Abstract
Metabolic reprogramming and altered bioenergetics have become emerged as a hallmark of cancer and an area of active basic and translational cancer research. Drastically upregulated glucose transport and metabolism in most cancers regardless the oxygen supply, a phenomenon called the Warburg effect, is one of major focuses of the research. Warburg speculated that cancer cells, due to defective mitochondrial oxidative phosphorylation (OXPHOS), switch to glycolysis for ATP synthesis, even in the presence of oxygen. Studies in the recent decade indicated that while glycolysis is indeed drastically upregulated in almost all cancer cells, mitochondrial respiration continues to operate normally at rates proportional to oxygen supply. There is no OXPHOS-to-glycolysis switch but rather upregulation of glycolysis. Furthermore, upregulated glycolysis appears to be for synthesis of biomass and reducing equivalents in addition to ATP production. The new finding that a significant amount of glycolytic intermediates are diverted to the pentose phosphate pathway (PPP) for production of NADPH has profound implications in how cancer cells use the Warburg effect to cope with reactive oxygen species (ROS) generation and oxidative stress, opening the door for anti-cancer interventions taking advantage of this. Recent findings in the Warburg effect and its relationship with ROS and oxidative stress controls will be reviewed. Cancer treatment strategies based on these new findings will be presented and discussed.
Keywords: The Warburg effect, metabolism reprogram, glucose transport, ROS, HIF, MYC
Introduction
A wave of new evidence indicates that not only gene mutations but also metabolic reprogramming play important roles in cancer [1–6]. In certain cases, the reprogramming of cell metabolism may even participate in the initiation of tumorigenesis [7–9]. The alterations of metabolism and energetics, within which glucose and adenosine triphosphate (ATP) are prominent players, have been recognized in recent years as an emerging hallmark of cancer [10]. Actually, the importance of metabolic alteration in cancer cells was recognized long ago. In the 1920s, Otto Warburg, a German biochemist, demonstrated that unlike normal tissues, cancer cells always upregulated glycolysis even when oxygen was abundant [11–13]. This phenomenon of so-called aerobic glycolysis became known as the Warburg effect [14–20].
Warburg hypothesized that existing mitochondrial dysfunction disrupts oxidative phosphorylation (OXPHOS) pathway therefore, cancer cells have to switch from OXPHOS to glycolysis for ATP generation [14, 18]. As glycolysis is much less efficient than OXPHOS for producing ATP, it has to be greatly upregulated so that sufficient ATP will be synthesized. However, this hypothesis has been challenged in recent years due to findings that upregulated glycolysis in many cancers is not accompanied by detectable mitochondrial defects or OXPHOS disruptions [21, 22]. In addition, new evidence revealed that the upregulation of glycolysis is not just for ATP synthesis, but also for synthesis of biomass such as ribonucleotides [23] and amino acids [24] as well as reduced nicotinamide adenine dinucleotide phosphate (NADPH) production [25], which can remove reactive oxygen species (ROS) generated by cancer cells’ accelerated metabolism under hypoxic conditions [25, 26]. Thus, the Warburg effect appears to be a strategic move made by cancer cells not only to cope with multiple urgent requirements simultaneously for growth, and proliferation in an ever-changing microenvironment under numerous material limitations, such as shortages of oxygen and nutrients; but also to reduce ROS and therefore oxidative stress in cancer cells.
Although the Warburg effect was specifically described for metabolic changes in cancer cells, the phenomenon (aerobic glycolysis) was also observed in rapidly proliferating normal cells such as stimulated lymphocytes and mitotic and proliferating fibroblasts [27–32]. This dramatic physiological change in normal cells is due to the temporary higher demands in metabolic material and energy for completing the cell proliferation process. The fact that aerobic glycolysis is present in E coli, yeasts, normal proliferating cells as well as almost all cancer cells [27–32], suggests that this is an evolution-selected metabolic strategy conserved among cells to meet special needs during cell proliferation and most cancer cells exploit this strategy because of their constant needs for rapid growth and proliferation.
Brief History And Current Interpretations Of The Warburg Effect
In the early 1920s, after partially elucidating the metabolic pathways of glycolysis and OXPHOS for ATP synthesis, Otto Warburg and his co-workers developed an ex vivo system to measure energy metabolism of cancer tissue slices with a thickness of approximate 200–300 µm isolated from Flexner-Jobling rat liver carcinoma using then newly developed quantitative measurement techniques. He and his coworkers meticulously measured O2 uptake and lactic acid production by the tumor slices and calculated the amount of glucose consumed by cancer slices. He observed that, compared to normal tissues, cancer slices used approximately 10 times more glucose in their energy metabolism and produced a large amount lactate from upregulated glycolysis. Interestingly and surprisingly, the approximately ten-fold upregulation of glycolysis persisted even when the cancer slices were assayed in the presence of normal O2 pressure [11–13, 18]. From this observation, Warburg concluded that the upregulated and persistent glycolysis was likely to be a forced action taken by cancer cells to switch to glycolysis for producing sufficient ATP to compensate for ATP loss due to dysfunctional OXPHOS resulting from mitochondrial defects [14, 18].
In recent decades, it has been recognized that cancer metabolism is an integral part of cancer biology. Research done in the last 10–15 years has confirmed the near-universal prevalence of the Warburg effect in cancers. What was observed and measured by Warburg more than 90 years ago was mostly and quantitatively correct. However, his theory regarding the reason for cancer cells to upregulate glycolysis has been challenged, because in many cancers, aerobic glycolysis is upregulated without mitochondrial dysfunction (no identifiable mitochondrial gene mutations) or OXPHOS disruption [18, 21, 22]. In these cancers, OXPHOS continues as normal and produces as much ATP as OXPHOS in normal tissue under the same oxygen pressures [18, 21, 22]. Therefore, the upregulation of glycolysis may be a strategic metabolic action made by cancer cells for the purpose of balancing the functional needs of cancer cells, primarily in synthesis of biomass: ribose for RNA and DNA [23], amino acids for proteins [24], fatty acids as precursors for components of plasma and intracellular membranes [27, 33] as well as reducing equivalents (NADPH) for reducing ROS and oxidative stress [25]. Glycolytic ATP synthesis seems to be of no higher priority because cancer cells, regardless of their oxygen supply, do not suffer an ATP shortage. The debate on the functional roles of upregulated glycolysis in the Warburg effect is ongoing, and interpretation is evolving alongside exciting new findings. Of note, the metabolic reprogramming observed in cancer cells is also found in normal proliferating cells for the same requirements in increased biosynthesis of nucleic acids, amino acids, and fatty acids [27–32].
Because of drastically upregulated glycolysis, more glucose is transported into cells and thus more pyruvate is produced in an average cancer cell than in normal cells. Limited by the capacity of OXPHOS and regulated by lactate dehydrogenase (LDH), pyruvate comparable to the amount in normal cells enters the mitochondrial TCA cycle. A lingering misconception about the Warburg effect is that OXPHOS in cancer cells is greatly reduced compared to normal cells. In fact, OXPHOS in most cancers is normal and a similar amount of ATP is produced by OXPHOS. Different from what Warburg theorized, there is no switch from OXPHOS to glycolysis in cancer cells, rather, glycolysis is upregulated even in the presence of normal oxygen pressure (normoxia) because of higher demands for biosynthesis. Under hypoxia, due to the limited oxygen availability, less pyruvate enters the TCA cycle and thus more pyruvate is converted into lactate. The excess lactate is secreted into the intratumoral space by hypoxic cancer cells [18, 20].
ROS Balancing And The Warburg Effect
ROS act as a double-edged sword for cancer cells. An elevated but controlled ROS level is required for cancer growth and proliferation [34]. ROS are involved in tumor angiogenesis [35, 36], in ligand-independent transactivation of receptor tyrosine kinase [37, 38], as well as in promoting invasion and metastasis of cancer cells [39, 40].
However, ROS are also a major contributor to oxidative damage [41]. Thus the cellular level of ROS must be vigorously maintained within certain ranges so that they will only promote cancer cell growth and proliferation without causing severe oxidative damage and even cell death. ROS production in cancer cells is elevated due to oncogenic stimulation and increased metabolic activities [34]. The Warburg effect, which leads to the production of NADPH and thus a proper redox status, becomes an important survival mechanism for cancer cells.
