Abstract
Background
Several cohort studies report associations between chronic exposure to ambient fine particles (PM2.5) and cardiovascular mortality. Uncertainty exists about biological mechanisms responsible for this observation, but systemic inflammation has been postulated. In addition, the subgroups susceptible to inflammation have not been fully elucidated.
Methods
We investigated whether certain subgroups are susceptible to the effects of long-term exposure to PM2.5 on C-reactive protein (CRP), a marker of inflammation directly linked to subsequent cardiovascular disease. We used data from the SWAN cohort of 1,923 mid-life women with up to five annual repeated measures of CRP. Linear mixed and GEE models accounting for repeated measurements within an individual were used to estimate the effects of prior-year PM2.5 exposure on CRP. We examined CRP as a continuous and as binary outcome for CRP greater than 3 mg/l, a level of clinical significance.
Results
We found strong associations between PM2.5 and CRP among several subgroups. For example a 10 µg/m3 increase in annual PM2.5 more than doubled the risk of CRP greater than 3 mg/l in older diabetics, smokers and the unmarried. Larger effects were also observed among those with low income, high blood pressure, or who were using hormone therapy, with indications of a protective effects for those using statins or consuming moderate amounts of alcohol.
Conclusions
In this study, we observed significant associations between long-term exposure to PM2.5 and CRP in several susceptible subgroups. This suggests a plausible pathway by which exposure to particulate matter may be associated with increased risk of cardiovascular disease.
Keywords: air pollution, PM2.5, C-reactive protein, cardiovascular diseases, susceptibility
1. Introduction
Studies of cohorts followed over several years have reported associations between long-term exposure to ambient fine particles (PM2.5 or particulate matter less than 2.5 microns) and cardiovascular disease (Lepeule et al., 2012; Lipsett et al., 2011; Miller et al., 2007; Ostro et al., 2010; Pope et al., 2002; Puett et al., 2009). Researchers have posited several mechanisms by which inhalation of fine particles could exacerbate cardiovascular disease (CVD) including systemic inflammation and oxidative stress (Brook et al., 2010). One marker indicating the presence of systemic inflammation is high-sensitivity C-reactive protein (CRP), an acute phase protein produced in the liver. In over 30 studies, CRP has been linked with subsequent CVD and death in both healthy men and women and in those with pre-existing cardiovascular disease (Calabro et al., 2009; Cushman et al., 2005; Pai et al., 2004; Ridker et al., 2008; Ridker et al., 2002).
Several epidemiological studies have reported associations between PM2.5 and cardiovascular biomarkers of inflammation, such as CRP and fibrinogen (Brook et al., 2010). To date, however, many of the existing studies of particulate matter and markers of CVD have been cross-sectional, with the attendant lack of temporality of exposure and the potential for residual confounding (Hoffmann et al., 2009). Also, existing prospective epidemiologic studies of CRP using repeated measures have generally examined only short-term exposures of several days to weeks (Chuang et al., 2007). While these studies are informative, the implications of chronic exposure to air pollution for markers of inflammation need to be examined, because longer-term exposures (i.e., one year or more) have been shown to have much larger effects on mortality than short-term (i.e., daily or multi-day) exposures (Brook et al., 2010), Similarly, studies that have examined the extent to which personal characteristics, such as body mass index (BMI) and pre-existing disease, modify the effects of air pollution on biomarkers of CVD have only examined relatively acute exposures consisting of a few days (Zeka et al., 2006a). Thus, the extent to which chronic exposure may differentially impact CRP in potentially sensitive subgroups is unknown.
Data from a prospective cohort enrolled in the Study of Women’s Health Across the Nation (SWAN) present a unique opportunity to examine the long-term chronic effects of air pollution exposure on inflammatory markers and to investigate the existence of susceptible subgroups. In this study commencing in 1996–1997, cardiovascular biomarkers of pre- and early peri-menopausal women have been collected on an annual basis. These repeated measures facilitate analyses of individual-level effects of long-term pollution while reducing the potential for confounding by other measured or unmeasured variables. In this paper, we examined effect modification to determine whether personal characteristics altered the associations between long-term exposure to PM2.5 and CRP.
2. Materials and Methods
2.1 Participants
SWAN is a multi-center, multi-racial/ethnic, longitudinal study initially designed to characterize the physical and psychosocial changes that occur during menopause. Details of the study design and recruitment methodology have been previously published (Matthews et al., 2007; Sowers et al., 2000). We used data from six of the seven SWAN study centers located in the metropolitan areas of Chicago, Detroit, Los Angeles, Newark, Pittsburgh and Oakland. A total of 2849 women aged 42 through 52 years from these sites were enrolled at the baseline visit. To ensure diversity, each SWAN site recruited a race/ethnic group in addition to white women. Specifically, African Americans were recruited from the sites at Chicago, IL, Detroit, MI and Pittsburgh, PA; Hispanics (including women of Central American, Mexican and Caribbean origin) were recruited in Newark, NJ; Chinese were recruited in Oakland, CA and Japanese were recruited in Los Angeles, CA. Women were enrolled from a wide region around each of the study centers.
