Abstract
The guanylyl cyclase/natriuretic peptide receptor-A (GC-A/NPRA), also referred to as GC-A, is a single polypeptide molecule having a critical function in blood pressure regulation and cardiovascular homeostasis. GC-A/NPRA, which resides in the plasma membrane, consists of an extracellular ligand-binding domain, a single transmembrane domain, and an intracellular cytoplasmic region containing a protein kinase-like homology domain (KHD) and a guanylyl cyclase (GC) catalytic domain. After binding with atrial and brain natriuretic peptides (ANP and BNP), GC-A/NPRA is internalized and sequestered into intracellular compartments. Therefore, GC-A/NPRA is a dynamic cellular macromolecule that traverses different subcellular compartments through its lifetime. This review describes the roles of short-signal sequences in the internalization, trafficking, and intracellular redistribution of GC-A/NPRA from cell surface to cell interior. Evidence indicates that, after internalization, the ligand-receptor complexes dissociate inside the cell and a population of GC-A/NPRA recycles back to the plasma membrane. Subsequently, the disassociated ligands are degraded in the lysosomes. However a small percentage of the ligand escapes the lysosomal degradative pathway and is released intact into culture medium. By using pharmacologic and molecular perturbants, emphasis has been placed on the cellular regulation and processing of ligand-bound GC-A/NPRA in terms of receptor trafficking and down-regulation in intact cells. The discussion is concluded by examining the functions of short-signal sequence motifs in the cellular life-cycle of GC-A/NPRA, including endocytosis, trafficking, metabolic processing, inactivation, and/or down-regulation in model cell systems.
Keywords: Atrial and brain natriuretic peptides, guanylyl cyclase/natriuretic peptide receptors, internalization and trafficking, short sequence signal motifs
Introduction
In a pioneering discovery, de Bold et al., [1] demonstrated that atrial extracts contained natriuretic and diuretic activity, which led them to isolate “atrial natriuretic factor/peptide (ANF/ANP). The biological actions of natriuretic peptide hormones are triggered by their interaction with highly selective and specific natriuretic peptide receptors (NPRs). The cardiac hormone ANP and two complementary related peptides, brain natriuretic peptide (BNP) and C-type natriuretic peptide (CNP), exert natriuretic, diuretic, vasorelaxant, antimitogenic, and antihypertrophic effects [2-9]. Under normal hemodynamic conditions, ANP is predominantly synthesized, stored, and secreted in regulated fashion by cardiac myocytes and ventricles [2-5, 10]. However, in response to hemodynamic overload, such as congestive heart failure, both plasma and cardiac levels of ANP and BNP content are greatly increased, contributing significantly to the circulating pool of these peptides [6]. On the other hand, CNP is mainly present in endothelial cells and is not released into the circulation. Three subtypes of natriuretic peptide receptors have been cloned and characterized: natriuretic peptide receptor-A (NPRA), natriuretic peptide receptor-B (NPRB), and natriuretic peptide receptor-C (NPRC). Both NPRA and NPRB contain a guanylyl cyclase (GC) catalytic domain and are also referred to as GC-A and GC-B, respectively, [5, 6, 11, 12]. Both ANP and BNP exert their effects by interacting with GC-A/NPRA, leading to the synthesis and accumulation of intracellular second-messenger cGMP [5, 6, 8, 13-18]. CNP exerts its functional effects by activating GC-B/NPRB and by increased production of intracellular cGMP [16, 19].
Studies with mice have demonstrated that genetic disruption of the Npr1 gene (coding for GC-A/NPRA) increases blood pressure and causes hypertension in these animals [6, 20-24]. On the other hand, the effect of ANP was increased linearly in Npr1 gene-duplicated mice in a manner consistent with gene copy numbers [6, 25-27]. The findings of earlier studies clearly indicated that the level of GC-A/NPRA expression determines the extent of the biological effects of ANP and BNP; however, the interventional strategies aimed at controlling GC-A/NPRA expression are limited by the paucity of studies in this area.
GC-A/NPRA is a dynamic cellular macromolecule that traverses different sub-cellular compartments throughout its life [5, 28-31]. It has been suggested that, after internalization, ANP/NPRA complexes dissociate and a population of receptors recycles back to the plasma membrane [32]. This is an interesting area of research because controversy prevails with regard to internalization of the members of GCs/NPRs. Indeed, it has been reported by the default, that among the three natriuretic peptide receptors only NPRC is internalized with bound ligand [33]. Hence, this review focuses on the cellular trafficking and processing of ligand-bound GC-coupled natriuretic peptide receptors, with an example of GC-A/NPRA internalization and trafficking in intact cells. The cellular life-cycle of GC-A/NPRA in the context of short-signal sequence-regulated internalization, recycling, down-regulation, and metabolic processing has been presented.
Functional domains of GC-A/NPRA
The primary structure of GC-A/NPRA is consistent with that of the GC receptor family, with at least four distinct regions comprising ligand-binding domain, transmembrane region, the protein kinase-like homology domain (protein KHD) and the GC catalytic domain. The integrity of these regions are conserved among humans, mice, and rats [34-36]. The GC-B/NPRB has an overall domain structure similar to that of GC-A/NPRA and is localized mainly in the brain and endothelial cells, which is thought to mediate the action of CNP in the central nervous system and vasculature [37, 38]. Comparison of the amino-acid sequence indicated a 62% identity between GC-A/NPRA and GC-B/NPRB, with the intracellular regions appearing to be more highly conserved than are the extracellular domains of these two receptors [36]. The extracellular domain of GC-A/NPRA is homologous to the NPRC, which contains a short (35-residue) cytoplasmic tail, that apparently is not coupled to GC activation [39]. GC-A/NPRA mediates most of the known actions of ANP and BNP because major cellular and physiological responsiveness is mimicked by cGMP and its cell permeable analogs [5, 6, 9, 14, 17, 40, 41]. The intracellular signaling mechanism of GC-A/NPRA elicits effects in a cGMP-dependent manner (Fig. 1). The protein KHD of GC-A/NPRA contains an approximately 280-amino acid region that immediately follows the transmembrane spanning domain of the receptor and is more closely related to protein tyrosine kinases than it is to protein serine/threonine kinases [36]. It has been demonstrated that the protein KHD has as an important mediatory role in transducing ligand-induced signals to activate the GC catalytic domain of the receptor [11, 12, 34, 42-45]. The GC catalytic domain of the receptor contains an approximately 250-amino acid region that constitutes the catalytic active site of the receptor [46-48]. The transmembrane GC receptors contain a single cyclase catalytic active site per polypeptide molecule, however, based on structure modeling data, two polypeptide chains seem to be required to activate GC-A/NPRA, and the receptor functions as a homodimer [49-52]. Initially, the dimerization region of the receptor was thought to be located between the protein KHD and GC domain, which has been suggested to form an amphipathic alpha helix structure [53]. It has been reported that the crystal packing of the extracellular ligand-binding of NPRA contains two possible dimer pairs, the head-to-head and tail-to-tail dimer pairs, respectively, associated through the membrane-distal and membrane-proximal subdomains [54, 55]. The tail-to-tail dimer of GC-A/NPRA has been proposed [52]. The crystal structure suggests that NPRC is dimerized in a head-to-head configuration bound with ligand [56, 57]. It has been proposed that a head-to-head dimer may represent the latent inactive state, while the tail-to-tail dimer could represent the hormone-activated state [58]. The ligand-dependent activation of NPRA stabilizes a membrane-distal dimer interface of this receptor protein, suggesting that ANP binding stabilizes the GC-A/NPRA dimer with more stringent spacing at the dimer interface [59]. Nevertheless, the results of site-directed mutagenesis and chemical modification studies suggest that the head-to-head dimmer structure reflects the physiological dimmer structure of GC-A/NPRA [54, 55].
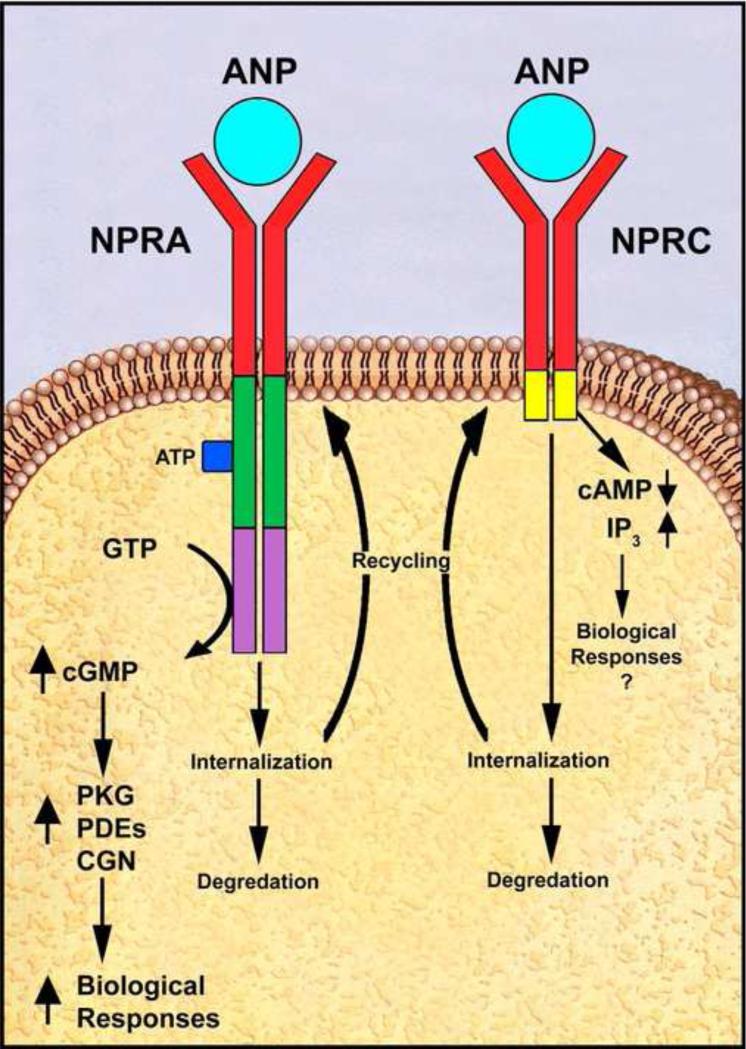
Fig. 1. Schematic representation of ANP-dependent activation and post-binding events of GC-A/NPRA and NPRC proteins.
ANP binding activates GC-A/NPRA in ATP-dependent manner, which leads to enhanced production of second messenger cGMP. An increased accumulation of intracellular cGMP activates cGMP-dependent protein kinase (PKG), which plays a critical role in ANP-dependent biological responsiveness. cGMP can also activate phosphodiesterases (PDEs) as well as cGMP-gated ion channels to activate ANP-dependent cellular and physiological functions. Finally, ligand-receptor complexes of GC-A/NPRA are internalized into the intracellular compartments and a larger proportion of ligand-receptor complexes are degraded in the lysosomal compartments. However, a small population of receptor is dissociated from the ligand and recycles back to the plasma membrane. ANP binding to NPRC is suggested to lower cAMP levels and to increase inositoltrisphosphate (IP3) in target cells. It is considered that ANP binding to NPRC leads the hydrolysis of phosphoinositides at very low dosages; however, its biological significance is not well defined. Finally, the bound ligand-receptor complexes of NPRC are internalized and degraded in the lysosomal compartments. However, a small population of receptor is also dissociated from the ligand in the intracellular compartments and receptor recycles back to the plasma membrane. The results are expressed as percentage maximum of bound/free hormone. Copyright @ 2005 Peptides. Used with permission.
The gene encoding GC-A/NPRA is designated as Npr1, which has been cloned and characterized in humans [35, 60, 61], mice [36, 62], rat [34, 63], bullfrogs [64], euryhaline eels [65], and medaka fish [66]. The primary structure of this gene is essentially similar in all these species, containing 22 exons interrupted by 21 introns [62]. The sequence analysis of the promoter region has identified putative cis-acting binding sites for the known transcription factors, but the functional significance of most of these sites remains to be elucidated. Although transcriptional regulation of the Npr1 gene is poorly understood, the activity and expression of NPRA is regulated by various factors, including natriuretic peptides [67-74], hormones such as endothelin [75], glucocorticoids [76-79], angiotensin II [80-85], growth factors [67, 86, 87], extracellular ion composition [88-93], pregnancy [94-98], physiological and pathophysiological conditions [99-108], and transcription factors [109-111]. A better understanding of the regulation of NPRA expression depends on more extensive functional characterization of its promoter region and elucidation of the functional significance of the potential cis-elements in this region, which are responsible for the binding of known transacting factors.
Receptor binding properties and ligand-mediated internalization of GC-A/NPRA
ANP binding to intact cells containing endogenous NPRA or to purified receptor preparations evaluated by scatchard plot analysis have revealed a single class of binding sites with a kD value of 1×10−10 −1×10−9M [28, 112-115]. Initial studies on the post-binding events of GC-A/NPRA were hampered by a lack of suitable primary cells predominantly containing this receptor protein. Nevertheless, our studies using a Leydig tumor (MA-10) cell line [28, 29], as well as the work of others with PC-12 cells [116] indicated that ANP/NPRA complexes are internalized and sequestered in cells. On the other hand, Koh et al., [117] indicated that ANP/NPRA complexes are not processed intracellularly in renomedullary interstitial cells. These authors suggested that rapid dissociation of receptor-ligand complexes takes place upon ANP binding to NPRA at 37°C on the plasma membrane. However, in those studies the dissociation of ligand was performed in cell culture medium containing very high concentrations of unlabeled ANP (1μM) to preclude the rebinding of dissociated ligand to receptors, which might have produced artifactual results. In addition, the cell lines used in those studies expressed more than one ANP receptor subtype, including both NPRA and NPRC. The kinetics of internalization and metabolic processing of ANP through NPRC, which does not contain GC catalytic activity, have been extensively studied [8, 113, 118-126]. Early studies of the post-binding events of NPRC were greatly facilitated due to its predominance in vascular smooth muscle cells, which provided a suitable model for study of the metabolic fate of NPRC. Studies using the MA-10 cell line containing exclusively high-density endogenous GC-A/NPRA, as well as COS-7 and human embryonic kidney-293 (HEK-293) cells expressing recombinant GC-A/NPRA, firmly established that ANP/NPRA complexes are rapidly internalized and intracellularly processed in intact cells [5, 6, 29, 67, 127]. Earlier, it has been indicated that in neuroblastoma cells bound-ligand to GC-A/NPRA was degraded and released by a neutral metalloendopeptidase [128], but further studies have not been carried out to confirm this finding.
