Abstract
Influenza viruses remain a critical global health concern. More efficacious vaccines are needed to protect against influenza virus, yet few adjuvants are approved for routine use. Specialized proresolving mediators (SPMs) are powerful endogenous bioactive regulators of inflammation, with great clinical translational properties. Here, we investigated the ability of the SPM 17-HDHA to enhance the adaptive immune response using an OVA immunization model and a pre-clinical influenza vaccination mouse model. Our findings revealed that mice immunized with OVA plus 17-HDHA or with H1N1-derived HA protein plus 17-HDHA increased antigen-specific antibody titers. 17-HDHA increased the number of antibody-secreting cells in vitro as well as the number of HA-specific antibody secreting cells present in the bone marrow. Importantly, the 17-HDHA-mediated increased antibody production was more protective against live pH1N1 influenza infection in mice. This is the first report on the biological effects of omega-3-derived SPMs on the humoral immune response. These findings illustrate a previously unknown biological link between proresolution signals and the adaptive immune system. Furthermore, this work has important implications for the understanding of B cell biology, as well as the development of new potential vaccine adjuvants.
Introduction
Vaccines against infectious agents, such as influenza viruses, rely on the ability of the adaptive immune system to generate long-term memory and protection. An enhanced antigen-specific immune response increases the ability of the immune system to eliminate pathogens and maintain homeostasis. Adjuvants increase a vaccine’s efficacy by enhancing the immune response to the introduced antigen. Currently, alum is the only approved adjuvant for routine use in vaccines in the United States (1). Influenza virus is responsible for seasonal flu outbreaks, as well as deadly flu pandemics, which have recurred throughout history, such as the latest 2009 H1N1 pandemic (2, 3). Current seasonal influenza vaccines include the inactivated influenza vaccine (IIV), live-attenuated influenza vaccine (LAIV) and the recently approved recombinant influenza vaccine (RIV) (4, 5). These vaccines are designed to confer immune protection against the most common seasonal influenza strains expected to circulate each season. Neither IIV, LAIV nor RIV use adjuvants in the United States. Efficient vaccination is particularly important for susceptible populations such as infants, the elderly and the immuno-suppressed (5). More efficacious vaccines are needed to protect against seasonal influenza and possible pandemic strains. The development of novel adjuvants could improve vaccines against influenza and other pathogens.
The acute inflammatory response is a self-limiting processcrucial to fight pathogens and for tissue repair and homeostasis (6, 7). Specialized proresolving mediators (SPMs) are newly identified lipid-derived molecules responsible for actively regulating the resolution phase of inflammation (8–10). These endogenous mediators are derived from either n-3 or n-6 poly unsaturated fatty acids (PUFA) obtained from dietary sources, and are found in the bone marrow, spleen, and blood among other tissues (11–13). SPMs are classified into lipoxins, resolvins, protectins and maresins (9, 10, 14). Docosahexaenoic acid (DHA) is a majorn-3 PUFA and a precursor to the protectins, maresins and D-series resolvins families. 17-hydroxydocosahexaenoic acid (17-HDHA) is an example of a DHA-derived SPM (10, 15).
SPMs have many functions, which can be cell and context dependent. These include, decrease of neutrophil cell transmigration, enhancement of non-phlogistic monocyte recruitment and increase of macrophage engulfment of apoptotic neutrophils (16–18). In addition, SPMs decrease production of proinflammatory mediators such as IL-12 and TNFα, and promote anti-inflammatory cytokine production such as IL-10(19–21). Little is known about the effects of SPMs on B cells and the adaptive immune system. We recently reported the presence of DHA-derived resolvin D1 (RvD1), 17-HDHA and protect in D1 in the spleen, and have discovered that RvD1 and 17-HDHAenhance human B cell antibody production (13). Furthermore, our study showed that 17-HDHA promoted human B cell differentiation towards an antibody-secreting phenotype, while not affecting proliferation nor cytotoxicity (13). Antibodies, produced solely by B cells, are pivotal for anti-viral immunity as they mediate faster pathogen clearance and promote long-term immune protection. The biological roles of SPMs during the adaptive immune response, specifically B cell-mediated immunity, are not known. Here, we used a preclinical influenza vaccination and infection in vivo mouse model to analyze the actions of the SPM 17-HDHA on antibody production.
Materials and methods
Mouse immunization and viral challenge
OVA protein immunizations were done using 10 μg OVA emulsified in complete Freund’s adjuvant (CFA) (Sigma-Aldrich, St. Louis, MO) (1:1 ratio by volume). C57BL/6J male mice, 8–10 weeks old (The Jackson Laboratory, Bar Harbor, ME), were immunized by intraperitoneal (i.p.) injection (22). Mice were immediately given a second injection in the same site (i.p.) containing either 1 μg 17R-hydroxy-4Z, 7Z, 10Z, 13Z, 15E, 19Z-docosahexaenoic acid (17-HDHA) (Cayman Chemical Company, Ann Harbor, MI) or vehicle control (defined as PBS with 0.4 % ethanol by volume). Sera were collected 2 weeks and 6 weeks after primary immunization and antibody levels were measured by ELISA. Ten weeks after primary immunization, mice were boosted by i.p. injection containing 10 μg OVA suspended in PBS. Sera were collected 2 weeks after boost and used for antibody ELISA.
Influenza hemagglutinin (HA) immunization was performed onC57BL/6J male mice, 8–10 weeks old (The Jackson Laboratory, Bar Harbor, ME). Mice were immunized with recombinant HA protein derived from influenza H1N1 A/Brisbane/59/2007 (5 μg) or A/California/04/2009 (2 μg)(BEI Resources, Manassas, VA), plus 1 μg 17-HDHAor vehicle control. Mock immunization injection was defined as PBS with 0.4 % ethanol by volume without HA protein nor SPMs. HA immunizations were delivered by intramuscular (i.m.) injection on the left flank. Mice were given an initial immunization at week 0 and boosted again at week 2 and week4(23, 24). Sera were collected2 weeks after each injection and used for analysis. Mice from experiments shown in Figure 2 were immunized with HA protein plus 10 μg CpG oligodeoxynucleotides (ODN) 1826, sequence 3′-TCCAATGAGCTTCCTGAGTCT-5′ (Integrated DNA technologies, Coralville, IA), plus 1 μg 17-HDHA or vehicle control. Immunizations were delivered by i.m. injection at week 0 and at week 3. Sera antibody titers were measured at week 3, 6 and 11.
Figure 2. 17-HDHA increases HA-specific IgG production.
C57Bl/6 mice were immunized i.m. with A/Brisbane/59/2007 recombinant HA plus CpG plus vehicle control (CpG + vehicle), or with A/Brisbane/59/2007 recombinant HA plus CpG and 1 μg 17-HDHA (CpG + 17-HDHA) and sera was collected as represented in A) immunization schematic. HA-specific IgM and HA-specific IgG antibody levels were measured by ELISA at B–C) week 3, D–E) week 6 and F–G) week 11 (n=6/group). Data shown as mean ±SEM. Statistical analysis done using unpaired Student’s t-test (*p≤0.05).
For live influenza infections, C57BL/6J male mice were anesthetized with Avert in (2,2,2-tribromoethanol), injected i.p., and inoculated intranasally with H1N1 A/California/04/E3/2009 as previously described (25). Weight and survival were monitored for 14 days following infection. All mouse experiments were approved by the University Committee on Animal Resources at the University of Rochester Medical Center.