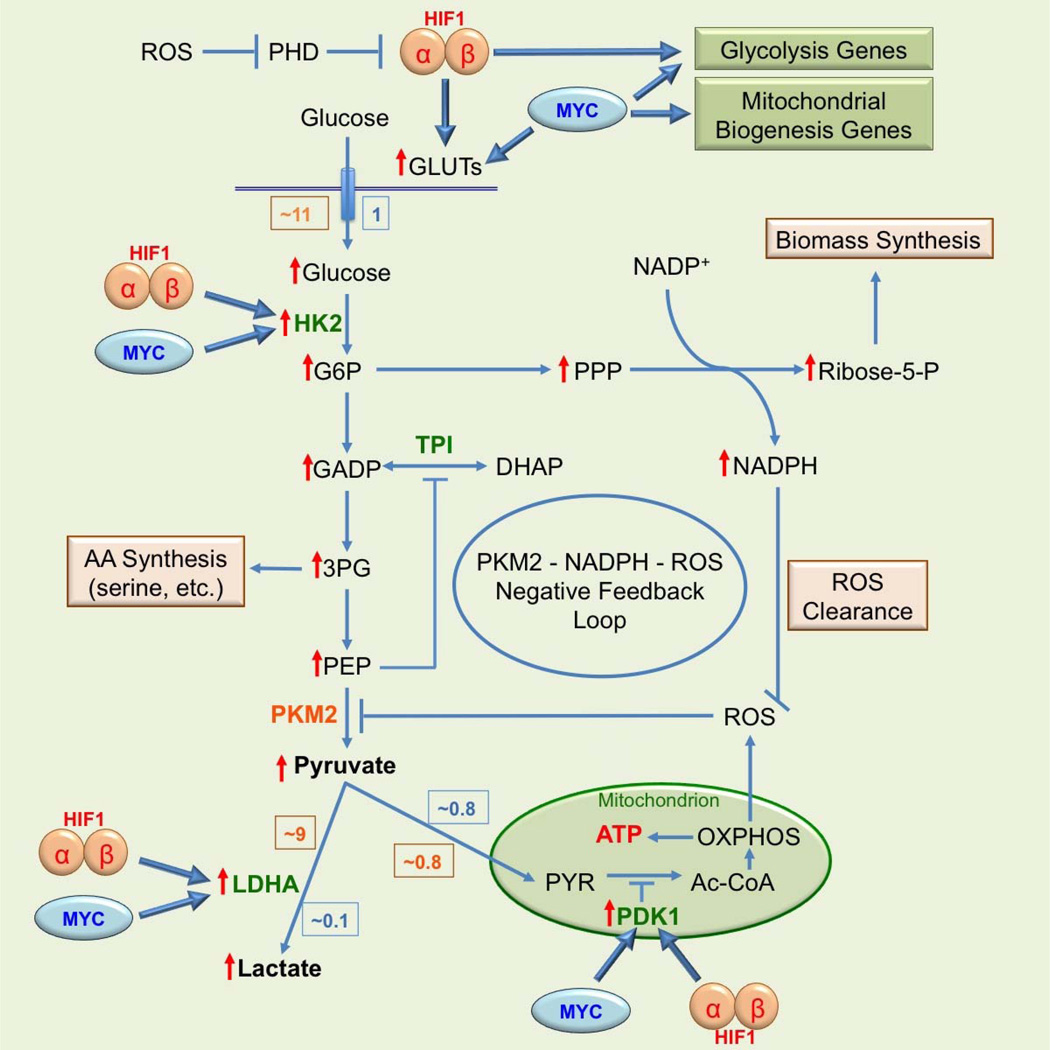
One important step of glycolysis reprogramming that leads to The Warburg effect is the switch in isoform of pyruvate kinase (PK), which catalyzes the conversion of phosphoenolpyruvate (PEP) to pyruvate as the last step of glycolysis. Many types of cancer cells use the M2 isoform of pyruvate kinase (PKM2) instead of the M1 isoform of the enzyme (PKM1) as normal tissues do [42–44]. This is surprising because cancer cells drastically upregulate glycolysis; yet PKM2 is less active than PKM1. This paradoxical phenomenon was explained in recent years. The major reasons for upregulating glycolysis in cancers are not only for synthesis of ATP but also for synthesis of biomass (nucleic acids and proteins) and NADPH [25, 26]. By using PKM2, cancer cells upregulate glucose transport and the early steps of glycolysis without overproducing pyruvate. Instead, using the slower PKM2 leads to accumulation of earlier glycolytic intermediates, diverting them to glycolysis-connected subsidiary biosynthesis pathways such as hexosamine, PPP, and amino acids. One major purpose for upregulating glucose transport and early steps of glycolysis appears to be for cell biomass production and achieving metabolic balance among ATP production, biomass synthesis, as well as the control of oxidative stress resulted from ROS generation [25, 26]. The switch to PKM2 results in accumulation of PEP, which functions as a feedback inhibitor of the glycolytic enzyme triosephosphate isomerase (TPI). This in turn activates PPP, increasing antioxidative metabolism by producing more NADPH, reducing ROS, and amplifying the inhibitory effect of PKM2 [25, 26]. In addition, ROS and PKM2 form a negative feedback loop to maintain ROS in a tolerable and functional range (Fig. 1). PKM2 can be specifically oxidized on cysteine 358 by hydrogen peroxide (H2O2), an ROS, which leads to reduction in its activity and pyruvate production as well as augmentation of flux of glycolytic intermediates into PPP [25, 26].
Figure 1. The Warburg effect with its extended functions and regulations.
Relative amount of glucose consumption and its metabolic products in normal (blue box) and cancer (orange box) under normoxic condition are shown and compared. Red ↑ indicates an elevated level in cancer cells. The enzymes in green function in both normal and cancer cells; and the enzyme in orange functions mainly in cancer cells.
Although the role of the Warburg effect in generating additional NADPH via the pentose phosphate pathway (PPP) for coping with higher ROS levels in cancer cells was elucidated in the past ten years [45], the higher steady-state levels of superoxide and H2O2 in cancer cells relative to normal cells were reported as early as 35 years ago [46–51]. Based on these earlier experimental results, it was hypothesized and has been validated that the increased glucose metabolism in cancer cells is for compensating increased fluxes of H2O2 produced in mitochondria by producing higher amounts of both NADPH as a co-factor for H2O2 metabolism and pyruvate for directly scavenging H2O2 in a deacetylation reaction to form acetic acid and H2O [46, 52–56].
Superoxide and H2O2, two common ROS, are known to be increasingly produced primarily by mitochondria in cancer cells. One possible mechanism for the increased ROS production in cancer cells is that some alterations in the assembly of electron transport chain complexes lead to stoichiometric mismatches. Such mismatches result in increased residence time of electrons on sites of the complexes that mediate electron reductions of O2 and production of increased amount of superoxide and H2O2 [57]. The increased fluxes and steady-state levels of ROS in cancer cells versus normal cells have significant impacts on tumorigenesis and metabolic reprogramming. It can not only lead to uncontrolled growth and the inability of cancer cells to differentiate but also to increased genomic instability. The resulting additional genetic mutations caused by ROS and other factors may significantly influence the progression of tumors and the evolution of metabolic reprogramming of cancer cells.
ROS-Involved Gene Regulation And The Warburg Effect
Besides direct impact, ROS can also indirectly contribute to the Warburg effect via its involvement in regulation of gene expression. One well-studied ROS-regulated gene is hypoxia-inducible factors (HIF) [58–60]. The uncontrollable growth and proliferation of cancer cells as well as abnormal vasculogenesis lead to deficiency of oxygen supply and local hypoxia in tumors [61]. The resulting condition triggers the increased expression of HIF [61, 62]. There are three members (isoforms) in the HIF family: HIF1, HIF2, and HIF3 with HIF1 and HIF2 better studied and HIF3’s functions poorly understood. Among the three, HIF1 is the only one that is ubiquitously expressed and the most relevant to cancer [61, 62]. HIF1, like all HIFs, consists of an oxygen-dependent α-subunit and a constitutively expressed β-subunit. Under normoxia, HIF1α is constitutively synthesized and hydroxylated by prolyl hydroxylases (PHDs). Hydroxylated HIF1α is recognized by the von Hippel-Lindau protein (VHL) and its associated ubiquitinase, resulting in proteolytic degradation in proteasomes [62, 63]. Under hypoxia, reduced oxygen supply diminishes the activity of PHDs, which are further inhibited by ROS released from stressed mitochondria that operate under reduced OXPHOS. ROS oxidize and inactivate the ferrous ion located in the active site of PHDs such that they become unable to modify and thus stabilize HIF1α, which binds to HIF1β to form a stabilized HIF1. HIF1 complex then binds and transactivates genes involved in glucose transport, glycolysis, pH regulation, and vasculogenesis, allowing cancer cells to rapidly adapt to hypoxia [61, 63, 64]. HIF functions as a master regulator for the initiation and maintenance of the Warburg effect at the level of gene expression. Recently, PKM2 is found to be a PHD-induced coactivator for HIF [65], adding another link between the Warburg effect and HIF.
Another well-documented gene that is regulated by HIF and contributes to the Warburg effect is the MYC proto-oncogene [66]. While it is not directly regulated by ROS, activated MYC, the protein product of the MYC gene, can either work together with HIF or independently in regulating glycolysis and OXPHOS. The MYC gene is a classical immediate early serum response proto-oncogene under vigorous transcriptional control [66, 67]. Approximately 30% of all human cancers show deregulated MYC gene expression [68]. MYC is a transcription factor that regulates genes involved in glucose metabolism by binding to and regulating virtually all glycolytic enzyme genes as well as numerous genes involved in mitochondrial biogenesis [61, 69]. One of the key roles of MYC in normal cells is to stimulate glycolytic flux for OXPHOS [66, 67]. In cancer cells, working together with HIF and PKM2, constitutively active MYC upregulates glycolysis to ensure sufficient metabolic intermediates for synthesis of biomass and reducing equivalents needed by cancer cells [25, 26, 70]. Compared to HIF, MYC appears to be more involved in the transcriptional regulation of genes participating in energy generation and cell growth and proliferation. Both HIF and MYC activate hexokinase 2 (HK2) and pyruvate dehydrogenase kinase 1 (PDK1), leading to augmented glycolytic rates and conversion of glucose to lactate [61]. Furthermore, HIF1 and MYC independently activate glucose transporter 1 (GLUT1) and lactate dehydrogenase A (LDHA), resulting in increased glucose influx and higher glycolytic rates [61]. The roles of HIF1 and MYC in the regulation of the Warburg effect are schematically shown in Figure 1.
