Abstract
Disruptions in GABAergic neurotransmission have been implicated in numerous CNS disorders, including epilepsy and neuropathic pain. Selective inhibition of neuronal and glial GABA transporter subtypes may offer unique therapeutic options for regaining balance between inhibitory and excitatory systems. The ability of two GABA transport inhibitors to modulate inhibitory tone via inhibition of mGAT1 (tiagabine) or mGAT2/BGT-1 (N-[4,4-bis(3-methyl-2-thienyl)-3-butenyl]-4-(methylamino-4,5,6,7-tetrahydrobenzo[d]isoxazol-3-ol), also known as EF1502) was evaluated using an in vitro model of spontaneous interictal-like bursting (SB). SBs were recorded extracellularly in combined mEC-HC horizontal brain slices (400 μm; 31 ± 1°C) obtained from KA-treated rats. Slice recordings demonstrated that EF1502 exhibited a concentration-dependent reduction in SB frequency. EF1502 significantly reduced SB rate to 32% of control at the 30 μM concentration, while reducing the area and duration of SB activity to 60% and 46% of control, respectively, at the 10 μM concentration. In contrast, the GAT1 selective inhibitor tiagabine (3, 10, and 30 μM) was unable to significantly reduce the frequency of SB activity in the mEC, despite significantly reducing both the duration (51% of control) and area (58% of control) of the SB at concentrations as low as 3 μM. The ability of EF1502, but not tiagabine, to inhibit SBs in the mEC suggests that this in vitro model of pharmacoresistant SB activity is useful to differentiate between novel anticonvulsants with similar mechanisms of action and suggests a therapeutic potential for non-GAT1 transport inhibitors.
Keywords: kainic acid, tiagabine, EF1502, Glia, combined mEC-HC slices
1. Introduction
γ-Aminobutyric acid (GABA) is the primary inhibitory neurotransmitter in the CNS and GABA has been implicated in numerous neurological disorders, including epilepsy, migraine, anxiety, depression, bipolar disorder, and neuropathic pain (Frediani, 2004; Frediani et al., 2004; Sinkkonen et al., 2005; Blackburn-Munro and Erichsen, 2005; Guidotti et al., 2005; Perucca, 2005). Therapeutic agents developed for these conditions have therefore been designed to target one or more of the following mechanisms in hopes of increasing the efficacy of GABAergic inhibition: increased GABA release (vigabatrin, VGB), enhanced GABA synthesis (gabapentin, GBP), decreased GABA metabolism (VGB), modulation of the postsynaptic receptors (benzodiazepines, barbiturates), and decreased GABA uptake (tiagabine, TGB) (Schachter, 2001; Treiman, 2001; White, 2003).
The action of neuronally released GABA is terminated by the uptake of GABA into neurons and astrocytes by selective GABA transporters (White et al., 2002). To date, four distinct plasma membrane transporters (GAT1−4) have been identified using molecular cloning techniques, each with regionally characteristic distributions in the CNS: GAT1 is preferentially located on neurons with less expression on astrocytes, while mGAT2/BGT-1, mGAT3 (rat GAT-2), and mGAT4 (rat GAT-3) are located mainly on non-neuronal elements (Gadea and Lopez-Colome, 2001; Sarup et al., 2003a,b; Olsen et al., 2005).
Efforts to develop specific inhibitors of the GABA transporters have led to the identification of multiple compounds that have been shown to possess anticonvulsant activity in preclinical animal seizure models (Yunger et al., 1984; Krogsgaard-Larsen et al., 1987; Swinyard et al., 1991; White et al., 1993; Borden et al., 1994; Suzdak, 1995; Bolvig et al., 1999; Schousboe et al., 2004a; White et al., 2005). EF1502 (N-[4,4-bis(3-methyl-2-thienyl)-3-butenyl]-4-(methylamino-4,5,6,7-tetrahydrobenzo[d]isoxazol-3-ol), the N-substituted analogue of exo-THPO (4-amino-4,5,6,7-tetrahydrobenzo[d]isoxazol-3-ol) noncompetitively inhibits both GAT1 and GAT2 (White et al., 2005; Clausen et al., 2006). EF1502 is devoid of activity at GABAergic, adrenergic, and dopaminergic receptors (White et al., 2005). Isobolographic analysis of results from combination studies in animal seizure models demonstrated that EF1502 had broad spectrum anticonvulsant activity as well as synergistic interaction with tiagabine, without increasing the behavioral toxicity (White et al., 2005). This result suggests a functional contribution of the mGAT2/BGT-1 transporter and supports a potential utility of nonGAT1 inhibitors as anticonvulsants. While the mechanisms involved remain to be elucidated, both osmotic and inhibitory effects are likely to contribute to the anticonvulsant effects of EF1502, since mGAT2/BGT-1 transporter also regulates the uptake of betaine, the osmolyte involved in maintaining osmolarity during conditions of hypertonic stress (Yancey et al., 1982; Burg, 1995; Zhu and Ong, 2004 ). Indeed, exposure to hypertonic media induces increased expression of mGAT2/BGT-1 in cultured astrocytes (Olsen et al., 2005) and previous KA-induced seizures induce enhanced expression of mGAT2/BGT-1 in the hippocampus (Zhu and Ong, 2004).