At enrollment, women were required to have an intact uterus and at least one ovary and could not be pregnant or breast-feeding. All participants were still menstruating, and women who used oral contraceptives or hormone therapy in the prior three months were excluded from the study. Data on demographics and personal health habits and risk factors were recorded at the baseline interview (three years prior to the start of our study period) and at every annual clinic visit. All participating institutions obtained approval from institutional review boards, and all women signed informed consent forms prior to participation.
Because routine national monitoring of PM2.5 began around 1998, the current study began at the third annual clinic visit, mostly during the years 1999–2000, so that a full year of exposure to PM2.5 prior to the first clinic visit could be measured. We focused on one year of exposure since the cohort studies linking PM2.5 to mortality use one or more years or exposure (Lipsett et al., 2011; Miller et al., 2007; Pope et al., 2002). Our study followed women through the seventh clinic visit, during the years 2004–2005, after which CRP data were not available. Women returned every year for an interview and clinic visit except at the Newark site where blood work was only collected for the first three years. At each SWAN site, a residential history was maintained for participants from the beginning of the study to the most recent visit. As a result, the participant’s geo-coded residence was used to assign exposure. The location of each residence was randomly moved up to 400 feet (about a block) to ensure confidentiality.
We excluded women and data with the following conditions: (1) no laboratory data; (2) observations with CRP > 10 mg/l, because this would likely be the result of an acute infection and other pathologies and not necessarily predictive of CVD; (3) no geocodable address, precluding assignment of exposure; (4) history of previous heart attack or stroke (with data also censored after an incident event during the study); (5) residence further than 20 km from a PM2.5 monitor to reduce exposure misclassification; and (6) missing information on education, smoking status, BMI, alcohol consumption within the last 24 hours, or PM2.5 exposure in the last year. Several variables were updated at the annual re-interviews, including BMI, age, smoking status, alcohol use, medication use, disease status, whether alcohol was consumed within the previous 24 hours of the laboratory visit and marital status.
2.2. CRP Assay
A 12-hour fasting blood sample (in menstruating women, targeted to the follicular phase of the menstrual cycle – days 2 to 5) was obtained at the annual visit, usually before 10 AM. If a timed sample could not be obtained, a fasting sample was taken within 90 days of the annual visit. Samples were stored at 4°C until separated and then were frozen at −80°C and shipped on dry ice to the Medical Research Laboratory (Lexington, KY), which is certified by the National Health, Lung, and Blood Institute, Centers for Disease Control and Prevention, Part III program. High sensitivity CRP was quantitated using an ultrasensitive rate immunonephelemetric method (hs-CRP on BN 100, Dade-Behring, Marburg, Germany).
2.3. Pollution Exposure Assessment
Daily PM2.5 data were acquired from the US EPA Air Quality System Data Mart (US Environmental Protection Agency. Air Quality System Data Mart [internet database] available at http://www.epa.gov/ttn/airs/aqsdatamart) (EPA, 2009). Most monitors provided data either every third or sixth day, with a few sites providing data on a daily schedule. Exposures were only assigned to participants with residences within 20 km of a monitor. Annual averages of PM2.5 utilized readings from the 360 days prior to the day of the SWAN blood draw. For each person, 12 thirty-day averages were calculated for the periods prior to the laboratory visit, with the requirement that at least three readings be available for each 30-day period. These monthly metrics were then averaged to create a cumulative average over the prior year. Only averages consisting of at least 10 of 12 30-day averages qualified for inclusion. For participants with multiple monitors within 20 km from the residence, selection was based on criteria balancing the number of women’s clinic visits that could be included versus the distance to the monitor. Preference was typically given to the closer monitor but, as an example, if a monitor was located twice as far away as the closest monitor, it would be chosen if it more than doubled the number of clinic visits that could be included in the study. Information on residential location was obtained from the annual re-interview. If a participant moved during the study period and a date of relocation was provided, PM2.5 was determined at each residential location pro-rated for the time at each residence. If there was a move but no date was provided, it was assumed that the participant spent equal time at each address.
2.4. Statistical Methods
Two different analyses were undertaken. First, to examine the effects of PM2.5 on CRP levels, we used a longitudinal linear mixed regression with person-specific random intercepts, with repeated measures and an autoregressive error term, an approach similar to that of Matthews et al. (Matthews et al., 2007). Thus, for each of the five blood draws, a unique prior one-year exposure to PM2.5 was assigned enabling us to focus on within-person changes and minimize the influence of potential confounders. CRP was log-transformed to approximate a more normal distribution. Besides pollution, the basic regression model included the following covariates: time-invariant binary variables for study site, race/ethnicity and education (having a college degree or more versus not), and time-varying variables for age, BMI, smoking status (current or not) at each clinic visit and whether alcohol was consumed in the 24-hours prior to the clinic visit.