Oxygen Supply, ATP Synthesis And The Warburg Effect
The Warburg effect is a dynamic process, in which the weight of OXPHOS relative to glycolysis in total ATP synthesis is constantly adjusted in response to cancer cells’ microenvironments, particularly oxygen supply rate. Oxygen pressures (pO2) in cancer cells are lower than those in normal cells of the same tissue origin and are different in different tumor types, ranging from very low mmHg to slightly above 10 mmHg as compared to 160 mmHg in the air and approximately 40 mmHg in the vein [71–73]. Normoxia and hypoxia are relative concepts without absolute standards because hypoxic pO2 in one cancer type may be normoxic in another type. Inside of a single tumor, pO2 is also different from one location to another depending upon the abundance of blood vessels to that location. Even more complicated, pO2 of one location of a tumor can also be changing during tumorigenesis. This is because the vascular structure inside of a tumor is constantly forming and dying, leading to reported phenomena of intermittent and cyclic hypoxia inside tumors [74–77]. Cancer cells in tumors are heterogeneous with respect to their oxygen supply and ATP synthesis rate. Normoxic cancer cells produce as much as 50% more ATP than normal cells by fully oxidizing a molecule of glucose through OXPHOS (with 36 ATP molecules produced), while simultaneously metabolizing approximately another 10 glucose molecules to lactate through upregulated glycolysis and producing an additional ~20 ATP molecules during the process [18]. By comparison, anoxic cancer cells metabolize about 13 glucose molecules to produce only 26 ATP molecules exclusively through glycolysis. However, a large proportion of cancer cells in tumors lives and grows in varied hypoxic conditions between these two extremes. These heterogeneous cells produce more ATP than anoxic ones but significantly less ATP than normoxic cancer even normal cells [18]. Exactly how much ATP they can produce depends on their oxygen and glucose availabilities. On average, cancer cells in a tumor produce about 10% more ATP than normoxic normal cells [18], a value indicative of the heterogeneity of cancer cells within a tumor. While a sub-population of cancer cells has reduced ATP production due to a lack of oxygen and severely depressed OXPHOS rates, these cells do not appear to suffer from a lack of ATP. It is presently unclear as to how these hypoxic cancer cells are capable of securing sufficient levels of ATP to maintain growth and proliferation. One possible explanation is that high ATP-producing normoxic cancer cells and possibly even stromal cells release ATP while low-ATP producing hypoxic cancer cells take up the released ATP from the intratumoral space to supplement their intracellular ATP pool (Fig. 2). As a result, the intracellular ATP concentration of hypoxic cancer cells are elevated to such levels that they are capable of performing all the biological functions required for survival, growth, proliferation and even cell movement required for invasion and metastasis [78, 79].
Figure 2. Potential drug targets in ATP-sharing model.
According to this model, a symbiotic relationship exists among cancer and stromal cells in a tumor. Normoxic cancer cells and stromal cells recruited by hypoxic cancer cells release ATP into intratumoral space, leading to a large intratumoral ATP concentration increase. Highly concentrated intraturmoral ATP is then internalized by hypoxic cancer cells through macropinocytosis and/or other endocytic processes, supplementing the intracellular ATP pool in hypoxic cancer cells. Meanwhile, cancer cells uptake and release of lactate through transporters MCT1 and MCT4. Potential targets for anticancer therapeutic intervention in this model are shown by symbol  .
.
Mammalian cells, including cancer cells, are known to release ATP under certain conditions [80–84]. Uptake of ATP by animal cells has been speculated [85–88] but has not been experimentally demonstrated. While interstitial ATP concentrations in normal tissues are maintained between 1–1000 nM depending upon tissue type [89–94], intratumoral ATP levels are in the range of several hundred M [95–98], which suggests ATP is readily available for use by hypoxic cancer cells. However, ATP is a charged and thus hydrophilic molecule, unable to cross the cell membrane without the help of a transporter. Since no plasma-membrane-associated ATP transporter has been found, it has long been presumed that extracellular ATP does not enter cells. There has been no direct evidence or identified mechanism for ATP uptake by cancer cells until a recent study demonstrated that normal pancreatic cells transformed with an oncogenic form of Kras gene (Krasonc) drastically upregulated macropinocytosis [99], a type of endocytosis that engulfs extracellular fluid and nonspecifically takes up extracellular molecules in the fluid. When the macropinocytosis inhibitor EIPA was used to treat nude mice with xenografted tumors of the transformed cells, it substantially reduced tumor growth [99] indicating that the transformed cells use macropinocytosis to take up extracellular nutrients to support their growth in vivo. Because macropinocytosis, commonly named “large-scale fluid drinking”, is nonspecific for the molecules it internalizes [100, 101], many extracellular molecules including the highly concentrated ATP should be taken up by this process. These reports [80–88, 95–99] and our prevous finding of that extracellular ATP increased intracellular ATP levels and increased cancer cell growth and survival [102] led to the hypothesis that low ATP-producing hypoxic cancer cells likely solve the problem of ATP deficiency by upregulating macropinocytosis to bypass the lack of an ATP transporter for acquiring extracellular ATP. They internalize intratumoral ATP, hypothetically released from stromal cells and/or normoxic cancer cells (Fig. 2). The uptake of extracellular ATP also theoretically reduces intracellular ATP synthesis, ROS production and oxidative stress as well as increases survival of cancer cells under conditions of hypoxia. The hypothesis is supported by our recent finding that treating cells with ATP, in the range of the reported extracellular ATP concentrations in several cancer types [60–65], induced intracellular ATP concentration by 50% to 100% via macropinocytosis and other endocytic processes [103]. The increase of ATP was not observed in their non-cancerous cell counterparts, suggesting the extracellular ATP-caused intracellular ATP elevation was a capability associated with these cancer cell lines. In addition, the extracellular ATP substantially contributes the cancer cells’ resistance to tyrosine kinase inhibitor (TKI) anticancer drugs such as sunitinib [103]. The mechanism of the resistance might be the direct competition between intracellular ATP, which was in higher concentration in the presence of extracellular ATP, and TKIs. All these indicate not only extracellular ATP can enter cancer cells but also significantly contribute to cancer cell survival, growth and drug resistance to TKIs.
Warburg Effect At The Tumor Microenvironment Scale
Cancer cells in a tumor nodule are far from a homogeneous population and several levels of heterogeneity exist among cancer cells in tumors. First, cancer cells in tumors are often genetically heterogeneous among tumors in the same individual or even within a single tumor. As tumors grow, cancer cells within a tumor can accumulate additional genetic mutations and create further genetic diversity [104, 105]. Second, cancer cells in a tumor are also metabolically heterogeneous primarily due to their distances to intratumoral blood vessels, which determines the relative levels of oxygen and nutrient supplies to the cells. Because of the differences in oxygen and nutrient supply, these cancer cells exhibit different rates of mitochondrial respiration and different degrees of reliance on aerobic glycolysis [18]. Third, a subpopulation of cancer stem cells (CSCs) have been identified and isolated from different cancers. Similar to normal stem cells (SCs), CSCs show full self-renewal capability and the Warburg effect (upregulated aerobic glycolysis) [106–109]. Different from SCs, CSCs can differentiate into only non-CSC cancer cells. Forth, the tumor heterogeneity is not only spatial but also temporal. Tumorigenesis is an ever-changing dynamic process. Oxygen and nutrient status of a region in a tumor can change during tumor development and normoxic cancer cells in a tumor at a given time can become hypoxic at another time and vice versa [74–77]. Genetic and metabolic heterogeneities can further communicate with each other, partially determining the fate of tumor development. These diversities exert differential metabolic pressures on heterogeneous cancer cells in a tumor. As a result, different cancer cells exhibit different levels of the Warburg effect depending upon their oxygen and nutrient status as well as their communications with other cancer cells and stromal cells.
Cancer is a mixed population of cancer cells and stromal cells, which include cells of hematopoietic and mesenchymal origins. All these cancer and non-cancer cells plus the extracellular matrix (ECM) form the tumor microenvironment [110–112]. Stromal cells of hematopoietic origin include T and B lymphocytes, natural killer (NK) cells as well as macrophages, neutrophils, and myeloid-derived suppressor cells (MDSCs) [111]. The role of T cells is either tumor-promotion [113] or tumor-elimination [114] depending upon the exact tumor microenvironmental context. For example, stimulated T lymphocytes are able to release large amount ATP into extracellular space, potentially modulating cancer metabolism through purinergic receptor-mediated signaling [115]. T cells and macrophages, interacting with cancer cells through cytokines, can launch tumor-protective and tumor growth-promoting inflammatory responses [116]. Similarly, each of the other constituent cell types of hematopoietic origin may have either a positive or negative effect on tumor development. Stromal cells of mesenchymal origin include fibroblasts, myofibroblasts, mesenchymal stem cells (MSCs), adipocytes and endothelial cells. Among these cells, adipocytes are found to secret hepatocyte growth factor (HGF) to promote tumor growth [117] and endothelial cells play roles in angiogenesis and cancer cell dissemination [118, 119]. Thus, all of these stromal cells directly or indirectly affect cancer metabolism and participate in metabolic reprogramming of cancer cells, including regulating the Warburg effect.
Although cancer cells are competing with stromal cells for limited resources such as oxygen and nutrients and energy in the form of ATP, they also form symbiotic and cooperative relationships with one another. The outcome of such overall competition and cooperation results in the formation of a commonly acceptable microenvironment for the best survival of the tumor by transforming normal stromal cells into tumor-friendly stromal cells to serve the needs of cancer cells and tumors. One such example is stimulated lymphocytes release large amounts of ATP into extracellular space [120], potentially creating an ATP-rich environment for both direct energy intake and for purinergic receptor-mediated signaling to regulated metabolisms of cancer cells. This theory is supported, at least in part, by the observation that intratumoral ATP concentrations are 103 to 104 times higher than those of normal tissues [95–98]. Also, immune stromal cells also use cell surface enzymes CD39 and CD73 to dephosphorylate ATP into AMP and adenosine, respectively [115, 121], creating an immunosuppressed environment within tumors. Working in coordination, CD39 and CD73 create and adjust a "purinergic halo" surrounding immune stromal cells to modulate signaling events mediated by purinergic receptors by regulating the duration, magnitude and composition of the halo [115, 121]. Through purinergic signaling, immune stromal cells mediate immunological and inflammatory responses such as immunoescape and cancer cell killing impairment in tumors. In addition, CD39/CD73 system is also found overexpressed on the surface of cancer cells [115], suggesting that this system also utilize high intratumoral ATP concentration to regulate cancer cell metabolism including the Warburg effect.