The stoichiometric kinetic analyses of ligand-binding, internalization, and sequestration of GC-A/NPRA have been studied to distinguish between cell-surface associated, internalized, and degraded ligand-receptor complexes in MA-10 cells containing endogenous receptors and both COS-7 and HEK-293 cells expressing recombinant receptors [29, 31, 32, 115]. Early observations suggested that after binding of 125I-ANP to GC-A/NPRA, ligand-receptor complexes were internalized at physiological temperatures and both the degraded and intact ligands were released into culture medium [29, 31]. The distribution of 125I-ANP radioactivity on the cell surface, in intracellular compartments, and in culture medium provided a dynamic equilibrium among the rates of 125I-ANP uptake, its degradation, and its extrusion in intact cells (Fig. 2). It was observed that a major portion of the internalized 125I-ANP was released into culture medium, which consisted of approximately 70%-75% degraded products and about 25%-30% intact ligand [29, 30, 115, 127]. During the initial incubation period, the release of both degraded and intact ligands was blocked by the lysosomotropic agents chloroquine, ammonium chloride, and monensin (Table 1). However, after longer incubation, chloroquine was only partially effective in blocking the release of both degraded and intact ligands [29, 31, 32, 115]. Previous studies have provided the evidence supporting the notion that most of the internalized 125I-ANP is processed through the degradative compartments in intact cells [30-32, 115]. Nevertheless, an alternate mechanism also seems to exist for the release of intact ligand to cell exterior. Dual pathways for the intracellular processing of ligand-receptor complexes of insulin have been suggested [129, 130]. Our previous studies have provided definitive evidence for the recycling of internalized GC-ANPRA to the plasma membrane [29-32]. Those studies also showed that majority of the internalized ligand (>70%) is degraded in lysosomes and released into culture medium. Nevertheless, 25%-30% of ligand-receptor complexes escape the lysosomal pathway and are extruded intact to the cell exterior. It is expected that the intact ligand can rebind recycled receptors on the cell surface and reenter the cell via repeated retroendocytosis.
Fig. 2. Surface-bound, internalized, and released 125I-ANP radioactivity in 293 cells stably expressing recombinant NPRA.
293 cells were pretreated in the absence (○, △, □) or presence (●, ▲, ■) of 200 μM chloroquine at 37 °C for 1 h. Both control and chloroquine-treated cells were allowed to bind 125I-ANP at 4 °C for 60 min, after which cells were washed four times with ice-cold assay medium (2 ml each wash) to remove the unbound ligand and then warmed at 37 °C. At the indicated time intervals, cell surface-associated (a), internalized (b), and released (c) 125I-ANP radioactivity levels were determined in acid eluates, cell extracts, and culture medium, respectively. Copyright @ 2002 the Journal of Biological Chemistry. Used with permission.
Table 1.
Cell surface-associated, internalized, and released 125I-ANP radioactivity in 293 cells after treatment with various agents known to inhibit intracellular degradation of ligand-receptor complexes in intact cells. HEK-293 cells expressing wild-type recombinant GC-A/NPRA were treated with various lysosomotropic agents namely; ammonium chloride, chloroquine, monensin, and nigericin and energy depleter dinitrophenol. Cell surface associated, internalized, and released 125I-ANP radioactivities were analyzed.
| Treatment |
125I-ANP radioactivity (% of Control) |
||
|---|---|---|---|
| Cell surface | 125I-ANP | Internalized 125I-ANP | Released 125I-ANP |
| None | 43±4 | 5±1 | 62±6 |
| Amonium Chloride | 24± 2 | 60±1 | 20±2 |
| Chloroquine | 20±3 | 58±7 | 24±2 |
| Dinitrophenol | 22±3 | 62±8 | 18±2 |
| Monensin | 18±2 | 68±5 | 24±3 |
| Nigericin | 28±3 | 56±6 | 17±2 |
Confluent 293 cells expressing recombinant NPRA were labeled with 125I-ANP. The radioactivity in acid eluate, cell extract, and culture medium was counted to determine the cell surface-associated, internalized, and released radioactivity, respectively.
It is envisioned that the homeostatic regulation of GC-A/NPRA and its cellular sensitivity to ANP are dependent on the dynamic equilibrium of endocytosis mechanisms and intracellular processing in a cell-specific manner. The rates of both the internalization and breakdown of 125I-ANP in MA-10 cells containing endogenous GC-A/NPRA, as well as in COS-7 and HEK-293 cells containing recombinant receptor, were markedly inhibited in the presence of lysosomtropic inhibitors [29, 31]. Chloroquine and monensin inhibit lysosomal degradation, while dinitrophenol disrupts the energy-dependent intracellular trafficking of various ligand-receptor complexes [29, 31, 32, 130-135]. Ligand-binding studies in intact cells demonstrated that internalized GC-A/NPRA recycles back to the plasma membrane [29, 31, 32]. Receptor binding in trypsin-treated solubilized cell preparations have indicated that most of the receptors were present on the plasma membrane; only 18%–20% were assigned to the preexisting intracellular pool [32, 115]. Since receptors can be inactivated by trypsin on the cell surface, it can be assumed that some receptors might be present on the cytoplasmic side of the plasma membrane, accounting for a significant proportion of the measured intracellular receptor pool. However, this possibility seems to be unlikely because only 20% of the total GC-A/NPRA population of receptors constituted the intracellular pool, which cannot account for the replacement of receptors lost during ANP treatment [32]. Previous studies using photoaffinity labeling of intact cells have also demonstrated that internalized GC-A/NPRA recycles back to the plasma membrane [29, 118]. Trypsin treatment of the photoaffinity-labeled cells produced the 68 kDa tryptic fragment, which increased in a time-dependent manner [17, 29]. The concomitant increase in the 68-kDa tryptic fragments of the receptor suggested that the internalized GC-A/NPRA recycles from intracellular compartments to the plasma membrane. The density of the intact 135-kDa receptor band decreased in the cell interior and the 68-kDa tryptic fragment increased, suggesting that internalized receptor molecules became trypsin-sensitive after returning to the plasma membrane [29]. A decrease in the receptor population from the intracellular pool was impaired by chloroquine and dinitrophenol, which further supported the notion that a decrease in the intracellular pool of GC-A/NPRA occurs due to recycling of receptor to the plasma membrane rather than as a result of trypsin entering the cell interior. The results of our earlier studies, as well as recent experimental findings, are consistent with the notion that if recycling is inhibited, internalization of the receptor continues, suggesting that the receptor will not be reinserted into the plasma membrane and that a rapid loss in the number of cell surface receptors will occur [29, 31, 32, 115].
ANP binding experiments have clearly demonstrated that in the presence of chloroquine the amount of 125I-ANP bound to GC-A/NPRA was significantly decreased [29, 31]. Those previous studies suggested that both the lysosomotropic agent chloroquine and the metabolic inhibitor dinitrophenol, which depletes cellular ATP, disrupted the internalization and recycling of GC-A/NPRA [29, 31, 32]. Although it has been reported that ATP is not required for the internalization of insulin receptor [136, 137], it is essential for the internalization of epidermal growth factor (EGF) receptor [131]. Studies using COS-7 and HEK-293 cells expressing recombinant GC-A/NPRA and MA-10 cells containing endogenous receptors suggested that the dissociation of bound ANP from the receptor is not a prerequisite for the recycling of GC-A/NPRA [29, 127]. The internalized hormone-receptor complexes of the low-density lipoprotein (LDL) enter the acidic vesicular compartments where the ligand dissociates from the receptor. Subsequently the dissociated LDL receptors recycle back to the plasma membrane and the ligand is degraded in the lysosomal compartments [138]. This indicates that the recycling of GC-A/NPRA probably occurs constitutively as well as via the ligand-dependent regulatory mechanisms. It has been postulated that after endocytosis of GC-A/NPRA, some of the internalized pool of receptors could be diverted to degradation, while the remainder are subject to regulated recycling [29, 31, 133]. Clearly, further studies are needed to clarify the mechanisms that control the endocytosis and recycling of GC-A/NPRA. The possibility that internalized GC-A/NPRA provides signals that contribute to the regulation of receptor gene expression should be considered. The ligand-evoked internalization and partial degradation of GABA receptors both enhance and repress receptor gene expression [139-142]. One Intriguing finding was that agonist-dependent endocytosis of ß2-adrenergic receptors is a necessary step in the activation of mitogenic signals [143]. The early studies support the view that a certain class of receptors may regulate their own biosynthesis through intracellular signals involving ligand-dependent endocytosis. At present, this notion remains speculative; however, it may provide a new direction for investigations of the trafficking and functional regulation of GC-A/NPRA, as well as other members of the GC family of receptors.
Down-regulation, desensitization, and metabolic processing of GC-A/NPRA
Receptors that are metabolically processed and degraded after internalization can have important physiological and pathophysiological implications, including an effect on promoting ligand-receptor internalization that would lead to the degradation of both ligand and receptor molecules in lysosomes. In the course of time, the increased rate of degradation can exceed the rate at which the receptors are replaced by de-novo synthesis, so that the total number of receptors is correspondingly reduced [32, 144]. This in turn, renders the cell refractory to the hormone. In this state of hormone resistance the prevailing ligand concentrations is not sufficient to cause biological responsiveness. After binding of ANP to GC-A/NPRA, ligand-receptor complexes are internalized and sequestered into intracellular compartments, while degraded products are released into culture medium [127, 133]. The pretreatment of HEK-293 cells with unlabeled ANP caused a substantial decrease in the 125I-ANP binding capacity of GC-A/NPRA in both time- and dose-dependent manner [32, 133]. Internalization of ANP/NPRA complexes seems to be important in receptor down-regulation [31, 32, 133]. The ligand-dependent down-regulation by receptor degradation is usually homologous, since it involves only the receptor of one particular ligand, even though other receptors may be present on the cell surface. This mechanism differs from that of sequestration since it involves the total loss of cellular receptors rather than receptor redistribution into a subcellular compartment, which may be inaccessible to extracellular ligands. Usually the mechanisms leading to the homologous down-regulation of receptors involve complete removal of ligand-bound receptors from the cell surface by an endocytic processes. There could be several variations on this theme, resulting in receptor internalization, ligand degradation, and recycling of the receptor to the cell surface; alternatively, both the receptor and the ligand can be degraded in lysosomal compartments. If a receptor recycles to the cell surface, the internalization can be compensated for to some extent by reappearance of the receptors in the plasma membrane. Thus, down-regulation can be delayed until receptors begin to be degraded. Essentially, down-regulation may result in a loss of cellular GC-A/NPRA by means of an accelerated rate constant for receptor internalization and, presumably, inactivation.
Desensitization of receptor can be defined as a process whereby the function of a receptor is lost, whether that function is ANP-binding affinity, autoregulatory efficacy of the protein KHD, and/or GC catalytic activity and intracellular accumulation of cGMP. In our metabolic processing studies of GC-A/NPRA, we have used ANP binding as the index of receptor activity [32, 115]. Degradation, on the other hand, has been considered to be the actual loss of the receptor protein from the cell surface by proteolysis into amino acids. Inactivation can precede degradation or both events can occur simultaneously. ANP-induced receptor down-regulation would be an invaluable experimental tool for elucidation of GC-A/NPRA inactivation or desensitization. A change in the receptor phosphorylation state has been implicated in the process of desensitization [16]. Interestingly, desensitization does not require large-scale removal of receptors from the cell surface and is probably achieved by a combination of both receptor phosphorylation and degradation. Desensitization and/or inactivation of GC-A/NPRA has been suggested by ANP-dependent dephosphorylation of receptor protein [145]. However, the exact mechanism of the dephosphorylation-dependent inactivation of GC-A/NPRA is not well understood. Both early and recent results have indicated that GC-A/NPRA phosphorylation seems to occur in various cell types [42, 146-150].
It is anticipated that the desensitization or inactivation of receptor occurs intracellularly and that inhibition of internalization should prevent this process. The stoichiometric analyses and metabolic processing of ANP/NPRA complexes in the MA-10 cell line and recombinant COS-7 and HEK-293 cells provided evidence that a large population of bound ANP/NPRA complexes enters into lysosomes and the degraded products are released into the culture medium [29, 31, 32, 133]. The lysosomotropic agent chloroquine and NH4Cl2 inhibited the degradation of ANP, providing direct evidence that ligand is metabolized in lysosomes. Specific endopeptidases are also known to degrade ANP, but their location in the endosomal compartments has not been established. The endosomal system requires ATP for the maintenance of a pH gradient across the endosomal membrane. Since acidification is believed to augment the release of ligand from receptors within the endosomes, it must be asked whether ANP-endopeptidase is active on ANP; bound to the receptor or ANP, acting as a substrate for endopeptidase; or has to be free in the lumen of the endosome. The localization of ANP-endopeptidase-like activity in the cytosol, plasma membrane, and endosomes would be of great significance. If the physiologically relevant location of the endopeptidase is the endosomal compartment and the enzymes in all three compartments are identical, a question can be raised about the mechanism whereby the cytosolic enzyme is inserted into the endosome. Furthermore, if this insertion process is a locus for cellular regulation of the degradation of ANP, that remains to be established. Inactivation of GC-A/NPRA might occur intracellularly, but inhibition of internalization should prevent this process. However, the molecular and biochemical aspects of the process of inactivation of GC-A/NPRA, as well as the subcellular localization of the events, have yet to be determined.