Inhibitors of GABA transport have also demonstrated anticonvulsant activity in an in vitro model of electrographic burst activity in the mEC. (Pfeiffer et al., 1996) demonstrated that nipecotic acid and beta-alanine suppressed electrographic activity at high concentration (1−5 mM), while tiagabine failed to block the late recurrent discharges observed in the mEC following prolonged exposure to magnesium-free medium. It has thus been proposed that the late bursting activity observed in the superficial mEC of combined slices bathed in low magnesium media may represent an in vitro model for pharmacoresistant events, since it has been demonstrated to be refractory to several AEDs: including, phenytoin, carbamazepine, phenobarbital, valproic acid, and midazolam (Armand et al., 2000; Dreier et al., 1998; Zhang et al., 1995) as well as tiagabine (Pfeiffer., 1996). Low-Mg2+-induced epileptiform activity recorded in the entorhinal cortex of combined slices from naïve rats slowly transition from seizure-like events (SLEs) to recurring epileptiform discharges. However, the delay from pharmacosensitivity to pharmacoresistance does not make this an ideal model from a drug screening perspective. Recent work by Smith et al. (2007) comparing extracellular field responses and spontaneous bursting in combined mEC-HC brain slices made from KA-treated rats versus those from age-matched naïve animals suggests that utilizing slices from seizure-experienced rats may offer several unique advantages to their nonseized controls as an in vitro model system for detecting novel anticonvulsant therapies (see discussion). Combined mEC-HC brain slices generated from rats that have experienced prolonged KA-induced seizures (>3.5 hours) demonstrate spontaneous, interictal-like discharges in layer II of the mEC that are resistant to both phenytoin and carbamazepine while maintaining sensitivity to the novel anticonvulsant compound, retigabine (Smith et al., 2007). The goal of the present study was to evaluate the effects of the two different GABA uptake inhibitors, EF1502 and tiagabine, alone and in combination on the SB activity recorded in brain slices of rats that have experienced KA-induced seizures.
2. Methods
(2.1) Animal Treatments
Adult, male Sprague-Dawley rats (150−200g) were obtained from Charles River Laboratories (Raleigh, NC) and group housed with free access to food and water in an Institutional Animal Care and Use Committee (IACUC)-approved facility. Animals were maintained in a 12:12 hour light dark cycle. All experimental procedures were in accordance with the guidelines set by the National Institute of Health and received the approval of the Institutional Animal Care and Use Committee (IACUC) of the University of Utah.
(2.2) KA-induced status epilepticus
At the time of KA administration, animals were singly housed in plexiglass observation cages. Systemic injections of KA (Ocean Produce International, Shelbourne, Nova Scotia; 5mg/kg, i.p. per hour) were given to each animal until the onset of behavioral seizures was observed (e.g., jaw clonus, head nodding, etc). Once seizure activity was observed, KA-treated rats received 2.5 mg/kg every 30 minutes until onset of stage IV/V seizures, at which time KA administration was terminated (Hellier et al., 1998a). Seizures were classified using the Racine scale (Racine et al., 1972). The number, stage, and time of each seizure were documented following the onset of Stage IV/V seizure activity. Previous work from our laboratory has demonstrated that KA-treated rats receive an average (± SEM) dose of 14.3 ± 2.1 mg/kg KA (i.p.) and demonstrated 22.3 ± 3.7 stage 4/5 seizures during the 3.5 hours of behavioral seizure scoring (Smith et al., 2007). The average latency to stage 4/5 seizure onset observed in vivo was 121.9 ± 20.5 minutes following initial KA administration to these animals (Smith et al., 2007). After 3.5 hours of observation, all rats received 2−4 ml 0.9% saline (s.c.) to prevent dehydration and were returned to the animal facility.
(2.3) Brain Slice Preparation
One-two weeks after KA-administration, animals were deeply anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and brains were rapidly removed and placed in ice-cold (4°C), oxygenated artificial cerebrospinal fluid (ACSF) solution (95%O2-5%CO2) containing (in mM): sucrose (124), KCl (3), NaH2PO4 (1.2), MgSO4 (2), NaHCO3 (26), glucose (10), CaCl2 (2) (Barton et al., 2004). Brains were mounted to the stage of a motorized Vibroslicer (Campden Instruments, Lafayette, IN) and horizontal brain slices (400 μM) containing both the mEC and HC were prepared. The combined slices were then incubated at room temperature for at least one hour prior to recording in ACSF containing NaCl (124mM) rather than sucrose. The oxygenated ACSF bath solution had a pH of 7.33−7.43 (ideal, ∼7.37) and osmolality of 290−310 (ideal, ∼309).
