Abstract
Genetic engineering of T cells for adoptive transfer by introducing a tumor-targeting chimeric antigen receptor (CAR) is a new approach to cancer immunotherapy. A challenge for the field is to define cell surface molecules that are both preferentially expressed on tumor cells and can be safely targeted with T cells. The orphan tyrosine kinase receptor ROR1 is a candidate target for T-cell therapy with CAR-modified T cells (CAR-T cells) since it is expressed on the surface of many lymphatic and epithelial malignancies and has a putative role in tumor cell survival. The cell surface isoform of ROR1 is expressed in embryogenesis but absent in adult tissues except for B-cell precursors, and low levels of transcripts in adipocytes, pancreas, and lung. ROR1 is highly conserved between humans and macaques and has a similar pattern of tissue expression. To determine if low-level ROR1-expression on normal cells would result in toxicity or adversely affect CAR-T cell survival and/or function, we adoptively transferred autologous ROR1 CAR-T cells into nonhuman primates. ROR1 CAR-T cells did not cause overt toxicity to normal organs and accumulated in bone marrow and lymph node sites where ROR1-positive B cells were present. The findings support the clinical evaluation of ROR1 CAR-T cells for ROR1+ malignancies and demonstrate the utility of nonhuman primates for evaluating the safety of immunotherapy with engineered T cells specific for tumor-associated molecules that are homologous between humans and nonhuman primates.
Keywords: Chimeric antigen receptor, ROR1, Animal model, Cellular immunotherapy, Leukemias and lymphomas
Introduction
The introduction of specific antigen receptors into T cells by gene transfer allows the rapid generation of tumor-reactive T cells from any cancer patient for adoptive T-cell therapy. A promising strategy involves engineering T cells with synthetic chimeric antigen receptors (CARs) comprising a single-chain antibody or other binding domain that is specific for a tumor cell surface molecule and is linked to one or more T-cell signaling molecules (1–3). Recent trials using CAR-modified T cells (CAR-T cells) specific for the CD19 molecule on B-cell malignancies demonstrated marked tumor regression in a subset of patients with advanced disease (3–6). Extending this therapy to common epithelial cancers poses several challenges, including the identification of molecules that are expressed on tumor cells and can be targeted safely with T cells. This is underscored by serious and even fatal toxicities that have been observed due to on-target/off-tumor effects of CAR-therapy on normal cells that express the target molecule (7, 8). Thus, identifying and validating molecules that are expressed selectively on tumor cells and can be targeted without serious toxicity to normal cells remain important areas of research.
The tyrosine kinase receptor gene ROR1 encodes two well-defined isoforms-a short 393 amino acid (aa) intracellular protein (isoform 2) and a long 937 aa type-1 transmembrane protein (isoform 1)(9, 10). The long cell surface isoform is expressed on primary human B-chronic lymphocytic leukemias (B-CLL) and mantle cell lymphomas (11), a subset of B-acute lymphocytic leukemia, and many epithelial tumors including breast cancer, where it has been associated with a metastatic phenotype (12–19). Experiments in which ROR1-expression is knocked-down in tumor cells or conversely expressed as a transgene, demonstrate that ROR1 provides pro-survival signals, suggesting that selection of tumor variants lacking ROR1 would be detrimental to tumor progression (17–22). In normal tissues, ROR1 protein is abundantly expressed during embryonic development, but absent in most adult tissues except a stage of immature B-cells in the bone marrow (BM). ROR1 mRNA is also detected in adipocytes, pancreas, and lung but at markedly lower levels than in tumor cells(11, 23, 24). A recent study used Western blot to analyze ROR1-expression in tissue lysates and identified a protein with the predicted molecular size of isoform 2 in several tissues. The full-length cell surface isoform 1 was not detected in normal tissues (12, 13). This differential expression of ROR1 in cancerous and normal tissue is encouraging; however it would be ideal to evaluate the safety of targeting ROR1 with CAR-T cells in an animal model to determine the potential for serious toxicity from recognition of rare normal tissue cells that might express ROR1.
We previously developed ROR1-specific CARs that when expressed in T cells confer potent tumor recognition of ROR1-expressing tumor cell lines in vitro and in NOD/SCID/γc−/− mice engrafted with human tumor xenografts(11, 25). The most active ROR1 CAR was constructed from the R12 single-chain variable fragment (scFv) that recognizes an epitope at the interface of the immunoglobulin-like and frizzled (Ig/Fz)-region of ROR1 (11, 25, 26). The aa sequences of the Ig/Fz-region of ROR1 are not completely conserved between mouse and humans, and the R12 scFv does not bind murine ROR1 (26). Human and macacamulatta (M. mulatta) ROR1 are completely homologous in the Ig/Fz-region and we found that the tissue expression of ROR1 in macaques and humans was similar. Thus, we evaluated the safety of autologous ROR1 CAR-T cells in adoptive T-cell transfer experiments in M. mulatta. We observed no overt clinical toxicity even after transferring high doses of ROR1 CAR-T cells. Moreover, ROR1 CAR-T cells trafficked to BM and lymph nodes (LNs), and were functional in vivo as demonstrated by elimination of endogenous ROR1+ B cells and response to challenge with ROR1 antigen. Our findings support the careful clinical evaluation of ROR1 CAR-T cells for ROR1+ malignancies, and suggest the nonhuman primate (NHP) model may be useful to examine safety of CAR-T cells for many candidate molecules expressed on human cancers and homologous between humans and macaques.
Materials and Methods
Human subjects
Peripheral blood mononuclear cells (PBMC) were obtained from donors or patients after written informed consent on protocols approved by the Institutional Review Board of the Fred Hutchinson Cancer Research Center (FHCRC).
Animal protocols and monitoring
The Institutional Animal Care and Use Committee of the University of Washington and FHCRC approved the animal protocols. M. mulatta were housed at the Washington National Primate Research Center under American Association for Accreditation of Laboratory Animal Care approved conditions. The study was performed according to recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Autologous macaque CD4+ and CD8+ T cells were modified by retrovirus gene transfer to introduce the ROR1 CAR and/or a surface marker, and infused intravenously in a CD4:CD8 ratio of 1:1 in each CAR T-cell transfer experiment. BM aspirates and LN biopsies were obtained before and after infusion. Veterinary staff monitored the animals for clinical toxicity. Complete blood count (CBC) and serum chemistry were measured in accredited clinical laboratories. The frequency of transferred T cells in blood, LNs, and BM was measured by flow cytometry using mAbs specific for truncated CD19 (tCD19), truncated EGFR (EGFRt), or truncated CD34 (tCD34) markers, and by quantitative real-time PCR (qPCR) for unique vector sequences within the R12-ROR1 CAR or marker gene using a qPCR-assay (TaqMan) as described (27).
