Abstract
MYC is one of the most frequently overexpressed oncogenes in human cancer and even modestly deregulated MYC can initiate ectopic proliferation in many post-mitotic cell types in vivo. Sensitization of cells to apoptosis limits MYC’s oncogenic potential. However, the mechanism through which MYC induces apoptosis is controversial: Some studies implicate p19ARF-mediated stabilization of p53, followed by induction of pro-apoptotic BH3 proteins NOXA and PUMA, while others argue for direct regulation of BH3 proteins, especially BIM. Here, we use a single experimental system to systematically evaluate the roles of p19ARF and BIM during MYC-induced apoptosis, in vitro, in vivo, and in combination with a widely used chemotherapeutic, Doxorubicin. We find a common specific requirement for BIM during MYC-induced apoptosis in multiple settings, which does not extend to the p53-responsive BH3 family member PUMA, and find no evidence of a role for p19ARF during MYC-induced apoptosis in the tissues examined.
Introduction
Oncogene-induced tumour suppression presents an efficient cell-autonomous restraint to tumour formation in the face of mitogenic signaling by deregulated proto-oncogenes (Evan et al., 2005). MYC serves as a paradigm example of this phenomenon (Askew et al., 1991, Evan et al., 1992, Strasser et al., 1990): deregulated MYC expression simultaneously drives both cell proliferation and apoptosis, with the relative rates of each ultimately determining whether the affected population expands or contracts. Importantly, the threshold level of MYC required to engage apoptosis is set higher than the level required to initiate cell proliferation, thereby enabling healthy cells to proliferate in response to physiological signaling, all the while maintaining an effective barrier to supra-physiological (ie. oncogenic) MYC expression (Murphy et al., 2008).
MYC induced apoptosis is widely thought to be mediated by the ARF-p53 pathway: Overexpression of Myc induces accumulation of p19ARF (p14ARF in human cells), which counteracts MDM2-mediated degradation of p53 (Kamijo et al., 1998, Stott et al., 1998, Zindy et al., 1998, Eischen et al., 1999, Schmitt et al., 1999). Activated p53 in turn induces apoptosis via transcriptional upregulation of BH3-only proteins PUMA and NOXA (Nakano and Vousden, 2001, Villunger et al., 2003). However, apoptosis is not the only possible outcome from p53 stabilization and recent work has shown that acetylation of K117 (K120 in human p53) is also specifically required for induction of NOXA, PUMA, and thereby apoptosis. Significantly, a K117R mutant p53 is still competent to induce cell cycle arrest, senescence and tumour suppression (Li et al., 2012). Thus, a dual-signal mechanism governs cell fate in response to p53 accumulation. Additionally, several groups have reported Myc-induced apoptosis in the absence of either p19ARF or p53, strongly suggesting the existence of an alternative pathway to MYC induced killing (Hsu et al., 1995, Blyth et al., 2000, Eischen et al., 2001, Finch et al., 2006).
A growing body of evidence supports a central role for the BCL2 homologous (BH) family of proteins in mediating MYC induced apoptosis. This family of proteins is subdivided into anti-apoptotic (eg. BCL2, BCLXL, MCL1, A1), pro-apoptotic BH3-only (including BIM, BID, BAD, PUMA and NOXA), and effector BH3 proteins, BAX and BAK. In response to pro-apoptotic stimuli, BAX and BAK oligomerize to form pores in the outer mitochondrial membrane (MOMP), triggering release of Cytochrome C, SMAC/DIABLO and consequent activation of effector Caspases, (Sarosiek et al., 2013b, Czabotar et al., 2014). Anti-apoptotic BH family proteins buffer against pore formation, while pro-apoptotic BH3-only proteins counteract this buffering and in some instances can directly stimulate BAX/BAK oligomerization (Llambi et al., 2011, Sarosiek et al., 2013a). MYC induced apoptosis requires BAX/BAK (Dansen et al., 2006, Juin et al., 2002), release of cytochrome C (Juin et al., 1999) and activation of effector Caspases, contingent upon tonic signaling through CD95 (Hueber et al., 1997), and is blocked by co-expression of anti-apoptotic family proteins (Bissonnette et al., 1992, Fanidi et al., 1992, Pelengaris et al., 2002). Conversely, MYC dependent suppression of BCLXL and BCL2 sensitizes cells to γ-irradiation-induced apoptosis (Eischen et al., 2001, Maclean et al., 2003). The recent identification of BIM as a transcriptional target of MYC suggests that this BH3-only protein may directly mediate MYC’s pro-apoptotic signal (Campone et al., 2011, Lee et al., 2013). MYC induces BIM accumulation in Burkitt’s Lymphoma and MYC point mutants that fail to induce BIM also fail to induce apoptosis. Notably, such mutants are fully competent to induce p19ARF, p53 and indeed accumulation of p21 downstream of p53 (Hemann et al., 2005). These observations prompted us to re-examine the relative contributions of p19ARF and BIM to MYC-induced apoptosis. We employed here a single transgenic model to systematically evaluate MYC-induced apoptosis in a variety of settings. Our data reveal that BIM is the primary mediator of MYC-induced apoptosis in multiple solid tissues.
