Humans heterozygous for a glucocerebrosidase (GBA) mutation show no signs of Gaucher disease, but are at increased risk of Parkinson’s disease. Fishbein et al. reveal that heterozygosity for the L444P Gba mutation disrupts α-synuclein degradation in mice, and exacerbates the phenotype of the A53T α-synuclein mouse model of Parkinson’s disease.
Keywords: Parkinson’s disease, mouse model, Gaucher disease, alpha-synuclein, lysosomal protein degradation
Abstract
The involvement of the protein α-synuclein (SNCA) in the pathogenesis of Parkinson’s disease is strongly supported by the facts that (i) missense and copy number mutations in the SNCA gene can cause inherited Parkinson’s disease; and (ii) Lewy bodies in sporadic Parkinson’s disease are largely composed of aggregated SNCA. Unaffected heterozygous carriers of Gaucher disease mutations have an increased risk for Parkinson’s disease. As mutations in the GBA gene encoding glucocerebrosidase (GBA) are known to interfere with lysosomal protein degradation, GBA heterozygotes may demonstrate reduced lysosomal SNCA degradation, leading to increased steady-state SNCA levels and promoting its aggregation. We have created mouse models to investigate the interaction between GBA mutations and synucleinopathies. We investigated the rate of SNCA degradation in cultured primary cortical neurons from mice expressing wild-type mouse SNCA, wild-type human SNCA, or mutant A53T SNCA, in a background of either wild-type Gba or heterozygosity for the L444P GBA mutation associated with Gaucher disease. We also tested the effect of this Gaucher mutation on motor and enteric nervous system function in these transgenic animals. We found that human SNCA is stable, with a half-life of 61 h, and that the A53T mutation did not significantly affect its half-life. Heterozygosity for a naturally occurring Gaucher mutation, L444P, reduced GBA activity by 40%, reduced SNCA degradation and triggered accumulation of the protein in culture. This mutation also resulted in the exacerbation of motor and gastrointestinal deficits found in the A53T mouse model of Parkinson’s disease. This study demonstrates that heterozygosity for a Gaucher disease-associated mutation in Gba interferes with SNCA degradation and contributes to its accumulation, and exacerbates the phenotype in a mouse model of Parkinson’s disease.
Introduction
Parkinson’s disease is a movement disorder characterized by tremor, rigidity, bradykinesia, and postural instability. It affects ∼2% of the population over the age of 65 years and is the second most common neurodegenerative disease after Alzheimer’s disease. Symptoms are caused by loss of dopaminergic neurons in the substantia nigra pars compacta projecting to the striatum. Parkinson’s disease-related neuronal death is also found throughout the nervous system, and is associated with a variety of non-motor disturbances including gastrointestinal dysfunction, hyposmia, depression, and cognitive decline (Korczyn and Gurevich, 2010; Ferrer et al., 2011). Currently available treatments are based on enhancing dopaminergic signalling or on surgical interventions such as deep brain stimulation. These treatments provide substantial relief, but do not prevent progressive loss of neurons.
The discovery of the α-synuclein gene (SNCA), and its association with autosomal dominant Parkinson’s disease (Polymeropoulos et al., 1997), has provided researchers with an important insight into the pathogenesis of Parkinson’s disease. The product of the SNCA gene is a 15 kDa protein expressed primarily in the nervous system and haematopoietic lineages and, although restricted to vertebrates, is highly conserved across these species. Although its physiological roles are not yet fully understood, evidence linking it with Parkinson’s disease has been continuously mounting since its original discovery. Five missense mutations in the SNCA gene cause autosomal dominant Parkinson’s disease (Corti et al., 2011; Lesage et al., 2013; Proukakis et al., 2013). In addition, duplication or triplication of the normal gene also causes a heritable form of the disease (Singleton, 2003). There is also a role for α-synuclein (SNCA) in the pathogenesis of the far more common sporadic Parkinson’s disease. First, intracellular neuronal inclusions, Lewy bodies and Lewy neurites, that are characteristic of the disease, are composed chiefly of aggregated SNCA (Mezey et al., 1998) and are found in the brains of all patients with the sporadic forms of Parkinson’s disease. Second, multiple genome-wide case-control association studies (GWAS) have replicated a significant association between common variants at the SNCA locus and sporadic Parkinson’s disease (Simon-Sanchez et al., 2009).
The most striking genetic contributor to sporadic Parkinson’s disease susceptibility, however, was not found by GWAS but instead was found in studies of patients and families with Gaucher disease. Gaucher disease is an autosomal recessive disease caused by mutations in the gene encoding the lysosomal enzyme glucocerebrosidase (GBA). The loss of enzyme function and subsequent accumulation of its glycolipid substrate glucosylceramide, compromises the lysosome, leading to Gaucher disease (Grabowski, 2008).
The first reports of a possible connection between Gaucher disease and Parkinson’s disease came from case studies of patients with Gaucher disease with early Parkinson’s disease onset (Neudorfer et al., 1996; Tayebi et al., 2001). These were followed by an association study done in an Ashkenazi Jewish population, indicating that even heterozygosity for Gaucher disease mutations may predispose their carriers to Parkinson’s disease (Aharon-Peretz et al., 2004). Finally, a large international case-control study of patients with sporadic Parkinson’s disease, confirmed a 5 to 6-fold increase in the frequency of GBA mutations in patients with Parkinson’s disease, compared to the control cases (Sidransky et al., 2009).
The relationship between Gaucher disease, particularly heterozygotes with Gaucher disease, and Parkinson’s disease, recently reviewed by Siebert et al. (2014), is not yet fully understood. It may arise from either the gain of a new toxic function by the mutated GBA protein, or by a decrease in its enzymatic efficiency, or by both. The second and third possibilities are supported by the fact that the lysosomal-autophagy pathway is considered to be the main pathway through which SNCA is degraded (Vogiatzi et al., 2008; Machiya et al., 2010). Although Gaucher disease is a recessive disorder and heterozygous carriers of the disease are asymptomatic, lysosomal function might not be optimal in Gaucher disease heterozygotes. A compromised neuronal lysosome as seen in Gaucher disease carriers could contribute to SNCA accumulation and an increased frequency of Parkinson’s disease, as suggested by Westbroek et al. (2011). Mazzuli et al. (2011) showed that a reduction in GBA protein levels to 50% of control, results in reduced lysosomal efficiency, and in a 2-fold accumulation of endogenous mouse SNCA. A more drastic reduction of GBA activity, to 10% of control, caused a 4-fold increase in total SNCA (Mazzulli et al., 2011). On the other hand, blocking GBA activity with the conduritol B epoxide (CBE) blocker have shown mixed results. For example, Cleeter et al. (2013) demonstrated in SHY-5Y cells a 59% SNCA increase, whereas Dermentzaki et al. (2013) did not find CBE treatment to affect SNCA levels in either SHY-5Y or primary cortical cultures. In any case, it is still unproven that heterozygosity for a naturally occurring human Gaucher disease allele would increase SNCA half-life and raise SNCA levels enough to trigger a Parkinson’s disease phenotype in vivo.
In this study, we used cortical neuronal cultures derived from transgenic mice to determine the half-life of human SNCA and mutated human SNCA (hSNCAA53T), as well as mouse SNCA. Once the baseline half-life was determined, we quantified SNCA half-life in neurons expressing both human SNCA and a single copy of a known Gaucher disease mutation, p.L444P. For this, we crossed the transgenic mice expressing human SNCA to knock-in mice carrying the Gba+/L444P mutation, and used this newly-created mouse strain to generate the neuronal cultures needed for half-life measurements. Finally, we evaluated the effect of the Gba+/L444P mutation in vivo by comparing hSnca A53T/A53T;Gba+/L444P mouse with hSNCAA53T/A53T mice for histopathological and behavioural signs related to Parkinson’s disease. We show that neuronal cultures from mice heterozygous for a mutation in the mouse Gba gene analogous to a known human mutation, L444P, showed an increased SNCA half-life and hSnca A53T/A53T;Gba+/L444P mice have both histopathological and behavioural evidence of SNCA accumulation and neuronal dysfunction beyond what is seen in hSnca A53T/A53T mice in a wild-type Gba background.