(2.4) Field Potential Electrophysiology
Following the incubation, slices were placed in a submersion chamber constantly perfused (2.5−3.0 ml/min.) with hyperexcitable bath solution containing in (mM): NaCl (124), KCl (6), NaH2PO4 (1.2), MgSO4 (0.1), NaHCO3 (26), glucose (10), CaCl2 (2) and 10 μM glycine at 31±1°C. Borosilicate glass recording electrodes were pulled to 3−6 MΩ resistance using a micropipette electrode puller (Sutter) and then filled with 2.0 M NaCl solution for all experiments. A concentric bipolar stimulating electrode was used to evoke population field responses via angular bundle inputs to the superficial layers of the mEC. Signals were filtered at 3 KHz, sampled at 10 KHz, and acquired for computer storage using a Digidata 1200 AD Converter (Axon Instruments). All SBs were recorded using a Gould Strip Chart Recorder (Akron, OH) for off-line analysis and determination of the SB frequency (SBs per minute, or bpm) before, during and after application of each transport inhibitor (Smith et al., 2007).
(2.5) Drug Preparation and Bath Perfusion
EF1502 (N-[4,4-bis(3-methyl-2-thienyl)-3-butenyl]-4-(methylamino-4,5,6,7-tetrahydrobenzo[d]isoxazol-3-ol) was synthesized as described previously (Clausen et al., 2006). Tiagabine was generously provided by Sanofi Synthelabo (Brǿndby, Denmark). Stock solutions (30mM) of EF1502 and tiagabine were first solubilized in dimethylsulfoxide (DMSO). Stocks were then diluted to the appropriate concentrations (3−30 μM) in hyperexcitable ACSF solution. Slices were incubated in hyperexcitable ACSF for at least 30 minutes prior to bath application of the transport inhibitors to obtain stable pre-drug baseline responses. Bath perfusion of the compounds was only initiated once the SB rate demonstrated consistent frequency over at least 6 minutes and 40 seconds and the amplitude of SB's recorded in layer II of the mEC measured at least 0.2 mV in amplitude. The effects of EF1502 and tiagabine alone at 3, 10, and 30 μM were tested. For the combination study, EF1502 and tiagabine were evaluated at two concentrations; i.e., 3 μM + 3 μM and 10 μM +10 μM. Following bath application of test compounds, the perfusion medium was switched back to the same hyperexcitable ACSF used for the predrug wash. The averages of pre- and post-drug wash values for each measure were determined and results are presented as the percent of control values.
(2.6) Data Analysis and Statistics
Data were acquired and analyzed using the pCLAMP 8.0 and CLAMPFIT software (Axon Instruments, Union City, CA). Statistical comparisons and graphs were generated using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego California). The frequency of SBs (bursts/minute), the duration of SBs (msec), and area (mV*msec) of SBs were then normalized to each of the slice's control values determined in the absence of transport inhibitor(s). Results are thus presented as percent of control (%) and were compared using one-way ANOVA with Tukey's multiple comparison post hoc analysis. Differences in results were considered statistically significant at p≤0.05.
3. Results
To determine the effects of inhibiting the mGAT2/BGT-1 transporter on electrographic activity, SBs were recorded in the mEC of brain slices from KA-treated rats in aCSF containing elevated potassium (6 mM), lowered magnesium (0.1 mM) and glycine (10 μM). Under these hyperexcitable recording conditions, SBs are readily recorded (Fig.1). The averages of pre- and post-drug wash values for each measure were determined and results are presented as the percent of control values. The present study uses the model characterized by Hellier et al. (1998) which utilizes multiple low-dose (5 mg/kg per hour ) injections of KA titrated to each animal's onset to stage 4/5 seizure activity. This protocol has been shown to reliably produce chronically epileptic rats and resulted in little, if any, mortality (hellier and Dudek, 1999.) For example, during the time frame of these investigations our laboratory observed minimal mortality using this technique (<2%, or 3 / 204 KA-treated rats) using KA obtained from Ocean Produce International (OPIKA™).
Fig 1.

Traces of SB activity recorded in Layer II of the mEC of brain slices from rats onetwo weeks after prolonged KA-induced seizure activity in vivo. Field recordings following bath application of either 10 μM EF1502 (A) or 10 μM tiagabine (B) were compared to control traces for each slice in the absence of drug. GAT Inhibitors were bath perfused for at least 30 minutes prior to recording. At the 10 μM concentration, both EF1502 and tiagabine reduced SB area and duration. However, neither compound was able to reduce SB frequency at the 10 μM concentration.
