Abstract
The effects of novel, selective adenosine (ADO) A3 receptor antagonists of diverse structure on cells of the human HL-60 leukemia and U-937 lymphoma cell lines were examined. Both 3-ethyl 5-benzyl 2-methyl-6-phenyl-4-phenylethynyl-1,4-(±)-dihydropyridine3,5-dicarboxylate (MRS 1191, 0.5µM) and 6-carboxymethyl-5,9-dihydro-9-methyl-2-phenyl-[1,2,4]-triazolo[5,1-a][2,7]naphthyridine (L-249313, 0.5 µM) induced apoptotic cell death and expression of bak protein. Low concentrations of the A3 receptor agonist 2-chloro-N6-(3-iodobenzyl)adenosine-5′-N-methyluxonamide (Cl-IB-MECA, 10 nM or 1 µM) protected against antagonist-induced cell death. At concentrations ≥ 10 µM, the agonist alone produced apoptosis and bak expression in various cell lines. It is suggested that there exists a tonic low level of A3 receptor activation, possibly induced by release of endogenous adenosine, that results in cell protection.
Agonists selective for three of the four subtypes of ADO receptors (A1, A2A, and A3) are known (1). Among these agonists, only A3 agonists, such as IB-MECA and Cl-IB-MECA consistently induce apoptosis (programmed cell death), and this occurs only at relatively high concentrations (≥ 10 µM) of the agents (2). The induction of apoptosis by A3 selective agonists was first demonstrated in promyelocytic human leukemia HL-60 cells (2), and was shown to be accompanied by a rise in intracellular calcium concentration. Similar effects have been noted in human peripheral blood eosinophils, which have been shown to express high levels of the A3 receptor (3). IB-MECA (≥ 10 µM) induces apoptosis in spontaneously contracting rat cardiac myocytes in culture (4). In CHO cells transfected with the human A3 receptor, cell death induced by high concentrations of Cl-IB-MEGA occured only in those cells expressing the A3 receptor and not in the corresponding wild-type clone, indicating that this is a receptor-mediated effect (5).
The in vitro and in vivo effects of A3 agonists are paradoxical, i.e. in some paradigms they cause increased damage and cell death and in others they are protective. Using an in vivo gerbil model of global ischemia, von Lubitz et al. observed that acute administration of IB-MECA immmediately prior to the ischemia clearly worsened the post-occlusive outcome (6). However chronic preadministration of the same agent over several weeks had a highly neuroprotective, post-ischemic effect. In astroglial cell cultures, nanomolar concentrations of selective A3 agonists cause protection against cell death, while high concentrations increased cell death (7). A3 agonists are also protective in the heart, as well as the brain. In a chick ventricular myocyte culture (8) and in the isolated rabbit heart (9) activation of A3 receptors by IB-MECA induced preconditioning, which greatly improved the outcome following an ischemic injury.
In the present study we have confirmed that A3 agonists in high concentrations induce apoptotic cell death with the characteristic DNA fragmentation in a variety of human cell lines of origin in the immune system. Clarification of the need for such high doses of agonists, thousands of fold higher than the Ki values at A3 receptors, has awaited the introduction of selective A3 receptor antagonists, which are now available for the human A3 receptor (10–13). We have developed A3 receptor antagonists (10–12) belonging to three distinct, non-purine chemical classes: flavonoids (e.g. MRS 1067, 3,6-dichloro-2′-isopropyloxy-4′-methyl-flavone), 1, 4-dihydropyridine derivatives (e.g. MRS 1191, which is 1300-fold selective for human A3 receptors with a Ki, value of 31 nM), and the triazoloquinazolines (e.g. MRS 1220, 9-chloro-2-(2-furyl)-5-phenylacetylamino[1,2,4]triazolo[1,5-c]quinazoline, which is highly potent with a Ki value of 0.65 nM but less selective). Another highly selective although non-competitive A3 receptor antagonist, 6-carboxymethyl-5, 9-dihydro-9-methyl-2-phenyl-[1,2,4]-triazolo[5,1-a][2,7]naphthyridine (L-249313, which has Ki value of 13 nM), have been identified through broad screening at Merck (13). This study is the first to demonstrate that A3 receptor antagonists alone cause apoptotic cell death, and that the cells may be rescued by subcytotoxic concentrations of selective A3 receptor agonists.