Results
BIM is required for MYC-induced apoptosis in the intestine
To investigate the mechanistic requirements of MYC-induced apoptosis in multiple tissues we used a previously described Rosa26-MycERT2 mouse line that ubiquitously expresses a latent, Tamoxifen-inducible, chimeric protein comprised of full-length human C-MYC fused to a modified ligand-binding domain from the human Estrogen Receptor (MycERT2). Deregulated MYC function can thus be induced acutely in Rosa26-MycERT2 mice by systemic injection of Tamoxifen (Tam). Owing to the relatively weak activity of the endogenous Rosa26 promoter, the level of MycERT2 expressed is sufficient to drive ectopic proliferation without triggering apoptosis in most adult R26MER/MER tissues, with the exception of the small and large intestine, wherein MycERT2 is expressed at somewhat higher levels as compared with other tissues. Consequently, activation of MycERT2 in the intestine breaches the threshold level of MYC deregulation required to drive apoptosis (Murphy et al., 2008).
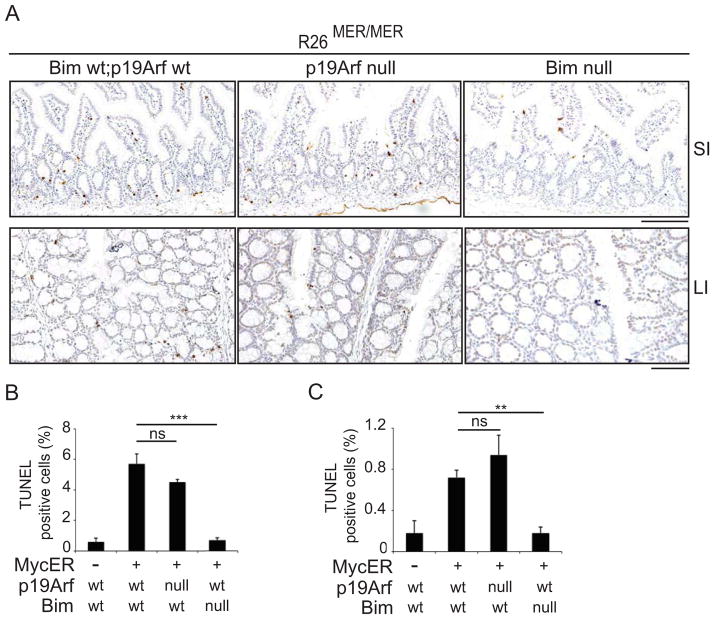
To assess the relative contributions of p19ARF and BIM to MYC-induced apoptosis in these tissues, we interbred Rosa26-MycERT2 mice with Cdkn2atm1(GFP)Cjs (ARFGFP) mice, wherein GFP is inserted into exon 1β of the Cdkn2a locus, abrogating p19ARF expression (Zindy et al., 2003), and Bcl2l11tm1.1Ast (BIM−/−) mice (Bouillet et al., 1999), to generate R26MER/MER;ARFGFP/GFP and R26MER/MER;BIM−/− progeny, respectively. MycERT2 was induced systemically in adult mice via daily injection with Tam for 3 days, by which time Myc-induced ectopic proliferation peaks, as previously shown (Supplemental Figure 1a) (Murphy et al., 2008). Tissues were harvested within 24hrs of the final injection and apoptotic cells were identified by nuclear TUNEL staining (Fig. 1). In the small intestine, MYC-induced apoptosis is largely restricted to the crypt region and was modestly reduced by Arf deletion. In the large intestine, where MYC-induced apoptosis is more widely distributed, apoptosis was unaffected by p19ARF loss. Deletion of Bim on the other hand abrogated MYC-induced apoptosis in these two tissues.
Figure 1.
(A) Representative images of TUNEL staining of apoptotic cells in small (SI) and large intestine (LI) of mice of the indicated genotypes, treated daily with Tamoxifen (50mg/kg) to activate MycERT2 for 3 days. Scale bars = 100μm. (B) Quantification (Mean, SEM) of TUNEL positive cells (% of total) in the small intestine of Tamoxifen treated wild-type control (n=3); R26MER/MER (n=3); R26MER/MER;Bim null (n=4); R26MER/MER;Arf null mice (n=3). (C) Quantification (Mean, SEM) of TUNEL positive cells in the large intestine of Tamoxifen treated wild-type control (n=3); R26MER/MER (n=3); R26MER/MER;Bim null (n=4); R26MER/MER;Arf null mice (n=3). T tests (2-tailed, unpaired) were used to determine statistical significance (* p<.05; ** p<.01; *** p<.001). See also Figure S1.