Using Clampfit software, the duration of the SB (msec) was measured as the latency from initial negative offset to when the response returned to baseline. The area of the SB (mV*msec) was measured as the negative excursion of the SB from baseline, determined as the negative area under the curve by Clampfit. SB frequency (bpm) was calculated from the strip chart recording for each experiment as the total number of SBs occurring over the last 6 minutes and 40 seconds of the recording in each bath perfusion condition (predrug, drug, wash). For both EF1502 and tiagabine, effects were assessed at 3, 10, and 30 μM concentrations. Slices were allowed to incubate in the presence of the transport inhibitor(s) for at least 25 minutes prior to wash-out. The minimal criteria for ‘stability’ was defined after at least 25 minutes in the drug(s) as consistent SB frequencies (± 5 bpm) over at least two consecutive 3 minute and 20 seconds epochs.
The SB frequency observed was significantly reduced (p < 0.05, n=10) at the 30 μM concentration of EF1502 by 32%, but not at 10 μM (Fig 2A). At both concentrations, the SB frequency returned to predrug rates (9 bursts per minute) following the final washout in hyperexcitable bath solution (10 and 9 bursts per minute, respectively). Interestingly, bath application of 10 μM EF1502 significantly reduced both the area (Fig 3A) and the duration (Fig 4A) of the SB (46% and 60% reductions at 10 μM, respectively), but failed to significantly reduce these measures following perfusion with 30 μM EF1502. At 30 μM EF1502, the washout period was insufficient to allow the return of the area and duration measures to predrug values. In contrast, tiagabine did not significantly reduce SB frequency at any of the concentrations tested (Fig 2B), although both area (Fig 3B) and duration (Fig 4B) were significantly reduced at concentrations ≥ 3μM (58% and 51% at 3μM, respectively). Following washout of tiagabine, burst rates remained unchanged, but area and duration both failed to return to the predrug values in a dose-dependent manner, with the 10 μM treated slices recovering more efficiently than the 30 μM treated slices.
Fig 2.

The effect of EF1502 (A) and tiagabine (B) at 3, 10, or 30 μM to inhibit the frequency of SBs recorded in the mEC of combined slices from KA-treated rats. The number of slices tested for each compound were 5, 9, and 10 slices, respectively at these doses, for EF1502 (A) and 5, 11, and 3 slices, respectively, for tiagabine (B). When administered individually, only 30 μM EF1502 (n=10) was able to significantly reduce SB frequency. For combination studies, drugs were coadministered via bath perfusion at either 3 + 3 μM (n=5) or 10 + 10 μM (n=7). Interestingly, when bath perfused in combination at 10 μM each, the transport inhibitors caused a significant reduction in SB rate, suggesting a contribution of extrasynaptic transporters in the inhibitory effect observed. Results (averages ± SEM, n = 3−11) are presented as percent of baseline SB rate (%) and were compared using one-way ANOVA with Tukey's multiple comparison post hoc analysis. Differences in results were considered statistically significant at p≤0.05 and indicated by asterisks.
Figure 3.
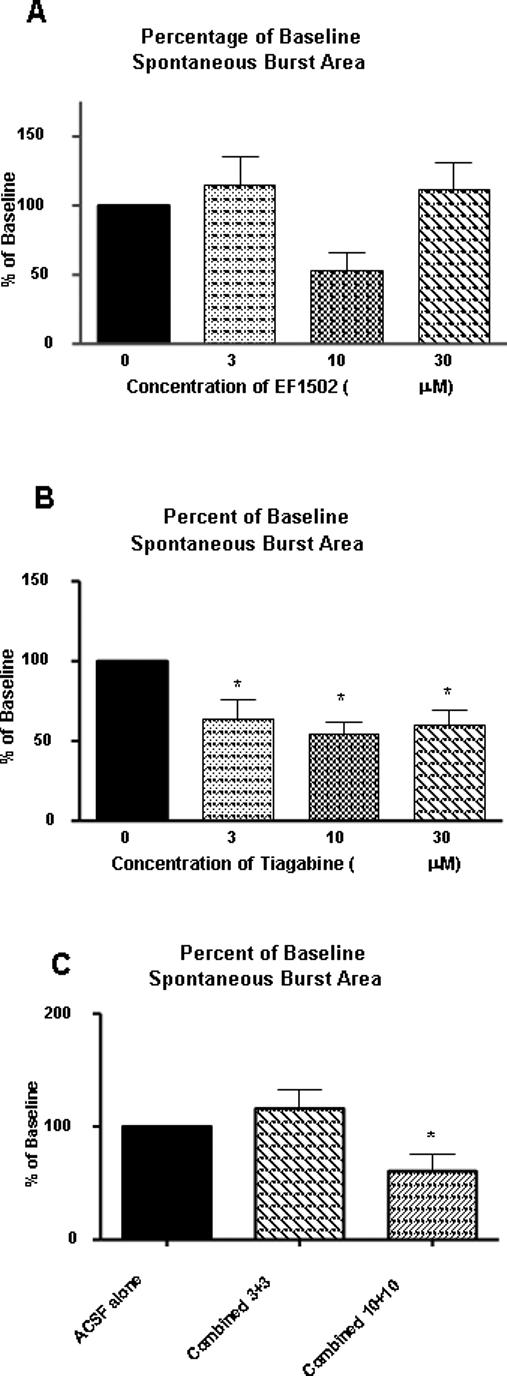
The effect of EF1502 (A) and tiagabine (B) at 3, 10, or 30 μM on area (mV/msec) of SBs recorded in layer II of the mEC in slices from KA-treated rats. Tiagabine demonstrated greater potency than EF1502, significantly reducing the SB area at doses as low as 3 μM. (C) Even so, following combined bath application of EF1502 and tiagabine, no significant reduction in SB area was observed. For combination studies, drugs were coadministered via bath perfusion at either 3 + 3 μM (n=5) or 10 + 10 μM (n=7). Results (averages ± SEM) are presented as percent of baseline SB area (%) and were compared using one-way ANOVA with Tukey's multiple comparison post hoc analysis. Differences were considered statistically significant at p≤0.05 and indicated by asterisks.
