Abstract
Individuals with Autism Spectrum Disorders (ASD) exhibit alterations in sensory processing, including changes in the integration of information across the different sensory modalities. In the current study, we used the sound-induced flash illusion to assess multisensory integration in children with ASD and typically-developing (TD) controls. Thirty-one children with ASD and 31 age and IQ matched TD children (average age = 12 years) were presented with simple visual (i.e., flash) and auditory (i.e., beep) stimuli of varying number. In illusory conditions, a single flash was presented with 2 to 4 beeps. In TD children, these conditions generally result in the perception of multiple flashes, implying a perceptual fusion across vision and audition. In the present study, children with ASD were significantly less likely to perceive the illusion relative to TD controls, suggesting that multisensory integration and cross-modal binding may be weaker in some children with ASD. These results are discussed in the context of previous findings for multisensory integration in ASD and future directions for research.
Keywords: Autism Spectrum Disorders, Sensory Processing, Audiovisual, Multisensory Integration, Audition, Vision
Autism Spectrum Disorders (ASD) are characterized by atypical social communication and by the presence of restricted interests and/or repetitive behaviors (APA & APA DSM-5 Task Force., 2013; APA, 2000). Individuals with ASD also frequently exhibit altered sensory processing, now recognized in the diagnostic criteria for ASD (APA & APA DSM-5 Task Force., 2013). In addition to hypo- and/or hyper-responsivity to sensory stimuli within individual modalities (e.g., vision, hearing, touch, proprioception), emerging evidence suggests children with ASD have selective deficits in integrating information across sensory modalities (APA & APA DSM-5 Task Force., 2013; and for review, see Iarocci & McDonald, 2006).
Multisensory integration (MSI) can be indexed in a variety of ways (Calvert & Thesen, 2004), and several approaches have been applied in the context of ASD. Multisensory illusions, in which the presentation of stimuli in one modality impacts perception in a second modality, can be powerful tools for assessing the strength of multisensory integration or binding. The most commonly used illusion to examine MSI in ASD is the McGurk effect (de Gelder, Vroomen, & Van der Heide, 1991; Iarocci, Rombough, Yager, Weeks, & Chua, 2010; Irwin, Tornatore, Brancazio, & Whalen, 2011; Irwin, Tornatore, Brancazio, & Whalen, 2011; Mongillo et al., 2008; Stevenson et al., In Press; Williams, Massaro, Peel, Bosseler, & Suddendorf, 2004; Woynaroski et al., 2013). The McGurk effect is an audiovisual illusion in which incongruent auditory and visual speech tokens (e.g., visual “ga” and auditory “ba”) are fused to generate a novel, illusory percept (e.g., “da” or “tha”) (McGurk & MacDonald, 1976). While the McGurk effect is the most often used, results are hard to interpret in ASD because they may result from differences in social-communication processing. To avoid this confounding variable, three studies have used the sound-induced flash illusion (SIFI). In this illusion, a single visual flash is presented concurrently with multiple auditory beeps (Shams, Kamitani, & Shimojo, 2000). When multiple beeps are presented, observers frequently report multiple flashes, reflecting perceptual integration of the visual and auditory cues (Calvert & Thesen, 2004). Thus, the SIFI indexes lower-level (i.e., simple, non-social) audiovisual MSI without confounding issues associated with social-communication processing.
To date, three studies have used the SIFI with individuals with ASD. Two failed to find differences between adults with ASD relative to typically developing (TD) controls (Keane, Rosenthal, Chun, & Shams, 2010; van der Smagt, van Engeland, & Kemner, 2007). A third study found differences between cognitively-able children with ASD and TD controls (Foss-Feig et al., 2010). Intriguingly, these data also suggested that children with ASD experienced an increased likelihood of integration relative to their TD peers, but only when flashes and beeps are presented asynchronously and at considerable temporal offsets of 200 ms or greater. To date, the original SIFI paradigm has not been used to investigate multisensory function in children with ASD. Hence, the current study sought to examine the susceptibility to the SIFI in a larger cohort of cognitively-able children with ASD and TD controls matched on age and IQ.
Methods
Participants
Participants included 31 children with ASD and 31 TD controls matched on chronological age and on verbal and performance IQ as measured with two-subtests of the Wechsler Abbreviated Scale of Intelligence, Second Edition (WASI-2; Wechsler, 1999) (Table 1). As all previous data suggests no gender differences in multisensory integration in ASD, gender distributions matched that of each population, with 26 of 31 in the ASD group being male, and 13 of 31 in the TD population being male. ASD diagnoses were based on the Autism Diagnostic Observation Schedule (Lord et al., 2000) and/or Autism Diagnostic Interview, Revised (Lord, Rutter, & Le Couteur, 1994) and clinical diagnosis by a practitioner familiar with ASD. Diagnoses were based on the Diagnostic and Statistical Manual of Mental Disorders IV-TR (Association, 2000). Individuals in the TD group had no diagnoses of ASD or any other developmental disorder or related medical diagnosis, including, but not limited to, Fragile X, tuberous sclerosis, or seizure disorders. Individuals in both groups were screened for normal or corrected- to-normal vision using a tumbling E chart and were reported to have normal hearing. All experimental protocols were approved by Vanderbilt University Medical Center’s Institutional Review Board.
