Abstract
Evidence for the important role for poly(ADP-ribose) polymerase (PARP) in the pathogenesis of diabetic nephropathy is emerging. We previously reported that PARP inhibitors counteract early Type 1 diabetic nephropathy. This study evaluated the role for PARP in kidney disease in long-term Type 1 diabetes. Control and streptozotocin-diabetic rats were maintained with or without treatment with the PARP inhibitor 10-(4-methyl-piperazin-1-ylmethyl)-2H-7-oxa-1,2-diaza-benzo[de] anthracen-3-one (GPI-15,427, Eisai Inc.), 30 mg kg−1d−1, for 26 weeks after first 2 weeks without treatment. PARP activity in the renal cortex was assessed by Western blot analysis of poly(ADP-ribosyl)ated proteins. Urinary albumin, isoprostane, and 8-hydroxy-2′-deoxyguanosine excretion, and renal concentrations of transforming growth factor-β1, vascular endothelial growth factor, soluble intercellular adhesion molecule-1, fibronectin, and nitrotyrosine were evaluated by ELISA, and urinary creatinine and renal lipid peroxidation products by colorimetric assays. PARP inhibition counteracted diabetes-associated increase in renal cortex poly(ADP-ribosyl)ated protein level. Urinary albumin, isoprostane, and 8-hydroxy-2′-deoxyguanosine excretions and urinary albumin/creatinine ratio were increased in diabetic rats, and all these changes were at least partially prevented by GPI-15,427 treatment. PARP inhibition counteracted diabetes-induced renal transforming growth factor-β1, vascular endothelial growth factor, and fibronectin, but not soluble intercellular adhesion molecule-1 and nitrotyrosine, accumulations. Lipid peroxidation product concentrations were indistinguishable among control and diabetic rats maintained with or without GPI-15,427 treatment. In conclusion, PARP activation plays an important role in kidney disease in long-term diabetes. These findings provide rationale for development and further studies of PARP inhibitors and PARP inhibitor-containing combination therapies, for prevention and treatment of diabetic nephropathy.
Keywords: Poly(ADP-ribose) polymerase, diabetic nephropathy, streptozotocin-diabetic rat, oxidative-nitrosative stress, vascular endothelial growth factor, transforming growth factor-β
1. INTRODUCTION
Diabetic nephropathy develops in 30% to 40% of patients with both Type 1 and Type 2 diabetes mellitus within 20–25 years after the onset of diabetes [1,2]. Diabetes now accounts for at least ~35% of all new cases of end-stage renal disease in the United States [3], and diabetic patients make up the fastest growing group of renal dialysis and transplant recipients. Diabetic nephropathy is also associated with elevated blood pressure, increased incidence of coronary heart disease, stroke, peripheral arterial occlusive disease, other microvascular complications of diabetes like diabetic retinopathy or diabetic foot, and premature mortality [4]. A recent study revealed that subjects with both Type 1 and Type 2 diabetes and initial stage of nephropathy (manifested by microalbuminuria) already have increased cardiovascular risk, morbidity and mortality [1,5].
The pathogenesis of diabetic nephropathy has extensively been studied in animal models of diabetes, and involves complex interactions between haemodynamic and metabolic factors [6]. The haemodynamic factors include increased systemic and intraglomerular pressure and activation of various vasoactive hormone pathways e.g., the renin-angiotensin and endothelin systems [6]. The metabolic mechanisms include increased sorbitol pathway activity [7,8], non-enzymatic glycation/glycoxidation [8–10], activation of protein kinase C, 12/15-lipoxygenase, and hexosamine pathway [11–14]. Evidence for the important role for oxidative stress in diabetic kidney disease is emerging [15–19]. Oxidative stress is linked to activation of mitogen-activated protein kinases (MAPKs) [20], the nuclear transcription factor NF-κB [21], and upregulation of growth factors such as cytokines [22], and vascular endothelial growth factor (VEGF) [23] implicated in diabetic renal disease [24].
One of the most important phenomena closely linked to oxidative stress is activation of poly(ADP-ribose) polymerase (PARP), known to lead to NAD+ depletion and energy failure [25], activation of non-enzymatic glycation and protein kinase C [26], impairment in signal transduction mechanisms [27], and changes in transcriptional regulation and gene expression [28]. Evidence for the important role for PARP activation in the pathogenesis of diabetic complications including endothelial and myocardial dysfunction, peripheral and autonomic neuropathy, retinopathy, and cataract is emerging [29–33]. PARP activation has been implicated in diabetic nephropathy in leptin receptor-deficient (db/db) mice, a model of Type 2 (non-insulin-dependent) diabetes [34]. Furthermore, we recently reported that PARP activation in the renal cortex contributes to enhanced production of transforming growth factor-β, endothelin-1, and vascular endothelial growth factor, as well as enhanced oxidative-nitrosative stress, advanced glycation end-product formation, and pro-inflammatory response, and plays an important role in albuminuria, podocyte loss, and mesangial expansion associated with early nephropathy in the streptozotocin-diabetic rat model [35]. The purpose of the present study was to evaluate the effect of PARP inhibition on indices of kidney disease associated with chronic Type 1 diabetes.
2. MATERIALS AND METHODS
2.1. Reagents
Unless otherwise stated, all chemicals were of reagent-grade quality, and were purchased from Sigma Chemical Co., St. Louis, MO. GPI-15,427 was obtained from Eisai Inc, Baltimore, MD. Mouse monoclonal anti-poly(ADP-ribose) antibody was purchased from Trevigen, Inc., Gaithersburg, MD.
2.2. Animals
The experiments were performed in accordance with regulations specified by the National Institutes of Health “Principles of Laboratory Animal Care, 1985 Revised Version” and Pennington Biomedical Research Center Protocol for Animal Studies. Male Wistar rats (Charles River, Wilmington, MA), body weight 250–300 g, were fed a standard rat chow (PMI Nutrition Int., Brentwood, MO) and had access to water ad libitum. STZ-diabetes was induced as described [35]. Blood samples for glucose measurements were taken from the tail vein ~48 h after the STZ injection and the day prior to the study termination. All rats with blood glucose levels ≥13.8 mM were considered diabetic. Diabetic rats were maintained on suboptimal doses of insulin (~1–2 U/every second day) to prevent ketoacidosis and weight loss. The experimental groups comprised control and diabetic rats treated with or without the PARP inhibitor GPI-15,427 (formulated as mesilate salt, 30 mgkg−1d−1, in the drinking water), for 26 wks after first 2 wks without treatment. An initial 2-wk period without treatment was introduced to avoid β–cell regeneration and alleviation of hyperglycemia which is known to occur when PARP inhibitors are administered together with streptozotocin or shortly after induction of diabetes [25]. At the end of the study, rats were placed in individual metabolic cages (Lab Products Inc., Seaford, Delaware) and urine collected for 24 h. Urine specimen were centrifuged at 12,000 g (4°C, 10 min) and frozen for subsequent assessment of albumin, isoprostane, and 8-hydroxy-2′-deoxyguanosine by ELISA and creatinine by spectrophotometric procedure based on the Jaffe reaction.