Figure 4.

The effect of EF1502 (A) and tiagabine (B) at 3, 10, or 30 μM on the duration of SBs recorded in layer II of the mEC of combined slices from KA-treated rats. Tiagabine demonstrated greater potency than EF1502, significantly reducing the SB area at doses as low as 3μM. (C) Combined application of 10 μM EF1502 and tiagabine resulted in significant reduction in SB duration, whereas lower concentrations had no effect on SB duration. For combination studies, drugs were coadministered via bath perfusion at either 3 + 3 μM (n=5) or 10 + 10 μM (n=7). Results (averages ± SEM) are presented as percent of baseline SB duration (%) and compared via one-way ANOVA with Tukey's multiple post hoc comparisons. Statistical significance was defined at p≤0.05 and indicated by asterisks.
Given the reported synergistic interactions observed following isobolographic analysis of the drug effects in animal seizure models in previous in vivo studies (White et al., 2005), the differential effects of EF1502 and tiagabine on SB in the present study led us to investigate the effect of combining EF1502 and tiagabine in vitro during bath perfusion of the combined mEC-HC slices.
Following bath perfusion of the bursting slices with the combination of 10 μM EF1502 and 10 μM tiagabine (n = 4 slices), significant reductions in SB frequency, area, and duration were observed (Fig. 2C-4C). Most notably, the average SB frequency was significantly reduced relative to the rate determined in hyperexcitable ACSF alone (18% reduction from control) (Fig. 2C). The effect of the drug combination on SB frequency is interesting given the observation that neither GAT inhibitor alone at the 10 μM concentration had an inhibitory effect on SB frequency. In addition to the reduction in SB frequency, the mean SB duration (Fig. 4C) and area (Fig. 3C) were both significantly reduced relative to the control values (33% and 30% reduction from control, respectively). Following washout in hyperexcitable bath solution, neither SB duration nor SB area recovered fully to the predrug values, although the SB frequency returned to the predrug rate, 11.7 versus 12.5 bursts per minute, respectively. No significant effects on SB frequency were observed when drugs were administered in combination at concentrations of 3μM (n = 3 slices), suggesting a concentration-dependent reduction in SB frequency (Fig. 2C). Furthermore, combining the low concentration (3μM) of EF1502 and tiagabine had somewhat facilitory effects on both SB area (Fig. 3C, 53% increase relative to baseline) and SB duration (Fig. 4C, 13%), but both enhancements failed to show statistical significance in light of the variability observed at the low dose. In both cases, the post-washout values for theses measures increased but failed to reach the predrug values during the final washout period. Figure 5 represents sample traces recorded in the presence of either 3 μM EF1502 + 3 μM tiagabine (Fig. 5A) or 10 μM EF1502 + 10 μM tiagabine (Fig. 5B) together with the examples of pre- and post-drug SB traces.
Figure 5.

SB traces recorded in layer II of the mEC following bath perfusion with either 3 μM EF1502 + 3 μM tiagabine (A) or 10 μM EF1502 + 10 μM tiagabine (B). The combination of GAT inhibitors were bath perfused at least 30 minutes prior to recording and compared to control traces for each slice in the absence of drug (average of pre-drug control and post-drug wash). Drugs were co-administered via bath perfusion at either 3 + 3 μM (n=5) or 10 + 10 μM (n=7). (A) Combined administration of 3 μM each had no significant effect on SB frequency, area or duration. (B) When administered at 10 μM each, EF1502 and tiagabine demonstrated a synergistic reduction in SB frequency and duration.