IgM and IgG ELISA and ELISpot
Mouse sera antigen-specific antibodies were measured by ELISA. Plates were pre-coated with HA protein (1ug/ml), OVA (10 μg/ml), capture IgM or capture IgG antibodies. Mouse-specific antibody ELISA kits were used to measure IgM and IgG (Bethyl Laboratories, Montgomery, TX) as suggested by manufacturer. For ELISpot analysis, cells were incubated in HA-coated ELISpot plates (Millipore, Billerica, MA). Alkaline phosphatase-conjugated goat anti-mouse IgM or IgG antibodies (Southern Biotech, Birmingham, AL) were used as recommended by the manufacturer. ELISpot plates were developed using Vector AP substrate kit III (Vector Laboratories, Burlingame, CA) and quantified on a CTL plate reader and ImmunoSpot software (Cellular Technologies, Shaker Heights, OH).
Cell staining
Bone marrow and spleen single cell suspensions were stained for dead cell exclusion using Live/Dead fixable violet dead cell staining kit (Invitrogen Life Technologies, Grand Island, NY). Surface markers were stained with a mixture of fluorochrome-conjugated antibodies, which included: CD19 (clone 6D5, BioLegend, San Diego, CA), B220 (clone RA3-6B2, eBioscience, San Diego, CA), CD138 (clone 281–2, BD bioscience, San Jose, CA), IgD (clone 11.26c.2a, BioLegend, San Diego, CA), IgM (clone II/41, eBioscience, San Diego, CA), IgG (eBioscience, San Diego, CA), MHC class II (clone 500A2, eBioscience, San Diego, CA), CD80 (clone I6-10A1, BD bioscience, San Jose, CA) CD86 (clone GL-1, BioLegend, San Diego, CA).
Fluorescence minus one (FMO) controls were included in each staining protocol and used to set specific gates (22, 26, 27). 6-peak validation beads were used for calibration during the time course analysis. Samples were run on a 12-color LSRII cytometer (BD bioscience, San Jose, CA) and analyzed by Flow Jo software (Tree Star Inc., Ashland, OR).
B lymphocyte isolation and in vitro culture conditions
Mouse spleens were harvested, single cell suspensions prepared and B cells were isolated using CD19 magnetic beads (Miltenyi; Auburn, CA). B cells (1 × 106 cells/ml, unless otherwise specified) were cultured in RPMI1640 media (Invitrogen Life Technologies, Grand Island, NY) supplemented with 5% FBS (Thermo Fisher Scientific, Pittsburgh, PA), 50 μM β-mercaptoethanol (Eastman Kodak, Rochester, NY), 10 mM HEPES (U.S. Biochemical, Cleveland, OH), 2 mM L-glutamine (Invitrogen Life Technologies, Carlsbad, CA), 50 μg/ml gentamicin (Invitrogen Life Technologies, Grand Island, NY). For CD138+ B cell depletion, purified CD19+ B cells were stained with anti-CD138 (clone 281–2, BD bioscience, San Jose, CA) antibody. Cells were then sorted using an 18-color FACSAria II (BD bioscience, San Jose, CA). Purified B cells were activated with CpG ODN 1826 (1 μg/mL) plus rabbit anti-mouse IgM antibody fragment (2 μg/mL) (Jackson ImmunoResearch Laboratories, West Grove, PA) and cultured for up to 6 days (22). 17-HDHA or vehicle control were added to cell culture 30 minutes before mitogen stimulation. Because 17-HDHA was suspended in ethanol, vehicle control was defined as PBS with 0.03% ethanol by volume, equivalent to 100 nM 17-HDHA. SPM treatments were added daily for the duration of the culture.
Proliferation analysis
Purified B cells (1×105 cells/ ml) were cultured in triplicate using 96-well round bottom plates for up to 6 days. B cell cultures were pulsed with [3H]-Thymidine (1 μCi/well) 24 hours prior to collection. [3H]-Thymidine incorporation was measured by scintillation spectroscopy using a Top count Luminometer (PerkinElmer, Boston, MA).
For CD138 depletion experiments, purified CD19+ CD138− B cells (1 × 106 cells/ml) were stained at day 0, using Cell Trace™ CFSE Cell Proliferation Kit (Invitrogen, Carlsbad, CA) as suggested by manufacturer. CFSE staining was then monitored over time using a 12-color LSRII cytometer (BD bioscience, San Jose, CA) and analyzed by FlowJo software (Tree Star Inc., Ashland, OR).
Real-time PCR
Total RNA was isolated using Qiagen RNA easy mini kit (Valencia, CA). RNA was reversed transcribed using Superscript III and random primers (Invitrogen, Carlsbad, CA). Steady-state levels of Bcl-6, Blimp-1 and 7S RNA were measured by real-time PCR using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). Bcl-6 and Blimp-1 mRNA steady-state levels were normalized to 7S. Primers used were:Bcl-6 sense 5′-AGACGCACAGTGACAAACCATACAA-3′, antisense 5′-GCTCCACAAATGTTACAGCGATAGG-3′; Blimp-1 sense 5′-TTCTTGTGTGGTATTGTCGGGACTT-3′, antisense 5′-TTGGGGACACTCTTTGGGTAGAGTT-3′, and 7S sense 5′-ACCACCAGGTTGCCTAAGGA-3′, and antisense 5′-CACGGGAGTTTTGACCTGCT-3 (22). Results were analyzed using Bio-Rad iCycler software (Hercules, CA).
Western blot
Protein was extracted from purified B cells using radio immunoprecipitation assay buffer (150mMNaCl, 1% Nonidet P-40, 0.5% Na deoxycholate, 50mMTris-base, 0.1% SDS [pH 8.0]). Protein was loaded onto gradient SDS-PAGE gels (Pierce/Thermo Fisher Scientific, Rockford, IL) and then transferred on to PDVF membranes (Millipore, Billerica, MA). Membranes were probed with antibodies against Blimp-1 (clone NB600-235, Novus, Littleton, CO), Bcl-6 (clone 4242, Cell Signaling Technologies, Danvers, MA), tubulin (Clone 2146, Cell Signaling Technologies, Danvers, MA), and HRP-conjugated secondary antibody. ECL reagents (Perkin Elmer Life Sciences, Boston, MA) was then used to develop Western blots.
Virus neutralization assay
Madin-Darby canine kidney (MDCK) cells (ATCC CCL-34) were maintained in Dulbecco’s modified Eagle’s medium (DMEM, Media tech, Inc.) supplemented with 10% fetal bovine serum (FBS, Atlanta biologicals), and 1% P-S-G (penicillin, 100 units/ml; streptomycin, 100 μg/ml; L-glutamine, 2 mM; Media tech, Inc.). Cells were grown at 37°C in a 5% CO2 atmosphere. MDCK cells constitutively expressing HA from influenza H1N1 A/WSN/33 (WSN) have been previously described (28). After viral infections, cells were maintained at 33°C in a 5% CO2 atmosphere in DMEM containing 0.3% bovine serum albumin (BSA), 1% P-S-G, and 0.3 or 1.0 μg/ml to syl-sulfonyl phenylalanyl chloromethyl ketone (TPCK)-treated trypsin (Sigma) for WSN and MDCK cells, respectively.