2.3. Anesthesia, euthanasia and tissue sampling
The animals were sedated by CO2, and immediately sacrificed by cervical dislocation. Glycosylated hemoglobin was measured using A1cNow INVIEW Multi-test A1C system (Metrika, Sunnyvale, CA). Kidneys were immediately frozen in liquid nitrogen for subsequent Western blot analysis of poly(ADP-ribosyl)ated proteins, ELISA measurements of renal transforming growth factor β1 (TGF-β1), vascular endothelial growth factor (VEGF), fibronectin, nitrotyrosine (NT), and soluble intercellular adhesion molecule-1 (iCAM-1) concentrations, and colorimetric assessment of renal malondialdehyde (MDA) and MDA plus 4-hydroxyalkenal (4-HA) concentrations.
2.4. Specific methods
2.4.1. Urinary albumin, creatinine, isoprostane and 8-hydroxy-2′-deoxyguanosine excretion
Urinary albumin, isoprostane, and 8-hydroxy-2′-deoxyguanosine excretions were assessed by ELISA. The Nephrat kit (Exocell, Philadelphia, PA), Urinary Isoprostane ELISA kit (Oxford Biomedical Research, Oxford, MI) and 8-hydroxy-2′-deoxy Guanosine EIA Kit (Cayman Chemical Company, Ann Arbor, MI) were used for measurements of albumin, isoprostane, and 8-hydroxy-2′-deoxyguanosine, respectively. Urinary creatinine was measured spectrophotometrically, using Creatinine Parameter assay kit (R&D Systems, Minneapolis, MN). The assays were performed in accordance with the manufacturers’ instructions.
2.4.2. TGF-β1, VEGF, fibronectin, MDA, MDA plus 4-HA, NT, and iCAM-1 concentrations
For measurements of TGF-β1, iCAM-1 and NT concentrations, renal cortex samples were homogenized on ice in RIPA buffer (1:10 w/v) containing 50 mM Tris-HCl, pH 7.2; 150 mM NaCl; 0.1% sodium dodecyl sulfate; 1% NP-40; 5 mM EDTA; 1 mM EGTA; 1% sodium deoxycholate and the protease/phosphatase inhibitors leupeptin (10 μg/ml), aprotinin (20 μg/ml), benzamidine (10 mM), phenylmethylsulfonyl fluoride (1 mM), sodium orthovanadate (1 mM). Homogenates were sonicated (3 × 5 s) and centrifuged at 14,000g (4°C, 20 min). TGF-β1 and NT concentrations were measured with the Quantikine mouse/rat/porcine/canine TGF-β1 kit (R&D Systems, Minneapolis, MN) and the OxiSelect Nitrotyrosine ELISA kit (Cell Biolabs, San Diego, CA).
For VEGF measurements, renal cortex samples were homogenized in 20 mM phosphate-buffered saline (PBS), pH 7.4 (1:5 w/v), on ice. Homogenate was used for VEGF measurements with the Quantikine Rat VEGF ELISA kit (R&D Systems, Minneapolis, MN).
For fibronectin, MDA, and MDA+HA measurements, renal cortex samples were homogenized in 20 mM PBS, pH 7.4 (1:10 w/v), on ice. Homogenate was centrifuged at 14,000g (4°C, 20 min). Supernatant fibronectin concentrations were measured with the AssayMax Rat Fibronectin ELISA kit (AssayPro, St. Charles, MO). Supernatant MDA and MDA+4-HA concentrations were measured with the Bioxytech LPO-586 kit (Oxis International, Inc, Foster City, CA). All measurements were performed according to the manufacturers’ instruction.
For iCAM-1 measurements, kidney tissue was homogenized (1:10 w/v) in 20 mM PBS, pH 7.4, containing the protease inhibitors (10 μg/ml leupeptin, 20 μg/ml aprotinin, 10 mM benzamidine, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium orthovanadate, 1 μg/ml pepstatin), on ice. Homogenates were centrifuged at 12,000g (4°C, 20 min). Supernatant soluble iCAM concentrations were measured with the Quantikine rat soluble iCAM kit (R&D Systems, Minneapolis, MN). Protein was measured with the bicinchoninic acid protein assay (Pierce Biotechnology, Rockford, IL).
2.4.3. Western blot analysis
Western blot analysis of poly(ADP-ribosyl)ated proteins was performed as described previously [36]. Protein bands were visualized with the Amersham ECL Western blotting detection reagents and analysis system (GE Healthcare, Buckinghampshire, UK). Membranes were then stripped and reprobed with β-actin antibody to verify equal protein loading. The data were quantified by densitometry (Quantity One 4.5.0 software, Bio-Rad Laboratories, Richmond, CA).
2.5. Statistical analysis
The results are expressed as Mean ± SEM. Data were subjected to equality of variance F test, and then to log transformation, if necessary, before one-way analysis of variance. Where overall significance (p<0.05) was attained, individual between group comparisons were made using the Student-Newman-Keuls multiple range test. Significance was defined at p ≤ 0.05. When between-group variance differences could not be normalized by log transformation (datasets for body weights and plasma glucose), the data were analyzed by the nonparametric Kruskal-Wallis one-way analysis of variance, followed by the Bonferroni/Dunn or Fisher’s PLSD tests for multiple comparisons.
3. RESULTS
3.1. PARP inhibition did not affect weight gain or glycemia in either non-diabetic or diabetic rats
The initial (prior to STZ administration) body weights were similar in control and diabetic rats maintained with or without GPI-15,427 treatment. The final body weights were similarly reduced in untreated and PARP inhibitor-treated diabetic rats compared with the control group (Table 1). Initial blood glucose concentrations were increased 4.1- and 4.2-fold in untreated and GPI-15,427-treated diabetic rats, respectively, compared with non-diabetic controls. Final blood glucose concentrations were 3.1- and 3.2-fold higher in untreated and GPI-15,427-treated diabetic rats than in non-diabetic controls. Final HbA1C concentrations were increased 2.2- and 2.1-fold in untreated and GPI-15,427-treated diabetic rats compared with non-diabetic controls. PARP inhibition did not affect either weight gain, or level of glycemia in non-diabetic rats.