Materials and methods
Transgenic mice
Animal care was in accordance with the guidelines of UCSF Animal Care and Use Committee.
B6;129S4-Gbatm1Rlp/Mmnc (000117-UNC) mice containing the Gaucher disease L444P mutation, introduced into the Gba gene by a knock-in procedure in which a part of the gene is duplicated, yet only the mutated copy is expressed (Liu et al., 1998). Mice were purchased from the Mutant Mouse Regional Resource Centres (MMRRC). Note these mice are wild-type for the glucosylceramide synthase (Ugcg) gene, and have an intact adjacent downstream metaxin gene (details in the online Supplementary material). Two independent lines, PAC-Tg(SNCAA53T);Snca−/− or PAC-Tg(SNCAWT);Snca−/− lines expressing A53T mutant human SNCA or wild-type human SNCA and both lacking endogenous mSNCA, were previously generated in the lab (Kuo et al., 2010) and bred with heterozygous Gbatm1Rlp/+. A breeding scheme and a summary table describing the genotypes and nomenclature of the mice are found in Supplementary Fig. 1 and details in the Supplementary material.
Neuronal cultures
Cultures were prepared as previously described (Fishbein and Segal, 2007), with modifications (Supplementary material). Antibodies used for immunohistochemistry were rabbit and mouse anti-MAP2 (SIgma) and anti-GFAP (DAKO).
Quantitative RT-PCR
RNA was purified from neuronal cultures using the PureLink® RNA mini kit (Ambion), according to manufacturer’s directions. cDNA was prepared and RT-PCR preformed as previously described (Kuo et al., 2010) (Supplementary material).
Pulse chase experiment
Metabolic labelling of SNCA
Ten days after plating, when neuronal cultures express SNCA robustly (Rideout et al., 2003; Clough et al., 2011), cultures were incubated in the presence of methionine-free and serum-free Dulbecco’s modified Eagle’s medium (Cellgro 17-204-Cl) for 1 h and then incubated for 1.5 h in methionine-free Dulbecco’s modified Eagle’s medium supplemented with 0.1 mCi/ml S35 methionine (Perkin Elmer). Metabolic labelling medium was then removed and replaced with modified Eagle’s medium supplemented with 10% horse serum. Cells were cultured for various time intervals (0–1, 24, 48, 96, 144 h), after which cells were lysed in RIPA buffer (50 mM Tris-HCl, pH 8, 150 mM NaCl, 0.1% SDS, 1.0% Nonidet P-40, 0.5% sodium deoxycholate and Protease Inhibitors (Roche)) and SNCA was immunoprecipitated from cell lysates with a rabbit SNCA antibody (Santa Cruz), whose specificity was confirmed (Supplementary material) using a mixture of magnetic Dynabead Protein A and G in equal amounts (Invitrogen), according to the manufacturer’s instructions. Eluted protein was then subjected to an SDS-PAGE, (12% gel, Bio-Rad) to resolve the immunoprecipitated SNCA. The gel was silver stained to confirm successful precipitation, dried and exposed in a phosphorimager (Fujifilm FLA-5100).
SNCA half-life analysis
Radioactivity counts over immunoprecipitated SNCA were entered into GraphPad Prism 6 software. A one-phase exponential decay trend line was fitted to the data, and the half-life for the specific experiment was determined. SNCA half-life was calculated separately for each experiment. Because exposure to methionine-free medium for labelling caused disturbances in cell viability, we used 24 h after labelling as the initial time point.
Western blotting
Neuronal cultures were homogenized in RIPA buffer, and brain samples were prepared as previously described (Kuo et al., 2010). The following antibodies were used: mouse anti-SNCA (BD Transduction Laboratories), mouse anti-phospho S129 SNCA (Wako), rabbit anti-GBA (Sigma), mouse anti-tubulin (Calbiochem), anti-neuronal specific enolase (NSE, Abcam), mouse anti-NeuN (Millipore) and goat anti-actin (Santa Cruz Biotech Inc). Horseradish peroxidase goat anti-mouse (GE Healthcare) or anti-rabbit (Cell Signaling) secondary antibodies and Clarity (BioRad) or Prime ECL kits were used. Analyses were prepared using ImageJ software.
GBA activity assay
GBA activity assay was based on Urban et al. (2008). One brain hemisphere (3-week-old mouse), was lysed in 1 ml of GBA activity assay buffer (50 mM citric acid, 176 mM K2HPO4, 10 mM sodium taurocholate, 0.01% Tween-20 pH 5.9). Brain lysate (5 μl) was incubated with either 5 μl of 20 mM CBE GBA blocker (Sigma) to assess non-specific activity, or 5 μl of assay buffer, for 15 min at room temperature. Twenty-five microlitres of 5 mM 4-methylumbelliferyl β-glucopyranoside (4MU-β-Glc) substrates were then added simultaneously to samples, which were then incubated at 37°C for 25 min. Samples were cooled on ice, and reactions stopped with 40 μl stop buffer (1 M NaOH, 1 M glycine pH 10). Relative fluorescent units (RFU) were measured at 450 nm using the NanoDrop 3300 Fluorospectrometer. Non-specific activity was subtracted from each reading, and final results normalized to the total protein concentration. Protein amounts and incubation times were chosen after being confirmed to be in linear range.
Motor and enteric nervous system tests
Motor and enteric nervous system tests were performed as previously described (Kuo et al., 2010) (Supplementary material).
Statistical analysis
A non-parametric two-tailed Mann-Whitney U-test was used to determine statistical significance when the data were not normally distributed or could only be ranked. Student’s t-test was used otherwise. All data were analysed by GraphPad Prism 6 statistical software (GraphPad Software).
Results
SNCA is expressed predominantly by neurons in a mixed neuronal-glial culture
To measure SNCA degradation kinetics in a neuronal cellular environment, we used primary neuronal cortical cultures derived from various lines of mice, expressing either wild-type mouse SNCA or human SNCA, as previously described (Kuo et al., 2010). As these cortical cultures also contain glial cells (Fig. 1A) (mainly astrocytes), we first confirmed that SNCA was produced predominantly by the neurons in the culture.
Figure 1.
SNCA is produced chiefly by neurons in the mixed, 11-day-old glial/neuronal culture. (A) A mixed glial/neuronal culture stained for both neurons in red (MAP2), and glia in green (GFAP). Cultures were counterstained with 4',6-diamidino-2-phenylindole (DAPI, blue). (B) Quantitative RT-PCR of mouse Snca mRNA in a pure wild-type glial culture revealed mouse Snca mRNA levels to be 4.4 ± 1% and 7.5 ± 0.5% (standard deviation) of the combined glial + neuronal (G+N) culture. ELAV14 and synaptophysin served as reference genes, respectively. (C) Western blots of mouse and human SNCA, for a combined glial + neuronal culture (G+N) or a pure glial culture (G). Westerns blots done for Snca−/−, wild-type (Snca+/+), hSNCAWT(Snca−/−) and hSNCAA53T(Snca−/−), confirmed that SNCA protein levels in the glial culture are a small fraction of total SNCA in all glial/neuronal cultures. Control proteins were the neuronal marker NeuN and actin. WT = wild-type.