Table 1.
Group demographics.
| Variable | ASD group | TD Group |
|---|---|---|
| N | 31ns | 31ns |
| Age | 12.1 ± 3.1 years (6-18)ns | 11.9 ± 2.9 years (6-18)ns |
| Gender | 26 male* | 13 male* |
| Verbal IQ | 56.4 ± 10.0 (45-78)ns | 61.1 ± 8.7 (48-78)ns |
| Performance IQ | 58.0 ± 5.5 (47-66)ns | 54.5 ± 7.5 (40-68)ns |
Groups did not significantly differ in chronological age, verbal IQ (T-scores), or performance IQ (T-scores).
non-significant
significant, p > 0.05.
Values reported include means and standard deviations with ranges in parentheses.
Stimuli
Visual stimuli consisted of a white ring on a black background with a duration of 10 ms, presented 60 cm from the participants. When multiple flashes were presented, they were separated by 43 ms intervals. Auditory stimuli consisted of a 3500 Hz, 7 ms tone, with the onset of the first beep concurrent with the visual flash. Trials included a single flash presented with 0-4 beeps or 1-4 flashes presented with no beep. Twenty-five trials per condition were presented in a randomized order. Visual stimuli were presented on a NEC MultiSync FE992 monitor at 100 Hz at a distance of approximately 60 cm from the participants at a luminance of 55.8 cd/m2. Auditory stimuli were presented binaurally via Phillips noise-cancelling SBC HN-110 headphones at 72 dB SPL. The duration of all visual and auditory stimuli, as well as the SOAs, was confirmed using a Hameg 507 oscilloscope with a photovoltaic cell and microphone.
Procedure
Participants sat inside an unlit, sound-attenuating WhisperRoom™ (Model SE 2000; Whisper Room Inc) that controlled for light and attenuated background noise. Task instructions were to fixated a central cross and report the number of visual flashes perceived via keyboard button press according to visual perception only, ignoring the beeps. Participants verbally confirmed that they understood the instructions. Trials comprised a fixation screen for 500 ms plus a random jitter ranging from 1 to 1000 ms, a stimulus presentation, a 250 ms fixation screen, and a response screen (“How many flashes did you see?”). Following a response, the fixation screen reappeared, and a subsequent trial was initiated. Participants were monitored by closed circuit infrared cameras to ensure compliance. Breaks were offered every 100 trials. The experimental SIFI procedure lasted approximately ten minutes. All participants were able to complete the task.
Analysis
Responses to the SIFI were calculated using trials in which a single flash was presented with a varying number of beeps (0-4). For each of these trial types, individuals’ mean response was calculated. Given that only a single flash was presented in these trials, any perception of multiple flashes indicates an illusory percept. To account for possible individual differences in response biases, responses to illusory conditions (one flash with 2-4 beeps) were normalized to each individual's responses to non-illusory conditions (one flash with 0 or 1 beep). Each individual's response to illusory trials was calculated relative to their mean response to these non-illusory conditions using:
where RCORn represents the corrected response, R0 and R1 represent responses to single flashes with zero and one beep respectively, and Rn represents the number of perceived flashes with n beeps (2-4) (R. A. Stevenson, Zemtsov, & Wallace, 2012). Group average responses were calculated as a mean of individuals’ means. Individuals’ mean responses were also calculated for each of the four visual-only conditions, and group average responses were calculated as a mean of individuals’ means. Thus, it should be noted that below, all results with illusory conditions are not discussed in absolute terms, but are discussed in terms of perceived illusory flashes relative to the individual's baseline responses in the non-illusory, one-flash with zero or one beep conditions.
