially entered the memory compartment, but underwent slow attrition due to decreased proliferative renewal. These results suggest that Bim is critical for limiting the numbers of T cells available to enter the memory compartment, but that Bim-independent mechanisms control the long-term maintenance of memory T cells. These data are critical for potential therapeutic manipulation of Bim to promote short-term enhancement of T cell memory.
Results
Specificity of MHC class II-tetrameric staining reagents
While CD8+ T cell responses to LCMV infection have been rigorously studied, anti-viral CD4+ T cell responses are less well characterized. To track virus-specific CD4+ T cells, we generated MHC class II tetramers [22, 23] specific for the LCMV-glycoprotein 61–80 epitope [24]. As a control, we generated a second I-Ab reagent displaying a synthetic epitope (e.g., 2W1S) expressed by a recombinant vaccinia virus (rVV-2W1S) [23, 25]. The specificity of these reagents was tested on spleen cells from uninjected mice or virally infected mice. In uninfected controls, both I-Abgp61–80 and I-Ab-2W1S tetramers stained roughly 0.08% of CD4+ T cells (Supplemental Fig. 1). After LCMV infection, a population of CD4+ T cells was detected with I-Abgp61–80 but not I-Ab-2W1S tetramer, whereas, after rVV infection, a population of CD4+ T cells was detected with I-Ab-2W1S but not I-Abgp61–80 tetramer (Supplemental Fig. 1). These results show the specificity of MHC class II-tetramers and their usefulness in tracking endogenous Ag-specific T cell responses in vivo.
Bim drives contraction of LCMV-specific T cell responses
Our previous data showed a critical role for Bim in the apoptotic deletion of superantigen reactive T cells in vivo [10]. However, studies on memory development were precluded because superantigens partially tolerize their responding Vβ-bearing T cells. To assess the role of Bim on memory development, we examined T cell responses following LCMV infection of H-2b mice, in which the kinetics of the CD4+ and CD8+ T cell responses have been characterized [1, 26–29].
Groups of either Bim+/+, Bim+/− or Bim−/− mice were infected with LCMV, and CD4+ and CD8+ T cell responses were tracked over time using MHC class I and class II tetramers. After LCMV infection, expansion of Db gp33–41-specific and Db np396–404-specific T cells was similar in Bim+/+, Bim+/−, and Bim−/− mice at days 8 and 10 after infection (Fig. 1A–D). On days 15, 21 and 38 after infection, the percentages and numbers of LCMV-specific CD8+ T cells decreased significantly in Bim+/+ and Bim+/− mice, but remained high in Bim−/− mice (Fig. 1A–D). Similar results were obtained for Kbnp205–214-specific T cells (data not shown). Later after infection, there was a slow attrition of the Bim−/− LCMV-specific T cells, and by day 104, their numbers of Dbgp33+ T cells and Dbnp396+ T cells approached the numbers found in either Bim+/− or Bim+/+ mice (Fig. 1A–D). Like LCMV-specific CD8+ T cells, CD4+ I-Abgp61+ T cells expanded similarly in all three strains of mice, reaching a peak on days 8–10 post infection (Fig. 1E, F). On days 15, 21 and 38 after infection, the percentages and total numbers of CD4+ I-Ab-gp61+ T cells decreased significantly in Bim+/+ mice, but remained increased in Bim−/− mice (Fig. 1E, F). Interestingly, unlike LCMV-specific CD8+ T cells, numbers of I-Ab-gp61+ T cells in Bim+/− mice were intermediate between those observed for Bim+/+ and Bim−/− mice, consistent with previously reported gene dosage effects of Bim [10, 30, 31]. By day 104 after infection, numbers of I-Ab-gp61+ T cells had decreased in Bim−/− mice approaching levels observed for either Bim+/+ or Bim+/− mice (Fig. 1E, F). Thus, Bim is critical for the initial contraction of LCMV-specific CD4+ and CD8+ T cells.
Figure 1.
Bim is critical for the initial apoptotic contraction of LCMV-specific CD4+ and CD8+ T cell responses. Groups of either Bim+/+ (open circles, light lines), Bim+/− (open squares, dashed lines), or Bim−/− (filled squares, dark lines) mice (n=3–5 mice/group/time point) were infected with LCMV. At the indicated times after infection, spleen cells were stained with MHC tetramers and numbers and percentages of Ag-specific T cells quantified by flow cytometry. Results show the percentages (A, C, E) and absolute number (B, D, F) ± SEM of CD8+ Db-NP396–404-specific T cells (A, B); of CD8+ Db-GP33–41-specific T cells (C, D); and of CD4+ I-Ab-GP61–80-specific T cells (E, F). Similar results were obtained for a third population of CD8+ T cells specific for Kb-NP205–212 (data not shown). Note: Bim+/− were not analyzed on day 10, and IAbgp61–80 MHC tetramer stains were not performed on day 67. Data are pooled from four independent experiments. * denotes a statistically significant difference between Bim−/− and Bim+/+ mice (at least p≤0.05 for the percentages and p≤0.002 for the total numbers; one-way ANOVA, Minitab for Windows).
It was possible that viral persistence or altered T cell trafficking contributed to the increased numbers of T cells observed in Bim−/− mice. However, we were unable to detect virus in the livers of Bim+/+, Bim+/−, and Bim−/− mice by plaque assay at any of the indicated days after infection (data not shown), suggesting that the increased numbers of T cells in Bim−/− mice is not due to prolonged viral persistence. We also measured LCMV-specific T cells in the livers of Bim+/+ and Bim−/− mice at 22 days after infection. Similar to our previous results [10], Bim−/− mice had a two- to fourfold increase in the numbers of LCMV-specific CD4+ and CD8+ T cells in the liver compared to Bim+/+ mice (data not shown). Taken together, these results indicate that the increased numbers of T cells observed in Bim−/− mice is likely not due to prolonged viral persistence or to selective accrual in the spleen.
Decreased apoptosis of LCMV-specific Bim−/− T cells
The above data suggested that effector T cells from Bim−/− mice underwent less apoptosis than their Bim+/+ counterparts. To further test this, we measured in vitro survival of LCMV-specific Bim−/− or Bim+/+ T cells using an assay we have described previously [10, 32–34]. Percentages of live LCMV-specific CD4+ and CD8+ T cells from Bim−/− mice were significantly elevated compared to T cells from Bim+/+ mice after 24 h in culture (Table 1). These data demonstrate that LCMV-specific CD4+ and CD8+ T cells from Bim−/− mice are resistant to death in vitro.
Table 1.
Increased survival of LCMV-specific Bim−/− T cells
| T cells | Mice | Survival in vitroa) | Survival in vivob) | |
|---|---|---|---|---|
| % alive at 24 h | Day 7 | Day 14 | ||
| I-Abgp61 sp. | Bim+/+ | 32.0 ± 8.5% | 49.1 ± 5.4% | 1.6 ± 2.0% |
| Bim−/− | 58.9 ± 11.7%* | 76.3 ± 5.5%** | 14.0 ± 3.6%* | |
| Dbnp396 sp. | Bim+/+ | 25.2 ± 4.3% | 35.1 ± 4.9% | 6.8 ± 1.3% |
| Bim−/− | 76.9 ± 4.2%** | 66.5 ± 4.8%** | 17.3 ± 7.8% | |
| Dbgp33 sp. | Bim+/+ | 27.9 ± 4.5% | 62.3 ± 7.9% | 11.7 ± 0.6% |
| Bim−/− | 87.1 ± 6.6%** | 80.2 ± 4.5%* | 34.5% ±13.0% |
To assess survival in vitro, spleen cells from Bim+/+ or Bim−/− LCMV-infected mice were cultured for 24 h and analyzed for death. Data show the percentage of tetramer+ T cells alive ± SD.