Retroviral vectors and cell lines
The R12-ROR1-specific CAR containing the IgG4 hinge, CD28 transmembrane domain, and a signaling module comprising the cytoplasmic domains of human 4–1BB and CD3ζ was described previously (25). A similar ROR1 CAR except containing the macaque 4–1BB and CD3ζ sequences was constructed. Both constructs encode a T2A ribosomal skip element and a rhesus tCD19 sequence downstream the CAR (27, 28). Codon-optimized nucleotide sequences (Life Technologies) were cloned into the MP71 retroviral vector (29). MP71 vectors encoding only macaque EGFRt (30), macaque tCD34 (31), and a codon-optimized truncated human ROR1 gene (tROR1) were constructed (32), and retroviruses were produced as described (27–29). K562 cells transfected to express ROR1 (K562/ROR1) were previously described (11, 25).
Retroviral transduction, expansion, and characterization of macaque T cells
Isolation of macaque PBMCs, retroviral transduction, T-cell expansion, and specificity analysis of ROR1 CAR-T cells was performed as described (11, 27). Transduced T cells were first enriched by immunomagnetic selection for expression of tCD19, EGFRt, or tCD34 (27, 30, 31), and at the end of the first stimulation cycle, CD4+ and CD8+ fractions were separated by immunomagnetic selection, and used as cell bank. For large-scale expansion for the CART-cell transfer experiments, T cells from each subset were stimulated separately with anti-CD3/CD28 mAbs in the presence of γ-irradiated human PBMCs and B-lymphoblastoid lymphocytes, and IL2 (27).
For analyses of cytokine secretion, ROR1 CAR-T cells were incubated for 24 hours at an effector-to-target (E/T) ratio of 2:1, and supernatants were assayed using a macaque-specific multiplex assay (Life Technologies). In some experiments, CAR-T cells were labeled with carboxyfluorescein-succinimidyl ester (CFSE, Life Technologies), plated in triplicate with K562/ROR1 cells, K562 cells, or phorbol-12-myristate 13-acetate (PMA) and Ionomycin, and examined after 72 hours by flow cytometry as described (25).
Flow cytometry
PBMCs and T cells were stained with fluorochrome-conjugated mAbs to macaque CD3, CD4, CD8, CD34, CD14, CD16, CD45 (BD Biosciences), CD19 (Beckman Coulter), ROR1 (2A2,Biolegend or Miltenyi), or isotype-controls. Fixable viability dyes were obtained from eBioscience. EGFRt-expression was examined using a biotinylated EGFR-specific antibody (30). Samples were analyzed on an LSR-II instrument and FlowJo software (Tree Star, Inc.).
CD107A assay
We used a CD107A-degranulation assay to examine the function of ROR1 CAR-T cells (4, 33). Samples of ROR1 CAR-T cells or PBMCs were stimulated for 4 hours at 37°C at a 4:1 ratio with K562/ROR1 cells, media, or PMA/Ionomycin, in the presence of anti-CD49d/CD28 mAbs, monensin, and a PE-conjugated CD107A mAb (eBioscience). Cells were then stained with fluorochrome-conjugated CD3, CD8, and CD19 mAbs and analyzed by flow cytometry.
Cytokine analysis of plasma samples
Pre- and post-infusion plasma samples were examined for IFNγ, IL6, and IL2 using a macaque-specific multiplex assay (Life Technologies). A macaque-specific adiponectin bioplex-kit (Bio-Rad) was used to detect plasma adiponectin.
Analysis for transgene product-specific T-cell responses
ROR1 CAR-specific CTL responses were examined as described (34, 35). Pre- and post-infusion PBMCs were stimulated twice one week apart with γ-irradiated autologous ROR1 CAR-T cells and examined for recognition of 51Cr-labeled unmodified or ROR1 CART cells.
Reverse transcriptase (RT)-qPCR
A RT-qPCR assay was used to determine ROR1-expression in human and macaque tissues as described (11). cDNA from most tissues was obtained from Biochain and additional cDNA samples were prepared using the SuperScript-III First-Strand-Synthesis kit starting with RNA (1μg) from human pancreas and colon (Clontech) and from primary B-CLL cells. 2μL of cDNA was used in a 10μL reaction using the Power-SYBR-Green-PCR mix on the 7900HT Fast Real-Time PCR-System (Life Technologies). ROR1-expression was normalized to the geometric mean of the housekeeping genes glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and TATA-binding protein (TBP) using gene-specific forward (F) and reverse (R) primers as previously described (11, 36) including human ROR1-F: 5′-AGCGTGCGATTCAAAGGATT-3′, human ROR1-R: 5′-GACTGGTGCCGACGATGACT-3′, human GAPDH-F: 5′-GAAGGTGAAGGTCGGAGTC-3′, human GAPDH-R: 5′-GAAGATGGTGATGGGATTTC-3′, human TBP-F: 5′-TGCACAGGAGCCAAGAGTGAA-3′, human TBP-R: 5′-CACATCACAGCTCCCCACCA-3′, rhesus ROR1-F: 5′-AGCTTGCGATTCAAAGGATT-3′, rhesus ROR1-R: 5′-GACTGGTGGTGATGATGACT-3′, rhesus GAPDH-F: 5′-GAAGGTGAAGGTCGGAGTC-3′, rhesus GAPDH-R: 5′-GAAGATGGTGATGGGGCTTC-3′, rhesus TBP-F: 5′-TGCACAGGAGCCAAGAGTGAA-3′, rhesus TBP-R: 5′-CACATCACAGCTCCCCACCA-3′. Primer-efficiency and fold-changes were determined using the Pfaffl method (37), and expression determined relative to B-CLL cells.