Table 1.
Initial and final body weights, blood glucose and glycosylated hemoglobin concentrations in control and diabetic rats maintained with and without PARP inhibitor treatment.
| Variable | Control | Control + GPI-15,427 | Diabetic | Diabetic + GPI-15427 |
|---|---|---|---|---|
| Initial body weight, g | 293 ± 3.4 | 296 ± 5.3 | 263 ± 4.2 | 264 ± 3.9 |
| Final body weight, g | 591 ± 23 | 545 ± 8 | 377 ± 19** | 362 ± 16** |
| Initial blood glucose, mmol/l | 5.58 ± 0.08 | 5.32 ± 0.1 | 22.7 ± 1.4** | 23.4 ± 0.4** |
| Final blood glucose, mmol/l | 5.99 ± 0.22 | 6.22 ± 0.11 | 18.4 ± 1** | 18.9 ± 2.6** |
| HbA1c, % | 5.34 ± 0.07 | 5.12 ± 0.06 | 11.8 ± 0.3** | 11.4 ± 0.5** |
Data are Means SEM, n = 8–10 per group.
p < 0.01 vs controls.
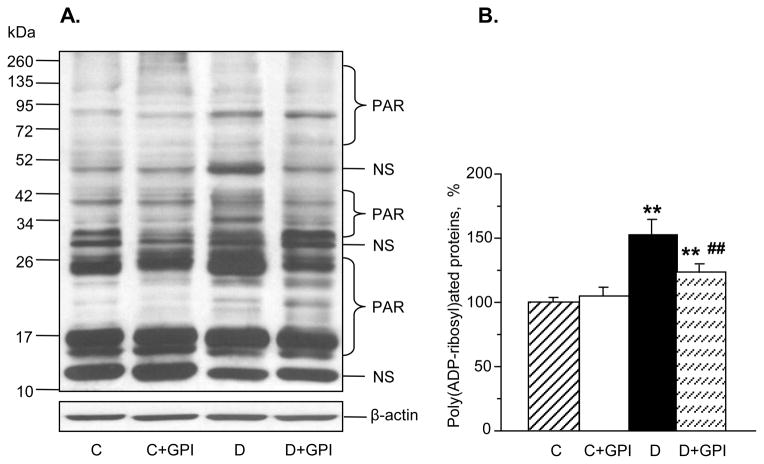
3.2. GPI-15,427 partially suppressed diabetes-induced poly(ADP-ribosyl)ated protein accumulation in the renal cortex
Poly(ADP-ribosyl)ated protein expression was increased 1.5-fold in untreated diabetic rats compared with non-diabetic controls, and this increase was partially prevented by GPI-15,427 treatment (to 123.8% of control value, Fig. 1, A and B). PARP inhibition did not affect poly(ADP-ribosyl)ated protein expression in non-diabetic control rats.
Fig. 1.
The PARP inhibitor GPI-15,427, at 30 mgkg−1d−1, partially suppresses diabetes-induced accumulation of poly(ADP-ribosyl)ated proteins in the renal cortex as manifested by representative Western blot analysis of renal poly(ADP-ribosyl)ated proteins (A) and poly (ADP-ribosyl)ated protein content (densitometry, B). C – control group; D – diabetic group. PAR – poly(ADP-ribosyl)ated proteins. NS –non-specific band. Mean ± SEM, n = 5–7 per group. ** - p < 0.01 vs control group; ## - p < 0.01 vs untreated diabetic group.
3.3. PARP inhibition counteracted diabetes-induced albuminuria
Urinary albumin excretion was increased 7.8-fold in untreated diabetic rats compared with non-diabetic controls, and this increase was partially prevented by GPI-15,427 (Fig. 2, A). In a similar fashion, urinary albumin/creatinine ratio was increased in untreated diabetic group (Fig. 2, B). This ratio was reduced by a PARP inhibitor treatment to the level that was not significantly different from those in either control or untreated diabetic rats (Fig. 2, B).
Fig. 2.

PARP inhibition reduces, but does not completely prevent, diabetes-associated albuminuria (A) and increase in urinary albumin/creatinine ratio. C – control group; D – diabetic group. Mean ± SEM, n = 8–10 per group. *, ** - p < 0.05 and 0.01 vs controls; # - p < 0.05 vs untreated diabetic group.
3.4. PARP inhibition counteracted diabetes-induced growth factor imbalances and fibronectin accumulation in the renal cortex
Diabetic rats displayed 2.1- and 1.8-fold increases in renal TGF-β1 and VEGF concentrations, respectively, compared with non-diabetic controls (Fig. 3, A and B). A PARP inhibitor treatment reduced the concentrations of both growth factors to the levels that were not significantly different from those in either control or untreated diabetic groups. GPI-15,427 did not significantly affect renal TGF-β1 and VEGF concentrations in non-diabetic rats. Renal fibronectin concentration was increased 1.6-fold in untreated diabetic rats compared with controls (Fig. 4), and this increase was essentially prevented by a PARP inhibitor treatment.
Fig. 3.

PARP inhibition counteracts diabetes-induced accumulation of TGF-β1 (A) and VEGF (B) in the renal cortex. C – control group; D – diabetic group. Mean ± SEM, n = 7–10 per group. *, ** - p < 0.05 and < 0.01 vs controls.
Fig. 4.
PARP inhibition essentially prevents diabetes-induced renal cortex fibronectin accumulation. C – control group; D – diabetic group. Mean ± SEM; n = 7–10 per group. ** - p <0.01 vs controls; ## - p < 0.01 vs untreated diabetic group.
3.5. PARP inhibition counteracted diabetes-induced systemic, but not renal, oxidative stress and did not affect renal iCAM accumulation
Urinary isoprostane (Fig. 5, A) and 8-hydroxy-2′-deoxyguanosine (Fig. 5, B) excretions were increased 17.5- and 1.5-fold in diabetic rats compared with controls, consistent with activation of the whole body lipid peroxidation and increased oxidative damage to DNA. GPI-15,427 treatment reduced urinary isoprostane and 8-hydroxy-2′-deoxyguanosine excretion in diabetic rats, without significantly affecting this variable in the non-diabetic controls.