We envision that receptor-mediated endocytosis of ANP/NPRA complexes may involve a number of sequential sorting steps through which ligand-receptor complexes could be eventually degraded, recycled back to the cell surface, or released into the cell exterior [5, 30-32]. Some of these events may take place sequentially. The first step would be the noncovalent binding of ligand to the cell surface receptor inserted into the plasma membrane. The receptors, through some intrinsic affinity or aggregation induced by protein binding, must cluster into coated pits on the plasma membrane. This proposed itinerary would be consistent with the notion that ANP/NPRA complexes are be delivered to lysosomal compartments. Acidification of lysosomes induces the dissociation of ligand from the receptor, after which a population of receptor molecules may recycle back to the plasma membrane, initiating the following events: i) as lysosomotropic agent such as chloroquine is unable completely to block the degradation of ANP/NPRA in lysosomes; ii) the release of intact ANP occurs through a lysosome-independent pathway; and iii) recycling of endocytosed GC-A/NPRA back to the plasma membrane occurs simultaneously with this process, leading to degradation of the majority of ligand-receptor complexes into the lysosomal compartments. Some findings suggest that after the internalization of ANP-receptor complexes, ligand bifurcates into a degradative pathway, through which the 70%-80% of internalized ANP of all incoming ligand is processed in lysosomes, and a retroendocytotic pathway that accelerates the release of intact ligand [29, 32]. An investigative approach based on these hypotheses should provide a direct assessment of ligand-bound receptor trafficking and sequestration into intracellular compartments.
It is conceivable that some remarkable differences do exist with regard to internalization, processing, and metabolic turnover among various types of membrane receptors. The phenomenon of ANP-NPRA degradation is similar to that reported for low-density lipoprotein receptors in human fibroblasts [151, 152], insulin receptors in adipocytes [129, 153, 154], transfected Chinese hamster ovary cells [155], and thyrotropin hormone receptors in GH3 cells [156]. However, the degradation of asialoglycoprotein and its receptor complexes is not observed until about 30 min after endocytosis in hepatoma cells [157]. Similarly, the degradation of EGF-receptor complexes is not detectable for at least 20 min in hepatocytes [158]. Although there is no apparent mechanistic evidence to account for such differences, several possibilities can be considered. Because the internalization of receptor-bound ligand should not be the limiting factor, there may be multiple pathways leading to the eventual metabolic turnover of ligand-receptor complexes, perhaps through the use of different intermediate vesicles for the transfer of ligand to the site of degradation. If this is the case, then it should be possible to determine the intracellular sequestration route by either intrinsic properties of ligand-receptor complexes or the pathways by which various cells process the incoming ligands. It is also possible that there is a single metabolic pathway composed of several distinct processing steps, which should be unique for a specific ligand-receptor complex. Dual pathways for the intracellular processing of ligand-receptor complexes have been proposed for insulin and epidermal growth factor (EGF) receptors [130, 159-162]. Similarly, the finding that chloroquine effectively interrupts the degradative processing of GC-A/NPRA without exerting a deleterious effect on the retroendocytotic pathway is novel and intriguing [32, 133], giving rise to the notion that after internalization, many types of ligand-receptor complexes can recycle through the chloroquine-insensitive pathway and finally be degraded via chloroquine-sensitive lysosomal compartments. Several types of ligand-receptor complexes, including EGF, insulin, and asialoglycoprotein receptors, recycle through the chloroquine-insensitive pathway, [129, 160, 163-165].
Short sequence motifs and internalization of GC-A/NPRA
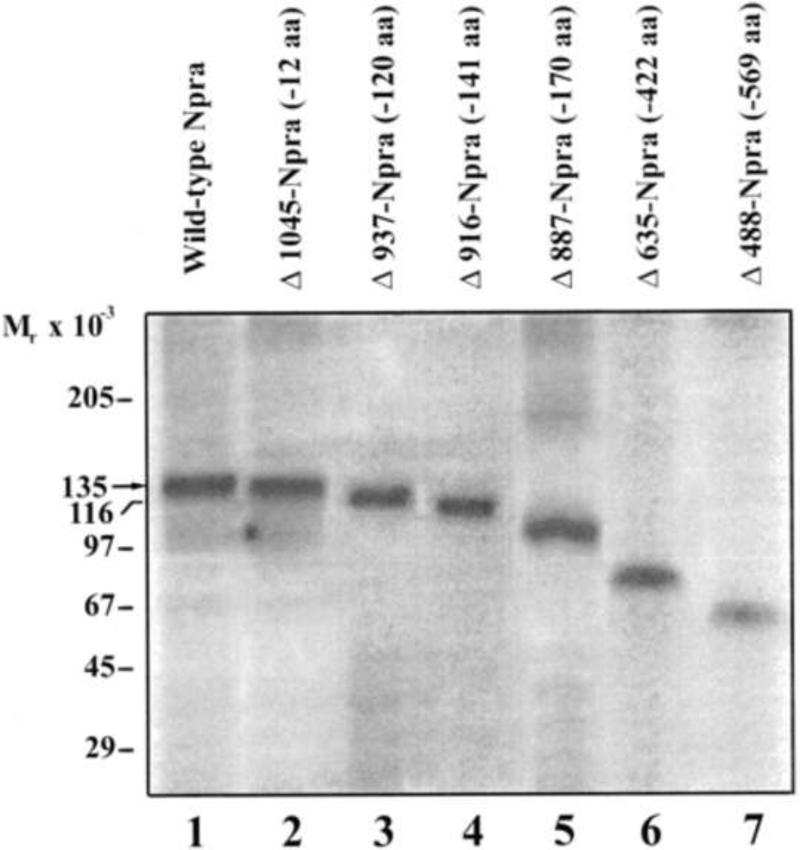
Using deletion mutagenesis, the specific regions in the intracellular domain of GC-A/NPRA have been determined for internalization, sequestration, and recycling [31, 133]. The loss of function of deletion and/or site-directed mutations have been utilized to identify the regions within the protein KHD and GC catalytic domains of this receptor protein (Fig. 3). The results of immunoprecipitation studies indicated that wild-type and carboxyl-terminal-deleted receptors are expressed on the cell surface without a significant decrease in the protein contents of mutant receptors (Fig. 4). It has been demonstrated that the truncation of GC-A/NPRA at the carboxyl-terminal end significantly reduced the hydrolysis and metabolic degradation of ligand-receptor complexes as compared with the wild-type receptor [31]. The complete deletion of both the protein KHD and GC catalytic domains abolished the internalization of GC-A/NPRA. The deletion of 170 amino acids at the carboxyl-terminal end of the receptor ( 937-NPRA, 916-NPRA, 887-NPRA) reduced the internalization of ligand-receptor complexes by 60% [31]. Our findings suggested that specific regions within the intracellular domains of GC-A/NPRA determine the extent of ligand binding efficiency, endocytosis, and intracellular sequestration of ligand-receptor complexes in transfected COS-7 cells [31]. Those previous studies indicated that with increasing deletions of amino acid residues at the carboxyl-terminal end of the receptor, a large proportion of ligand-receptor complexes did not internalize and remained on the cell surface (Fig. 5). Analysis of the intact and degraded ligands demonstrated that the release of 125I-ANP consisted of a higher amount of degraded products and a lesser amount of intact ligand in the culture medium of cells expressing either wild-type or carboxyl-terminal truncated mutant GC-A/NPRA [31]. Interestingly, most of the internalization signals have been reported to reside in the cytoplasmic domains of endocytosed receptors [30, 166-169]. Evidence suggests that a majority of the receptors that undergo endocytosis contain internalization signals such as Asn-Pro-x-Tyr (NPXY) sequence motif in the cytoplasmic portion, near the transmembrane domain of the receptor. However, NPRA including other members of the GC-A receptor family do not seem to contain an intracellular NPXY sequence motif near the transmembrane domain. Nevertheless, examples exist where this motif is not essential for endocytosis and processing of ligand-receptor complexes; insulin-receptor complexes in CHO cells are one such case [170]. Therefore, the internalization of ligand-receptor complexes may not be solely dependent on the NPXY motif located near the transmembrane domain of the receptor protein. Although it has been suggested that the protein KHD of GC-A/NPRA is critical to the functional ability of the receptor, the exact mechanisms whereby it controls GC catalytic activity and other functions of GC-A/NPRA are not well understood.
Fig. 3. Binding of 125I-ANP in COS-7 cells expressing the with wild-type and carboxyl-terminal-deleted mutant receptors.
Transfection of wild-type (full-length) and carboxyl-terminal- deleted NPRA cDNAs was carried out by electroporation. Forty-eight hours after transfection, cells were washed with assay medium and incubated with 1 nM 125I-ANP in the presence or absence of 100 nM unlabeled ANP for 1 h at 4°C. At the end of the incubation period, cells were washed four times with ice-cold HBSS, dissolved in 0.5N NaOH, and cell-bound radioactivities were determined. Copyright @ 2000 Molecular Pharmacology. Used with permission.
Fig. 4. Autoradiograph showing the 35S-labeled wild-type and carboxyl-terminal-deleted receptors.
COS-7 cells were transfected with wild-type and carboxyl-terminal-deleted NPRA cDNAs and labeled with 35S-methionine. Cell lysates were immunoprecipitated using polyclonal antibodies against the NPRA. Lanes 1-7 show the correspondingly labeled protein bands of expressed wild-type and carboxyl-terminal-deleted mutant receptors. Arrow indicates the molecular weight of wild-type receptor. Copyright @ 2000 Molecular Pharmacology. Used with permission.
Fig. 5. Quantitative analysis of cell-surface-associated, internalized and released 125I-ANP radioactivity in COS-7 cells expressing the wild-type and carboxyl-terminal truncated receptors.
Forty-eight hours after transfection, cells in culture dishes were allowed to bind 125I-ANP at 4°C for 1 h, after which cell monolayers were washed four times with ice-cold assay medium to remove the unbound ligand and then reincubated in fresh medium at 37°C for indicated time periods. The distribution of 125I-ANP radioactivity is represented as (a) cell-surface associated, (b) internalized into the intracellular compartments, and (c) released into culture media. Copyright @ 2000 Molecular Pharmacology. Used with permission.
A variety of structures, known collectively as the endosomal apparatus, are considered to be involved in the uptake of extracellular substances. The endosomal apparatus is nonlysosomal and is characterized by subcellular fractionation, morphological markers, and enzyme activities. It has been suggested that the endosomal apparatus may contain three separate compartments: the early stage is close to plasma membrane; the larger intracellular structures contain endocytosed ligand and/or receptor, which is vesicular and tubular; and a structural compartment contains acid phosphatase activity, much as do the lysosomes [171]. Most likely, it is this third compartment that accumulates the weak base chloroquine, maintains low pH, and contains an ATP-dependent pump. Presumably, the discharge of ANP from the bound ligand-receptor complexes occurs in this compartment.
Role of GDXY motif in the internalization and trafficking of GC-A/NPRA
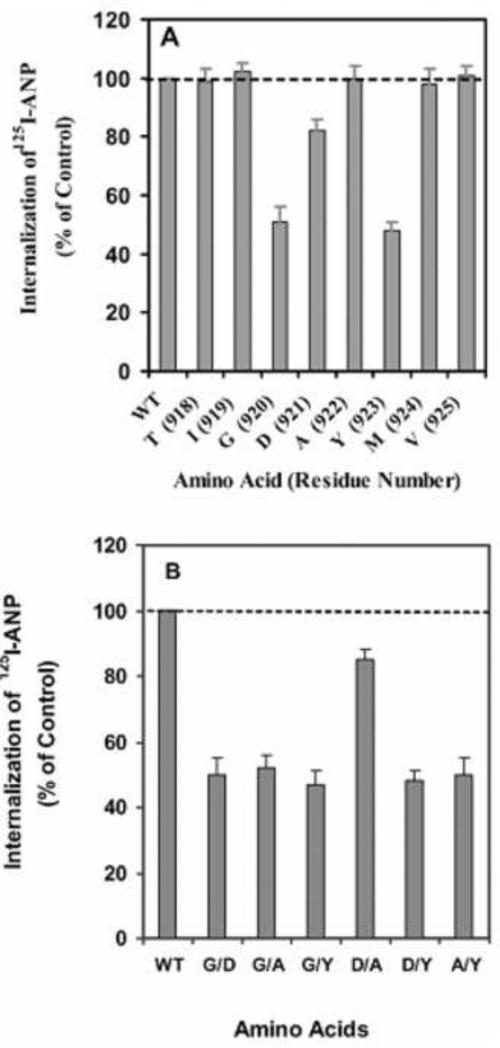
The sequence motif GDAY in the carboxyl terminal-domain of GC-A/NPRA serves as an internalization signal for endocytosis and trafficking of this receptor protein [115]. The residues Gly920 and Tyr923 constitute the important elements in the GDAY internalization signal sequence motif (Fig. 6). However, it is thought that the residue Asp921 provides an acidic environment for efficient signaling of the GDAY motif during GC-A/NPRA internalization. The mutation of Asp921 to alanine did not have a major effect on internalization, but significantly attenuated the recycling of internalized receptors back to the plasma membrane [30, 115]. On the other hand, mutation of Gly920 and Tyr923 residues to alanines inhibited the internalization of NPRA, although these residues had no discernible effect on the receptor recycling. These findings suggested that the tyrosine-based GDAY motif modulates the early internalization of GC-A/NPRA, whereas Asp residue in the GDAY sequence seems to mediate recycling or later sorting of the receptor. Two overlapping motifs within the GDAY sequence may exert different but specific effects on endocytosis and subsequent trafficking of GC-A/NPRA. More studies are needed to define the dual role of GDAY motif in receptor internalization and recycling [6, 115].