BIM is thought to act primarily via broad antagonism of pro-survival BH3-family proteins including BCL2, BCLXL and MCL1, although direct activation of the effector BH3 protein BAX likely contributes to BIM’s potency (Sarosiek et al., 2013a). PUMA is the primary BH3-only effector of p53-mediated apoptosis in the intestine (Yu et al., 2003). Like BIM, PUMA is a broad specificity antagonist of pro-survival BH3 proteins and, like BIM deletion, PUMA deletion accelerates MYC-induced B cell lymphomagenesis (Michalak et al., 2009). We asked therefore if loss of PUMA (in Bbc3tm1Ast mice) suppresses MYC-induced apoptosis in the intestine. Acute activation of MycERT2 in R26MER/MER;Puma−/− mice revealed modestly reduced MYC-driven apoptosis in the small intestine, relative to PUMA replete controls, while deletion of Puma had no effect on MYC-induced apoptosis in the large intestine (Supplemental Figure 1b–d). Thus, amongst the pro-apoptotic BH3-only proteins, there is a specific requirement for BIM during MYC-induced apoptosis in these tissues.
BIM mediates pro-apoptotic signaling by MYC in multiple tissues
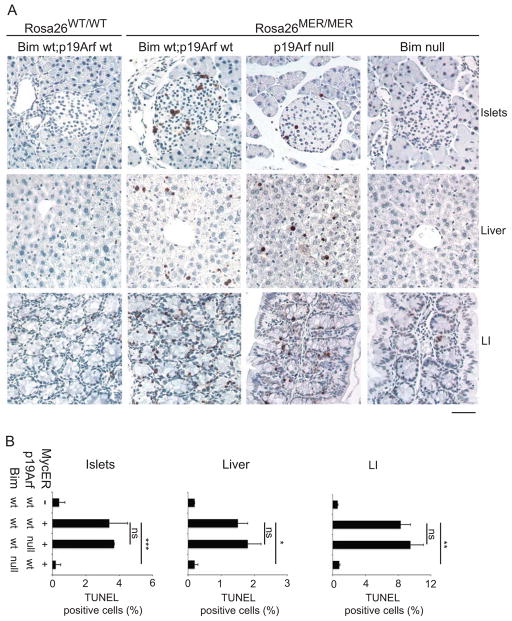
The level of MycERT2 expressed in other tissues of R26MER/MER mice is insufficient to alone breach the apoptotic threshold but does sensitize cells to additional pro-apoptotic stimuli, such as Doxorubicin (Murphy et al., 2008). To determine if sub-threshold apoptotic signaling by MYC is mediated by p19ARF or BIM, we primed R26MER/MER;Arf GFP/GFP and R26MER/MER;Bim−/− mice by activating MycERT2 for 3 days, then treated mice with a dose of Doxorubicin that alone fails to drive apoptosis in most tissues. The combination of deregulated MYC and Doxorubicin drove measurable apoptosis in liver and pancreatic islets of Langerhans, and enhanced MYC-induced killing in colonic epithelium (Figure 2). Again, deletion of Bim abrogated the apoptotic response in all three tissues while disruption of Arf had no effect. Deletion of PUMA protected all three tissues to the same extent as Bim loss, confirming the cooperative nature of death in response to these two pro-apoptotic stimuli (Supplemental Figure 2a). Notably however, death induced by Doxorubicin alone, confined in these experiments to the small intestine, is unaffected by Bim deletion but does require PUMA, as expected for a canonical p53-mediated response to DNA damage (Supplemental Figure 2b). Moreover, Doxorubicin neither induces BIM nor augments MYC induction of BIM protein and Bim is not required for Doxorubicin-induced killing in MEFs (Supplemental Figure 2c and d). Thus, MYC and Doxorubicin elicit apoptotic signaling via induction of distinct pro-apoptotic BH3 family proteins.
Figure 2.
(A) Representative images of TUNEL staining of apoptotic cells in tissues of mice of the indicated genotypes treated daily with Tamoxifen (50mg/kg) for 3 days and Doxorubicin (10mg/kg) on the 3rd day. Scale bar = 50μm. (B) Quantification (Mean, SEM) of TUNEL positive cells (% of total) in the indicated tissues of untreated control R26MER/MER (n=3); and Tamoxifen treated R26MER/MER (n=3); R26MER/MER;Bim null (n=3); R26MER/MER;Arf null mice (n=5). T tests (2-tailed, unpaired) were used to determine statistical significance (* p<.05; ** p<.01; *** p<.001). See also Figure S2.
