Abstract
Objective:
To investigate whether certain CSF biomarkers at baseline can predict future progression of motor symptoms and cognitive decline in patients with Parkinson disease (PD).
Methods:
Patients and controls were recruited from hospitals in southern Sweden as part of the prospective and longitudinal Swedish BioFinder Study. In the present study, we included 42 patients with PD and 69 controls who had clinical assessment and lumbar puncture at baseline. Baseline CSF samples were analyzed for α-synuclein (αSyn), β-amyloid 1–42 (Aβ42), tau, phosphorylated tau, and neurofilament light. Associations between CSF markers at baseline and change in clinical characteristics after 2 years of follow-up were investigated using multivariate models adjusting for age, sex, disease duration, and levodopa-equivalent daily dose.
Results:
Higher levels of αSyn within the PD group were associated with progression of motor symptoms and cognitive decline over 2 years, indicated by significant relationships between αSyn and change in Hoehn and Yahr (β = 0.394, p = 0.043), Unified Parkinson's Disease Rating Scale, Part III (UPDRS-III) (β = 0.449, p = 0.013), Timed Up and Go (β = 0.406, p = 0.023), and A Quick Test of Cognitive Speed (β = 0.423, p = 0.018). Lower levels of Aβ42 were associated with worsening of performance on delayed memory recall (F = 5.834, p = 0.022). Finally, high levels of phosphorylated tau were associated with worsening in motor symptoms (UPDRS-III, β = 0.350, p = 0.045; Hoehn and Yahr, β = 0.366, p = 0.038).
Conclusion:
We found evidence of a link between higher levels of αSyn at baseline and worsening of motor symptoms and cognitive speed over 2 years in PD. Increased αSyn might be a marker of more intense synaptic degeneration in PD. The results indicate that cortical amyloid pathology (low CSF Aβ42) is associated with memory decline.
There is a need for diagnostic and/or prognostic biomarkers for Parkinson disease (PD). CSF levels of α-synuclein (αSyn) are decreased in patients with PD, as well as in patients with other synucleinopathies, e.g., dementia with Lewy bodies and multiple system atrophy.1–5 Regarding CSF Alzheimer disease (AD) biomarkers (i.e., tau, phosphorylated tau [p-tau], and β-amyloid 1–42 [Aβ42]), most studies have found normal or slightly decreased levels in PD.2,3,5
The clinical presentation and the prognostic outcome of PD is highly variable both regarding progression of motor symptoms and cognitive performance. It is important to develop biomarkers that can predict future progression of motor and cognitive deficits in patients with PD without dementia. Yet, there are few longitudinal studies assessing the association between baseline CSF biomarkers and disease progression. A longitudinal study on patients with early PD found that the CSF levels of the ratios p-tau/tau and p-tau/β-amyloid at baseline correlate with progression of motor symptoms in PD.6 A few studies show that CSF Aβ42 might be a predictor of subsequent development of cognitive decline in patients with PD without dementia.7–10 However, another recent study found that increased baseline levels of αSyn, but not β-amyloid, are associated with future decline in cognitive function.11
The objective of the present study was to perform a prospective and longitudinal study designed to investigate the associations between all of the most established CSF biomarkers, including αSyn, Aβ42, tau, p-tau, and neurofilament light (NFL, a marker of injury to or degeneration of large caliber myelinated axons) and subsequent progression of both motor symptoms as well as cognitive decline in PD.12
METHODS
Participants.
