Abstract
Peptides presented by MHC class I molecules are derived mostly from proteins synthesized by the antigen-presenting cell itself, while peptides presented by MHC class II molecules are derived predominantly from materials acquired by endocytosis. External antigens can also be presented by MHC class I molecules in a process referred to as cross-presentation. We report that mouse dendritic cell engagement of a phagocytic target alters endocytic processing and inhibits their proteolytic activities. During phagocytosis, endosome maturation is delayed, shows less progression towards the lysosome, and the endocytosed soluble antigen is targeted for MHC class I cross-presentation. The antigen processing in these arrested endosomes is under the control of NAPDH oxidase associated ROS. We also show that cathepsin S is responsible for the generation of the MHC class I epitope. Our results suggest that in addition to solid structure uptake, DC phagocytosis simultaneously modifies the kinetics of endosomal trafficking and maturation. As a consequence, external soluble antigens are targeted into the MHC class I cross-presentation pathway.
Keywords: Dendritic Cells, Phagocytosis, Cross-presentation, Antigen Processing, MHC class I
Introduction
Cross-presentation is thought to be the main immune detection mechanism for pathogens that do not directly target antigen presenting cells (APCs) [1]. Antigens contained in cellular debris/particulate substances are phagocytosed by APCs, especially dendritic cells (DCs). This often results in a CD8 response with antigenic peptides being presented on MHC class I molecules. In this case, antigens enter the cell via phagocytosis and reside in the phagosomes, topologically separated from the cytosol. For phagocytosed particulate substances, the consensus is that antigens are mostly included in phagosomes; hence there is considerable debate about how they gain access to the MHC class I pathway that mainly processes antigens available in the cytoplasm [2]. A few models have been proposed. An intensely discussed scenario combines work from several labs [3–6]. It was found that ER components appeared in the phagosomes, possibly via fusion between the two. In this case, Sec61, an ER resident membrane transporter, is potentially responsible for translocation of antigens across the phagosomal membrane, to gain access to the conventional MHC class I antigen-processing machinery, such as TAP and proteasomes. In addition, other ER components have been found on the phagosome, leading to the suggestion that phagosome alone could mediate cross-presentation [7]. Recent work suggests that in the presence of Toll-like receptor activation, Rab11a+ endocytoic recycling compartments (ERCs) can contribute MHC class I to phagosomes for peptide loading via SNAP23 [8]. These models are adding complexity to the mechanism of cross-presentation [9].
Particulate antigens are cross-presented with higher efficiency than their soluble counterpart [10, 11]. Soluble antigens alone can be cross-presented although the resulting MHC class I presentation is not very robust [12]. Receptor-mediated endocytosis enhances the efficiency. It has been reported that antigens endocytosed via mannose receptor were biased towards MHC class I [13]. In other cases, antigens targeted to cell surface receptors, such as DNGR-1 (also called Clec9A), and Dectin-1 (Clec7a) were also cross-presented well [14–16]. Fc receptor-based phagocytosis had a similar effect [14]. More recently, Chatterjee et al. directed soluble antigen to different receptors on human dendritic cells. They found that antigens internalized via CD40 and mannose receptor were targeted to EEA1 positive endosomes, and were cross-presented with high efficiency. The same antigens internalized via DEC205 resulted in Lamp-1-positive late endosome/lysosome targeting and were poorly cross-presented to antigen-specific CD8+ T cells [17]. The same group also suggested that certain DCs with reduced lysosomal proteolytic degradation, in contrast with other DCs, might permit better egress of antigens into the cytosol to access the MHC class I pathway. Regardless of the difference, targeting antigens into early endosomal compartments led to efficient cross-presentation in both cell types [18]. These results suggest that the presence of soluble antigens in early endosomes strongly favors their cross-presentation.
Nearly twenty years ago, Germain’s group reported that in a subset of macrophages, adding solid structures enhanced soluble antigen presentation via the MHC class I pathway [19, 20]. They reported that the amount of soluble antigen uptake was not altered by the presence of the solid structures. But the exact mechanism for the enhanced cross-presentation was not revealed.
Extracellular soluble antigens gathered in the endosomes are translocated into MHC class II compartments (MIIC) [21] that have enriched MHC class II molecules, proteolytic enzymes and H-2DM/H-2DO, two MHC class II-like molecules that facilitate and modulate peptide loading. The MHC/peptide complexes are then displayed on the cell surface. Phagocytosis and endocytosis are similar in their maturation processes [22]. A question therefore arises whether phagocytosis will affect the steady state processing of soluble antigens as endosomal maturation is dependent on the same microtubules for their movement and requires the same fusion partners and donors to gain proteolytic enzymes and to achieve acidification [23]. Since professional APCs are responsible for both MHC class II antigen presentation and cross-presentation, whether or how much these two processes affect each other could have immunological implications.
We started our investigation from a set of observations similar to the ones made by Germain’s group. We observed that upon phagocytic activation with non-receptor-based targets, such as processed fine monosodium urate (MSU) crystals and latex beads, external soluble antigens that normally target the MHC class II pathway were also distributed into the MHC class I pathway. Surprisingly, this effect is unrelated to costimulatory molecule expression and is independent of innate immune signaling. Our studies show that, during phagocytosis cathepsin S processed the antigen for the MHC class I antigen presentation. In addition, reactive oxygen species (ROS) generated during phagocytosis reduced the activity of proteases present in these vesicles. To demonstrate biological relevance, we showed that triggering phagocytosis with MSU in mice results in soluble antigen-specific CD8 activation. Our finding represents a scenario in the routing and processing of external soluble antigens under the influence of phagocytosis, and may suggest a new method in controlling antigen-specific immune activation, particularly CD8 T-cell immunity.