To assess survival in vivo, purified T cells from the above LCMV-infected Bim+/+ or Bim−/− mice were transferred into Ly5.1 congenic recipient mice. Donor tetramer+Ly5.2+CD4+ or CD8+ T cells were quantified in the spleen of recipient mice on days 1, 7 and 14 after transfer. On day 1, recipients of either Bim+/+ or Bim−/− T cells had similar numbers of CD8+ Ly5.2+ tetramer+ cells, but recipients of Bim+/+ cells had more CD4+Ly5.2+ tetramer+ cells compared to Bim−/− recipients. Data show the percent (± SD) of donor (Ly5.2+) tetramer+ T cells remaining in recipient mice relative to the number of donor cells in recipients at day 1. Statistically significant differences were observed between Bim+/+ and Bim−/− mice (*denotes p<0.05, **denotes p<0.001, Student's two-sample t-test).
To determine whether LCMV-specific Bim−/− T cells were resistant to apoptosis in vivo, an adoptive transfer experiment was performed. Groups of either Bim+/+ or Bim−/− mice were infected with LCMV, and 10 days later purified splenic T cells were injected into groups of B6.SJL congenic recipient mice. Numbers of tetramer+ donor (Ly5.2+) spleen cells in recipient mice were quantified by flow cytometry at days 1, 7, 14 post transfer. Donor (Ly5.2+) CD4+ I-Abgp61+ T cells were significantly increased at days 7 and 14 in recipients of Bim−/− T cells compared to recipients of Bim+/+ T cells (Table 1). Further, donor Dbnp396+ and Dbgp33+ CD8+ T cells that survived for 7 days were significantly increased in mice that received Bim−/− instead of Bim+/+ T cells (Table 1). By day 14 post-transfer, numbers of LCMV-specific CD4+ and CD8+ T cells decreased in both groups of recipients, although Bim−/− recipients still had an approximately threefold increase in the percentage of remaining LCMV-specific T cells (Table 1). Thus, both the in vivo and in vitro results suggest that Bim plays a critical and likely T cell intrinsic role in the apoptotic contraction of LCMV-specific CD4+ and CD8+ T cell responses.
Bim levels are not increased in LCMV-specific T cells at the peak of the response
Previous reports have shown that antibody-driven TCR stimulation increases Bim levels, which correlate with increased apoptosis [30, 35]. However, we showed that Bim levels were not increased in superantigen-activated T cells [10]. To determine whether or not Bim levels changed within LCMV-specific T cells in vivo, we examined Bim levels within tetramer+ T cells ex vivo, using a Bim-specific mAb. Although there was some background staining, the anti-Bim antibody clearly stained Bim+/+ T cells more than it did Bim−/− T cells (Fig. 2 upper panels). At the peak of the response, just prior to their massive death in vivo, Bim levels within tetramer+ CD4+ or CD8+ T cells were not significantly increased following LCMV infection (Fig. 2). In fact, for CD8+ T cells the mean fluorescence intensity of the Bim signal is actually somewhat decreased within tetramer+ CD8+ T cells compared to their naïve counterparts (Fig. 2). These data suggest that up-regulation of Bim expression following T cell activation in vivo is unlikely to be the mechanism by which the pro-apoptotic activity of Bim is unleashed.
Figure 2.
Bim levels are not increased in LCMV-specific T cells at the peak of the response. The top panels show a representative histogram of Bim staining intensity in CD4+ (upper left panel) and CD8+ (upper right panel) from either Bim+/+ (dark lines) or Bim−/− (dashed lines) mice. As a control, cells from Bim−/− mice were stained with an isotype control antibody (light lines). To assess Bim levels after infection, spleen cells from day 9 LCMV-infected Bim+/+ mice (n=3) were stained with MHC tetramers and intracellularly for Bim. Histograms are from representative animals and show the Bim staining intensity of MHC-tetramer-gated events. The mean fluorescence intensity (MFI) of anti-Bim staining on Dbnp396+; Dbgp33+; or I-Abgp61+ T cells is shown in the upper right corner. This experiment is one of two independent experiments with similar results.
Massive loss of LCMV-specific CD127lo T cells in Bim+/+ mice is prevented in Bim−/− mice
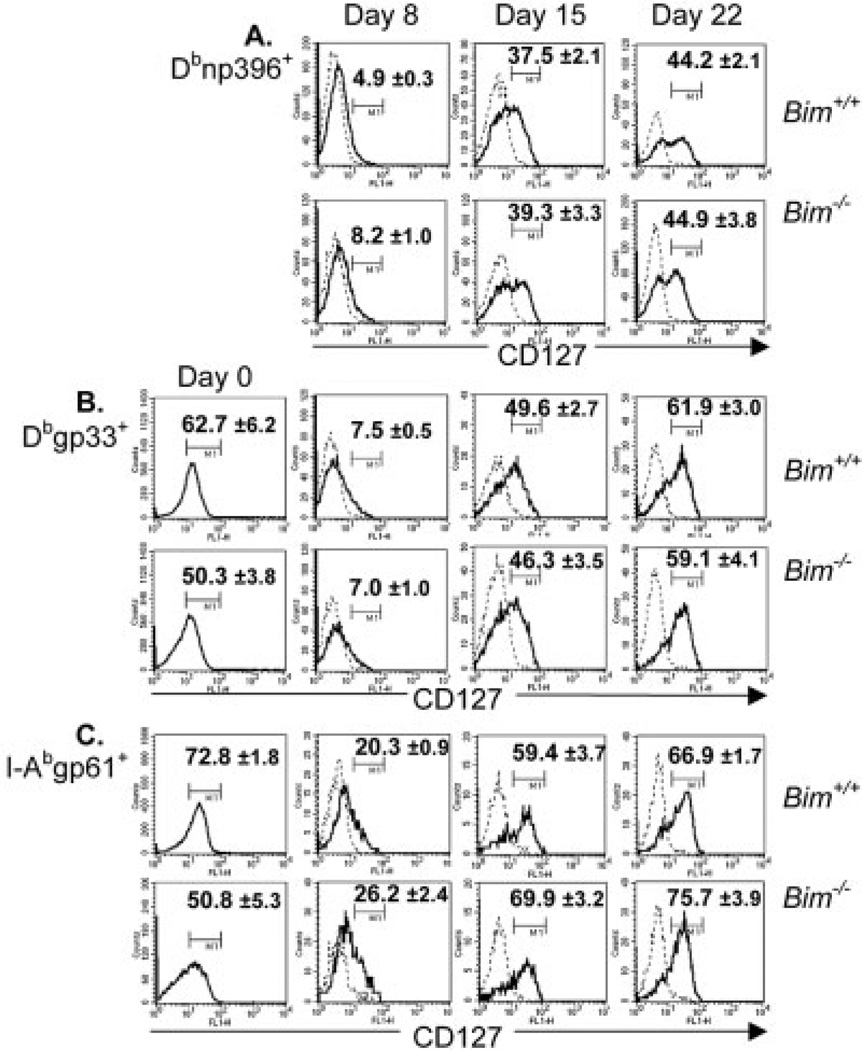
Most LCMV-specific CD8+ T cells decrease expression of CD127 at the peak of the response and CD127hi T cells are enriched during the apoptotic decline of the response [19, 20]. To test whether the absence of Bim would promote survival of CD127lo T cells, we infected Bim+/+ and Bim−/− mice with LCMV and, on days 8, 15, and 22, measured the percentages and total numbers of LCMV-specific CD4+ and CD8+ T cells that expressed CD127. First, in uninjected Bim−/− mice, the percentage of CD127hi CD4+ and CD8+ T cells were significantly decreased compared to Bim+/+ mice (Fig. 3, day 0). By day 8 after LCMV infection, CD127 expression decreased on both Dbnp396+ and Dbgp33+ CD8+ T cells (Fig. 3A, B), as reported previously [19]. Similarly, many CD4+ I-Abgp61+ T cells also decreased their expression of CD127 by day 8, although the percent of CD4+ I-Abgp61+ CD127hi T cells was two- to threefold higher than observed for tetramer+ CD8+ T cells (Fig. 3C). Surprisingly, on days 15 and 22 after infection, the percentages of CD4+ and CD8+ tetramer+ T cells that were CD127hi increased in both Bim+/+ and Bim−/− mice to a similar extent (Fig. 3A–C). Further, a significant percentage of I-Abgp61+, Dbnp396+, and Dbgp33+ T cells failed to express CD127 by day 22 (Fig. 3A). Thus, despite the fact that contraction of the response is largely complete by day 22 after infection, a substantial percentage of LCMV-specific CD127lo T cells (30–50%) persists.