Results
Macaque and human ROR1 homologues exhibit similar tissue expression
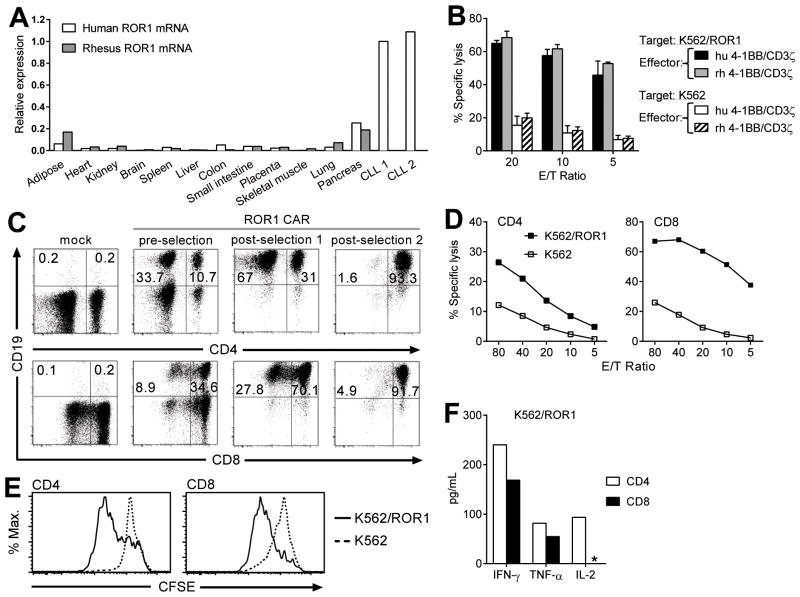
Mouse ROR1 is not recognized by the R12 scFv due to differences in the protein sequence in the Ig/Fz-region, which precluded safety evaluation of ROR1 CAR-T cells in mice (26). In contrast, the region targeted by R12 is entirely conserved between human ROR1 (NCBI: NP_005003) and rhesus macaques (NCBI: EHH14854). To determine if the expression pattern of ROR1 was similar in tissues of macaques and humans, we performed RT-qPCR on aliquots of cDNA from a panel of human and macaque tissues, and two primary B-CLL samples that expressed cell-surface ROR1 by flow cytometry (11). The level of ROR1 mRNA was similar in human and macaque tissues with low levels detected in adipose tissue, pancreas, and lung (Fig. 1A). These data suggest that the NHP model is relevant for evaluating the safety of targeting ROR1.
Figure 1.
A, RT-qPCR of ROR1 mRNA expression in B-CLL cells and a panel of human and rhesus macaque tissues. ROR1-expression was normalized to the geometric mean of the housekeeping genes GAPDH and TBP. B-CLL sample #1 was used as the reference control (ROR1-expression=1). B, cytolytic activity of rhesus T cells modified to express a R12-ROR1 CAR with human (hu) or rhesus macaque (rh) 4–1BB and CD3ζ domains against 51Cr-labeled K562/ROR1 or K562 cells. Results (mean, SEM) are from 3 independent experiments in 3 animals (A11047, A13011, A13002). C, flow cytometric analysis of purity and phenotype of ROR1 CAR-modified CD4+ and CD8+ T cells showing expression of tCD19 in CD4+ (top panels) or CD8+ (bottom panels) T cells either mock-transduced, after transduction with the ROR1 CAR (pre-selection), after selection for tCD19-expression (post-selection 1), and after selection for CD4+ or CD8+ cells, respectively (post-selection 2). All samples are gated on CD3+ cells. D, cytolytic activity of CD4+ and CD8+ ROR1 CAR-T cells against 51Cr-labeled K562/ROR1 or K562 cells. E, proliferation of CFSE-labeled ROR1 CAR-T cells 72 hours after stimulation with K562/ROR1 cells or unmodified K562 cells. CFSE-dye dilution is shown after gating on CD3+CD4+tCD19+ or CD3+CD8+tCD19+T cells. F, Luminex cytokine assay of supernatants obtained after 24 hours from triplicate co-cultures of 5×104 CD4+ or CD8+ROR1 CAR-T cells with K562/ROR1 cells or media alone as described in Methods. The asterisk (*) demarks cytokine levels below detection level. Cytokine levels in media controls were below detection level. Data in C-F is shown for macaque A13002 and representative of results in 3 animals.
Expansion and characterization of ROR1 CAR-T cells for adoptive transfer
The human and macaque 4–1BB and CD3ζ-signaling molecules are highly conserved (95%), and there is 100% identity of the immunoreceptor tyrosine-based activation motifs of CD3ζ. However, to ensure the ROR1 CARs were functional in macaque T cells, we transduced T cells with ROR1 CARs encoding human or macaque 4–1BB and CD3ζ-signaling domains, and evaluated the recognition of ROR1+ target cells. To provide a selection marker to purify transduced T cells, we incorporated a sequence encoding rhesus tCD19 in the vector downstream of a T2A ribosomal skip element (27). The recognition of K562/ROR1 tumor cells by macaque T cells transduced with either the ROR1 CAR containing human 4–1BB and CD3ζ or the ROR1 CAR encoding macaque 4–1BB and CD3ζ was not significantly different (Fig. 1B). We elected to perform the in vivo safety studies using the construct containing the human signaling domains to support the clinical translation of this vector.
For adoptive transfer experiments, we obtained samples of peripheral blood from 3 adult rhesus macaques, stimulated PBMCs with anti-CD3/CD28 mAbs, and transduced the cells with the ROR1 CAR vector. CD4+ and CD8+ T cells were efficiently transduced in vitro, as measured by tCD19-expression, and transduced cells were enriched to >90% purity by immunomagnetic selection 4–5 days after transduction (Fig. 1C). Following expansion, a second immunomagnetic selection was performed to purify CD4+ and CD8+ CAR-T cells (Fig. 1C), and each subset was then expanded separately to formulate a1:1 mixture and ensure uniform composition of CAR-T cells in each transfer experiment. The function of the ROR1 CAR in CD4+ and CD8+ T cells was confirmed by cytotoxicity, proliferation, and cytokine-release assays after co-culture with K562/ROR1 cells (Fig. 1D-F). Using the same strategy, we generated control autologous CD4+ and CD8+ T cells modified to express only a marker gene and co-infused these with ROR1 CAR-T cells into each animal to compare migration and persistence.
Adoptive transfer of a low dose of ROR1 CAR-T cells is safe
To evaluate the safety of infusing autologous ROR1 CAR-T cells and analyze their persistence and migration in vivo, we co-administered 1×108 ROR1 CAR-T cells/kg and 1×108EGFRt+ T cells/kg (CD4:CD8-ratio of 1:1) to a single macaque. These cell doses are less than the dose of CMV-specific T cells we have administered safely to macaques, but equals or exceeds the dose of CD19 CAR-T cells used in clinical trials to treat CD19+ B-cell malignancies (38). We monitored the animal after the T-cell infusions for fever, respiratory distress, appetite, diarrhea, and weight loss, and examined pre- and post-infusion blood samples for CBC, serum chemistry, and cytokine levels. We observed no immediate or delayed clinical abnormalities at this cell dose. Body weight, CBC, and serum chemistry remained within normal limits (39, 40), apart from a transient increase in the muscle-derived creatine-phosphokinase (CPK)(Fig. 2A). The increase in CPK is also seen in control animals not treated with ROR1 CAR-T cells and attributed to intra-muscular (i.m.) ketamine injections given for sedation before the transfer of autologous gene-marked T cells (Supplementary Fig. S1A and B) (41). Plasma levels of IFNγ and IL6 were increased on day 1 after ROR1 CAR-T cells, but returned to normal by day 3 (Fig. 2B). We did not observe increases in IFNγ and IL6 after transferring a 5-times higher dose of gene-marked T cells into a separate animal (Supplementary Fig. S1C), suggesting that the elevated cytokine levels after infusing ROR1 CAR-T cells may have reflected transient activation of CAR-T cells in vivo.