We compared the levels of mouse Snca RNA using quantitative PCR in wild-type mixed neuronal-glial cultures, and in pure glial cultures where neurons were eliminated by exposure to glutamate. We found that the amount of Snca mRNA expressed in pure glial cultures was negligible (Fig. 1B). Additionally, western blots in both wild-type and the transgenic lines revealed mouse and human SNCA levels to be minimal in pure glial cultures, as opposed to glial/neuronal cultures, although some human SNCA is still being made in hSNCAWT and hSNCAA53T glial cultures (Fig. 1C).
SNCA half-life measurement
We metabolically labelled 10-day-old cultures with radioactive methionine, and used pulse chase to measure the degradation of neuronal SNCA over 144 h. This culture age was chosen because Snca expression is high, yet neuronal cultures have not become so old that they cannot tolerate the frequent media changes required for pulse labelling. Some neuronal cell death was observed in the first few hours after labelling. After that, neuronal numbers remained stable throughout the rest of the experiment. As some of the decrease in incorporated radioactivity in the first 24 h could be attributed to neuronal death rather than protein degradation, measurements taken immediately after labelling were not used to calculate SNCA half-life. Instead, radioactivity incorporated at 24 h after labelling (when cultures had stabilized), were used as the first time point and all readings from later time points were normalized to it. Data were fit to a one-phase exponential decay and SNCA half-life determined (Fig. 2A and B).
Figure 2.
SNCA rate of degradation is species-specific, and is not affected by the A53T mutation, in cortical neuron. (A) Protein degradation kinetics of SNCA in neuronal cultures derived from wild-type and two transgenic lines of mice, expressing human SNCA, with or without the A53T mutation. A one-phase exponential decay tread line was fitted to the results of a pulse/chase metabolic labelling experiment. Wild-type mouse SNCA degradation is substantially slower than that of human SNCA. (B) Representative examples of phosphorimager images taken after pulse/chase experiments done on cultures from each of the three genotypes. Time after labelling is denoted as real and (relative to the reference time point of 24 h) under each lane. (C) SNCA half-life (t1/2) was calculated separately for each experiment and averaged for each of the three genotypes. Red denotes higher radioactive signal intensity and green lower intensity.
We found that wild-type human SNCA turns over slowly in cortical neurons with a half-life (t1/2) of 61.2 ± 6 h (n = 7, Fig. 2A–C). To determine whether the A53T mutation contributes to the stability of the protein and protects it from degradation, we repeated the experiment in neuronal cultures derived from a second transgenic line expressing human SNCA with the A53T mutation and found t1/2 = 66.4 ± 6 h (n = 5) (Kuo et al., 2010). There was no significant difference in the rate of degradation between the wild-type and mutated forms of human SNCA (P > 0.05, Fig. 2A and C), indicating that the A53T mutation by itself is not sufficient to influence the protein rate of degradation in neuronal cultures. Finally, we also measured the half-life of mouse SNCA and found its rate of degradation to be slower than either the wild-type or A53T human SNCA, with t1/2 = 145 ± 12 h, a 2.3-fold difference (n = 4, P < 0.01, Fig. 2A–C).
GBA expression and enzymatic activity in the brains of SNCA transgenic mice
Prompted by the suggestion for a reciprocal relation between the GBA enzyme and SNCA (Mazzulli et al., 2011; Yap et al., 2013), we asked how different degrees of SNCA expression might affect GBA protein levels and enzyme activity. Using western blot analyses and enzyme assays, we measured the relative protein levels and activity of GBA in the brains of Snca knockout mice that expressed no endogenous SNCA (Snca−/−) and two transgenic lines expressing human SNCA in a Snca−/− background (hSNCAWT; Snca−/− or hSNCAA53T;Snca−/−). These transgenic lines showed a modest 1.3 to 2-fold increase in steady-state human SNCA protein compared to wild-type mouse SNCA (Kuo et al., 2010). We could not detect a significant difference in whole brain GBA protein by western blot analyses between any of these four lines (Fig. 3A). However, GBA enzyme activity in brain samples taken from the same mice showed a significant 35% increase in the GBA activity in SNCA-knockout mice as compared to control SncaWT mice (from 100 ± 5% to 135 ± 8% P < 0.01, n = 10 for both; Fig. 3B). However, the reciprocal effect was not seen, because the hSNCAWT and an hSNCAA53T mice, showed no decrease in GBA activity, despite the 1.3 to 2-fold increase in human SNCA expression over wild-type mouse SNCA.
Figure 3.
GBA enzymatic activity is affected by loss of Snca expression. (A) GBA protein levels are not significantly affected by different expression levels of SNCA as revealed by western blots (top), and by quantification (bottom). From left to right; Snca−/−, hSNCAWT and hSNCAA53T mice are not significantly different from wild-type (WT) mice expressing mouse endogenous SNCA (Snca+/+) (P > 0.05). (B) GBA activity in the brains of mice with different expression levels of SNCA. GBA activity is reduced by the +/L444P mutation to 68 ± 1 of control activity level (P < 0.01, n = 3), while the absence of SNCA expression (in the Snca−/−) increases GBA activity to 135% of control (P < 0.01, n = 10). However, a 1.5 to 2-fold overexpression of human SNCA in the two transgenic mouse lines has no significant effect on GBA activity (P > 0.05, n = 10 for SncaWT, n = 5 for SNCAWT and SNCAA53T).
Heterozygosity for the Gba L444P mutation decreases the rate of SNCA degradation
To test the hypothesis that a common Gaucher disease mutation in the heterozygous state is sufficient to influence the rate of SNCA degradation in neurons, we obtained a knock-in mouse carrying the L444P Gaucher mutation in its Gba gene (Liu et al., 1998). These mice were wild-type for the glucosylceramide synthase gene (Ugcg), enabling normal glucosylceramide synthesis. When homozygous for the mutation, GBA activity is reduced to 20% of control yet, presumed glucosylceramide accumulation is small and detectable only in the mouse epidermis (Liu et al., 1998; Mizukami et al., 2002). After confirming decreased GBA enzymatic activity in the heterozygous mouse (Supplementary Fig. 2A), we crossed the GbaL444P allele on to each of the human SNCA transgenic lines. These mice were healthy, fertile and had a normal life span (26 months). By visual inspection, they showed no gross motor dysfunction such as ataxia, tremor or paralysis. As expected, these double mutant mice also exhibited a decrease in GBA activity (Fig. 3B), as well as lower GBA protein levels in brain tissue (Supplementary Fig. 2B).