The Warburg Effect As A Potential Target For Cancer Treatment
As presented in the Figure 1, some major characteristics of the Warburg effect are: (i) increased expression of glucose transporters and thus an increased uptake of glucose. (ii) Increased PPP-catalyzed NADPH production. (iii) Altered activities of glycolytic or glycolysis-related enzymes (such as HIF/MYC induced activation of HK2, LDHA and PDK1; and the switch from PKM1 to a less active PKM2). (iv) Increased lactate production. Some of these characteristics have been or could potentially be targeted for developing therapeutics for cancer treatments (Fig. 2) (Table 1). For example, inhibiting glucose transport should lead to shortage of glucose supply to cancer cells, thus slowing down cancer metabolism and biomass synthesis and forcing cancer cells to stop growing and undergo apoptosis. Up to 90% of all cancers substantially upregulate GLUTs and glucose metabolism as demonstrated by PET-scans of cancer patients [122–126]. Cancer cells are “addicted” to glucose and are more sensitive to changes in glucose transport and glucose supply than normal cells [102, 127]. At least twelve glucose transporters (GLUTs) have been identified [128–133]. Among them, GLUT1[134–139], GLUT2 [140, 141] and GLUT3 [142] are the most relevant to cancer. In fact, many GLUT inhibitors have been in studies. More inhibitors for GLUT1 have been developed than for other GLUTs due to its near-universal upregulation in cancer and better knowledge of its protein structure (Fig. 2). Reported GLUT1 inhibitors include GLUT1 antibody [143], fasentin [144, 145], apigenin [146–148], genestein [149–152], oxime-based inhibitors [153], STF-31 [154], WZB117 [67] and shRNA interfering expression of GLUT1 [155]. While all these GLUT1 inhibitors showed some inhibitory effect in different cultured cancer cell lines, STF-31 [154], WZB117 [102], and GLUT1 RNAi [155] also inhibited cancer growth in vivo. STF-31 inhibited tumor growth in a renal cell carcinoma animal model [154]; WZB117 reduced cancer growth rate by more than 60% in nude mice bearing human lung cancer (A549 cells) [102], and RNAi inhibition of GLUT1 prevented myeloproliferation [155]. These three in vivo studies clearly demonstrate anticancer efficacy of GLUT1 inhibitors in animal models.
Table 1.
Therapeutics targeting the Warburg effect in cancers
| Process | Target | Compound | Effect | Status | References |
|---|---|---|---|---|---|
| Glucose transport | GLUT1 | WZB117, STF-31 | Inhibits GLUT1 | Preclinical | [102, 154] |
| Glycolysis | HK | 2DG | Inhibits HK | Clinical trials discontinued | [156] NCT00633087 NCT00096707 NCT00247403 |
| PKM2 | TEPP-46 | Activates PKM2 and inhibits PPP | Preclinical | [157, 158] | |
| LDHA | FX11 | Inhibits LDHA | Preclinical | [159] | |
| PPP | G6PD | 6-AN | Induces oxidative stress | Preclinical | [160] |
| Lactate transport | MCT1 | AZD3965 | Inhibits uptake of extracellular lactate | Phase I | [161] NCT01791595 |
| Mitochondrial function | PDK1 | DCA | Inhibits PDK1 | Phase I-II | [162, 163] NCT00566410 NCT01111097 NCT00540176 |
| AKT signaling pathway | AKT | AZD5363 | Inhibits AKT activity | Phase I-II | [164] NCT01895946 NCT01353781 NCT01692262 NCT02077569 NCT01226316 |
| GDC0068 | Phase I | [165] NCT01090960 |
|||
| GSK2141795 | Phase I completed | [166] NCT00920257 NCT01266954 |
|||
| GSK2110183 | Phase I–II completed Phase II | [166] NCT00881946 NCT01531894 |
|||
| MK-2206 | Phase I-II | [167] NCT01283035 NCT01277757 NCT01253447 NCT01231919 NCT01604772 NCT01258998 NCT01307631 NCT01349933 NCT01481129 NCT01802320 NCT01260701 NCT01425879 NCT01319539 NCT01226316 |
In addition to GLUT inhibitors, drugs targeting enzymes involved in regulation of glycolysis are also in development. 2-deoxy-glucose (2DG) is a hexokinase inhibitor competing with glucose and 2DG has been widely studied as a potential anti-cancer drug [156]. As detailed previously, PKM2 used in cancer cells is less active than PKM1 used in normal tissues [42–44]. Thus, multiple activators of PKM2 have been studied for their ability in reducing upstream PPP-mediated anabolism and suppressing tumorigenesis [157, 158]. The activity of LDHA is closely related with NADH consumption. FX-11, as a selective inhibitor of LDHA, induced oxidative stress and inhibited tumor progression [159]. PPP not only produces metabolic intermediates for biomass synthesis, but also generates NADPH as reducing agents. Glucose-6-phosphate dehydrogenase (G6PD) catalyzes the critical step of generating NADPH in PPP. 6-aminocicotinamide (6-AN), as an inhibitor of G6PD, induced oxidative stress and inhibited the growth of cancer cells [160]. AZD3965, as an inhibitor of MCT1, blocked the transport of lactate into cancer cells and therefore inhibited the lactate-fueled respiration in cancer cells [161]. Dichloroacetate (DCA), an inhibitor of PDK1, indirectly activated mitochondrial respiration and inhibited cancer growth [162]. A recent clinical trial of DCA showed some durable anticancer efficacy in the treatment of non-Hodgkin’s lymphoma [163]. Finally a group of inhibitors suppress tumor growth by targeting PI3K/AKT signaling pathway [164–167], which can regulate glycolysis and the Warburg effect by altering levels and activities of key glycolytic proteins and enzymes such as GLUTs, HK, and PFK [2, 16, 87].
Many of above-described agents could inhibit cancer cell growth by decreasing metabolic precursors for glycolysis and synthesis of biomass, reducing equivalents, and generating “absolute nutritional hunger”; their “side effect” on ROS production should not be ignored. Overproduction of ROS leads to excessive oxidative stress and cancer cell death. Inhibiting glucose transport reduces glucose supply, forcing cancer cells to reduce OXPHOS and resulting in increased ROS generation. Starvation and oxidative stress induced by glucose transport inhibition produce severe one-two punches to cancer. The key for the success of this approach is to produce more harm to cancer cells than benefits. An initial assault must be severe such that cancer cells suffer irreversible damage and cell death before they can shift to other energy and/or carbon sources such as glutamine. Tolerable ROS and oxidative stress ranges are also relative and can be adjusted by cancer cells. The details of the regulation of these two processes must be learned before we can identify and formulate a more effective anticancer strategy by combining inhibition of glucose transport and glucose metabolism as well as ROS dysregulation. Besides potential side effect from ROS, targeting the Warburg effect may encounter some other challenges. First, CSCs have at least three known features that may interfere cancer therapy targeting glucose metabolism or the Warburg effect. (a) CSCs show upregulated aerobic glycolysis as non-CSC cancer cells [51–54]. (b) CSCs are slower cycling compared to the non-CSC cancer cells in the same tumors [33]. Thus, it is likely that CSCs have lower glucose metabolic rate or reliance on glucose metabolism relative to non-CSC cancer cells. (c) CSCs overexpress the ATP-binding cassette transporters (ABCs), which play crucial roles in multi-drug resistance (MDR) [33]. As ABCs are ATP-binding and ATP-consuming, reducing ATP production or ATP internalization processes such as macropinocytosis may contribute to fighting drug resistance and the overall strategy of targeting CSCs. Simultaneous reduction of intratumoral (extracellular) ATP concentration and intracellular ATP synthesis and creation of an ATP-poor microenvironment should be a novel anticancer and anti-CSC strategy. Second, both normal proliferating cells and cancer cells upregulate aerobic glycolysis [27–29]. Targeting the Warburg effect (glycolytic proteins and/or enzymes) will not only inhibit cancer cells, but also normal proliferating cells. The latter (lymphocytes under clonal expansion for example) perform some important normal and urgently needed physiological functions. For this reason, infant and child cancer patients should not be treated with the Warburg effect-targeting therapeutics, as they are growing with large quantities of proliferating cells in their body. For similar reasons, cancer patients undergoing immunotherapy (with potential immune cell clonal expansion going on) should be excluded from the simultaneous anti-Warburg effect therapy. Last, the strategy may not be very effective for those cancers that rely more on glutamine rather than glucose for their metabolic needs. Profiles of glucose metabolism of tumors from cancer patients should be determined before the anti-Warburg effect therapy strategy is adopted.
Conclusion
Cancer metabolism research in the past decade has substantially enhanced our understanding and changed the interpretations of the Warburg effect. Much more than Warburg initially speculated and in addition to glycolytic ATP synthesis, aerobic glycolysis also contributes to synthesis of biomass and reducing equivalents and plays a significant and varied role in cancer biology. The connection between the Warburg effect and cancer cell redox homeostasis has been established. The regulation of glycolysis and other glycolysis-connected metabolic pathways are well understood. However, many differences among various cancer types are recognized, with respect to their different genetic mutations; the heterogeneity among cancer cells within a single tumor; and their oxygen and nutritional supplies. Although still in an exploratory stage, targeting the Warburg effect nevertheless already has yielded promising results. With improved knowledge and better understanding of the Warburg effect, a therapy in combination with oncogenic and metabolic targets with redox manipulation is likely to generate synergistic anticancer effects and become one future anti-cancer strategy.
Highlights.