The prototypic influenza pandemic H1N1 A/California/04_NYICE_E3/2009 (pH1N1/E3) virus was prepared in eggs as described previously (25). Virus titers were determined by standard plaque assay (plaque forming units, PFU) in MDCK cells as described (29). Single-cycle infectious Influenza A Virus (sciIAV) containing the pH1N1 backbone (pH1N1/E3-sciIAV) was generated as previously published (30) and has the ORF of GFP instead of the fourth viral segment, and was amplified on MDCK cells constitutively expressing pH1N1/E3 HA. SciIAV titers were determined by plaque assay as above, but in MDCK-HA cells (28). For antiviral analyses, viral titers were calculated by immunofocus assay (focus forming units, FFU/mL). Briefly, influenza NP positive cells from serial supernatant dilutions were detected by indirect immunofluorescence with 1 μg/mL of the primary antibody HT103 (a gift from Dr. Thomas M. Moran at Mount Sinai School of Medicine), and a secondary anti-mouse FITC (1:140, Dako) using a fluorescence microscope (DM IRB; Leica) as previously described (30).
Virus neutralizing antibody titers in mice sera were determined using a GFP-based microneutralization assay as previously described (28). Briefly, sera were heat-inactivated for 45 min at 57°C, and triplicates were serially diluted 2-fold in a 96-well plate. Two hundred PFU of pH1N1/E3-sciIAV virus were then added to equivalent volumes of sera dilutions and incubated for 1 hour at room temperature. Confluent monolayers of MDCK WSN-HA cells (96-well plate format, 4 × 104 cells) were then infected for 1 hour at room temperature with virus/sera mixture, and incubated for 24 hr at 33°C. Cells were then washed twice with PBS and GFP was quantified for relative fluorescence units using a plate reader (DTX-880; Beckman Coulter). Infection in the absence of sera was used to normalize GFP expression to 100%. Neutralization titers represent the highest dilution at which the %GFP expression (+/− standard deviation) was below 50%.
Viral replication assay
To assess the inhibition of influenza replication in vitro (31) monolayers of MDCK cells (12-well plate format, 5 × 105 cells, triplicates) were infected with pH1N1/E3 at a multiplicity of infection (MOI) of 0.001 for 48 hours. Post infection media was uniformly supplemented with <0.2% (v/v) EtOH alone (vehicle) or additionally with 5-fold serial dilutions of 17-HDHA, or oseltamivir acid (Toronto Research Chemicals, Inc.). Tissue culture supernatants were removed at 24 and 48 hours post infection (hpi), and viral titers calculated by immunofocus assay.
Statistical analysis
Results are expressed as mean ± SEM. Significance was determined by statistical analysis using a two-tailed unpaired Student t-test where applicable, or one-way ANOVA when comparing three or more groups. Two-way ANOVA with a Bonferroni post-test was used where two or more variables were analyzed. All tests were carried out using Graph Pad Prism 5 (Graph Pad Software, La Jolla, CA). Probability values of p ≤ 0.05 were considered statistically significant.
Results
SPM 17-HDHA enhances HA-specific antibody production
The in vivo effects of 17-HDHA on the humoral response are not known. Therefore, an OVA immunization mouse model was initially used to study the effects of 17-HDHA under physiological conditions. Mice were immunized with OVA protein plus 17-HDHA or vehicle control. OVA-specific IgM and IgG titers in serum were measured following primary and secondary challenges (Figure 1). OVA immunization induced a strong OVA-specific IgM and OVA-specific IgG levels. Mice that received 17-HDHA along with OVA had significantly higher OVA-specific IgM and IgG levels 6 weeks after primary challenge. OVA-specific IgM production was further increased following secondary OVA exposure as measured at week 12. OVA-specific IgE was also measured in sera. Interestingly, 17-HDHA induced a decreasing trend in IgE levels (data not shown).
Figure 1. 17-HDHA increases OVA-specific antibody production.

C57Bl/6 mice were immunized i.p. at week 0 and at week 10 with OVA plus vehicle control (vehicle) or with OVA plus 1 μg 17-HDHA (17-HDHA). Sera were collected at weeks 2, 6 and 12. OVA-specific A) IgM and B) IgG titers were measured by ELISA. Here is shown mean ±SEM (n=6/group). Statistical analysis done using two-way ANOVA (*p≤0.05).
Next, the effects of 17-HDHA were analyzed in a highly translational preclinical influenza vaccination mouse model. To this end, recombinant hemagglutinin (HA) protein, derived from H1N1 influenza virus, was used to elicit an antigen-specific humoral response. Mice were immunized and challenged with HA and CpG ODN 1826 (a commonly used adjuvant) plus17-HDHA or vehicle control (Figure 2A). HA-specific IgM and IgG serum levels were measured 3 weeks after primary immunization (Figure 2B–C), and again after secondary challenge (Figure 2D–G). In all cases, mice that received immunizations containing 17-HDHA, had significantly higher HA-specific IgG titers compared to control group. These results show that 17-HDHA enhances antigen-specific antibody levels in both HA- and OVA-immunization mouse models. Therefore, the 17-HDHA-enhanced antigen-specific humoral response is not limited to the HA protein.
17-HDHA enhances HA-specific antibody production and plasma cell differentiation
In the absence of adjuvants, recombinant HA induces a poor humoral response (24). Current seasonal influenza vaccines (IIV, LAIV or RIV) do not use adjuvants (4, 5). Considering that 17-HDHA increased antigen-specific antibody levels (Figure 1 and Figure 2), we asked whether 17-HDHA could increase the HA-specific humoral response without the use of an adjuvant, such as CpG ODN 1826. Based on the dose-dependent HA-specific antibody titers from 17-HDHA immunized animals (data not shown), mice were injected with PBS (mock), or given immunizations containing recombinant HA plus 1 μg 17-HDHA (17-HDHA) or HA without 17-HDHA (vehicle control) (Figure 3). After primary immunization, vehicle treated mice had no significant differences in the HA-specific IgM and IgG titers compared to mock immunized mice. Interestingly, 17-HDHA-treated mice had a 2-foldincrease in HA-specific IgM levels compared to mock treated group and a 9-fold increase in HA-specific IgG compared to mock and vehicle treated groups (Figure 3B and 3C respectively).
Figure 3. 17-HDHA enhances HA-specific antibody production without the use of classic adjuvants.
A) C57Bl/6 mice were immunized i.m. at week 0, 2 and 4 with PBS (mock), A/California/04/2009 recombinant HA plus vehicle control (vehicle) or with A/California/04/2009 recombinant HA plus 1 μg 17-HDHA (17-HDHA). HA-specific IgM and HA-specific IgG antibody levels were measured by ELISA at B–C) week 2, DE) week 4 and F–G) week 6 (n=10/group). Data shown as mean ±SEM. Statistical analysis done using one-way ANOVA (*p≤0.05, **p≤0.01, ***p≤0.001).
Antigen experienced B cells undergo somatic hypermutation and class switch recombination, which promotes antigen-specific antibody affinity maturation, isotype switching, and improves the immune response upon antigen re-encounter (32, 33). Therefore, we measured the antibody response following antigen challenge (Figure #D–G). HA-specific IgM levels did not change between vehicle and 17-HDHA treated groups. However, HA-IgG titers were 100-fold higher compared to HA-IgM levels, reflective of antibody isotype switching. Remarkably, mice immunized with HA plus 17-HDHA had a 3-fold increase in HA-specific IgG titers by week 4, and a 9-fold increase by week 6 compared to vehicle control group (Figure 3E and G). Total antibody levels were not different between vehicle and 17-HDHA immunized groups (data not shown).