We then ran a number of stratified analyses to examine possible effect modifiers. Stratifications were created for several socioeconomic measures that might directly impact susceptibility or be associated with pollution “hot spots” such as traffic and point sources of emissions (Brulle and Pellow, 2006; Mohai et al., 2009). These measures include education (high school graduation or less versus college degree or more), race/ethnicity, family income (below $50,000 versus greater than or equal $100,000 was used to provide contrast) and marital status (unmarried or divorced versus married/partnered). We also subdivided health indicators, conditions, and behaviors that might enhance inflammation, specifically age at baseline (42 to 47.5 versus 47.51 to 52 years), BMI at blood draw (below versus equal to and above 25 kg/m2), diabetes, hypertension (using antihypertensive medication), current menopause transition stage (early and late peri-menopausal versus post-menopausal; premenopausal omitted due to rarity), and current smoking. Particular interest was paid to the use of possible pharmacological inhibitors of inflammation, specifically statins, hormone therapy, and alcohol (none versus 2 or more glasses/wk; these were the only classifications available for all of the years). Lastly, distance from residence to pollution monitor (above versus equal or below the median 8.96 km) was considered as a potential indicator of exposure misclassification.
Stratifying variables were omitted from these models. Effect modification was determined to exist if either the difference in effects between two subgroups of each of the personal characteristic variables was statistically significant at p < 0.05 using a t-test or the effect estimate for one group was more than two times that of another (Schenker and Gentleman, 2001; Zeka et al., 2006b). The latter was used because some of the subgroups had small sample sizes and therefore larger confidence intervals, which reduced power to detect statistically significant difference. Because we observed, in our initial analysis, a stronger association between PM2.5 and CRP for women with higher ages (47.51 through 52 years at baseline), we conducted an additional examination of effect modification among this higher age subgroup. For these analyses, continuous age was omitted from the regression model. These analyses were conducted using xtregar in Stata, version 12 (StataCorp LP, 2011).
In the final set of analyses, we examined the probability of a CRP greater than 3 mg/l using repeated measures logistic regression. The 3 mg/l cutpoint was used because this level is often used as a clinical indicator for medical intervention (Ridker, 2003). Similar covariates and stratification by subgroups were used as described above. We also tested for effect modification due to various characteristics using the sample of higher aged participants. We used generalized estimated equations (xtgee in Stata) specified with a Poisson family, log link model with robust variance estimators. Such a model directly provides relative risks and unbiased standard errors when the outcome is common (Yelland et al., 2011; Zou and Donner, 2011).
Linear regression results were presented as the percent change in CRP and the binary model results were presented as the percent change in probability of CRP above 3 mg/l where both were calculated by [exp(10*β) − 1] × 100. All effect estimates were evaluated for a 10 µg/m3 increase in annual PM2.5 exposure.
3. Results
After the exclusions from the study sample described above, a total of 1,923 women with 6,047 observations were available for analysis, although the counts among subgroups varied due to missing variables. For the full sample of those included in the study, approximately 66% had three or more annual clinic visits with blood draws and 17% attended all clinic visits possible in our study period. The average of the annual mean PM2.5 ranged from 12.1 µg/m3 in Oakland to 20.9 µg/m3 in Los Angeles, while the other 4 sites ranged from 15.4 to 16.2 µg/m3 (Table 1). Median CRP ranged from 1.9 mg/l at the Oakland site to 3.4 mg/l at the Detroit site with an overall mean of 2.3 mg/l. Twenty-nine percent of study observations had a CRP level above 3 mg/l. At the third clinic visit (the first year of our study) 60% had a BMI of 25 or above, 5% reported taking statins, 9% were diabetic and 12% were smokers (Table 2)..
Table 1.
Grand Means and Distributions of PM2.5 and CRP Concentrations by Study Site Over Visits 3 Through 7.
| Study Site | PM2.5 Annual Average (µg/m3) | CRP (mg/l) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | mean | sd | min | max | IQR | mean | sd | min | max | median | |
| Oakland | 1181 | 12.1 | 1.2 | 8.3 | 18.3 | 1.6 | 1.7 | 2.0 | 0.04 | 10 | 0.9 |
| Detroit | 1041 | 15.4 | 1.7 | 9.4 | 20.5 | 1.8 | 3.4 | 2.7 | 0.05 | 10 | 2.7 |
| Newark | 286 | 16.0 | 1.3 | 11.8 | 18.8 | 1.8 | 2.8 | 2.5 | 0.11 | 10 | 1.9 |
| Pittsburgh | 1109 | 16.0 | 1.6 | 10.2 | 23.2 | 1.8 | 2.7 | 2.4 | 0.06 | 10 | 1.9 |
| Chicago | 838 | 16.2 | 1.3 | 10.8 | 20.4 | 1.9 | 2.7 | 2.5 | 0.09 | 10 | 1.8 |
| Los Angeles | 1592 | 20.9 | 2.3 | 7.7 | 26.1 | 3.2 | 1.6 | 2.0 | 0.04 | 10 | 0.8 |
Note: sd = standard deviation; IQR = interquartile range
Table 2.