ROS Balancing And The Warburg Effect
ROS-Involved Gene Regulation And The Warburg Effect
Oxygen Supply, ATP Synthesis And The Warburg Effect
Warburg Effect At The Tumor Microenvironment Scale
The Warburg Effect As A Potential Target For Cancer Treatment
Acknowledgments
We thank Dr. Athena Chen for critical reading of the manuscript. This work was partially supported by Student Enhancement Award (to Y. Qian), Graduate Student Senate Original Work Grant (to Y. Qian), the Donald Clippinger Graduate Fellowship from Ohio University (to Y. Qian), HCOM RSAC research award (to X. Chen) and NIH 2RO1CA086928 (to S. Wu).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 2.Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Levine AJ, Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science. 2010;330:1340–1344. doi: 10.1126/science.1193494. [DOI] [PubMed] [Google Scholar]
- 4.Schulze A, Harris AL. How cancer metabolism is tuned for proliferation and vulnerable to disruption. Nature. 2012;491:364–373. doi: 10.1038/nature11706. [DOI] [PubMed] [Google Scholar]
- 5.Soga T. Cancer metabolism: key players in metabolic reprogramming. Cancer Sci. 2013;104:275–281. doi: 10.1111/cas.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang M, Soga T, Pollard PJ. Oncometabolites: linking altered metabolism with cancer. J Clin Invest. 2013;123:3652–3658. doi: 10.1172/JCI67228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundaram S, Johnson AR, Makowski L. Obesity, metabolism and the microenvironment: Links to cancer. J Carcinog. 2013;12:19. doi: 10.4103/1477-3163.119606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taubes G. Cancer research. Unraveling the obesity-cancer connection. Science. 2012;335:28, 30–22. doi: 10.1126/science.335.6064.28. [DOI] [PubMed] [Google Scholar]
- 9.Yun J, Rago C, Cheong I, Pagliarini R, Angenendt P, Rajagopalan H, Schmidt K, Willson JK, Markowitz S, Zhou S, Diaz LA, Jr., Velculescu VE, Lengauer C, Kinzler KW, Vogelstein B, Papadopoulos N. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325:1555–1559. doi: 10.1126/science.1174229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Warburg O. The Chemical Constitution of Respiration Ferment. Science. 1928;68:437–443. doi: 10.1126/science.68.1767.437. [DOI] [PubMed] [Google Scholar]
- 12.Warburg O, Wind F, Negelein E. The Metabolism of Tumors in the Body. Journal of General Physiology. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warburg OH. The classic: The chemical constitution of respiration ferment. Clinical Orthopaedics and Related Research. 2010;468:2833–2839. doi: 10.1007/s11999-010-1534-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 15.Bayley JP, Devilee P. The Warburg effect in 2012. Curr Opin Oncol. 2012;24:62–67. doi: 10.1097/CCO.0b013e32834deb9e. [DOI] [PubMed] [Google Scholar]
- 16.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 18.Koppenol WH, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 19.Upadhyay M, Samal J, Kandpal M, Singh OV, Vivekanandan P. The Warburg effect: insights from the past decade. Pharmacol Ther. 2013;137:318–330. doi: 10.1016/j.pharmthera.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 22.Moreno-Sanchez R, Rodriguez-Enriquez S, Marin-Hernandez A, Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;274:1393–1418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- 23.Tong X, Zhao F, Thompson CB. The molecular determinants of de novo nucleotide biosynthesis in cancer cells. Curr Opin Genet Dev. 2009;19:32–37. doi: 10.1016/j.gde.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Locasale JW, Grassian AR, Melman T, Lyssiotis CA, Mattaini KR, Bass AJ, Heffron G, Metallo CM, Muranen T, Sharfi H, Sasaki AT, Anastasiou D, Mullarky E, Vokes NI, Sasaki M, Beroukhim R, Stephanopoulos G, Ligon AH, Meyerson M, Richardson AL, Chin L, Wagner G, Asara JM, Brugge JS, Cantley LC, Vander Heiden MG. Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet. 2011;43:869–874. doi: 10.1038/ng.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, Thomas CJ, Vander Heiden MG, Cantley LC. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamanaka RB, Chandel NS. Cell biology. Warburg effect and redox balance. Science. 2011;334:1219–1220. doi: 10.1126/science.1215637. [DOI] [PubMed] [Google Scholar]
- 27.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 28.Christen S, Sauer U. Intracellular characterization of aerobic glucose metabolism in seven yeast species by 13C flux analysis and metabolomics. FEMS Yeast Res. 2011;11:263–272. doi: 10.1111/j.1567-1364.2010.00713.x. [DOI] [PubMed] [Google Scholar]
- 29.Darzynkiewicz Z, Staiano-Coico L, Melamed MR. Increased mitochondrial uptake of rhodamine 123 during lymphocyte stimulation. Proc Natl Acad Sci U S A. 1981;78:2383–2387. doi: 10.1073/pnas.78.4.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hedeskov CJ. Early effects of phytohaemagglutinin on glucose metabolism of normal human lymphocytes. Biochem J. 1968;110:373–380. doi: 10.1042/bj1100373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang T, Marquardt C, Foker J. Aerobic glycolysis during lymphocyte proliferation. Nature. 1976;261:702–705. doi: 10.1038/261702a0. [DOI] [PubMed] [Google Scholar]
- 32.Munyon WH, Merchant DJ. The relation between glucose utilization, lactic acid production and utilization and the growth cycle of L strain fibroblasts. Exp Cell Res. 1959;17:490–498. doi: 10.1016/0014-4827(59)90069-2. [DOI] [PubMed] [Google Scholar]
- 33.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 34.Fiaschi T, Chiarugi P. Oxidative stress, tumor microenvironment, and metabolic reprogramming: a diabolic liaison. Int J Cell Biol. 2012;2012:762–825. doi: 10.1155/2012/762825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maulik N. Redox signaling of angiogenesis. Antioxid Redox Signal. 2002;4:805–815. doi: 10.1089/152308602760598963. [DOI] [PubMed] [Google Scholar]
- 36.Ushio-Fukai M, Urao N. Novel role of NADPH oxidase in angiogenesis and stem/progenitor cell function. Antioxid Redox Signal. 2009;11:2517–2533. doi: 10.1089/ars.2009.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881–1896. doi: 10.2741/1667. [DOI] [PubMed] [Google Scholar]
- 38.Pani G, Galeotti T, Chiarugi P. Metastasis: cancer cell's escape from oxidative stress. Cancer Metastasis Rev. 2010;29:351–378. doi: 10.1007/s10555-010-9225-4. [DOI] [PubMed] [Google Scholar]
- 39.Svineng G, Ravuri C, Rikardsen O, Huseby NE, Winberg JO. The role of reactive oxygen species in integrin and matrix metalloproteinase expression and function. Connect Tissue Res. 2008;49:197–202. doi: 10.1080/03008200802143166. [DOI] [PubMed] [Google Scholar]
- 40.Toullec A, Gerald D, Despouy G, Bourachot B, Cardon M, Lefort S, Richardson M, Rigaill G, Parrini MC, Lucchesi C, Bellanger D, Stern MH, Dubois T, Sastre-Garau X, Delattre O, Vincent-Salomon A, Mechta-Grigoriou F. Oxidative stress promotes myofibroblast differentiation and tumour spreading. EMBO Mol Med. 2010;2:211–230. doi: 10.1002/emmm.201000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blokhina O, Virolainen E, Fagerstedt KV. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot. 2003;91:179–194. doi: 10.1093/aob/mcf118. Spec No. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 43.Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC. Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature. 2008;452:181–186. doi: 10.1038/nature06667. [DOI] [PubMed] [Google Scholar]
- 44.Hitosugi T, Kang S, Vander Heiden MG, Chung TW, Elf S, Lythgoe K, Dong S, Lonial S, Wang X, Chen GZ, Xie J, Gu TL, Polakiewicz RD, Roesel JL, Boggon TJ, Khuri FR, Gilliland DG, Cantley LC, Kaufman J, Chen J. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal. 2009;2:ra73. doi: 10.1126/scisignal.2000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anastasiou D, Poulogiannis G, Asara JM, Boxer MB, Jiang JK, Shen M, Bellinger G, Sasaki AT, Locasale JW, Auld DS, Thomas CJ, Vander Heiden MG, Cantley LC. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science. 2011;334:1278–1283. doi: 10.1126/science.1211485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aykin-Burns N, Ahmad IM, Zhu Y, Oberley LW, Spitz DR. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochem J. 2009;418:29–37. doi: 10.1042/BJ20081258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bize IB, Oberley LW, Morris HP. Superoxide dismutase and superoxide radical in Morris hepatomas. Cancer Res. 1980;40:3686–3693. [PubMed] [Google Scholar]
- 48.Oberley LW, Bize IB, Sahu SK, Leuthauser SW, Gruber HE. Superoxide dismutase activity of normal murine liver, regenerating liver, and H6 hepatoma. J Natl Cancer Inst. 1978;61:375–379. [PubMed] [Google Scholar]
- 49.Oberley LW, Buettner GR. Role of superoxide dismutase in cancer: a review. Cancer Res. 1979;39:1141–1149. [PubMed] [Google Scholar]
- 50.Oberley LW, Oberley TD, Buettner GR. Cell differentiation, aging and cancer: the possible roles of superoxide and superoxide dismutases. Med Hypotheses. 1980;6:249–268. doi: 10.1016/0306-9877(80)90123-1. [DOI] [PubMed] [Google Scholar]