MYC directly regulates BIM expression
Elevated expression of Bim-EL is evident in RNA and protein derived from the small intestine of Tamoxifen treated R26MER/MER mice (Supplemental Figure 3a & b). We generated embryonic fibroblasts (MEFs) from Rosa26-MycERT2 mice and acutely activated MycERT2 with 4-hydroxy-Tamoxifen (4-OHT) in vitro to determine if MYC directly regulates Bim expression. Increased expression of Bim-EL mRNA was found after 6 hrs of MycERT2 activation and was followed by clear accumulation of BIM-EL protein (Figure 3a). Whole-genome chromatin immunoprecipitation-coupled deep sequencing (ChIP-SEQ) analysis revealed 4-OHT-induced binding of MycER to the BIM (BCL2L11) promoter in human MCF10A cells, and constitutive occupation of the Bim promoter by endogenous MYC in murine KPC pancreatic tumour cells (Morton et al., 2010), comparable to binding at the canonical MYC target gene ODC1 (Figure 3b and Supplemental Figure 3c). Gene-specific ChIP with the N262 antibody, which recognizes both endogenous murine MYC and exogenous MycERT2, revealed increased occupancy of the Bim promoter in R26MER/WT MEFs in response to 4-OHT (Figure 3c), while ChIP analysis in HeLa cells confirmed binding of MYC to the human BIM promoter region (Supplemental Figure 3d), consistent with previous reports (Campone et al., 2011, Lee et al., 2013).
Figure 3.
(A) Upper panel: Q-RT PCR analysis on mRNA from R26MER/WT MEFs, treated with 4-OHT for the indicated durations (hrs). Odc1, an established MYC target, was used as a positive control. Mean and SEM shown (N=3). Lower panel: Immunoblot of BIM-EL expression in a parallel time-course. (B) ChIP-sequencing read-counts documenting binding of endogenous MYC to the promoter region of murine Bcl2l11 encoding BIM. N262 denotes specifically immunoprecipitated protein/DNA complexes. (C) Chromatin immunoprecipitation on R26MER/WT MEFs treated with or without 4-OHT using IgG control or MYC antibody, followed by Q-RT PCR with primers amplifying the BIM promoter region and control fragment. (D & E) Early passage (p<5) R26MER/WT MEFs, wt or nullizygous for Bim or Arf were treated with 4-OHT (100nm) for 30hrs under low (0.2%) serum conditions. The graph shows the percentage of cells stained positive for Annexin V-only (Black) or both Annexin V and propidium iodide (red). Mean±SEM from representative experiments performed in biological triplicate are shown. Consistent results were obtained in MEFs derived from at least 2 embryos for each genotype. (F) Clonogenic assay on R26MER/MER;Bim null and R26MER/MER;Bimwt early passage MEFs cultured in growth media + 100nM OHT. See also Figures S3 and S4.
Activation of MycERT2 in R26MER/WT MEFs cultured in low serum rapidly leads to apoptosis. We generated MEFs from multiple R26MER/WT;Arf GFP/GFP and R26MER/WT;Bim−/− embryos to assess the contribution of p19ARF and BIM to MYC-induced apoptosis in this system, and measured apoptosis 30 and 72 hrs after addition of 4-OHT to cells in low serum. Again, deletion of Bim suppressed MYC-induced apoptosis while deletion of Arf did not (Figure 3d & e and Supplemental Figure 3e & f). Although serum deprivation alone did induce low levels of BIM expression, further induction of BIM by MYC was unaffected by serum levels (Supplemental Figure 3g) and, notably, Bim deletion had no effect on the basal level of apoptosis induced by culturing MEFs in low serum. Moreover, deletion of Bim suppressed MYC sensitization of MEFs cultured in growth media to both γ-irradiation- and ABT-737-induced apoptosis (Supplemental Figure 4) and Bim null MEFs showed increased clonogenic survival in the presence of deregulated MYC (Figure 3f).
A threshold level of BIM is required for MYC-induced apoptosis
Activation of MycERT2 in heterozygous R26MER/WT mice fails to induce enterocyte apoptosis whereas apoptosis is readily detected in the same tissue of Tam-treated homozygous R26MER/MER mice, indicating that a threshold level of MYC deregulation is required for this effect (Murphy et al., 2008). We therefore asked if reducing Bim expression would attenuate the pro-apoptotic signal emanating from MYC deregulation to a level below this threshold, and thereby protect R26MER/MER intestines from apoptosis. Comparing levels of apoptosis across R26MER/MER;Bim−/−, R26MER/MER;Bim+/− and R26MER/MER;BimWT mice, it is clear that haplo-insufficiency for Bim rescues R26MER/MER intestines from MYC-induced apoptosis (Figure 4). This result is in broad agreement with a previous report that haplo-insufficiency for Bim accelerates MYC-induced lymphomagenesis and supports a model where a threshold level of BIM is required to mediate MYC’s apoptotic signal (Egle et al., 2004).
Figure 4.