Activated B cells can differentiate into plasma cells, which are responsible for antibody production (34, 35). Long-lived plasma cells reside mainly in the bone marrow where they confer long-term immune protection (36, 37). 17-HDHA promotes human B cell differentiation towards an antibody secreting cell in vitro (13). Thus, the effects of 17-HDHA on B cell differentiation were analyzed using the influenza immunization mouse model (Figure 4). Two weeks after completion of the HA-immunization protocol, B cell populations were analyzed in the spleen and bone marrow by flow cytometry and CD138 expression was used to identify the plasma cell population (Figure 4A–C). Mice immunized with HA plus 17-HDHA had a two-fold increase in the percentage of CD138+B cells present in the bone marrow compared to vehicle control group (Figure 4A–B). Furthermore, the distribution of CD138+B cells in the spleen showed no differences (Figure 4C).
Figure 4. 17-HDHA enhances plasma cell frequency in the bone marrow.
C57Bl/6 mice were immunized i.m. with PBS (mock), A/California/04/2009 HA plus vehicle control (vehicle) or A/California/04/2009 HA plus 17-HDHA (17-HDHA). Six weeks later, bone marrow and spleen were collected. Cells were isolated and stained for flow cytometry analysis (n=10/group). A) Gating strategy (left side) and representative histogram of CD138 expression on CD19+ bone marrow B cells (right side). Quantification of B) bone marrow plasma cells (CD19+ CD138+) and C) spleen plasma cells (CD19+ CD138+). D) Quantification of HA-specific IgG secreting bone marrow cells. Analysis done by ELISpot (n=6/groups). Data shown as mean ±SEM. Statistical analysis done using one-way ANOVA (*p≤0.05, ***p≤0.001).
Next, the number of HA-specific antibody secreting cells present in the bone marrow was measured (Figure 4D). ELISpot analysis showed that HA-specific IgG secreting cells were increased 2-fold in the 17-HDHA treated group compared to mock and vehicle controls, consistent with flow cytometry results. Vehicle control group had low numbers of HA-specific antibody secreting cells, comparable to those of the mock immunized control. Furthermore, HA-specific IgM secreting cells were not detectable. These results are the first to show that 17-HDHA strongly enhances the HA-specific antibody response, in part by increasing plasma cell differentiation in vivo. Thus, 17-HDHA has adjuvant-like properties.
17-HDHA-mediated HA-specific antibodies are protective against live influenza infection
Achieving an efficacious number of plasma cells and protective antibodies are crucial elements for an effective anti-viral immune response and are important goals in vaccine development (38, 39). We tested whether or not the17-HDHA-mediated antibody increase also enhanced long-term immune protection against influenza viral infection (Figure 5). To this end, mice were injected with PBS (mock) or immunized with recombinant HA plus 17-HDHA (17-HDHA) or vehicle control (vehicle) at weeks 0, 2 and 4 (see immunization schematic, Figure 3A). Sera were collected at week 6 and the neutralizing properties of the HA-specific antibodies were tested in vitro (Figure 5A). Results showed that 44% of mice immunized with HA plus 17-HDHA had neutralizing antibodies circulating in serum compared to 0% for both mock and vehicle controls, though one of the nine mock-immunized mice displayed low levels of non-specific neutralization (below 1:20 sera dilution).
Figure 5. 17-HDHA-mediated HA-specific antibodies are protective against live influenza infection.
C57Bl/6 mice were immunized i.m. with PBS (mock), A/California/04/2009 HA plus vehicle control (vehicle), or A/California/04/2009 HA plus 17-HDHA (17-HDHA). A) Neutralizing antibody titers in mice. Serum from mice collected 14 days post final vaccination was used in a GFP microneutralization assay. The neutralization titer was determined as the highest dilution providing >50% reduction in GFP expression from triplicate wells. Columns represent the geometric mean neutralization titer from nine mice. A dotted line represents the limit of detection (<20) for antigen-specific antibodies. Negative samples were arbitrarily assigned a value of 10. The number of mice that had a microneutralization titer equal to or greater than 40 is shown in parentheses. B–C) Twenty-eight days after the last immunization, mice were infected intra-nasally with 300 infectious units of H1N1 influenza virus (A/California/04/E3/2009) (n=10/group). B) Weight and C) survival were monitored daily for 14 days following infection. Results expressed as mean ± SEM. For weight loss analysis, statistical analysis was performed by calculating the area under the curve followed by a Chi-square test (***p≤0.001). Survival rates were analyzed by doing a log-rank (Mantel-Cox) test (*p≤0.05, **p≤0.01). D) Direct antiviral activity of 17-HDHA. MDCK cells were infected with pH1N1/E3 at an MOI of 0.001 for 48 hours in the presence of 5-fold serial dilutions of 17-HDHA, oseltamivir or vehicle control (<0.2% v/v; dotted line). Virus titers in triplicate wells were determined by immunofocus assay. Results expressed as mean ± SEM. Significance was determined against vehicle using unpaired two-tailed Student’s t test (***P≤0.001, **P≤0.01).
Next, the protective qualities of the 17-HDHA-mediated antibody response were tested in vivo. Twenty-eight days after the last immunization, mice were infected with the mouse adapted live influenza A/California/04/E3/2009 H1N1 virus as previously described (25). Weight and survival were monitored for 14 days following influenza infection (Figure 5B–C). The mock-immunized group, which received no HA, had rapid weight loss and a 30% survival rate. By comparison, 17-HDHA-treated mice had minimal weight changes compared to the dramatic weight loss seen in both mock and vehicle-treated mice (Figure 5B). Furthermore, 17-HDHA and vehicle control groups had significantly higher survival rates of 100% and 80%, respectively (Figure 5C).
A recent report indicated that the SPM protect in D1, exhibited direct anti-viral activity against influenza A virus (40). However, we found that 17-HDHA, which is produced by the same biosynthetic pathway (10), did not inhibit the accumulation of live influenza virus in tissue culture supernatants, unlike oseltamivir, a well-described anti-viral medication (Figure 5D). Overall, these results show that the 17-HDHA-mediated antibody-increase is functional and protects against live influenza virus infection and thus holds great potential as a vaccine adjuvant.
17-HDHA directly promotes B cell differentiation towards an antibody-secreting phenotype
To interrogate the mechanisms by which 17-HDHA enhances the humor al immune response in vivo, the effects of 17-HDHA were tested on purified B cells. Following B cell activation, the expression of CD80, CD86 and MHC class II are upregulated on the B cell surface as these activation markers are involved in antigen presentation and the development of germinal center reaction within secondary lymphoid organs (41–44). Interestingly, 17-HDHA strongly upregulated CD80 and CD86 expression on B cells 6 days after CpG plus anti-IgM stimulation (Figure 6A–B). However, 17-HDHA did not affect MHC class II expression seen at day 6 after activation in this system (Figure 6C). 17-HDHA was not cytotoxic, nor did it enhance viability of B cells (data not shown). Of interest, flow cytometry analysis of the draining lymph nodes of mice challenged with either HA plus 17-HDHA or vehicle control showed no detectable changes above background in the frequency of GC B cells (CD19+ B220+ GL7+ CD95+) and Tfh cells (CD3+ CD4+ CXCR5+ PD-1+), at 6 or 10 days after challenge, potentially due to a low response against HA (data not shown).
Figure 6. 17-HDHA increases B cell expression of CD80 and CD86, but not MHC class II.