Results
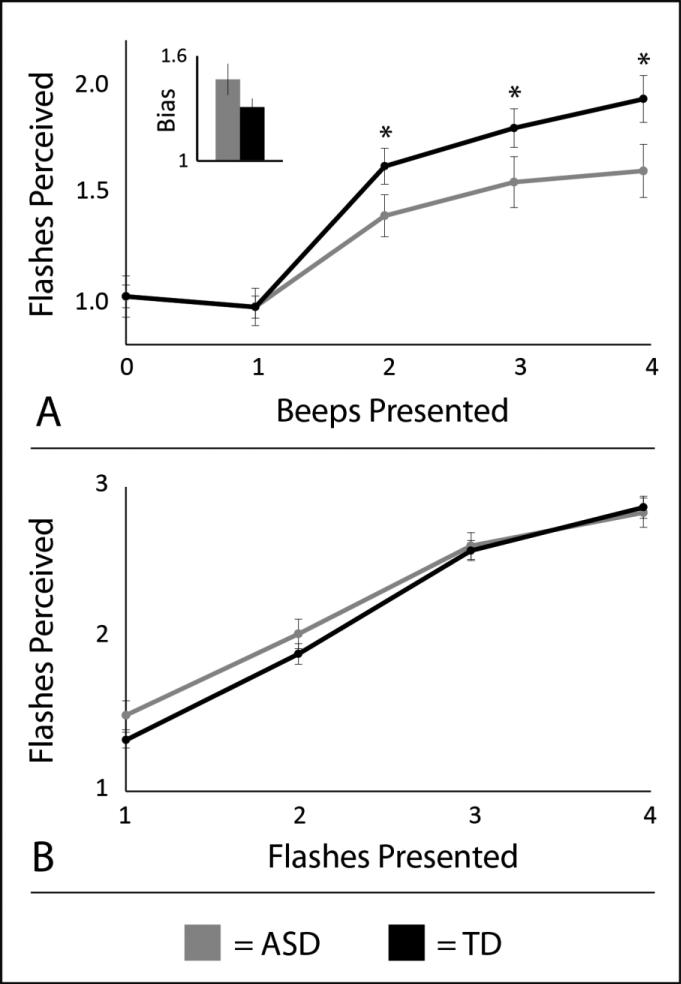
A two-way (# beeps X group) ANOVA was performed using individuals’ responses to the audiovisual conditions (one flash paired with 0 to 4 beeps). A significant main effect of number of beeps was found. Individuals perceived an increased number of illusory flashes on average with more beeps (F(60,4) = 132.18, p < 0.001, partial-η2 = 0.70). A significant main effect of diagnostic group was also found (F(60,1) = 9.43, p = 0.003, partial-η2 = 0.14). Participants with ASD perceived fewer flashes on average than controls. Finally, a significant interaction was found (F(60,4) = 8.71, p < 0.05, partial-η2 = 0.13). Individuals with ASD were more likely to perceive fewer flashes relative to their TD peers as the number of beeps increased. Follow-up, protected t-tests were conducted to compare diagnostic groups in each of the three illusory conditions (one flash paired with 2, 3 or 4 beeps). Individuals with ASD reported fewer flashes than TD controls when a single flash was accompanied by two beeps (t = 3.37, p = 0.002, d = 0.85), three beeps (t = 2.76, p = 0.010, d = 0.71), and four beeps (t = 2.97, p = 0.006, d = 0.76). See Figure 1A.
Figure 1.
Perceptions of the sound-induced flash illusion (SIFI) in individuals with and without ASD. ASD = Autism spectrum disorders. TD = Typically developing control group. In illusory trials with a single flash and multiple beeps (Panel A), differences were found between groups with ASD (gray) and age-matched TD controls (black). The number of perceived flashes was normalized to each individual's rate of perceived flashes in the no illusory conditions with one flash paired with one beep or no beeps (inset). No group differences were seen in response biases. Visual-only control conditions revealed no group differences in the ability to perceive visual only stimuli (Panel B).
Raw responses indicating the number of perceived flashes to conditions in which individuals were presented with a single flash paired with zero or one beeps (i.e., the trials used for individualized response bias correction). These raw responses, were also compared across groups (Figure 1A, insert). No significant difference was seen with these trials (t = 1.55, p = 0.12, d = 0.39). Individuals with ASD reported an average of 1.47 (SD = 0.50, range = 1.02-3.30) perceived flashes, while those without ASD reported 1.31 (SD = 0.28, range = 1.00-2.02). These raw data provide assurance that the observed group differences were not caused by one group reaching ceiling.
To examine differences in visual performance, a two-way (# flashes X group) ANOVA was performed with individuals’ responses to visual-only conditions (1, 2, 3 and 4 flashes with no beeps). A significant main effect of number of flashes was found (F(60,3) = 237.72, p < 0.001, partial-η2 = 0.80), but no main effect of diagnostic group (F(60,1) = 0.35, p = 0.56, partial-η2 = 0.01) or interaction effect was observed (F(60,3) = 1.38, p = 0.25, partial-η2 = 0.02). See Figure 1B.
To examine for possible gender differences, a two way, repeated-measures ANOVA (gender X number of beeps) was performed using individuals’ responses to the audiovisual conditions (one flash paired with 0 to 4 beeps). No significant main effect of gender was found (F(60,1) = 2.39, p = 0.13, partial-η2 < 0.04). Likewise, no significant interaction was found (F(60,4) = 2.29, p = 0.14, partial-η2 < 0.04). In order to be conservative in this control, individual t-tests were conducted for each individual audiovisual condition (0-4 flashes) despite a non-significant interaction effect. Even with no correction for multiple comparisons (which makes these statistics more conservative in terms of ensuring no impact of gender), no effect of gender was seen in any condition (0 beeps, t = 1.48, p = 0.14, d = 0.39; 1 beep, t = 1.48, p = 0.14, d = 0.39; 2 beeps, t = 1.36, p = 0.18, d = 0.37; 3 beeps, t = 1.53, p = 0.13, d = 0.41; 4 beeps, t = 1.57, p = 0.12, d = 0.42).