Descriptive Statistics and Estimated Effect of Prior 12-month PM2.5 for Full Cohort and Subgroups (n = 1923)
| Characteristic/subgroup | Effect and 95% CI (% per 10 µg/m3) |
Proportion in subgroup# |
|||
|---|---|---|---|---|---|
| Central | Low | High | |||
| Full cohort | 25.5 | 10.2 | 42.9 | 1.00 | |
| Age, baseline (yrs)* | Low (age = 42 – 47.5) | 10.6 | −6.4 | 30.7 | 0.67 |
| High (age = 47.6 – 52) | 51.8 | 21.6 | 89.5 | 0.33 | |
| Alcohol | None | 25.5 | 4.0 | 51.4 | 0.53 |
| Some (2+ drinks/week) | 20.2 | −9.3 | 59.3 | 0.22 | |
| BMI (kg/m2) | Low (<25) | 22.4 | −2.0 | 52.8 | 0.40 |
| High (≥25) | 27.4 | 8.8 | 49.1 | 0.60 | |
| Diabetes* | No | 22.5 | 7.1 | 40.1 | 0.91 |
| Yes | 72.1 | 2.9 | 187.8 | 0.09 | |
| Distance to monitor* | Close (≤9.0 km) | 43.2 | 15.5 | 77.5 | 0.50 |
| Far (> 9.0 km) | 17.1 | −1.0 | 38.5 | 0.50 | |
| Education | Low (HSG or less) | 55.7 | 13.9 | 112.7 | 0.20 |
| High (College or more) | 31.2 | 8.8 | 58.2 | 0.48 | |
| HBP* | No | 15.5 | −1.8 | 35.9 | 0.69 |
| Yes | 46.6 | 17.0 | 83.8 | 0.31 | |
| HRM Use* | No | 14.0 | −1.6 | 32.0 | 0.81 |
| Yes | 41.0 | 6.0 | 87.6 | 0.19 | |
| Annual household income* | Low (< $50k) | 36.6 | 4.4 | 78.9 | 0.41 |
| High (≥$100k) | 6.5 | −15.2 | 33.7 | 0.32 | |
| Married/partnered* | No | 37.8 | 6.2 | 78.8 | 0.30 |
| Yes | 17.7 | 1.1 | 36.9 | 0.70 | |
| Menopausal stage* | Peri | 7.4 | −12.0 | 31.1 | 0.56 |
| Post | 49.7 | 19.7 | 87.2 | 0.21 | |
| Current Smoker | No | 25.3 | 9.0 | 44.0 | 0.88 |
| Yes | 24.8 | −14.9 | 83.2 | 0.12 | |
| Statins* | No | 21.7 | 6.3 | 39.3 | 0.95 |
| Yes | 11.2 | −34.1 | 87.6 | 0.05 | |
Note:
statistics based on the first year of the study unless indicated otherwise;
Effect modification indicated;
BMI = body mass index, HSG = high school graduate, HBP = high blood pressure, HRM = hormone therapy.
Regression model includes prior 12 months PM2.5, BMI, binary variables for smoker, age at clinic visit, alcohol use in the previous 24 hours, college or more education, site and race/ethnicity.
In the analysis of the full cohort, a 10 µg/m3 increment in annual PM2.5 exposure was associated with a 25.5% (95% CI = 10.2, 42.9) increase in CRP (note: all effect estimates were evaluated for a change in annual PM2.5 from 0 to 10 µg/m3) (Table 2). For many of the subgroups, associations between PM2.5 and CRP were observed (Table 2 and Figure 1). The strongest relation to CRP was observed for diabetics (72.1%, 95% CI =2.9, 187.8 for a 10 µg/m3 change in PM2.5), and associations greater than 40% were noted for the high-age group and several other subgroups such as those living close to a monitor, low education, high blood pressure, on hormone therapy and post-menopausal. Effect modification was observed for all of the above characteristics except education, but with the addition of marital status, and income level.
Figure 1.
Percent Change in CRP due to a 10 µg/m3 Annual Change in PM2.5 (central estimate and 95% confidence interval; triangles and squares indicate effect modification based on p-value or doubling of effect estimate, respectively). Number in parenthesis indicates upper confidence interval. See Table 2 for definitions of strata.