- 51.Oberley LW, Oberley TD, Buettner GR. Cell division in normal and transformed cells: the possible role of superoxide and hydrogen peroxide. Med Hypotheses. 1981;7:21–42. doi: 10.1016/0306-9877(81)90018-9. [DOI] [PubMed] [Google Scholar]
- 52.Lee YJ, Galoforo SS, Berns CM, Chen JC, Davis BH, Sim JE, Corry PM, Spitz DR. Glucose deprivation-induced cytotoxicity and alterations in mitogen-activated protein kinase activation are mediated by oxidative stress in multidrug-resistant human breast carcinoma cells. J Biol Chem. 1998;273:5294–5299. doi: 10.1074/jbc.273.9.5294. [DOI] [PubMed] [Google Scholar]
- 53.Blackburn RV, Spitz DR, Liu X, Galoforo SS, Sim JE, Ridnour LA, Chen JC, Davis BH, Corry PM, Lee YJ. Metabolic oxidative stress activates signal transduction and gene expression during glucose deprivation in human tumor cells. Free Radic Biol Med. 1999;26:419–430. doi: 10.1016/s0891-5849(98)00217-2. [DOI] [PubMed] [Google Scholar]
- 54.Spitz DR, Sim JE, Ridnour LA, Galoforo SS, Lee YJ. Glucose deprivation-induced oxidative stress in human tumor cells. A fundamental defect in metabolism? Ann N Y Acad Sci. 2000;899:349–362. doi: 10.1111/j.1749-6632.2000.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 55.Ahmad IM, Aykin-Burns N, Sim JE, Walsh SA, Higashikubo R, Buettner GR, Venkataraman S, Mackey MA, Flanagan SW, Oberley LW, Spitz DR. Mitochondrial O2*- and H2O2 mediate glucose deprivation-induced stress in human cancer cells. J Biol Chem. 2005;280:4254–4263. doi: 10.1074/jbc.M411662200. [DOI] [PubMed] [Google Scholar]
- 56.Simons AL, Ahmad IM, Mattson DM, Dornfeld KJ, Spitz DR. 2-Deoxy-D-glucose combined with cisplatin enhances cytotoxicity via metabolic oxidative stress in human head and neck cancer cells. Cancer Res. 2007;67:3364–3370. doi: 10.1158/0008-5472.CAN-06-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Granger DL, Lehninger AL. Sites of inhibition of mitochondrial electron transport in macrophage-injured neoplastic cells. J Cell Biol. 1982;95:527–535. doi: 10.1083/jcb.95.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zepeda AB, Pessoa A, Jr., Castillo RL, Figueroa CA, Pulgar VM, Farias JG. Cellular and molecular mechanisms in the hypoxic tissue: role of HIF-1 and ROS. Cell Biochemistry and Function. 2013;31:451–459. doi: 10.1002/cbf.2985. [DOI] [PubMed] [Google Scholar]
- 59.Hwang AB, Lee SJ. Regulation of life span by mitochondrial respiration: the HIF-1 and ROS connection. Aging (Albany NY) 2011;3:304–310. doi: 10.18632/aging.100292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Niecknig H, Tug S, Reyes BD, Kirsch M, Fandrey J, Berchner-Pfannschmidt U. Role of reactive oxygen species in the regulation of HIF-1 by prolyl hydroxylase 2 under mild hypoxia. Free Radic Res. 2012;46:705–717. doi: 10.3109/10715762.2012.669041. [DOI] [PubMed] [Google Scholar]
- 61.Dang CV, Kim JW, Gao P, Yustein J. The interplay between MYC and HIF in cancer. Nat Rev Cancer. 2008;8:51–56. doi: 10.1038/nrc2274. [DOI] [PubMed] [Google Scholar]
- 62.Gordan JD, Simon MC. Hypoxia-inducible factors: central regulators of the tumor phenotype. Curr Opin Genet Dev. 2007;17:71–77. doi: 10.1016/j.gde.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia signalling controls metabolic demand. Curr Opin Cell Biol. 2007;19:223–229. doi: 10.1016/j.ceb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 64.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 65.Luo W, Hu H, Chang R, Zhong J, Knabel M, O'Meally R, Cole RN, Pandey A, Semenza GL. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–744. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu J, Levens D. Making myc. Curr Top Microbiol Immunol. 2006;302:1–32. doi: 10.1007/3-540-32952-8_1. [DOI] [PubMed] [Google Scholar]
- 67.Chung HJ, Levens D. c-myc expression: keep the noise down. Mol Cells. 2005;20:157–166. [PubMed] [Google Scholar]
- 68.Nesbit CE, Tersak JM, Prochownik EV. MYC oncogenes and human neoplastic disease. Oncogene. 1999;18:3004–3016. doi: 10.1038/sj.onc.1202746. [DOI] [PubMed] [Google Scholar]
- 69.Li F, Wang Y, Zeller KI, Potter JJ, Wonsey DR, O'Donnell KA, Kim JW, Yustein JT, Lee LA, Dang CV. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol Cell Biol. 2005;25:6225–6234. doi: 10.1128/MCB.25.14.6225-6234.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 71.Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 72.Sridhar KS, Plasse TF, Holland JF, Shapiro M, Ohnuma T. Effects of physiological oxygen concentration on human tumor colony growth in soft agar. Cancer Res. 1983;43:4629–4631. [PubMed] [Google Scholar]
- 73.Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med. 2011;15:1239–1253. doi: 10.1111/j.1582-4934.2011.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bhaskara VK, Mohanam I, Rao JS, Mohanam S. Intermittent hypoxia regulates stem-like characteristics and differentiation of neuroblastoma cells. PLoS One. 2012;7:e30905. doi: 10.1371/journal.pone.0030905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsumoto S, Yasui H, Mitchell JB, Krishna MC. Imaging cycling tumor hypoxia. Cancer Res. 2010;70:10019–10023. doi: 10.1158/0008-5472.CAN-10-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cardenas-Navia LI, Mace D, Richardson RA, Wilson DF, Shan S, Dewhirst MW. The pervasive presence of fluctuating oxygenation in tumors. Cancer Res. 2008;68:5812–5819. doi: 10.1158/0008-5472.CAN-07-6387. [DOI] [PubMed] [Google Scholar]
- 77.Toffoli S, Michiels C. Intermittent hypoxia is a key regulator of cancer cell and endothelial cell interplay in tumours. FEBS J. 2008;275:2991–3002. doi: 10.1111/j.1742-4658.2008.06454.x. [DOI] [PubMed] [Google Scholar]
- 78.Semenza GL. Molecular mechanisms mediating metastasis of hypoxic breast cancer cells. Trends Mol Med. 2012;18:534–543. doi: 10.1016/j.molmed.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wong CC, Gilkes DM, Zhang H, Chen J, Wei H, Chaturvedi P, Fraley SI, Wong CM, Khoo US, Ng IO, Wirtz D, Semenza GL. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc Natl Acad Sci U S A. 2011;108:16369–16374. doi: 10.1073/pnas.1113483108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ahmad S, Ahmad A, McConville G, Schneider BK, Allen CB, Manzer R, Mason RJ, White CW. Lung epithelial cells release ATP during ozone exposure: signaling for cell survival. Free Radic Biol Med. 2005;39:213–226. doi: 10.1016/j.freeradbiomed.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 81.Chaudry IH, Gould MK. Evidence for the uptake of ATP by rat soleus muscle in vitro. Biochim Biophys Acta. 1970;196:320–326. doi: 10.1016/0005-2736(70)90019-2. [DOI] [PubMed] [Google Scholar]
- 82.Grygorczyk R, Furuya K, Sokabe M. Imaging and characterization of stretch-induced ATP release from alveolar A549 cells. J Physiol. 2013;591:1195–1215. doi: 10.1113/jphysiol.2012.244145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Israel M, Lesbats B, Meunier FM, Stinnakre J. Postsynaptic release of adenosine triphosphate induced by single impulse transmitter action. Proc R Soc Lond B Biol Sci. 1976;193:461–468. doi: 10.1098/rspb.1976.0058. [DOI] [PubMed] [Google Scholar]
- 84.Pearson JD, Gordon JL. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature. 1979;281:384–386. doi: 10.1038/281384a0. [DOI] [PubMed] [Google Scholar]
- 85.Chaudry IH. Does ATP cross the cell plasma membrane. Yale J Biol Med. 1982;55:1–10. [PMC free article] [PubMed] [Google Scholar]
- 86.Chaudry IH, Baue AE. Further evidence for ATP uptake by rat tissues. Biochim Biophys Acta. 1980;628:336–342. doi: 10.1016/0304-4165(80)90383-9. [DOI] [PubMed] [Google Scholar]
- 87.Pant HC, Terakawa S, Yoshioka T, Tasaki I, Gainer H. Evidence for the utilization of extracellular [gamma-32P]ATP for the phosphorylation of intracellular proteins in the squid giant axon. Biochim Biophys Acta. 1979;582:107–114. doi: 10.1016/0304-4165(79)90293-9. [DOI] [PubMed] [Google Scholar]
- 88.Weidemann MJ, Hems DA, Krebs HA. Effects of added nucleotides on renal carbohydrate metabolism. Biochem J. 1969;115:1–10. doi: 10.1042/bj1150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Corriden R, Insel PA, Junger WG. A novel method using fluorescence microscopy for real-time assessment of ATP release from individual cells. Am J Physiol Cell Physiol. 2007;293:C1420–C1425. doi: 10.1152/ajpcell.00271.2007. [DOI] [PubMed] [Google Scholar]
- 90.Goldman N, Chen M, Fujita T, Xu Q, Peng W, Liu W, Jensen TK, Pei Y, Wang F, Han X, Chen JF, Schnermann J, Takano T, Bekar L, Tieu K, Nedergaard M. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nat Neurosci. 2010;13:883–888. doi: 10.1038/nn.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li J, King NC, Sinoway LI. ATP concentrations and muscle tension increase linearly with muscle contraction. J Appl Physiol (1985) 2003;95:577–583. doi: 10.1152/japplphysiol.00185.2003. [DOI] [PubMed] [Google Scholar]
- 92.Li J, King NC, Sinoway LI. Interstitial ATP and norepinephrine concentrations in active muscle. Circulation. 2005;111:2748–2751. doi: 10.1161/CIRCULATIONAHA.104.510669. [DOI] [PubMed] [Google Scholar]