This prospective and longitudinal study was performed at the Clinic of Neurology, Skåne University Hospital, Sweden (part of the Swedish BioFinder Study). The study participants are primarily recruited from the southern region of Sweden. Study participants have been recruited since 2008 and they are followed with repeated neurologic, psychiatric, and cognitive assessments and collection of CSF and blood samples. In this first substudy, we included 42 patients with PD (without dementia) with lumbar puncture at baseline who had been followed for a minimum of 2 years. We also included 69 neurologically healthy controls with lumbar puncture. The controls were primarily spouses or relatives of patients in the study. Controls were only used for baseline comparisons of biomarker levels. Patients with PD met the National Institute of Neurological Disorders and Stroke Diagnostic Criteria for PD.13 At baseline and during the follow-up after 2 years, the patients were examined by a physician experienced in movement disorders and registered nurse using, among other scales, the Unified Parkinson's Disease Rating Scale, Part III (UPDRS-III), the Hoehn and Yahr Scale, and the Timed Up and Go (TUG) test.14–16 The study participants' cognitive function was assessed using the Mini-Mental State Examination (MMSE), Alzheimer's Disease Assessment Scale (ADAS) items 1–3 (measure of episodic memory delayed recall), A Quick Test of Cognitive Speed (AQT; measure of cognitive processing speed), and the 1-minute Animal Fluency test.17–20 At each study visit, a thorough medical history was taken and the patients underwent extensive testing in both motor symptoms and cognition. The baseline data of the study participants in the present study have previously been described in Hall et al.,5 2012, where they were part of a larger cohort.
Standard protocol approvals, registrations, and patient consents.
All individuals gave informed written consent. The study procedure was approved by the local ethics committee at Lund University Sweden and conducted according to the Helsinki Declaration.
CSF samples.
CSF samples were obtained by lumbar puncture in the L3-4 or L4-5 interspace with the patient sitting, nonfasting. The samples was collected in polypropylene tubes and gently mixed to avoid gradient effects. All samples were centrifuged within 30 minutes at +4°C at 2,000g for 10 minutes to remove cells and debris, and then stored in aliquots at −80°C pending biochemical analysis. The levels of Aβ42, tau, p-tau, αSyn, NFL, and hemoglobin were determined as previously described.5
Statistical analysis.
The statistical analyses were accomplished with SPSS for Windows, version 20.0 (IBM Corp., Armonk, NY). To compare demographic and CSF baseline data between groups, Mann–Whitney U test was used for continuous variables. Pearson χ2 test was used to compare proportions. Univariate associations between 2 continuous variables were analyzed using the Pearson r (normally distributed variables) or Spearman ρ (skewed variables and/or ordinal data). We used standard linear regressions to test associations between scores on clinical assessment scales (dependent variables) and CSF biomarkers, adjusting for age, sex, disease duration, and levodopa-equivalent daily dose (LEDD).21 Because of the bimodal distribution of Aβ42, this measure was also dichotomized using 550 ng/L as cutoff.22 Here, Student t test was used to compare change in scores on clinical assessment scales, and analysis of covariance was used to adjust for covariates. Before parametric analyses, skewed data were normalized using log-transformation or, in the case of change on clinical assessment scores, using Blom's method.23 When analyzing αSyn, all subjects with hemoglobin levels >1,000 ng/L were excluded.
RESULTS
Demographics.
Demographics, clinical characteristics, and CSF biomarker levels of study participants are given in tables 1 and 2. Patients with PD had significantly lower levels of αSyn, tau, and p-tau compared with controls (table 2). In the control group, there were no sex differences in any of the biomarker levels. In the control group, higher age correlated with NFL (Rs = 0.580, p < 0.001) but not with any of the other CSF biomarkers in the control group. None of the biomarkers correlated significantly with education level. In the patients with PD, longer disease duration and a higher LEDD correlated with increased CSF levels of Aβ42 (Rs = 0.29–0.31, p < 0.05), but there were no significant correlations between disease duration or LEDD and tau, p-tau, NFL, or αSyn.
Table 1.