(A) Representative images of TUNEL staining of apoptotic cells in small (SI) and large intestine (LI) of mice of the indicated genotypes, treated daily with Tamoxifen (50mg/kg) to activate MycERT2 for 3 days. Scale bars = 100μm. (B) Quantification (Mean, SEM) of TUNEL positive cells in the small intestine of untreated control R26MER/MER (n=3); and Tamoxifen treated R26MER/MER (n=3); R26MER/MER;Bim null (n=4), R26MER/MER;Bim heterozygous mice (n=3). (C) Quantification (Mean, SEM) of TUNEL positive cells in the large intestine of untreated control R26MER/MER (n=3); and Tamoxifen treated R26MER/MER (n=3); R26MER/MER;Bim null (n=4); R26MER/MER;Bim heterozygous mice (n=3). T tests (2-tailed, unpaired) were used to determine statistical significance (* p<.05; ** p<.01; *** p<.001).
Discussion
We present here evidence that the BH3-only protein BIM is the primary mediator of MYC-induced apoptosis in vivo and in vitro. We show MYC binding to the BIM promoter, acutely elevating BIM expression, and provide genetic evidence that BIM is required for MYC-induced apoptosis under multiple circumstances and in multiple solid tissues. Strikingly, deletion of Puma fails to phenocopy Bim deletion when apoptosis is induced by MYC alone, despite the fact that loss of PUMA, like loss of BIM, has been shown to accelerate MYC-induced lymphomagenesis and despite similarities in their mechanism of action (Egle et al., 2004, Czabotar et al., 2014). Conversely, Bim deletion fails to phenocopy PUMA loss during doxorubicin-induced apoptosis in the small intestine. However, both BIM and PUMA are required for the synthetic induction of apoptosis driven by the combination of MYC and Doxorubicin in tissues where either alone is insufficient to trigger cell death. The differential sensitivities of various tissue to experimental apoptotic stimuli is partly explained by differences in MycERT2 expression levels (Murphy et al., 2008) but also likely reflects the level of apoptotic priming intrinsic to each tissue, where differential expression of specific anti-apoptotic BH3 proteins may determine the relative potency of individual pro-apoptotic BH3 proteins (Ni Chonghaile et al., 2011). Thus, these results argue for a model wherein individual BH3-only proteins have evolved to transduce specific pro-apoptotic signals yet can combine to overcome anti-apoptotic buffering. See Graphical Abstract.
Therapeutic implications
MYC family oncogenes are among the most frequently overexpressed genes across a broad spectrum of human cancers. Effective MYC-centric therapies could therefore have a tremendous impact on cancer survival rates. Several strategies to target MYC are being actively pursued, including direct suppression of MYC protein (Soucek et al., 2008, Popov et al., 2010), transcriptional suppression (Zuber et al., 2011) and synthetic lethality (Goga et al., 2007, Liu et al., 2012, Kemp and Grandori, 2013) and it is likely that combinatorial strategies will ultimately prove the most effective. A rational strategy to exploit the intrinsic apoptotic potential of MYC-overexpressing cells would complement these efforts greatly. BH3 mimetics suppress lymphomagenesis in Eμ-MYC mice, even in the absence of a functional p53 pathway (Kelly et al., 2014, Kelly et al., 2013). Given the prevalence of MYC overexpression in human cancers, our data support the approach of augmenting the intrinsic apoptotic response through the use of BH3 mimetics and suggests that this strategy may elicit therapeutic benefits in a spectrum of solid tumour types.
Materials and Methods
Genetically Engineered Mice & Mouse Procedures
Rosa26-MycERT2 (Murphy et al., 2008); Cdkn2atm1(GFP)Cjs (ARFGFP; NCI Mouse Repository; Zindy et al., 2003); Bcl2l11tm1.1Ast (BimNull; Jackson Labs; Bouillet et al., 1999) and Bbc3tm1Ast (PUMANull; Jackson Labs, distributed by Charles River, Europe; Villunger et al., 2003) mice were maintained on a C57/bl6 background, housed on a 12hr light cycle and fed and watered ad libitum. Procedures involving mice were performed in accordance with protocol numbers AN 076148 (UCSF IACUC, USA); 55.2-2531.01-30/11 (University of Wuerzburg, Germany) and Home Office licence number 60/4183 (CRUK BICR, UK) and approved by the local animal research committees at all three locations. All treatments were performed on mice aged 8–12 wks. Tamoxifen (Sigma) dissolved in peanut oil was administered once a day by intraperitoneal injection for 3 days at 50mg/kg. Doxorubicin (LC labs) dissolved in 0.9% NaCl was administered once by intraperitoneal injection at 10mg/kg. Tissues were fixed overnight in zinc buffered formalin (Thermo Scientific 5701ZF).