Fig. 6. Sequence requirements for internalization of NPRA in HEK-293 cells.
(A) Alanine substitutions at amino acid positions 918–925. (B) Alanine substitutions indicating two residues in different combinations in the GDAY motif. Confluent HEK-293 cells in 6 cm2 dishes expressing either wild-type (WT) or mutant receptors were washed twice with 2 ml of assay medium (DMEM containing 0.1% BSA) and then treated with 125I-ANP at 4 °C for 1 h in the absence or presence of unlabelled ANP. Then, the cells were washed four times with assay medium and re-incubated in 2 ml of fresh medium at 37 °C. After 10 min of internalization and incubation, the internalization of ligand–receptor complexes was quantified. Copyright @ 2005 the Biochemical Journal. Used with permission.
It is conceivable that the GDAY motif has a dual function in receptor internalization and in subsequent recycling of internalized receptor back to the plasma membrane. The mutations of Gly920 and Tyr923 to alanine in NPRA attenuated the internalization of mutant receptors by almost 50% as compared with wild-type receptor [115]. However, the mutation of Asp921 to alanine had only a minimal effect. It is possible that the regulation of receptor internalization relies largely on residues Gly920 and Tyr923 in the carboxyl-terminal domain of the receptor protein. On the other hand, mutation of Asp921 to alanine significantly attenuated the recycling of internalized receptor back to the plasma membrane. Thus, the functional role of the cytoplasmic tail of GC-A/NPRA is important, being comparable to the thyrotropin-stimulating hormone receptor in that its disruption attenuates receptor internalization [172]. The point and deletion mutations within the carboxyl-terminal region of GC-A/NPRA also seem to have a major effect on internalization [31]. Replacement of the selected Gly920, Asp921, and Try923 residues with alanine in the GDAY showed that the internalization of mutant receptor was significantly reduced compared with wild-type receptor [115]. The GDAY/AAAA mutant receptor displayed a dose-response curve similar to that of wild-type receptor. The recombinant HEK-293 cells harboring both wild-type and GDAY/AAAA mutant receptors, respectively, expressed 1.87×106 and1.89×106 receptor sites/cell and the binding affinity was comparable in both cell lines, with Kd = 2.4 × 10−8 M [115].
The GC-A/NPRA is internalized into subcellular compartments in the ligand-dependent manner [28, 29, 116, 118, 127, 133]. The ligand-dependent endocytosis and sequestration of GC-A/NPRA involves a series of sequential sorting steps through which ligand-receptor complexes can eventually be degraded, receptor recycled back to the plasma membrane, and intact ligand released into the cell exterior [29, 31, 127, 133]. The recycling of endocytosed receptor to the plasma membrane and the release of intact ANP into the cell exterior occur simultaneously with processes leading to degradation of the majority of ligand-receptor complexes into lysosomes, which appears to be controlled by receptor sequences in the carboxyl-terminal domain of GC-A/NPRA [31, 115]. Furthermore, a return in 125I-ANP binding in trypsin-treated cells indicated that 125I-ANP binding in trypsinized cells was due to recycling of the receptor protein [115, 127]. As shown in Fig. 7, after trypsin treatment, the greater return of 125I-ANP binding in HEK-293 cells expressing wild-type receptors was due to a large population of internalized receptors and subsequent recycling to the plasma membrane compared with cells expressing GDAY mutant receptors. These findings provided direct evidence that treatment of cells with unlabeled ANP accelerates the disappearance of surface receptors, indicating that ANP-dependent down-regulation of GC-A/NPRA involves the internalization of the receptor [115]. The internalization of platelet-activating factor is also regulated by DPXXY motif [173] and that of type-2 vasopressin receptor by the NPXXY sequence [174]. It has been suggested that, similarly, the YXXL motif functions in endocytosis of LDL receptor-related protein [175]. A common feature of these internalization signal motifs, including NPXY and GDAY, is the presence of a tyrosine residue at the end of the tetrapeptide sequence motif [115, 173]. Moreover, tyrosine residues in the mannose-6-phosphate receptor and the influenza virus hemagglutinin are also involved in endocytosis and trafficking, even though they are not present in the context of NPXY or YXRF consensus sequence motifs. Therefore, if a universal internalization signal exists, it may not be based on a universal amino acid sequence [173, 176-179].
Fig. 7. ANP-induced receptor down-regulation in HEK-293 cells expressing wild-type or mutant receptors.
Confluent HEK-293 cells expressing wild-type, G920A, D921A, Y923A or GDAY/AAAA mutant receptors in 6 cm2 culture dishes were pretreated with unlabelled ANP (10 nM) for the indicated time periods. Cells were then washed with glycine acidic buffer (pH 3.8) to remove any surface-bound 125I-ANP. After washing the cells four times with assay medium (2 ml each), cells were reincubated in 2 ml of fresh assay medium. The specific 125I-ANP binding was determined and expressed as a percentage of the initial radioligand 125I-ANP binding. Copyright @ 2005 the Biochemical Journal. Used with permission.
The critical features of the internalization sequences might be their specification of a particular conformation, such as a tight beta-turn in protein structure [180]. A compilation of some of the membrane receptors with known signal sequence has been provided in Table 2. The Gly920 residue constitutes the important element in conjunction with the Tyr923 residue of the GDAY internalization signal motif [30, 115]. Similarly, the Gly950-Pro951-Leu952-Tyr953 motif has been implicated in internalization of the insulin receptor, in which Gly950 and Tyr953 residues have been shown to be the critical components [181]. A common internalization motif consists of four amino acids with an aromatic residue, specifically a tyrosine in the fourth position. The tyrosine recognition signals form a small surface loop [182], but one differing in terms of the positioning of tyrosine in the loop structure [179, 183]. Earlier studies have provided direct evidence that the NPXY sequence in the LDL receptor forms a beta-turn structure, but that peptides containing the NPXY motif assume a reverse-turn conformation with Tyr in the fourth position of the turn [184]. Using residues known to be inactive in receptor endocytosis to substitute for Tyr resulted in disruption of the beta-turn conformation. A similar approach was used to obtain evidence that the PPGY sequence of the acid phosphatase in the cytoplasmic tail forms a type 1 beta turn with Tyr in the fourth position [185]. Thus, the presence of Tyr in the fourth position of internalization signals is critical for receptor endocytosis. Similarly, seven transmembrane G-protein-linked receptors have a homologous motif, NPXXY. Mutation of this sequence to NPXXA resulted in a complete loss of agonist-induced receptor sequestration [186, 187]. The conserved Tyr was required for internalization of vasopressin receptor [174]. However, there are exceptions to this rule. For example, in YXXF (Tyr-X-Arg-Phe) motif, the critical Tyr residue is included at the amino-terminal first position and provides general consensus of YXXphi motifs for internalization of insulin-like growth factor receptors, mannose-6-phosphate receptors, and transferrin receptors [179, 180, 182].
Table 2.
Important short-sequence motifs for the internalization and trafficking of various membrane receptors. The degenerate short sequence motifs usually contain tyrosine or phenylalanine residues, followed by hydrophobic or aromatic residues. Some sequences also contain acidic residues in conjunction with required tyrosine. Certain membrane receptors make use of the dileucine-type of signal motifs.
| Signal Motifs | Membrane Receptor/Protein | |
|---|---|---|
| DSLL | (Asp-Ser-Leu-Leu) | Beta-adrenergic receptor |
| YENPTY | (Tyr-Glu-Asn-Pro-Thr-Tyr) | Beta-amyloid precursor protein |
| YKYSKV | (Tyr-Lys-Tyr-Ser-Lys-Val) | CD-Mannose-6-phosphate receptor |
| YSKV | (Tyr-Ser-Lys-Val) | CI-Mannose-6-phosphate receptor |
| GDAY | (Gly-Asp-Ala-Tyr) | Guanylyl cyclase receptor-A |
| FDNPVY | (Phe-Asp-Asn-Pro-Val-Tyr) | LDL receptor |
| GTALL | (Gly-Thr-Ala-Leu-Leu) | LH receptor |
| FENTLY | (Phe-Glu-Asn-Thr-Leu-Tyr) | Mannose phosphate receptor |
| YEQGL | (Tyr-Glu-Gln-Gly-Leu) | P2x receptor (ATP-gated ion channel) |
| YTRF | (Tyr-Thr-Arg-Phe) | Transferrin receptor |
| YQPL | (Tyr-Gln-Pro-Leu) | T-cell receptor (CD3) |
| LL | (Leu-Leu) | Fc receptor |
GLUT4, glucose transporter 4; LDL, low density lipoprotein; LH, leutinizing hormone; TGN, Trans-Golgi network. Copyright @ 2009 Frontiers in BioScience. Used with permission.
Internalization of GC-B/NPRB
Interestingly, Brackmann et al. 2005 [188] have shown that guanylyl cyclase-B/natriuretic peptide receptor-B (GC-B/NPRB) is internalized and recycled in hippocampus neurons and C6 glioma cells cultures.. These authors suggested that the trafficking of GC-B/NPRB occurs ligand-dependently in response to CNP binding and stimulation of the receptor protein. The internalization and trafficking of GC-B/NPRB has been suggested to involve a clathrin-dependent mechanism. Our recent work indicates that the internalization of GC-A/NPRA also involves clathrin-dependent pathways [189]. Receptor internalization is severely diminished by inhibitors of clathrin proteins such as chlorpromazine and monodensyl cadaverine. However, interaction of the GDAY motif in GC-A/NPRA and GC-B/NPRB with clathrin adaptor proteins remains to be established.
Conclusions and perspectives
In recent years, much has been learned about the function and mechanisms of endocytic pathways responsible for the trafficking and molecular sorting of membrane receptors and their ligands into intracellular compartments. These signals usually consist of short linear amino-acid sequences, which are recognized by adaptor coat proteins along the endocytic and sorting pathways. It is thought that complex arrays of signals and recognition proteins ensure the dynamic movement, accurate trafficking, and designated distribution of transmembrane receptors and ligands into intracellular compartments, particularly the endosomal-lysosomal system. In this review, we have given major emphasis to the functions of short-sequence signal motifs responsible for the itinerary and destination of GC-A/NPRA into subcellular compartments. Substantial evidence supports the premise that expression and cellular regulation of GC-A/NPRA activity is accomplished by the insertion of receptor on the plasma membrane, ligand binding, and movement of the receptor protein through multiple subcellular compartments. Assessment of the stoichiometric distribution of 125I-ANP bound to GC-A/NPRA from plasma membrane to the intracellular compartments and into culture medium has provided the definitive means of directly determining the dynamics of ANP-mediated translocation and redistribution of biologically active GC-A/NPRA in intact cells. In this process, ligand-bound GC-A/NPRA is rapidly internalized and delivered to the endosomes, while a majority of ligand-receptor complexes are degraded in lysosomal compartments. However, a small population of receptor is dissociated from the ligand in intracellular compartments and recycles back to the plasma membrane, and intact ligand is released into culture medium. Recent findings have established that specific intracellular cytoplasmic domain, as well as the specific sequence motif GDAY promotes the internalization and trafficking of GC-A/NPRA.
Experimental data support the notion that various components may influence the internalization, intracellular trafficking, and redistribution of GC-A/NPRA. Among these components are the binding kinetics and interaction of ligand receptor complexes of GC-A/NPRA, which govern the rate at which receptor traverses through the intracellular compartments. Another is the receptor protein, which should be adequately processed for appropriate routing and redistribution in various subcellular compartments. Then there are the specific short- sequence signals, which inherently control the internalization, routing, and trafficking of ligand-receptor complexes in the intracellular compartments. The specific routing path and itinerary followed by the receptor may be cell-specific. These possibilities have ensured that cellular regulation and expression of GC-A/NPRA activity involves cellular endocytosis, trafficking, and the movement of activated receptor into subcellular compartments. Nevertheless, we have not yet attained a full understanding of the cellular pathway and routing of receptor trafficking or the rate at which receptors traverse the cell interior and influence the sensitivity of the cells to ligand. Similarly, receptor biosynthesis and the subcellular assembly that is responsible for receptor expression and function remain to be defined in a cell-specific manner. More importantly, studies are needed to focus on the biosynthetic assemblies of GC-coupled receptors and the regulatory functions of these assemblies in receptor endocytosis, trafficking, desensitization or inactivation, down-regulation, and metabolic degradation of the GC family of receptors. Also waiting for thorough investigation is the molecular basis of endocytic processes, as well as its relationship to the GC-A/NPRA phosphorylation, dephosphorylation, and/or glycosylation in the context of the internalization, trafficking and metabolic redistribution of receptors in the subcellular compartments.
Acknowledgments
I thank my wife Kamala Pandey for her assistance in preparing this manuscript. I also offer my special thanks to Dr. Bharat B. Aggarwal, Department of Experimental Therapeutics and Cytokine Research Laboratory at MD Anderson Cancer Center, Houston, TX, and Dr. Susan L. Hamilton, Department of Molecular Physiology and Biophysics at Baylor College of Medicine, Houston, TX, who made their facilities available to us during our displacement due to Hurricane Katrina. The work in the author's laboratory was supported by grants from the National Institutes of Health (HL57531 and R56 HL57531).