4. Discussion
The present study investigated the ability of two GABA transport inhibitors to modulate SB activity recorded in layer II of the mEC in combined mEC-HC slices obtained from KA-treated rats. Previous work from our laboratories have shown that the spontaneous bursts recorded in combined mEC-HC slices from KA-treated rats (Smith et al., 2007) can be established more rapidly than late recurrent discharges of the low-Mg+ model in naive rats, which can often exceed 1.5 hours. Furthermore, the spontaneous interictal bursts in slices from KA-treated rats may provide further insight into the transitions taking place in the animal in the initial weeks following prolonged seizure activity. Using the multiple, low-dose (5 mg/kg per hour) paradigm for injections of KA, the amount of KA administered is titrated to each individual animal's onset of stage 4/5 seizure activity to improve upon the limitations of the traditional model (Hellier et al., 1998b; Hellier and Dudek, 1999). This paradigm was shown to reliably produce chronically epileptic rats and resulted in little, if any, mortality (Hellier and Dudek, 1999). In fact, in rats treated with kainic acid purchased from Ocean Produce International (OPIKA) during the time frame of the present study, a mortality rate of approximately 1.5%, or 3 deaths out of 204 KA-treated rats was observed using in this multiple, low-dose KA paradigm.
It should be noted that only modest differences were observed in PHT, CBZ, and RGB effects between the KA-treated and control groups in Smith et al (2007). Nevertheless, this work demonstrated that slices obtained from KA-treated rats offer significant experimental advantages when compared to slices from naive rats. First, slices from KA-treated rats showed endogenous spontaneous discharge activity and prolonged evoked potentials indicative of hyperexcitability in baseline ACSF recording conditions. Second, under hyperexcitable bath conditions, the latency to spontaneous bursting was significantly shorter in slices from KA-treated rats versus controls. This data suggest that seizure-induced alterations in the mEC-HC circuit may facilitate the epileptogenic cascade as slices progress to pharmacoresistant electrographic activity within the mEC and support further development of this model for the differentiation of novel AEDs efficacious against drug-resistant electrographic activity in the mEC.
Additional studies from our laboratory have also suggested a functional role for mGAT2/BGT-1 among inhibitors of GAT1 and GAT2 for the treatment of seizure activity (White et al., 2005). In the present in vitro study, both tiagabine and EF1502 had inhibitory effects on the SB activity observed. Bath perfusion of slices with tiagabine, but not EF1502, significantly reduced both SB duration and SB area at concentrations as low as 3 μM, suggesting that tiagabine is more potent than EF1502. This finding is in agreement with the relative potency of the two inhibitors of GAT-1 as well as with their relative potencies as anticonvulsants (Sarup et al., 2003a,b; White et al., 2005; Clausen et al., 2006). Indeed, the potent inhibitory effects demonstrated by tiagabine on SB area and duration indicate that the selective inhibition of GAT-1 has important modulatory effects on electrographic events recorded in the mEC.
Perhaps the most interesting finding of the study was that when given in combination at concentrations that individually were ineffective at altering SB frequency, the transport inhibitors demonstrated a greater than additive (i.e. synergistic) inhibitory effect consistent with previous reports on their anticonvulsant profile (White et al., 2002; White et al., 2005). Immunohistochemical evidence suggests that the mGAT2/BGT-1 transporter is primarily localized to the cerebral cortex and hippocampus in monkeys (Zhu and Ong, 2004). Following KA-treatment, upregulation of the mGAT2/BGT-1 transporter in astrocytes within the degenerating CA subfields of the hippocampus have been also been reported in the rat (Borden et al., 1994; Zhu and Ong, 2004). Furthermore, Ahn et al. (1996) have provided additional evidence for the somatodendritic localization of mGAT2/BGT-1 from studies in cultured hippocampal neurons. Inhibition of mGAT2/BGT-1 could potentially increase extrasynaptic levels of GABA sufficiently enough to modulate extrasynaptic GABAA and GABAB receptors to enhance inhibitory tone (Schousboe et al., 2004b; White et al., 2005) . Such changes could be functionally expressed through regulation of SB activity recorded in vitro. Thus, the synergistic effects observed in the present in vitro mEC-slice preparation support the hypothesis that effects at extrasynaptic GABA transporters could play an important role in controlling of extrasynaptic levels of GABA. Given the enhanced expression of the mGAT2/BGT-1 transporters within the mEC-HC circuit in rats one week after prolonged KA-induced seizure activity (unpublished results), it is probable that mGAT2/BGT-1 is involved in the transition to epileptogenesis and thus represents a potential target for therapeutic intervention. Collectively, these findings support the continued evaluation and development of selective inhibitors for mGAT2/BGT-1.