Results
Phagocytosis enhances cross-presentation of independently endocytosed soluble antigens
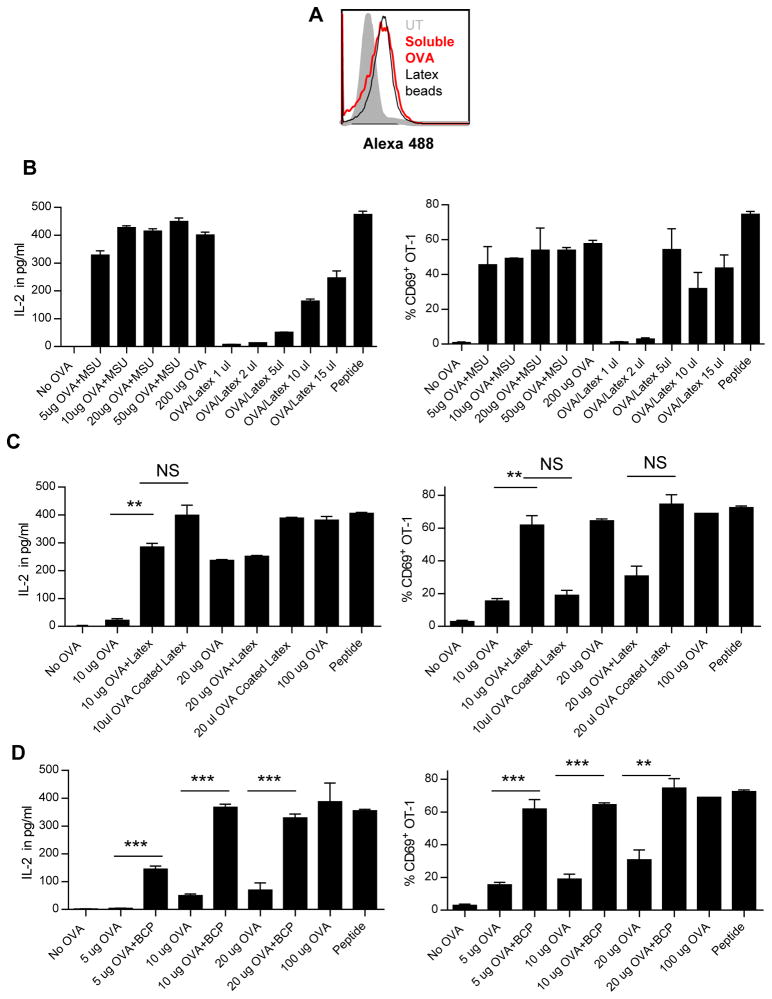
We first studied the presentation of an antigen in the endocytic/MHC class II pathway during phagocytosis. We used OT-1/OT-II T-cell activation assays. DC2.4 cells (a C57BL/6 DC line), or C57BL/6 bone marrow DCs (BMDCs) were incubated with soluble OVA, in the presence or absence of MSU. We ascertained that adding solid structures did not alter OVA uptake by DCs (Fig 1A) within the first 2 hours of MSU/OVA/DC incubation. Extended presence of MSU or latex beads for 4 hours showed a similar degree of OVA uptake (Supporting Information Fig 1A). Non-specific binding of OVA to the DCs at 4°C was minimal, although some absorption was seen after 4 hours (Supporting Information Fig 1B). Antigen loading was then stopped by washing after 4 hours, and DCs were then incubated with OT-1 T cells that specifically recognize OVA peptide SIINFEKL/H-2Kb complex. CD69 expression and IL-2 production in response to soluble OVA in the presence of MSU were increased, regardless if DC2.4 (Fig 1B) or BMDCs (Fig 1C, percentage of CD69-positive cells are shown in bar graphs) were used as APCs. To see if this event was associated with DCs only, we also tested FLT3L (FMS-related tyrosine kinase 3 Ligand)-stimulated BMDCs and cultured primary macrophages. While FLT3L DCs (Supporting Information Fig 1C) behaved similarly to GM-CSF/IL-4 BMDCs and DC2.4 cells, macrophages, on the other hand, showed high variability in results from assay to assay in response to MSU, and were therefore not pursued further (data not shown). Adding MSU 20 minutes before or after OVA incubation with washings in between did not change the outcome, ruling out nonspecific binding of OVA to MSU (Supporting Information Fig 1D). In addition, we did not detect any binding of OVA to MSU under the microscope even when they were mixed (not shown). MSU treatment marginally decreased OT-II T-cell activation, although the difference was not statistically significant in multiple experiments (Fig 1D). We used a new preparation of finer MSU crystals made by one week of mechanical grinding with stainless steel balls. The resulting crystals were smaller (around 0.5–1 um in length) in comparison with crude crystals (10 to 20 um in average length). The fine MSU crystals had a much milder DC stimulation capacity and caused little cell death. This preparation, used in the duration of our assays, did not cause any change in apoptosis (Supporting Information Fig 2A) or MHC class I, class II, CD40, CD80, and CD86 expression in 6 hours (Fig 1E). Treatment of MSU crystals and latex beads did not cause any T-cell activation in the absence of OVA (Supporting Information Fig 2B). To demonstrate that our MSU preparation was not contaminated by microbial products or triggered innate immune response that led to the observed enhanced cross-presentation, we performed the cross-presentation assay with Nlrp3−/−, Myd88−/−/Ticam1−/−, as well as Nfkb1−/− BMDCs. The enhanced cross-presentation in response to MSU crystal remained intact despite these deficiencies (Supporting Information Fig 2C–E). Cross-presentation of soluble antigens can be mediated by mannose receptor (MR) [13]. To see if solid structure-treated cells have enhanced expression of MR, they were stained with an anti MR antibody. We detected no enhance expression of this receptor (Supporting Information Fig 2F).
Figure 1. Soluble OVA can be diverted into cross presentation pathway in the presence of phagocytic signals.
(A) Representative histogram of uptake of Alexa-488-conjugated OVA by DC2.4 cells after 2 hours incubation measured by FACS with or without MSU stimulation. Two-hour loading was chosen because of the minimal interference by MSU at this time point. See Supporting Information Figure 1 for additional loading durations. (B) IL-2 (left panel) and CD69 expression (right panel) in OT1 T cells after being stimulated by DC2.4 cells mixed with soluble OVA in the presence or absence of fine MSU crystals (250 ug/ml) was measured by ELISA and FACS, respectively. (C) Production of IL-2 (left panel) by OT-1 T cells and the percentage of CD69 positive OT-1 T cells (right panel) upon stimulation by C57BL/6 BMDCs mixed with soluble OVA in the presence or absence of MSU was measured by ELISA and FACS, respectively. (D) Expression of activation marker CD69 by OT-II T cells upon stimulation with CB57BL/6 BMDCs mixed with soluble OVA in the presence of MSU was measured by FACS. (E) Representative histograms of CD40, CD80, CD86, MHC class I and MHC class II expression after 6 hour stimulation of fine MSU crystals in comparison with killed E.coli (BAC), 3 um latex beads (0.02% total volume), BCP crystals (250 ug/ml), measured by FACS. (A–E) Data are representative of at least three independent experiments, and (B–D) are shown as mean + SEM (3/group). In this and the subsequent figures, statistical analyses are shown as: *p <0.05, ** p<0.01, *** p<0.001, and N.S. not significant by student T test.
Cross-presentation of endosomal antigen in the presence of solid structure is efficient
Our results raised two questions: is this re-routing effect common to other crystals and how efficient is the endosomal cross-presentation compared with the phagosomal? To compare the two routes of cross-presentation, we fed DCs with either soluble or latex-bound Alexa-488 OVA. Considering these two routes of antigen uptake have different efficiencies, it was necessary to determine the amounts of antigen inside the cells. Due to extensive and different levels of proteolysis in cell lysates (not shown), western blotting-based quantitation was ruled out. We therefore selected FACS to provide a crude estimation of antigen uptake in BMDCs. Two hours into the incubation, 200 ug/ml soluble OVA was roughly equivalent to 15 ul of OVA beads as determined by similar mean fluorescence intensity (Fig 2A). Similar results were obtained from four-hour incubation (Supporting Information Fig 2G). Using this crude estimate as a starting point, we titrated both preparations for incubation with BMDCs, stopped the loading after 4 hours by washing, and mixed the treated cells with OT-1 T cells. Using CD69 and IL-2 as readouts, the same amount of OVA was cross-presented more efficiently via endosome than via phagosome (Fig 2B), i.e. undiluted latex OVA beads were cross-presented to a similar extent as the soluble antigen after a 40-fold dilution. In this assay, the particles used to deliver the antigen and for phagocytic stimulation were different (latex vs MSU), and the experiments were therefore imperfectly controlled. Some crystals such as MSU and CPPD (calcium pyrophosphate dehydrate) cannot be coated with OVA. We therefore studied latex beads both as phagocytic target carrying OVA and as solid structure that stimulated phagocytosis when OVA was given separately. Figure 2C shows that latex beads were fully capable of stimulating the soluble antigen cross-presentation. Because latex beads are an artificial stimulant, we therefore tested basic calcium phosphate (BCP), another pathogenic crystal, in addition to MSU. Figure 2D shows that BCP-stimulated cross-presentation was similar to that of fine MSU crystals and latex beads, suggesting that phagocytosis-induced soluble antigen presentation into the MHC class I pathway may be a common occurrence.