References
- 1.de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981;28:89–94. doi: 10.1016/0024-3205(81)90370-2. [DOI] [PubMed] [Google Scholar]
- 2.Brenner BM, Ballermann BJ, Gunning ME, Zeidel ML. Diverse biological actions of atrial natriuretic peptide. Physiol Rev. 1990;70:665–699. doi: 10.1152/physrev.1990.70.3.665. [DOI] [PubMed] [Google Scholar]
- 3.de Bold AJ. Atrial natriuretic factor: a hormone produced by the heart. Science. 1985;230:767–770. doi: 10.1126/science.2932797. [DOI] [PubMed] [Google Scholar]
- 4.Levin ER, Gardner DG, Samson WK. Natriuretic peptides. N Engl J Med. 1998;339:321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 5.Pandey KN. Biology of natriuretic peptides and their receptors. Peptides. 2005;26:901–932. doi: 10.1016/j.peptides.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 6.Pandey KN. Emerging roles of antriuretic peptides and their receptors in pathophysiology of hypertension and cardiovascular regulation. J Am Soc Hypert. 2008;2:210–226. doi: 10.1016/j.jash.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellmers LJ, Scott NJ, Piuhola J, Maeda N, Smithies O, Frampton CM, Richards AM, Cameron VA. Npr1-regulated gene pathways contributing to cardiac hypertrophy and fibrosis. J Mol Endocrinol. 2007;38:245–257. doi: 10.1677/jme.1.02138. [DOI] [PubMed] [Google Scholar]
- 8.Pandey KN. Vascular action Natriuretic peptide receptor. In: Sowers JR, editor. Contemporary Endocrinology: Endocrinology of the Vasculature. Humana Press Inc.; Totawa, NJ: 1996. pp. 255–267. [Google Scholar]
- 9.Khurana ML, Pandey KN. Receptor-mediated stimulatory effect of atrial natriuretic factor, brain natriuretic peptide, and C-type natriuretic peptide on testosterone production in purified mouse Leydig cells: activation of cholesterol side-chain cleavage enzyme. Endocrinology. 1993;133:2141–2149. doi: 10.1210/endo.133.5.8404664. [DOI] [PubMed] [Google Scholar]
- 10.McGrath MF, de Bold ML, de Bold AJ. The endocrine function of the heart. Trends Endocrinol Metab. 2005;16:469–477. doi: 10.1016/j.tem.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Garbers DL. Guanylyl cyclase receptors and their endocrine, paracrine and autocrine ligands. Cell. 1992;71:1–4. doi: 10.1016/0092-8674(92)90258-e. [DOI] [PubMed] [Google Scholar]
- 12.Koller KJ, deSauvage FJ, Lowe DG, Goeddel DV. Conservation of the kinase-like regulatory domain is essential for activation of the natriuretic peptide receptor guanylyl cyclase. Mol Cell Biol. 1992;12:2581–2590. doi: 10.1128/mcb.12.6.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drewett JG, Garbers DL. The family of guanylyl cyclase receptors and their ligands. Endocr Rev. 1994;15:135–162. doi: 10.1210/edrv-15-2-135. [DOI] [PubMed] [Google Scholar]
- 14.Sharma RK. Evolution of the membrane guanylate cyclase transduction system. Mol Cell Biochem. 2002;230:3–30. [PubMed] [Google Scholar]
- 15.Duda T, Venkataraman V, Ravichandran S, Sharma RK. ATP-regulated module (ARM) of the atrial natriuretic factor receptor guanylate cyclase. Peptides. 2005;26:969–984. doi: 10.1016/j.peptides.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 16.Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA. Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev. 2000;52:375–414. [PubMed] [Google Scholar]
- 17.Pandey KN. Physiology of the natriuretic peptides gonadal function. Humana Press; Totawa, NJ: 1997. pp. 171–191. [Google Scholar]
- 18.Koller KJ, Lowe DG, Bennett GL, Minamino N, Kangawa K, Matsuo H, Goeddel DV. Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP). Science. 1991;252:120–123. doi: 10.1126/science.1672777. [DOI] [PubMed] [Google Scholar]
- 19.Suga S, Nakao K, Itoh H, Komatsu Y, Ogawa Y, Hama N, Imura H. Endothelial production of C-type natriuretic peptide and its marked augmentation by transforming growth factor-beta possible existence of vascular natriuretic peptide system. J Clin Invest. 1992;90:1145–1149. doi: 10.1172/JCI115933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez MJ, Wong SK, Kishimoto I, Dubois S, Mach V, Friesen J, Garbers DL, Beuve A. Salt-resistant hypertension in mice lacking the guanylyl cyclase-A receptor for atrial natriuretic peptide. Nature. 1995;378:65–68. doi: 10.1038/378065a0. [DOI] [PubMed] [Google Scholar]
- 21.Oliver PM, Fox JE, Kim R, Rockman HA, Kim HS, Reddick RL, Pandey KN, Milgram SL, Smithies O, Maeda N. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor. A. Proc Natl Acad Sci U S A. 1997;94:14730–14735. doi: 10.1073/pnas.94.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sekiguchi T, Miyamoto K, Mizutani T, Yamada K, Yazawa T, Yoshino M, Minegishi T, Takei Y, Kangawa K, Minamino N, Saito Y, Kojima M. Molecular cloning of natriuretic peptide receptor A from bullfrog (Rana catesbeiana) brain and its functional expression. Gene. 2001;273:251–257. doi: 10.1016/s0378-1119(01)00585-6. [DOI] [PubMed] [Google Scholar]
- 23.Shi SJ, Nguyen HT, Sharma GD, Navar LG, Pandey KN. Genetic disruption of atrial natriuretic peptide receptor-A alters renin and angiotensin II levels. Am J Physiol. 2001;281:F665–673. doi: 10.1152/ajprenal.2001.281.4.F665. [DOI] [PubMed] [Google Scholar]
- 24.Vellaichamy E, Khurana ML, Fink J, Pandey KN. Involvement of the NF-kappa B/matrix metalloproteinase pathway in cardiac fibrosis of mice lacking guanylyl cyclase/natriuretic peptide receptor A. J Biol Chem. 2005;280:19230–19242. doi: 10.1074/jbc.M411373200. [DOI] [PubMed] [Google Scholar]
- 25.Oliver PM, John SW, Purdy KE, Kim R, Maeda N, Goy MF, Smithies O. Natriuretic peptide receptor 1 expression influences blood pressures of mice in a dose-dependent manner. Proc Natl Acad Sci U S A. 1998;95:2547–2551. doi: 10.1073/pnas.95.5.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi SJ, Vellaichamy E, Chin SY, Smithies O, Navar LG, Pandey KN. Natriuretic peptide receptor A mediates renal sodium excretory responses to blood volume expansion. Am J Physiol. 2003;285:F694–702. doi: 10.1152/ajprenal.00097.2003. [DOI] [PubMed] [Google Scholar]
- 27.Pandey KN, Oliver PM, Maeda N, Smithies O. Hypertension associated with decreased testosterone levels in natriuretic peptide receptor-A gene-knockout and gene-duplicated mutant mouse models. Endocrinology. 1999;140:5112–5119. doi: 10.1210/endo.140.11.7121. [DOI] [PubMed] [Google Scholar]
- 28.Pandey KN, Inagami T, Misono KS. Atrial natriuretic factor receptor on cultured Leydig tumor cells: ligand binding and photoaffinity labeling. Biochemistry. 1986;25:8467–8472. doi: 10.1021/bi00374a022. [DOI] [PubMed] [Google Scholar]
- 29.Pandey KN. Stoichiometric analysis of internalization, recycling, and redistribution of photoaffinity-labeled guanylate cyclase/atrial natriuretic factor receptors in cultured murine Leydig tumor cells. J Biol Chem. 1993;268:4382–4390. [PubMed] [Google Scholar]
- 30.Pandey KN. Functional roles of short sequence motifs in the endocytosis of membrane receptors. Front Biosci. 2009;14:5339–5360. doi: 10.2741/3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pandey KN, Kumar R, Li M, Nguyen H. Functional domains and expression of truncated atrial natriuretic peptide receptor-A: the carboxyl-terminal regions direct the receptor internalization and sequestration in COS-7 cells. Mol Pharmacol. 2000;57:259–267. [PubMed] [Google Scholar]
- 32.Pandey KN, Nguyen HT, Sharma GD, Shi SJ, Kriegel AM. Ligand-regulated internalization, trafficking, and down-regulation of guanylyl cyclase/atrial natriuretic peptide receptor-A in human embryonic kidney 293 cells. J Biol Chem. 2002;277:4618–4627. doi: 10.1074/jbc.M106436200. [DOI] [PubMed] [Google Scholar]
- 33.Maack T. The role of atrial natriuretic factor in volume control. Kidney Int. 1996;49:1732–1737. doi: 10.1038/ki.1996.257. [DOI] [PubMed] [Google Scholar]
- 34.Chinkers M, Garbers DL, Chang MS, Lowe DG, Chin HM, Goeddel DV, Schulz S. A membrane form of guanylate cyclase is an atrial natriuretic peptide receptor. Nature. 1989;338:78–83. doi: 10.1038/338078a0. [DOI] [PubMed] [Google Scholar]
- 35.Lowe DG, Chang M-S, Hellmis R, Chen E, Singh S, Garbers DL, Goeddel DV. Human atrial natriuretic peptide receptor defines a new paradigm for second messenger signal transduction. EMBO J. 1989;8:1377–1384. doi: 10.1002/j.1460-2075.1989.tb03518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandey KN, Singh S. Molecular cloning and expression of murine guanylate cyclase/atrial natriuretic factor receptor cDNA. J Biol Chem. 1990;265:12342–12348. [PubMed] [Google Scholar]
- 37.Schulz S, Singh S, Bellet RA, Singh G, Tubb DJ, Chin H, Garbers DL. The primary structure of a plasma membrane guanylate cyclase demonstrates diversity within this new receptor family. Cell. 1989;58:1155–1162. doi: 10.1016/0092-8674(89)90513-8. [DOI] [PubMed] [Google Scholar]
- 38.Chang MS, Lowe DG, Lewis M, Hellmiss R, Chen E, Goeddel DV. Differential activation by atrial and brain natriuretic peptides of two different receptor guanylate cyclases. Nature. 1989;341:68–72. doi: 10.1038/341068a0. [DOI] [PubMed] [Google Scholar]
- 39.Fuller F, Porter JG, Arfsten AE, Miller J, Schilling JW, Scarborough RM, Lewicki JA, Schenk DB. Atrial natriuretic peptide clearance receptor. Complete sequence and functional expression of cDNA clones. J Biol Chem. 1988;263:9395–9401. [PubMed] [Google Scholar]
- 40.Anand-Srivastava MB, Trachte GJ. Atrial natriuretic factor receptor and signal transduction mechanisms. Pharmacol Rev. 1993;45:455–497. [PubMed] [Google Scholar]
- 41.Foster DC, Garbers DL. Dual role for adenine nucleotides in the regulation of the atrial natriuretic peptide receptor, guanylyl cyclase-A. J Biol Chem. 1998;273:16311–16318. doi: 10.1074/jbc.273.26.16311. [DOI] [PubMed] [Google Scholar]
- 42.Duda T, Goraczniak RM, Sharma RK. Core sequence of ATP regulatory module in receptor guanylate cyclases. FEBS Lett. 1993;315:143–148. doi: 10.1016/0014-5793(93)81151-o. [DOI] [PubMed] [Google Scholar]
- 43.Koller KJ, Lipari MT, Goeddel DV. Proper glycosylation and phosphorylation of the type A natriuretic peptide receptor are required for hormone-stimulated guanylyl cyclase activity. J Biol Chem. 1993;268:5997–6003. [PubMed] [Google Scholar]
- 44.Goraczniak RM, Duda T, Sharma RK. A structural motif that defines the ATP-regulatory module of guanylate cyclase in atrial natriuretic factor signalling. Biochem J. 1992;282(Pt 2):533–537. doi: 10.1042/bj2820533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burczynska B, Duda T, Sharma RK. ATP signaling site in the ARM domain of atrial natriuretic factor receptor guanylate cyclase. Mol Cell Biochem. 2007;301:93–107. doi: 10.1007/s11010-006-9400-7. [DOI] [PubMed] [Google Scholar]
- 46.Liu Y, Ruoho ER, Rao VD, Hurley JH. Catalytic mechanism of the adenylyl cyclase Modeling and mutational analysis. Proc Natl Acad Sci USA. 1997;94:13414–13419. doi: 10.1073/pnas.94.25.13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sunahara RK, Beuve A, Tesmer JJG, Sprang SR, Garbers DL, Gilman AG. Exchange of substrate and inhibitor specificities between adenylyl and guanylyl cyclase. J Biol Chem. 1998;273:16332–16338. doi: 10.1074/jbc.273.26.16332. [DOI] [PubMed] [Google Scholar]
- 48.Tucker CL, Hurley JH, Miller TR, Hurley JB. Two amino acid substitutions convert a guanylyl cyclase, Ret GC-1 into an adenylyl cyclase. Proc Natl Acad Sci USA. 1998;95:5993–5997. doi: 10.1073/pnas.95.11.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Labrecque J, McNicoll N, Marquis M, De Lean A. A disulfide-bridged mutant of natriuretic peptide receptor-A displays constitutive activity. Role of receptor dimerization in signal transduction. J Biol Chem. 1999;274:9752–9759. doi: 10.1074/jbc.274.14.9752. [DOI] [PubMed] [Google Scholar]
- 50.Wilson EM, Chinkers M. Identification of sequences mediating guanylyl cyclase dimerization. Biochemistry. 1995;34:4696–4701. doi: 10.1021/bi00014a025. [DOI] [PubMed] [Google Scholar]