Acknowledgements
The authors would like to acknowledge the financial support provided by the Lundbeck Foundation, The Danish Medical Research Council 22-03-0250 (AS), NO1-NS-4-2359 (HSW), 1-R21-NS-49624-01(HSW & KSW), and RO1-NS-44210 (KSW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barton ME, White HS, Wilcox KS. The effect of CGX-1007 and CI-1041, novel NMDA receptor antagonists, on NMDA receptor-mediated EPSCs. Epilepsy Res. 2004;59:13–24. doi: 10.1016/j.eplepsyres.2003.12.011. [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G, Erichsen HK. Antiepileptics and the treatment of neuropathic pain: evidence from animal models. Curr Pharm Des. 2005;11:2961–2976. doi: 10.2174/1381612054865000. [DOI] [PubMed] [Google Scholar]
- Bolvig T, Larsson OM, Pickering DS, Nelson N, Falch E, Krogsgaard-Larsen P, Schousboe A. Action of bicyclic isoxazole GABA analogues on GABA transporters and its relation to anticonvulsant activity. Eur J Pharmacol. 1999;375:367–374. doi: 10.1016/s0014-2999(99)00263-0. [DOI] [PubMed] [Google Scholar]
- Borden LA, Murali Dhar TG, Smith KE, Weinshank RL, Branchek TA, Gluchowski C. Tiagabine, SK&F 89976-A, CI-966, and NNC-711 are selective for the cloned GABA transporter GAT-1. Eur J Pharmacol. 1994;269:219–224. doi: 10.1016/0922-4106(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Clausen RP, Frolund B, Larsson OM, Schousboe A, Krogsgaard-Larsen P, White HS. A novel selective gamma-aminobutyric acid transport inhibitor demonstrates a functional role for GABA transporter subtype GAT2/BGT-1 in the CNS. Neurochem Int. 2006;48:637–642. doi: 10.1016/j.neuint.2005.12.031. [DOI] [PubMed] [Google Scholar]
- Frediani F. Anticonvulsant drugs in primary headaches prophylaxis. Neurol Sci. 2004;25(Suppl 3):S161–166. doi: 10.1007/s10072-004-0278-4. [DOI] [PubMed] [Google Scholar]
- Frediani T, Pelliccia A, Aprile A, Ferri E, Lucarelli S. Partial idiopathic epilepsy: recovery after allergen-free diet. Pediatr Med Chir. 2004;26:196–197. [PubMed] [Google Scholar]
- Gadea A, Lopez-Colome AM. Glial transporters for glutamate, glycine, and GABA: II. GABA transporters. J Neurosci Res. 2001;63:461–468. doi: 10.1002/jnr.1040. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Dong E, Grayson DR, Veldic M, Zhang X, Costa E. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology (Berl) 2005;180:191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Dudek FE. Spontaneous motor seizures of rats with kainate-induced epilepsy: effect of time of day and activity state. Epilepsy Res. 1999;35:47–57. doi: 10.1016/s0920-1211(98)00127-2. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Res. 1998a;31:73–84. doi: 10.1016/s0920-1211(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Hellier JL, Patrylo PR, Buckmaster PS, Dudek FE. Recurrent spontaneous motor seizures after repeated low-dose systemic treatment with kainate: assessment of a rat model of temporal lobe epilepsy. Epilepsy Research. 1998b;31:73–84. doi: 10.1016/s0920-1211(98)00017-5. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P, Falch E, Larsson OM, Schousboe A. GABA uptake inhibitors: relevance to antiepileptic drug research. Epilepsy Res. 1987;1:77–93. doi: 10.1016/0920-1211(87)90012-x. [DOI] [PubMed] [Google Scholar]
- Olsen M, Sarup A, Larsson OM, Schousboe A. Effect of hyperosmotic conditions on the expression of the betaine-GABA-transporter (BGT-1) in cultured mouse astrocytes. Neurochem Res. 2005;30:855–865. doi: 10.1007/s11064-005-6879-3. [DOI] [PubMed] [Google Scholar]
- Perucca E. An introduction to antiepileptic drugs. Epilepsia. 2005;46(Suppl 4):31–37. doi: 10.1111/j.1528-1167.2005.463007.x. [DOI] [PubMed] [Google Scholar]
- Pfeiffer M, Draguhn A, Meierkord H, Heinemann U. Effects of gamma-aminobutyric acid (GABA) agonists and GABA uptake inhibitors on pharmacosensitive and pharmacoresistant epileptiform activity in vitro. Br J Pharmacol. 1996;119:569–577. doi: 10.1111/j.1476-5381.1996.tb15710.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ, Gartner JG, Burnham WM. Epileptiform activity and neural plasticity in limbic structures. Brain Res. 1972;47:262–268. doi: 10.1016/0006-8993(72)90268-5. [DOI] [PubMed] [Google Scholar]