Given the significantly higher effect estimates for the older age group (aged 47.51 to 52 years at baseline) of 48.7% (95% CI =21.0, 82.7), the stratified analyses were repeated for this sub-cohort (Figure 2). The effect estimates for most of the subgroups in this age range were generally higher, often twice that of the all-age cohort, with many effects statistically different from the null. The highest associations on CRP from a 10 µg/m3 change in PM2.5 were observed for diabetics at 301% and smokers at 105%, with additional effects above 60% for several subgroups. Effect modification was detected by diabetic status (based on the t-test) and by distance to the monitor, hormone therapy use, marital and smoking status and statin use (based on a doubling of the effect size). Of note, no association was observed between PM2.5 and CRP for those consuming some alcohol or on statins.
Figure 2.
Percent Change in CRP due to a 10 µg/m3 annual change in PM2.5 for the Higher Aged Subgroup (central estimate and 95% confidence interval; triangles and squares indicate effect modification based on p-value or doubling of effect estimate, respectively). Number in parenthesis indicates upper confidence interval. See Table 2 for definitions of strata.
To investigate whether other body fat metrics demonstrated effect modification, we reran the model after stratifying the sample by waist-hip ratio (greater or less than 0.85) and then for BMI of 30 or above. No effect modification was observed for these strata. Also, adjusting for the season of blood draw had very little effect on the results.
The next analysis examined the effect of PM2.5 on the probability of having a CRP > 3 mg/l. For the full cohort, no association was observed between annual average PM2.5 and elevated CRP. Among subgroups, associations were observed only for the higher age group and post-menopausal women with an estimated increase in the probability of CRP > 3 mg/l of 55% (95% CI = 12, 115) and 60% (95% CI = 12, 128), respectively (results not shown).
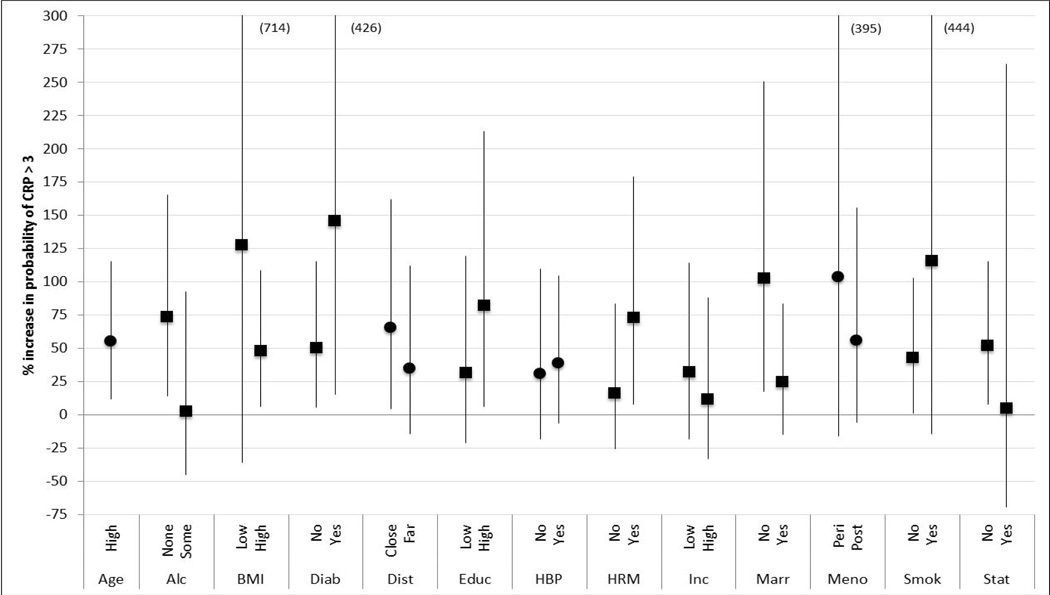
The final analysis examined the effects of PM2.5 on the probability of CRP > 3 mg/l for the older aged sub-cohort (Figure 3). Again, associations between PM2.5 and CRP were demonstrated for many of the subgroups. The greatest increases in probability were observed in diabetics at 146% (95% CI= 15, 426) and smokers at 116% (95% CI = −14, 444). Statistically significant associations with PM2.5 were observed for many of the subgroups and effect modification was noted for stratifications based on alcohol consumption, BMI, diabetes, education, use of hormone therapy, income level, marital status, smoking and statins. Again, while associations between PM2.5 and CRP were detected for alcohol abstainers and those not on statins, no association was observed for those consuming alcohol or on statins.
Figure 3.
Percent Change in the Probability of CRP Greater than 3 mg/l due to a 10 µg/m3 annual change in PM2.5 for the Higher Aged Subgroup (central estimate and 95% confidence interval; squares indicate effect modification based on doubling of effect estimate). Number in parenthesis indicates upper confidence interval. See Table 2 for definitions of strata.