Demographic information and comparisons of clinical outcomes at baseline
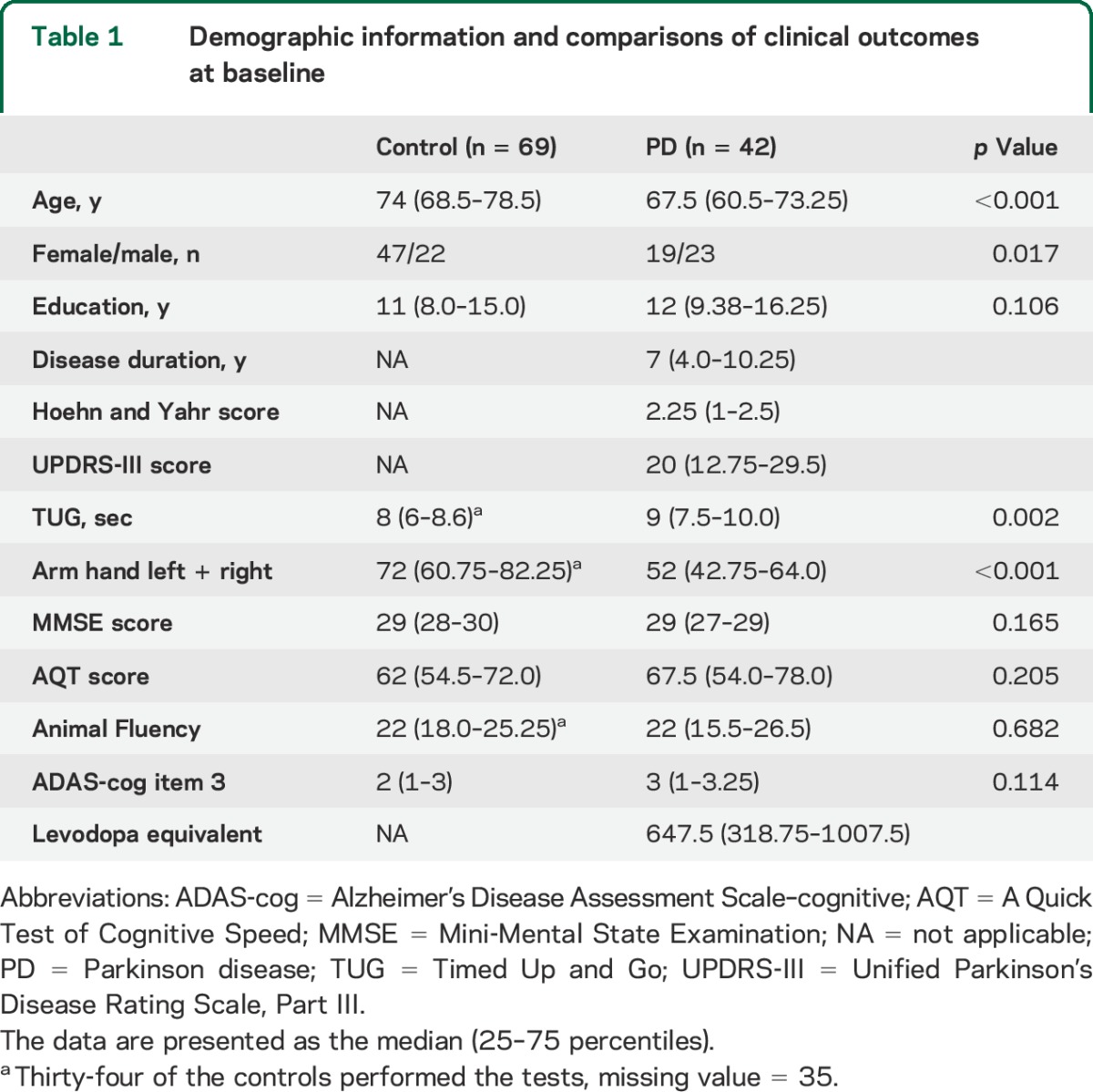
Table 2.
Comparisons of CSF biomarker levels at baseline between PD and control groups

Correlations between baseline biomarkers.
In the PD group, CSF αSyn correlated with tau (Rs = 0.508, p = 0.001), p-tau (Rs = 0.597, p < 0.001), and NFL (Rs = 0.380, p = 0.019) (figure 1). p-Tau correlated with tau (Rs = 0.620, p < 0.001). NFL correlated with lower levels of Aβ42 (Rs = −0.308, p = 0.050).
Figure 1. Correlations of CSF levels of αSyn with tau, p-tau, and NFL.

Correlations of CSF levels of αSyn with tau (A), p-tau (B), and NFL (C) in the Parkinson disease group. Black line indicates a linear regression, blue lines 95% confidence intervals. αSyn = α-synuclein; NFL = neurofilament light; p-tau = phosphorylated tau.