MATERIALS AND METHODS
Reagents
HL-60 and U-937 cells were obtained from the ATCC (Bethesda, MD). RPMI 1640 medium and fetal bovine serum were obtained from Gibco BRL (Gaithersburg, MD). IB-MEGA and Cl-IB-MECA were obtained from RBI (Natick, MA) through the NIMH Synthesis Program. The selective ADO A3 antagonists MRS 1191 (11) and MRS 1220 (12) were synthesized in our laboratory. L-249313 supplied as the mesylate salt was the gift of Dr. Marlene Jacobson at Merck (West Point, PA). Apoptosis Detection System was from Promega (Madison, WI), and the mouse monoclonal anti-bak antibody was from Calbiochem (La Jolla, CA). Rhodamine (TRITC)-conjugated AffiniPure Goat Anti-mouse IgG (H+L) is from Jackson ImmunoReseaarch Laboratories, Inc. Chromomycin A3 was purchased from Sigma Chemical (St. Louis, MO).
Cell cultures and preparations
The HL-60 cells were maintained in RPMI 1640 supplemented with 10% fetal calf serum, 100 units/ml penicillin, 100 µg/ml streptomycin and 2 mM L-glutamine. The cells were split every third day, and 2 days before each experiment cultures were diluted to 2 × 105 cells/ml. Live cell counting was carried out using 0.1% trypan blue.
DNA content analysis by flow cytometry
For analysis of DNA content, aliquots of 2 ml of cell suspension were placed into 12-well flat-bottomed plates (Costar, Cambridge, MA, USA) containing 2~6 µl test-compound solutions at defined concentrations or 6 µl DMSO (diluting medium). Cells were fixed by adding ~107 cells suspended in 1 ml of PBS to 1 ml 80% ethanol at 4°C and stored for 24–48 hrs. After the cells were washed twice with PBS, the cells were stained with 20 mg/ml chromomycin A3 dissolved in PBS containing 2 mM MgCl2 by incubation in subdued light (2 hrs; 4°C). The cells were then analyzed using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA) as previously described (14).
In situ hybridization
Cells were fixed by immersing slides in 4% methanol-free formaldehyde in PBS at pH 7.4, 4°C, for 25 min. Fixed cells were permeabilized in 0.2% Triton® X-100 in PBS at 4°C, for 5 min. After preincubation with 50 µl Promega equilibration buffer for 10 min at room temperature, the cells were covered with 50µl TdT incubation buffer and a coverslip at 37°C for 60 min inside the dark humidified chamber to protect from dry and direct light. For stop the reaction, remove the coverslip and immerse the slides in 2×SSC for 15 min at room temperature. The cells were restained by propidium iodide 1µg/ml in PBS for 15 min. After every step, rinse cells 2–3 times with fresh PBS for 5 min each. Finaly, the glass slides were sealed with nail polish. Store the slides at 4°C in the dark (15).
Immunofluorescent staining
Cells were fixed as following steps: 10 min with 1% formalin in PBS, 5 min with −10°C methanol and 2 min with ice cold acetone, then permeblilized with 0.1% Nonidet P-40 for 20 min, After the preparetion, the specimens were incubated step by step: 10% goat serum in PBS for 20 min, mouse monoclonal anti-bak antibody (5µg,/ml PBS-BSA) for 60 min, Rhodamine (TRITC)-conjugated AffiniPure Goat Anti-mouse IgG (H+L) in PBS-BSA for 60 min, after every step, wash 3–5 times with PBS. Store the glass slides at 4°C in the dark (16,17).