CD19+B cells were isolated from the spleens of naïve C57Bl/6 mice (n=6). Purified B cells were cultured and stimulated with CpG 1826 ODN (1 μg/ml) plus anti-IgM (2 μg/ml). Cells were treated with 17-HDHA or vehicle control for 30 minutes prior to B cell activation and treatments were repeated daily for 6 days. Cells were collected and stained for A) CD80, B) CD86 and C) MHC class II, and analyzed by flow cytometry at day 6. Results showed mean fluorescence intensity (MFI) ± SEM. Statistical analysis was performed using a one-way ANOVA with a Bonferroni post-test (*p≤0.05, **p≤0.01, ***p≤0.001).
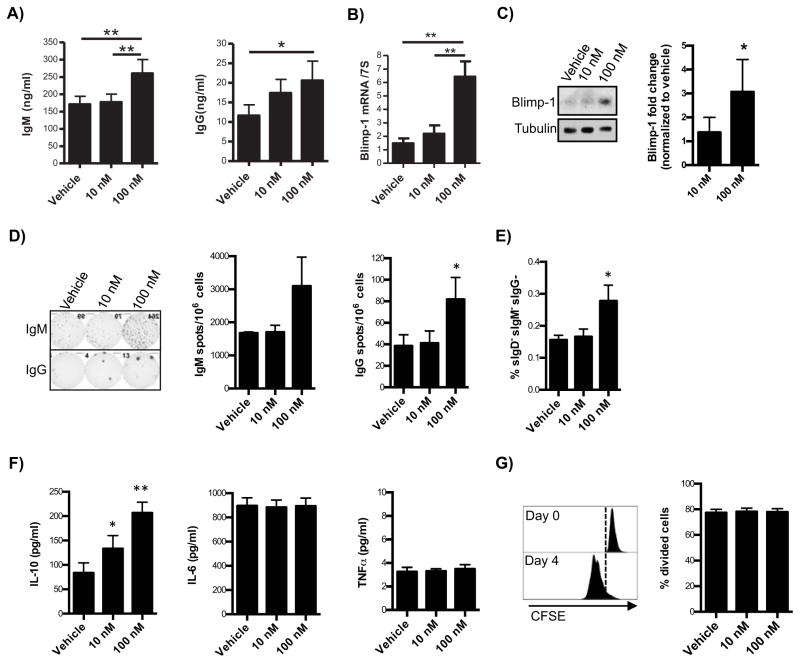
Activated B cells can further differentiate into antibody-secreting cells or plasma cells. Therefore, the effects of 17-HDHA on mouse B cell differentiation were analyzed (Figure 7). Activated B cells treated with 17-HDHA nearly doubled IgM and IgG production compared to vehicle controls (Figure 7A–B). Blimp-1 mRNA and protein levels were also increased by a factor of 3 in 17-HDHA-treated B cells (Figure 7B–C)). Blimp-1 is the master regulator of B cell differentiation of towards a plasma cell phenotype (45, 46). Therefore, to determine if the increased antibody levels were due to enhanced antibody-secreting B cell differentiation, or due to a direct effect of 17-HDHA on existing plasma cells, CD138+ B cells were sorted out of purified CD19+ B cells. The remaining CD19+ CD138− B cells were pre-treated with 17-HDHA and then stimulated with CpG plus anti-IgM (Figure 7D–H). 17-HDHA induced a 2-fold increase in the number of IgM and IgG secreting cells (Figure 7D. No changes were seen in the spot size between the vehicle and17-HDHA-treated samples, suggesting that 17-HDHA does not affect the amount of antibody produced per cell (data not shown). In addition, flow cytometry analysis showed that 17-HDHA increased the frequency of plasmablasts (IgD− IgM− IgG−)(Figure 7E). These results show that 17-HDHA increases antibody production by promoting B cell differentiation towards an antibody-secreting B cell phenotype.
Figure 7. 17-HDHA increases B cell antibody production and promotes B cell differentiation towards an antibody-secreting B cell phenotype.
A–C) CD19+B cells were isolated from the spleens of naïve C57Bl/6 mice (n=6). Purified B cells were cultured in triplicate and stimulated with CpG 1826 ODN (1 μg/ml) plus anti-IgM (2 μg/ml). Cells were treated with 17-HDHA at 10 nM and 100 nM, or vehicle control for 30 minutes prior to B cell activation. 17-HDHA or vehicle control treatments were added daily for up to 6 days. Culture supernatants were collected and A) IgM and IgG were measured by ELISA. B) Blimp-1 steady levels were measured by real-time quantitative PCR and normalized to 7S expression. C) Blimp-1 protein levels were measured by Western blot, representative blot images (left panels) and densitometry (right panels) are shown. D–H) Purified CD19+B cells isolated from naïve mouse spleens were depleted of plasma cells (CD19+ CD138+) via FACS (n=6). CD19+ CD138− B cells were then stimulated with CpG 1826 ODN plus anti-IgM. D) IgM and IgG-secreting cells were quantified after 5 days of activation using ELISpot analysis. E) Plasmablasts (IgD− IgM− IgG−) differentiation was measured by flow cytometry after 6 days of activation. F) Supernatants were collected from 6 day activated cultures and IL-10, IL-6and TNFα levels were measured by ELISA.G) The percent of dividing cells was measured using CFSE tracing and flow cytometry analysis. Representative histogram (left panel) and quantification of dividing cells (right panel) are shown. Results showed as mean ± SEM. Statistical analysis was performed using a one-way ANOVA with a Bonferroni post-test (*p≤0.05, **p≤0.01, ***p≤0.001).
SPMs have been shown to affect cytokine production on different immune cells, which can help resolve inflammation while avoiding immune-suppression (19–21). Therefore, the effects of 17-HDHA on CD19+ CD138− B cell cytokine production were analyzed. Interestingly, we discovered that17-HDHA increased production of IL-10. However, 17-HDHA did not affect IL-6 nor TNFα production (Figure 7F). The increase in IL-10 is suggestive of a pro-resolution cytokine profile, while maintaining IL-6 and TNFα, which are important in B cell differentiation and survival (47, 48). Lastly, 17-HDHA did not affect B cell proliferation (Figure 7G) nor was cytotoxic (data not shown). Overall, these new findings show that 17-HDHA increased the humoral response, in part through promotion of B cell differentiation towards an antibody-secreting phenotype.
Discussion
Here, we report the novel finding that 17-HDHA strongly enhances the antigen-specific antibody response in a pre-clinical influenza immunization mouse model. Mice vaccinated with HA plus 17-HDHA had an enhanced antibody mediated immune response, which was protective against live influenza virus infection. This is the first report on the in vivo effects of DHA-derived lipid mediators on the adaptive immune response, which provides a link between pro resolution pathways and adaptive immunity.
Long-lived plasma cells have a transient state in circulation before they establish permanent residence in the bone marrow. Results showed an increase in the number of bone marrow CD138+ B cells and HA-specific antibody secreting cells in mice that received 17-HDHA. Although, the number of HA-specific antibody-secreting B cells and CD138+ cells could not be determined simultaneously, 17-HDHA induced a 2-fold increase in the percentage of CD138+ B cells, and a 2-fold increase in the number of HA-specific antibody secreting cells. These results suggest that the increase in HA-specific serum antibody levels is due to enhanced plasma cell frequency. Furthermore, no changes were observed in the number of splenic CD138+ or HA antibody secreting cells at week 6, an expected observation given the transient nature of plasma cells. Furthermore, in vitro analysis revealed that 17-HDHA directly increasedBlimp-1 expression and B cell differentiation towards an antibody-secreting cell phenotype. Considering the direct effects of 17-HDHA on B cells in vitro, it is possible that during the humoral response, 17-HDHA, as well as other immuno-stimulatory SPMs, promote B cell activation and differentiation. These new results highlight the potential use of 17-HDHA and other immune-stimulatory SPMs as a vaccine adjuvant, as it strongly promotes antigen-specific antibody production and plasma cell differentiation.