Fig. 5.
PARP inhibition counteracts diabetes-induced urinary isoprostane (A) and 8-hydroxy-2′-deoxyguanosine (B) excretion. C – control group; D – diabetic group. Mean ± SEM, n = 8–10 per group. ** - p < 0.01 vs controls; #, ## - p < 0.05 and 0.01 compared with untreated diabetic group.
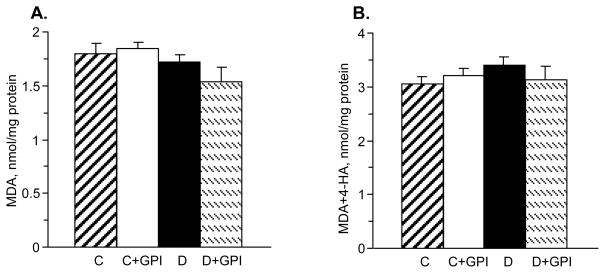
Renal MDA and MDA plus 4-HA concentrations were indistinguishable among control and diabetic rats maintained with or without GPI-15,427 treatment (Fig. 6, A and B).
Fig. 6.
Neither diabetes nor PARP inhibition affects renal malondialdehyde (A) or malondialdehyde plus 4-hydroxyalkenal (B) concentrations in control or diabetic rats. C – control group; D – diabetic group. Mean ± SEM, n = 6–10. * - p < 0.05 vs controls.
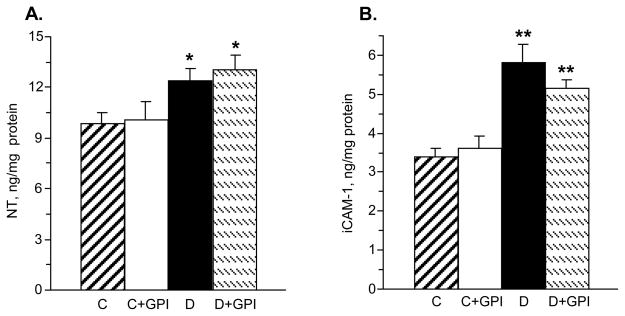
Renal NT concentration was increased 1.3-fold in diabetic rats compared with controls, and this increase was not affected by a PARP inhibitor treatment (Fig. 7, A). iCAM-1 concentrations were increased 1.7-fold in the renal cortex of diabetic rats compared with controls, and this increase was not significantly affected by a PARP inhibitor treatment (Fig. 7, B).
Fig. 7.
PARP inhibition does not affect diabetes-induced renal cortex nitrotyrosine (A) or soluble intercellular adhesion molecule-1 (B) accumulations. C – control group; D – diabetic group. Mean ± SEM, n = 7–10. ** - p < 0.01 compared with controls.
4. DISCUSSION
The findings described herein provide the first evidence of the important role for PARP activation in multiple manifestations of kidney disease associated with long-term Type 1 diabetes. PARP activation manifest by accumulation of poly(ADP-ribosyl)ated proteins is clearly manifest in the renal cortex of rats with 28-wk duration of STZ-diabetes, and triggers multiple mechanisms implicated in diabetic nephropathy.
Albuminuria is a hallmark of diabetic kidney disease and an important predictor of a decline in renal function [37]. Understanding the pathogenesis of urinary albumin excretion in diabetic nephropathy is important to improve methods for early diagnosis and treatment. Multiple pathogenetic mechanisms including, but not limited by, non-enzymatic glycation/glyocoxidation [9,10], activation of angiotensin-converting enzyme [9,38], aldose reductase [7,8,39], protein kinase C [11,40], and 12/15-lipoxygenase [12,41], mesangial ion channel dysregulation [42], growth factor imbalances [43–46], impaired fatty acid metabolism [47], and oxidative stress [17,48–50] have been implicated in albuminuria associated with diabetes. Recently, we reported that PARP activation plays an important role in albuminuria associated with early nephropathy in STZ-diabetic rats [35]. The results of the present study, consistent with findings of others in the leptin receptor-deficient (db/db) mouse model of Type 2 diabetes [34], suggest that PAR activation is involved in albuminuria in kidney disease in chronic diabetes. A partial inhibition of diabetes-associated PARP activation in the renal cortex with GPI-15,427, 30 mgkg−1d−1, was associated with alleviation of albuminuria and blunting of the elevation of the urinary albumin/creatinine ratio.
Several growth factors and, among them, TGF-β, VEGF, pigment epithelium-derived factor, connective tissue growth factor, insulin-like growth factor, and basic fibroblast growth factor have been implicated in the pathogenesis of diabetic nephropathy [43–46,51,52]. TGF-β and VEGF accumulate in the kidney at both early [35,48] and advanced (the present study) stages of diabetes. TGF-β, through its Smad3 signaling pathway, is involved in diabetes-associated accumulation of mesangial extracellular matrix [24]. The important role for TGF-β in diabetic kidney disease is supported by studies with neutralizing anti-TGF-β antibodies, antisense TGF-β oligodeoxynucleotides, and Smad3 knockout mice in which renal TGF-β binding, knockdown, or knockout prevented or even reversed renal hypertrophic and profibrotic changes in diabetic mice [24]. VEGF, another important player in diabetic kidney disease, has been implicated in renal hyperfiltration, albuminuria, and glomerular hypertrophy in animal models of both Type 1 [44] and Type 2 [53,54] diabetes. A neutralizing VEGF antibody was found effective against the afore-mentioned changes [44,53,54]. We recently found that PARP inhibition counteracted renal VEGF accumulation in early diabetes [35], consistent with previous findings of our group and others implicating PARP activation in diabetes- and hypoxia-induced retinal VEGF formation [55] as well as angiogenesis [56,57]. The present study revealed that an effect of the PARP inhibitor GPI-15,427 on renal VEGF accumulation persists with prolongation of diabetes indicative of the important role for PARP activation in upregulation of VEGF associated with advanced diabetic nephropathy.