Figure 2. Efficient soluble antigen cross-presentation in the presence of solid structures.
(A) Representative histogram of BMDCs fed with 200 ug/ml Alexa 488-OVA or 15ul Alexa 488-OVA latex beads were analyzed by FACS after 2 hours of incubation. See Supporting Information Figure 2G for additional loading durations. (B–D) The levels of IL-2 and CD69 expression by OT-1 cells were measured by ELISA and FACS, respectively, after stimulation with BMDCs treated with OVA along with (B) MSU, (C) uncoated latex beads, or (B and C) with OVA-coated latex beads alone at the indicated concentrations, or (D) with or without BCP crystals (250 ug/ml). (A–D) Data are representative of at least three independent experiments, and (B–D) are shown as mean + SEM (n=3/group).
Phagocytosis reduces endocytic maturation and proteolysis
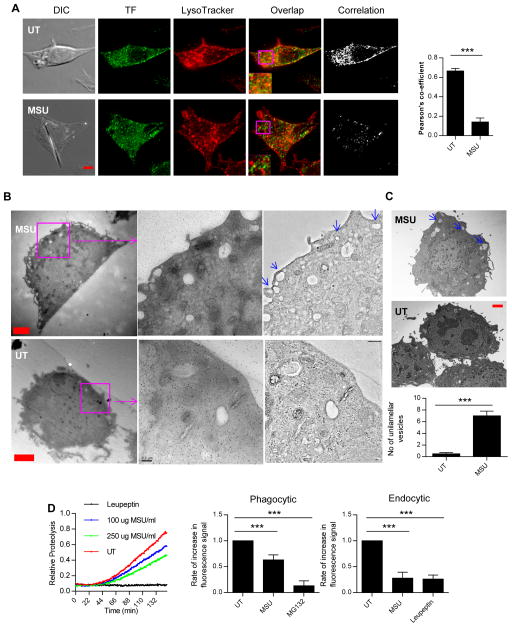
In our settings, phagocytosis appeared to alter endocytic MHC class II antigen processing. The maturation processes and proteolytic activities in both routes are similar given extensive cross talk and the shared fusion partners between the two vesicular systems [24]. It is possible that phagocytosis influences endosomal behavior. Endosomes and lysosomes can be traced by transferrin (TF) receptor and Lysotracker, respectively. Figure 3A shows that in the presence of MSU, endosome/lysosome overlapping was reduced by about 70%, suggesting a lack of progression in the endosomal maturation. The reduced co-localization was similarly detected with latex beads (Supporting Information Fig 3A). We used high resolution Electron Tomography (Fig 3B) and TEM (Fig 3C) to study the morphology and distribution of endosomes in the cytoplasm [25–27]. Interestingly, with MSU stimulation, there was an increased presence of unilamellar endocytic vesicles closely aligned with the plasma membrane. From the lack of any internal structures, these endocytic vesicles appeared to be at the beginning stage of endosomal fusion. These results suggested that in the presence of MSU there was an increase of early endosomes (or endocytic vesicles). Using TEM, we collected more representative pictures (6 images each group) from MSU-treated or -untreated DCs, and counted the numbers of these early vesicles defined as being smaller than 500 nm in diameter, within 1 um from the plasma membrane, and a void of internal structures. The comparison is shown in Figure 3C and the increase in the number of plasma membrane-proximal endosomes in the presence of MSU was apparent.
Figure 3. Endosomal maturation is slowed in the presence of MSU with a simultaneous drop in proteolytic activity.
(A) Distribution of Lysotracker (red) and transferrin receptor (TF, green) in the presence and absence of MSU crystals determined by immunofluorescence staining assay. The colocalization plot was generated using ImageJ. Right panel is the quantification of the colocalization correlation. Red bar is 2 um. (B) Right upper and lower panels: electron tomography sections of DCs showing distribution of early endosomes (unilamellar, empty vesicles, blue arrows) in treated and untreated cells. Left and middle panels are raw EM files showing the gold particles that were used as fiducial markers for tomogram tracking. Sections used in magnification are labeled in magenta, and the red bars are 2 um in length. (C) TEM images of similar samples from (B) with blue arrows showing the early endosomes/endocytic vesicles. Red bar is 1 um. Lower panel: quantification of the number of vesicles seen at the same magnification and resolution in both conditions. Bars are generated from six TEM images similar to the ones shown. (D) Left panel: relative fluorescence units obtained to measure phagosomal proteolytic activity over time as described in the methods. Middle and right panels: quantification of the linear range of the curves for phagocytic (middle) and endocytic (right) proteolysis assays. (A–D) Data are representative of three independent experiments, and are (A, C and D) shown as mean + SEM (3/group).
To see how phagocytosis altered intracellular proteolysis, we used standard fluorescent probes (DQ OVA) to measure protease activities. Figure 3D shows that in the presence of MSU, phagocytic (the time plot and the middle panel) and endocytic compartments (right panel) had reduced proteolysis particularly in the exponential range of the slope, which indicates the steady state proteolysis is reduced upon phagocytosis. Interestingly, the presence of MSU did not alter the acidification progression in the endocytic compartment (Supporting Information Fig 3B), suggesting that the accumulation of proteolytic enzymes through fusion is independent from the acquisition of vacuolar proton pumps (V-ATPase). The lack of strong proteolysis is regarded as a hallmark of DCs in comparison with macrophages and neutrophils [2]. Our results indicate that the “arrested” early endosomes have features that favor cross-presentation.
ROS and cathepsin S are involved in soluble antigen cross-presentation
Our data suggested that OVA can be processed in the endocytic compartment. To rule out the possibility of antigen translocation to the cytosol, we tested whether the redirected MHC class I antigen required TAP for presentation. Figure 4A shows that TAP-deficient BMDCs sensed MSU crystals and showed the enhanced cross-presentation, suggesting that cytosolic antigen processing was not essential for the soluble antigen cross-presentation. In DCs, phagocytosis activates NOX2 (NADPH oxidase), and its associated ROS inhibits endosomal peptidases [28, 29]. This step is critical in protecting antigenic MHC class I epitopes from destruction. To see if this mechanism was employed here, we generated DCs from CYBB (cytochrome B β chain) and NCF (Neutrophil cytosolic factor 1) deficient (Cybb−/− and Ncf1−/−) mice. CYBB (Fig 4B) and NCF (Supporting Information Fig 4A) deficient DCs showed a reduced cross-presentation. These DCs when pulsed with OVA peptide showed no defect in activating OT-1 cells. Importantly, the effect of MSU treatment on the cross-presentation was diminished. This suggests that ROS generation during phagocytosis is important in dampening proteolysis and MSU uptake actively utilizes this effect to enhanced cross-presentation.