In the control group, CSF αSyn correlated with tau (Rs = 0.744, p < 0.001), p-tau (Rs = 0.652, p < 0.001), and NFL (Rs = 0.421, p < 0.001). p-Tau also correlated with tau (Rs = 0.689, p < 0.001), Aβ42 (R = −0.510, p < 0.001), and NFL (Rs = 0.349, p = 0.003). NFL also correlated with tau (Rs = 0.245, p = 0.043).
Correlations between CSF biomarkers and cognitive decline over 2 years in the PD group.
Patients with PD who had baseline CSF Aβ42 levels <550 ng/L deteriorated more in delayed memory recall (ADAS–cognitive [ADAS-cog] item 3) over 2 years of follow-up compared to those with normal Aβ42 levels (t = 3.40, p = 0.002). This result remained significant when adjusting for age, sex, disease duration, and LEDD (F = 5.834, p = 0.022) (figure 2).
Figure 2. CSF Aβ42 and worsening on ADAS-cog item 3 over 2 years.
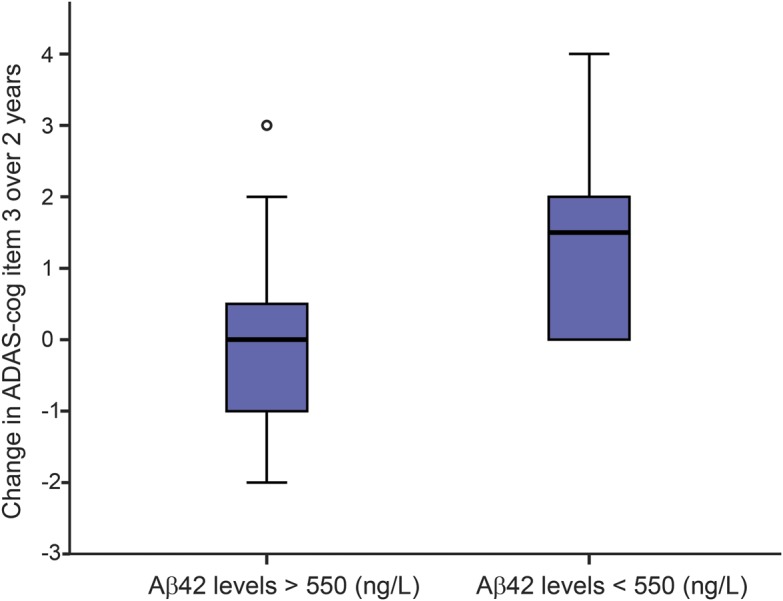
Boxplot showing change in number of correct answers on ADAS-cog item 3 over 2 years in Aβ42 >550 ng/L compared with Aβ42 <550 ng/L in PD patients. Aβ42 = β-amyloid 1–42; ADAS-cog = Alzheimer's Disease Assessment Scale–cognitive.
Higher baseline levels of CSF αSyn correlated with worsening in cognitive processing speed (AQT) over the subsequent 2-year period (Rs = 0.373, p = 0.030) and this correlation remained significant when correcting for age, sex, disease duration, and LEDD (β = 0.423, p = 0.018) (figure 3A).
Figure 3. Correlations of CSF αSyn with change in AQT and UPDRS-III, and CSF p-tau with UPDRS-III change.

Correlations of (A) αSyn with worsening in AQT over 2 years (change in AQT is normalized using Blom's method), (B) αSyn with worsening in UPDRS-III over 2 years, and (C) p-tau with increase in UPDRS-III over 2 years in patients with Parkinson disease. αSyn and p-tau are log-transformed. Black line indicates a linear regression, blue lines 95% confidence intervals. αSyn = α-synuclein; AQT = A Quick Test of Cognitive Speed; p-tau = phosphorylated tau; UPDRS-III = Unified Parkinson's Disease Rating Scale, Part III.
We found no associations between baseline CSF levels of tau, p-tau, or NFL and subsequent cognitive decline when adjusting associations for confounding factors.