Figure 3.
Emergence of LCMV-specific CD127hi T cells during contraction of the T cell response. Groups of Bim+/+ and Bim−/− mice (n=5–6 mice/group/time point) were infected i.p. with LCMV and killed at days 8, 15, and 22 after infection. CD127 expression on spleen cells from either naïve or LCMV-infected Bim+/+ and Bim−/− mice was analyzed by flow cytometry. Histograms show CD127 expression (dark lines) or isotype control staining (dashed lines) on gated MHC tetramer+ T cells or on total CD8+ or CD4+ T cells in naïve mice. Histogram markers were drawn according to isotype control stain. Graphs show the percentage of (A) Dbnp396+ cells; (B) Dbgp33+; or (C) I-Abgp61–80+ T cells that were CD127hi ± SEM. This experiment is representative of three independent experiments with similar results.
We next assessed the total number of LCMV-specific T cells in both Bim+/+ and Bim−/− mice as the response contracted between days 8 and 22 after infection. Similar to Fig. 1, substantial numbers of LCMV-specific CD4+ and CD8+ T cells were lost in Bim+/+, but not Bim−/− mice between days 8 and 22 after infection (Fig. 4, top row). The numbers of CD127hi LCMV-specific T cells in Bim+/+ mice was nearly identical between days 8 and 15 after infection and decreased slightly (1.5–2.5-fold) from days 15 to 22 after infection (Fig. 4, middle row). In contrast, numbers of CD127hi LCMV-specific T cells in Bim−/− mice increased from days 8 to 15 (I-Abgp61+, 2.3-fold; Dbnp396+, 5-fold; Dbgp33+, 3.9-fold), and remained elevated on day 22 after infection (Fig. 4, middle row).
Figure 4.
Bim mediates apoptosis of LCMV-specific CD127lo T cells during contraction of the T cell response. Numbers of total (top row), CD127hi (middle row), and CD127lo (bottom row) tetramer+ T cells fromBim+/+ (□) and Bim−/− (■) mice described in Fig. 3 were calculated. Results show the total numbers of CD8+np396+ (left panels) CD8+gp33+ (middle panels) and CD4+ I-Abgp61+ (right panels) T cells that were CD127hi or CD127lo for both Bim+/+ and Bim−/− mice ± SEM. * denotes statistically significant difference between Bim+/+ and Bim−/− mice (at least p≤0.05, one-way ANOVA). This experiment is representative of three independent experiments with similar results.
Numbers of CD127lo T cells declined dramatically in Bim+/+ mice between days 8 and 22 after infection (I-Abgp61+ 11-fold; Dbnp396+, 33-fold; Dbgp33+, 47-fold decreases) (Fig. 4, bottom row). Thus, many of the CD127lo T cells died during contraction of the response. Between days 8 to 22 after infection, numbers of LCMV-specific CD127lo T cells declined more gradually in Bim−/− mice (I-Abgp61+, 4-fold; Dbnp396+, 2.8-fold; Dbgp33+ 4.5-fold decreases) (Fig. 4, bottom row). Compared to Bim+/+ mice, greater numbers of CD127lo CD8+ Dbgp33+, Dbnp396+, and CD4+ I-Abgp61+ T cells were detected in Bim−/− mice on days 15 and day 22 after infection (Fig. 4, bottom row). Thus, the absence of Bim affords significant survival of LCMV-specific, CD127lo CD4+ and CD8+ T cells.
CD127 re-expression on Bim−/− T cells
It was possible that the increased numbers of LCMV-specific T cells in Bim−/− mice was due to increased proliferation rather than survival. We next measured proliferation by labeling T cells in LCMV-infected mice with BrdU between days 8 and 15 after infection. Compared to Bim+/+ mice, in vivo proliferation of I-Abgp61+ T cells was actually lower in Bim−/− mice compared to Bim+/+ controls (Fig. 5A). Percentages of CD8+ Dbnp396+ and Dbgp33+ T cells that were BrdU+ were not significantly different between Bim−/− and Bim+/+ mice (Fig. 5A). These data suggest that the increased numbers of tetramer+ T cells in Bim−/− mice was due to survival rather than proliferation.
Figure 5.
Increased numbers of LCMV-specific CD127hi T cells in Bim−/− mice is due to survival and re-expression not proliferation. (A) Groups of Bim+/+ (open bars) and Bim−/− (filled bars) mice (n=5 mice/group)were infected with LCMV and were labeled in vivo with BrdU between days 8 to 14 after infection. On day 15, mice were killed and proliferation of tetramer+ spleen cells was assessed by flow cytometry. Results show the percentage of MHC tetramer+ T cells that were BrdU+ ± SEM. * denotes a statistically significant difference between Bim+/+ and Bim−/− mice (p<0.01, Student's two-sample t-test). (B) Groups of Bim+/+ and Bim−/− mice (n=3 mice/group) were infected with LCMV and 9 days later were killed; CD127lo spleen cells were purified and cultured at 37°C. CD127 re-expression was assessed after 3 and 40 h in vitro, by flow cytometry. Results show (B) the percent of tetramer+ cells that were CD127hi at 3 and 40 h after culture ± SD; or (C) the fold increase in live CD127hi tetramer+ T cells between 3 and 40 h of culture. *denotes significant differences in the fold increases of CD127hi cells between Bim+/+ and Bim−/− mice (at least p≤0.02 one-way ANOVA Minitab for windows). This experiment is one of two independent experiments with similar results.
The numbers of CD127hi LCMV-specific T cells increased in Bim−/− mice between days 8 and 15 after infection without significant differences in proliferation between Bim−/− and Bim+/+ mice. We next reasoned that such increases in CD127hi T cells could be due to re-expression of CD127 on cells that did not undergo apoptosis in Bim−/− mice. To test this, we infected groups Bim−/− and Bim+/+ mice with LCMV and at day 8 after infection purified CD127lo spleen cells, cultured them in vitro, and assessed their ability to re-express CD127 over time. Notably, an increased percentage of I-Abgp61+ T cells from Bim−/− mice were CD127hi after culture compared to Bim+/+ T cells (Fig. 5B). Also, I-Abgp61+ T cells from Bim−/− and Bim+/+ mice had a greater capacity to re-express CD127 than did either Dbgp33+ or Dbnp396+ T cells (Fig. 5B). Although the percentage of CD127hi Dbgp33+ or Dbnp396+ T cells after culture was not different between Bim−/− and Bim+/+ mice; significantly more CD127hi cells accumulated in cultures of Bim−/− cells (Fig. 5C). These data suggest that accumulation of CD127hi T cells in Bim−/− mice is due to re-expression on previously CD127lo cells.