Fibronectin is a high-molecular weight extracellular matrix glycoprotein that binds to membrane-spanning receptor proteins, integrins. In addition to integrins, fibronectin also binds to extracellular matrix components such as collagen, fibrin and heparan sulfate proteoglycans (e.g. syndecans). Several factors in the diabetic milieu including a highly reactive dicarbonyl compound methylglyoxal [58], superoxide [59], nitric oxide [60], activated Ras (GTP-binding proteins of small-molecular-weight) [59], and increased aldose reductase activity [61,62] have been reported to increase fibronectin expression in renal mesangial and proximal tubular cells. Renal fibronectin accumulation has been identified in several animal models of diabetic nephropathy, and has been found amenable to correction by pharmacological agents counteracting expansion of the mesangial extracellular matrix [9,62]. The non-specific PARP inhibitor 3-aminobenzamide has been reported to reduce fibronectin mRNA expression and to prevent mesangial expansion in STZ-diabetic rats [63]. However, the study does not contain quantitation of the mesangial expansion data and, furthermore, evidence of a robust inhibition of renal poly(ADP-ribosyl)ation by 3-aminobenzamide. In our previous study, fibronectin concentration was increased in the kidney of rats with 3-mo duration of STZ-diabetes, and this increase was counteracted, although not completely prevented by two structurally unrelated PARP inhibitors, 1,5-isoquinolinediol and GPI-15,427 [35]. The present findings suggest that inhibitory effect of GPI-15,427 on renal fibronectin accumulation persists in the chronic STZ-diabetic model. This provides the rationale for further, more detailed, evaluation of PARP inhibitors and PARP inhibitor-containing drug combinations as potential new therapies for the prevention of mesangial expansion and resulting obliteration of capillary lumen, glomerulosclerosis, and the end-stage renal disease often associated with advanced Type 1 diabetic nephropathy.
Growing evidence suggests that the relationship between diabetes-induced oxidative-nitrosative stress and PARP activation is bi-, rather than unidirectional, i.e., reactive oxygen (ROS) and nitrogen species induce PARP activation, whereas PARP activation, in turn, leads to oxidative damage. Such relationship between the two phenomena has been demonstrated for several tissue-sites for diabetic complications [9,34,35,64]. In particular, we reported that PARP activation contributes to systemic oxidative stress manifest by increased urinary isoprostane and 8-hydroxy-2′-deoxyguanosine excretions as well as renal oxidative-nitrosative stress manifest by elevated renal MDA plus HA and NT concentrations [35]. Others [34] implicated PARP activation in ROS generation in high glucose-exposed cultured glomerular podocytes. The present findings indicating that GPI-15,427 counteracts increases in urinary isoprostane and 8-hydroxy-2′-deoxyguanosine excretion, but not renal NT accumulation, in rats with 28-mo duration of STZ-diabetes, may suggest that whereas PARP activation plays an important role in the whole body oxidative damage to lipids and DNA, its role in renal peroxynitrite formation in long-term Type 1 diabetes is minor. Note, however, that GPI-15,427, at the dose of 30 mg kg−1d−1, only partially inhibited renal poly(ADP-ribosyl)ated protein overexpression in the chronic STZ-diabetic model. Evidently, further studies with higher doses of PARP inhibitors that would result in a robust inhibition of poly(ADP-ribosyl)ation, are required to sort out the relationship between nitrosative stress and PARP activation in advanced diabetic nephropathy. In contrast to our previous studies in rats with shorter (4-wk and 12-wk [35, 65]) durations of diabetes, no differences in renal lipid peroxidation product accumulation were found between control and 28-wk STZ-diabetic rats treated with or without GPI-15,427. A potential explanation may be related to a protracted treatment with suboptimal doses of insulin, as insulin reduces renal lipid peroxidation product accumulation in chronic diabetes [50]. Renal iCAM-1 concentration was increased in diabetic rats, consistent with low-grade inflammation recently implicated in the pathogenesis of diabetic nephropathy [66,67] as well as other diabetic complications. This inflammatory marker was not affected by a PARP inhibitor treatment. Note, that other studies demonstrated that 1) PARP inhibition suppressed adhesion molecule (iCAM-1, vascular cell adhesion molecule-1, E-selectin) overexpression in intermittent high glucose-exposed endothelial cells [68]; and 2) PARP gene deficiency protected from lipopolysaccharide-induced iCAM-1 overexpression in glial cells [28]. Apparently, further studies are needed to determine whether renal cortex iCAM-1 expression would be suppressed under conditions of a more robust PARP inhibition, or mechanisms, other than PARP activation, play a major role in renal iCAM-1 accumulation in chronic Type 1 diabetes.
In conclusion, PARP activation plays an important role in multiple manifestations of kidney disease associated with long-term insulin-dependent diabetes. These findings provide rationale for development and further studies of PARP inhibitors and PARP inhibitor-containing combination therapies, for prevention and treatment of diabetic nephropathy.
Acknowledgments
The study was supported by the Juvenile Diabetes Research Foundation International Grant 1-2005-223, National Institutes of Health Grants DK070720, DK074517, and DK077141, and American Diabetes Association Grant (all to I.G.O.). The authors thank Dr. Ivan A. Pavlov for expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFRENCES