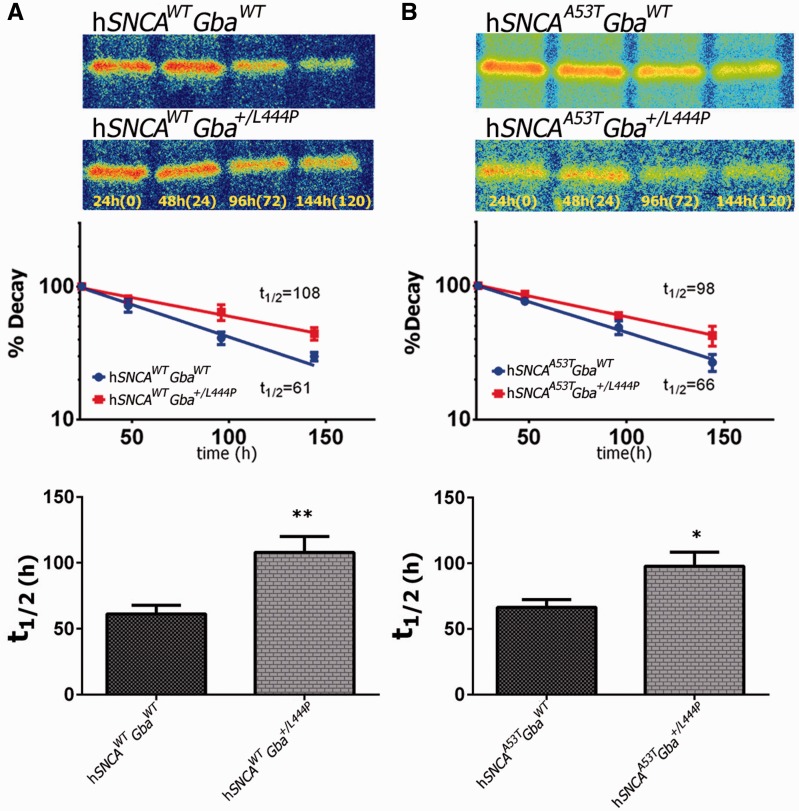
We then repeated pulse chase experiments in neurons derived from neurons expressing human SNCA in both Snca−/− and Gba+/L444P backgrounds. We found that L444P heterozygosity significantly affected the rate of human SNCA degradation, with the hSNCAWT half-life increasing by 77% from 61.2 ± 6 h to 108 ± 12 h (P < 0.01, n = 5, Fig. 4A), whereas hSNCAA53T half-life increased by 46% from 67 ± 6 h to 98 ± 11 h P < 0.05, n = 3, Fig. 4B). As might be expected from the prolongation of half-life in a Gba+/L444P background, cultures derived from the brains of mice heterozygous for the Gaucher L444P mutation, tended to show higher SNCA steady state levels, as revealed by western blot. SNCA protein levels increased 57 ± 7% in hSNCAA53T;Gba+/L444P cultures versus hSNCAA53T GbaWT cultures (n = 3, P < 0.05, Fig. 5A) whereas SNCA levels in hSNCAWT;Gba+/L444P cultures increased 58% ± 27% versus hSNCAWT;GbaWT cultures (n = 3 and 5, respectively, P = 0.058, Fig. 5B), which just failed to reach significance at the P < 0.05 level.
Figure 4.
Heterozygosity for the Gba L444P mutation decreases the rate of SNCA degradation. (A) Top: Representative phosphorimager images of pulse/chase experiments from cultures with and without the Gba+/L444P mutation. Middle: One-phase exponential decay trend-line fitted to the results of a pulse/chase metabolic labelling experiment carried out in neuronal cultures expressing hSNCAWT, with or without the Gba L444P mutation. Bottom: hSNCAWT half-life increased by 77%, from 61.2 ± 6 h to 108 ± 12 h (P < 0.01 n = 5) when neurons carried the L444P mutation. (B) Top: Representative phosphorimager images of hSNCAA53T, with or without the Gba +/L444P mutation. Middle: One-phase exponential decay trend-line fitted to the results of a pulse/chase metabolic labelling experiment carried out in a neuronal cultures expressing hSNCAA53T, with or without the L444P mutation. Bottom: hSNCAA53T half-life increased by 46% from 67 ± 6 to 98 ± 11 (P < 0.05 n = 3).
Figure 5.
Human SNCA accumulates in neuronal cultures carrying the Gaucher L444P mutation. (A) Western blot analysis revealed that 3-week-old neuronal cultures derived from SNCAA53T;Gba+/L444P mice contained significantly higher levels of the human SNCA protein than their SNCAA53T;GbaWT counterpart (157 ± 7% in SNCAA53T;Gba+/L444P when compared to hSNCAA53T;GbaWT cultures, n = 3, P < 0.05). (B) Three-week-old neuronal cultures derived from SNCAWT;Gba+/L444P mice tend to show higher levels of the human SNCA protein when compared to their SNCAA53T;GbaWT counterparts (158% ± 27% in hSNCAWT;Gba+/L444P cultures when compared to hSNCAWT;GbaWT cultures (n = 3 and 5, respectively P = 0.058). WT = wild-type; NSE = neuron-specific enolase.
The GBA L444P mutation causes an exacerbation of a Parkinson’s disease-related phenotype
We then tested whether this slower degradation of human SNCA would have an effect in vivo. As the hSNCAA53T transgenic mouse exhibit both enteric dysfunction by 3 months, and abnormal motor function starting at 6 months of age (Kuo et al., 2010), we hypothesized that the slower degradation and increase in human SNCA accumulation would exacerbate these phenotypes in the hSNCAA53T;Gba+/L444P double-mutant mouse. To test this hypothesis we characterized enteric and motor dysfunction in the hSNCAA53T;Gba+/L444P double transgenic mice in comparison to hSNCAA53T;GbaWT mice.
Motor function
We examined motor function in the hSNCAA53T;Gba+/L444P mice against an age-matched cohort of hSNCAA53T;GbaWT mice using the accelerating Rotarod and Open Field at 7 and 14 months of age. Cohorts were matched for age and showed no significant difference in body weight that could affect motor performance (Supplementary Fig. 3). In the accelerating Rotarod test (Fig. 6A), both sets of mice at 7 months of age had similar latency times that increased consistently, as expected, over four trials per day carried out over three consecutive days as the animals were trained to stay on the rotating apparatus. We also confirmed that the latency for both lines was significantly shorter than age-matched SncaWT and Snca−/− control lines (data not shown). However, when tested at 14 months of age the hSNCAA53T;Gba+/L444P mice had consistently reduced latency times (Fig. 6B, P-values ranging from P < 0.05 at Day 2 to P < 0.002 with consecutive testing on Day 3).
Figure 6.
Augmentation of Parkinson’s disease-related behavioural symptoms in 14-month-old but not 7-month-old hSNCAA53T GbaL444P/+ mice. Rotarod latency (time to fall) in the Rotarod test at 7 months (A), and 14 months of age (B). hSNCAA53T = Dbl-PAC-Tg(SNCAA53T)+/+; Snca−/− and hSNCAA53T;Gba+/L444P = Dbl-PAC-Tg(SNCAA53T)+/+;Snca−/−; Gba+/L444P. Error bars are ±1 standard errors of the mean (n = 20 for each genotype at 7 months, and n = 31 and 34 for hSNCAA53T and hSNCAA53T; Gba+/L444Pat 14 months of age, respectively). Total number of rearings (C), time in rearing position (D), and total distance covered (E) in open field at 14 months of age. Enteric nervous system dysfunction: colonic motility plotted for males at 7 and 14 months (F) as assessed by time (in seconds) required for a bead to be expelled from the rectum. The number of mice of each genotype tested at each age are: n = 16 and 24 for hSNCAA53T;GbaWT and hSNCAA53T;Gba+/L444P, respectively at 7 months of age, and n = 14 and 19 at 14 months of age. Whole-gut transit time (G) at 15 months as determined by time (in minutes) required for a non-absorbable dye, introduced by gavage, to appear in the stool. Mice were not tested at 7 months as there were no differences in colonic motility at that age. Males demonstrated a prolonged whole-gut transit time compared to hSNCAA53T;GbaWT (P < 0.05) by t-test. The increase in transit times was not seen in female mice, and failed to reach significance by one-way ANOVA testing of both sexes.
In the open field apparatus, total distance (Fig. 6E), centre and periphery distances travelled were similar in both lines at each time point. However, both the total time spent in the rearing position and the number of rearings was reduced in the hSNCAA53T;Gba+/L444P mice at 14 months (Fig. 6C and D). Rearing differences can indicate alterations in exploratory behaviour or hind limb weakness, but there was no generalized reduction in movement.