- 51.Yang RB, Garbers DL. Two eye guanylyl cyclase are expressed in the same photoreceptor cells and form homomers in preference to heteromers. J Biol Chem. 1997;272:13738–13742. doi: 10.1074/jbc.272.21.13738. [DOI] [PubMed] [Google Scholar]
- 52.van den Akker F, Zang X, Miyagi H, Huo X, Misono KS, Yee VC. Structure of the dimerized hormone-binding domain of a guanylyl cyclase-coupled receptor. Nature. 2000;406:101–104. doi: 10.1038/35017602. [DOI] [PubMed] [Google Scholar]
- 53.Garbers DL, Lowe DG. Guanylyl cyclase receptors. J Biol Chem. 1994;269:30714–30744. [PubMed] [Google Scholar]
- 54.Qiu Y, Ogawa H, Miyagi M, Misono KS. Constitutive activation and uncoupling of the atrial natriuretic peptide receptor by mutations at the dimer interface: role of the dimer structure in signaling. J Biol Chem. 2004;279:6115–6123. doi: 10.1074/jbc.M310225200. [DOI] [PubMed] [Google Scholar]
- 55.Misono KS, Ogawa H, Qiu Y, Ogata CM. Structural studies of the natriuretic peptide receptor: a novel hormone-induced rotation mechanism for transmembrane signal transduction. Peptides. 2005;26:957–968. doi: 10.1016/j.peptides.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 56.He X, Chow D, Martick MM, Garcia KC. Allosteric activation of a spring-loaded natriuretic peptide receptor dimer by hormone. Science. 2001;293:1657–1662. doi: 10.1126/science.1062246. [DOI] [PubMed] [Google Scholar]
- 57.He XL, Dukkipati A, Wang X, Garcia KC. A new paradigm for hormone recognition and allosteric receptor activation revealed from structural studies of NPR-C. Peptides. 2005;26:1035–1043. doi: 10.1016/j.peptides.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 58.van den Akker F. Structural insights into the ligand binding domains of membrane bound guanylyl cyclases and natriuretic peptide receptors. J Mol Biol. 2001;311:923–937. doi: 10.1006/jmbi.2001.4922. [DOI] [PubMed] [Google Scholar]
- 59.De Lean A, McNicoll N, Labrecque J. Natriuretic peptide receptor A activation stabilizes a membrane-distal dimer interface. J Biol Chem. 2003;278:11159–11166. doi: 10.1074/jbc.M212862200. [DOI] [PubMed] [Google Scholar]
- 60.Sun JZ, Oparil S, Lucchesi P, Thompson JA, Chen YF. Tyrosine kinase receptor activation inhibits NPR-C in lung arterial smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L155–163. doi: 10.1152/ajplung.2001.281.1.L155. [DOI] [PubMed] [Google Scholar]
- 61.Nakayama T, Soma M, Takahashi Y, Rehemudula D, Sato M, Uwabo J, Izumi Y, Kanmatsuse K. Nucleotide sequence of the 5'-flanking region of the type A human natriuretic peptide receptor gene and association analysis using a novel microsatellite in essential hypertension. Am J Hypertens. 1999;12:1144–1148. doi: 10.1016/s0895-7061(99)00135-1. [DOI] [PubMed] [Google Scholar]
- 62.Garg R, Oliver PM, Maeda N, Pandey KN. Genomic structure, organization, and promoter region analysis of murine guanylyl cyclase/atrial natriuretic peptide receptor-A gene. Gene. 2002;291:123–133. doi: 10.1016/s0378-1119(02)00589-9. [DOI] [PubMed] [Google Scholar]
- 63.Yamagami S, Suzuki K, Suzuki N. Expression and exon/intron organization of two medaka fish homologs of the mammalian guanylyl cyclase A. J Biochem. 2001;130:39–50. doi: 10.1093/oxfordjournals.jbchem.a002960. [DOI] [PubMed] [Google Scholar]
- 64.Schramek H, Gstraunthaler G, Willinger CC, Pfaller W. Hyperosmolality regulates endothelin release by Madin-Darby canine kidney cells. J Am Soc Nephrol. 1993;4:206–213. doi: 10.1681/ASN.V42206. [DOI] [PubMed] [Google Scholar]
- 65.Kashiwagi M, Miyamoto K, Takei Y, Hirose S. Cloning, properties and tissue distribution of natriuretic peptide receptor-A of euryhaline eel, Anguilla japonica. Eur J Biochem. 1999;259:204–211. doi: 10.1046/j.1432-1327.1999.00023.x. [DOI] [PubMed] [Google Scholar]
- 66.Woodard GE, Zhao J, Rosado JA, Brown J. A-type natriuretic peptide receptor in the spontaneously hypertensive rat kidney. Peptides. 2002;23:1637–1647. doi: 10.1016/s0196-9781(02)00106-7. [DOI] [PubMed] [Google Scholar]
- 67.Pandey KN, Nguyen HT, Li M, Boyle JW. Natriuretic peptide receptor-A negatively regulates mitogen-activated protein kinase and proliferation of mesangial cells: role of cGMP-dependent protein kinase. Biochem Biophys Res Commun. 2000;271:374–379. doi: 10.1006/bbrc.2000.2627. [DOI] [PubMed] [Google Scholar]
- 68.Cao L, Chen SC, Cheng T, Humphreys MH, Gardner DG. Ligand-dependent regulation of NPR-A gene expression in inner medullary collecting duct cells. Am J Physiol. 1998;275:F119–125. doi: 10.1152/ajprenal.1998.275.1.F119. [DOI] [PubMed] [Google Scholar]
- 69.Cao L, Wu J, Gardner DG. Atrial natriuretic peptide suppresses the transcription of its guanylyl cyclase-linked receptor. J Biol Chem. 1995;270:24891–24897. doi: 10.1074/jbc.270.42.24891. [DOI] [PubMed] [Google Scholar]
- 70.Hum D, Besnard S, Sanchez R, Devost D, Gossard F, Hamet P, Tremblay J. Characterization of a cGMP-response element in the guanylyl cyclase/natriuretic peptide receptor A gene promoter. Hypertension. 2004;43:1270–1278. doi: 10.1161/01.HYP.0000126920.93207.53. [DOI] [PubMed] [Google Scholar]
- 71.Placier S, Bretot X, Ardaillou N, Dussaule JC, Ardaillou R. Regulation of ANP clearance receptors by EGF in mesangial cells from NOD mice. Am J Physiol Renal Physiol. 2001;281:F244–254. doi: 10.1152/ajprenal.2001.281.2.F244. [DOI] [PubMed] [Google Scholar]
- 72.Khurana ML, Pandey KN. Modulation of guanylate cyclase-coupled atrial natriuretic factor receptor activity by mastoparan and ANF in murine Leydig tumor cells: role of G-proteins. Biochim Biophys Acta. 1994;1224:61–67. doi: 10.1016/0167-4889(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 73.Kumar R, Cartledge WA, Lincoln TM, Pandey KN. Expression of guanylyl cyclase-A/atrial natriuretic peptide receptor blocks the activation of protein kinase C in vascular smooth muscle cells. Role of cGMP and cGMP-dependent protein kinase. Hypertension. 1997;29:414–421. doi: 10.1161/01.hyp.29.1.414. [DOI] [PubMed] [Google Scholar]
- 74.Kumar R, von Geldern TW, Calle RA, Pandey KN. Stimulation of atrial natriuretic peptide receptor/guanylyl cyclase- A signaling pathway antagonizes the activation of protein kinase C-alpha in murine Leydig cells. Biochim Biophys Acta. 1997;1356:221–228. doi: 10.1016/s0167-4889(96)00168-1. [DOI] [PubMed] [Google Scholar]
- 75.Yasunari K, Kohno M, Murakawa K, Yokokawa K, Takeda T. Glucocorticoids and atrial natriuretic factor receptors on vascular smooth muscle. Hypertension. 1990;16:581–586. doi: 10.1161/01.hyp.16.5.581. [DOI] [PubMed] [Google Scholar]
- 76.Yasunari K, Kohno M, Murakawa K, Yokokawa K, Takeda T. Effect of glucocorticoid on prostaglandin E1 mediated cyclic AMP formation by vascular smooth muscle cells. J Hypertens. 1988;6:1023–1028. doi: 10.1097/00004872-198812000-00011. [DOI] [PubMed] [Google Scholar]
- 77.Yang T, Terada Y, Nonoguchi H, Ujiie K, Tomita K, Marumo F. Effect of hyperosmolality on production and mRNA expression of ET-1 in inner medullary collecting duct. Am J Physiol. 1993;264:F684–689. doi: 10.1152/ajprenal.1993.264.4.F684. [DOI] [PubMed] [Google Scholar]
- 78.Lanier-Smith KL, Currie MG. Glucocorticoid regulation of atrial natriuretic peptide receptors on cultured endothelial cells. Endocrinology. 1991;129:2311–2317. doi: 10.1210/endo-129-5-2311. [DOI] [PubMed] [Google Scholar]
- 79.Nuglozeh E, Mbikay M, Stewart DJ, Legault L. Rat natriuretic peptide receptor genes are regulated by glucocorticoids in vitro. Life Sci. 1997;61:2143–2155. doi: 10.1016/s0024-3205(97)00630-9. [DOI] [PubMed] [Google Scholar]
- 80.Bottari SP, King IN, Reichlin S, Dahlstroem I, Lydon N, de Gasparo M. The angiotensin AT2 receptor stimulates protein tyrosine phosphatase activity and mediates inhibition of particulate guanylate cyclase. Biochem Biophys Res Commun. 1992;183:206–211. doi: 10.1016/0006-291x(92)91629-5. [DOI] [PubMed] [Google Scholar]
- 81.Garg R, Pandey KN. Angiotensin II-mediated negative regulation of Npr1 promoter activity and gene transcription. Hypertension. 2003;41:730–736. doi: 10.1161/01.HYP.0000051890.68573.94. [DOI] [PubMed] [Google Scholar]
- 82.Haneda M, Kikkawa R, Maeda S, Togawa M, Koya D, Horide N, Kajiwara N, Shigeta Y. Dual mechanism of angiotensin II inhibits ANP-induced mesangial cGMP accumulation. Kidney Int. 1991;40:188–194. doi: 10.1038/ki.1991.199. [DOI] [PubMed] [Google Scholar]
- 83.Smale ST, Schmidt MC, Berk AJ, Baltimore D. Transcriptional activation by Sp1 as directed through TATA or initiator: specific requirement for mammalian transcription factor IID. Proc Natl Acad Sci U S A. 1990;87:4509–4513. doi: 10.1073/pnas.87.12.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sudhir K, Jennings GL, Esler MD, Korner PI, Blombery PA, Lambert GW, Scoggins B, Whitworth JA. Hydrocortisone-induced hypertension in humans: pressor responsiveness and sympathetic function. Hypertension. 1989;13:416–421. doi: 10.1161/01.hyp.13.5.416. [DOI] [PubMed] [Google Scholar]
- 85.Arise KK, Pandey KN. Inhibition and down-regulation of gene transcription and guanylyl cyclase activity of NPRA by angiotensin II involving protein kinase C. Biochem Biophys Res Commun. 2006;349:131–135. doi: 10.1016/j.bbrc.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 86.Agui T, Xin X, Cai Y, Shim G, Muramatsu Y, Yamada T, Fujiwara H, Matsumoto K. Opposite actions of transforming growth factor-beta 1 on the gene expression of atrial natriuretic peptide biological and clearance receptors in a murine thymic stromal cell line. J Biochem. 1995;118:500–507. doi: 10.1093/oxfordjournals.jbchem.a124936. [DOI] [PubMed] [Google Scholar]
- 87.Fujio N, Gossard F, Bayard F, Tremblay J. Regulation of natriuretic peptide receptor A and B expression by transforming growth factor-beta 1 in cultured aortic smooth muscle cells. Hypertension. 1994;23:908–913. doi: 10.1161/01.hyp.23.6.908. [DOI] [PubMed] [Google Scholar]
- 88.Berl T, Mansour J, Veis JH. Regulation of atrial natriuretic peptide-stimulated cGMP production in the inner medulla. Kidney Int. 1992;41:37–42. doi: 10.1038/ki.1992.5. [DOI] [PubMed] [Google Scholar]
- 89.Chen S, Cao L, Intengan HD, Humphreys M, Gardner DG. Osmoregulation of endothelial nitric-oxide synthase gene expression in inner medullary collecting duct cells. Role in activation of the type A natriuretic peptide receptor. J Biol Chem. 2002;277:32498–32504. doi: 10.1074/jbc.M202321200. [DOI] [PubMed] [Google Scholar]
- 90.Chen S, Gardner DG. Osmoregulation of natriuretic peptide receptor signaling in inner medullary collecting duct. A requirement for p38 MAPK. J Biol Chem. 2002;277:6037–6043. doi: 10.1074/jbc.M111117200. [DOI] [PubMed] [Google Scholar]
- 91.Iimura O, Kusano E, Ishida F, Oono S, Ando Y, Asano Y. Hyperosmolality rapidly reduces atrial-natriuretic-peptide-dependent cyclic guanosine monophosphate production in cultured rat inner medullary collecting duct cells. Pflugers Arch. 1995;430:81–87. doi: 10.1007/BF00373842. [DOI] [PubMed] [Google Scholar]
- 92.Katafuchi T, Mizuno T, Hagiwara H, Itakura M, Ito T, Hirose S. Modulation by NaCl of atrial natriuretic peptide receptor levels and cyclic GMP responsiveness to atrial natriuretic peptide of cultured vascular endothelial cells. J Biol Chem. 1992;267:7624–7629. [PubMed] [Google Scholar]
- 93.Shields PP, Dixon JE, Glembotski CC. The secretion of atrial natriuretic factor-(99-126) by cultured cardiac myocytes is regulated by glucocorticoids. J Biol Chem. 1988;263:12619–12628. [PubMed] [Google Scholar]
- 94.Itoh H, Bird IM, Nakao K, Magness RR. Pregnancy increases soluble and particulate guanylate cyclases and decreases the clearance receptor of natriuretic peptides in ovine uterine, but not systemic, arteries. Endocrinology. 1998;139:3329–3341. doi: 10.1210/endo.139.7.6093. [DOI] [PubMed] [Google Scholar]