RESULTS AND DISCUSSION
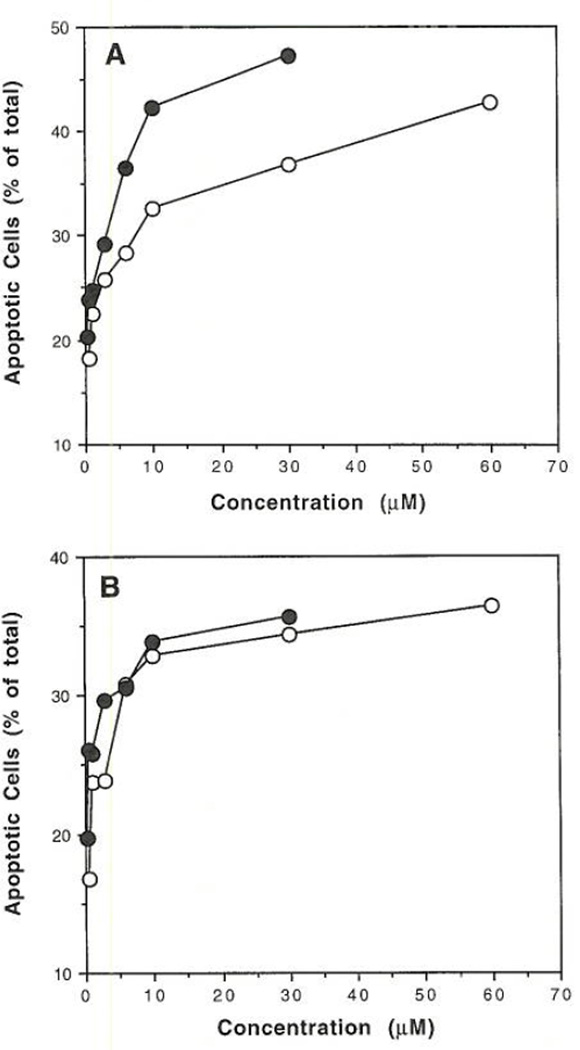
As shown in our previous study (2), flow cytometry (Figure 1) indicated that high concentrations (≥ 10 µM) of the A3 receptor agonists, IB-MECA and Cl-IB-MECA, caused apoptosis in HL-60 promyelocytic leukemia cells. In the present study a similar response was observed in U-937 histiocytic lymphoma cells. In Figure 1, the percent of apoptotic cells was estimated from the percent cells having hypodiploid DNA content in a DNA frequency histogram. Upon incubation with the agonist for 48 hours, a plateau in the percent of apoptotic cells within the range of 30–50% of total was observed beginning at approximately 10 µM for either agonist in HL-60 (Figure 1A) and in U-937 cells (Figure 1B).
FIG. 1.
Flow cytometric DNA analysis of A) HL-60 and B) U-937 cells determined by fluorescent cell sorting. DNA frequency histograms (similar to those shown in ref. 2) were generated using chromomycin A3-stained cells that had been pretreated for 48 h with various ADO agonists. The concentrations of A3 agonists (IB-MECA (○) or Cl-IB-MECA (●)) used were 0.3, 0.6, 1, 3, 6, 10, 30, and 60 µM. Apoptotic cell death was measured by the integration of the hypodiploid region of the histogram as a percentage of total cells.
The more highly A3 receptor selective agonist, Cl-IB-MECA, at lower concentrations (≤1 µM) did not significantly promote apoptosis, as determined using flow cytometry (Figure 1), or general cell death, as determined using trypan blue staining (Figure 2). In the latter assay, cell death was studied in HL-60 cells (Figures 2A,B) and in U-937 cells (Figures 2C,D) over a time course of 9 days. Cells in culture receiving no drug treatment proliferated geometrically until nearly the end of the experiment. In the presence of 0.01 or 1.0 µM Cl-IB-MECA alone, no deviation from this growth curve was observed (data not shown).
FIG. 2.
Effects A3 antagonists and low concentration of Cl-IB-MECA on growth curves for HL60 (A and B) and U-937 (C and D) cells. The antagonists used were MRS 1191 (A and C) and L-249313 (B and D), each at a concentration of 0.5 µM. The antagonist was added at day 0, and the cells remained in the same medium until the termination of the experiment. For each set of curves, control (--), antagonist alone (Δ), or antagonist in the presence of 10 nM (○) or 1 µM (●) Cl-IB-MECA are shown.
Cl-IB-MECA at a concentration of 20 or 40 µM caused death of >90% of HL-60 cells in cultutre after 4 days followed by a very slow increase in cell number. U-937 cells were slightly less sensitive to this agonist, with 40 µM Cl-IB-MECA required to obtain a similar effect (data not shown). Either of the selective A3 receptor antagonists, MRS 1191 or L-249313, at a concentration of 0.5 µM, alone caused a dramatic change in cell number during this time period. An initial reduction in the number of cells during the first 4–5 days was followed by highly impeded growth. However, in the presence of both an A3 antagonist and either 0.01 or 1.0 µM Cl-IB-MECA, the growth curves were nearly coincident with the control curves. Thus, this A3 agonist at sublethal concentrations, including the very low concentration of 10 nM, reversed the decrease of cell number induced by either of the selective antagonists in both HL-60 cells (Figures 2A,B) and U-937 cells (Figures 2C,D).