Mice were also tested on both the Elevated Plus and Zero Maze at 20 months of age (Supplementary Fig. 3B–F). The hSNCAA53T;GbaWT mice spent a higher percentage of time in the open areas as measured by both the Plus and Zero Mazes compared to the hSNCAA53T;Gba+/L444P mice (P < 0.01 and P = 0.045, respectively). hSNCAA53T;GbaWT mice also had significantly more open area entries (P < 0.05 by Plus Maze) and spent more time in the centre of the Plus Maze (P < 0.01), although the closed area entries and total distance travelled were similar in both sets of mice in both tests. The difference in the rearing changes seen by Open Field further implies possible altered exploratory behaviours. In summary, hSNCAA53T;Gba+/L444P mice, demonstrated a consistent reduced motor activity, with abnormal endurance and coordination on the Rotarod, as compared to hSNCAA53T;GbaWT mice.
Enteric nervous system function
We tested the motility of the distal colon in the hSNCAA53T;Gba+/L444P animals. In accordance with our previous observations, the expulsion time was normally longer in males than in females. This sex difference, however, was greatly increased in male Gaucher hSNCAA53T;Gba+/L444P mice, in which bead expulsion time was increased 42% compared to the hSNCAA53T;GbaWT mice. The increased expulsion time was not observed at 7 months of age but was present at 14 months of age (P < 0.01, Fig. 6F).
We then examined whole-gut transit time, a global indicator of enteric nervous system function. At 15 months of age, male hSNCAA53T;Gba+/L444P mice demonstrated a prolonged whole-gut transit time compared to hSNCAA53T;GbaWT (P < 0.05) by t-test (Fig. 6G). Consistent with the bead expulsion times, the increase in transit times was not seen in female mice, and thus failed to reach significance by one-way ANOVA testing of both males and females.
We conclude that the reduced colonic motility, and comparably prolonged whole-gut transit time in the hSNCAA53T;Gba +/L444P transgenic male mice indicate that the dysfunction in these mice has been exacerbated by the presence of one copy of the L444P Gba allele.
Histological characterization of SNCA CNS pathology
The exacerbation of these Parkinson’s disease-related phenotypes in the hSNCAA53T;Gba+/L444P double transgenic mouse, motivated us to search for histological evidence of synucleinopathies in these mouse brains. Although we could not detect stereotypical Lewy body pathology, immunohistochemistry of hSNCAA53T;Gba+/L444P brains at 15 months of age revealed localized accumulations of human SNCA phosphorylated at serine 129 (pSer129), typical of pathologically aggregated SNCA (Giasson et al., 2000; Waxman et al., 2008; Waxman and Giasson, 2010). This build-up of pSer129 SNCA staining, which appeared reminiscent of synaptic terminals, was seen primarily in the hippocampi (mainly at the CA1 region) but was absent in hSNCAA53T;GbaWT mice (Fig. 7A and B, n = 2 for each genotype). By 19 months of age, however, some pSer129 SNCA accumulations also appeared in the hippocampi of hSNCAA53T;GbaWT mice, yet none were detected in wild-type mice (Fig. 7C and D, n = 2 for wild-type mice, and n = 3 for each transgene). Similar results were also obtained by western blot analysis of 19 month hippocampal lysates with a different commercial antibody against pSer129 (Wako) (S3). This indicates that heterozygosity for the L444P mutation caused an earlier onset of a pathological process that would eventually also appear in the hSNCAA53T;GbaWT mice. Nevertheless, when both sets of histological data were combined, the hSNCAA53T;Gba+/L444P mice still exhibited a 70% increase in pSer129 SNCA staining (from 100 ± 28% to 170 ± 15%, P < 0.05 one-tailed t-test).
Figure 7.
Local accumulations of phosphorylated SNCA in the CA1 region of the hippocampus of hSNCAA53T;Gba+/L444P and hSNCAA53T;GbaWT mice. (A.1–2) The hippocampus of a 15-month-old hSNCAA53T;GbaWT mouse stained for phosphorylated S129 SNCA. The boxed area is magnified in A.2. No accumulation of abnormal (brownish) staining can be seen. (A.3–4) The hippocampus of a 15-month-old hSNCAA53T;Gba+/L444P mouse. Increased staining for phosphorylated S129 SNCA, reminiscent of synaptic accumulations, can be seen in multiple locations in the CA1 region. (B) Quantification of the positive pixels in the hippocampus of 15-month-old mice as analysed by ImageScope software (Aperio) (n = 2 for each genotype). (C.1–2) The hippocampus of 18-month-old wild-type mouse. (C.3–4) The hippocampus of a 19-month-old hSNCAA53T;GbaWT mouse. (C.5–6) The hippocampus of a 19-month-old hSNCAA53T;Gba+/L444P mouse. Positive staining can be detected in the CA1 region of both genotypes. (D) Quantification of the positive pixels in the hippocampus of 18 to 19-month-old mice (n = 2 for wild-type control mice, and n = 3 for each of the transgene genotypes). When the two sets of data were combined, the hSNCAA53T;Gba+/L444P mice exhibited a 70% increase in pSer129 SNCA staining (from 100 ± 28% to 170 ± 15%, P < 0.05 one-tailed t-test). WT = wild-type.
Other SNCA antibodies, not selective for pSer129, did not show increased SNCA staining (data not shown) in the hippocampi. We therefore concluded that these local accumulations of pSer129 SNCA are quantitatively insignificant relative to total SNCA in the hippocampus, but are important markers of pathological aggregation.
No increase in absolute levels of SNCA in hSNCAA53TGba+/L444P brain
Given the effect of the L444P mutant allele on increasing SNCA levels in neuronal cultures and the histological findings of hippocampal phosphorylated SNCA, we examined total protein levels in whole brain homogenates. We were surprised that we could not detect any increase in the overall levels of SNCA in the hSNCAA53T;Gba+/L444P mice, at 15 months of age compared to hSNCAA53T;GbaWT brains (n = 4 for each group, P = 0.12, Fig. 8). Even when we probed blots against phosphorylated human SNCA, as seen in the hippocampal sections from the hSNCAA53T;Gba+/L444P brains, no absolute increase was detected in whole brain homogenates (whereas both hSNCAA53T;Gba +/L444P and hSNCAA53T exhibited higher amounts of phosphorylated human SNCA compared to wild-type controls, data not shown). We therefore concluded that although the Gba+/L444P genotype interferes with SNCA degradation in cultured cortical neurons, and has a demonstrable effect on the histology of the brain and on the behaviour of mice, the western blot approach is not sensitive enough to detect localized changes in different subregions of the brain.
Figure 8.
No increase in the total amount of human SNCA in the brains of symptomatic SNCAA53T;Gba+/L444P mice. Top: Western blot of whole brains lysates, of 14-month-old SNCAA53T;GbaWT and SNCAA53T;Gba+/L444P mice. Blots were probed against human SNCA and tubulin. Bottom: Quantification of human SNCA protein normalized to tubulin revealed no significant difference between the two genotypes (P = 0.18).
Discussion
Human SNCA is a highly stable protein in cultured murine neurons with a half-life of 61 h. The half-life of mouse SNCA, is twice as long (145 h), measured under precisely the same conditions. In contrast to previous reports (Cuervo et al., 2004; Li et al., 2004), we found no evidence that the SNCAA53T mutation increases the stability of SNCA. In addition, we confirmed that heterozygosity for a common Gaucher disease mutation is indeed sufficient to affect the rate of human SNCA degradation, and that this can have an effect on brain pathology and animal behaviour in vivo.