Correlations between CSF biomarkers and motor progression over 2 years in the PD group.
Higher baseline levels of CSF αSyn within the PD group were associated with worsening in motor symptoms over 2 years. We found that higher CSF αSyn correlated with increase in Hoehn and Yahr score over 2 years (Rs = 0.361, p = 0.033) and this result remained significant when correcting for age, sex, disease duration, and LEDD (β = 0.394, p = 0.043). Higher CSF αSyn also correlated with an increase in UPDRS-III score during follow-up (Rs = 0.503, p = 0.002), even after correcting for age, sex, disease duration, and LEDD (β = 0.449, p = 0.013) (figure 3B). Finally, higher αSyn levels were associated with a prolonged TUG test (Rs = 0.515, p = 0.002), which remained significant when correcting for age, sex, disease duration, and LEDD (β = 0.406, p = 0.023).
Furthermore, higher baseline levels of CSF p-tau were associated with worsening in motor symptoms over 2 years. We found that higher CSF p-tau correlated with an increase in Hoehn and Yahr score (Rs = 0.378, p = 0.018) and this result remained significant when correcting for age, sex, disease duration, and LEDD (β = 0.366, p = 0.038). CSF levels of p-tau also correlated with an increase in UPDRS-III score (Rs = 0.332, p = 0.039), which was still significant after correcting for age, sex, disease duration, and LEDD (β = 0.350, p = 0.045) (figure 3C). Tau showed a tendency toward correlating with change in UPDRS score when correcting for age, sex, disease duration, and LEDD (β = 0.326, p = 0.053).
We found no significant associations between baseline CSF levels of Aβ42 or NFL and subsequent deterioration in motor symptoms when adjusting associations for confounding factors (data not shown).
DISCUSSION
In the present prospective and longitudinal study, we found that high levels of αSyn at baseline were associated with subsequent worsening of cognitive processing speed (AQT) and motor symptoms (UPDRS-III, Hoehn and Yahr, and TUG) over 2 years in patients with PD. Also, increased CSF p-tau levels were associated with worsening in motor performance (UPDRS-III and Hoehn and Yahr). Finally, decreased baseline CSF levels of Aβ42 (reflecting cerebral amyloid pathology) were associated with worsening of memory functioning during follow-up (ADAS-cog delayed memory recall).
A quite expected result was that lower levels of Aβ42 predicted worsening of delayed memory recall. In line with this finding, a few cross-sectional studies indicate that Aβ42 is decreased in PD dementia, and longitudinal studies have shown that reduced CSF Aβ42 is a predictor of cognitive decline/dementia in PD.7–10,24–26 Recently, Parnetti et al.10 showed that low CSF Aβ42 was associated with a higher rate of decline in global cognition. It is interesting to note that in the present study, reduced CSF Aβ42 predicted subsequent memory impairment, a feature associated with a more AD-like phenotype, and not decline in other cognitive measures (e.g., measures of cognitive processing speed). Reduced Aβ42 levels in CSF most likely reflect increased cortical accumulation of Aβ aggregates, as shown in healthy elderly individuals and cases with AD or PD.27–29 Indeed, neuropathologic studies have revealed that cerebral amyloid pathology is frequent in many cases with PD dementia.30,31 The lack of association of tau-related markers with memory impairment speaks against AD as the underlying process explaining the Aβ42–memory impairment relationship in our cohort.
Several studies, including the present one, have shown that CSF αSyn is decreased in patients with PD compared with controls, which can be explained by the cerebral accumulation of αSyn in, e.g., Lewy bodies.2,4,5 However, we show that increased levels of αSyn in CSF within the PD group might predict future worsening in cognitive processing speed and motor symptoms. αSyn is a protein localized in the presynaptic nerve terminal. It is associated with synaptic vesicles and is believed to have a role in the regulation of transmitter release, synaptic function, and plasticity.32,33 We therefore hypothesize that a greater synaptic degeneration, as one would expect to be present in the brain of patients with PD with more rapid disease progression, could lead to a greater release of αSyn into the interstitial fluid and CSF. This hypothesis is supported by previous studies showing increased CSF levels of αSyn in AD as well as Creutzfeldt-Jakob disease.2,4,5,34 It is also supported by the observed correlations between CSF levels of αSyn and markers reflecting neurodegeneration, such as tau and NFL (figure 1).3,6 However, animal and cell studies are needed to show whether increased CSF levels of αSyn merely reflect ongoing neurodegeneration or more complex changes in the cerebral metabolism of αSyn, such as changed active secretion of the protein from affected neurons.