Discussion
The current study used the SIFI to examine lower-level MSI in high-functioning children with ASD. Children with ASD showed less susceptibility to the SIFI relative to TD controls matched on chronological age and general intellectual ability, suggesting a weakness in their ability to bind low-level auditory and visual signals. Similar performance for children with ASD and controls in visual-only conditions indicates that these results are not a result of differences in unisensory visual processes associated with flash perception.
Only a single study to date has used the SIFI to investigate MSI in children with ASD (Foss-Feig et al., 2010). This study was designed to explore differential temporal processing by presenting a single flash with two beeps at varying temporal offsets, and was framed from the perspective that individuals with ASD have a greater width of their multisensory temporal binding window. Although this hypothesis was supported, a secondary finding of this study was that individuals with ASD were more likely to report an illusory flash than their TD peers. While we cannot definitively say what drives the differences between these results and those of the current study, there are a number of possibilities. Most obvious are the differences in experimental design, as the previous study's manipulated variable of interest was the timing between auditory and visual presentations. Additionally, the current study takes into account differential response biases across groups, while the prior study did not. Also, as with all studies of ASD, the difference in results may be partly attributable to differences in the characteristics of the population at study, where individuals with ASD can vary greatly in their degree of social and communicative dysfunction (Kuhl, Coffey-Corina, Padden, & Dawson, 2005) and severity of other sensory symptoms (Mongillo et al., 2008). Each of these possible explanations warrants investigation.
The SIFI has also been used in two studies of adults with ASD (Keane et al., 2010 - 18 to 49 years old; van der Smagt et al., 2007 - mean age 20.5 years old, range not reported). In these studies, no difference was found in the perception of the SIFI between groups with and without ASD. This discrepancy in results is not surprising, as individuals’ abilities to benefit from integrating audiovisual sensory input shows increases with development well into adolescence (Hillock, Powers, & Wallace, 2011; Ross et al., 2011), and previous studies have shown divergent developmental trajectories between populations with and without ASD, specifically with audiovisual illusions such as the McGurk effect (Taylor, Isaac, & Milne, 2010) as well as audiovisual speech-in-noise perception (Foxe et al., 2013), where it has been shown that while individuals show weaker integration in childhood, they “catch up” to their peers in adulthood (but see Ryan A Stevenson et al., In Press).
Findings of atypical multisensory processing fit well within the framework of a number of prevalent theories concerning the neurobiological bases of autism. For example, weak central coherence proposes that children with autism have difficulty integrating discrete information into combined, holistic perceptions and experiences (Happé & Frith, 2006); a view that is readily extended to include the integration of information across the different sensory modalities. In the context of the current results, the lessened susceptibility to the SIFI in ASD is interpreted to reflect weaknesses in this integration across the different sensory streams. While these results suggest atypical integration across the different streams, they could also be interpreted as an enhanced ability to segregate these streams, or an improved ability to actively ignore the auditory signal (it should be noted however, that the interpretations of decreased sensory integration and increased unisensory focus are not mutually exclusive). Indeed, previous research has shown unimpaired or enhanced processing of simple stimuli in ASD (Bertone, Mottron, Jelenic, & Faubert, 2005; Minshew & Hobson, 2008), including for unisensory visual (Bertone et al., 2005; Joseph, Keehn, Connolly, Wolfe, & Horowitz, 2009; O'Riordan M, 2004; M. O'Riordan & Plaisted, 2001; M. A. O'Riordan, Plaisted, Driver, & Baron-Cohen, 2001; Plaisted, O'Riordan, & Baron-Cohen, 1998a, 1998b) and auditory (Bonnel et al., 2003; M. O'Riordan & Passetti, 2006) stimuli.
Another theory of autism that maps onto the current data is the Bayesian “hypo-priors” explanation (Pellicano & Burr, 2012). Bayesian models describe a decision making process by which individuals process incoming sensory information based on its reliability and within the context of their prior experiences with the world. The hypo-priors hypothesis proposes that individuals with ASD weight their prior experiences very lightly, and instead rely upon the raw sensory information more heavily. Throughout typical development, individuals learn through experience that auditory and visual events that occur at around the same time (Hillock-Dunn & Wallace, 2012; Hillock et al., 2011; Lewkowicz, 2000, 2010, 2012; Lewkowicz & Flom, 2014) and in the same location (Neil, Chee-Ruiter, Scheier, Lewkowicz, & Shimojo, 2006) are very likely to originate from the same external event, and can thus be processed as a single, unified percept, resulting in more efficient processing (Altieri, Stevenson, Wallace, & Wenger, 2013). Under-weighting such prior probabilities based on previous experiences would lead to a more literal perception of the world in which individuals components of sensory inputs may be more accurately perceived (such as the number of flashes in the SIFI), but at the cost of a reduced ability to make perceptual inferences (Gregory, 1980; von Helmholtz, 2005).