The evidence that DNA strand breaks, characteristic of apoptosis, are induced by the A3 antagonists was obtained using in-situ end-labeling in-situ of fragmented nuclear DNA by terminal deoxynucleotidyl transferase (TUNEL method, 15), which incorporates a fluorescent label at the 3′-ends. In HL-60 and U-937 cells (Table 1), a 24 h incubation with either 50 nM MRS 1191 or 50 nM L-249313 produced widespread (approximately 30–40% of the cells present) fluorescent labeling of apoptotic cells. In the control cultures and in those treated with 10 nM Cl-IB-MECA alone, spontaneous apoptosis occured in only 5–7% of the cells (Table 1). Coadministration of 10 nM Cl-IB-IVIECA with either antagonist suppressed the increase in apoptotic cells. The reversal of antagonist-induced apoptosis by the agonist is similar to that observed for the growth curves, except that both agonist and antagonist were applied at lower concentrations. High concentrations of Cl-IB-MECA (30 µM) also caused extensive DNA fragmentation in both HL-60 and U-937 cells, as indicated by the TUNEL method, with approximately 50% percent labeling.
TABLE 1.
Percentage of Apoptotic Cell in HL60 and U-937 after 48 Hour Treatment with A3 Antagonists (50 nM) and a Low Concentration of CI-IB-MECA (10 nM), Determined Using End-labeling in Situ of Fragmented Nuclear DNA by Terminal Deoxynucleotidyl Transferase
| % Labeled | ||||||
|---|---|---|---|---|---|---|
| Cell | Control | CI-IB-MECA | MRS 1191 | MRS 1191 + CI-IB-MECA |
L-249313 | L-249313 + CI-IB-MECA |
| HL-60 | 6.8 | 7.1 | 39.7 | 11.6 | 31.2 | 12.0 |
| U-937 | 5.1 | 5.6 | 27.3 | 10.4 | 30.8 | 10.9 |
Both small and large cells that stained positive were counted manually.
The mechanism of the apoptosis observed in response to either A3 antagonists or high concentrations of A3 agonists was investigated. Expression of the apoptosis-inducing protein bak in response to ADO receptor ligands was studied using an immunofluorescent method (16). Bak labeling was absent in control HL-60 cells (Figures 3A) or U-937 cells (Figures 3C), but clearly present in the cells treated for 24 hours with either L-249313 (Figure 3B) or MRS 1191 (Figure 3D). The expression of bak was also induced in both HL-60 cells and U-937 cells using the triazoloquinazoline A3 antagonist MRS 1220 (10 nM). Cl-IB-MECA, at 30 µM but not concentrations ≤ 1 µM, induced a level of expression of bak similar to those shown in Figures 3B and D in both HL-60 cells and in U-937 cells (data not shown). Various other cell lines were investigated for the expression of bak in response to the A3 agonist. Cl-IB-MECA at 10 µM was found to induce the expression of bak in MCF7 breast adenocarcinoma cells and 1321N1 human astrocytoma cells, but not in U373 astrocytoma cells (data not shown), so the upregulation of bak expression by A3 agonists appears to be a widespread but not universal phenomenon.
FIG. 3.
Fluorescent micrographs (40× enlarged) showing expression of bak in HL-60 (A and B) or U-937 cells (C and D), either in control cells (A and C) or following treatment with the A3 antagonists MRS 1191 (B) and L-249313 (D), each at a concentration of 0.5 µM. The incubation period with antagonist was 24 hours. The immunofluorescent staining used an anti-bak antibody.
The fact that A3 antagonists representing three diverse chemical classes elicited a biological effect, i.e. apoptosis, suggests that there exists a tonic state of activation of this receptor, and that this possible low level of receptor activation has a protective role. The tonic activation could arise conceivably through the action of endogenous ADO or via constitutive activity of the A3 receptor, which has not yet been demonstrated. If indeed a tonic A3 receptor activation exists, the apoptotic effects of A3 antagonists may simply be explained on the basis of a blockade of such adenosine mediated protective action. Moreover, it is speculated that the very high doses of agonist alone induce apoptosis through desensitization of this endogenously activated state. These hypotheses will require further investigation, which is presently limited by the lack of a high affinity antagonist radioligand for the A3 receptor. Other mediators of A3 receptor-induced cell death or protection may include the cytokines. Sajjadi et al. (19) have shown that the activation of A3 receptors with the agonist IB-MECA in U-937 cells inhibits the release of TNF-α, which in turn may induce apoptosis. Apoptosis is accompanied by the appearance of bak protein (18). Bak, a member of the bcl-2 family, was found to accelerate cell death in neurons deprived of nerve growth factor and may also be related to the effects of TNF-α (18). These results suggest that both A3 agonists and antagonists, by virtue of regulating programmed cell death, may have application in treating diseases either in which cytotoxicity is undesirable, such as neurodegeneration, or desirable, such as cancer and inflammation.