Increased numbers of functional memory T cells in Bim−/− mice
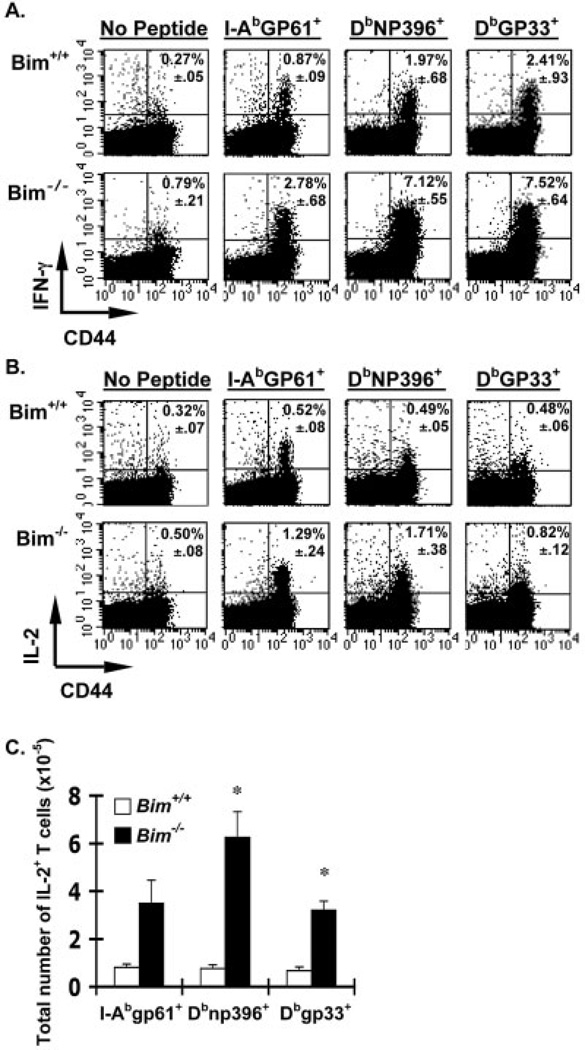
It is possible that the cells that normally undergo apoptosis are functionally different or inactivated compared to the cells that remain and become memory T cells. Clearance of LCMV by Bim−/− mice (data not shown), suggested that their CD8+ T cells were functional because clearance of LCMV requires perfor-in-dependent CTL activity [36]. We next assessed the functionality of peptide-specific Bim−/− T cells using intracellular cytokine staining. At day 38 after infection, Bim−/− mice had significantly increased LCMV-specific CD4+ and CD8+ IFN-γ-producing T cells compared to Bim+/+ mice (Fig. 6A). Further, similar numbers of T cells in Bim−/− mice were observed whether measured by tetramer or by intracellular IFN-γ staining (data not shown), suggesting that the cells that had avoided apoptosis in Bim−/− mice were functional.
Figure 6.
Increased functional memory T cells in the absence of Bim. (A) Groups (n=4–5/group) of Bim+/+ (upper panels) or Bim−/− (lower panels) mice were infected with LCMV, killed at either 38 (A) or 67 (B, C) days later, and spleen cells were stimulated ± LCMV peptides and IFN-γ or IL-2 production assessed by intracellular cytokine staining. Representative dot plots are shown; numbers in upper right quadrant are the average percentage of CD4+ or CD8+ cells that are IFN-γ+ ± SEM. No peptide control plots show gated CD4+ T cells. (B) Representative dot plots are shown; numbers in upper right quadrant indicate the average percentage of CD4+ or CD8+ cells that are IL-2+ ± SEM. No peptide control plots show gated CD8+ T cells. Note: GP33–41 plots also contain T cells that are specific for GP34–41 and restricted to Kb. (C) Total numbers of IL-2-producing, LCMV-specific T cells were significantly increased in Bim−/− mice. * denotes statistically significant difference between Bim+/+ and Bim−/− mice in the numbers of IL-2-producing T cells that are Dbgp33+ (p<0.008) and Dbnp396+ (p<0.015, Student's two-sample t-test). Numbers of IL-2-producing I-Abgp61+ T cells were increased in Bim−/− mice (p<0.07, Student's two-sample t-test).
Recent studies in the LCMV model have shown that IL-2 production after peptide stimulation ex vivo is a marker of CD8+ T cell memory [37, 38]. To test whether the absence of Bim-mediated apoptosis could affect numbers of IL-2-producing memory T cells, we performed intracellular IL-2 staining on Bim−/− and Bim+/+ T cells at 67 days after infection. Both the percentages and absolute number of IL-2-producing LCMV-specific CD4+ and CD8+ T cells were increased in Bim−/− compared to Bim+/+ mice (Fig. 6B, C). Taken together, these data show that the absence of Bim significantly increases the numbers of functional IL-2-secreting memory T cells.
Decreased proliferative renewal of Bim−/− T cells
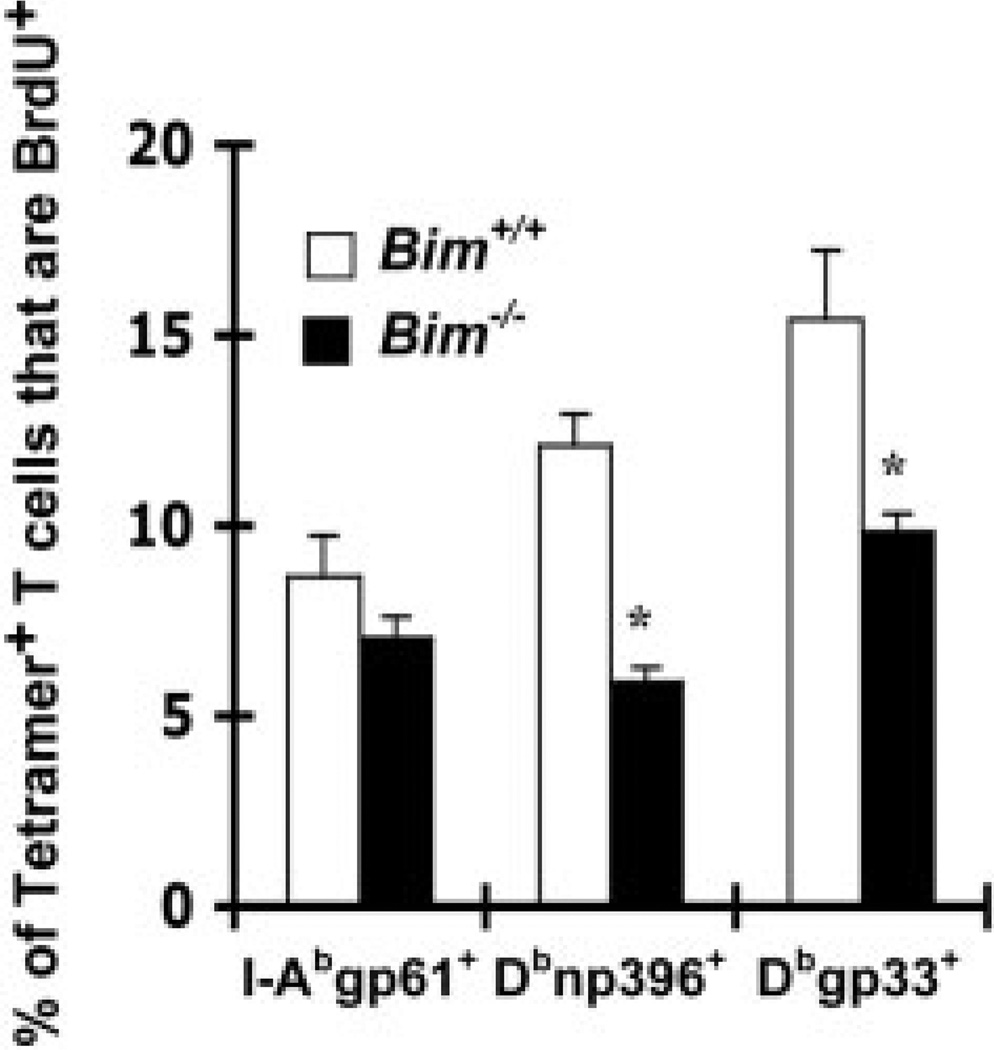
A major mechanism by which memory T cells are maintained is through their ability to slowly divide in response to available cytokines [27, 39–41]. Because of the slow attrition of memory T cells in Bim−/− mice, we assessed their turnover in vivo relative to memory T cells in Bim+/+ mice. We infected groups of Bim+/+ and Bim−/− mice with LCMV, allowed memory cells to develop for 214 days, labeled cells with BrdU for 2 weeks, then measured in vivo proliferation by flow cytometry. The percent of I-Abgp61+ T cells that were BrdU+ was slightly decreased in Bim−/− compared to Bim+/+ mice (Fig. 7). Further, the percentages of Dbgp33+ and Dbnp396+ T cells that were BrdU+ were significantly decreased in Bim−/− compared to Bim+/+ mice (Fig. 7). These data suggest that decreased proliferative renewal of memory T cells in Bim−/− mice contributes to their slow attrition.