Differences between how individual with and without ASD bind stimuli into a unified perceptual representation has also been described as a possible result of impaired temporal processing. The temporal binding deficit hypothesis posits that timing-related deficits are a core feature of autism (Brock, Brown, Boucher, & Rippon, 2002). The timing of sensory inputs and their processing times is vital to the ability to integrate sensory information within and across sensory modalities (Conrey & Pisoni, 2006; Dixon & Spitz, 1980; Stevenson, Altieri, Kim, Pisoni, & James, 2010; Stevenson, VanDerKlok, Pisoni, & James, 2011; Stevenson, Zemtsov, et al., 2012; van Wassenhove, Grant, & Poeppel, 2007; for review, see Vroomen & Keetels, 2010). Disruptions in timing–related circuits could give rise to supramodal or multisensory processing deficits. In fact, while our results run counter to those found in adults with ASD, the findings of atypical multisensory processing reported here are consistent with a number of recent studies investigating the role of temporal processing in ASD. A decrease in multisensory temporal precision has been found in virtually all studies using speech-related stimuli (Bebko, Weiss, Demark, & Gomez, 2006; de Boer-Schellekens, Eussen, & Vroomen, 2013; Kwakye, Foss-Feig, Cascio, Stone, & Wallace, 2011; Stevenson et al., 2014; Woynaroski et al., 2013), but has been found to be more variable for more simple, non-speech stimuli. Here, whereas two studies have found decreases in such precision or acuity (de Boer-Schellekens et al., 2013; Foss-Feig et al., 2010), two others have not (Bebko et al., 2006; Stevenson et al., 2014). Even in cases where no multisensory temporal difference was observed with simple, non-speech stimuli however, individuals with ASD's multisensory temporal processing abilities were significantly correlated with their ability to perceptually bind audiovisual sensory inputs (Stevenson et al., 2014).
Given these theoretical relations, future work should seek to characterize multisensory integration in ASD more thoroughly. Specific emphases should be placed on elucidating how these processes differentially develop in populations with and without ASD. Given the previously identified age-related changes in multisensory function, coupled with the discrepant findings for the SIFI across age groups, mapping the developmental trajectory of multisensory processing in individuals with ASD relative to their TD peers is vital. Recent studies have sought to examine the relationship between chronological age and multisensory function, specifically in terms of speech processing (Foxe et al., 2013; Stevenson et al., In Press; Taylor et al., 2010). In contrast, little-to-no work has addressed the development of lower-level audiovisual processing. Second, relating multisensory abilities to clinical symptoms represents an important gap in the literature, and future work is needed to begin to elucidate how multisensory function maps onto the core impacted domains such as social and communicative function. Finally, the findings in this study, and the prevalent pattern of inconsistencies reported in multisensory studies in ASD are intriguing and showcase the need to determine whether these differences are specific to certain stimulus types (e.g. social or speech stimuli), and what other factors (e.g. temporal/spatial) play a central role (Stevenson, Fister, Barnett, Nidiffer, & Wallace, 2012; Stevenson & Wallace, 2013; Vatakis & Spence, 2006).
Acknowledgements
Funding for this work was provided by a Banting Postdoctoral Fellowship, National Institutes of Health grant F32 DC011993, Multisensory Integration and Temporal Processing in ASD, a grant from the Vanderbilt Institute for Clinical and Translational Research, VICTR VR5807.1, Development and Modulation of Multisensory Integration, National Institutes of Health grant R34 DC010927, Evaluation of Sensory Integration Treatment in ASD, a Vanderbilt Kennedy Center MARI/Hobbs Award, the Vanderbilt Brain Institute, and the Vanderbilt University Kennedy Center. We also acknowledge the help of Walter Lee and Zachary Barnett for technical assistance, and Magali Segers for clinical input.
Contributor Information
Ryan A. Stevenson, Department of Hearing and Speech Sciences, Vanderbilt Brain Institute, Vanderbilt Kennedy Center, Vanderbilt University Medical Center; Psychology Department, University of Toronto
Justin K. Siemann, Neuroscience Graduate Program, Vanderbilt University
Tiffany G. Woynaroski, Department of Hearing and Speech Sciences, Vanderbilt University Medical Center
Brittany C. Schneider, Program in Neuroscience, Vanderbilt University
Haley E. Eberly, Program in Neuroscience, Vanderbilt University
Stephen M. Camarata, Department of Hearing and Speech Sciences, Vanderbilt University Medical Center