- 1.Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, et al. American Diabetes Association: Nephropathy in diabetes. Diabetes Care. 2004;27:S79–83. doi: 10.2337/diacare.27.2007.s79. [DOI] [PubMed] [Google Scholar]
- 2.Strippoli GF, Craig M, Deeks JJ, Schena FP, Craig JC. Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review. BMJ. 2004;329:828. doi: 10.1136/bmj.38237.585000.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nelson R, Knowler W, Pettitt D, Bennett P. National Institutes of Diabetes and Digestive and Kidney Diseases, editor. Diabetes in America. Bethesda, MD: 1995. Kidney diseases in diabetes; pp. 349–85. NIH Publication No.95-1468. [Google Scholar]
- 4.Parving HH, Gall MA, Nielsen FS. Dyslipidaemia and cardiovascular disease in non-insulin-dependent diabetic patients with and without diabetic nephropathy. J Intern Med Suppl. 1994;736:89–94. [PubMed] [Google Scholar]
- 5.Yokoyama H, Aoki T, Imahori M, Kuramitsu M. Subclinical atherosclerosis is increased in type 2 diabetic patients with microalbuminuria evaluated by intima-media thickness and pulse wave velocity. Kidney Int. 2004;66:448–54. doi: 10.1111/j.1523-1755.2004.00752.x. [DOI] [PubMed] [Google Scholar]
- 6.Cooper ME. Interaction of metabolic and haemodynamic factors in mediating experimental diabetic nephropathy. Diabetologia. 2001;44:1957–72. doi: 10.1007/s001250100000. [DOI] [PubMed] [Google Scholar]
- 7.Oates PJ, Mylari BL. Aldose reductase inhibitors: therapeutic implications for diabetic complications. Expert Opin Investig Drugs. 1999;8:2095–119. doi: 10.1517/13543784.8.12.2095. [DOI] [PubMed] [Google Scholar]
- 8.Dan Q, Wong RL, Yin S, Chung SK, Chung SS, Lam KS. Interaction between the polyol pathway and non-enzymatic glycation on mesangial cell gene expression. Nephron Exp Nephrol. 2004;98:e89–99. doi: 10.1159/000080684. [DOI] [PubMed] [Google Scholar]
- 9.Forbes JM, Thallas V, Thomas MC, Founds HW, Burns WC, Jerums G, et al. The breakdown of preexisting advanced glycation end products is associated with reduced renal fibrosis in experimental diabetes. FASEB J. 2003;17:1762–4. doi: 10.1096/fj.02-1102fje. [DOI] [PubMed] [Google Scholar]
- 10.Beisswenger PJ, Drummond KS, Nelson RG, Howell SK, Szwergold BS, Mauer M. Susceptibility to diabetic nephropathy is related to dicarbonyl and oxidative stress. Diabetes. 2005;54:3274–81. doi: 10.2337/diabetes.54.11.3274. [DOI] [PubMed] [Google Scholar]
- 11.Koya D, Haneda M, Nakagawa H, Isshiki K, Sato H, Maeda S, et al. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. FASEB J. 2000;14:439–47. doi: 10.1096/fasebj.14.3.439. [DOI] [PubMed] [Google Scholar]
- 12.Ohshiro Y, Ma RC, Yasuda Y, Hiraoka-Yamamoto J, Clermont AC, Isshiki K, et al. Reduction of diabetes-induced oxidative stress, fibrotic cytokine expression, and renal dysfunction in protein kinase Cbeta-null mice. Diabetes. 2006;55:3112–20. doi: 10.2337/db06-0895. [DOI] [PubMed] [Google Scholar]
- 13.Yuan H, Lanting L, Xu ZG, Li SL, Swiderski P, Putta S, et al. Effects of cholesterol-tagged small interfering RNAs targeting 12/15-lipoxygenase on parameters of diabetic nephropathy in a mouse model of type 1 diabetes. Am J Physiol Renal Physiol. 2008;295:F605–17. doi: 10.1152/ajprenal.90268.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schleicher E, Weigert C. Role for hexoseamine biosynthetic pathway in diabetic nephropathy. Kidney Int. 2000;(Suppl 77):S13–8. doi: 10.1046/j.1523-1755.2000.07703.x. [DOI] [PubMed] [Google Scholar]
- 15.DeRubertis FR, Craven PA, Melhem MF. Acceleration of diabetic renal injury in the superoxide dismutase knockout mouse: effects of tempol. Metabolism. 2007;56:1256–64. doi: 10.1016/j.metabol.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 16.Thallas-Bonke V, Thorpe SR, Coughlan MT, Fukami K, Yap FY, Sourris KC, et al. Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha-dependent pathway. Diabetes. 2008;57:460–9. doi: 10.2337/db07-1119. [DOI] [PubMed] [Google Scholar]
- 17.Zheng S, Carlson EC, Yang L, Kralik PM, Huang Y, Epstein PN. Podocyte-specific overexpression of the antioxidant metallothionein reduces diabetic nephropathy. J Am Soc Nephrol. 2008;19:2035–7. doi: 10.1681/ASN.2007080967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eid AA, Gorin Y, Fagg BM, Maalouf R, Barnes JL, Block K, et al. Mechanisms of podocyte injury in diabetes: role of cytochrome P450 and NADPH oxidases. Diabetes. 2009;58:1201–11. doi: 10.2337/db08-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A. 2009;106:14385–90. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greene EL, Houghton O, Collinsworth G, Garnovskaya MN, Nagai T, Sajjad T, et al. 5-HT(2A) receptors stimulate mitogen-activated protein kinase via H(2)O(2) generation in rat renal mesangial cells. Am J Physiol Renal Physiol. 2000;278:F650–8. doi: 10.1152/ajprenal.2000.278.4.F650. [DOI] [PubMed] [Google Scholar]
- 21.Morcos M, Schlotterer A, Sayed AA, Kukudov G, Oikomonou D, Ibrahim Y, et al. Rosiglitazone reduces angiotensin II and advanced glycation end product-dependent sustained nuclear factor-kappaB activation in cultured human proximal tubular epithelial cells. Horm Metab Res. 2008;40:752–9. doi: 10.1055/s-0028-1082039. [DOI] [PubMed] [Google Scholar]
- 22.Montero A, Munger KA, Khan RZ, Valdivielso JM, Morrow JD, Guasch A, et al. F2-isoprostanes mediate high glucose-induced TGF-beta synthesis and glomerular proteinuria in experimental type 1 diabetes. Kidney Int. 2000;58:1963–72. doi: 10.1111/j.1523-1755.2000.00368.x. [DOI] [PubMed] [Google Scholar]
- 23.Obrosova IG, Minchenko AG, Marinescu V, Fathallah L, Kennedy A, Stockert CM, et al. Antioxidants attenuate early up regulation of retinal vascular endothelial growth factor in streptozotocin-diabetic rats. Diabetologia. 2001;44:1102–10. doi: 10.1007/s001250100631. [DOI] [PubMed] [Google Scholar]