Figure 2.
Toxicity and in vivo persistence of ROR1 CAR-T cells. A, body weight and serum chemistry before and at the indicated days after the T-cell infusion. The grey shaded area demarks the normal range for each parameter in rhesus macaques. B, plasma cytokine levels measured prior to and post-infusion. C, frequency of transferred T cells (%) within the CD3+CD4+ and CD3+CD8+ subsets in blood after infusion of ROR1 CAR and control EGFRt+ T cells. Stainings are with mAbs specific for CD3, CD4, CD8, and CD19 or EGFRt. D, absolute numbers of ROR1 CAR+ and EGFRt+T cells in the blood measured by flow cytometry and calculated based on the results of a CBC on the indicated days in an accredited clinical laboratory. E, real-time qPCR for the presence of transgene vector-specific DNA sequences in samples of PBMC obtained before and after T-cell transfer. The arrow indicates the T-cell infusion (D, E).
ROR1 CAR-T cells are functional in vivo but exhibit altered migration
Analysis of the frequency of ROR1 CAR and control T cells in the blood revealed a difference beginning on day 1 after infusion. ROR1 CAR-T cells were detected in the blood one day after the infusion at a frequency of 0.3% of CD4+ and 0.8% of CD8+ T cells (7 cells/μL and 6 cells/μL), and persisted at levels of 4–11 cells/μL over the following 2 weeks. The frequencies of control CD4+ and CD8+EGFRt+ T cells were higher on day 1 (4.6% of CD4+ and 6.2% of CD8+ T cells corresponding to 102 cells/μL and 48 cells/μL respectively). EGFRt+ T cells gradually declined to stable levels of 6–13 cells/μL during the 4 weeks of follow-up (Fig. 2C-D). qPCR analysis for transgene-specific sequences confirmed the distinct pattern of in vivo persistence (Fig. 2E).
The persistence data was consistent with the ROR1 CAR-T cells migrating from the blood, perhaps into tissues where ROR1 is expressed. We previously showed in humans that a subset of immature B cells in the BM expresses ROR1, and that adoptively transferred macaque T cells migrate to BM and LNs (11, 27, 28). There fore, we examined BM and LN samples obtained prior to, and on day 5 post-infusion, for the presence of transferred T cells and ROR1+ B cells. EGFRt+ and ROR1 CAR-T cells were present in the post-infusion BM and LN samples; however the ROR1 CAR-T cells were present at 1.2–2.5-fold higher frequency than the control EGFRt+ T cells (Fig. 3A). In humans, the pre-BII-large stage of B cells in the BM that express ROR1 have a CD19+CD10+CD45intermediate phenotype (11), however CD10 does not serve as a marker to detect immature B cells in macaques (42). Therefore, we gated only on CD19+CD45intermediate B cells and identified ROR1-expression on a small fraction of these cells in the BM obtained prior to the T-cell infusion (Supplementary Fig. S2). The ROR1+ fraction was greatly reduced on day 5 after transfer of ROR1 CAR-T cells (Fig. 3B). Enumeration of the peripheral blood CD19+ B cells, which are ROR1-, demonstrated that the ROR1 CAR-T cells had no effect on the mature CD19+ B-cell pool (Fig. 3C). These results demonstrate that transferred ROR1 CAR-T cells migrated to the BM, eliminated ROR1+ B-cell precursors, but did not cause organ toxicity or deplete circulating mature B cells.
Figure 3.
In vivo migration and function of ROR1 CAR-T cells. A, frequency of ROR1 CART cells in BM and LN samples obtained on day 5 after the T-cell infusion. Stainings were performed with mAbs specific for CD3, CD4, CD8, and CD19 or EGFRt, and gating on CD3+CD4+ or CD3+CD8+T cells. B, frequency of ROR1+ B cells in the BM before and on day 5 after ROR1 CAR-T cell infusion. The gating strategy for CD19+CD45intermediate B cells is shown in Supplementary Fig. S2. C, absolute number of CD19+ B cells in blood samples obtained before and after the T-cell infusion determined by staining with mAbs specific for CD19, CD3, CD4, and CD8 and flow cytometry to detect CD19+CD3- B cells. Absolute numbers were determined based on the lymphocyte count of a CBC obtained at the same time and determined in an accredited clinical laboratory. D, CD107A-degranulation assay on PBMC obtained before and at day 7 post-infusion, stimulated ex vivo with K562/ROR1 cells or PMA and Ionomycin. Expression of CD107A was determined by flow cytometry. Cultured ROR1 CAR-T cells served as positive control. E, detection of a transgene product-specific T-cell response after transfer of ROR1 CAR-T cells. Pre- and post-infusion PBMC were stimulated twice one week apart with γ-irradiated ROR1 CAR-T cells and autologous feeder cells. Aliquots of these cultures were evaluated in a cytotoxicity assay for recognition of autologous 51Cr-labeled ROR1 CAR-T cells or unmodified T cells.
To confirm that the ROR1 CAR-T cells remained functional in vivo, PBMC obtained one week post-infusion were stimulated for 4 hours with K562/ROR1 cells, PMA/Ionomycin, or media alone, and analyzed for expression of CD107A, which is a marker of degranulation after antigen recognition by CD8+ T cells (33). Pre-infusion PBMCs and an aliquot of ROR1 CAR-T cells served as controls. After stimulation, CD107A-expression increased in the subset of CD8+ ROR1 CAR-T cells persisting in vivo similar to that observed with ROR1 CAR-T cells prior to infusion (Fig. 3D), suggesting the lack of observed organ toxicity was not due to dysfunction of ROR1 CAR-T cells.
ROR1 CAR-T cells but not control gene-marked T cells became undetectable on day 28 (Fig. 2D and E). Because the R12 scFv is derived from a rabbit antibody and is fused to human IgG4 hinge, 4–1BB, and CD3ζ molecules, foreign sequences in the transgene product could potentially elicit a T-cell immune response against the transgene-expressing T cells. To address this possibility, we stimulated samples of pre- and post-infusion PBMCs with autologous ROR1 CAR-T cells in vitro, and examined these lines for recognition of autologous ROR1 CAR- and unmodified T cells. We observed specific lysis of ROR1 CAR-T cells by the post-infusion, but not pre-infusion T-cell line (Fig. 3E). Thus, the transferred CAR-T cells elicited a CTL response in vivo that coincided with their elimination 4 weeks post-infusion, which limited analysis of long-term toxicity.