Figure 7.
Bim−/− memory T cells exhibit decreased proliferative renewal. Groups (n=4–5/group) of Bim+/+ (open squares) or Bim−/− (closed squares) mice were infected with LCMV and labeled in vivo with BrdU between days 215 and 228 after infection. Mice were killed 229 days after infection and the percent of tetramer+ T cells that were BrdU+ was assessed by flow cytometry. *Significant differences were observed between Bim−/− and Bim+/+ mice for Dbnp396+ and Dbgp33+ T cells (at least p<0.03, Student's two-sample t-test).
Discussion
Maintenance of T cell homeostasis is critical for normal functioning of the adaptive immune system. Transient disruption of homeostasis occurs when naïve T cells undergo Ag-driven expansion and acquire effector functions. Effector T cells then make a fate decision by either undergoing apoptosis (i.e., contraction at the population level) or survive and become memory cells. This process is crucial: it resets homeostasis, promotes protective immunity, and limits autoimmunity. Our data clearly show that the intrinsic pathway and more specifically, the pro-apoptotic Bcl-2 family member, Bim, plays a critical role in this process. These data confirm and extend previous work by us in a model of superantigen-driven deletion [10] and by others examining the “shut-down” of HSV-specific CD8+ T cells [12]. In these previous studies, the effect of Bim on survival of CD127-expressing T cells and on long-term T cell memory was not investigated. Importantly, here we have shown that the numbers of LCMV-specific CD127lo CD4+ and CD8+ T cells die during the contraction of the response and the absence of Bim largely prevents their loss. Further, in the absence of Bim-mediated apoptosis, many of these CD127lo T cells become CD127hi, resulting in significant increases in LCMV-specific, IL-2-producing memory T cells. Thus, Bim limits the numbers of effector T cells available for entry into the memory compartment.
There are also subtle differences in contraction of LCMV-specific CD4+ versus CD8+ T cell responses. Similar to a previous report [26], we found that the initial apoptotic decline of tetramer+ CD4+ T cells is less than that of tetramer+ CD8+ T cells. Interestingly, we found that more IAbgp61+ T cells were CD127hi at 8 days after infection compared to either tetramer+ CD8+ population. It is possible that the decreased magnitude of the decline of the LCMV-specific CD4+ T cell response is due to less “bim-susceptible” LCMV-specific CD4+ CD127lo T cells at the peak of the response. Another non-mutually exclusive possibility is that expression and regulation of anti-apoptotic molecules may be different between LCMV-specific CD4+ and CD8+ T cells. Three observations support the notion of differences in antiapoptotic molecules between CD4+ and CD8+ T cells. First, we and others have shown that, naïve CD4+ T cells express lower levels of Bcl-2 compared to CD8+ T cells [10, 26], but both CD4+ and CD8+ T cells express similar levels of Bim. Second, we have found that CD8+ T cells are significantly more dependent upon Bcl-2 than are CD4+ T cells for survival in vivo (data not shown). Third, two alleles of Bim are required for complete contraction of CD4+ T cell responses but one allele is sufficient to contract CD8+ responses, suggesting that CD4+ T cells are more efficient at restraining Bim. Thus, CD4+ T cells have a somewhat greater ability to counteract Bim-mediated apoptosis potentially through greater redundancy in their anti-apoptotic arsenal compared to CD8+ T cells. Although further work is necessary to identify the range of anti- and pro-apoptotic Bcl-2 family members expressed by CD4+ and CD8+ T cells, it is clear that Bcl-2 plays a critical role in regulating Bim-mediated apoptosis.
Decreased Bcl-2 expression is observed in both superantigen-activated T cells [10] and in LCMV-specific CD4+ and CD8+ T cells [17, 26], just prior to the onset of apoptosis. Notably, TCR transgenic CD8+ LCMV-specific CD127lo T cells contain decreased levels of Bcl-2 compared to their CD127hi counterparts [19], an observation that we have repeated in non-TCR Tg LCMV-specific T cells (data not shown). Because Bim is bound to Bcl-2 in resting T cells [15] and such interactions promote Bim inactivation, it is likely that decreased expression of Bcl-2, rather than increased expression of Bim, is a mechanism involved in unleashing the pro-apoptotic activity of Bim. An increased amount of Bim that is not complexed to Bcl-2 could then activate Bax and/or Bak, which are required to complete Bim-mediated apoptosis [42, 43].
Interestingly, while the numbers of CD127lo T cells were spared in Bim−/− mice, when assessed on a percentage basis, the percent of tetramer+ T cells expressing high levels of CD127 was not different between Bim+/+ and Bim−/− mice. This was because, in Bim−/− mice, numbers of both CD127hi and CD127lo T cells were increased relative to Bim+/+ mice. The lack of proliferation differences between tetramer+ Bim−/− and Bim+/+ T cells in vivo between days 8 and 15, suggests that at least some of the cells that were CD127lo and have avoided Bim-mediated apoptosis, can increase their expression of CD127. Indeed, our in vitro data clearly show substantial increases in the numbers of CD127hi T cells preferentially in Bim−/− mice. As IL-7 can induce down-regulation of its own receptor [44], it is possible that the increased numbers of T cells surviving in the absence of Bim drives increased expression of CD127 due to increased competition for IL-7. On the other hand, our data showing that naïve Bim−/− T cells actually have decreased levels of CD127 compared to naïve Bim+/+ T cells despite having more T cells in the periphery argues against this as a potential mechanism. However, as loss of Bim rescues a block in T cell development that occurs in CD127-deficient mice [45], it is possible that some of the peripheral T cells in Bim−/− mice are CD127lo cells that would normally have been deleted in the thymus. We are currently investigating mechanism(s) underlying control CD127 expression in Bim−/− mice.
