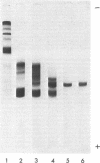
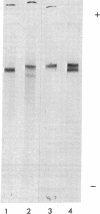
Abstract
Serum contains a polypeptide with insulin-like activity not suppressible by insulin antibodies (NSILA). A large-scale isolation procedure for NSILA is described, starting from an acid ethanol extract of a Cohn fraction (precipitate B) obtained from human plasma. Two homogenous polypeptides with insulin-like and cell-growth promoting activities could be isolated by gel filtration, ion exchange chromatography, and preparative polyacrylamide gel electrophoresis. Both components are slightly basic polypeptides with a minimal molecular weight of 5800 +/- 400. Both are single-chain molecules with two intrachain disulfide bridges each and no free sulfhydryl groups. NSILA I and II differ, however, in their amino acid compositions. The N-terminal amino acid sequences are Gly-Pro-Glu- in NSILA I, and Ala-Tyr-Arg- and Tyr-Arg- in NSILA II. Both NSILA I and II enhance net gas exchange in adipose tissue with a specific activity 60 times lower than that of insulin. In the range of 1-50 ng/ml, both substances stimulate [3H]thymidine incorporation into DNA of chick embryo fibroblasts. The same effect can be obtained with insulin but only at concentrations 50-100 times higher than those of NSILA. These results suggest that NSILA I and II are two forms of an insulin-like hormone with predominating effects on cell and tissue growth parameters.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daughaday W. H., Hall K., Raben M. S., Salmon W. D., Jr, van den Brande J. L., van Wyk J. J. Somatomedin: proposed designation for sulphation factor. Nature. 1972 Jan 14;235(5333):107–107. doi: 10.1038/235107a0. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- FROESCH E. R., BUERGI H., RAMSEIER E. B., BALLY P., LABHART A. ANTIBODY-SUPPRESSIBLE AND NONSUPPRESSIBLE INSULIN-LIKE ACTIVITIES IN HUMAN SERUM AND THEIR PHYSIOLOGIC SIGNIFICANCE. AN INSULIN ASSAY WITH ADIPOSE TISSUE OF INCREASED PRECISION AND SPECIFICITY. J Clin Invest. 1963 Nov;42:1816–1834. doi: 10.1172/JCI104866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish W. W., Mann K. G., Tanford C. The estimation of polypeptide chain molecular weights by gel filtration in 6 M guanidine hydrochloride. J Biol Chem. 1969 Sep 25;244(18):4989–4994. [PubMed] [Google Scholar]
- Jakob A., Hauri C., Froesch E. R. Nonsuppressible insulin-like activity in human serum. 3. Differentiation of two distinct molecules with nonsuppressible ILA. J Clin Invest. 1968 Dec;47(12):2678–2688. doi: 10.1172/JCI105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morell B., Froesch E. R. Fibroblasts as an experimental tool in metabolic and hormone studies. II. Effects of insulin and nonsuppressible insulin-like activity (NSILA-S) on fibroblasts in culture. Eur J Clin Invest. 1973 Mar;3(2):119–123. doi: 10.1111/j.1365-2362.1973.tb00338.x. [DOI] [PubMed] [Google Scholar]
- Schlumpf U., Heimann R., Zapf J., Froesch E. R. Non-suppressible insulin-like activity and sulphaton activity in serum extracts of normal subjects, acromegalics and pituitary dwarfs. Acta Endocrinol (Copenh) 1976 Jan;81(1):28–42. doi: 10.1530/acta.0.0810028. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Van Montagu M. Sequence analysis of fluorescamine-stained peptides and proteins purified on a nanomole scale. Application to proteins of bacteriophage MS2. Eur J Biochem. 1974 May 2;44(1):279–288. doi: 10.1111/j.1432-1033.1974.tb03483.x. [DOI] [PubMed] [Google Scholar]
- Vesterberg O., Svensson H. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. IV. Further studies on the resolving power in connection with separation of myoglobins. Acta Chem Scand. 1966;20(3):820–834. doi: 10.3891/acta.chem.scand.20-0820. [DOI] [PubMed] [Google Scholar]
- Zapf J., Waldvogel M., Froesch E. R. Binding of nonsuppressible insulinlike activity to human serum. Evidence for a carrier protein. Arch Biochem Biophys. 1975 Jun;168(2):638–645. doi: 10.1016/0003-9861(75)90296-9. [DOI] [PubMed] [Google Scholar]
- Zingg A. E., Froesch E. R. Effects of partially purified preparations with nonsuppressible insulin-like activity (NSILA-S) on sulfate incorporation into rat and chicken cartilage. Diabetologia. 1973 Dec;9(6):472–476. doi: 10.1007/BF00461691. [DOI] [PubMed] [Google Scholar]