Immunohistochemistry
TUNEL staining was performed on paraffin-embedded sections (4μm thick) using the ApopTag peroxidase labeling kit (Millipore-S7100). An additional blocking step (1% BSA for 1hr at RT) was incorporated prior to addition of peroxidase-conjugated anti-digoxigenin. Tissues were counterstained in Gil 1 hematoxylin followed by blueing solution. For quantification, the number of TUNEL positive nuclei (pyknotic or intact) and the total number of cells per 20x field were counted manually. Five representative fields per tissue sample from each mouse were scored, yielding a percent apoptosis value. Graphs represent the Mean ± SEM percent apoptosis across N number of mice as indicated in the figure legends.
Supplementary Material
Footnotes
Accession Numbers
Complete ChIP-SEQ datasets can be accessed on the Gene Expression Omnibus accession numbers GSE44672 (mouse) and GSE59001 (human).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ASKEW DS, ASHMUN RA, SIMMONS BC, CLEVELAND JL. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991;6:1915–22. [PubMed] [Google Scholar]
- BISSONNETTE RP, ECHEVERRI F, MAHBOUBI A, GREEN DR. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992;359:552–4. doi: 10.1038/359552a0. [DOI] [PubMed] [Google Scholar]
- BLYTH K, STEWART M, BELL M, JAMES C, EVAN G, NEIL JC, CAMERON ER. Sensitivity to myc-induced apoptosis is retained in spontaneous and transplanted lymphomas of CD2-mycER mice. Oncogene. 2000;19:773–82. doi: 10.1038/sj.onc.1203321. [DOI] [PubMed] [Google Scholar]
- BOUILLET P, METCALF D, HUANG DC, TARLINTON DM, KAY TW, KONTGEN F, ADAMS JM, STRASSER A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–8. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- CAMPONE M, NOEL B, COURIAUD C, GRAU M, GUILLEMIN Y, GAUTIER F, GOURAUD W, CHARBONNEL C, CAMPION L, JEZEQUEL P, BRAUN F, BARRE B, COQUERET O, BARILLE-NION S, JUIN P. c-Myc dependent expression of pro-apoptotic Bim renders HER2-overexpressing breast cancer cells dependent on anti-apoptotic Mcl-1. Mol Cancer. 2011;10:110. doi: 10.1186/1476-4598-10-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CZABOTAR PE, LESSENE G, STRASSER A, ADAMS JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- DANSEN TB, WHITFIELD J, ROSTKER F, BROWN-SWIGART L, EVAN GI. Specific requirement for Bax, not Bak, in Myc-induced apoptosis and tumor suppression in vivo. J Biol Chem. 2006;281:10890–5. doi: 10.1074/jbc.M513655200. [DOI] [PubMed] [Google Scholar]
- EGLE A, HARRIS AW, BOUILLET P, CORY S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci U S A. 2004;101:6164–9. doi: 10.1073/pnas.0401471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISCHEN CM, WEBER JD, ROUSSEL MF, SHERR CJ, CLEVELAND JL. Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev. 1999;13:2658–69. doi: 10.1101/gad.13.20.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISCHEN CM, WOO D, ROUSSEL MF, CLEVELAND JL. Apoptosis triggered by Myc-induced suppression of Bcl-X(L) or Bcl-2 is bypassed during lymphomagenesis. Mol Cell Biol. 2001;21:5063–70. doi: 10.1128/MCB.21.15.5063-5070.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVAN GI, CHRISTOPHOROU M, LAWLOR EA, RINGSHAUSEN I, PRESCOTT J, DANSEN T, FINCH A, MARTINS C, MURPHY D. Oncogene-dependent tumor suppression: using the dark side of the force for cancer therapy. Cold Spring Harb Symp Quant Biol. 2005;70:263–73. doi: 10.1101/sqb.2005.70.054. [DOI] [PubMed] [Google Scholar]
- EVAN GI, WYLLIE AH, GILBERT CS, LITTLEWOOD TD, LAND H, BROOKS M, WATERS CM, PENN LZ, HANCOCK DC. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–28. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- FANIDI A, HARRINGTON EA, EVAN GI. Cooperative interaction between c-myc and bcl-2 proto-oncogenes. Nature. 1992;359:554–6. doi: 10.1038/359554a0. [DOI] [PubMed] [Google Scholar]
- FINCH A, PRESCOTT J, SHCHORS K, HUNT A, SOUCEK L, DANSEN TB, SWIGART LB, EVAN GI. Bcl-xL gain of function and p19 ARF loss of function cooperate oncogenically with Myc in vivo by distinct mechanisms. Cancer Cell. 2006;10:113–20. doi: 10.1016/j.ccr.2006.06.017. [DOI] [PubMed] [Google Scholar]