- 95.Mulay S, Omer S, Vaillancourt P, D'Sylva S, Singh A, Varma DR. Hormonal modulation of atrial natriuretic factor receptors and effects on adrenal glomerulosa cells of female rats. Life Sci. 1994;55:PL169–176. doi: 10.1016/0024-3205(94)00682-2. [DOI] [PubMed] [Google Scholar]
- 96.Omer S, Vaillancourt P, Peri KG, Varma DR, Mulay S. Downregulation of renal atrial natriuretic factor receptors and receptor mRNAs during rat pregnancy. Am J Physiol. 1997;272:F87–93. doi: 10.1152/ajprenal.1997.272.1.F87. [DOI] [PubMed] [Google Scholar]
- 97.Tucker RF, Volkenant ME, Branum EL, Moses HL. Comparison of intra- and extracellular transforming growth factors from nontransformed and chemically transformed mouse embryo cells. Cancer Res. 1983;43:1581–1586. [PubMed] [Google Scholar]
- 98.Vaillancourt P, Omer S, Deng XF, Mulay S, Varma DR. Differential effects of rat pregnancy on uterine and lung atrial natriuretic factor receptors. Am J Physiol. 1998;274:E52–56. doi: 10.1152/ajpendo.1998.274.1.E52. [DOI] [PubMed] [Google Scholar]
- 99.Bonhomme MC, Garcia R. Heterogeneous regulation of renal atrial natriuretic factor receptor subtypes in one-kidney, one clip hypertensive rats. J Hypertens. 1993;11:389–397. doi: 10.1097/00004872-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 100.Brown LA, Nunez DJ, Wilkins MR. Differential regulation of natriuretic peptide receptor messenger RNAs during the development of cardiac hypertrophy in the rat. J Clin Invest. 1993;92:2702–2712. doi: 10.1172/JCI116887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Leon H, Garcia R. Regulation of glomerular atrial natriuretic factor receptor subtypes by renal sympathetic nerves. Am J Physiol. 1991;260:R1043–1050. doi: 10.1152/ajpregu.1991.260.6.R1043. [DOI] [PubMed] [Google Scholar]
- 102.Dessi-Fulgheri P, Sarzani R, Tamburrini P, Moraca A, Espinosa E, Cola G, Giantomassi L, Rappelli A. Plasma atrial natriuretic peptide and natriuretic peptide receptor gene expression in adipose tissue of normotensive and hypertensive obese patients. J Hypertens. 1997;15:1695–1699. doi: 10.1097/00004872-199715120-00074. [DOI] [PubMed] [Google Scholar]
- 103.Goto M, Itoh H, Tanaka I, Suga S, Ogawa Y, Kishimoto I, Nakagawa M, Sugawara A, Yoshimasa T, Mukoyama M, et al. Altered gene expression of natriuretic peptide receptor subtypes in the kidney of stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol Suppl. 1995;22:S177–179. doi: 10.1111/j.1440-1681.1995.tb02871.x. [DOI] [PubMed] [Google Scholar]
- 104.Kapasi AA, Kumar R, Pauly JR, Pandey KN. Differential expression and autoradiographic localization of atrial natriuretic peptide receptor in spontaneously hypertensive and normotensive rat testes: diminution of testosterone in hypertension. Hypertension. 1996;28:847–853. doi: 10.1161/01.hyp.28.5.847. [DOI] [PubMed] [Google Scholar]
- 105.Tremblay J, Hum DH, Sanchez R, Dumas P, Pravenec M, Krenova D, Kren V, Kunes J, Pausova Z, Gossard F, Hamet P. TA repeat variation, Npr1 expression, and blood pressure: impact of the Ace locus. Hypertension. 2003;41:16–24. doi: 10.1161/01.hyp.0000042664.75193.1b. [DOI] [PubMed] [Google Scholar]
- 106.Vera N, Tse MY, Watson JD, Sarda S, Steinhelper ME, John SW, Flynn TG, Pang SC. Altered expression of natriuretic peptide receptors in proANP gene disrupted mice. Cardiovasc Res. 2000;46:595–603. doi: 10.1016/s0008-6363(00)00038-9. [DOI] [PubMed] [Google Scholar]
- 107.Yoshimoto T, Naruse K, Shionoya K, Tanaka M, Seki T, Hagiwara H, Hirose S, Kuen LS, Demura H, Naruse M, Muraki T. Angiotensin converting enzyme inhibitor normalizes vascular natriuretic peptide type A receptor gene expression via bradykinin-dependent mechanism in hypertensive rats. Biochem Biophys Res Commun. 1996;218:50–53. doi: 10.1006/bbrc.1996.0010. [DOI] [PubMed] [Google Scholar]
- 108.Yoshimoto T, Naruse M, Naruse K, Fujimaki Y, Tanabe A, Muraki T, Itakura M, Hagiwara H, Hirose S, Demura H. Modulation of vascular natriuretic peptide receptor gene expression in hypertensive and obese hyperglycemic rats. Endocrinology. 1995;136:2427–2434. doi: 10.1210/endo.136.6.7750464. [DOI] [PubMed] [Google Scholar]
- 109.Kumar P, Arise KK, Pandey KN. Transcriptional regulation of guanylyl cyclase/natriuretic peptide receptor-A gene. Peptides. 2006;27:1762–1769. doi: 10.1016/j.peptides.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 110.Kumar P, Bolden G, Arise KK, Krazit ST, Pandey KN. Regulation of natriuretic peptide receptor-A gene expression and stimulation of its guanylate cyclase activity by transcription factor Ets-1. Biosci Rep. 2009;29:57–70. doi: 10.1042/BSR20080094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kumar P, Pandey KN. Cooperative activation of Npr1 gene transcription and expression by interaction of Ets-1 and p300. Hypertension. 2009;54:172–178. doi: 10.1161/HYPERTENSIONAHA.109.133033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kuno T, Andresen JW, Kamisaki Y, Waldman SA, Chang LY, Saheki S, Leitman DC, Nakane M, Murad F. Co-purification of an atrial natriuretic factor receptor and particulate guanylate cyclase from rat lung. J Biol Chem. 1986;261:5817–5823. [PubMed] [Google Scholar]
- 113.Pandey KN, Pavlou SN, Inagami T. Identification and characterization of three distinct atrial natriuretic factor receptors. Evidence for tissue-specific heterogeneity of receptor subtypes in vascular smooth muscle, kidney tubular epithelium, and Leydig tumor cells by ligand binding, photoaffinity labeling, and tryptic proteolysis. J Biol Chem. 1988;263:13406–13413. [PubMed] [Google Scholar]
- 114.Takayanagi R, Snajdar RM, Imada T, Tamura M, Pandey KN, Misono KS, Inagami T. Purification and characterization of two types of atrial natriuretic factor receptors from bovine adrenal cortex: guanylate cyclase-linked and cyclase-free receptors. Biochem Biophys Res Commun. 1987;144:244–250. doi: 10.1016/s0006-291x(87)80502-8. [DOI] [PubMed] [Google Scholar]
- 115.Pandey KN, Nguyen HT, Garg R, Khurana ML, Fink J. Internalization and trafficking of guanylyl (guanylate) cyclase/natriuretic peptide receptor A is regulated by an acidic tyrosine-based cytoplasmic motif GDAY. Biochem J. 2005;388:103–113. doi: 10.1042/BJ20041250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rathinavelu A, Isom GE. Differential internalization and processing of atrialnatriuretic-factor B and C receptor in PC12 cells. Biochem J. 1991;276(Pt 2):493–497. doi: 10.1042/bj2760493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Koh GY, Nussenzweig DR, Okolicany J, Price DA, Maack T. Dynamics of atrial natriuretic factor-guanylate cyclase recetpors and receptor-ligand complexes in cultured glomerular mesangial and renomedullary interstitial cells. J Biol Chem. 1992;267:11987–11994. [PubMed] [Google Scholar]
- 118.Pandey KN. Dynamics of internalization and sequestration of guanylyl cyclase/atrial natriuretic peptide receptor-A. Can J Physiol Pharmacol. 2001;79:631–639. [PubMed] [Google Scholar]
- 119.Anand-Srivastava MB. Downregulation of atrial natriuretic peptide ANP-C receptor is associated with alterations in G-protein expression in A10 smooth muscle cells. Biochemistry. 2000;39:6503–6513. doi: 10.1021/bi992660q. [DOI] [PubMed] [Google Scholar]
- 120.Cahill PA, Redmond EM, Keenan AK. Vascular atrial natriuretic factor receptor subtypes are not independently regulated by atrial peptides. J Biol Chem. 1990;265:21896–21906. [PubMed] [Google Scholar]
- 121.Cohen D, Koh GY, Nikonova LN, Porter JG, Maack T. Molecular determinants of the clearance function of type-C receptor of natriuretic peptides. J Biol Chem. 1996;271:9863–9869. doi: 10.1074/jbc.271.16.9863. [DOI] [PubMed] [Google Scholar]
- 122.Hirata Y, Takata S, Tomita M, Takaichi S. Binding, internalization, and degradation of atrial natriuretic peptide in cultured vascular smooth muscle cells of rat. Biochem Biophys Res Commun. 1985;132:976–984. doi: 10.1016/0006-291x(85)91903-5. [DOI] [PubMed] [Google Scholar]
- 123.Murthy KK, Thibault G, Cantin M. Binding and intracellular degradation of atrial natriuretic factor by cultured vascular smooth muscle cells. Mol Cell Endocrinol. 1989;67:195–206. doi: 10.1016/0303-7207(89)90210-4. [DOI] [PubMed] [Google Scholar]
- 124.Napier M, Arcuri K, Vandlen R. Binding and internalization of atrial natriuretic factor by high-affinity receptors in A10 smooth muscle cells. Arch Biochem Biophys. 1986;248:516–522. doi: 10.1016/0003-9861(86)90504-7. [DOI] [PubMed] [Google Scholar]
- 125.Nussenzveig DR, Lewicki JA, Maack T. Cellular mechanisms of the clearance function of type-C receptors of atrial natriuretic factor. J Biol Chem. 1990;265:20952–20958. [PubMed] [Google Scholar]
- 126.Pandey KN. Kinetic analysis of internalization, recycling and redistribution of atrial natriuretic factor-receptor complex in cultured vascular smooth-muscle cells. Ligand-dependent receptor down-regulation. Biochem J. 1992;288:55–61. doi: 10.1042/bj2880055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pandey KN. Intracellular trafficking and metabolic turnover of ligand-bound guanylyl cyclase/atrial natriuretic peptide receptor-A into subcellular compartments. Mol Cell Biochem. 2002;230:61–72. [PubMed] [Google Scholar]
- 128.Delporte C, Poloczek P, Tastenoy M, Winard J, Christopher J. Atrial natriuretic peptide binds to ANP-R 1 receptors in neuroblastoma cells or is degraded extracellularly at the Ser-Phe bond. Eur J Pharmacol. 1992;227:247–256. doi: 10.1016/0922-4106(92)90002-d. [DOI] [PubMed] [Google Scholar]
- 129.Marshall S. Dual pathways for the intracellular processing of insulin: Relationship between retroendocytosis of intact hormone and the recycling of insulin receptors. J BiolChem. 1985;260:13524–13531. [PubMed] [Google Scholar]
- 130.Tietze C, Schlesinger P, Stahl P. Mannose-specific endocytosis receptor of alveolar macrophages: Demonstration of two functionally distinct intracellular pools of receptor and their roles in receptor recycling. J Cell Biol. 1982;92:417–424. doi: 10.1083/jcb.92.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Carpenter G, Cohen S. 125I-labeled human epidermal growth factor: Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976;71:159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Maxifeld FR, Willingram MC, Davis PJ, Pastan I. Amines inhibit the clustering of alpha 2-macroglobulin and EGF on the fibroblast cell surface. Nature. 1979;227:661–663. doi: 10.1038/277661a0. [DOI] [PubMed] [Google Scholar]
- 133.Pandey KN. Internalization and trafficking of guanylyl cyclase/natriuretic peptide receptor-A. Peptides. 2005;26:985–1000. doi: 10.1016/j.peptides.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 134.Ganzalez-Noriega A, Grubb A, Talkad JT, Sly WS. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980;85:839–852. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Van Leuvan F, Cassiman JJ, Van Den Berghe H. Primary amines inhibit recycling of α2 M receptors in fibroblasts. Cell. 1980;20:37–43. doi: 10.1016/0092-8674(80)90232-9. [DOI] [PubMed] [Google Scholar]
- 136.Backer JM, Kahn CR, White MF. Tyrosine phosphorylation of the insulin receptor is not required for receptor internalization: Studies in 2,4-dinitrophenol-treated cells. Pro Natl Acad Sci USA. 1989;86:3201–3213. doi: 10.1073/pnas.86.9.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Smith RM, Jarett L. Differences in adenosine triphosphate dependency of receptor-mediated endocytosis of α2 macroglobulin and insulin correlate with separate routes of ligand-receptor complex internalization. Endocrinology. 1990;126:1551–1560. doi: 10.1210/endo-126-3-1551. [DOI] [PubMed] [Google Scholar]
- 138.Brown MS, Anderson RGW, Goldstein JL. Recycling receptors: The round-trip itinerary of migrant membrane proteins. Cell. 1983;32:663–667. doi: 10.1016/0092-8674(83)90052-1. [DOI] [PubMed] [Google Scholar]