A previous report has suggested that the CD127hi T cells on day 8 are “selected” to become memory cells [19]. The implication of this is that that the cells that are CD127hi on day 8 after infection are the same cells (or descendents thereof) as the CD127hi cells observed on days 15 and 21 after infection. While this may be true, we note that our data does not necessarily support this conclusion. First, we show that after contraction of the response, a substantial number of CD127lo T cells are present. Second, expression of CD127 is dynamic and there is substantial evidence of interconversion between CD127hi and CD127lo T cells. Recent studies, including ours here, have shown that simply removing T cells from mice and placing them in culture results in re-expression of CD127 [44, 46]. Additionally, we provide evidence that CD127lo to CD127hi transitions can occur in vitro and in vivo, at least in the absence of Bim. Further, we also recently showed that, during infection of wild-type mice with LCMV clone 13, LCMV-specific CD4+ and CD8+ CD127hi T cells emerge after viral clearance [47], and another group has shown CD127lo to CD127hi transition on adoptively transferred CD8+ T cells [21]. The data suggesting that selection of CD127hi T cells occurs during T cell contraction came from adoptive transfer experiments in which LCMV-specific CD127hi versus CD127lo T cells were transferred into naïve recipients [19, 21]. We note that the use of naïve mice as recipients does not necessarily mimic conditions that occur in vivo during LCMV infection. Thus, while our data clearly demonstrate that many CD127lo T cells succumb to Bim-mediated apoptosis in vivo, further work is required to determine whether CD127hi T cells are selected for or can emerge out of the CD127lo pool during acute LCMV infection.
Our data also suggest that Bim plays an important role in limiting initial entry of T cells into the memory compartment. Indeed, Bim−/− mice had significantly more LCMV-specific, IL-2-producing T cells than Bim+/+ mice. Further, many of the CD127lo cells that likely would have died in Bim+/+ mice survived and re-expressed CD127 in Bim−/− mice. Although LCMV-specific CD4+ and CD8+ T cell responses were significantly prolonged in Bim−/− mice, eventually however, the number of these cells at later time points decreased. Similar results were observed in LCMV-infected mice treated during the contraction phase with IL-2 [48]. Treatment of LCMV-infected mice with IL-2 prolonged survival of effector CD8+ T cells, likely through up-regulation of Bcl-2 and/or Bcl-xL molecules [48], which would help block Bim-mediated apoptosis. Similar to our results, the numbers of LCMV-specific CD8+ T cells in IL-2-treated mice eventually declined to levels found in control treated mice [48]. In LCMV infection, memory CD8+ and CD4+ T cells are maintained by proliferative renewal driven by IL-15 and IL-7, respectively [27, 39]. The decreased proliferation observed for Bim−/− memory T cells along with their slow attrition suggests that the available levels of IL-15 can only support a certain number of memory T cells. Thus, while Bim limits the numbers of effectors available for memory, other mechanisms, such as cytokine availability, control their long-term survival.
Finally, these data have implications for the ability to manipulate Ag-specific T cell responses in vivo. Interference with contraction of the T cell response can lead to increased T cell memory in vivo, and could potentially be exploited to increase vaccine efficiency. However, our data suggest that such protective immunity may be relatively short-lived as the numbers of memory T cells eventually decline. Nonetheless, for vaccinations that are suboptimal or require several rounds of “boosting”, limiting Bim-mediated apoptosis during immunization may provide enhanced immunity under conditions where less stimulation may be needed.
Materials and methods
Mice and injections
Bim-deficient mice (a generous gift of Drs. Andreas Strasser and Philippe Bouillet, WEHI, Melbourne, Australia) were backcrossed 15 times to C57BL/6 mice [10, 31]. Bim+/+ mice used for experiments were either C57BL/6 purchased from Jackson Labs, Taconic Farms, or were from our Bim colony. B6.SJL-Ptprca/BoAiTac mice used as recipients in adoptive transfer experiments were purchased from Taconic Farms or were bred in house. Bim+/+, Bim+/−, Bim−/− mice were identified by PCR from tail DNA and by the death rate of their LN or splenic T cells in vitro. All mice were used between 8 and 16 weeks of age. All animals were housed under specific pathogen-free conditions in the Animal Facility at Cincinnati Children's Hospital Research Foundation. Experimental procedures were reviewed and approved by the institutional animal care and use committee.
Viruses
The Armstrong-3 strain of LCMV, a kind gift from Dr. Rafi Ahmed (Emory University, Atlanta, GA.), was grown in BHK-21 cells; the number of plaque forming units (PFU) was assayed on Vero cells as previously described [49]. Mice were injected i.p. with 0.25 mL LCMV (2 × 105 PFU) diluted in balanced salt solution (BSS). rVV expressing I-Ab with the covalently bound I-Eα mutant peptide EAWGALANWAVDSA, referred to as “2W1S” [23, 25] was a generous gift of Dr. Thomas Mitchell (University of Louisville).
Generation of MHC tetrameric staining reagents
MHC class II tetrameric staining reagents were created as described [22, 23]. Briefly, recombinant baculoviral plasmids encoding I-Ab plus genetically linked peptide was transfected into SF-9 insect cells to produce baculoviral stocks. Baculoviral SF-9 supernatant was harvested and used to infect Hi-5 insect cells (Invitrogen). After infection, Hi-5 cells produced and secreted assembled monomeric I-Ab peptide complexes. Hi-5 cell supernatants were harvested, monomers purified by affinity chromatography using anti-I-Ab antibody (M5–114), and eluted using a high pH buffer. After purification of monomeric MHC-peptide complexes, the complexes were buffer exchanged into biotinylation buffer and biotinylated with the recombinant enzyme BirA (Avidity, Inc.). After biotinylation, unbound biotin was removed and buffered exchanged into PBS using centricon concentrators. Biotinylated monomers were coupled to streptavidin-phycoerythrin and subsequently subjected to size exclusion FPLC chromatography on Superdex-200 to separate large multimeric complexes from excess free monomeric complexes.
The methodology for preparation of MHC class I tetramers was modified from the protocol described by Altman and coworkers [50]. Recombinant MHC class I molecules fused to a BirA substrate peptide and recombinant β2-microglobulin were produced in Escherichia coli BL21 (DE3) cells as described [51]. Recombinant proteins were solubilized in 8 M urea, 10 mM EDTA, 0.1 mM DTT, 25 mM MES pH 6.0, aliquoted and snapfrozen. MHC-peptide complexes were generated by folding class I heavy chains in vitro with β2-microglobulin and defined LCMV-derived peptide epitopes. Soluble MHC class I peptide complexes were concentrated using a stirred ultrafiltration cell (Amicon, Bedford, MA) and enzymatic biotinylated with recombinant BirA enzyme. Biotinylated MHC monomers were purified over a Sepharose-G75 column (Amersham-Pharmacia) and fractions containing biotinylated class I-β2-microglobulin-peptide complexes were pooled and exchanged into 20 mM Tris pH 8.0 using centrifugal filtration devices (Millipore, Bedford, MA). The complexes were further purified by ion exchange chromatography using a QM-Sepharose column (Amersham-Pharmacia). Appropriate fractions were concentrated and dialyzed against PBS containing 2 mM EDTA, 1 µg/mL leupeptin, 1 µM pepstatin, and 200 µM PMSF (Sigma, St. Louis, MO). The complexes were tetramerized by the step-wise addition of allophycocyanin (APC)-conjugated streptavidin (Molecular Probes, Eugene, OR).
Flow cytometric staining of Ag-specific T cells
At various days after infection, mice were killed and lymph nodes or spleens from individual mice were removed. To detect LCMV-specific CD4+ and CD8+ T cells, 2 × 106 LN or spleen cells/well were stained with Dbgp33, Dbnp396, or Kbnp205 tetrameric staining reagents for 2 h at 37°C (I-Abgp61–80 tetramer) or 90 min at 4°C (class I tetramers). During the last 45 min of incubation, cells were stained with various combinations of cell surface marker antibodies (e.g., anti-CD4, -CD8, -CD16/32, -CD44, -CD62L, -CD127 from either BD Pharmingen, E Biosciences, or produced in house), then washed and fixed with 2% paraformaldehyde (PFA). A minimum of 5 × 105 events were acquired on a FACSCalibur flow cytometer and analyzed using CellQuest software.