4. Discussion
Based on a community-based subsample of women from the SWAN cohort without pre-existing cardiovascular events, our analysis indicates that several subgroups experience strong inflammatory responses following long-term exposure to PM2.5. Many results persisted when CRP was examined as either a continuous variable or as a binary variable using a threshold of 3 mg/l. In particular, those in the upper half of the age distribution at baseline visit (and therefore aged approximately 50.5 to 55 years at the start of our study three years after baseline) had notably greater effects of chronic exposure to PM2.5. In addition, higher aged subgroups (e.g., alcohol abstainers, diabetics, etc.) often demonstrated doubled risk estimates relative to those of a lower age. Diabetics and smokers exhibited the largest associations with PM2.5. Large and statistically significant effects or effect modification were also observed for those living closer to the monitor, using hormone therapy or unmarried. Among the full cohort (all ages), effects were also apparent for those with high blood pressure and those with lower family income. Finally, consumption of alcohol and the use of statins appeared to be protective in terms of the effects of PM2.5 on CRP. However, only 5% of the sample were taking statins so some caution about interpreting this result is necessary. It is also noteworthy that several modifiable risk factors for CVD in women, such as smoking, high blood pressure, diabetes and non-consumption of alcohol are factors that increase susceptibility to PM2.5 as mediated by CRP (Tice et al., 2006).
To date, there is only limited information about the extent to which personal characteristics modify the effect of air pollution through inflammation. Among the characteristics that we examined, effect modification by diabetes and BMI has probably been most extensively studied, albeit from mostly shorter-term exposures, with greater effects observed among diabetics and conflicting results for high BMI (Alexeeff et al., 2011; Dubowsky et al., 2006; Hertel et al., 2010; Hoffmann et al., 2009; Zanobetti and Schwartz, 2002). We also observed much greater effects among diabetics. While we found somewhat higher effects for those with higher BMI in the full and older cohorts using the continuous measure of CRP, we saw no evidence of effect modification for the binary measure of CRP > 3 mg/l. In fact, in the latter analysis, those with lower BMI had a much greater (though not statistically significant) effect. This may be due to the within-group distribution of CRP because approximately 44% of the high BMI subgroup had CRP levels greater than 3 mg/l and 22% were above 5. In contrast, of those with low BMI, only 13% were above 3 mg/l. High effect estimates for those with higher education may also have occurred for this reason.
On the other extreme, we observed protective characteristics indicating no effects of PM2.5 on CRP for several subgroups, including those on statins and moderate users of alcohol. These results are supported by previous studies on CRP (O'Neill et al., 2007; Zanobetti et al., 2004). While not as well documented as the known anti-inflammatory effects of statins, substantial evidence supports the anti-inflammatory aspect of moderate alcohol consumption (Imhof et al., 2004; Pai et al., 2006).
While several previous epidemiologic studies have examined the effects of PM2.5 on CRP and other inflammatory markers, to our knowledge few, if any, have examined exposures of a year or more on repeated measures of CRP. While previous epidemiologic findings are not fully consistent, they provide evidence of a potential inflammatory effect from exposure to PM2.5 or other particle metrics. One of the few studies of long-term exposure was a cross-sectional analysis of 4,800 adults in Germany (average age of 60) where the effect of the previous year’s exposure to PM2.5 of 3.9 µg/m3 was associated with a 24% increase in CRP (Hoffmann et al., 2009). This amounts to a 60% change per 10 µg/m3 compared to our estimated effect on the older age subgroup of 49%.
In contrast to prior studies, we were able to evaluate the effects of long-term exposures using five years of repeated measurements of CRP to facilitate analysis of within-person effects rather than effects across individuals or geographic areas. As a result, our models controlled for several time-varying factors (i.e., age, BMI, smoking, medication use) and eliminated observations after an incident stroke or myocardial infarction before or during the study period. Cross-sectional studies are likely to experience some residual confounding or omitted variable bias. Furthermore, the median distance between residence and pollution monitor in our study was 8.9 km, which was much less than studies in which a single monitor was used to represent PM2.5 exposure over an entire county or metropolitan area. Thus, we may have significantly reduced random exposure misclassification and the likelihood of biasing our effect estimate toward the null.