The generation of long-lived plasma cells during an immune response is fundamental for a strong and long-term protection, which is a highly desired outcome during vaccination. Furthermore, the duration of immune memory protection is also an important measure of a vaccine’s efficacy. In this study, vaccinated mice were infected with influenza 28 days after the last immunization. Infections at later time points have not been tested. Future work should analyze the actions of 17-HDHA using longer vaccination protocols in order to assess the long-term protective properties of the 17-HDHA-mediated humoral response.
Taking advantage of the pre-clinical influenza vaccination mouse model, the efficacy of the HA vaccination was tested by challenging mice with influenza virus. An important and exciting finding is that mice vaccinated with HA plus 17-HDHA had minimal weight loss and complete survival as compared to vehicle and mock vaccinated control groups. Vaccinating mice with recombinant HA alone, even though it did not produce detectable neutralizing antibody levels, it did generate a protective immune response (80% survival rate), which has been previously reported (24). Notably, 17-HDHA vaccinated mice had a 100% survival rate. No statistical significance was seen in the survival rate of vehicle control versus 17-HDHA vaccinated animals, both of which received recombinant HA. It is possible that infection with a higher viral titer would further differentiation the level of immune protection between 17-HDHA and vehicle control group. Remarkably, 17-HDHAvaccinated mice, unlike the vehicle control, had significantly decreased morbidity, as shown by minimal weight loss. This discovery is of great importance as it demonstrates that 17-HDHA-mediated humoral response is functional and provides the host with a greater level of protection against influenza infection. Therefore, 17-HDHA possesses magnificent potential as a novel adjuvant candidate.
17-HDHA enhanced the humoral response in both, OVA and HA, immunization models. These results show that the 17-HDHA-mediated enhanced humoral response is not unique to the immunization model or antigen used. An interesting finding is the ability of 17-HDHA to increase antigen-specific IgG in both the OVA and HA models while also inducing a decreasing trend in IgE levels. Whether 17-HDHA specifically regulates B cell isotype switching remains to be addressed.
Others have reported on the direct anti-viral properties of SPMs, including protect in D1 (40, 49, 50). 17-HDHA, although highly bioactive, can also be biosynthesized into D-series resolvins and only its 17-HpDHA precursor is converted to PD1(51, 52). The present results showed that 17-HDHA alone did not inhibit H1N1 viral replication. It is possible that in vivo, 17-HDHA is converted into other SPMs, which in turn, could have anti-viral properties. However, this does not detract from the increased antibody titers uncovered herein. More importantly, for the focus of our study, the 17-HDHA-mediated increase in antibody levels were found to have virus-neutralizing properties and remarkably, conferred strong protection against live influenza virus infection. Thus in view of the direct anti-viral actions of PD1, our findings suggest that activation of the DHA metabolome pathways is critical to novel and previously unappreciated, viral host defense pathways.
SPMs are endogenous mediators found in tissues such as the bone marrow, spleen, tonsil and blood (11–13, 53–55). These tissues provide an ideal environment under which SPMs can directly influence immune cells, such as B cells. Here, we discovered that 17-HDHA increased B cell antibody production, increases Blimp-1 expression and promotes B cell differentiation towards an antibody-secreting cell. In addition, molecular analysis revealed that 17-HDHA upregualtesCD80 and CD86 expression on B cells. These co-stimulatory molecules are upregulated following B cell activation and play a crucial role during antigen presentation and the germinal center reaction (41–43). The 17-HDHA-mediated increase in co-stimulatory molecules could increase the immunological synapse signaling and, with it the T cell-dependent immune response. Overall, the evidence presented here shows that 17-HDHA enhances the humoral response in part by upregulation B cell activation and differentiation markers.
Interestingly, 17-HDHA did not affect the levels of TNFα nor IL-6. Although these two cytokines are generally considered pro-inflammatory, they are involved in early B cell activation, proliferation and antibody production (47, 48). In addition, 17-HDHA increased IL-10 production, which has anti-inflammatory functions. The increase of IL-10 raises questions regarding the effects of 17-HDHA on IL-10-producing B cells, as well as the effects of 17-HDHA on other B cell subsets (56). Whether, the increase in IL-10 and maintenance of IL-6 and TNFα by 17-HDHA takes place within the B cell microenvironment in vivo remains to be addressed. Importantly, the B cell cytokine profile induced by 17-HDHA does not increase or decrease proinflammatory signals, but it does enhance anti-inflammatory signals, while still promoting antibody-production.
Alum is the only FDA-approved adjuvant currently in routine use in the United States, with modest success. Current influenza vaccines do not even include adjuvants. New adjuvants are needed to improve efficacy of many types of vaccines. This is particularly critical for susceptible populations such as the elderly, the immuno-suppressed and infants. We propose that SPM 17-HDHA is a new class of adjuvant. Our results show that 17-HDHA directly promotes B cell differentiation towards an antibody-secreting phenotype in vitro and in vivo. Whether the 17-HDHA-mediated effects are due to specific receptor-binding or further conversion of 17-HDHA into D-series resolvins is not known. It is also possible that 17-HDHA directly affects immune cell functions, such as APCs’ antigen processing capabilities. Determining the in vivo bio availability of exogenous 17-HDHAin the local environment is critical yet, challenging. One difficulty lays on the properties of 17-HDHA to rapidly undergo enzymatic conversion into D-series resolvins (15, 57). In turn, resolvins can signal cells or been zymatically inactivated by oxidoreductases (as reviewed in (52)). Understanding how 17-HDHA and its derivatives enhance the adaptive immune response is of great interest for the development of novel and more efficient adjuvants and clearly guarantees future studies.
The present results provide new evidence for the immuno-stimulatory properties of DHA-derived SPMs on the adaptive immune response, particularly on B cells. Overall, 17-HDHA has great potential as an adjuvant considering that it is a naturally occurring small molecule, it is non-cytotoxic and has a low production cost. Using SPMs to treat chronic inflammatory diseases has proven beneficial in models of periodontitis, asthma, arthritis and cancer (58–63). Further study of the mechanisms regulating the resolution of inflammation and how they are linked to adaptive immunity has important implications for the understanding of B cell biology, vaccine development and the potential generation of a new class of adjuvant.
Footnotes
This work was supported by NIH grants AI103690 (to RPP), ES01247 (to RPP), GM038765 (to CNS), RO1 AI077719 (to LM-S), R21NS075611 (to LM-S), R03AI099681(to LM-S), University of Rochester Center for Biodefense Immune Modeling HHSN272201000055C, NIAID Center of Excellence for Influenza Research and Surveillance HHSN266200700008C and training grants T90 DE021985, T32 AI007285 and T32 HL066988(to the University of Rochester Medical Center).
References
- 1.Administration, U. S. F. a. D. Common Ingredients in U.S. Licensed Vaccines. 2013 http://www.fda.gov.