Mark T. Wallace, Department of Hearing and Speech Sciences, Psychology and Psychiatry, Vanderbilt Kennedy Center, Vanderbilt rain Institute, Vanderbilt University Medical Center
References
- Altieri N, Stevenson RA, Wallace MT, Wenger MJ. Learning to Associate Auditory and Visual Stimuli: Behavioral and Neural Mechanisms. Brain Topography. 2013 doi: 10.1007/s10548-013-0333-7. doi: 10.1007/s10548-013-0333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, & American Psychiatric Association DSM-5 Task Force . Diagnostic and statistical manual of mental disorders : DSM-5. 5th ed. American Psychiatric Association; Arlington, Va.: 2013. [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders-IV-TR. APA; Washington, D.C.: 2000. [Google Scholar]
- Bebko JM, Weiss JA, Demark JL, Gomez P. Discrimination of temporal synchrony in intermodal events by children with autism and children with developmental disabilities without autism. J Child Psychol Psychiatry. 2006;47(1):88–98. doi: 10.1111/j.1469-7610.2005.01443.x. doi: JCPP1443 [pii] 10.1111/j.1469-7610.2005.01443.x. [DOI] [PubMed] [Google Scholar]
- Bertone A, Mottron L, Jelenic P, Faubert J. Enhanced and diminished visuo-spatial information processing in autism depends on stimulus complexity. Brain. 2005;128(Pt 10):2430–2441. doi: 10.1093/brain/awh561. doi: awh561 [pii] 10.1093/brain/awh561. [DOI] [PubMed] [Google Scholar]
- Bonnel A, Mottron L, Peretz I, Trudel M, Gallun E, Bonnel AM. Enhanced pitch sensitivity in individuals with autism: a signal detection analysis. J Cogn Neurosci. 2003;15(2):226–235. doi: 10.1162/089892903321208169. doi: 10.1162/089892903321208169. [DOI] [PubMed] [Google Scholar]
- Brock J, Brown CC, Boucher J, Rippon G. The temporal binding deficit hypothesis of autism. Dev Psychopathol. 2002;14(2):209–224. doi: 10.1017/s0954579402002018. [DOI] [PubMed] [Google Scholar]
- Calvert GA, Thesen T. Multisensory integration: methodological approaches and emerging principles in the human brain. J Physiol Paris. 2004;98(1-3):191–205. doi: 10.1016/j.jphysparis.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Conrey B, Pisoni DB. Auditory-visual speech perception and synchrony detection for speech and nonspeech signals. J Acoust Soc Am. 2006;119(6):4065–4073. doi: 10.1121/1.2195091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer-Schellekens L, Eussen M, Vroomen J. Diminished sensitivity of audiovisual temporal order in autism spectrum disorder. Front Integr Neurosci. 2013;7:8. doi: 10.3389/fnint.2013.00008. doi: 10.3389/fnint.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gelder B, Vroomen J, Van der Heide L. Face recognition and lip-reading in autism. European Journal of Cognitive Psychology. 1991;3(1):69–86. [Google Scholar]
- Dixon NF, Spitz L. The detection of auditory visual desynchrony. Perception. 1980;9(6):719–721. doi: 10.1068/p090719. [DOI] [PubMed] [Google Scholar]
- Foss-Feig JH, Kwakye LD, Cascio CJ, Burnette CP, Kadivar H, Stone WL, Wallace MT. An extended multisensory temporal binding window in autism spectrum disorders. Exp Brain Res. 2010;203(2):381–389. doi: 10.1007/s00221-010-2240-4. doi: 10.1007/s00221-010-2240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxe JJ, Molholm S, Del Bene VA, Frey HP, Russo NN, Blanco D, Ross LA. Severe Multisensory Speech Integration Deficits in High-Functioning School-Aged Children with Autism Spectrum Disorder (ASD) and Their Resolution During Early Adolescence. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht213. doi: 10.1093/cercor/bht213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory RL. Perceptions as hypotheses. Philos Trans R Soc Lond B Biol Sci. 1980;(290):181–197. doi: 10.1098/rstb.1980.0090. [DOI] [PubMed] [Google Scholar]
- Happé F, Frith U. The Weak Coherence Account: Detail-focused Cognitive Style in Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. 2006;36(1):5–25. doi: 10.1007/s10803-005-0039-0. doi: 10.1007/s10803-005-0039-0. [DOI] [PubMed] [Google Scholar]
- Hillock-Dunn A, Wallace MT. Developmental changes in the multisensory temporal binding window persist into adolescence. Developmental Science. 2012;15(5):688–696. doi: 10.1111/j.1467-7687.2012.01171.x. doi: 10.1111/j.1467-7687.2012.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillock AR, Powers AR, Wallace MT. Binding of sights and sounds: age-related changes in multisensory temporal processing. Neuropsychologia. 2011;49(3):461–467. doi: 10.1016/j.neuropsychologia.2010.11.041. doi: S0028-3932(10)00521-X [pii] 10.1016/j.neuropsychologia.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iarocci G, McDonald J. Sensory integration and the perceptual experience of persons with autism. J Autism Dev Disord. 2006;36(1):77–90. doi: 10.1007/s10803-005-0044-3. doi: 10.1007/s10803-005-0044-3. [DOI] [PubMed] [Google Scholar]