Abbreviations
- ADO, ADO; CGS 21680
2-[4-[(2-carboxyethyl)phenyl]ethylamino]-5′-N-ethylcarboxamidoADO
- Cl-IB-MECA
N6-(3-iodobenzyl)-2-chloro-ADO-5′-N-methyluronamide
- CPA
N6-cyclopentylADO
- IB-MECA
N6-(3-iodobenzyl)ADO-5′-N-methyluronamide
- L-249313
6-carboxymethyl-5,9-dihydro-9-methyl-2-phenyl-[1,2,4]-triazolo[5,1-a][2,7]-naphthyridine
- MRS 1191
3-ethyl 5-benzyl 2-methyl-6-phenyl-4-phenylethynyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate
- MRS 1220
9-chloro-2-(2-furyl)-5-phenylacetyl-amino[1,2,4]triazolo[1,5-c]quinazoline
- PBS
phosphate buffered saline
- TNF
tumor necrosis factor
- TUNEL
TdT-mediated dUTP Nick End Labeling
REFERENCES
- 1.Jacobson KA, Suzuki F. Drug Devel. Res. 1997 doi: 10.1002/(sici)1098-2299(199611/12)39:3/4<289::aid-ddr8>3.0.co;2-n. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kohno V, Sei Y, Koshiba M, Kim HO, Jacobson KA. Biochem. Biophys. Res. Commun. 1996;219:904–910. doi: 10.1006/bbrc.1996.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohno Y, Ji Xd, Mawhorter SD, Koshiba M, Jacobson KA. Blood. 1996;88:3569–3574. [PMC free article] [PubMed] [Google Scholar]
- 4.Shneyvays V, Jacobson KA, Shainberg A. J. Basic Clin. Physiol. Pharmacol. 1997 in press. [Google Scholar]
- 5.Brambilla R, Abbracchio MP, Ceruti S, Franceschi C, Malorni W, Giammarioli AM, Rainaldi G, von Lubitz DKJE, Jacobson KA, Lohse MJ, Klotz KN, Cattabeni F. Drug Devel. Res. 1996;37:181. [Google Scholar]
- 6.von Lubitz D, Lin RC, Popik P, Carter MF, Jacobson KA. Eur J. Pharmacol. 1994;263:59–67. doi: 10.1016/0014-2999(94)90523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbracchio MP, Malorni W, Ceruti S, Brambilla R, Barbieri D, Franceschi C, Giammarioli AM, Rainaldi G, von Lubitz DKJE, Jacobson KA, Cattabeni F. J. Neurosci. 1997 doi: 10.1006/bbrc.1997.7705. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strickler J, Jacobson KA, Liang BT. J. Clin. Invest. 1996;98:1773–1779. doi: 10.1172/JCI118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tracey WR, Magee W, Masamune H, Kennedy SP, Knight DR, Buchholz RA, Hill RJ. Cardiovasc. Res. l997 doi: 10.1016/s0008-6363(96)00240-4. in press. [DOI] [PubMed] [Google Scholar]
- 10.Rarton Y, Jiang J-l, Ji Xd, Melman N, Olah ME, Stiles GL, Jacobson KA. J. Med. Chem. 1996;39:2293–2301. doi: 10.1021/jm950923i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang J-l, van Rhee AM, Melman N, Ji Xd, Jacobson KA. J. Med. Chem. 1996;39:4667–4675. doi: 10.1021/jm960457c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim Y-C, Ji Xd, Jacobson KA. J. Med. Chem. 1996;39:4142–4148. doi: 10.1021/jm960482i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobson M, Chakravarty PK, Johnson RG, Norton R. Drug Devel. Res. 1996;37:131. [Google Scholar]
- 14.Gorczyca W, Gong J, Ardelt B, Traganos F, Darzynkiewicz Z. Cancer Res. 1993;53:3186–3192. [PubMed] [Google Scholar]
- 15.Cooke H. Trends in Biotech. 1992;10:23. doi: 10.1016/0167-7799(92)90162-o. [DOI] [PubMed] [Google Scholar]
- 16.Towbin H, Staehelin T, Gordon J. Proc. Natl. Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franke WW, Heid HW, Grund C, Winter S, Freudenstein C, Schmid E, Jarasch E, Keenan TW. J. Cell Biol. 1981;89:485–494. doi: 10.1083/jcb.89.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farrow SN, White JHM, Martinou T, Raven T, Pun K-T, Grinham CJ, Martinou J-C, Brown R. Nature. 1995;374:731–733. doi: 10.1038/374731a0. [DOI] [PubMed] [Google Scholar]
- 19.Sajjadi FG, Firestein GS. Biochim. Biophys. Acta. 1993;1179:105–107. doi: 10.1016/0167-4889(93)90077-3. [DOI] [PubMed] [Google Scholar]