- 2.Neumann G, Noda T, Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 2009;459:931–939. doi: 10.1038/nature08157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prevention, C. f. D. C. a. Lessons from a Virus: Public Health Laboratories Respond to the H1N1 Pandemic. Center for Disease Control and Prevention; 2012. CDC.gov. [Google Scholar]
- 4.Treanor JJ, El Sahly H, King J, Graham I, Izikson R, Kohberger R, Patriarca P, Cox M. Protective efficacy of a trivalent recombinant hemagglutinin protein vaccine (FluBlok(R)) against influenza in healthy adults: a randomized, placebo-controlled trial. Vaccine. 2011;29:7733–7739. doi: 10.1016/j.vaccine.2011.07.128. [DOI] [PubMed] [Google Scholar]
- 5.Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, Iskander JK, Wortley PM, Shay DK, Bresee JS, Cox NJ. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. 2010;59:1–62. [PubMed] [Google Scholar]
- 6.Janeway C. Immunobiology : the immune system in health and disease. Garland Science; New York: 2005. [Google Scholar]
- 7.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 8.Hsiao HM, Sapinoro RE, Thatcher TH, Croasdell A, Levy EP, Fulton RA, Olsen KC, Pollock SJ, Serhan CN, Phipps RP, Sime PJ. A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PloS one. 2013;8:e58258. doi: 10.1371/journal.pone.0058258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature reviews. Immunology. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annual review of immunology. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 11.Dona M, Fredman G, Schwab JM, Chiang N, Arita M, Goodarzi A, Cheng G, von Andrian UH, Serhan CN. Resolvin E1, an EPA-derived mediator in whole blood, selectively counter regulates leukocytes and platelets. Blood. 2008;112:848–855. doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poulsen RC, Gotlinger KH, Serhan CN, Kruger MC. Identification of inflammatory and proresolving lipid mediators in bone marrow and their lipidomic profiles with ovariectomy and omega-3 intake. American journal of hematology. 2008;83:437–445. doi: 10.1002/ajh.21170. [DOI] [PubMed] [Google Scholar]
- 13.Ramon S, Gao F, Serhan CN, Phipps RP. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J Immunol. 2012;189:1036–1042. doi: 10.4049/jimmunol.1103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent anti inflammatory and proresolving actions. The Journal of experimental medicine. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. The Journal of experimental medicine. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee TH, Horton CE, Kyan-Aung U, Haskard D, Crea AE, Spur BW. Lipoxin A4 and lipoxin B4 inhibit chemotactic responses of human neutrophils stimulated by leukotriene B4 and N-formyl-L-methionyl-L-leucyl-L-phenylalanine. Clin Sci (Lond) 1989;77:195–203. doi: 10.1042/cs0770195. [DOI] [PubMed] [Google Scholar]
- 17.Maddox JF, Serhan CN. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. The Journal of experimental medicine. 1996;183:137–146. doi: 10.1084/jem.183.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fierro IM, Colgan SP, Bernasconi G, Petasis NA, Clish CB, Arita M, Serhan CN. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit human neutrophil migration: comparisons between synthetic 15 epimers in chemotaxis and transmigration with microvessel endothelial cells and epithelial cells. J Immunol. 2003;170:2688–2694. doi: 10.4049/jimmunol.170.5.2688. [DOI] [PubMed] [Google Scholar]
- 19.Souza DG, Fagundes CT, Amaral FA, Cisalpino D, Sousa LP, Vieira AT, Pinho V, Nicoli JR, Vieira LQ, Fierro IM, Teixeira MM. The required role of endogenously produced lipoxin A4 and annexin-1 for the production of IL-10 and inflammatory hyporesponsiveness in mice. J Immunol. 2007;179:8533–8543. doi: 10.4049/jimmunol.179.12.8533. [DOI] [PubMed] [Google Scholar]
- 20.Aliberti J, Hieny S, Reis e Sousa C, Serhan CN, Sher A. Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nature immunology. 2002;3:76–82. doi: 10.1038/ni745. [DOI] [PubMed] [Google Scholar]
- 21.Ariel A, Chiang N, Arita M, Petasis NA, Serhan CN. Aspirin-triggered lipoxin A4 and B4 analogs block extracellular signal-regulated kinase-dependent TNF-alpha secretion from human T cells. J Immunol. 2003;170:6266–6272. doi: 10.4049/jimmunol.170.12.6266. [DOI] [PubMed] [Google Scholar]
- 22.Ramon S, Bancos S, Thatcher TH, Murant TI, Moshkani S, Sahler JM, Bottaro A, Sime PJ, Phipps RP. Peroxisome proliferator-activated receptor gamma B cell-specific-deficient mice have an impaired antibody response. J Immunol. 2012;189:4740–4747. doi: 10.4049/jimmunol.1200956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kistner O, Crowe BA, Wodal W, Kerschbaum A, Savidis-Dacho H, Sabarth N, Falkner FG, Mayerhofer I, Mundt W, Reiter M, Grillberger L, Tauer C, Graninger M, Sachslehner A, Schwendinger M, Bruhl P, Kreil TR, Ehrlich HJ, Barrett PN. A whole virus pandemic influenza H1N1 vaccine is highly immunogenic and protective in active immunization and passive protection mouse models. PloS one. 2010;5:e9349. doi: 10.1371/journal.pone.0009349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santiago FW, Fitzgerald T, Treanor JJ, Topham DJ. Vaccination with drifted variants of avian H5 hemaglutinin protein elicits a broadened antibody response that is protective against challenge with homologous or drifted live H5 influenza virus. Vaccine. 2011;29:8888–8897. doi: 10.1016/j.vaccine.2011.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo H, Santiago F, Lambert K, Takimoto T, Topham DJ. T cell-mediated protection against lethal 2009 pandemic H1N1 influenza virus infection in a mouse model. Journal of virology. 2011;85:448–455. doi: 10.1128/JVI.01812-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hulspas R, O’Gorman MR, Wood BL, Gratama JW, Sutherland DR. Considerations for the control of background fluorescence in clinical flow cytometry. Cytometry. Part B, Clinical cytometry. 2009;76:355–364. doi: 10.1002/cyto.b.20485. [DOI] [PubMed] [Google Scholar]
- 27.Herzenberg LA, Tung J, Moore WA, Herzenberg LA, Parks DR. Interpreting flow cytometry data: a guide for the perplexed. Nature immunology. 2006;7:681–685. doi: 10.1038/ni0706-681. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Sobrido L, Cadagan R, Steel J, Basler CF, Palese P, Moran TM, Garcia-Sastre A. Hemagglutinin-pseudotyped green fluorescent protein-expressing influenza viruses for the detection of influenza virus neutralizing antibodies. Journal of virology. 2010;84:2157–2163. doi: 10.1128/JVI.01433-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapman TJ, Castrucci MR, Padrick RC, Bradley LM, Topham DJ. Antigen-specific and non-specific CD4+ T cell recruitment and proliferation during influenza infection. Virology. 2005;340:296–306. doi: 10.1016/j.virol.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 30.Baker SF, Guo H, Albrecht RA, Garcia-Sastre A, Topham DJ, Martinez-Sobrido L. Protection against Lethal Influenza with a Viral Mimic. Journal of virology. 2013;87:8591–8605. doi: 10.1128/JVI.01081-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bauman JD, Patel D, Baker SF, Vijayan RS, Xiang A, Parhi AK, Martinez-Sobrido L, Lavoie EJ, Das K, Arnold E. Crystallographic Fragment Screening and Structure-Based Optimization Yields a New Class of Influenza Endonuclease Inhibitors. ACS chemical biology. 