Previously published measurements of human SNCA half-life were made under a variety of experimental conditions including the use of primary versus transfected, dividing cells, different times in culture of primary neurons, the presence of glia in the culture or serum in the medium, the use of stable versus transient transfection, and the use of expression plasmids to overexpress native versus tagged forms of SNCA. Not surprisingly, these different conditions produced conflicting results, with SNCA half-life varying from 2–36 h (Bennett et al., 1999; Cuervo et al., 2004; Vogiatzi et al., 2008; Machiya et al., 2010; Alvarez-Castelao and Castano, 2011). The most similar experiments to ours were performed by Li et al. (2004), although their culture method differed from ours in that they used prenatal neurons in serum-free glia-free conditions. They measured SNCA in cortical neurons and reported that the half-life of endogenous mouse SNCA increased with time in culture with half-life rising from 54 to 160 h. One important difference between their study and ours is that they proposed a biphasic turnover, with a fast degradation phase occurring in the first 24 h after labelling. We made a similar observation, but attributed the initial drop in SNCA labelling to the observed cell death seen in the first few hours after labelling and thus excluded it from the final analysis. We hold that only the later phase of degradation, after cultures have stabilized, should be considered as physiologically relevant. In any event, the higher stability of the SNCA protein in primary neurons, compared to immortalized cells, might reflect a general characteristic of neurons. Neurons do not divide and are in cell cycle arrest. Such slower intracellular metabolism might extend to other proteins, making neurons more vulnerable to misfolded proteins.
When speculating on the origin of the differences in the half-life of mouse and human SNCA, we cannot dismiss the possibility that this difference is due to the latter being a foreign protein introduced into the transgenic mouse. Nevertheless, it is also possible that it reflects a real difference in the innate properties of mouse versus human SNCA. The amino acid sequence of the mouse and human SNCA proteins differs at only 7 of 140 residues. Yet, this small divergence is enough to produce significant structural differences (Wu et al., 2008). Notably, in the mouse, position 53 is occupied by threonine rather than alanine as in the human A53T mutation. Thus, from an evolutionary perspective, one could reason that the murine relative short life span makes it more tolerant to mouse SNCA accumulation and aggregation, and thus a longer half-life for SNCA, could be tolerated in a mouse, but not in the human brain.
Whatever the reason for the mouse SNCA prolonged half-life, it cannot be attributed solely to the threonine at position 53, because the A53T mutation by itself was not enough to trigger a significant prolongation of human SNCA half-life. In our view this is an indication that this mutation does not act through prolonging the half-life of soluble SNCA protein but rather it may have its pathogenic effect through promoting the aggregation of the protein.
Determining the mechanism by which an individual heterozygous for a disease-causing GBA mutation would have increased susceptibility to Parkinson’s disease without any clinical signs of Gaucher disease itself could provide important insights into the pathogenesis of sporadic Parkinson’s disease. Using a viral delivered knock-down in cultured mouse cortical neurons, Mazzuli et al. (2011) demonstrated that a 50% reduction in the levels of the GBA enzyme can result in 80% increase in SNCA levels. On the other hand, Cleeter et al. (2013) working in neuroblastoma cells, demonstrated that a direct inhibition of GBA enzymatic activity triggered the accumulation of SNCA, but suppression of Gba expression did not result in protein accumulation that was statistically significant. In comparison, introduced mutations in the Gba gene more closely emulates the human disease scenario as the presence of a mutant protein decreases enzymatic activity as well as affecting other protein–protein interactions. This approach was taken by Sardi et al. (2011), who studied a transgenic mouse homozygous for an artificial (not found in human patients) GBA mutation (D409V). Homozygosity for the mutation reduced GBA activity to ∼15% of controls (Sun et al., 2012) caused SNCA aggregation in the mouse hippocampus, and triggered hippocampus-related memory deficiencies. A heterozygous mouse on the other hand exhibited some SNCA aggregation but no behavioural phenotype, whereas a mouse heterozygous for a Gba deletion did not exhibit either sign (Sardi et al., 2011). Similarly, the accumulation of SNCA was seen in the striatum of two distinct Gaucher models homozygous to either the L444P or the R463C mutations. This accumulation was accompanied by neuroinflammation and synaptic dysfunction. Additionally, blocking GBA in vivo with the CBE blocker achieved similar results (Ginns et al., 2014).
Nevertheless until today, it remained unclear whether heterozygosity for a known Gaucher mutation would be sufficient to have any influence on SNCA half-life. If not, GBA mutations might cause a toxic gain of function unrelated to lysosomal enzyme deficiency. In this study we showed that in a line of mice with a genotype heterozygous for p.L444P, a common Gba missense mutation that results in a 40% reduction in enzyme activity increased hSNCAWT half-life by 77%. This finding supports the hypothesis that even a partially compromised lysosome could underlie the Gaucher-Parkinson’s disease association. Nevertheless, a role for a gain of toxic function by the mutant GBA protein cannot be excluded. For example, a plausible mechanism is that the expression of GBAL444P alters trafficking and localization of SNCA, leading to its longer half-life. Supporting this hypothesis is the observation made by Cullen et al. (2011), who reported that overexpression of various GBA mutations (including the D409V and L444P) in SNCA producing cell lines, increases SNCA concentration without affecting GBA activity. On the other hand, it should be considered that artificial overexpression of mutant GBA can have a dominant-negative effect in the cell. Such an effect may not be detectable in standard activity assays done in cell lysates. Thus this question will remain open until a mouse model bearing a mutant Gba allele in addition to two non-mutated Gba alleles can be studied.
Given that heterozygosity for a GBA mutation does not always result in Parkinson’s disease but simply raises the relative risk ∼4-fold, and much shorter life span of mice versus humans, the difficulty in reproducing a Parkinson’s disease-related phenotype in a heterozygous Gaucher mouse model is to be expected. We addressed this problem by crossing the Gba+/L444P mutant to the established SNCAA53T Parkinson’s disease model that develops early-onset enteric nervous system dysfunction and later onset motor function abnormalities, similar to what is seen in sporadic Parkinson’s disease in humans. We then looked for the exacerbation of the Parkinson’s disease-related phenotypes rather than the development of new signs of Parkinson’s disease. With this approach we were able to demonstrate that heterozygosity for this Gaucher mutation was indeed sufficient to augment motor deficits, and to exacerbate abnormal gastrointestinal motility. In addition, it triggered an increase of phosphorylated SNCA in the hippocampus CA1 region, and thus resembles the GbaD409V/D409V mouse (Sardi et al., 2011) as well as human Gaucher carriers with Parkinson’s disease (Wong et al., 2004). Although the exacerbated motor and enteric phenotypes are unlikely to be directly linked to the increased immunostaining of the hippocampal CA1 region with the pSer129/81A antibody, we interpret it to indicate the start of human SNCA aggregation. The fact that positive staining was detected in both of the A53T lines with or without the Gba+/L444P mutation at 19 months of age, but not in wild-type mice (Fig. 7 and Supplementary Fig. 4), demonstrates that the hSNCAA53T mutation increases the probability for human SNCA aggregation. The question remains as to the possible contribution of the Gba+/L444P mutation to this process, as seen at 15 months. One possible cause could be the local accumulation of SNCA. We could not detect such accumulation in either hippocampal or whole brain lysates by western blot, but these may still occur locally in small compartments, but were not detected by crude western analyses. Another possibility is that different lipid compositions or direct interactions between these two proteins promote SNCA aggregation independently of lysosome dysfunction. Indeed, erythrocytes (which lack lysosomes) from patients with Gaucher disease exhibit abnormal lipid composition accompanied with high levels of polymeric SNCA (Argyriou et al., 2012). Yet a third possibility, is that due to poor trafficking of L444P GBA to the lysosome, an unfolded protein response is elicited leading to phosphorylated SNCA accumulation. However other Gaucher mutations, which allow GBAs to properly traffic into the lysosome, do not elicit an unfolded protein response, and still appear to be associated with an increased risk of Parkinson’s disease. Additionally, CBE-treated neurons did not show an increase in unfolded protein response markers (Farfel-Becker et al., 2009; reviewed in Siebert et al., 2014).