Bim levels were measured by intracellular immunofluorescence staining and flow cytometric analysis. First, anti-Bim antibody was purified from culture supernatants of Ham149.51 hybridoma cells (a generous gift of Dr. Philippa Marrack, Howard Hughes Medical Institute, Denver, CO) over Sepharose protein G columns, dialyzed into sodium bicarbonate buffer and coupled to AlexaFluor 488 (Molecular Probes). Single spleen cell suspensions from day 9-infected Bim+/+ mice were stained with MHC-tetrameric staining reagents as described above. Cells were then permeabilized with 0.03% saponin in BSS and stained with either anti-Bim-AlexaFluor 488 or hamster-IgG-FITC control antibody for 45 min, then washed and fixed with 2% PFA. Data were acquired using a FACSCalibur flow cytometer and analyzed using CellQuest software.
To assess T cell viability, spleen cells from LCMV-infected Bim+/+ or Bim−/− mice (n=4/group) were first stained with either MHC class I or class II staining reagent to determine the number of live Ag-specific T cells. Then 2 × 106 spleen cells from each mouse were cultured for 24 h in vitro at 37°C. After culture, cells were harvested and stained with either MHC class I or class II staining reagent and the number of live tetramer+ cells quantified by flow cytometric analysis. Live cells were gated based on their light scatter properties as described previously [10, 33].
For CD127 re-expression experiments, CD127lo spleen cells from day 9 LCMV infected Bim+/+ or Bim−/− mice (n=3 mice/group) were purified using a MACS kit (Miltenyi Biotech) in combination with an anti-CD127-biotin (produced in house) and anti-Biotin beads. CD127lo spleen cells (1.5 × 106) were cultured (in triplicate for each tetramer stain) at 37°C for either 3 or 40 h. After culture, cells were counted on a hemocytometer and stained with MHC tetramers and antibodies against CD4, CD8, CD127, CD16/32 and data acquired on a FACSCalibur flow cytometer.
Intracellular cytokine staining was performed as described [51]. Briefly, spleen cells from LCMV-infected or control uninfected mice were cultured at 37°C for 4–5 h with or without various LCMV peptides (GP61–80 10 µg/mL; GP33–41, NP396–404, NP205–214; all at 1 µg/mL) and BrefeldinA at 10 µg/mL. After culture, cells were stained for cell surface markers (CD4, CD8, CD44, antibodies from BD Pharmingen) for 45 min at 4°C. Cells were then washed, fixed and permeabilized with 0.03% saponin and stained intracellularly with PE-labeled anti-IFN-γ or anti-IL-2 antibody (BD Pharmingen). A minimum of 5 × 105 events were acquired on a FACSCalibur flow cytometer and analyzed using CellQuest software.
In vivo proliferation was determined by injecting mice i.p. with 100 µL BrdU (10 mg/mL) daily. Incorporation of BrdU In LCMV-specific CD8+ T cells was assessed by flow cytometry using a kit (BD Biosciences). Incorporation of BrdU in LCMV-specific CD4+ T cells was assessed by staining splenocytes with MHC class II tetramers, followed by overnight incubation in 1% PFA/0.05% igepal. Cells were then resuspended in 4.2 mM MgCl2 buffer containing 50 kunitz units/mL DNase I (Sigma) for 30 min at 37°C, washed and stained with anti-BrdU-FITC. Data were acquired using a FACSCalibur flow cytometer and analyzed using CellQuest software.
Adoptive transfer experiments
T cells were purified from spleens of day 10 LCMV-infected Bim+/+ or Bim−/− using a MACS pan T cell isolation kit (Miltenyi Biotech). Purity of T cells prior to adoptive transfer was almost always >90% for both Bim+/+ mice and Bim−/− mice. Purified T cells from either Bim+/+ or Bim−/− donors were washed twice with BSS, and 4 × 106 cells were injected separately into groups of recipient B6.SJL congenic mice. Groups of four recipient mice that had received either Bim+/+ or Bim−/− T cells were killed on days 1, 7, and 14 after injection and numbers of tetramer+Ly5.2+CD4+ or CD8+ T cells quantified in the spleens of individual recipient mice by flow cytometry.
Statistical analyses
Statistical analyses were performed using either a Student's two-sample t-test or an unstacked one-way analysis of variance (ANOVA) with Minitab for Windows Software (Release 14), State College, Pennsylvania.
Acknowledgements
We thank Dr. Philippa Marrack for her generous gift of Ham149.51 an anti-Bim secreting B cell hybridoma. We also thank Drs. Joerg Kohl, Claire Chougnet, Yasmine Belkaid, Thomas Mitchell, and Chris Karp for critical review of the manuscript. This work was supported by generous start-up funds from the Division of Immunobiology, a Trustee Grant from Cincinnati Children's Hospital Research Foundation, and Public Health Service Grant AI057753 (D.A.H.).
Abbreviations
- Bim
Bcl-2 interacting mediator of death
- BSS
balanced salt solution
- LCMV
lymphocytic choriomeningitis virus
- PFA
paraformaldehyde
- VV
vaccinia virus
Footnotes
Supporting information for this article is available at http://www.wiley-vch.de/contents/jc_2040/2006/35897_s.pdf
References
- 1.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 2.Butz EA, Bevan MJ. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis–immune regulation in a dynamic and unpredictable antigenic environment. Annu. Rev. Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 4.Peter ME, Krammer PH. Mechanisms of CD95 (APO-1/Fas)-mediated apoptosis. Curr. Opin. Immunol. 1998;10:545–551. doi: 10.1016/s0952-7915(98)80222-7. [DOI] [PubMed] [Google Scholar]
- 5.Cohen GM. Caspases: the executioners of apoptosis. Biochem. J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suresh M, Singh A, Fischer C. Role of tumor necrosis factor receptors in regulating CD8 T-cell responses during acute lymphocytic choriomeningitis virus infection. J. Virol. 2005;79:202–213. doi: 10.1128/JVI.79.1.202-213.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suresh M, Gao X, Fischer C, Miller NE, Tewari K. Dissection of antiviral and immune regulatory functions of tumor necrosis factor receptors in a chronic lymphocytic choriomeningitis virus infection. J. Virol. 2004;78:3906–3918. doi: 10.1128/JVI.78.8.3906-3918.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonfoco E, Stuart PM, Brunner T, Lin T, Griffith TS, Gao Y, Nakajima H, et al. Inducible nonlymphoid expression of Fas ligand is responsible for superantigen-induced peripheral deletion of T cells. Immunity. 1998;9:711–720. doi: 10.1016/s1074-7613(00)80668-8. [DOI] [PubMed] [Google Scholar]
- 9.Singer GG, Abbas AK. The Fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994;1:365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 10.Hildeman DA, Zhu Y, Mitchell TC, Bouillet P, Strasser A, Kappler J, Marrack P. Activated T cell death in vivo mediated by proapoptotic bcl-2 family member bim. Immunity. 2002;16:759–767. doi: 10.1016/s1074-7613(02)00322-9. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen LT, McKall-Faienza K, Zakarian A, Speiser DE, Mak TW, Ohashi PS. TNF receptor 1 (TNFR1) and CD95 are not required for T cell deletion after virus infection but contribute to peptide-induced deletion under limited conditions. Eur. J. Immunol. 2000;30:683–688. doi: 10.1002/1521-4141(200002)30:2<683::AID-IMMU683>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Pellegrini M, Belz G, Bouillet P, Strasser A. Shutdown of an acute T cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim. Proc. Natl. Acad. Sci. USA. 2003;100:14175–14180. doi: 10.1073/pnas.2336198100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mogil RJ, Radvanyi L, Gonzalez-Quintial R, Miller R, Mills G, Theofilopoulos AN, Green DR. Fas (CD95) participates in peripheral T cell deletion and associated apoptosis in vivo. Int. Immunol. 1995;7:1451–1458. doi: 10.1093/intimm/7.9.1451. [DOI] [PubMed] [Google Scholar]