- Iarocci G, Rombough A, Yager J, Weeks DJ, Chua R. Visual influences on speech perception in children with autism. Autism. 2010;14(4):305–320. doi: 10.1177/1362361309353615. doi: 10.1177/1362361309353615. [DOI] [PubMed] [Google Scholar]
- Irwin JR, Tornatore LA, Brancazio L, Whalen DH. Can children with autism spectrum disorders “hear” a speaking face? Child Dev. 2011;82(5):1397–1403. doi: 10.1111/j.1467-8624.2011.01619.x. doi: 10.1111/j.1467-8624.2011.01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin JR, Tornatore LA, Brancazio L, Whalen DH. Can children with autism spectrum disorders “hear” a speaking face? Child Development. 2011;82(5):1397–1403. doi: 10.1111/j.1467-8624.2011.01619.x. doi: 10.1111/j.1467-8624.2011.01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RM, Keehn B, Connolly C, Wolfe JM, Horowitz TS. Why is visual search superior in autism spectrum disorder? Developmental Science. 2009;12(6):1083–1096. doi: 10.1111/j.1467-7687.2009.00855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane BP, Rosenthal O, Chun NH, Shams L. Audiovisual integration in high functioning adults with autism. Research in Autism Spectrum Disorders. 2010;4(2):276–289. [Google Scholar]
- Kuhl P, Coffey-Corina S, Padden D, Dawson G. Links between social and linguistic processing of speech in preschool children with autism: Behavioral and electrophysiological measures. Developmental Science. 2005;8(1):F1–F12. doi: 10.1111/j.1467-7687.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- Kwakye LD, Foss-Feig JH, Cascio CJ, Stone WL, Wallace MT. Altered auditory and multisensory temporal processing in autism spectrum disorders. Front Integr Neurosci. 2011;4:129. doi: 10.3389/fnint.2010.00129. doi: 10.3389/fnint.2010.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewkowicz DJ. The development of intersensory temporal perception: an epigenetic systems/limitations view. Psychol Bull. 2000;126(2):281–308. doi: 10.1037/0033-2909.126.2.281. [DOI] [PubMed] [Google Scholar]
- Lewkowicz DJ. Infant perception of audio-visual speech synchrony. Developmental Psychology. 2010;46(1):66–77. doi: 10.1037/a0015579. doi: 10.1037/a0015579. [DOI] [PubMed] [Google Scholar]
- Lewkowicz DJ. Development of Multisensory Temporal Perception. In: Murray MM, Wallace MT, editors. The Neural Bases of Multisensory Processes. Boca Raton (FL): 2012. [PubMed] [Google Scholar]
- Lewkowicz DJ, Flom R. The audiovisual temporal binding window narrows in early childhood. Child Dev. 2014;85(2):685–694. doi: 10.1111/cdev.12142. doi: 10.1111/cdev.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, DiLavore PC, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- McGurk H, MacDonald J. Hearing lips and seeing voices. Nature. 1976;264(5588):746–748. doi: 10.1038/264746a0. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Hobson JA. Sensory sensitivities and performance on sensory perceptual tasks in high-functioning individuals with autism. J Autism Dev Disord. 2008;38(8):1485–1498. doi: 10.1007/s10803-007-0528-4. doi: 10.1007/s10803-007-0528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongillo E, Irwin J, Whalen D, Klaiman C, Carter A, Schultz R. Audiovisual processing in children with and without autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38(7):1349–1358. doi: 10.1007/s10803-007-0521-y. doi: 10.1007/s10803-007-0521-y. [DOI] [PubMed] [Google Scholar]
- Neil PA, Chee-Ruiter C, Scheier C, Lewkowicz DJ, Shimojo S. Development of multisensory spatial integration and perception in humans. Developmental Science. 2006;9(5):454–464. doi: 10.1111/j.1467-7687.2006.00512.x. doi: 10.1111/j.1467-7687.2006.00512.x. [DOI] [PubMed] [Google Scholar]
- O'Riordan M,A. Superior visual search in adults with autism. Autism. 2004;8(3):229–248. doi: 10.1177/1362361304045219. doi: 10.1177/13623613040452198/3/229 [pii] [DOI] [PubMed] [Google Scholar]
- O'Riordan M, Passetti F. Discrimination in autism within different sensory modalities. J Autism Dev Disord. 2006;36(5):665–675. doi: 10.1007/s10803-006-0106-1. doi: 10.1007/s10803-006-0106-1. [DOI] [PubMed] [Google Scholar]
- O'Riordan M, Plaisted K. Enhanced discrimination in autism. Q J Exp Psychol A. 2001;54(4):961–979. doi: 10.1080/713756000. [DOI] [PubMed] [Google Scholar]
- O'Riordan MA, Plaisted KC, Driver J, Baron-Cohen S. Superior visual search in autism. J Exp Psychol Hum Percept Perform. 2001;27(3):719–730. doi: 10.1037//0096-1523.27.3.719. [DOI] [PubMed] [Google Scholar]
- Pellicano E, Burr D. When the world becomes ‘too real’: a Bayesian explanation of autistic perception. Trends in Cognitive Sciences. 2012;16(10):504–510. doi: 10.1016/j.tics.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Plaisted K, O'Riordan M, Baron-Cohen S. Enhanced discrimination of novel, highly similar stimuli by adults with autism during a perceptual learning task. J Child Psychol Psychiatry. 1998a;39(5):765–775. [PubMed] [Google Scholar]