High doses of ROR1 CAR-T cells respond to antigen in vivo and lack overt toxicity
The infusion of higher CAR-T cell doses may be necessary to treat ROR1+ malignancies and might reveal toxicities to normal tissues, particularly if the CAR-T cells were activated in vivo by recognition of ROR1+tumor cells. During analysis of ROR1-expression on B cells in macaques, we discovered that unlike humans, some macaques have a high frequency of mature ROR1+B cells in LNs (Supplementary Fig. S3). We selected two animals with high levels of ROR1+ B cells in LNs for analysis of the safety of a higher dose (5×108/kg) of ROR1 CAR-T cells, since data in the first animal showed that reduction of ROR1+ B cells provided a surrogate for in vivo function of CAR-T cells. We used tCD34 as a marker gene in the control T cells in these animals because CD34-enrichment was more efficient than the EGFRt-selection, making it easier to achieve the higher T-cell dose. We also planned an infusion of autologous T cells transfected to express cell-surface tROR1 (ROR1+ T-APC) five days after administration of CAR-T cells to determine if activation of persisting CAR-T cells in vivo might reveal toxicity (Fig. 4A).
Figure 4.
Persistence, migration, and safety of a high dose of ROR1 CAR-T cells. A, schematic design of the T-cell infusions. B, frequency of transferred T cells (%) within the CD3+CD4+ and CD3+CD8+ subsets in blood on day 1 after the infusion of ROR1 CAR and control tCD34+ T cells. Staining is performed with mAbs specific for CD3, CD4, CD8, and CD19 or CD34. C, frequency of ROR1 CAR and control tCD34+ T cells in PBMC, BM, and LN samples obtained on day 3 after the T-cell infusion. Staining was performed with mAbs specific for CD3, CD4, CD8, and CD19 or CD34, and gating on CD3+CD4+ or CD3+CD8+T cells. The frequency of ROR1 CAR and control tCD34+ T cells in each subset is shown in the bar graphs for each animal. D, frequency of ROR1+ B cells in the BM before and 3 days after infusion of ROR1 CAR-T cells. Shown are representative staining of macaque A13011 gated on CD19+ cells. The staining and gating strategy is shown in Supplementary Fig. S2. E, frequency of ROR1+ B cells in the LN before and 3 days after infusion of ROR1 CAR-T cells. The gating strategy is shown in Supplementary Fig. S3. Shown are data from macaque A13011.
The higher dose of ROR1 CAR-T cells was well tolerated. We again observed a reduced frequency of ROR1 CAR-T cells compared to control tCD34+ T cells in the blood one day after transfer (Fig. 4B). The difference in the peak frequency did not reflect unique properties of the marker genes that might influence migration since co-infusion of T cells marked only with tCD19 or tCD34 in a control animal provided equivalently high levels of both populations in vivo (Supplementary Fig. S4A-D). Analysis of cell suspensions from PBMCs, BM and LNs obtained from each animal 3 days after infusion showed that ROR1 CAR-T cells, but not control tCD34+ T cells were present at a higher frequency in the BM than in PBMCs, and the frequency of ROR1 CAR-T cells in the LN exceeded that of control tCD34+ T cells in both animals (Fig. 4C). The accumulation of ROR1 CAR-T cells in BM and LNs at day 3 after adoptive transfer coincided with a 72.5% and 80.9% reduction of ROR1+ B cells compared to the pre-treatment BM and LNs, respectively (Fig. 4D and E). A 72.4% and 78.9% reduction of ROR1+ B cells compared to the pre-treatment BM and LNs was observed in macaque A13002 (data not shown). This result is consistent with what we observed in the first animal treated with a lower dose of ROR1 CAR-T cells and shows that transferred ROR1 CAR-T cells migrate out of the blood and accumulate at sites where ROR1+ B cells reside.
To determine if the ROR1 CAR-T cells could be activated in vivo, we infused autologous tROR1+ T-APC into each animal (Fig. 4A). In vitro, tROR1-modified T cells were recognized as efficiently as K562/ROR1 cells by autologous ROR1 CAR-T cells (Supplementary Fig. S5A). The infusion of tROR1-expressing T-APC did not cause acute side effects in either animal, and we observed increases in CD4+ and CD8+ ROR1 CAR-T cells to 29 cells/μL and 52 cells/μL (A13011), and to 21 cells/μL and 34 cells/μL (A13002) respectively over 5–7 days (Fig. 5A). This represented an up to 7.7-fold (A13011) and 4.3-fold (A13002) increase in ROR1 CAR-T cells without substantial changes in numbers of endogenous or tCD34-marked CD4+ and CD8+ T cells (Fig. 5A and B). We examined samples of PBMCs obtained on day 1, 5, and 7 after T-APC challenge to determine if the ROR1 CAR-T cells eliminated the tROR1+ T-APC in vivo. In A13011, we found that the tROR1+ T cells were present at a frequency of 0.9% of CD3+ T cells one day after transfer and the majority of these cells displayed only low-level ROR1-expression compared to the input tROR1+ T-APC (Fig. 5C). Importantly, the ROR1low T cells declined further to a frequency of 0.3% and <0.1% of CD3+ T cells by day 5 and 7, respectively (Fig. 5C). A similar pattern of T-APC persistence was observed in A13002, with only a few ROR1low T-APC detected at day 5 (data not shown). In contrast, the frequencies of tROR1+ T cells in the blood at days 1, 5, and 7 were 5.5%, 8.6%, and 8.0% of CD3+ T cells after infusion of the same dose to an animal without detectable ROR1 CAR-T cells (Fig. 5D).
Figure 5.
Effect of T-APC challenge on transferred ROR1 CAR-T cells in vivo. A, absolute numbers of ROR1 CAR and tCD34+ T cells in CD3+CD4+ and CD3+CD8+ subsets of PBMC in A13011 and A13002 at the indicated days after T-cell transfer. Staining was performed with mAbs specific for CD3, CD4, CD8, and CD19 or CD34. The CBC was determined in an accredited clinical laboratory. The arrows indicate the day of the T-cell infusion. B, fold-change in the absolute numbers of ROR1 CAR-T cells in the blood after T-APC challenge. The number of endogenous or transferred CD3+CD4+ and CD3+CD8+ T cells/μL of blood was measured by flow cytometry before and on indicated days after the T-APC administration. C, presence of tROR1+ T cells before and after the T-APC infusion in PBMC obtained at days 1, 5, and 7 from an animal (A13011) with persisting ROR1 CAR-T cells. PBMC were stained with anti-CD3 and anti-ROR1 mAbs. D, presence of tROR1+ T cells in PBMC samples obtained before and at the indicated days after T-APC infusion in a control animal (A12022) without ROR1 CAR-T cells.
We monitored toxicity in both animals throughout the experiment and found no clinical symptoms or changes in CBC and serum chemistry, except a transient CPK increase attributed to the i.m. injections (Fig. 6A, Supplementary Fig.S1A and B). An increased level of IFNγ and IL6 was observed on day 1 after ROR1 CAR-T cell transfer and on day 6 (one day after tROR1+ T-APC) in both animals, consistent with activation of CAR-T cells in vivo (Fig. 6B, and data not shown). Macaque A13011 developed an episode of fever (103°F) 12 days after ROR1 CAR-T cell transfer that coincided with inflammation at the LN-biopsy site.