Our results are in line with a recent study from the DATATOP cohort in which increased CSF αSyn predicted worsening of cognitive function, although the authors did not find any correlations between αSyn and progression of motor symptoms.11 The somewhat divergent results might be explained by differences in study design. For example, the disease duration of the cases included in the present study was longer, and motor deterioration often becomes more evident in patients with longer disease duration. Another recent study on baseline biomarkers in relation to cognitive decline by Parnetti et al.10 did not find an association between the levels of αSyn and future global cognitive decline as measured by the MMSE and Montreal Cognitive Assessment. In the present study, we observed an association between change in AQT over time and higher baseline levels of αSyn within the PD group. AQT primarily measures cognitive processing speed, which is affected early in PD, but not specifically measured by MMSE or Montreal Cognitive Assessment.
In the present study, motor progression was associated not only with increased CSF αSyn, but also increased CSF p-tau. The tau-encoding gene (MAPT) and the αSyn-encoding gene (SNCA) have been linked to PD through genome-wide associations studies.35 A recent study found PD risk alleles of MAPT to be associated with overall parkinsonism.36 There seem to be molecular interactions between αSyn and tau pathology. Tau can be found in Lewy bodies in sporadic PD brains.37 Indeed, there is evidence that certain αSyn fibrils might induce aggregation of tau by cross-seeding.38 Furthermore, αSyn has been shown to contribute to the phosphorylation of tau in various mouse and human models, and increased levels of p-tau can be found in the striatum of patients with PD and PD dementia.39,40 In this study, we show that αSyn levels correlate with p-tau, which is in line with previous studies and connect well with the notion that tau pathology can be associated with synucleinopathy in PD.3 One could argue that in a more aggressive disease with higher levels of αSyn, there is also a higher rate of phosphorylation of tau leading to increased levels of p-tau, which in turn might aggravate the disease, leading to a vicious cycle. It is interesting to note that elevated CSF p-tau, which is firmly linked to cognitive decline in AD, correlated to worsening of motor symptoms in PD, which, again, suggests that the pathogenic pathways are distinct in PD and AD, at least regarding the brain regions that are affected.
In this study, we show that higher CSF levels of αSyn and p-tau in the PD group are associated with faster progression of motor symptoms in PD. Furthermore, increased αSyn and decreased Aβ42 in CSF predict different aspects of cognitive decline. One limitation of the present prospective and longitudinal study is the relatively small group of patients who have been followed for at least 2 years, and our results need to be replicated in a larger cohort.
ACKNOWLEDGMENT
The authors thank the patients, research nurse Ann Johansson, and research nurse Katarina Johansson. Without their invaluable contribution, this study would not have been possible.
GLOSSARY
- Aβ42
β-amyloid 1–42
- AD
Alzheimer disease
- ADAS
Alzheimer's Disease Assessment Scale
- ADAS-cog
Alzheimer's Disease Assessment Scale–cognitive
- αSyn
α-synuclein
- AQT
A Quick Test of Cognitive Speed
- LEDD
levodopa-equivalent daily dose
- MMSE
Mini-Mental State Examination
- NFL
neurofilament light
- PD
Parkinson disease
- p-tau
phosphorylated tau
- TUG
Timed Up and Go
- UPDRS-III
Unified Parkinson's Disease Rating Scale, Part III
AUTHOR CONTRIBUTIONS
Study concept and design: O.H. Acquisition, analysis, and interpretation of data: S.H., Y.S., A.Ö., H.Z., D.L., O.H. Drafting of the manuscript: S.H., O.H. Critical revision of the manuscript for important intellectual content: Y.S., A.Ö., H.Z., D.L. Statistical analysis: S.H., D.L. Obtaining funding: O.H., H.Z. Administrative, technical, and material support: S.H., Y.S., A.Ö., H.Z., D.L., O.H. Study supervision: O.H.