- Plaisted K, O'Riordan M, Baron-Cohen S. Enhanced visual search for a conjunctive target in autism: a research note. J Child Psychol Psychiatry. 1998b;39(5):777–783. [PubMed] [Google Scholar]
- Ross LA, Molholm S, Blanco D, Gomez-Ramirez M, Saint-Amour D, Foxe JJ. The development of multisensory speech perception continues into the late childhood years. Eur J Neurosci. 2011;33(12):2329–2337. doi: 10.1111/j.1460-9568.2011.07685.x. doi: 10.1111/j.1460-9568.2011.07685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams L, Kamitani Y, Shimojo S. Illusions. What you see is what you hear. Nature. 2000;408(6814):788. doi: 10.1038/35048669. [DOI] [PubMed] [Google Scholar]
- Stevenson RA, Altieri NA, Kim S, Pisoni DB, James TW. Neural processing of asynchronous audiovisual speech perception. Neuroimage. 2010;49(4):3308–3318. doi: 10.1016/j.neuroimage.2009.12.001. doi: S1053-8119(09)01288-9 [pii] 10.1016/j.neuroimage.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Fister JK, Barnett ZP, Nidiffer AR, Wallace MT. Interactions between the spatial and temporal stimulus factors that influence multisensory integration in human performance. Exp Brain Res. 2012 doi: 10.1007/s00221-012-3072-1. doi: 10.1007/s00221-012-3072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Siemann JK, Schneider BC, Eberly HE, Woynaroski TG, Camarata SM, Wallace MT. Multisensory Temporal Integration in Autism Spectrum Disorders. The Journal of Neuroscience. 2014;34(3):691–697. doi: 10.1523/JNEUROSCI.3615-13.2014. doi: 10.1523/jneurosci.3615-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Siemann JK, Woynaroski TG, Schneider BC, Eberly HE, Camarata SM, Wallace MT. Brief Report: Arrested Development of Audiovisual Speech Perception in Autism Spectrum Disorders. Journal of Autism and Developmental Disorders. :1–8. doi: 10.1007/s10803-013-1992-7. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, VanDerKlok RM, Pisoni DB, James TW. Discrete neural substrates underlie complementary audiovisual speech integration processes. Neuroimage. 2011;55(3):1339–1345. doi: 10.1016/j.neuroimage.2010.12.063. doi: 10.1016/j.neuroimage.2010.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Wallace MT. Multisensory temporal integration: task and stimulus dependencies. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 2013;227(2):249–261. doi: 10.1007/s00221-013-3507-3. doi: 10.1007/s00221-013-3507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson RA, Zemtsov RK, Wallace MT. Individual Differences in the Multisensory Temporal Binding Window Predict Susceptibility to Audiovisual Illusions. J Exp Psychol Hum Percept Perform. 2012 doi: 10.1037/a0027339. doi: 2012-05374-001 [pii] 10.1037/a0027339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N, Isaac C, Milne E. A comparison of the development of audiovisual integration in children with autism spectrum disorders and typically developing children. J Autism Dev Disord. 2010;40(11):1403–1411. doi: 10.1007/s10803-010-1000-4. doi: 10.1007/s10803-010-1000-4. [DOI] [PubMed] [Google Scholar]
- van der Smagt MJ, van Engeland H, Kemner C. Brief report: can you see what is not there? low-level auditory-visual integration in autism spectrum disorder. J Autism Dev Disord. 2007;37(10):2014–2019. doi: 10.1007/s10803-006-0346-0. doi: 10.1007/s10803-006-0346-0. [DOI] [PubMed] [Google Scholar]
- van Wassenhove V, Grant KW, Poeppel D. Temporal window of integration in auditory-visual speech perception. Neuropsychologia. 2007;45(3):598–607. doi: 10.1016/j.neuropsychologia.2006.01.001. doi: S0028-3932(06)00011-X [pii] 10.1016/j.neuropsychologia.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Vatakis A, Spence C. Audiovisual synchrony perception for music, speech, and object actions. Brain Res. 2006;1111(1):134–142. doi: 10.1016/j.brainres.2006.05.078. doi: S0006-8993(06)01590-3 [pii] 10.1016/j.brainres.2006.05.078. [DOI] [PubMed] [Google Scholar]
- von Helmholtz H. Treatise on physiological optics (Vol. 3) Courier Dover Publications; 2005. [Google Scholar]
- Vroomen J, Keetels M. Perception of intersensory synchrony: a tutorial review. Atten Percept Psychophys. 2010;72(4):871–884. doi: 10.3758/APP.72.4.871. doi: 72/4/871 [pii] 10.3758/APP.72.4.871. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. Harcourt Brace; San Antonio: 1999. [Google Scholar]
- Williams J, Massaro DW, Peel NJ, Bosseler A, Suddendorf T. Visual-auditory integration during speech imitation in autism. Research in Developmental Disabilities. 2004;25(6):559–575. doi: 10.1016/j.ridd.2004.01.008. doi: S0891422204000757 [pii] 10.1016/j.ridd.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Woynaroski TG, Kwakye LD, Foss-Feig JH, Stevenson RA, Stone WL, Wallace MT. Multisensory Speech Perception in Children with Autism Spectrum Disorders. J Autism Dev Disord. 2013 doi: 10.1007/s10803-013-1836-5. doi: 10.1007/s10803-013-1836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]