Figure 4. Soluble antigen cross-presentation in the presence of phagocytic stimulus is dependent on NOX2 and cathepsin S but does not involve TAP.
(A–E) Production of IL-2 (left panel) by OT-1 T cells upon stimulation with (A) TAP−/− BMDCs (B) Cybb−/−, (C) WT and Ctsb−/−, (D) Ctsl−/−, (E) Ctss−/− BMDCs mixed with soluble OVA in the presence or absence of MSU. Right panel: as in the left, the percentage of CD69 positive OT-1 T cells. (A–E) Data are shown as mean + SEM (3/group) and are representative of at least three independent experiments.
From endosomes, there are potentially several ways whereby antigenic peptides can be loaded onto MHC class I molecules. For vacuolar antigen processing, endosomal cathepsins are critical. cathepsin L and B-deficient DCs showed no reduction in this mode of antigen presentation as both OT-1 CD69 expression and IL-2 production were intact in response to MSU (Fig 4C and D). However, cathepsin S deficiency led to reduced OT-1 IL-2 production and CD69 expression (Fig 4E). Importantly, cathepsin S-deficient cells no longer responded to particle stimulation for higher levels of cross-presentation (Fig 4E and Supporting Information Fig 4B). In some experiments and for unknown reasons, cathepsin S-deficient DCs stimulated slightly stronger CD69 expression but not IL-2 activities at the basal level, yet they still failed to show an enhanced response to MSU stimulation in both cases (Supporting Information Fig 4B). The results were consistent with the understanding that cathepsin S is the only major protease active in early endosomes (pH range 6.0–7.5) and is responsible for antigen processing [30].
Phagocytosis triggers MHC class I cross-presentation of soluble antigen in vivo
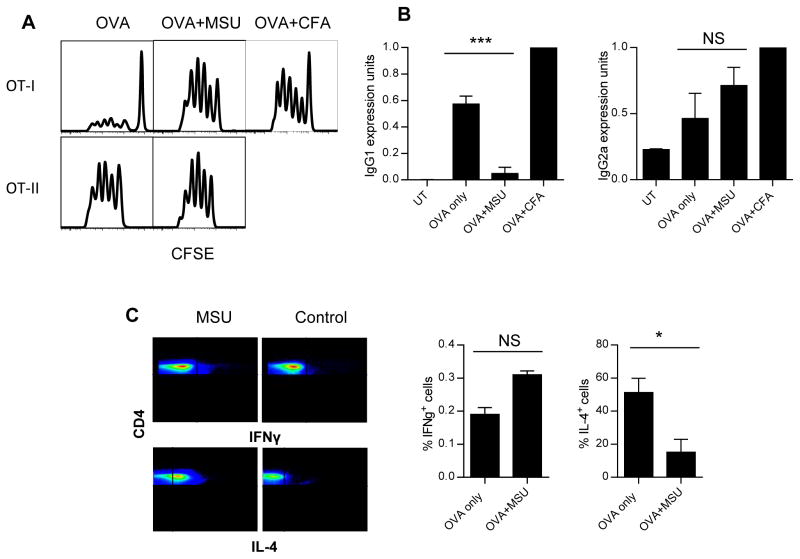
To study the in vivo relevance of our findings, we tested this phenomenon in mice. C57BL/6 CFSE-labeled OT-1 (Thy1.1) and OT-II (Ly5.1) cells with congenic marker mismatch were injected i.v. into C57BL/6 hosts (Thy1.2 or Ly5.2) and their proliferation was analyzed 6 days later. Injection of MSU s.c. triggered strong proliferation of OT-1 cells when OVA was delivered i.p. (Fig 5A), and this proliferation was absent without OVA (Supporting Information Fig 5A). OT-II activation was not altered (Fig 5A). To see how cathepsin S deficiency affected this phenomenon, we used Ctss−/− mice to perform the same assay. Supporting Information Figure 5B shows that as in the in vivo setting, Ctss−/− mice had a slightly higher basal OT-1 proliferation, however adding MSU did not result in a higher response, similar to the in vitro observation. We also tested whether antibody production, a result of CD4 T-cell activation, was affected by MSU. Figure 5B shows that in the presence of MSU, IgG1 antibody production was reduced. Interestingly, IgG2a response remained intact. To test this change in CD4 T cells directly, we injected OT-II cells (Ly5.1) i.v. into recipient mice (Ly5.2) and delivered OVA with or without MSU s.c.. After two weeks, isolated OT-II cells were gated via Ly5.1 and stained for intracellular IL-4 and IFN-γ production. With MSU injection, while IFN-γ expression was slightly higher, the overall number of positive cells remained very small. The IL-4 positive cells were reduced by about 80% (Fig 5C). This shift away from a dominant Th2 phenotype was in line with the notion that a balancing CD8 activation was in place.
Figure 5. In vivo soluble antigen cross-presentation is enhanced by the presence of MSU and is dependent on cathepsin S.
(A) CFSE-labeled OT-1 (top panels) and OT-II (bottom panels) were injected i.v. into C57BL/6 recipients, MSU crystals and soluble OVA were injected s.c. the day before. Splenic and lymph node T cells bearing congenic markers for OT-1 and OT-II cells were gated and analyzed for division after 6 days. OT-II + CFA were over divided on day 6 (not shown). Representative plots of three independent experiments are shown. (B) C57BL/6 mice were immunized with OVA alone, and with MSU or CFA as described in the in vivo assays section of the Materials and methods. Anti-OVA IgG1 (left) or IgG2A titers (right panel) were determined after 4 weeks. All antibody levels are expressed in I.U/ml normalized against CFA mouse levels. (C) C57BL/6 recipients of OVA/MSU injection in the foot pads as described were boosted with the same regimen after 1 week of initial injections. At the end of two weeks, spleen and draining lymph nodes were collected and CD4+ cells were isolated. The intracellular staining for IFN-γ and IL-4 was performed. Representative gated CD4+ cells are shown in the panel. Right: the percentage of IL-4 positive cells from the plots. (B and C) Data are shown as mean + SEM (n=3/group) and are representative of at least three independent experiments.
Discussion
Many particulate structures evoke innate immune responses. Silica, alum and crude MSU stimulate macrophages and DCs to produce inflammatory cytokines such as TNF-α, IL-6, and IL-1β and IL-18 and to express costimulatory molecules on their surface. It was proposed that these crystals entered APCs via phagocytosis, and caused the rupture of lysosomal membrane and the release of cathepsin B into the cytosol [31]. Cathespin B activated NLRP3/ASC/Caspase-1 inflammasome activation and IL-1β release. It was soon followed by a report that NLRP3 inflammasome-deficient mice failed to mount a strong antibody response to a protein admixed with alum [32]. Therefore, NLRP3 inflammasome complex appeared to be the central mechanism of sensing alum and potentially other particulate adjuvants. However, we and several other labs, using NLRP3/ASC-deficient mice, reported normal antibody responses to antigen/alum immunization [33–36]. While the role of NLRP3 in the adjuvanticity of solid particles remains a topic of debate, it seems possible that additional mechanisms may explain the adjuvant effect of particulate structures. We proposed previously that inflammatory phagocytosis of crude MSU and alum triggered Syk dependent activation of PI3Ks and the activation of DCs, resulting in TNF-α production and CD86/CD80/CD40 expression [33, 37]. However, whether other crystals that fail to induce DC activation, such as fine MSU, latex beads and BCP also modulate DC’s functions remains unknown. Here we reported that these “silent” particles may regulate the progression and maturation of DC endosomes, thereby redirecting endocytosed external soluble antigens into the MHC class I cross-presentation pathway. It is likely that “activating” solid structures (crude MSU, CPPD, and silica etc.) have similar effects, although additional efforts will be required to separate this enhanced antigen presentation from the enhanced DC activation that occurs simultaneously.