- GOGA A, YANG D, TWARD AD, MORGAN DO, BISHOP JM. Inhibition of CDK1 as a potential therapy for tumors over-expressing MYC. Nat Med. 2007;13:820–7. doi: 10.1038/nm1606. [DOI] [PubMed] [Google Scholar]
- HEMANN MT, BRIC A, TERUYA-FELDSTEIN J, HERBST A, NILSSON JA, CORDON-CARDO C, CLEVELAND JL, TANSEY WP, LOWE SW. Evasion of the p53 tumour surveillance network by tumour-derived MYC mutants. Nature. 2005;436:807–11. doi: 10.1038/nature03845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSU B, MARIN MC, EL-NAGGAR AK, STEPHENS LC, BRISBAY S, MCDONNELL TJ. Evidence that c-myc mediated apoptosis does not require wild-type p53 during lymphomagenesis. Oncogene. 1995;11:175–9. [PubMed] [Google Scholar]
- HUEBER AO, ZORNIG M, LYON D, SUDA T, NAGATA S, EVAN GI. Requirement for the CD95 receptor-ligand pathway in c-Myc-induced apoptosis. Science. 1997;278:1305–9. doi: 10.1126/science.278.5341.1305. [DOI] [PubMed] [Google Scholar]
- JUIN P, HUEBER AO, LITTLEWOOD T, EVAN G. c-Myc-induced sensitization to apoptosis is mediated through cytochrome c release. Genes Dev. 1999;13:1367–81. doi: 10.1101/gad.13.11.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUIN P, HUNT A, LITTLEWOOD T, GRIFFITHS B, SWIGART LB, KORSMEYER S, EVAN G. c-Myc functionally cooperates with Bax to induce apoptosis. Mol Cell Biol. 2002;22:6158–69. doi: 10.1128/MCB.22.17.6158-6169.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMIJO T, WEBER JD, ZAMBETTI G, ZINDY F, ROUSSEL MF, SHERR CJ. Functional and physical interactions of the ARF tumor suppressor with p53 and Mdm2. Proc Natl Acad Sci U S A. 1998;95:8292–7. doi: 10.1073/pnas.95.14.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY GL, GRABOW S, GLASER SP, FITZSIMMONS L, AUBREY BJ, OKAMOTO T, VALENTE LJ, ROBATI M, TAI L, FAIRLIE WD, LEE EF, LINDSTROM MS, WIMAN KG, HUANG DC, BOUILLET P, ROWE M, RICKINSON AB, HEROLD MJ, STRASSER A. Targeting of MCL-1 kills MYC-driven mouse and human lymphomas even when they bear mutations in p53. Genes Dev. 2014;28:58–70. doi: 10.1101/gad.232009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY PN, GRABOW S, DELBRIDGE AR, ADAMS JM, STRASSER A. Prophylactic treatment with the BH3 mimetic ABT-737 impedes Myc-driven lymphomagenesis in mice. Cell Death Differ. 2013;20:57–63. doi: 10.1038/cdd.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEMP CJ, GRANDORI C. Functional genomics to identify unforeseen cancer drug targets. Future Oncol. 2013;9:473–6. doi: 10.2217/fon.13.26. [DOI] [PubMed] [Google Scholar]
- LEE YY, MOUJALLED D, DOERFLINGER M, GANGODA L, WESTON R, RAHIMI A, DE ALBORAN I, HEROLD M, BOUILLET P, XU Q, GAO X, DU XJ, PUTHALAKATH H. CREB-binding protein (CBP) regulates beta-adrenoceptor (beta-AR)-mediated apoptosis. Cell Death Differ. 2013;20:941–52. doi: 10.1038/cdd.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI T, KON N, JIANG L, TAN M, LUDWIG T, ZHAO Y, BAER R, GU W. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012;149:1269–83. doi: 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU L, ULBRICH J, MULLER J, WUSTEFELD T, AEBERHARD L, KRESS TR, MUTHALAGU N, RYCAK L, RUDALSKA R, MOLL R, KEMPA S, ZENDER L, EILERS M, MURPHY DJ. Deregulated MYC expression induces dependence upon AMPK-related kinase 5. Nature. 2012;483:608–12. doi: 10.1038/nature10927. [DOI] [PubMed] [Google Scholar]
- LLAMBI F, MOLDOVEANU T, TAIT SW, BOUCHIER-HAYES L, TEMIROV J, MCCORMICK LL, DILLON CP, GREEN DR. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44:517–31. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLEAN KH, KELLER UB, RODRIGUEZ-GALINDO C, NILSSON JA, CLEVELAND JL. c-Myc augments gamma irradiation-induced apoptosis by suppressing Bcl-XL. Mol Cell Biol. 2003;23:7256–70. doi: 10.1128/MCB.23.20.7256-7270.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICHALAK EM, JANSEN ES, HAPPO L, CRAGG MS, TAI L, SMYTH GK, STRASSER A, ADAMS JM, SCOTT CL. Puma and to a lesser extent Noxa are suppressors of Myc-induced lymphomagenesis. Cell Death Differ. 2009;16:684–96. doi: 10.1038/cdd.