- 139.Elster L, Hansen GH, Belhage BO, Fritschy JM, Mohler H, Schousboe A. Differential distribution of GABA receptor subunits in soma and processes of cerebellular granule cells: Effects of maturation and a GABA agonist. J Dev Neurosci. 1995;13:417–428. doi: 10.1016/0736-5748(95)00024-b. [DOI] [PubMed] [Google Scholar]
- 140.Barnes EM. Intracellular trafficking of GABAA receptors. Int Rev Neurobiol. 1996;39:53–76. doi: 10.1016/s0074-7742(08)60663-7. [DOI] [PubMed] [Google Scholar]
- 141.Kim HY, Sapp DE, Olsen RW, Tobin AJ. GABA alters GABAA receptor mRNAs and increases ligand binding. J Neurochem. 1993;61:2334–2337. doi: 10.1111/j.1471-4159.1993.tb07481.x. [DOI] [PubMed] [Google Scholar]
- 142.Miranda JD, Barnes EM. Repression of γ-aminobutyric acid type A receptor a 1 polypeptide biosynthesis requires chronic agonist exposure. J Biol Chem. 1997;272:16288–16294. doi: 10.1074/jbc.272.26.16288. [DOI] [PubMed] [Google Scholar]
- 143.Lefkowitz RJ. G protein-coupled receptors. J Biol Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- 144.Tsao P, Cao T, Von Zastrow M. Role of endocytosis in mediating down-regulation of G-protein-coupled receptor. Trends Pharmacol Sci. 2001;22:91–96. doi: 10.1016/s0165-6147(00)01620-5. [DOI] [PubMed] [Google Scholar]
- 145.Potter LR, Garbers DL. Protein kinase C-dependent desensitization of the atrial natriuretic peptide receptor is mediated by dephosphorylation. J Biol Chem. 1994;269:14636–14642. [PubMed] [Google Scholar]
- 146.Potter LR, Hunter T. Identification and characterization of the major phosphorylation sites of the B-type natriuretic peptide receptor. J Biol Chem. 1998;273:15533–15539. doi: 10.1074/jbc.273.25.15533. [DOI] [PubMed] [Google Scholar]
- 147.Duda T, Yadav P, Jankowska A, Venkataraman V, Sharma RK. Three dimensional atomic model and experimental validation for the ATP-regulated module (ARM) of the atrial natriuretic factor receptor guanylate cyclase. Mol Cell Biochem. 2001;217:165–172. doi: 10.1023/a:1007236917061. [DOI] [PubMed] [Google Scholar]
- 148.Larose L, Rondeau JJ, Ong H, De Lean A. Phosphorylation of atrial natriuretic factor R1 receptor by serine /threonine protein kinases. Evidence for receptor regulation. Mol Cell Biochem. 1992;115:203–211. doi: 10.1007/BF00230332. [DOI] [PubMed] [Google Scholar]
- 149.Pandey KN. Stimulation of protein phosphorylation by atrial natriuretic factor in plasma membranes of bovine adrenal cortical cells. Biochem Biophys Res Commun. 1989;163:988–994. doi: 10.1016/0006-291x(89)92319-x. [DOI] [PubMed] [Google Scholar]
- 150.Sharma RK, Duda T. Plasma membrane guanylate cyclase: A multimodule transduction system. Adv Exp Med Biol. 1997;407:271–279. doi: 10.1007/978-1-4899-1813-0_41. [DOI] [PubMed] [Google Scholar]
- 151.Brown MS, Goldstein JL. Receptor-mediated endocytosis: Insights from the lipoprotein receptor system. Pro Natl Acad Sci USA. 1979;76:3330–3337. doi: 10.1073/pnas.76.7.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen WJ, Goldstein JL, Brown MS. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein. J Biol Chem. 1990;265:3116–3123. [PubMed] [Google Scholar]
- 153.Berhanu P. Internalized insulin-receptor complexes are unidirectionally translocated to chloroquine-senstitive degradative sites. J Biol Chem. 1988;263:5961–5969. [PubMed] [Google Scholar]
- 154.Garza LA, Birnbaum MJ. Insulin-responsive aminopeptidease trafficking in 3T3-L1 adipocytes. J Biol Chem. 2000;275:2560–2567. doi: 10.1074/jbc.275.4.2560. [DOI] [PubMed] [Google Scholar]
- 155.Paccuad JP, Siddle K, Carpentier JL. Internalization of the human insulin receptor: the insulin-independent pathway. J Biol Chem. 1992;267:13101–13106. [PubMed] [Google Scholar]
- 156.Ashworth R, Yu R, Nelson EJ, Dermer S, Gershengorn MC. Visualization of the thyrotropin-releasing hormone receptor and its ligand during endocytosis and recycling. Proc Natl Acad Sci U S A. 1995;92:512–516. doi: 10.1073/pnas.92.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hartford J, Bridges K, Ashwell G, Klausner RD. Intracellular dissociation of receptor-bound asialoglycoproteins in cultured hepatocytes. J Biol Chem. 1983;258:3191–3197. [PubMed] [Google Scholar]
- 158.Dunn WA, Hubbard AL. Receptor-mediated endocytosis of epidermal growth factor by hepatocytes in the perfused rat liver: Ligand and receptor dynamics. J Biol Chem. 1984;249:5153–5162. doi: 10.1083/jcb.98.6.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Marshall S, Green A, Olefsky JM. Evidence for recycling of insulin receptors in isolated rat adipocytes. J Biol Chem. 1981;256:11464–11470. [PubMed] [Google Scholar]
- 160.Well A, Wellsh JB, Lazer CS, Wiley HS, Rosenfeld MG. Ligand-induced transformation by a noninternalizing epidermal growth factor receptor. Science. 1990;247:962–964. doi: 10.1126/science.2305263. [DOI] [PubMed] [Google Scholar]
- 161.Sorkin A, Westermark B, Heldin CH, Claesson-Welsh L. Effect of receptor kinase inactivation on the rat of internalization and degradation of PDGF and the PDGF beta-receptor. J Cell Biol. 1991;112:469–478. doi: 10.1083/jcb.112.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sorkin A, von Zastrow M. Signal transduction and endocytosis close encounters of many kinds. Nature Rev Mol Cell Biol. 2002;3:600–614. doi: 10.1038/nrm883. [DOI] [PubMed] [Google Scholar]
- 163.Burwen SJ, Barker ME, Goldman IS, Hradek GT, Raper SE, Jones AL. Transport of epidermal growth factor by rat liver: Evidence for a nonlysosomal pathway. J Cell Biol. 1984;99:1259–1265. doi: 10.1083/jcb.99.4.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chang T-M, Kullberg DW. Diacytosis of 125I-asialoorosomucoid by rat hepatocytes: a non-lysosomal pathway insensitive to inhbibition by inhibitors of ligand degradation. Biochim Biophys Acta. 1984;805:268–276. doi: 10.1016/0167-4889(84)90082-x. [DOI] [PubMed] [Google Scholar]
- 165.Sorkin A, Krolenkom S, Kudrjavtceva N, Lazebnik J, Teslenko L, Soderquist AM, Nikolsky N. Recycling of epidermal grwoth factor-receptor complexes in A431cells: identification of dual pathways. J Cell Biol. 1991;112:55–63. doi: 10.1083/jcb.112.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Haft CR, Klausner RD, Taylor SI. Involvement of dileucine motifs in the internalization and degradation of the insulin receptors. J Biol Chem. 1994;269:26286–26294. [PubMed] [Google Scholar]
- 167.Huang Z, Chen Y, Nissenson RA. The cytoplasmic tail of the G-protein-coupled receptor for parathyroid hormone and parathyroid hormone-related protein contains positive and negative signals for endocytosis. J Biol Chem. 1995;270:151–156. doi: 10.1074/jbc.270.1.151. [DOI] [PubMed] [Google Scholar]
- 168.Perez HD, Holmes R, Vilander LR, Adams RR, Manzana W, Jolley D, Andrews WH. Formyl peptide receptor chimeras define domains involved in ligand binding. J Biol Chem. 1993;268:2292–2295. [PubMed] [Google Scholar]
- 169.Sorkin A, Mohammadi M, Huang J, Slessinger J. Internalization of fibroblast growth factor receptor is inhibited by a point mutation at tyrosine 766. J Biol Chem. 1994;269:17056–17061. [PubMed] [Google Scholar]
- 170.Berhanu P, Iabrahim-Schneck H, Anderson C, Wood WM. The NPEY sequence is not necessary for endocytosis and processing of insulin-receptor complexes. Mol Endocrinol. 1991;5:1827–1835. doi: 10.1210/mend-5-12-1827. [DOI] [PubMed] [Google Scholar]
- 171.Bergeron JJM, Cruz J, Khan MN, Posher BI. Uptake of insulin and other ligands in receptor rich endocytic components of target cells: The endosomal apparatus. Ann Rev Physiol. 1985;47:383–403. doi: 10.1146/annurev.ph.47.030185.002123. [DOI] [PubMed] [Google Scholar]
- 172.Nussenzveig DR, Heinflink M, Gershengorn MC. Agonist-stimulated internalization of the thyrotropin-releasing hormone receptor is dependent on two domains in the receptor carboxyl terminus. J Biol Chem. 1993;268:2389–2392. [PubMed] [Google Scholar]
- 173.Chen Z, Dupre DJ, Le Gouill C, Rola-Pleszczynski M, Stankova J. Agonist-induced internalization of the platelet-activating factor receptor is dependent on arrestins but independent of G-protein activation. Role of the C terminus and the (D/N)PXXY motif. J Biol Chem. 2002;277:7356–7362. doi: 10.1074/jbc.M110058200. [DOI] [PubMed] [Google Scholar]
- 174.Bouley R, Sun TX, Chenard M, McLaughlin M, McKee M, Lin HY, Brown D, Ausiello DA. Functional role of the NPxxY motif in internalization of the type 2 vasopressin receptor in LLC-PK1 cells. Am J Physiol Cell Physiol. 2003;285:C750–762. doi: 10.1152/ajpcell.00477.2002. [DOI] [PubMed] [Google Scholar]
- 175.Li Y, Marzolo MP, van Kerkhof P, Strous GJ, Bu G. The YXXL motif, but not the two NPXY motifs, serves as the dominant endocytosis signal for low density lipoprotein receptor-related protein. J Biol Chem. 2000;275:17187–17194. doi: 10.1074/jbc.M000490200. [DOI] [PubMed] [Google Scholar]
- 176.Thies RS, Webster NJ, McClain DA. A domain of the insulin receptor required for endocytosis in rat fibroblasts. J Biol Chem. 1990;265:10132–10137. [PubMed] [Google Scholar]
- 177.Lazarovits J, Roth M. A single amino acid change in the cytoplasmic domain allows the influenza virus hemagglutinin to be endocytosed through coated pits. Cell. 1988;53:743–752. doi: 10.1016/0092-8674(88)90092-x. [DOI] [PubMed] [Google Scholar]
- 178.Stolt PC, Bock HH. Modulation of lipoprotein receptor functions by intracellular adaptor proteins. Cell Signal. 2006;18:1560–1571. doi: 10.1016/j.cellsig.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 179.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 180.Collawn JF, Stangel M, Kuhn LA, Esekogwu V, Jing SQ, Trowbridge IS, Tainer JA. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell. 1990;63:1061–1072. doi: 10.1016/0092-8674(90)90509-d. [DOI] [PubMed] [Google Scholar]
- 181.Rajagopalan M, Neidigh JL, McClain DA. Amino acid sequences Gly-Pro-Leu-Tyr and Asn-Pro-Glu-Tyr in the submembranous domain of the insulin receptor are required for normal endocytosis. J Biol Chem. 1991;266:23068–23073. [PubMed] [Google Scholar]
- 182.Ktistakis NT, Thomas D, Roth MG. Characteristics of the tyrosine recognition signal for internalization of transmembrane surface glycoproteins. J Cell Biol. 1990;111:1393–1407. doi: 10.1083/jcb.111.4.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Collawn JF, Kuhn LA, Liu LF, Tainer JA, Trowbridge IS. Transplanted LDL and mannose-6-phosphate receptor internalization signals promote high-efficiency endocytosis of the transferrin receptor. Embo J. 1991;10:3247–3253. doi: 10.1002/j.1460-2075.1991.tb04888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bansal A, Gierasch LM. The NPXY internalization signal of the LDL receptor adopts a reverse-turn conformation. Cell. 1991;67:1195–1201. doi: 10.1016/0092-8674(91)90295-a. [DOI] [PubMed] [Google Scholar]
- 185.Eberle W, Sander C, Klaus W, Schmidt B, von Figura K, Peters C. The essential tyrosine of the internalization signal in lysosomal acid phosphatase is part of a beta turn. Cell. 1991;67:1203–1209. doi: 10.1016/0092-8674(91)90296-b. [DOI] [PubMed] [Google Scholar]
- 186.Gabilondo AM, Krasel C, Lohse MJ. Mutations of Tyr326 in the beta 2-adrenoceptor disrupt multiple receptor functions. Eur J Pharmacol. 1996;307:243–250. doi: 10.1016/0014-2999(96)00247-6. [DOI] [PubMed] [Google Scholar]
- 187.Barak LS, Tiberi M, Freedman NJ, Kwatra MM, Lefkowitz RJ, Caron MG. A highly conserved tyrosine residue in G protein-coupled receptors is required for agonist-mediated beta 2-adrenergic receptor sequestration. J Biol Chem. 1994;269:2790–2795. [PubMed] [Google Scholar]
- 188.Brackmann M, Schuchmann S, Anand R, Braunewell KH. Neuronal Ca2+ sensor protein VILIP-1 affects cGMP signalling of guanylyl cyclase B by regulating clathrin-dependent receptor recycling in hippocampal neurons. J Cell Sci. 2005;118:2495–2505. doi: 10.1242/jcs.02376. [DOI] [PubMed] [Google Scholar]
- 189.Somanna NK, Arise KK, Pandey KN. Analysis of natriuretic peptide receptor A Internalization by ribonucleic acid interference. J Am Investig Med. 2007;55:S262. [Google Scholar]