Figure 6.
Toxicity monitoring after adoptive transfer of a high dose of ROR1 CAR-T cells. A, serum chemistry of macaque A13011 and A13002 before and at the indicated days after infusion of ROR1 CAR-T cells (5×108/kg) and tROR1+T-APC challenge. Pancreatic, liver, and muscle enzymes, and glucose were measured in an accredited laboratory. The grey shaded area demarks the macaque specific normal range for each parameter. B, plasma IFNγ and IL6 levels before and at the indicated days after the T-cell infusion. Shown are data from macaque A13011. C, plasma adiponectin levels in animals A11047, A13011, and A13002 on the indicated days after the ROR1 CAR-T cell infusion. D, plasma adiponectin levels in control animals undergoing blood draw procedures alone (A12012) or receiving control gene-modified T cells (A12022, A13003).
Adipose tissue expresses ROR1 mRNA and we had previously shown that human adipocytes differentiated from pre-adipocytes in vitro express a low level of cell-surface ROR1 (11). We did not observe weight loss or any clinical signs of inflammation in adipose tissue, and therefore sought a biomarker that might indicate recognition of adipocytes. Adiponectin is an abundant adipocyte-specific adipokine that is prevalent in human plasma (43), and we hypothesized plasma levels might serve as a marker of adipocyte recognition. Adiponectin levels were examined in plasma samples obtained pre and post T-cell infusion in all three ROR1 CAR-T cell treated animals and in control animals receiving infusions of autologous gene-modified T cells or undergoing blood-draw procedures alone. We observed increased adiponectin levels within 3–7 days after ROR1 CAR-T cell transfer (Fig. 6C). In contrast, total plasma protein levels remained stable during this time and adiponectin levels were not increased in control animals (Fig. 6D, and data not shown). These results suggest the increased adiponectin levels observed after ROR1 CAR-T cells might be indicative of low-level recognition of adipocytes in vivo. Unfortunately, long-term assessment of toxicity to adipocytes was not possible because ROR1 CAR-T cells declined to undetectable levels by weeks 4–7 in both animals (Fig. 2D and E, and Fig. 5A), due to the development of transgene product-specific T-cell responses (Fig. 3E, and data not shown).
Discussion
The success of clinical trials of adoptive T-cell therapy with CD19 CAR-T cells for B-cell malignancies has encouraged efforts to identify target molecules expressed by common epithelial malignancies to broaden therapeutic applications of CAR-T cells. The expression of CD19 on normal B cells results in long-term depletion of normal B cells and hypo-gamma globulinemia in some patients receiving CD19 CAR-T cells, but this complication can be managed by intravenous immunoglobulin treatment (2–4, 6). Many candidate target molecules for epithelial tumors are also expressed at some level on normal tissues, and can pose a greater risk of serious toxicity. This was illustrated by the fatal pulmonary and cardiac toxicity that began within 15 minutes after infusion of 1010 T cells modified to express an ERBB2 CAR (8). Toxicity was attributed to cytokine release and recognition of normal lung epithelium that expresses low amounts of ERBB2 (8). Thus, it would be ideal to have animal models to evaluate the initial safety of novel targets, particularly because analysis of mRNA and protein expression cannot exclude low-level expression on critical but minor subsets of normal cells or determine the level of tissue expression that might confer serious adverse reactions.
There is increasing interest in targeting ROR1 for cancer therapies due to its cell surface expression on many lymphatic and epithelial malignancies and its role in maintaining tumor cell survival (12–22). We previously demonstrated that human ROR1 CAR-T cells conferred tumor-recognition in vitro and in NOD/SCID/γc−/−-xenograft models (11, 25). Here we extend this work and demonstrate the safety of adoptively transferring T cells expressing a second generation (4–1BB/CD3ζ) ROR1 CAR in macaques. Human and macaque ROR1 are highly homologous and exhibit similar low-level mRNA expression on a subset of normal tissues, including adipocytes and pancreas. Our study demonstrates that the adoptive transfer of autologous ROR1 CAR-T cells into macaques in doses equivalent to or exceeding those used in most human cancer immunotherapy trials is not associated with overt acute clinical or laboratory toxicity. The transferred ROR1 CAR-T cells were functional in vivo as demonstrated by CD107A-degranulation in response to antigen, reduction in endogenous ROR1+ B cells in BM and LNs, and in vivo expansion after infusing autologous T cells transfected to express cell-surface ROR1 to provide antigen stimulation. Despite the lack of overt toxicity, the ROR1 CAR-T cells persisted at lower levels compared to control T cells, in part reflecting preferential migration to BM and LNs where ROR1+ B cells are present. We have previously shown that a low level of cell-surface ROR1 is expressed on adipocytes that are differentiated from pre-adipocytes in vitro(11), and plasma adiponectin levels were elevated after ROR1 CAR-T cells suggesting there might have been low-level lysis of adipocytes, although not associated with any clinical toxicity or weight loss.
Our study has limitations and clinical trials targeting ROR1 should be undertaken cautiously. First, we evaluated a construct that contained a 4–1BB costimulatory domain and CD3ζ, and it is possible that other costimulatory domains alone or in tandem could cause toxicity. Second, long-term safety could not be established in our study since all animals developed a T-cell immune response after >3–4 weeks that coincided with elimination of the CAR-T cells. Transgene product-specific immune responses are likely to occur in humans as well since the R12 scFv is derived from a rabbit antibody, and later immune rejection might serve as a safety mechanism to avoid long-term toxicity. However, the duration that CAR-T cells must persist to eliminate tumors is not yet established, and it may be beneficial for tumor therapy to reduce immunogenicity by using a human or humanized scFv specific for ROR1. Third, the animals that were treated in our study did not have a ROR1+ tumor, which could result in persistent T-cell activation and proliferation in vivo and enhance recognition of some normal cells by promoting egress into tissue sites (44). We attempted to mimic this situation by infusing autologous ROR1-expressing T cells; however the burden of antigen-positive cells that was achieved may not be sufficient to reveal toxicity. Despite these limitations, our data support proceeding with clinical trials of ROR1 CAR-T cells in appropriate patients, particularly if the T cells were modified with a suicide gene such as EGFRt or inducible caspase-9(30, 45). Many other candidate targets for CAR-T cell therapy that are expressed on solid tumors are highly conserved between macaques and humans including but not limited to the L1-cell adhesion molecule (46), EGFRv806 (47), folate receptor (48), fibroblast activation protein (49, 50). Our results suggest that initial safety testing in NHP may be useful to identify potential on-target/off-tumor toxicities and prioritize molecules that can be targeted safely with CAR-T cells.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by National Institutes of Health grants CA114536, P50 CA138293, AI053193 (S.R.R.), P51-OD010425 (S.R.R., C.B), and RR00166 (Washington National Primate Research Center). D.S. and M.H. were supported by the German Research Foundation (Deutsche Forschungsgemeinschaft). M.H. was supported by the Leukemia and Lymphoma Society.