2013 doi: 10.1021/cb400400j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McHeyzer-Williams LJ, Driver DJ, McHeyzer-Williams MG. Germinal center reaction. Current opinion in hematology. 2001;8:52–59. doi: 10.1097/00062752-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nature reviews. Immunology. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 34.O’Connor BP, Gleeson MW, Noelle RJ, Erickson LD. The rise and fall of long-lived humoral immunity: terminal differentiation of plasma cells in health and disease. Immunol Rev. 2003;194:61–76. doi: 10.1034/j.1600-065x.2003.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nature reviews. Immunology. 2005;5:230–242. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- 37.Tangye SG, Avery DT, Hodgkin PD. A division-linked mechanism for the rapid generation of Ig-secreting cells from human memory B cells. J Immunol. 2003;170:261–269. doi: 10.4049/jimmunol.170.1.261. [DOI] [PubMed] [Google Scholar]
- 38.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevens TL, Bossie A, Sanders VM, Fernandez-Botran R, Coffman RL, Mosmann TR, Vitetta ES. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988;334:255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- 40.Morita M, Kuba K, Ichikawa A, Nakayama M, Katahira J, Iwamoto R, Watanebe T, Sakabe S, Daidoji T, Nakamura S, Kadowaki A, Ohto T, Nakanishi H, Taguchi R, Nakaya T, Murakami M, Yoneda Y, Arai H, Kawaoka Y, Penninger JM, Arita M, Imai Y. The lipid mediator protectin D1 inhibits influenza virus replication and improves severe influenza. Cell. 2013;153:112–125. doi: 10.1016/j.cell.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 41.Lenschow DJ, Sperling AI, Cooke MP, Freeman G, Rhee L, Decker DC, Gray G, Nadler LM, Goodnow CC, Bluestone JA. Differential up-regulation of the B7-1 and B7-2 costimulatory molecules after Ig receptor engagement by antigen. J Immunol. 1994;153:1990–1997. [PubMed] [Google Scholar]
- 42.Jacobson KL, Song E, Anderson S, Sharpe AH, Shlomchik MJ. CD80 expression on B cells regulates murine T follicular helper development, germinal center B cell survival, and plasma cell generation. J Immunol. 2012;188:4217–4225. doi: 10.4049/jimmunol.1102885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cyster JG. B cell follicles and antigen encounters of the third kind. Nature immunology. 2010;11:989–996. doi: 10.1038/ni.1946. [DOI] [PubMed] [Google Scholar]
- 44.Salek-Ardakani S, Choi YS, Rafii-El-Idrissi Benhnia M, Flynn R, Arens R, Shoenberger S, Crotty S, Croft M, Salek-Ardakani S. B cell-specific expression of B7-2 is required for follicular Th cell function in response to vaccinia virus. J Immunol. 2011;186:5294–5303. doi: 10.4049/jimmunol.1100406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin KI, Angelin-Duclos C, Kuo TC, Calame K. Blimp-1-dependent repression of Pax-5 is required for differentiation of B cells to immunoglobulin M-secreting plasma cells. Mol Cell Biol. 2002;22:4771–4780. doi: 10.1128/MCB.22.13.4771-4780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Crotty S, Johnston RJ, Schoenberger SP. Effectors and memories: Bcl-6 and Blimp-1 in T and B lymphocyte differentiation. Nature immunology. 2010;11:114–120. doi: 10.1038/ni.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones SA. Directing transition from innate to acquired immunity: defining a role for IL-6. J Immunol. 2005;175:3463–3468. doi: 10.4049/jimmunol.175.6.3463. [DOI] [PubMed] [Google Scholar]
- 48.Rieckmann P, Tuscano JM, Kehrl JH. Tumor necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6) in B-lymphocyte function. Methods. 1997;11:128–132. doi: 10.1006/meth.1996.0396. [DOI] [PubMed] [Google Scholar]
- 49.Baillie JK, Digard P. Influenza--time to target the host? The New England journal of medicine. 2013;369:191–193. doi: 10.1056/NEJMcibr1304414. [DOI] [PubMed] [Google Scholar]
- 50.Tam VC, Quehenberger O, Oshansky CM, Suen R, Armando AM, Treuting PM, Thomas PG, Dennis EA, Aderem A. Lipidomic profiling of influenza infection identifies mediators that induce and resolve inflammation. Cell. 2013;154:213–227. doi: 10.1016/j.cell.2013.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends Immunol. 2007;28:176–183. doi: 10.1016/j.it.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 52.Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chemical reviews. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneider C, Keeney DS, Boeglin WE, Brash AR. Detection and cellular localization of 12R-lipoxygenase in human tonsils. Arch Biochem Biophys. 2001;386:268–274. doi: 10.1006/abbi.2000.2217. [DOI] [PubMed] [Google Scholar]
- 54.Bafica A, Scanga CA, Serhan C, Machado F, White S, Sher A, Aliberti J. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. The Journal of clinical investigation. 2005;115:1601–1606. doi: 10.1172/JCI23949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hong S, Porter TF, Lu Y, Oh SF, Pillai PS, Serhan CN. Resolvin E1 metabolome in local inactivation during inflammation-resolution. J Immunol. 2008;180:3512–3519. doi: 10.4049/jimmunol.180.5.3512. [DOI] [PubMed] [Google Scholar]
- 56.Lund FE, Randall TD. Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nature reviews. Immunology. 2010;10:236–247. doi: 10.1038/nri2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jin Y, Arita M, Zhang Q, Saban DR, Chauhan SK, Chiang N, Serhan CN, Dana R. Anti-angiogenesis effect of the novel anti-inflammatory and pro-resolving lipid mediators. Investigative ophthalmology & visual science. 2009;50:4743–4752. doi: 10.1167/iovs.08-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y, Hao H, He S, Cai L, Li Y, Hu S, Ye D, Hoidal J, Wu P, Chen X. Lipoxin A4 and its analogue suppress the tumor growth of transplanted H22 in mice: the role of antiangiogenesis. Molecular cancer therapeutics. 2010;9:2164–2174. doi: 10.1158/1535-7163.MCT-10-0173. [DOI] [PubMed] [Google Scholar]
- 60.Zhang B, Jia H, Liu J, Yang Z, Jiang T, Tang K, Li D, Huang C, Ma J, Shen GX, Ye D, Huang B. Depletion of regulatory T cells facilitates growth of established tumors: a mechanism involving the regulation of myeloid-derived suppressor cells by lipoxin A4. J Immunol. 2010;185:7199–7206. doi: 10.4049/jimmunol.1001876. [DOI] [PubMed] [Google Scholar]
- 61.Zhang L, Zhang X, Wu P, Li H, Jin S, Zhou X, Li Y, Ye D, Chen B, Wan J. BML-111, a lipoxin receptor agonist, modulates the immune response and reduces the severity of collagen-induced arthritis. Inflammation research : official journal of the European Histamine Research Society [et al.] 2008;57:157–162. doi: 10.1007/s00011-007-7141-z. [DOI] [PubMed] [Google Scholar]
- 62.Hasturk H, Kantarci A, Ohira T, Arita M, Ebrahimi N, Chiang N, Petasis NA, Levy BD, Serhan CN, Van Dyke TE. RvE1 protects from local inflammation and osteoclast- mediated bone destruction in periodontitis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:401–403. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- 63.Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, Israel E, Haley KJ, Serhan CN. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]