Cross-presentation was initially discovered with cell-associated antigens. Phagosome to cytosol antigen transfer was proposed based on the observation that OVA-coated latex beads required TAP and proteasomes to be presented on the MHC class I [38, 39]. With this concept, phagocytosis was considered mostly as a means of antigen delivery. Here, we suggest that phagocytosis is not only for antigen delivery; it instead drives a set of seemingly coordinated events in DCs that target soluble antigen into MHC class I cross-presentation.
As endosomes mature with gradually increased proteolytic activities and reduced pH, the contained antigens are processed and loaded onto MHC class II molecules [21]. At the beginning of this process, small endocytic/pinocytic vesicles first fuse to become early endosomes. To maintain surface and volume equilibrium, the majority of the endocytic contents, as well as the lipid membrane are recycled back to the surface via two selection systems (rapid recycling and slow recycling) [40, 41]. Early endosomes first rapidly sort their cargos and send some of its protein and lipid contents back to the plasma membrane. They then sort and transport some cargos to endocytic recycling compartment (ERC) while the remaining endosomes become late endosomes/MVBs (multivesicular bodies). Tubular ERC then further sorts its cargo and sends it back to the cell surface, a process likely dependent on ADP-ribosylation factor 6 (ARF6) [40]. Progression towards late endosome/lysosome can be viewed as a “side pathway”. Endosomes rely on the MT system to drive the cargos centripetally towards the minus end of microtubular rods [23]. For an engulfed soluble antigen, its fate to become a MHC class II antigen is not determined until it reaches late endo/lysosome or MIIC. The destination of those antigens that are sent back to the cell surface before they meet MHC class II molecules remains a question.
It appears that several events in the MHC class II antigen-processing pathway are altered by phagocytosis. The most unexpected are the membrane-proximal localization of endocytic vesicles and reduced progression to lysosomes. Presumably, endocytic vesicles and early endosomes are subjected to the robust recycling that brings most of the endocytic contents back to the plasma membrane. The cross-presentation efficiency of endosomal antigens appears to be strong. In our settings, during phagocytosis similar amounts of endosomal antigen appeared to be more efficiently cross-presented than the phagocytic counterpart, although this comparison should be stated with caution, as it has not been tested in other systems. Nonetheless, the observation raises the possibility that in physiological conditions soluble substances in the extracellular environment may serve as an important source of cross-presenting antigens even without being targeted to receptors that facilitate their internalization.
Regarding the debate about how antigens are targeted to the MHC class I pathway during cross-presentation, a few proposals have been raised, including translocation of antigen from phagosome to the cytosol [42] and ER fusion with phagosome [43]. An additional suggestion implicated the role of recycling cell surface MHC molecules [44, 45]. Within phagosome-based cross-presentation, there are at least two pathways. One relies on TAP and proteasomes, and is evidently the conventional cytosolic pathway. The other is cathepsin S dependent and mostly TAP independent [30]. Although in the original report, the authors did not study the role of endocytic recycling, work from Jefferies’s group clearly shows that the antigens rely on the recycling pathway for the presentation [46].
Results of cathepsin S and ROS regulation fit well with known DC characteristics that favor cross-presentation [2, 47]. While endocytic vesicles stay surface proximal, cathepsin S accumulates in the same location [48, 49]. In the delayed and accumulated early endosomes, cathepsin S is likely the main proteolytic enzyme [30]. Furthermore, our results seem to support the earlier findings that a milder proteolysis in DCs was associated with the Redox state as ROS generated during the process blocks extensive proteolytic activities in these organelles [50–53]. Interestingly, a recent paper revealed that Clec12A, a CLR, acts as a receptor for MSU that downregulates the inflammation in neutrophils and in vivo [54]. It is perceivable that such an inhibition, if exerted on DCs, could negatively regulate their activation and prolong the endocytic processing. Whether such a scenario is of relevance from the perspectives of antigen presentation will be an interesting topic.
A logical next step is the molecular understanding why endosomes fail to mature in the presence of solid structures. It is possible that phagosome development involves changes in the distribution of sorting proteins in the endocytic system. Because the early endosomes accumulated following phagocytosis appear to be nascent, it is likely that they do not involve ARF6-mediated slow recycling from the ERC [40]. Whether other small GTPases, such as Rab4 involved in the fast recycling, [55] are preferentially used to sort soluble antigens will be an interesting question. In all, our work suggests that soluble endosomal antigens are not always destined to result in MHC class II activation and presents a scenario whereby cellular changes during phagocytosis may target soluble antigens into the MHC class I cross-presentation.
Materials and Methods
Mice and media
C57BL/6, Tap1−/−, OT-II and OT-1 mice were purchased from Jackson laboratories and Ctss−/−, Ctsl−/−, Ctsb−/−, Cybb−/−, Ncf1−/−, Myd88−/−/Ticam1−/−, Nlrp3−/− and Nfkb1−/− mice were housed at University of Calgary Animal Research Centre [33, 37]. Cathepsin-deficient mice were gifts from Dr. Kenneth Rock of UMass Medical School. All mouse experiments were approved by the Animal Protocol Committee of University of Calgary. DC2.4 cells were maintained with 5% FBS media. BMDCs were grown with IL-4 and GM-CSF (Biosource and Gibco inc, Invitrogen) (51). On day 6 of DC culture, cells were gently washed and replaced with fresh cell culture media before use.
Reagents
Ovalbumin (OVA) was purchased from Sigma. Monosodium urate and basic calcium phosphate crystals, both used at 250 ug/ml for antigen presentation assays, were made from materials from Sigma as described previously [33, 37]. For MSU, the crude crystal preparation was tumbled with 5 mm stainless steel beads in a 50 ml conical tube for one week to produce finer crystals about 1um in length. Latex beads (3 um) were purchased from Polyscience Inc. Complete Freund’s adjuvant (CFA) and incomplete Freund’s adjuvant (IFA) were from Rockland. All IL-2 ELISA Ready-Set-Go kits were from eBioscience. Alexa-488 OVA, Lysotracker (deep red and green) and Alexa-594 Transferrin receptor were bought from Invitrogen. Anti mouse CD4 was from BD. Anti mouse antibodies for CD8, CD40, CD45.1, CD69, CD80, CD86, CD90.1, MHC I (H2Db), MHC II (Ia/Ie), IL-4 and IFN-γ were from eBioscience Inc. Secondary antibodies and Prolong Gold Antifade were bought from Molecular Probes (Invitrogen).