STUDY FUNDING
The study was supported by the European Research Council, the Swedish Research Council, The Parkinson Foundation of Sweden, the Crafoord Foundation, the Swedish Brain Foundation, the Swedish Brain Power consortium, the Johan and Jakob Söderberg's Foundation, the Swedish Federal Government under the ALF Agreement, and the Knut and Alice Wallenberg Foundation. The funding sources had no role in the design and conduct of the study; in the collection, analysis, interpretation of the data; or in the preparation, review, or approval of the manuscript.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Zetterberg H, Petzold M, Magdalinou N. Cerebrospinal fluid alpha-synuclein levels in Parkinson's disease—changed or unchanged? Eur J Neurol 2014;21:365–367. [DOI] [PubMed] [Google Scholar]
- 2.Mollenhauer B, Locascio JJ, Schulz-Schaeffer W, Sixel-Doring F, Trenkwalder C, Schlossmacher MG. Alpha-synuclein and tau concentrations in cerebrospinal fluid of patients presenting with parkinsonism: a cohort study. Lancet Neurol 2011;10:230–240. [DOI] [PubMed] [Google Scholar]
- 3.Kang JH, Irwin DJ, Chen-Plotkin AS, et al. Association of cerebrospinal fluid beta-amyloid 1-42, t-tau, p-tau181, and alpha-synuclein levels with clinical features of drug-naive patients with early Parkinson disease. JAMA Neurol 2013;70:1277–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong Z, Shi M, Chung KA, et al. DJ-1 and alpha-synuclein in human cerebrospinal fluid as biomarkers of Parkinson's disease. Brain 2010;133:713–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall S, Ohrfelt A, Constantinescu R, et al. Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or parkinsonian disorders. Arch Neurol 2012;69:1445–1452. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Mattison HA, Liu C, et al. Longitudinal assessment of tau and amyloid beta in cerebrospinal fluid of Parkinson disease. Acta Neuropathol 2013;126:671–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siderowf A, Xie SX, Hurtig H, et al. CSF amyloid β 1-42 predicts cognitive decline in Parkinson disease. Neurology 2010;75:1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alves G, Lange J, Blennow K, et al. CSF Abeta42 predicts early-onset dementia in Parkinson disease. Neurology 2014;82:1784–1790. [DOI] [PubMed] [Google Scholar]
- 9.Compta Y, Pereira JB, Rios J, et al. Combined dementia-risk biomarkers in Parkinson's disease: a prospective longitudinal study. Parkinsonism Relat Disord 2013;19:717–724. [DOI] [PubMed] [Google Scholar]
- 10.Parnetti L, Farotti L, Eusebi P, et al. Differential role of CSF alpha-synuclein species, tau, and Abeta42 in Parkinson's disease. Front Aging Neurosci 2014;6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart T, Liu C, Ginghina C, et al. Cerebrospinal fluid alpha-synuclein predicts cognitive decline in Parkinson disease progression in the DATATOP cohort. Am J Pathol 2014;184:966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Constantinescu R, Rosengren L, Johnels B, Zetterberg H, Holmberg B. Consecutive analyses of cerebrospinal fluid axonal and glial markers in Parkinson's disease and atypical parkinsonian disorders. Parkinsonism Relat Disord 2010;16:142–145. [DOI] [PubMed] [Google Scholar]
- 13.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol 1999;56:33–39. [DOI] [PubMed] [Google Scholar]
- 14.Fahn S, Elton R; UPDRS Program Members. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan Healthcare Information; 1987:153–163. [Google Scholar]
- 15.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology 1967;17:427–442. [DOI] [PubMed] [Google Scholar]
- 16.Morris S, Morris ME, Iansek R. Reliability of measurements obtained with the Timed “Up & Go” test in people with Parkinson disease. Phys Ther 2001;81:810–818. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 18.Mohs RC, Cohen L. Alzheimer's Disease Assessment Scale (ADAS). Psychopharmacol Bull 1988;24:627–628. [PubMed] [Google Scholar]