We thank the staff of the Washington National Primate Research Center for their expert handling of the animals, and Rick Lawler (Shared Resources, FHCRC) for help with the Luminex analysis. We also thank Dr. Wolfgang Uckert for the MP71 vector (Max-Delbrueck-Center, Berlin, Germany) and Meei-Li Huang (FHCRC) for design of the qPCR assays.
Footnotes
Disclosure of Potential Conflicts of Interest
S.R.R. is co-founder and equity holder of Juno Therapeutics, which has licensed certain technologies from the FHCRC related to adoptive T-cell therapy. C.R. is inventor on a patent application (PCT/US2011/062670) that claims anti-ROR1 mAb R12 and has been filed by the National Institutes of Health. No potential conflicts of interest related to this work were disclosed by the other authors.
Authors’ Contributions
Conception and design: S.R. Riddell, C. Berger
Development of methodology: C. Berger, C. Rader, S.R. Riddell, D. Sommermeyer, M. Hudecek.
Acquisition of data (provided animals, acquired or managed patients, provided facilities, etc): C. Berger, M. Berger, D. Sommermeyer, A. Balakrishnan, P. J. Paszkiewicz, P.L. Kosasih, S.R. Riddell
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): C. Berger, M. Berger, D. Sommermeyer, A. Balakrishnan, P. J. Paszkiewicz, C. Rader, S.R. Riddell
Writing, review and/or revision of the manuscript: C. Berger, M. Berger, D. Sommermeyer, M. Hudecek, C. Rader, S.R. Riddell
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): M. Berger, P.L. Kosasih
Study supervision: S.R. Riddell
References
- 1.Turtle CJ, Hudecek M, Jensen MC, Riddell SR. Engineered T cells for anti-cancer therapy. Curr Opin Immunol. 2012;24:633–9. doi: 10.1016/j.coi.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–98. doi: 10.1158/2159-8290.CD-12-0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. Annu Rev Med. 2014;65:10.1–10.15. doi: 10.1146/annurev-med-060512-150254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kochenderfer JN, Rosenberg SA. Treating B-cell cancer with T cells expressing anti-CD19 chimeric antigen receptors. Nat Rev Clin Oncol. 2013;10:267–76. doi: 10.1038/nrclinonc.2013.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamers CH, Sleijfer S, van Steenbergen S, van Elzakker P, van Krimpen B, Groot C, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21:904–12. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masiakowski P, Carroll RD. A novel family of cell surface receptors with tyrosine kinase-like domain. J Biol Chem. 1992;267:26181–90. [PubMed] [Google Scholar]
- 10.Katoh M, Katoh M. Comparative genomics on ROR1 and ROR2 orthologs. Oncol Rep. 2005;14:1381–4. doi: 10.3892/or.14.5.1381. [DOI] [PubMed] [Google Scholar]
- 11.Hudecek M, Schmitt TM, Baskar S, Lupo-Stanghellini MT, Nishida T, Yamamoto TN, et al. The B-cell tumor associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor. Blood. 2010;116:4532–41. doi: 10.1182/blood-2010-05-283309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baskar S, Kwong KY, Hofer T, Levy JM, Kennedy MG, Lee E, et al. Unique cell surface expression of receptor tyrosine kinase ROR1 in human B-cell chronic lymphocytic leukemia. Clin Cancer Res. 2008;14:396–404. doi: 10.1158/1078-0432.CCR-07-1823. [DOI] [PubMed] [Google Scholar]
- 13.Dave H, Anver MR, Butcher DO, Brown P, Khan J, Wayne AS, et al. Restricted cell surface expression of receptor tyrosine kinase ROR1 in pediatric B-lineage acute lymphoblastic leukemia suggests targetability with therapeutic monoclonal antibodies. PLoS ONE. 2012;7:e52655. doi: 10.1371/journal.pone.0052655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang S, Chen L, Wang-Rodriguez J, Zhang L, Cui B, Frankel W, et al. The onco-embryonic antigen ROR1 is expressed by a variety of human cancers. Am J Pathol. 2012;181:1903–10. doi: 10.1016/j.ajpath.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang S, Chen L, Cui B, Chuang HY, Yu J, Wang-Rodriguez J, et al. ROR1 is expressed in human breast cancer and associated with enhanced tumor-cell growth. PLoS ONE. 2012;7:e31127. doi: 10.1371/journal.pone.0031127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borcherding N, Kusner D, Liu GH, Zhang W. ROR1, an embryonic protein with an emerging role in cancer biology. Protein Cell. 2014;5:496–502. doi: 10.1007/s13238-014-0059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bicocca VT, Chang BH, Masouleh BK, Muschen M, Loriaux MM, Druker BJ, et al. Crosstalk between ROR1 and the pre-B cell receptor promotes survival of t(1;19) acute lymphoblastic leukemia. Cancer Cell. 2012;22:656–67. doi: 10.1016/j.ccr.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui B, Zhang S, Chen L, Yu J, Widhopf GF, Fecteau JF, et al. Targeting ROR1 inhibits epithelial-mesenchymal transition and metastasis. Cancer Res. 2013;73:3649–60. doi: 10.1158/0008-5472.CAN-12-3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi T, Yanagisawa K, Sugiyama R, Hosono Y, Shimada Y, Arima C, et al. NKX2–1/TITF1/TTF-1-induced ROR1 is required to sustain EGFR survival signaling in lung adenocarcinoma. Cancer Cell. 2012;21:348–61. doi: 10.1016/j.ccr.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Gentile A, Lazzari L, Benvenuti S, Trusolino L, Comoglio PM. Ror1 is a pseudokinase that is crucial for Met-driven tumorigenesis. Cancer Res. 2011;71:3132–41. doi: 10.1158/0008-5472.CAN-10-2662. [DOI] [PubMed] [Google Scholar]
- 21.Choudhury A, Derkow K, Daneshmanesh AH, Mikaelsson E, Kiaii S, Kokhaei P, et al. Silencing of ROR1 and FMOD with siRNA results in apoptosis of CLL cells. Br J Haematol. 2010;151:327–35. doi: 10.1111/j.1365-2141.2010.08362.x. [DOI] [PubMed] [Google Scholar]
- 22.Widhopf GF, Cui B, Ghia EM, Chen L, Messer K, Shen Z, et al. ROR1 can interact with TCL1 and enhance leukemogenesis in Eμ-TCL1 transgenic mice. Proc Natl Acad Sci USA. 2014;111:793–8. doi: 10.1073/pnas.1308374111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuda T, Nomi M, Ikeya M, Kani S, Oishi I, Terashima T, et al. Expression of the receptor tyrosine kinase genes, Ror1 and Ror2, during mouse development. Mech Dev. 2001;105:153–6. doi: 10.1016/s0925-4773(01)00383-5. [DOI] [PubMed] [Google Scholar]