Flow cytometry
For the OVA uptake experiments, DCs were incubated for indicated durations with and without crystals (250 ug/ml) along with Alexa-488-conjugated OVA. In some assays, DCs were directly incubated with Alexa-488 conjugated OVA-coated latex beads. The cells were scrapped, washed with PBS and fixed using 2% PFA and read using an Attune® Acoustic Focusing Flow Cytometer. Apoptotic assays were done using apoptotic detection kits based on manufacturer’s instructions (eBioscience). All analyses were done using Treestar Flowjo 10.0.
Antigen presentation assays
OVA antigen (soluble or OVA coated beads) was added with or without phagocytic stimuli to BMDCs grown in 24 well plates. After 4 hours, cells were given fresh media and 2 × 106 OT-1 or OT-II cells and allowed to interact. 18–24 hours later the cells were collected and stained for CD8/CD4 and CD69. The supernatant collected at the same time was used to measure IL-2 levels. A similar protocol was followed for all gene deficient BMDCs.
Electron microscopy
For TEM analyses, the cultured cells were processed in situ for fixation, dehydration, infiltration and embedding in the culture dish. The cells were prefixed with 1.6% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.3 for 1 hour and post fixed with cacodylate buffered 1% osmium tetroxide for 1 hour at room temperature. Cells were then dehydrated through graded ethanol and embedded in Epon mixture. After polymerizing the hardened Epon layer containing the embedded cells was separated from the plastic culture dish. Under a light microscope a representative area was selected, trimmed and glued to resin stub for sectioning. Ultra-thin sections were cut with a diamond knife on an ultramicrotome (Ultracut E, Reichert-Jung, Vienna, Austria) and collected on single hole grids with Formvar supporting film. The sections were stained with aqueous uranyl acetate and Reynolds’s lead citrate and observed under a Hitachi H7650 TEM at 80 kV. The images were acquired with an AMT16000 digital camera mounted on the microscope. Unilamellar vesicles lacking electron dense contents and that were under the appropriate size of early endosomes (300–500 nm diameter) were visually counted and quantified using Graphpad Prism 6.0. For SEM, dried crystals were laid on prefabricated standard aluminum stubs with carbon tape. The samples were coated with gold (Techniques Hummer II, Anatech). Samples were then inspected under a Scanning Electron Microscope (ESEM– XL30, FEI).
Electron Tomography
Thick sections (about 250 nm) were cut and stained with 2% aqueous uranyl acetate and Reynold’s lead citrate and then placed on one side of a TEM Slot Grid (1 × 2 mm slot) that was covered with a ~40 nm thin continuous formvar film (EMS, Hatfield, Pennsylvania) and left to dry for several minutes. Colloidal gold particles (10 nm diameter) were placed on the both sides of the grid to serve as fiducial markers. Finally, a thin carbon coating was applied to both sides of the grid for mechanical stabilization and to reduce electric charging in the microscope. The images were captured on a Gatan UltraScan 4000 CCD (Gatan, Pleasanton, California, USA) at 2048x2048 pixels. Dual axis TEM tomography was carried out by taking one image every degree for a range between 120 and 130 degrees with the program Serial EM. The tomographic reconstruction was done by weighted back-projection with the IMOD software package, resulting in a tomographic dataset of approximately 4 nm resolution [56, 57].
Proteolysis assays
These assays were performed as previously described [29, 58]. For phagosomal analyses, IgG- DQ OVA conjugated particles used to evaluate proteolysis were prepared as previously described [59]. To measure endocytic proteolysis, soluble DQ OVA (Molecular probes) was used as the substrate. Relative fluorescent units (RFUs) were obtained from the equation: RFU=SFRT/CF, where SFRT is substrate fluorescence in real time, while CF represents average calibration fluorescence and was represented relative to time expressed in minutes. After normalization against the control fluor, the range of the curve where the signal increased exponentially was selected. For the plot in Figure 3 D showing phagosomal proteolysis, this range corresponds to 60–80 min. Relative rates, as defined by the increase in RFU over time, were calculated for each assay. Hydrolytic capacities were evaluated by plotting the slopes (as described by the equation y=mx+c, where y=RFU, m=slope and x=time) of the linear portion of the relative substrate fluorescence. The numbers obtained from six independent wells were averaged as the hydrolytic capacity for the conditions listed. The process was repeated for all treatments and plotted relative to untreated/negative controls. The selected range of the experimental groups plotted this way was compared by one-way analysis of variance (ANOVA) with Sidak’s multiple comparisons test.
Immunostaining
Endosomal tracking: DCs were plated at low density (less than 50% confluence) on sterile cover slips and grown overnight. 1 ul/ml of Alexa 594 tagged Transferrin receptor and 1 ul/ml of Lysotracker DND 26 were added to cells in the presence and absence of crystals or latex beads. After 5 or 10 min of incubation, the cells were washed and fixed. Cells were fixed in 2% PFA in PBS for 15 minutes, permeabilized in methanol and mounted with Prolong Gold with DAPI. All imaging was done on a Delta vision microscope followed by deconvolution of the images. Image J was used for the correlation of Lysotracker and Transferrin. More than 50% pixel overlap was documented as a positive correlation.
In vivo assays
200 ug OVA with or without 1 mg MSU crystals were injected s.c. in footpads and thighs of C57BL/6 and Ctss−/− mice. BL6 control mice were injected with CFA admixed with 200 ug OVA. After 24 hours, isolated OT-1 and OT-II T cells (using CD8 and CD4 kits from Stemcell Technologies.) labeled with CFSE (Invitrogen) were injected intravenously. Local draining lymph nodes and the spleen were collected on day 6 and analyzed for CFSE proliferation. The populations were selected based on congenic markers. The data was collected on BD LSR-II flow cytometer and analyzed with Flowjo 10.0.
Intra-cellular staining was done with a similar protocol: the mice were injected with OVA (with and without MSU crystals) s.c. in footpads and thighs. After 24 hours, isolated CD4 cells from OT2 mice were injected intravenously. The mice were boosted with the same regimen after 1 week of initial injections. At the end of two weeks, spleen and draining lymph nodes were collected and CD4 cells were isolated as described earlier. The cells were processed with permeabilization buffer and IC fixation buffer from eBioscience according to manufacturer’s instructions. The cells were then stained with CD4, Ly5.1, IL-4 and IFN-γ and read with BD LSR II.
Antibody induction was performed as previously described [33], the mice were boosted after 2 weeks with the same regimen except incomplete Freund’s adjuvant was used instead of CFA. Two weeks after final immunization, sera were collected and OVA specific antibody titers were determined using ELISA. IgG1 titers were analyzed as described [33]; IgG2a titers were measured using a kit from Alpha Diagnostics International.
Statistical analysis
All experiments were repeated at least three times with no less than three replicates per sample and three mice per group. All statistical analysis was done using Graphpad Prism 6.0. A one-way ANOVA was done to compare all data sets when there were more than 3 sets. Sidak’s multiple comparisons test was done as a posttest on selected columns. In cases where there were only 2 sets of data, student T test was used. P value legend *: <0.05, **:<0.01, ***”<0.001, and N.S: not significant.