- 24.Ziyadeh FN. Different roles for TGF-beta and VEGF in the pathogenesis of the cardinal features of diabetic nephropathy. Diabetes Res Clin Pract. 2008;82:S38–41. doi: 10.1016/j.diabres.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Jagtap P, Szabó C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–40. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- 26.Du X, Matsumura T, Edelstein D, Rossetti L, Zsengellér Z, Szabó C, et al. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112:1049–57. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Veres B, Radnai B, Gallyas F, Jr, Varbiro G, Berente Z, Osz E, et al. Regulation of kinase cascades and transcription factors by a poly(ADP-ribose) polymerase-1 inhibitor, 4-hydroxyquinazoline, in lipopolysaccharide-induced inflammation in mice. J Pharmacol Exp Ther. 2004;310:247–55. doi: 10.1124/jpet.104.065151. [DOI] [PubMed] [Google Scholar]
- 28.Ha HC, Hester LD, Snyder SH. Poly(ADP-ribose) polymerase-1 dependence of stress-induced transcription factors and associated gene expression in glia. Proc Natl Acad Sci U S A. 2002;99:3270–5. doi: 10.1073/pnas.052712399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia Soriano F, Virág L, Jagtap P, Szabó E, Mabley JG, Liaudet L, et al. Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat Med. 2001;7:108–13. doi: 10.1038/83241. [DOI] [PubMed] [Google Scholar]
- 30.Pacher P, Liaudet L, Soriano FG, Mabley JG, Szabó E, Szabó C. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes. 2002;51:514–21. doi: 10.2337/diabetes.51.2.514. [DOI] [PubMed] [Google Scholar]
- 31.Obrosova IG, Xu W, Lyzogubov VV, Ilnytska O, Mashtalir N, Vareniuk I, et al. PARP inhibition or gene deficiency counteracts intraepidermal nerve fiber loss and neuropathic pain in advanced diabetic neuropathy. Free Radic Biol Med. 2008;44:972–81. doi: 10.1016/j.freeradbiomed.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng L, Szabó C, Kern TS. Poly(ADP-ribose) polymerase is involved in the development of diabetic retinopathy via regulation of nuclear factor-kappaB. Diabetes. 2004;53:2960–7. doi: 10.2337/diabetes.53.11.2960. [DOI] [PubMed] [Google Scholar]
- 33.Drel VR, Xu W, Zhang J, Kador PF, Ali TK, Shin J, et al. Poly (ADP-ribose) polymerase inhibition counteracts cataract formation and early retinal changes in streptozotocin-diabetic rats. Invest Ophthalmol Vis Sci. 2009;50:1778–90. doi: 10.1167/iovs.08-2191. [DOI] [PubMed] [Google Scholar]
- 34.Szabó C, Biser A, Benko R, Böttinger E, Suszták K. Poly(ADP-ribose) polymerase inhibitors ameliorate nephropathy of type 2 diabetic Leprdb/db mice. Diabetes. 2006;55:3004–12. doi: 10.2337/db06-0147. [DOI] [PubMed] [Google Scholar]
- 35.Drel VR, Xu W, Zhang J, Pavlov IA, Shevalye H, Slusher B, et al. Poly(ADP-ribose) polymerase (PARP) inhibition counteracts multiple manifestations of experimental type 1 diabetic nephropathy. Endocrinology. 2009 doi: 10.1210/en.2009-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drel VR, Pacher P, Stevens MJ, Obrosova IG. Aldose reductase inhibition counteracts nitrosative stress and poly(ADP-ribose) polymerase activation in diabetic rat kidney and high-glucose-exposed human mesangial cells. Free Radic Biol Med. 2006;40:1454–65. doi: 10.1016/j.freeradbiomed.2005.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krolewski AS, Poznik GD, Placha G, Canani L, Dunn J, Walker W, et al. A genome-wide linkage scan for genes controlling variation in urinary albumin excretion in type II diabetes. Kidney Int. 2006;69:129–36. doi: 10.1038/sj.ki.5000023. [DOI] [PubMed] [Google Scholar]
- 38.Barit D, Cooper ME. Diabetic patients and kidney protection: an attainable target. J Hypertens. 2008;(Suppl 26):S3–7. doi: 10.1097/01.hjh.0000320752.31109.f5. [DOI] [PubMed] [Google Scholar]
- 39.Demaine AG. Polymorphisms of the aldose reductase gene and susceptibility to diabetic microvascular complications. Curr Med Chem. 2003;10:1389–98. doi: 10.2174/0929867033457359. [DOI] [PubMed] [Google Scholar]
- 40.Tuttle KR. Protein kinase C-beta inhibition for diabetic kidney disease. Diabetes Res Clin Pract. 2008;82:S70–4. doi: 10.1016/j.diabres.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 41.Ma J, Natarajan R, LaPage J, Lanting L, Kim N, Becerra D, et al. 12/15-lipoxygenase inhibitors in diabetic nephropathy in the rat. Prostaglandins Leukot Essent Fatty Acids. 2005;72:13–20. doi: 10.1016/j.plefa.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Grimm PR, Sansom SC. BK channels in the kidney. Curr Opin Nephrol Hypertens. 2007;16:430–6. doi: 10.1097/MNH.0b013e32826fbc7d. [DOI] [PubMed] [Google Scholar]
- 43.Zhu Y, Usui HK, Sharma K. Regulation of transforming growth factor beta in diabetic nephropathy: implications for treatment. Semin Nephrol. 2007;27:153–60. doi: 10.1016/j.semnephrol.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Vriese AS, Tilton RG, Elger M, Stephan CC, Kriz W, Lameire NH. Antibodies against vascular endothelial growth factor improve early renal dysfunction in experimental diabetes. J Am Soc Nephrol. 2001;12:993–1000. doi: 10.1681/ASN.V125993. [DOI] [PubMed] [Google Scholar]
- 45.Wang JJ, Zhang SX, Mott R, Knapp RR, Cao W, Lau K, et al. Salutary effect of pigment epithelium-derived factor in diabetic nephropathy: evidence for antifibrogenic activities. Diabetes. 2006;55:1678–85. doi: 10.2337/db05-1448. [DOI] [PubMed] [Google Scholar]
- 46.Mason RM. Connective tissue growth factor (CCN2), a pathogenic factor in diabetic nephropathy. What does it do? How does it do it? J Cell Commun Signal. 2009;3:95–104. doi: 10.1007/s12079-009-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garman JH, Mulroney S, Manigrasso M, Flynn E, Maric C. Omega-3 fatty acid rich diet prevents diabetic renal disease. Am J Physiol Renal Physiol. 2009;296:F306–16. doi: 10.1152/ajprenal.90326.2008. [DOI] [PubMed] [Google Scholar]