- 24.Broome HE, Rassenti LZ, Wang HY, Meyer LM, Kipps TJ. ROR1 is expressed on hematogones (non-neoplastic human B-lymphocyte precursors) and a minority of precursor-B acute lymphoblastic leukemia. Leuk Res. 2011;35:1390–4. doi: 10.1016/j.leukres.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hudecek M, Lupo-Stanghellini MT, Kosasih PL, Sommermeyer D, Jensen MC, Rader C, et al. Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin Cancer Res. 2013;19:3153–64. doi: 10.1158/1078-0432.CCR-13-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Baskar S, Kwong KY, Kennedy MG, Wiestner A, Rader C. Therapeutic potential and challenges of targeting receptor tyrosine kinase ROR1 with monoclonal antibodies in B-cell malignancies. PLoS ONE. 2011;6:e21018. doi: 10.1371/journal.pone.0021018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger C, Berger M, Anderson DE, Riddell SR. A non-human primate model for analysis of safety, persistence, and function of adoptively transferred T cells. J Med Primatol. 2011;40:88–103. doi: 10.1111/j.1600-0684.2010.00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leisegang M, Engels B, Meyerhuber P, Kieback E, Sommermeyer D, Xue S-A, et al. Enhanced functionality of T cell receptor-redirected T cells is defined by the transgene cassette. J Mol Med. 2008;86:573–83. doi: 10.1007/s00109-008-0317-3. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Chang W-C, Wong CW, Colcher D, Sherman M, Ostberg JR, et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood. 2011;118:1255–63. doi: 10.1182/blood-2011-02-337360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rettig MP, Ritchey JK, Meyerrose TE, Haug JS, Dipersio JF. Transduction and selection of human T cells with novel CD34/thymidine kinase chimeric suicide genes for the treatment of graft-versus-host disease. Mol Ther. 2003;8:29–41. doi: 10.1016/s1525-0016(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 32.Forouzesh F, Tabarian SS, Emami S, Tehrani M-J, Hadavi R, Rabbani H. Construction and stable expression of a truncated human receptor tyrosine kinase Ror1 (Ror1-ECD) Avicenna J Med Biotech. 2012;4:41–5. [PMC free article] [PubMed] [Google Scholar]
- 33.Chan KS, Kaur A. Flow cytometric detection of degranulation reveals phenotypic heterogeneity of degranulating CMV-specific CD8+ T lymphocytes in rhesus macaques. J Immunol Methods. 2007;325:20–34. doi: 10.1016/j.jim.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berger C, Huang M-L, Gough M, Greenberg PD, Riddell SR, Kiem H-P. Nonmyeloablative immunosuppressive regimen prolongs in vivo persistence of gene-modified autologous T cells in a nonhuman primate model. J Virol. 2001;75:799–808. doi: 10.1128/JVI.75.2.799-808.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riddell SR, Elliott M, Lewinsohn DA, Gilbert MJ, Wilson L, Manley SA, et al. T-cell mediated rejection of gene-modified HIV-specific cytotoxic T lymphocytes in HIV-infected patients. Nat Med. 1996;2:216–23. doi: 10.1038/nm0296-216. [DOI] [PubMed] [Google Scholar]
- 36.Ahn K, Huh J-W, Park S-J, Kim D-S, Ha H-S, Kim Y-J, et al. Selection of internal reference genes for SYBR green qRT-PCR studies of rhesus monkey (Macaca mulatta) tissues. BMC Mol Biol. 2008;9:78. doi: 10.1186/1471-2199-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maus MV, Grupp SA, Porter DL, June CH. Antibody modified T cells: CARs take the front seat for hematologic malignancies. Blood. 2014;123:2625–35. doi: 10.1182/blood-2013-11-492231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGeachin RL, Akin JR. Amylase levels in the tissue and body fluids of several primate species. Comp Biochem Physiol. 1982;72A:267–9. doi: 10.1016/0300-9629(82)90045-7. [DOI] [PubMed] [Google Scholar]
- 40.Lee J-I, Shin J-S, Lee J-E, Jung W-Y, Lee G, Kim M-S, et al. Reference values of hematology, chemistry, electrolytes, blood gas, coagluation time, and urinalysis in the Chinese rhesus macaques (Macaca mulatta) Xenotransplant. 2012;19:244–8. doi: 10.1111/j.1399-3089.2012.00713.x. [DOI] [PubMed] [Google Scholar]
- 41.Lefebvre HP, Laroute V, Braun JP, Lassourd V, Toutain PL. Non-invasive and quantitative evaluation of post-injection muscle damage by pharmacokinetic analysis of creatine kinase release. Vet Res. 1996;27:343–61. [PubMed] [Google Scholar]
- 42.Demberg T, Brocca-Cofano E, Xiao P, Venzon D, Vargas-Inc, Lee EM, et al. Dynamics of memory B-Cell populations in blood, lymph nodes, and bone marrow during antiretroviral therapy and envelope boosting in simian immunodeficiency virus SIVmac251-infected rhesus macaques. J Virol. 2012;86:12591–604. doi: 10.1128/JVI.00298-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–8. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 45.Di Stasi A, Tey S-K, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673–83. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hong H, Stastny M, Brown C, Chang W-C, Ostberg JR, Forman SJ, et al. Diverse solid tumors expressing a restricted epitope of L1-CAM can be targeted by chimeric antigen receptor redirected T lymphocytes. J Immunother. 2014;37:93–104. doi: 10.1097/CJI.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 47.Gan HK, Burgess AW, Clayton AHA, Scott AM. Targeting of a conformationally exposed, tumor-specific epitope of EGFR as a strategy for cancer therapy. Cancer Res. 2012;72:2924–30. doi: 10.1158/0008-5472.CAN-11-3898. [DOI] [PubMed] [Google Scholar]
- 48.Kandalaft L, Powell D, Coukos G. A phase I clinical trial of adoptive transfer of folate receptor-alpha redirected autologous T cells for recurrent ovarian cancer. J Transl Med. 2012;10:157. doi: 10.1186/1479-5876-10-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tran E, Chinnasamy D, Yu Z, Morgan RA, Lee CCR, Restifo NP, et al. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J Exp Med. 2013;210:1125–35. doi: 10.1084/jem.20130110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang LC, Lo A, Scholler J, Sun J, Majumdar RS, Kapoor V, et al. Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res. 2014;2:154–66. doi: 10.1158/2326-6066.CIR-13-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.