Supplementary Material
Acknowledgments
We would like to thank Dr. Li Yu of Tsinghua University for his comments and suggestions. We thank Dale Balce, Joanna Rybicka, Pankaj Tailor and Wanqing Du for their extensive technical advice in proteolysis, pH and in vivo assays. We would like to acknowledge Michael Schoel for help with SEM imaging. We also would like to thank Sue Tsai and Javier Casares-Clementes, Poornima Ambalavannan, Laurie Kennedy, Yiping Liu, Pina Colarusso, Ailian Yang and Barry Congdon for technical help. The Microscopy and Imaging facility and live cell imaging facilities at the University of Calgary are funded by Canada Foundation for Innovation. Y.S is supported by Peking-Tsinghua University Center for Life Sciences, Gates Foundation and Bayer. This work was supported by grants from US National Institutes of Health (R01AI098995 and R21AI089963), Natural Sciences and Engineering Research Council of Canada (RGPIN-355350/396037) and Canadian Institutes for Health Research (MOP-119295) to Y.S.
Abbreviations
- ARF
ADP-ribosylation factor
- BCP
Basic calcium phosphate
- CLR
C-type lectin
- CYBB
Cytochrome B β chain
- DC
Dendritic cells
- ERC
Endosome recycling compartment
- MIIC
MHC class II compartment
- MSU
Monosodium urate
- NCF
Neutrophil cytosolic factor 1
- Rab
Ras-related proteins in brain
Footnotes
Conflict of interest disclosure
The authors declare no financial or commercial conflicts of interest.
References
- 1.Rock KL, Shen L. Cross-presentation: underlying mechanisms and role in immune surveillance. Immunol Rev. 2005;207:166–183. doi: 10.1111/j.0105-2896.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- 2.Mantegazza AR, Magalhaes JG, Amigorena S, Marks MS. Presentation of Phagocytosed Antigens by MHC class I and II. Traffic. 2013;14:135–152. doi: 10.1111/tra.12026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackerman AL, Cresswell P. Cellular mechanisms governing cross-presentation of exogenous antigens. Nat Immunol. 2004;5:678–684. doi: 10.1038/ni1082. [DOI] [PubMed] [Google Scholar]
- 4.Gagnon E, Duclos S, Rondeau C, Chevet E, Cameron PH, Steele-Mortimer O, Paiement J, et al. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell. 2002;110:119–131. doi: 10.1016/s0092-8674(02)00797-3. [DOI] [PubMed] [Google Scholar]
- 5.Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, Van Endert P, Amigorena S. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425:397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- 6.Wiertz EJ, Tortorella D, Bogyo M, Yu J, Mothes W, Jones TR, Rapoport TA, et al. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature. 1996;384:432–438. doi: 10.1038/384432a0. [DOI] [PubMed] [Google Scholar]
- 7.Houde M, Bertholet S, Gagnon E, Brunet S, Goyette G, Laplante A, Princiotta MF, et al. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425:402–406. doi: 10.1038/nature01912. [DOI] [PubMed] [Google Scholar]
- 8.Nair-Gupta P, Baccarini A, Tung N, Seyffer F, Florey O, Huang Y, Banerjee M, et al. TLR Signals Induce Phagosomal MHC-I Delivery from the Endosomal Recycling Compartment to Allow Cross-Presentation. Cell. 2014;158:506–521. doi: 10.1016/j.cell.2014.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Touret N, Paroutis P, Terebiznik M, Harrison RE, Trombetta S, Pypaert M, Chow A, et al. Quantitative and dynamic assessment of the contribution of the ER to phagosome formation. Cell. 2005;123:157–170. doi: 10.1016/j.cell.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Thacker RI, Janssen EM. Cross-presentation of cell-associated antigens by mouse splenic dendritic cell populations. Front Immunol. 2012;3:41. doi: 10.3389/fimmu.2012.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harding CV, Song R. Phagocytic processing of exogenous particulate antigens by macrophages for presentation by class I MHC molecules. J Immunol. 1994;153:4925–4933. [PubMed] [Google Scholar]
- 12.Norbury CC, Hewlett LJ, Prescott AR, Shastri N, Watts C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity. 1995;3:783–791. doi: 10.1016/1074-7613(95)90067-5. [DOI] [PubMed] [Google Scholar]
- 13.Burgdorf S, Kautz A, Bohnert V, Knolle PA, Kurts C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science. 2007;316:612–616. doi: 10.1126/science.1137971. [DOI] [PubMed] [Google Scholar]
- 14.Amigorena S. Fc gamma receptors and cross-presentation in dendritic cells. J Exp Med. 2002;195:F1–3. doi: 10.1084/jem.20011925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zelenay S, Keller AM, Whitney PG, Schraml BU, Deddouche S, Rogers NC, Schulz O, et al. The dendritic cell receptor DNGR-1 controls endocytic handling of necrotic cell antigens to favor cross-priming of CTLs in virus-infected mice. J Clin Invest. 2012;122:1615–1627. doi: 10.1172/JCI60644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weck MM, Appel S, Werth D, Sinzger C, Bringmann A, Grunebach F, Brossart P. hDectin-1 is involved in uptake and cross-presentation of cellular antigens. Blood. 2008;111:4264–4272. doi: 10.1182/blood-2006-10-051375. [DOI] [PubMed] [Google Scholar]
- 17.Chatterjee B, Smed-Sorensen A, Cohn L, Chalouni C, Vandlen R, Lee BC, Widger J, et al. Internalization and endosomal degradation of receptor-bound antigens regulate the efficiency of cross presentation by human dendritic cells. Blood. 2012;120:2011–2020. doi: 10.1182/blood-2012-01-402370. [DOI] [PubMed] [Google Scholar]
- 18.Cohn L, Chatterjee B, Esselborn F, Smed-Sorensen A, Nakamura N, Chalouni C, Lee BC, et al. Antigen delivery to early endosomes eliminates the superiority of human blood BDCA3+ dendritic cells at cross presentation. J Exp Med. 2013;210:1049–1063. doi: 10.1084/jem.20121251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reis e Sousa C, Germain RN. Major histocompatibility complex class I presentation of peptides derived from soluble exogenous antigen by a subset of cells engaged in phagocytosis. J Exp Med. 1995;182:841–851. doi: 10.1084/jem.182.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castellino F, Boucher PE, Eichelberg K, Mayhew M, Rothman JE, Houghton AN, Germain RN. Receptor-mediated uptake of antigen/heat shock protein complexes results in major histocompatibility complex class I antigen presentation via two distinct processing pathways. J Exp Med. 2000;191:1957–1964. doi: 10.1084/jem.191.11.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berger AC, Roche PA. MHC class II transport at a glance. J Cell Sci. 2009;122:1–4. doi: 10.1242/jcs.035089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kinchen JM, Ravichandran KS. Phagosome maturation: going through the acid test. Nat Rev Mol Cell Biol. 2008;9:781–795. doi: 10.1038/nrm2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gohre V, Vollmeister E, Bolker M, Feldbrugge M. Microtubule-dependent membrane dynamics in Ustilago maydis: Trafficking and function of Rab5a-positive endosomes. Commun Integr Biol. 2012;5:485–490. doi: 10.4161/cib.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flannagan RS, Jaumouille V, Grinstein S. The cell biology of phagocytosis. Annu Rev Pathol. 2012;7:61–98. doi: 10.1146/annurev-pathol-011811-132445. [DOI] [PubMed] [Google Scholar]