It is instructive to compare our particulate effect estimates on CRP with estimates for those exposed to particles from second-hand smoke (SHS). Studies in Greece and Italy both reported that regular exposure to SHS increased CRP by approximately 50% (Kiechl et al., 2002; Panagiotakos et al., 2004). Our results for the full cohort indicated that a 10 µg/m3 change in annual PM2.5 was associated with a 25.5% change in CRP. Thus, our estimate for the effect of ambient PM2.5 on CRP is half of the effect of low to moderate exposure to SHS on CRP. Pope et al. (Pope et al., 2009) estimated that being exposed to 10 µg/m3 of ambient PM2.5 generated a daily dose (in mg) that is roughly half that of being exposed to low to moderate SHS (i.e., 0.18 versus 0.33 mg). Therefore, our estimated effect of PM2.5 on CRP that is one-half of the estimated effect of SHS is consistent with the estimated doses of PM2.5 and SHS as determined by Pope et al. (Pope et al., 2009)
Several caveats are important in interpreting our results. First, the subset of SWAN participants in our study is not representative of the U.S. population of midlife women as a whole. In particular, the racial/ethnic mix was very different from that of the U.S. population by study design. Second, the present study was restricted to women aged 45–55 years at the time PM2.5 was assessed. Given our stronger results for the older sub-cohort, it is likely that we would observe even larger effect estimates of PM2.5 in an older cohort. Third, exposure to ambient PM2.5 was assigned by residence, and we could not account for non-residential exposures. Thus, while we attempted to minimize exposure misclassification by using a 20 km buffer around each monitor, some measurement error will likely persist, leading to an attenuation of the estimated effect. In addition, the composition of PM2.5 may vary significantly by location and may have an impact on its toxicity. Fourth, some of the subgroup characteristics will be correlated with other factors, both measured and unmeasured, which could bias the estimates. For example, we found a greater effect among those living closer to a monitor possibly representing reduced exposure misclassification to PM2.5. However, monitor proximity might also serve as a proxy for other risk factors, although this is less certain since closer distance was associated with both positive (more diabetes, more unmarried and less education) and negative risk factors (less pollution and more statin use). However, participants from the two California sites were much more likely to be living further from a monitor. Fifth, differences in model specifications in terms of included covariates could alter the results. However, analysis of alternative model specifications has demonstrated that the results were fairly robust to different model specifications.
5. Summary and Conclusion
Our analyses indicated that annual exposure to PM2.5, a ubiquitous pollutant in the urban environment, was associated with an important marker of systemic inflammation. The study is unique in that it involved five years of repeated annual measures of CRP, used an extensive network of PM2.5 monitors so that exposures could be determined within 20 km of participants’ residence and detailed residential history to record changes in address during the study period. This facilitated an examination of long-term exposures and within-person effects, while minimizing potential confounding. As such, it provides evidence of a plausible pathway by which exposure to particulate matter may be responsible for exacerbation of cardiovascular disease and subsequent increases in CVD mortality.
Highlights.
We examined the effects of chronic exposure to PM2.5 on C-reactive Protein (CRP)
We had repeated measures of CRP, a marker of inflammation that predicts cardiovascular events
We examined susceptibility among several subgroups in a cohort of women
We found effects on CRP for diabetics, smokers, older women, unmarried and low SES.
Inflammation may explain the previously observed effects of PM2.5 on mortality
Acknowledgements
The opinions expressed in this paper are solely those of the authors and do not represent the policy or position of the State of California or the California Environmental Protection Agency
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH. This publication was also supported in part by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1 RR024131.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012 - present; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
SWAN Repository: University of Michigan, Ann Arbor – Siobán Harlow 2013; Dan McConnell 2011 – 2013; MaryFran Sowers 2000 – 2011.
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN. Finally, we would also like to acknowledge Nick Mangus for providing the air pollution data and Francesco Forastiere, Massimo Stafoggia and Michael Lipsett for helpful comments on earlier drafts of this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexeeff SE, et al. Medium-term exposure to traffic-related air pollution and markers of inflammation and endothelial function. Environ Health Perspect. 2011;119:481–486. doi: 10.1289/ehp.1002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, et al. Particulate Matter Air Pollution and Cardiovascular Disease. An Update to the Scientific Statement From the American Heart Association. Circulation. 2010 doi: 10.1161/CIR.0b013e3181dbece1. CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Brulle RJ, Pellow DN. Environmental justice: human health and environmental inequalities. Annu Rev Public Health. 2006;27:103–124. doi: 10.1146/annurev.publhealth.27.021405.102124. [DOI] [PubMed] [Google Scholar]
- Calabro P, et al. CRP and the risk of atherosclerotic events. Semin Immunopathol. 2009;31:79–94. doi: 10.1007/s00281-009-0149-4. [DOI] [PubMed] [Google Scholar]