- Sarup A, Larsson OM, Schousboe A. GABA transporters and GABA-transaminase as drug targets. Curr Drug Targets CNS Neurol Disord. 2003a;2:269–277. doi: 10.2174/1568007033482788. [DOI] [PubMed] [Google Scholar]
- Sarup A, Larsson OM, Bolvig T, Frolund B, Krogsgaard-Larsen P, Schousboe A. Effects of 3-hydroxy-4-amino-4,5,6,7-tetrahydro-1,2-benzisoxazol (exo-THPO) and its N-substituted analogs on GABA transport in cultured neurons and astrocytes and by the four cloned mouse GABA transporters. Neurochem Int. 2003b;43:445–451. doi: 10.1016/s0197-0186(03)00033-0. [DOI] [PubMed] [Google Scholar]
- Schachter SC. Pharmacology and clinical experience with tiagabine. Expert Opin Pharmacother. 2001;2:179–187. doi: 10.1517/14656566.2.1.179. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Larsson OM, Sarup A, White HS. Role of the betaine/GABA transporter (BGT-1/GAT2) for the control of epilepsy. Eur J Pharmacol. 2004a;500:281–287. doi: 10.1016/j.ejphar.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Schousboe A, Sarup A, Larsson OM, White HS. GABA transporters as drug targets for modulation of GABAergic activity. Biochem Pharmacol. 2004b;68:1557–1563. doi: 10.1016/j.bcp.2004.06.041. [DOI] [PubMed] [Google Scholar]
- Sinkkonen ST, Rabe H, Luddens H, Korpi ER. Evidence for a reduction of coupling between GABAA receptor agonist and ionophore binding sites by inorganic phosphate. Neurochem Res. 2005;30:1471–1482. doi: 10.1007/s11064-005-8824-x. [DOI] [PubMed] [Google Scholar]
- Smith M, Adams A, Saunders GW, White H, Wilcox KS. Phenytoin- and carbamazepine-resistant spontaneous bursting in rat entorhinal cortex is blocked by retigabine in vitro. Epilepsy Res. 2007:1–10. doi: 10.1016/j.eplepsyres.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Swinyard EA, White HS, Wolf HH, Bondinell WE. Anticonvulsant profiles of the potent and orally active GABA uptake inhibitors SK&F 89976-A and SK&F 100330-A and four prototype antiepileptic drugs in mice and rats. Epilepsia. 1991;32:569–577. doi: 10.1111/j.1528-1157.1991.tb04694.x. [DOI] [PubMed] [Google Scholar]
- Treiman DM. GABAergic mechanisms in epilepsy. Epilepsia. 2001;42(Suppl 3):8–12. doi: 10.1046/j.1528-1157.2001.042suppl.3008.x. [DOI] [PubMed] [Google Scholar]
- White HS. Mechanism of action of newer anticonvulsants. J Clin Psychiatry. 2003;64(Suppl 8):5–8. [PubMed] [Google Scholar]
- White HS, Hunt J, Wolf HH, Swinyard EA, Falch E, Krogsgaard-Larsen P, Schousboe A. Anticonvulsant activity of the gamma-aminobutyric acid uptake inhibitor N-4,4-diphenyl-3-butenyl-4,5,6,7-tetrahydroisoxazolo[4,5-c]pyridin-3-ol. Eur J Pharmacol. 1993;236:147–149. doi: 10.1016/0014-2999(93)90238-d. [DOI] [PubMed] [Google Scholar]
- White HS, Sarup A, Bolvig T, Kristensen AS, Petersen G, Nelson N, Pickering DS, Larsson OM, Frolund B, Krogsgaard-Larsen P, Schousboe A. Correlation between anticonvulsant activity and inhibitory action on glial gamma-aminobutyric acid uptake of the highly selective mouse gamma-aminobutyric acid transporter 1 inhibitor 3-hydroxy-4-amino-4,5,6,7-tetrahydro-1,2-benzisoxazole and its N-alkylated analogs. J Pharmacol Exp Ther. 2002;302:636–644. doi: 10.1124/jpet.102.034819. [DOI] [PubMed] [Google Scholar]
- White HS, Watson WP, Hansen SL, Slough S, Perregaard J, Sarup A, Bolvig T, Petersen G, Larsson OM, Clausen RP, Frolund B, Falch E, Krogsgaard-Larsen P, Schousboe A. First demonstration of a functional role for central nervous system betaine/{gamma}-aminobutyric acid transporter (mGAT2) based on synergistic anticonvulsant action among inhibitors of mGAT1 and mGAT2. J Pharmacol Exp Ther. 2005;312:866–874. doi: 10.1124/jpet.104.068825. [DOI] [PubMed] [Google Scholar]
- Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN. Living with water stress: evolution of osmolyte systems. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- Yunger LM, Fowler PJ, Zarevics P, Setler PE. Novel inhibitors of gamma-aminobutyric acid (GABA) uptake: anticonvulsant actions in rats and mice. J Pharmacol Exp Ther. 1984;228:109–115. [PubMed] [Google Scholar]
- Zhu XM, Ong WY. Changes in GABA transporters in the rat hippocampus after kainate-induced neuronal injury: decrease in GAT-1 and GAT-3 but upregulation of betaine/GABA transporter BGT-1. J Neurosci Res. 2004;77:402–409. doi: 10.1002/jnr.20171. [DOI] [PubMed] [Google Scholar]