- 14.Zhou S, Ou R, Huang L, Moskophidis D. Critical role for perforin-, Fas/FasL-, and TNFR1-mediated cytotoxic pathways in down-regulation of antigen-specific T cells during persistent viral infection. J. Virol. 2002;76:829–840. doi: 10.1128/JVI.76.2.829-840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu Y, Swanson BJ, Wang M, Hildeman DA, Schaefer BC, Liu X, Suzuki H, et al. Constitutive association of the proapoptotic protein Bim with Bcl-2-related proteins on mitochondria in T cells. Proc. Natl. Acad. Sci. USA. 2004;101:7681–7686. doi: 10.1073/pnas.0402293101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 17.Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J. Immunol. 2000;164:3950–3954. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell T, Kappler J, Marrack P. Bystander virus infection prolongs activated T cell survival. J. Immunol. 1999;162:4527–4535. [PubMed] [Google Scholar]
- 19.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 20.Huster KM, Busch V, Schiemann M, Linkemann K, Kerksiek KM, Wagner H, Busch DH. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Natl. Acad. Sci. USA. 2004;101:5610–5615. doi: 10.1073/pnas.0308054101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang KS, Recher M, Navarini AA, Harris NL, Lohning M, Junt T, Probst HC, et al. Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur. J. Immunol. 2005;35:738–745. doi: 10.1002/eji.200425828. [DOI] [PubMed] [Google Scholar]
- 22.Crawford F, Kozono H, White J, Marrack P, Kappler J. Detection of antigen-specific T cells with multivalent soluble class II MHC covalent peptide complexes. Immunity. 1998;8:675–682. doi: 10.1016/s1074-7613(00)80572-5. [DOI] [PubMed] [Google Scholar]
- 23.Rees W, Bender J, Teague TK, Kedl RM, Crawford F, Marrack P, Kappler J. An inverse relationship between T cell receptor affinity and antigen dose during CD4(+) T cell responses in vivo and in vitro. Proc. Natl. Acad. Sci. USA. 1999;96:9781–9786. doi: 10.1073/pnas.96.17.9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oxenius A, Bachmann MF, Ashton-Rickardt PG, Tonegawa S, Zinkernagel RM, Hengartner H. Presentation of endogenous viral proteins in association with major histocompatibility complex class II: on the role of intracellular compartmentalization, invariant chain and the TAP transporter system. Eur. J. Immunol. 1995;25:3402–3411. doi: 10.1002/eji.1830251230. [DOI] [PubMed] [Google Scholar]
- 25.Hildeman DA, Zhu Y, Mitchell TC, Kappler J, Marrack P. Molecular mechanisms of activated T cell death in vivo. Curr. Opin. Immunol. 2002;14:354–359. doi: 10.1016/s0952-7915(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 26.Homann D, Teyton L, Oldstone MB. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 2001;7:913–919. doi: 10.1038/90950. [DOI] [PubMed] [Google Scholar]
- 27.Lenz DC, Kurz SK, Lemmens E, Schoenberger SP, Sprent J, Oldstone MB, Homann D. IL-7 regulates basal homeostatic proliferation of antiviral CD4+ T cell memory. Proc. Natl. Acad. Sci. USA. 2004;101:9357–9362. doi: 10.1073/pnas.0400640101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 29.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 30.Bouillet P, Purton JF, Godfrey DI, Zhang LC, Coultas L, Puthalakath H, Pellegrini M, et al. BH3-only Bcl-2 family member Bim is required for apoptosis of autoreactive thymocytes. Nature. 2002;415:922–926. doi: 10.1038/415922a. [DOI] [PubMed] [Google Scholar]
- 31.Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 32.Hildeman DA, Mitchell T, Teague TK, Henson P, Day BJ, Kappler J, Marrack PC. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999;10:735–744. doi: 10.1016/s1074-7613(00)80072-2. [DOI] [PubMed] [Google Scholar]
- 33.Marrack P, Kappler J, Mitchell T. Type I interferons keep activated T cells alive. J. Exp. Med. 1999;189:521–530. doi: 10.1084/jem.189.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitchell TC, Hildeman D, Kedl RM, Teague TK, Schaefer BC, White J, Zhu Y, et al. Immunological adjuvants promote activated T cell survival via induction of Bcl-3. Nat. Immunol. 2001;2:397–402. doi: 10.1038/87692. [DOI] [PubMed] [Google Scholar]
- 35.Sandalova E, Wei CH, Masucci MG, Levitsky V. Regulation of expression of Bcl-2 protein family member Bim by T cell receptor triggering. Proc. Natl. Acad. Sci. USA. 2004;101:3011–3016. doi: 10.1073/pnas.0400005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 37.Masopust D, Kaech SM, Wherry EJ, Ahmed R. The role of programming in memory T-cell development. Curr. Opin. Immunol. 2004;16:217–225. doi: 10.1016/j.coi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 38.Kristensen NN, Christensen JP, Thomsen AR. High numbers of IL-2-producing CD8+ T cells during viral infection: correlation with stable memory development. J. Gen. Virol. 2002;83:2123–2133. doi: 10.1099/0022-1317-83-9-2123. [DOI] [PubMed] [Google Scholar]
- 39.Becker TC, Wherry EJ, Boone D, Murali-Krishna K, Antia R, Ma A, Ahmed R. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 2002;195:1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldrath AW, Sivakumar PV, Glaccum M, Kennedy MK, Bevan MJ, Benoist C, Mathis D, Butz EA. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J. Exp. Med. 2002;195:1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ku CC, Murakami M, Sakamoto A, Kappler J, Marrack P. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 42.Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15:1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rathmell JC, Lindsten T, Zong WX, Cinalli RM, Thompson CB. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat. Immunol. 2002;3:932–939. doi: 10.1038/ni834. [DOI] [PubMed] [Google Scholar]
- 44.Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, Singer A. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 45.Pellegrini M, Bouillet P, Robati M, Belz GT, Davey GM, Strasser A. Loss of Bim increases T cell production and function in interleukin 7 receptor-deficient mice. J. Exp. Med. 2004;200:1189–1195. doi: 10.1084/jem.20041328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J. Exp. Med. 2003;198:1807–1815. doi: 10.1084/jem.20030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fuller MJ, Hildeman DA, Sabbaj S, Gaddis DE, Tebo AE, Shang L, Goepfert PA, Zajac AJ. Cutting edge: Emergence of CD127high functionally competent memory T cells is compromised by high viral loads and inadequate T cell help. J. Immunol. 2005;174:5926–5930. doi: 10.4049/jimmunol.174.10.5926. [DOI] [PubMed] [Google Scholar]
- 48.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat. Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 49.Hildeman D, Yanez D, Pederson K, Havighurst T, Muller D. Vaccination against persistent viral infection exacerbates CD4+ T-cell-mediated immunopathological disease. J. Virol. 1997;71:9672–9678. doi: 10.1128/jvi.71.12.9672-9678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 51.Fuller MJ, Zajac AJ. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 2003;170:477–486. doi: 10.4049/jimmunol.170.1.477. [DOI] [PubMed] [Google Scholar]