- 93.Trabanelli S, Ocadlikova D, Gulinelli S, Curti A, Salvestrini V, Vieira RP, Idzko M, Di Virgilio F, Ferrari D, Lemoli RM. Extracellular ATP exerts opposite effects on activated and regulatory CD4+ T cells via purinergic P2 receptor activation. J Immunol. 2012;189:1303–1310. doi: 10.4049/jimmunol.1103800. [DOI] [PubMed] [Google Scholar]
- 94.Trautmann A. Extracellular ATP in the immune system: more than just a "danger signal". Sci Signal. 2009;2:pe6. doi: 10.1126/scisignal.256pe6. [DOI] [PubMed] [Google Scholar]
- 95.Falzoni S, Donvito G, Di Virgilio F. Detecting adenosine triphosphate in the pericellular space. Interface Focus. 2013;3:20120101. doi: 10.1098/rsfs.2012.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Michaud M, Martins I, Sukkurwala AQ, Adjemian S, Ma Y, Pellegatti P, Shen S, Kepp O, Scoazec M, Mignot G, Rello-Varona S, Tailler M, Menger L, Vacchelli E, Galluzzi L, Ghiringhelli F, di Virgilio F, Zitvogel L, Kroemer G. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science. 2011;334:1573–1577. doi: 10.1126/science.1208347. [DOI] [PubMed] [Google Scholar]
- 97.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased Level of Extracellular ATP at Tumor Sites: In Vivo Imaging with Plasma Membrane Luciferase. Plos One. 2008:3. doi: 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wilhelm K, Ganesan J, Muller T, Durr C, Grimm M, Beilhack A, Krempl CD, Sorichter S, Gerlach UV, Juttner E, Zerweck A, Gartner F, Pellegatti P, Di Virgilio F, Ferrari D, Kambham N, Fisch P, Finke J, Idzko M, Zeiser R. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat Med. 2010;16:1434–1438. doi: 10.1038/nm.2242. [DOI] [PubMed] [Google Scholar]
- 99.Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB, Rabinowitz JD, Metallo CM, Vander Heiden MG, Bar-Sagi D. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Amyere M, Payrastre B, Krause U, Van Der Smissen P, Veithen A, Courtoy PJ. Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation of phosphoinositide 3-kinase and phospholipase C. Mol Biol Cell. 2000;11:3453–3467. doi: 10.1091/mbc.11.10.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- 102.Liu Y, Cao Y, Zhang W, Bergmeier S, Qian Y, Akbar H, Colvin R, Ding J, Tong L, Wu S, Hines J, Chen X. A small-molecule inhibitor of glucose transporter 1 downregulates glycolysis, induces cell-cycle arrest, and inhibits cancer cell growth in vitro and in vivo. Mol Cancer Ther. 2012;11:1672–1682. doi: 10.1158/1535-7163.MCT-12-0131. [DOI] [PubMed] [Google Scholar]
- 103.Qian Y, Wang X, Liu Y, Li Y, Colvin RA, Tong L, Wu S, Chen X. Extracellular ATP is internalized by macropinocytosis and induces intracellular ATP increase and drug resistance in cancer cells. Cancer Lett. 2014;351:242–251. doi: 10.1016/j.canlet.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 104.Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12:323–334. doi: 10.1038/nrc3261. [DOI] [PubMed] [Google Scholar]
- 105.Swanton C. Intratumor heterogeneity: evolution through space and time. Cancer Res. 2012;72:4875–4882. doi: 10.1158/0008-5472.CAN-12-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pacini N, Borziani F. Cancer stem cell theory and the warburg effect, two sides of the same coin? Int J Mol Sci. 2014;15:8893–8930. doi: 10.3390/ijms15058893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Siggins RW, Zhang P, Welsh D, Lecapitaine NJ, Nelson S. Stem cells, phenotypic inversion, and differentiation. Int J Clin Exp Med. 2008;1:2–21. [PMC free article] [PubMed] [Google Scholar]
- 108.Kondoh H, Lleonart ME, Bernard D, Gil J. Protection from oxidative stress by enhanced glycolysis; a possible mechanism of cellular immortalization. Histol Histopathol. 2007;22:85–90. doi: 10.14670/HH-22.85. [DOI] [PubMed] [Google Scholar]
- 109.Kondoh H, Lleonart ME, Nakashima Y, Yokode M, Tanaka M, Bernard D, Gil J, Beach D. A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid Redox Signal. 2007;9:293–299. doi: 10.1089/ars.2006.1467. [DOI] [PubMed] [Google Scholar]
- 110.Goubran HA, Kotb RR, Stakiw J, Emara ME, Burnouf T. Regulation of tumor growth and metastasis: the role of tumor microenvironment. Cancer Growth Metastasis. 2014;7:9–18. doi: 10.4137/CGM.S11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells - what challenges do they pose? Nat Rev Drug Discov. 2014;13:497–512. doi: 10.1038/nrd4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Place AE, Jin Huh S, Polyak K. The microenvironment in breast cancer progression: biology and implications for treatment. Breast Cancer Res. 2011;13:227. doi: 10.1186/bcr2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 114.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Antonioli L, Pacher P, Vizi ES, Hasko G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19:355–367. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, Garrido I, Escourrou G, Valet P, Muller C. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71:2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 118.Butler JM, Kobayashi H, Rafii S. Instructive role of the vascular niche in promoting tumour growth and tissue repair by angiocrine factors. Nat Rev Cancer. 2010;10:138–146. doi: 10.1038/nrc2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 120.Filippini A, Taffs RE, Sitkovsky MV. Extracellular ATP in T-lymphocyte activation: possible role in effector functions. Proc Natl Acad Sci U S A. 1990;87:8267–8271. doi: 10.1073/pnas.87.21.8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Regateiro FS, Cobbold SP, Waldmann H. CD73 and adenosine generation in the creation of regulatory microenvironments. Clin Exp Immunol. 2013;171:1–7. doi: 10.1111/j.1365-2249.2012.04623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–693. doi: 10.1038/nrc882. [DOI] [PubMed] [Google Scholar]
- 123.Higashi T, Tamaki N, Torizuka T, Nakamoto Y, Sakahara H, Kimura T, Honda T, Inokuma T, Katsushima S, Ohshio G, Imamura M, Konishi J. FDG uptake, GLUT-1 glucose transporter and cellularity in human pancreatic tumors. J Nucl Med. 1998;39:1727–1735. [PubMed] [Google Scholar]
- 124.Jadvar H. Molecular imaging of prostate cancer with PET. J Nucl Med. 2013;54:1685–1688. doi: 10.2967/jnumed.113.126094. [DOI] [PubMed] [Google Scholar]
- 125.Kelloff GJ, Hoffman JM, Johnson B, Scher HI, Siegel BA, Cheng EY, Cheson BD, O'Shaughnessy J, Guyton KZ, Mankoff DA, Shankar L, Larson SM, Sigman CC, Schilsky RL, Sullivan DC. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res. 2005;11:2785–2808. doi: 10.1158/1078-0432.CCR-04-2626. [DOI] [PubMed] [Google Scholar]
- 126.Kurokawa T, Yoshida Y, Kawahara K, Tsuchida T, Okazawa H, Fujibayashi Y, Yonekura Y, Kotsuji F. Expression of GLUT-1 glucose transfer, cellular proliferation activity and grade of tumor correlate with [F-18]-fluorodeoxyglucose uptake by positron emission tomography in epithelial tumors of the ovary. Int J Cancer. 2004;109:926–932. doi: 10.1002/ijc.20057. [DOI] [PubMed] [Google Scholar]
- 127.Liu Y, Zhang W, Cao Y, Liu Y, Bergmeier S, Chen X. Small compound inhibitors of basal glucose transport inhibit cell proliferation and induce apoptosis in cancer cells via glucose-deprivation-like mechanisms. Cancer Lett. 2010;298:176–185. doi: 10.1016/j.canlet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 128.Hruz PW, Mueckler MM. Structural analysis of the GLUT1 facilitative glucose transporter (review) Mol Membr Biol. 2001;18:183–193. doi: 10.1080/09687680110072140. [DOI] [PubMed] [Google Scholar]
- 129.Jung CY. Proteins that interact with facilitative glucose transporters: implication for function. Exp Physiol. 1998;83:267–273. doi: 10.1113/expphysiol.1998.sp004112. [DOI] [PubMed] [Google Scholar]
- 130.Lachaal M, Rampal AL, Lee W, Shi Y, Jung CY. GLUT1 transmembrane glucose pathway. Affinity labeling with a transportable D-glucose diazirine. J Biol Chem. 1996;271:5225–5230. doi: 10.1074/jbc.271.9.5225. [DOI] [PubMed] [Google Scholar]
- 131.Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013;34:121–138. doi: 10.1016/j.mam.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wood IS, Trayhurn P. Glucose transporters (GLUT and SGLT): expanded families of sugar transport proteins. Br J Nutr. 2003;89:3–9. doi: 10.1079/BJN2002763. [DOI] [PubMed] [Google Scholar]
- 133.Zeng H, Parthasarathy R, Rampal AL, Jung CY. Proposed structure of putative glucose channel in GLUT1 facilitative glucose transporter. Biophys J. 1996;70:14–21. doi: 10.1016/S0006-3495(96)79560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cho H, Lee YS, Kim J, Chung JY, Kim JH. Overexpression of glucose transporter-1 (GLUT-1) predicts poor prognosis in epithelial ovarian cancer. Cancer Invest. 2013;31:607–615. doi: 10.3109/07357907.2013.849722. [DOI] [PubMed] [Google Scholar]
- 135.Kang SS, Chun YK, Hur MH, Lee HK, Kim YJ, Hong SR, Lee JH, Lee SG, Park YK. Clinical significance of glucose transporter 1 (GLUT1) expression in human breast carcinoma. Jpn J Cancer Res. 2002;93:1123–1128. doi: 10.1111/j.1349-7006.2002.tb01214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kunkel M, Reichert TE, Benz P, Lehr HA, Jeong JH, Wieand S, Bartenstein P, Wagner W, Whiteside TL. Overexpression of Glut-1 and increased glucose metabolism in tumors are associated with a poor prognosis in patients with oral squamous cell carcinoma. Cancer. 2003;97:1015–1024. doi: 10.1002/cncr.11159. [DOI] [PubMed] [Google Scholar]