- 25.Pelissier A, Chauvin JP, Lecuit T. Trafficking through Rab11 endosomes is required for cellularization during Drosophila embryogenesis. Curr Biol. 2003;13:1848–1857. doi: 10.1016/j.cub.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Lloyd TE, Atkinson R, Wu MN, Zhou Y, Pennetta G, Bellen HJ. Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell. 2002;108:261–269. doi: 10.1016/s0092-8674(02)00611-6. [DOI] [PubMed] [Google Scholar]
- 27.Ganley IG, Carroll K, Bittova L, Pfeffer S. Rab9 GTPase regulates late endosome size and requires effector interaction for its stability. Mol Biol Cell. 2004;15:5420–5430. doi: 10.1091/mbc.E04-08-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Honey K, Rudensky AY. Lysosomal cysteine proteases regulate antigen presentation. Nat Rev Immunol. 2003;3:472–482. doi: 10.1038/nri1110. [DOI] [PubMed] [Google Scholar]
- 29.Rybicka JM, Balce DR, Chaudhuri S, Allan ER, Yates RM. Phagosomal proteolysis in dendritic cells is modulated by NADPH oxidase in a pH-independent manner. EMBO J. 2012;31:932–944. doi: 10.1038/emboj.2011.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen L, Sigal LJ, Boes M, Rock KL. Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity. 2004;21:155–165. doi: 10.1016/j.immuni.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flach TL, Ng G, Hari A, Desrosiers MD, Zhang P, Ward SM, Seamone ME, et al. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat Med. 2011;17:479–487. doi: 10.1038/nm.2306. [DOI] [PubMed] [Google Scholar]
- 34.Franchi L, Nunez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38:2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marichal T, Ohata K, Bedoret D, Mesnil C, Sabatel C, Kobiyama K, Lekeux P, et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med. 2011;17:996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- 36.McKee AS, Munks MW, MacLeod MK, Fleenor CJ, Van Rooijen N, Kappler JW, Marrack P. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol. 2009;183:4403–4414. doi: 10.4049/jimmunol.0900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ng G, Sharma K, Ward SM, Desrosiers MD, Stephens LA, Schoel WM, Li T, et al. Receptor-independent, direct membrane binding leads to cell-surface lipid sorting and Syk kinase activation in dendritic cells. Immunity. 2008;29:807–818. doi: 10.1016/j.immuni.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kovacsovics-Bankowski M, Rock KL. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science. 1995;267:243–246. doi: 10.1126/science.7809629. [DOI] [PubMed] [Google Scholar]
- 39.Bevan MJ. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J Exp Med. 1976;143:1283–1288. doi: 10.1084/jem.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grant BD, Donaldson JG. Pathways and mechanisms of endocytic recycling. Nat Rev Mol Cell Biol. 2009;10:597–608. doi: 10.1038/nrm2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huotari J, Helenius A. Endosome maturation. EMBO J. 2011;30:3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ackerman AL, Giodini A, Cresswell P. A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity. 2006;25:607–617. doi: 10.1016/j.immuni.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 43.Guermonprez P, Amigorena S. Pathways for antigen cross presentation. Springer Semin Immunopathol. 2005;26:257–271. doi: 10.1007/s00281-004-0176-0. [DOI] [PubMed] [Google Scholar]
- 44.Di Pucchio T, Chatterjee B, Smed-Sorensen A, Clayton S, Palazzo A, Montes M, Xue Y, et al. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat Immunol. 2008;9:551–557. doi: 10.1038/ni.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gromme M, Uytdehaag FG, Janssen H, Calafat J, van Binnendijk RS, Kenter MJ, Tulp A, et al. Recycling MHC class I molecules and endosomal peptide loading. Proc Natl Acad Sci U S A. 1999;96:10326–10331. doi: 10.1073/pnas.96.18.10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lizee G, Basha G, Tiong J, Julien JP, Tian M, Biron KE, Jefferies WA. Control of dendritic cell cross-presentation by the major histocompatibility complex class I cytoplasmic domain. Nat Immunol. 2003;4:1065–1073. doi: 10.1038/ni989. [DOI] [PubMed] [Google Scholar]
- 47.Savina A, Amigorena S. Phagocytosis and antigen presentation in dendritic cells. Immunol Rev. 2007;219:143–156. doi: 10.1111/j.1600-065X.2007.00552.x. [DOI] [PubMed] [Google Scholar]
- 48.Lennon-Dumenil AM, Bakker AH, Maehr R, Fiebiger E, Overkleeft HS, Rosemblatt M, Ploegh HL, et al. Analysis of protease activity in live antigen-presenting cells shows regulation of the phagosomal proteolytic contents during dendritic cell activation. J Exp Med. 2002;196:529–540. doi: 10.1084/jem.20020327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kirschke H, Wiederanders B, Bromme D, Rinne A. Cathepsin S from bovine spleen. Purification, distribution, intracellular localization and action on proteins. Biochem J. 1989;264:467–473. doi: 10.1042/bj2640467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cross AR, Segal AW. The NADPH oxidase of professional phagocytes--prototype of the NOX electron transport chain systems. Biochim Biophys Acta. 2004;1657:1–22. doi: 10.1016/j.bbabio.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leto TL, Geiszt M. Role of Nox family NADPH oxidases in host defense. Antioxid Redox Signal. 2006;8:1549–1561. doi: 10.1089/ars.2006.8.1549. [DOI] [PubMed] [Google Scholar]
- 52.Yates RM. Redox considerations in the phagosome: current concepts, controversies, and future challenges. Antioxid Redox Signal. 2013;18:628–629. doi: 10.1089/ars.2012.4898. [DOI] [PubMed] [Google Scholar]
- 53.Yates RM, Hermetter A, Taylor GA, Russell DG. Macrophage activation downregulates the degradative capacity of the phagosome. Traffic. 2007;8:241–250. doi: 10.1111/j.1600-0854.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 54.Neumann K, Castineiras-Vilarino M, Hockendorf U, Hannesschlager N, Lemeer S, Kupka D, Meyermann S, et al. Clec12a is an inhibitory receptor for uric acid crystals that regulates inflammation in response to cell death. Immunity. 2014;40:389–399. doi: 10.1016/j.immuni.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 55.van der Sluijs P, Hull M, Webster P, Male P, Goud B, Mellman I. The small GTP-binding protein rab4 controls an early sorting event on the endocytic pathway. Cell. 1992;70:729–740. doi: 10.1016/0092-8674(92)90307-x. [DOI] [PubMed] [Google Scholar]
- 56.Mastronarde DN. Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol. 2005;152:36–51. doi: 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 57.Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 58.Rybicka JM, Balce DR, Khan MF, Krohn RM, Yates RM. NADPH oxidase activity controls phagosomal proteolysis in macrophages through modulation of the lumenal redox environment of phagosomes. Proc Natl Acad Sci U S A. 2010;107:10496–10501. doi: 10.1073/pnas.0914867107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yates RM, Russell DG. Real-time spectrofluorometric assays for the lumenal environment of the maturing phagosome. Methods Mol Biol. 2008;445:311–325. doi: 10.1007/978-1-59745-157-4_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.