- 48.Melhem MF, Craven PA, Derubertis FR. Effects of dietary supplementation of alpha-lipoic acid on early glomerular injury in diabetes mellitus. J Am Soc Nephrol. 2001;12:124–33. doi: 10.1681/ASN.V121124. [DOI] [PubMed] [Google Scholar]
- 49.Craven PA, Melhem MF, Phillips SL, DeRubertis FR. Overexpression of Cu2+/Zn2+ superoxide dismutase protects against early diabetic glomerular injury in transgenic mice. Diabetes. 2001;50:2114–25. doi: 10.2337/diabetes.50.9.2114. [DOI] [PubMed] [Google Scholar]
- 50.Melhem MF, Craven PA, Liachenko J, DeRubertis FR. Alpha-lipoic acid attenuates hyperglycemia and prevents glomerular mesangial matrix expansion in diabetes. J Am Soc Nephrol. 2002;13:108–16. doi: 10.1681/ASN.V131108. [DOI] [PubMed] [Google Scholar]
- 51.Davis LK, Rodgers BD, Kelley KM. Angiotensin II- and glucose-stimulated extracellular matrix production: mediation by the insulin-like growth factor (IGF) axis in a murine mesangial cell line. Endocrine. 2008;33:32–9. doi: 10.1007/s12020-008-9055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vasko R, Koziolek M, Ikehata M, Rastaldi MP, Jung K, Schmid H, et al. Role of basic fibroblast growth factor (FGF-2) in diabetic nephropathy and mechanisms of its induction by hyperglycemia in human renal fibroblasts. Am J Physiol Renal Physiol. 2009;296:F1452–63. doi: 10.1152/ajprenal.90352.2008. [DOI] [PubMed] [Google Scholar]
- 53.Flyvbjerg A, Dagnaes-Hansen F, De Vriese AS, Schrijvers BF, Tilton RG, Rasch R. Amelioration of long-term renal changes in obese type 2 diabetic mice by a neutralizing vascular endothelial growth factor antibody. Diabetes. 2002;51:3090–4. doi: 10.2337/diabetes.51.10.3090. [DOI] [PubMed] [Google Scholar]
- 54.Schrijvers BF, Flyvbjerg A, Tilton RG, Lameire NH, De Vriese AS. A neutralizing VEGF antibody prevents glomerular hypertrophy in a model of obese type 2 diabetes, the Zucker diabetic fatty rat. Nephrol Dial Transplant. 2006;21:324–9. doi: 10.1093/ndt/gfi217. [DOI] [PubMed] [Google Scholar]
- 55.Obrosova IG, Minchenko AG, Frank RN, Seigel GM, Zsengeller Z, Pacher P, et al. Poly(ADP-ribose) polymerase inhibitors counteract diabetes- and hypoxia-induced retinal vascular endothelial growth factor overexpression. Int J Mol Med. 2004;14:55–64. [PubMed] [Google Scholar]
- 56.Rajesh M, Mukhopadhyay P, Godlewski G, Bátkai S, Haskó G, Liaudet L, et al. Poly(ADP-ribose)polymerase inhibition decreases angiogenesis. Biochem Biophys Res Commun. 2006;350:1056–62. doi: 10.1016/j.bbrc.2006.09.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tentori L, Lacal PM, Muzi A, Dorio AS, Leonetti C, Scarsella M, et al. Poly(ADP-ribose) polymerase (PARP) inhibition or PARP-1 gene deletion reduces angiogenesis. Eur J Cancer. 2007;43:2124–33. doi: 10.1016/j.ejca.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 58.Ho C, Lee PH, Huang WJ, Hsu YC, Lin CL, Wang JY. Methylglyoxal-induced fibronectin gene expression through Ras-mediated NADPH oxidase activation in renal mesangial cells. Nephrology (Carlton) 2007;12:348–56. doi: 10.1111/j.1440-1797.2007.00809.x. [DOI] [PubMed] [Google Scholar]
- 59.Lin CL, Wang FS, Kuo YR, Huang YT, Huang HC, Sun YC, et al. Ras modulation of superoxide activates ERK-dependent fibronectin expression in diabetes-induced renal injuries. Kidney Int. 2006;69:1593–1600. doi: 10.1038/sj.ki.5000329. [DOI] [PubMed] [Google Scholar]
- 60.Noh H, Ha H, Yu MR, Kang SW, Choi KH, Han DS, et al. High glucose increases inducible NO production in cultured rat mesangial cells. Possible role in fibronectin production. Nephron. 2002;90:78–85. doi: 10.1159/000046318. [DOI] [PubMed] [Google Scholar]
- 61.Jiang T, Che Q, Lin Y, Li H, Zhang N. Aldose reductase regulates TGF-beta1-induced production of fibronectin and type IV collagen in cultured rat mesangial cells. Nephrology (Carlton) 2006;11:105–12. doi: 10.1111/j.1440-1797.2006.00553.x. [DOI] [PubMed] [Google Scholar]
- 62.Morrisey K, Steadman R, Williams JD, Phillips AO. Renal proximal tubular cell fibronectin accumulation in response to glucose is polyol pathway dependent. Kidney Int. 1999;55:2548–72. doi: 10.1046/j.1523-1755.2002.t01-1-00454.x. [DOI] [PubMed] [Google Scholar]
- 63.Xu B, Chiu J, Feng B, Chen S, Chakrabarti S. PARP activation and the alteration of vasoactive factors and extracellular matrix protein in retina and kidney in diabetes. Diabetes Metab Res Rev. 2008;24:404–12. doi: 10.1002/dmrr.842. [DOI] [PubMed] [Google Scholar]
- 64.Obrosova IG, Drel VR, Pacher P, Ilnytska O, Wang ZQ, Stevens MJ, et al. Oxidative-nitrosative stress and poly(ADP-ribose) polymerase (PARP) activation in experimental diabetic neuropathy: the relation is revisited. Diabetes. 2005;54:3435–41. doi: 10.2337/diabetes.54.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Obrosova IG, Fathallah L, Liu E, Nourooz-Zadeh J. Early oxidative stress in the diabetic kidney: effect of DL-alpha-lipoic acid. Free Radic Biol Med. 2003;34:186–95. doi: 10.1016/s0891-5849(02)01195-4. [DOI] [PubMed] [Google Scholar]
- 66.Maeda S. Do inflammatory cytokine genes confer susceptibility to diabetic nephropathy? Kidney Int. 2008;74:413–5. doi: 10.1038/ki.2008.291. [DOI] [PubMed] [Google Scholar]
- 67.Brosius FC., 3rd New insights into the mechanisms of fibrosis and sclerosis in diabetic nephropathy. Rev Endocr Metab Disord. 2008;9:245–54. doi: 10.1007/s11154-008-9100-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Piconi L, Quagliaro L, Da Ros R, Assaloni R, Giugliano D, Esposito K, Szabó C, Ceriello A. Intermittent high glucose enhances ICAM-1, VCAM-1, E-selectin and interleukin-6 expression in human umbilical endothelial cells in culture: the role of poly(ADP-ribose) polymerase. J Thromb Haemost. 2004;2:1453–9. doi: 10.1111/j.1538-7836.2004.00835.x. [DOI] [PubMed] [Google Scholar]