The observed symptoms are unlikely to be the result of the Gaucher mutation alone, because mice homozygous to the L444P mutation did not exhibit any form of neuronal pathology, up to 12 months of age, despite having multisystem inflammatory pathology (Mizukami et al., 2002). This is in contrast with the CNS inflammation observed in a different homozygous L444P line (Ginns et al., 2014).
Of the described phenotypes, we found the increased gastrointestinal transit time most compelling because, in patients with Parkinson’s disease, it precedes motor symptoms and thus represents the earliest stages of the disease. Likewise, the late manifestation, at 14 months but not at 7 months, of the augmented motor phenotype also recapitulates the typical late-onset of Parkinson’s disease in humans.
The fact that we could detect elevated levels of SNCA in neuronal cultures form Gba+/L444P mice but not in total SNCA extracted from brain, suggests that extraneuronal protein clearance mechanisms (as reviewed by Deleidi and Maetzler, 2012) might be acting in vivo, to delay or mask SNCA accumulation. Such mechanisms might not be present in the neuronal culture model. For example, microglial cells, which are not supported in the neuronal culture used in this study, were shown to play a role in the clearance of extracellular SNCA in the brains of mice overexpressing SNCA (Bae et al., 2012). A disruption of such mechanisms (either local or systemic), could trigger Parkinson’s disease over time. This could explain the relative low, but persistent penetrance of Parkinson’s disease in Gaucher carriers.
Our results also support the possibility of direct protein–protein interaction between GBA and SNCA, as samples from Snca−/− brains exhibited a 35% increase in GBA activity without showing an elevation in the overall amount of the enzyme. This observation supports the in vitro observation by Yap et al. (2013) of the inhibitory effect of membrane-bound SNCA on GBA activity. Nevertheless, we could not detect differences in GBA enzyme activity between the samples taken from wild-type mice, and those taken from transgenic mice with slightly higher SNCA protein levels (Kuo et al., 2010) and confirmed in neuronal cultures (data not shown). This—in our view—may indicate a relatively low saturation level after which an increase in SNCA concentration will not affect GBA activity. Nevertheless, it is possible that specific brain regions are differently affected by higher levels of human SNCA, and that some of these areas would have presented GBA activity deficits had they been explored separately. It has been shown that patients with Parkinson’s disease exhibit lower GBA activity in various brain areas, but not in the cortex (Gegg et al., 2012). Given the predominance of cortical matter, it is possible that such differences were lost in the whole brain lysates used.
In conclusion, our data indicate that within the neuron, human SNCA is a highly stable protein. Its optimal degradation thus is essential for the prevention of its accumulation and aggregation. Perturbation of SNCA degradation by even a modest decrease in GBA enzymatic activity has demonstrable effects on the enteric nervous system and motor activity in vivo in transgenic mice. These findings point to the possibility that a moderate decrease in GBA activity may contribute to the onset of non-familial Parkinson’s disease and supports the idea that the glucocerebroside degradation pathway in the lysosome should be explored as a potential target for prevention or treatment of Parkinson’s disease.
Acknowledgements
We thank Ellen Sidransky for verifying antibody specificity and sending Gba knockout mouse pups. We thank Xuefeng Sun and Xue Yang for their help with mice genotyping by PCR and cell cultures preparations and Mieu Brooks for help with histological analysis.
Funding
NIH/NIEHS R01ES017793 and NIH/NIA R21AG033941 to R.L.N. Parkinson’s Disease Foundation Fellowship PDF-FBS-1232 to I.F.
Supplementary material
Supplementary material is available at Brain online.
References
- Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R. Mutations in the glucocerebrosidase gene and Parkinson's disease in Ashkenazi Jews. N Engl J Med. 2004;351:1972–7. doi: 10.1056/NEJMoa033277. [DOI] [PubMed] [Google Scholar]
- Alvarez-Castelao B, Castano JG. Synphilin-1 inhibits alpha-synuclein degradation by the proteasome. Cell Mol Life Sci. 2011;68:2643–54. doi: 10.1007/s00018-010-0592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyriou A, Dermentzaki G, Papasilekas T, Moraitou M, Stamboulis E, Vekrellis K, et al. Increased dimerization of alpha-synuclein in erythrocytes in Gaucher disease and aging. Neurosci Lett. 2012;528:205–9. doi: 10.1016/j.neulet.2012.08.069. [DOI] [PubMed] [Google Scholar]
- Bae EJ, Lee HJ, Rockenstein E, Ho DH, Park EB, Yang NY, et al. Antibody-aided clearance of extracellular alpha-synuclein prevents cell-to-cell aggregate transmission. J Neurosci. 2012;32:13454–69. doi: 10.1523/JNEUROSCI.1292-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MC, Bishop JF, Leng Y, Chock PB, Chase TN, Mouradian MM. Degradation of alpha-synuclein by proteasome. J Biol Chem. 1999;274:33855–8. doi: 10.1074/jbc.274.48.33855. [DOI] [PubMed] [Google Scholar]
- Cleeter MW, Chau KY, Gluck C, Mehta A, Hughes DA, Duchen M, et al. Glucocerebrosidase inhibition causes mitochondrial dysfunction and free radical damage. Neurochem Int. 2013;62:1–7. doi: 10.1016/j.neuint.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough RL, Dermentzaki G, Haritou M, Petsakou A, Stefanis L. Regulation of alpha-synuclein expression in cultured cortical neurons. J Neurochem. 2011;117:275–85. doi: 10.1111/j.1471-4159.2011.07199.x. [DOI] [PubMed] [Google Scholar]
- Corti O, Lesage S, Brice A. What genetics tells us about the causes and mechanisms of Parkinson's disease. Physiol Rev. 2011;91:1161–218. doi: 10.1152/physrev.00022.2010. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–5. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- Cullen V, Sardi SP, Ng J, Xu YH, Sun Y, Tomlinson JJ, et al. Acid beta-glucosidase mutants linked to Gaucher disease, Parkinson disease, and Lewy body dementia alter alpha-synuclein processing. Ann Neurol. 2011;69:940–53. doi: 10.1002/ana.22400. [DOI] [PubMed] [Google Scholar]
- Deleidi M, Maetzler W. Protein clearance mechanisms of alpha-synuclein and amyloid-Beta in lewy body disorders. Int J Alzheimers Dis. 2012;2012:391438. doi: 10.1155/2012/391438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermentzaki G, Dimitriou E, Xilouri M, Michelakakis H, Stefanis L. Loss of beta-glucocerebrosidase activity does not affect alpha-synuclein levels or lysosomal function in neuronal cells. PLoS One. 2013;8:e60674. doi: 10.1371/journal.pone.0060674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farfel-Becker T, Vitner E, Dekel H, Leshem N, Enquist IB, Karlsson S, et al. No evidence for activation of the unfolded protein response in neuronopathic models of Gaucher disease. Hum Mol Genet. 2009;18:1482–8. doi: 10.1093/hmg/ddp061. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Martinez A, Blanco R, Dalfo E, Carmona M. Neuropathology of sporadic Parkinson disease before the appearance of parkinsonism: preclinical Parkinson disease. J Neural Transm. 2011;118:821–39. doi: 10.1007/s00702-010-0482-8. [DOI] [PubMed] [Google Scholar]
- Fishbein I, Segal M. Miniature synaptic currents become neurotoxic to chronically silenced neurons. Cereb Cortex. 2007;17:1292–306. doi: 10.1093/cercor/bhl037. [DOI] [PubMed] [Google Scholar]