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON JP, TIMPSON P, KARIM SA, RIDGWAY RA, ATHINEOS D, DOYLE B, JAMIESON NB, OIEN KA, LOWY AM, BRUNTON VG, FRAME MC, EVANS TR, SANSOM OJ. Mutant p53 drives metastasis and overcomes growth arrest/senescence in pancreatic cancer. Proc Natl Acad Sci U S A. 2010;107:246–51. doi: 10.1073/pnas.0908428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURPHY DJ, JUNTTILA MR, POUYET L, KARNEZIS A, SHCHORS K, BUI DA, BROWN-SWIGART L, JOHNSON L, EVAN GI. Distinct thresholds govern Myc’s biological output in vivo. Cancer Cell. 2008;14:447–57. doi: 10.1016/j.ccr.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKANO K, VOUSDEN KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–94. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- NI CHONGHAILE T, SAROSIEK KA, VO TT, RYAN JA, TAMMAREDDI A, DEL MOORE VG, DENG J, ANDERSON KC, RICHARDSON P, TAI YT, MITSIADES CS, MATULONIS UA, DRAPKIN R, STONE R, DEANGELO DJ, MCCONKEY DJ, SALLAN SE, SILVERMAN L, HIRSCH MS, CARRASCO DR, LETAI A. Pretreatment mitochondrial priming correlates with clinical response to cytotoxic chemotherapy. Science. 2011;334:1129–33. doi: 10.1126/science.1206727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PELENGARIS S, KHAN M, EVAN GI. Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell. 2002;109:321–34. doi: 10.1016/s0092-8674(02)00738-9. [DOI] [PubMed] [Google Scholar]
- POPOV N, SCHULEIN C, JAENICKE LA, EILERS M. Ubiquitylation of the amino terminus of Myc by SCF(beta-TrCP) antagonizes SCF(Fbw7)-mediated turnover. Nat Cell Biol. 2010;12:973–81. doi: 10.1038/ncb2104. [DOI] [PubMed] [Google Scholar]
- SAROSIEK KA, CHI X, BACHMAN JA, SIMS JJ, MONTERO J, PATEL L, FLANAGAN A, ANDREWS DW, SORGER P, LETAI A. BID preferentially activates BAK while BIM preferentially activates BAX, affecting chemotherapy response. Mol Cell. 2013a;51:751–65. doi: 10.1016/j.molcel.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAROSIEK KA, NI CHONGHAILE T, LETAI A. Mitochondria: gatekeepers of response to chemotherapy. Trends Cell Biol. 2013b;23:612–9. doi: 10.1016/j.tcb.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMITT CA, MCCURRACH ME, DE STANCHINA E, WALLACE-BRODEUR RR, LOWE SW. INK4a/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev. 1999;13:2670–7. doi: 10.1101/gad.13.20.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUCEK L, WHITFIELD J, MARTINS CP, FINCH AJ, MURPHY DJ, SODIR NM, KARNEZIS AN, SWIGART LB, NASI S, EVAN GI. Modelling Myc inhibition as a cancer therapy. Nature. 2008;455:679–83. doi: 10.1038/nature07260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOTT FJ, BATES S, JAMES MC, MCCONNELL BB, STARBORG M, BROOKES S, PALMERO I, RYAN K, HARA E, VOUSDEN KH, PETERS G. The alternative product from the human CDKN2A locus, p14(ARF), participates in a regulatory feedback loop with p53 and MDM2. EMBO J. 1998;17:5001–14. doi: 10.1093/emboj/17.17.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRASSER A, HARRIS AW, BATH ML, CORY S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990;348:331–3. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- VILLUNGER A, MICHALAK EM, COULTAS L, MULLAUER F, BOCK G, AUSSERLECHNER MJ, ADAMS JM, STRASSER A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–8. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- YU J, WANG Z, KINZLER KW, VOGELSTEIN B, ZHANG L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci U S A. 2003;100:1931–6. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINDY F, EISCHEN CM, RANDLE DH, KAMIJO T, CLEVELAND JL, SHERR CJ, ROUSSEL MF. Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev. 1998;12:2424–33. doi: 10.1101/gad.12.15.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINDY F, WILLIAMS RT, BAUDINO TA, REHG JE, SKAPEK SX, CLEVELAND JL, ROUSSEL MF, SHERR CJ. Arf tumor suppressor promoter monitors latent oncogenic signals in vivo. Proc Natl Acad Sci U S A. 2003;100:15930–5. doi: 10.1073/pnas.2536808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZUBER J, SHI J, WANG E, RAPPAPORT AR, HERRMANN H, SISON EA, MAGOON D, QI J, BLATT K, WUNDERLICH M, TAYLOR MJ, JOHNS C, CHICAS A, MULLOY JC, KOGAN SC, BROWN P, VALENT P, BRADNER JE, LOWE SW, VAKOC CR. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478:524–8. doi: 10.1038/nature10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.