- Chuang KJ, et al. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176:370–376. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- Cushman M, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the cardiovascular health study. Circulation. 2005;112:25–31. doi: 10.1161/CIRCULATIONAHA.104.504159. [DOI] [PubMed] [Google Scholar]
- Dubowsky SD, et al. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect. 2006;114:992–998. doi: 10.1289/ehp.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA. U. S., Air Quality System Data Mart. 2009 [Google Scholar]
- Hertel S, et al. Influence of short-term exposure to ultrafine and fine particles on systemic inflammation. European Journal of Epidemiology. 2010;25:581–592. doi: 10.1007/s10654-010-9477-x. [DOI] [PubMed] [Google Scholar]
- Hoffmann B, et al. Chronic residential exposure to particulate matter air pollution and systemic inflammatory markers. Environ Health Perspect. 2009;117:1302–1308. doi: 10.1289/ehp.0800362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof A, et al. Overall alcohol intake, beer, wine, and systemic markers of inflammation in western Europe: results from three MONICA samples (Augsburg, Glasgow, Lille) Eur Heart J. 2004;25:2092–2100. doi: 10.1016/j.ehj.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Kiechl S, et al. Active and passive smoking, chronic infections, and the risk of carotid atherosclerosis: prospective results from the Bruneck Study. Stroke. 2002;33:2170–2176. doi: 10.1161/01.str.0000027209.59821.98. [DOI] [PubMed] [Google Scholar]
- Lepeule J, et al. Chronic exposure to fine particles and mortality: an extended follow-up of the Harvard Six Cities study from 1974 to 2009. Environ Health Perspect. 2012;120:965–970. doi: 10.1289/ehp.1104660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsett MJ, et al. Long-term exposure to air pollution and cardiorespiratory disease in the California teachers study cohort. Am J Respir Crit Care Med. 2011;184:828–835. doi: 10.1164/rccm.201012-2082OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews KA, et al. Associations between depressive symptoms and inflammatory/hemostatic markers in women during the menopausal transition. Psychosom Med. 2007;69:124–130. doi: 10.1097/01.psy.0000256574.30389.1b. [DOI] [PubMed] [Google Scholar]
- Miller KA, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- Mohai P, et al. Annual Reviews. Palo Alto: 2009. Environmental Justice. Annual Review of Environment and Resources; pp. 405–430. [Google Scholar]
- O'Neill MS, et al. Air pollution and inflammation in type 2 diabetes: a mechanism for susceptibility. Occup Environ Med. 2007;64:373–379. doi: 10.1136/oem.2006.030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostro B, et al. Long-term exposure to constituents of fine particulate air pollution and mortality: results from the california teachers study. Environ Health Perspect. 2010;118:363–369. doi: 10.1289/ehp.0901181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai JK, et al. Moderate alcohol consumption and lower levels of inflammatory markers in US men and women. Atherosclerosis. 2006;186:113–120. doi: 10.1016/j.atherosclerosis.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Pai JK, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- Panagiotakos DB, et al. Effect of exposure to secondhand smoke on markers of inflammation: the ATTICA study. Am J Med. 2004;116:145–150. doi: 10.1016/j.amjmed.2003.07.019. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, et al. Cardiovascular Mortality and Exposure to Airborne Fine Particulate Matter and Cigarette Smoke. Shape of the Exposure-Response Relationship. Circulation. 2009;120:941–948. doi: 10.1161/CIRCULATIONAHA.109.857888. [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. Jama. 2002;287:1132–1141. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puett RC, et al. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses' Health Study. Environ Health Perspect. 2009;117:1697–1701. doi: 10.1289/ehp.0900572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- Ridker PM, et al. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118:2243–2251. doi: 10.1161/CIRCULATIONAHA.108.814251. 4p following 2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- Schenker N, Gentleman JF. On judging the significance of differences by examining the overlap between confidence intervals. The American Statistician. 2001;55:182–186. [Google Scholar]
- Sowers MF, et al. SWAN: A multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo R, et al., editors. Menopause: Biology and Pathology. San Diego, CA: Academic Press; 2000. pp. 175–188. [Google Scholar]
- StataCorp LP, STATA, version 12. StataCorp: College Station, TX; 2011. [Google Scholar]
- Tice JA, et al. Risk factors for mortality in middle-aged women. Arch Intern Med. 2006;166:2469–2477. doi: 10.1001/archinte.166.22.2469. [DOI] [PubMed] [Google Scholar]
- Yelland LN, et al. Performance of the modified Poisson regression approach for estimating relative risks from clustered prospective data. Am J Epidemiol. 2011;174:984–992. doi: 10.1093/aje/kwr183. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. Cardiovascular damage by airborne particles: are diabetics more susceptible? Epidemiology. 2002;13:588–592. doi: 10.1097/00001648-200209000-00016. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, et al. Air Pollution and Markers of Cardiovascular Risk. Epidemiology. 2004;15:S22. [Google Scholar]
- Zeka A, et al. Inflammatory markers and particulate air pollution: characterizing the pathway to disease. Int J Epidemiol. 2006a;35:1347–1354. doi: 10.1093/ije/dyl132. [DOI] [PubMed] [Google Scholar]
- Zeka A, et al. Individual-level modifiers of the effects of particulate matter on daily mortality. Am J Epidemiol. 2006b;163:849–859. doi: 10.1093/aje/kwj116. [DOI] [PubMed] [Google Scholar]
- Zou GY, Donner A. Extension of the modified Poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res. 2011;22:661–670. doi: 10.1177/0962280211427759. [DOI] [PubMed] [Google Scholar]