- 19.Andersson M, Wiig EH, Minthon L, Londos E. A quick test for cognitive speed: a measure of cognitive speed in dementia with Lewy bodies. Am J Alzheimers Dis Other Demen 2007;22:313–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sebaldt R, Dalziel W, Massoud F, et al. Detection of cognitive impairment and dementia using the animal fluency test: the DECIDE study. Can J Neurol Sci 2009;36:599–604. [DOI] [PubMed] [Google Scholar]
- 21.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–2653. [DOI] [PubMed] [Google Scholar]
- 22.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer's disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol 2006;5:228–234. [DOI] [PubMed] [Google Scholar]
- 23.Blom G. Statistical Estimates and Transformed Beta-Variables. New York: John Wiley & Sons; 1958. [Google Scholar]
- 24.Montine TJ, Shi M, Quinn JF, et al. CSF Abeta(42) and tau in Parkinson's disease with cognitive impairment. Mov Disord 2010;25:2682–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mollenhauer B, Trenkwalder C, von Ahsen N, et al. Beta-amyloid 1-42 and tau-protein in cerebrospinal fluid of patients with Parkinson's disease dementia. Dement Geriatr Cogn Disord 2006;22:200–208. [DOI] [PubMed] [Google Scholar]
- 26.Parnetti L, Tiraboschi P, Lanari A, et al. Cerebrospinal fluid biomarkers in Parkinson's disease with dementia and dementia with Lewy bodies. Biol Psychiatry 2008;64:850–855. [DOI] [PubMed] [Google Scholar]
- 27.Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol 2009;65:403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strozyk D, Blennow K, White LR, Launer LJ. CSF Abeta 42 levels correlate with amyloid-neuropathology in a population-based autopsy study. Neurology 2003;60:652–656. [DOI] [PubMed] [Google Scholar]
- 29.Maetzler W, Liepelt I, Reimold M, et al. Cortical PIB binding in Lewy body disease is associated with Alzheimer-like characteristics. Neurobiol Dis 2009;34:107–112. [DOI] [PubMed] [Google Scholar]
- 30.Compta Y, Parkkinen L, O'Sullivan SS, et al. Lewy- and Alzheimer-type pathologies in Parkinson's disease dementia: which is more important? Brain 2011;134:1493–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irwin DJ, White MT, Toledo JB, et al. Neuropathologic substrates of Parkinson disease dementia. Ann Neurol 2012;72:587–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bendor JT, Logan TP, Edwards RH. The function of alpha-synuclein. Neuron 2013;79:1044–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of alpha-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci 2013;14:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mollenhauer B, Cullen V, Kahn I, et al. Direct quantification of CSF alpha-synuclein by ELISA and first cross-sectional study in patients with neurodegeneration. Exp Neurol 2008;213:315–325. [DOI] [PubMed] [Google Scholar]
- 35.International Parkinson Disease Genomics Consortium; Nalls MA, Plagnol V, Hernandez DG, et al. Imputation of sequence variants for identification of genetic risks for Parkinson's disease: a meta-analysis of genome-wide association studies. Lancet 2011;377:641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shulman JM, Yu L, Buchman AS, et al. Association of Parkinson disease risk loci with mild parkinsonian signs in older persons. JAMA Neurol 2014;71:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol 2003;62:389–397. [DOI] [PubMed] [Google Scholar]
- 38.Guo JL, Covell DJ, Daniels JP, et al. Distinct alpha-synuclein strains differentially promote tau inclusions in neurons. Cell 2013;154:103–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duka T, Duka V, Joyce JN, Sidhu A. Alpha-synuclein contributes to GSK-3beta-catalyzed tau phosphorylation in Parkinson's disease models. FASEB J 2009;23:2820–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wills J, Jones J, Haggerty T, Duka V, Joyce JN, Sidhu A. Elevated tauopathy and alpha-synuclein pathology in postmortem Parkinson's disease brains with and without dementia. Exp Neurol 2010;225:210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]