- 137.Mori Y, Tsukinoki K, Yasuda M, Miyazawa M, Kaneko A, Watanabe Y. Glucose transporter type 1 expression are associated with poor prognosis in patients with salivary gland tumors. Oral Oncol. 2007;43:563–569. doi: 10.1016/j.oraloncology.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 138.Ravazoula P, Batistatou A, Aletra C, Ladopoulos J, Kourounis G, Tzigounis B. Immunohistochemical expression of glucose transporter Glut1 and cyclin D1 in breast carcinomas with negative lymph nodes. Eur J Gynaecol Oncol. 2003;24:544–546. [PubMed] [Google Scholar]
- 139.Younes M, Brown RW, Mody DR, Fernandez L, Laucirica R. GLUT1 expression in human breast carcinoma: correlation with known prognostic markers. Anticancer Res. 1995;15:2895–2898. [PubMed] [Google Scholar]
- 140.Eisenberg ML, Maker AV, Slezak LA, Nathan JD, Sritharan KC, Jena BP, Geibel JP, Andersen DK. Insulin receptor (IR) and glucose transporter 2 (GLUT2) proteins form a complex on the rat hepatocyte membrane. Cell Physiol Biochem. 2005;15:51–58. doi: 10.1159/000083638. [DOI] [PubMed] [Google Scholar]
- 141.Watanabe T, Nagamatsu S, Matsushima S, Kondo K, Motobu H, Hirosawa K, Mabuchi K, Kirino T, Uchimura H. Developmental expression of GLUT2 in the rat retina. Cell Tissue Res. 1999;298:217–223. doi: 10.1007/s004419900099. [DOI] [PubMed] [Google Scholar]
- 142.Younes M, Lechago LV, Somoano JR, Mosharaf M, Lechago J. Immunohistochemical detection of Glut3 in human tumors and normal tissues. Anticancer Res. 1997;17:2747–2750. [PubMed] [Google Scholar]
- 143.Rastogi S, Banerjee S, Chellappan S, Simon GR. Glut-1 antibodies induce growth arrest and apoptosis in human cancer cell lines. Cancer Lett. 2007;257:244–251. doi: 10.1016/j.canlet.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 144.Schimmer AD, Thomas MP, Hurren R, Gronda M, Pellecchia M, Pond GR, Konopleva M, Gurfinkel D, Mawji IA, Brown E, Reed JC. Identification of small molecules that sensitize resistant tumor cells to tumor necrosis factor-family death receptors. Cancer Res. 2006;66:2367–2375. doi: 10.1158/0008-5472.CAN-05-1061. [DOI] [PubMed] [Google Scholar]
- 145.Wood TE, Dalili S, Simpson CD, Hurren R, Mao X, Saiz FS, Gronda M, Eberhard Y, Minden MD, Bilan PJ, Klip A, Batey RA, Schimmer AD. A novel inhibitor of glucose uptake sensitizes cells to FAS-induced cell death. Mol Cancer Ther. 2008;7:3546–3555. doi: 10.1158/1535-7163.MCT-08-0569. [DOI] [PubMed] [Google Scholar]
- 146.Melstrom LG, Salabat MR, Ding XZ, Milam BM, Strouch M, Pelling JC, Bentrem DJ. Apigenin inhibits the GLUT-1 glucose transporter and the phosphoinositide 3-kinase/Akt pathway in human pancreatic cancer cells. Pancreas. 2008;37:426–431. doi: 10.1097/MPA.0b013e3181735ccb. [DOI] [PubMed] [Google Scholar]
- 147.Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise (review) Int J Oncol. 2007;30:233–245. [PubMed] [Google Scholar]
- 148.Shukla S, Gupta S. Apigenin: a promising molecule for cancer prevention. Pharm Res. 2010;27:962–978. doi: 10.1007/s11095-010-0089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li QS, Li CY, Li ZL, Zhu HL. Genistein and its synthetic analogs as anticancer agents. Anticancer Agents Med Chem. 2012;12:271–281. doi: 10.2174/187152012800228788. [DOI] [PubMed] [Google Scholar]
- 150.Nagaraju GP, Zafar SF, El-Rayes BF. Pleiotropic effects of genistein in metabolic, inflammatory, and malignant diseases. Nutr Rev. 2013;71:562–572. doi: 10.1111/nure.12044. [DOI] [PubMed] [Google Scholar]
- 151.Tarkowski M, Kokocinska M, Latocha M. [Genistein in chemoprevention and treatment] Pol Merkur Lekarski. 2013;34:54–57. [PubMed] [Google Scholar]
- 152.Vera JC, Reyes AM, Carcamo JG, Velasquez FV, Rivas CI, Zhang RH, Strobel P, Iribarren R, Scher HI, Slebe JC, et al. Genistein is a natural inhibitor of hexose and dehydroascorbic acid transport through the glucose transporter, GLUT1. J Biol Chem. 1996;271:8719–8724. doi: 10.1074/jbc.271.15.8719. [DOI] [PubMed] [Google Scholar]
- 153.Tuccinardi T, Granchi C, Iegre J, Paterni I, Bertini S, Macchia M, Martinelli A, Qian Y, Chen X, Minutolo F. Oxime-based inhibitors of glucose transporter 1 displaying antiproliferative effects in cancer cells. Bioorg Med Chem Lett. 2013;23:6923–6927. doi: 10.1016/j.bmcl.2013.09.037. [DOI] [PubMed] [Google Scholar]
- 154.Chan DA, Sutphin PD, Nguyen P, Turcotte S, Lai EW, Banh A, Reynolds GE, Chi JT, Wu J, Solow-Cordero DE, Bonnet M, Flanagan JU, Bouley DM, Graves EE, Denny WA, Hay MP, Giaccia AJ. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002394. 94ra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gautier EL, Westerterp M, Bhagwat N, Cremers S, Shih A, Abdel-Wahab O, Lutjohann D, Randolph GJ, Levine RL, Tall AR, Yvan-Charvet L. HDL and Glut1 inhibition reverse a hypermetabolic state in mouse models of myeloproliferative disorders. J Exp Med. 2013;210:339–353. doi: 10.1084/jem.20121357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Raez LE, Papadopoulos K, Ricart AD, Chiorean EG, Dipaola RS, Stein MN, Rocha Lima CM, Schlesselman JJ, Tolba K, Langmuir VK, Kroll S, Jung DT, Kurtoglu M, Rosenblatt J, Lampidis TJ. A phase I dose-escalation trial of 2-deoxy-D-glucose alone or combined with docetaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2013;71:523–530. doi: 10.1007/s00280-012-2045-1. [DOI] [PubMed] [Google Scholar]
- 157.Anastasiou D, Yu Y, Israelsen WJ, Jiang JK, Boxer MB, Hong BS, Tempel W, Dimov S, Shen M, Jha A, Yang H, Mattaini KR, Metallo CM, Fiske BP, Courtney KD, Malstrom S, Khan TM, Kung C, Skoumbourdis AP, Veith H, Southall N, Walsh MJ, Brimacombe KR, Leister W, Lunt SY, Johnson ZR, Yen KE, Kunii K, Davidson SM, Christofk HR, Austin CP, Inglese J, Harris MH, Asara JM, Stephanopoulos G, Salituro FG, Jin S, Dang L, Auld DS, Park HW, Cantley LC, Thomas CJ, Vander Heiden MG. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem Biol. 2012;8:839–847. doi: 10.1038/nchembio.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kung C, Hixon J, Choe S, Marks K, Gross S, Murphy E, DeLaBarre B, Cianchetta G, Sethumadhavan S, Wang X, Yan S, Gao Y, Fang C, Wei W, Jiang F, Wang S, Qian K, Saunders J, Driggers E, Woo HK, Kunii K, Murray S, Yang H, Yen K, Liu W, Cantley LC, Vander Heiden MG, Su SM, Jin S, Salituro FG, Dang L. Small molecule activation of PKM2 in cancer cells induces serine auxotrophy. Chem Biol. 2012;19:1187–1198. doi: 10.1016/j.chembiol.2012.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107:2037–2042. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bhardwaj R, Sharma PK, Jadon SP, Varshney R. A combination of 2-deoxy-D-glucose and 6-aminonicotinamide induces cell cycle arrest and apoptosis selectively in irradiated human malignant cells. Tumour Biol. 2012;33:1021–1030. doi: 10.1007/s13277-012-0335-1. [DOI] [PubMed] [Google Scholar]
- 161.Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF, Kelley MJ, Gallez B, Wahl ML, Feron O, Dewhirst MW. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Michelakis ED, Webster L, Mackey JR. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer. 2008;99:989–994. doi: 10.1038/sj.bjc.6604554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Strum SB, Adalsteinsson O, Black RR, Segal D, Peress NL, Waldenfels J. Case report: Sodium dichloroacetate (DCA) inhibition of the "Warburg Effect" in a human cancer patient: complete response in non-Hodgkin's lymphoma after disease progression with rituximab-CHOP. J Bioenerg Biomembr. 2013;45:307–315. doi: 10.1007/s10863-012-9496-2. [DOI] [PubMed] [Google Scholar]
- 164.Addie M, Ballard P, Buttar D, Crafter C, Currie G, Davies BR, Debreczeni J, Dry H, Dudley P, Greenwood R, Johnson PD, Kettle JG, Lane C, Lamont G, Leach A, Luke RW, Morris J, Ogilvie D, Page K, Pass M, Pearson S, Ruston L. Discovery of 4-amino-N-[(1S)-1-(4-chlorophenyl)-3-hydroxypropyl]-1-(7H-pyrrolo[2,3-d]pyrimidin -4-yl)piperidine-4-carboxamide (AZD5363), an orally bioavailable, potent inhibitor of Akt kinases. J Med Chem. 2013;56:2059–2073. doi: 10.1021/jm301762v. [DOI] [PubMed] [Google Scholar]
- 165.Lin J, Sampath D, Nannini MA, Lee BB, Degtyarev M, Oeh J, Savage H, Guan Z, Hong R, Kassees R, Lee LB, Risom T, Gross S, Liederer BM, Koeppen H, Skelton NJ, Wallin JJ, Belvin M, Punnoose E, Friedman LS, Lin K. Targeting activated Akt with GDC-0068, a novel selective Akt inhibitor that is efficacious in multiple tumor models. Clin Cancer Res. 2013;19:1760–1772. doi: 10.1158/1078-0432.CCR-12-3072. [DOI] [PubMed] [Google Scholar]
- 166.Dumble M, Crouthamel MC, Zhang SY, Schaber M, Levy D, Robell K, Liu Q, Figueroa DJ, Minthorn EA, Seefeld MA, Rouse MB, Rabindran SK, Heerding DA, Kumar R. Discovery of novel AKT inhibitors with enhanced anti-tumor effects in combination with the MEK inhibitor. PLoS One. 2014;9:e100880. doi: 10.1371/journal.pone.0100880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, Tsujioka K, Ueno Y, Hatch H, Majumder PK, Pan BS, Kotani H. MK-2206, an allosteric Akt inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol Cancer Ther. 2010;9:1956–1967. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]