- Gegg ME, Burke D, Heales SJ, Cooper JM, Hardy J, Wood NW, et al. Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Ann Neurol. 2012;72:455–63. doi: 10.1002/ana.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giasson BI, Jakes R, Goedert M, Duda JE, Leight S, Trojanowski JQ, et al. A panel of epitope-specific antibodies detects protein domains distributed throughout human alpha-synuclein in Lewy bodies of Parkinson's disease. J Neurosci Res. 2000;59:528–33. doi: 10.1002/(SICI)1097-4547(20000215)59:4<528::AID-JNR8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Ginns EI, Mak SK, Ko N, Karlgren J, Akbarian S, Chou VP, et al. Neuroinflammation and alpha-synuclein accumulation in response to glucocerebrosidase deficiency are accompanied by synaptic dysfunction. Mol Genet Metab. 2014;111:152–62. doi: 10.1016/j.ymgme.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Grabowski GA. Phenotype, diagnosis, and treatment of Gaucher's disease. Lancet. 2008;372:1263–71. doi: 10.1016/S0140-6736(08)61522-6. [DOI] [PubMed] [Google Scholar]
- Korczyn AD, Gurevich T. Parkinson's disease: before the motor symptoms and beyond. J Neurol Sci. 2010;289:2–6. doi: 10.1016/j.jns.2009.08.032. [DOI] [PubMed] [Google Scholar]
- Kuo YM, Li Z, Jiao Y, Gaborit N, Pani AK, Orrison BM, et al. Extensive enteric nervous system abnormalities in mice transgenic for artificial chromosomes containing Parkinson disease-associated alpha-synuclein gene mutations precede central nervous system changes. Hum Mol Genet. 2010;19:1633–50. doi: 10.1093/hmg/ddq038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage S, Anheim M, Letournel F, Bousset L, Honore A, Rozas N, et al. G51D alpha-synuclein mutation causes a novel parkinsonian-pyramidal syndrome. Ann Neurol. 2013;73:12. doi: 10.1002/ana.23894. [DOI] [PubMed] [Google Scholar]
- Li W, Lesuisse C, Xu Y, Troncoso JC, Price DL, Lee MK. Stabilization of alpha-synuclein protein with aging and familial parkinson's disease-linked A53T mutation. J Neurosci. 2004;24:7400–9. doi: 10.1523/JNEUROSCI.1370-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Suzuki K, Reed JD, Grinberg A, Westphal H, Hoffmann A, et al. Mice with type 2 and 3 Gaucher disease point mutations generated by a single insertion mutagenesis procedure. Proc Natl Acad Sci USA. 1998;95:2503–8. doi: 10.1073/pnas.95.5.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machiya Y, Hara S, Arawaka S, Fukushima S, Sato H, Sakamoto M, et al. Phosphorylated alpha-synuclein at Ser-129 is targeted to the proteasome pathway in a ubiquitin-independent manner. J Biol Chem. 2010;285:40732–44. doi: 10.1074/jbc.M110.141952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, et al. Gaucher disease glucocerebrosidase and alpha-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell. 2011;146:37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezey E, Dehejia AM, Harta G, Tresser N, Suchy SF, Nussbaum RL, et al. Alpha synuclein is present in Lewy bodies in sporadic Parkinson's disease. Mol Psychiatry. 1998;3:493–9. doi: 10.1038/sj.mp.4000446. [DOI] [PubMed] [Google Scholar]
- Mizukami H, Mi Y, Wada R, Kono M, Yamashita T, Liu Y, et al. Systemic inflammation in glucocerebrosidase-deficient mice with minimal glucosylceramide storage. J Clin Invest. 2002;109:1215–21. doi: 10.1172/JCI14530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neudorfer O, Giladi N, Elstein D, Abrahamov A, Turezkite T, Aghai E, et al. Occurrence of Parkinson's syndrome in type I Gaucher disease. QJM. 1996;89:691–4. doi: 10.1093/qjmed/89.9.691. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–7. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Proukakis C, Dudzik CG, Brier T, MacKay DS, Cooper JM, Millhauser GL, et al. A novel alpha-synuclein missense mutation in Parkinson disease. Neurology. 2013;80:1062–4. doi: 10.1212/WNL.0b013e31828727ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout HJ, Dietrich P, Savalle M, Dauer WT, Stefanis L. Regulation of alpha-synuclein by bFGF in cultured ventral midbrain dopaminergic neurons. J Neurochem. 2003;84:803–13. doi: 10.1046/j.1471-4159.2003.01574.x. [DOI] [PubMed] [Google Scholar]
- Sardi SP, Clarke J, Kinnecom C, Tamsett TJ, Li L, Stanek LM, et al. CNS expression of glucocerebrosidase corrects alpha-synuclein pathology and memory in a mouse model of Gaucher-related synucleinopathy. Proc Natl Acad Sci USA. 2011;108:12101–6. doi: 10.1073/pnas.1108197108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. N Engl J Med. 2009;361:1651–61. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert M, Sidransky E, Westbroek W. Glucocerebrosidase is shaking up the synucleinopathies. Brain. 2014;137(Pt 5):1304–22. doi: 10.1093/brain/awu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon-Sanchez J, Schulte C, Bras JM, Sharma M, Gibbs JR, Berg D, et al. Genome-wide association study reveals genetic risk underlying Parkinson's disease. Nat Genet. 2009;41:1308–12. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- Sun Y, Liou B, Xu YH, Quinn B, Zhang W, Hamler R, et al. Ex vivo and in vivo effects of isofagomine on acid beta-glucosidase variants and substrate levels in Gaucher disease. J Biol Chem. 2012;287:4275–87. doi: 10.1074/jbc.M111.280016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayebi N, Callahan M, Madike V, Stubblefield BK, Orvisky E, Krasnewich D, et al. Gaucher disease and parkinsonism: a phenotypic and genotypic characterization. Mol Genet Metab. 2001;73:313–21. doi: 10.1006/mgme.2001.3201. [DOI] [PubMed] [Google Scholar]
- Urban DJ, Zheng W, Goker-Alpan O, Jadhav A, Lamarca ME, Inglese J, et al. Optimization and validation of two miniaturized glucocerebrosidase enzyme assays for high throughput screening. Comb Chem High Throughput Screen. 2008;11:817–24. doi: 10.2174/138620708786734244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogiatzi T, Xilouri M, Vekrellis K, Stefanis L. Wild type alpha-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J Biol Chem. 2008;283:23542–56. doi: 10.1074/jbc.M801992200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman EA, Duda JE, Giasson BI. Characterization of antibodies that selectively detect alpha-synuclein in pathological inclusions. Acta Neuropathol. 2008;116:37–46. doi: 10.1007/s00401-008-0375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman EA, Giasson BI. A novel, high-efficiency cellular model of fibrillar alpha-synuclein inclusions and the examination of mutations that inhibit amyloid formation. J Neurochem. 2010;113:374–88. doi: 10.1111/j.1471-4159.2010.06592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westbroek W, Gustafson AM, Sidransky E. Exploring the link between glucocerebrosidase mutations and parkinsonism. Trends Mol Med. 2011;17:485–93. doi: 10.1016/j.molmed.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K, Sidransky E, Verma A, Mixon T, Sandberg GD, Wakefield LK, et al. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol Genet Metab. 2004;82:192–207. doi: 10.1016/j.ymgme.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Wu KP, Kim S, Fela DA, Baum J. Characterization of conformational and dynamic properties of natively unfolded human and mouse alpha-synuclein ensembles by NMR: implication for aggregation. J Mol Biol. 2008;378:1104–15. doi: 10.1016/j.jmb.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap TL, Velayati A, Sidransky E, Lee JC. Membrane-bound alpha-synuclein interacts with glucocerebrosidase and inhibits enzyme activity. Mol Genet Metab. 2013;108:56–64. doi: 10.1016/j.ymgme.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]