Abstract
Cellular damage triggers rapid resealing of the plasma membrane and repair of the cortical cytoskeleton. Plasma membrane resealing results from calcium-dependent fusion of membranous organelles and the plasma membrane at the site of the damage. Cortical cytoskeletal repair results from local assembly of actin filaments (F-actin), myosin-2 and microtubules into an array that closes around the original wound site. Control of the cytoskeletal response is exerted by local activation of the small GTPases, Rho and Cdc42. Recent work has given insight into both the membrane fusion and cytoskeletal responses to plasma membrane damage and we propose that Rho GTPase activation results at least in part from the events that drive membrane repair.
Introduction
“The thing about those holes, they won't fill themselves in and neither will the gophers” — Carl Spackler.
Over the past two decades, it has become apparent that holes made in cells do not repair themselves spontaneously, but rather are healed by an active, calcium-dependent process [1]. This process has two functional components: plasma membrane resealing by fusion of intracellular membrane compartments with each other and with the plasma membrane at the damage site, and cytoskeletal reorganization [1,2]. In this review we discuss ideas and issues emerging from the study of cellular wound repair, with an emphasis on findings from the last two years.
The medical relevance of cellular wound healing
Although wound healing is typically studied at the tissue level, tissue damage from physical or chemical insults, therapeutic interventions, or as a natural consequence of tissue function is inevitably accompanied by individual cell damage [3]. If the damage is not repaired, cell death follows from loss of cytoplasm and unabated calcium influx, resulting directly in loss of tissue integrity. Negative consequences can also ensue indirectly, from release of proteases that attack neighboring cells [4] or provocation of an inflammatory response [5].
Improper plasma membrane repair is a feature of certain genetic diseases, most notably the muscular dystrophies. Extensive muscle plasma membrane damage is a hallmark of muscular dystrophy, and two recent findings indicate that plasma membrane damage is likely to be responsible for some of the symptoms of muscular dystrophies. First, mutations in the gene encoding dysferlin, a 230 kD plasma membrane protein essential for muscle plasma membrane repair [6,7], result in limb-girdle muscular dystrophy [8]. Second, one of the more devastating consequences of muscular dystrophy, cardiomyopathy, can be alleviated in mice by treatment with agents that artificially repair plasma membrane damage by coating wound sites [9••]. Remarkably, a similar result may be obtained naturally in some cells: plasma membrane damage in gastric mucosa and colon results in mucus deposition at the wound site, where it may limit further damage [10••].
The membrane repair response
Thus, impaired cellular wound healing is both a cause of pathologies and a promising target for treatments. Understanding the normal mechanisms of cellular healing is therefore essential. The first event in cellular wound repair is plasma membrane resealing, to stem the inrush of calcium and loss of cytoplasm (Figure 1). Two membrane fusion events fulfill this role: vesicle–vesicle fusion to create a `patch' in the middle of the wound, and vesicle–plasma membrane fusion to link the patch and plasma membrane [11•]. These membrane fusion events are highly conserved and require extracellular calcium: when external calcium concentration is reduced to less than ~0.3 mM, membrane resealing fails [12]. This fact and the dependence of membrane resealing on exocytosis and core exocytosis machinery such as SNAREs indicate that mechanisms responsible for calcium-dependent exocytosis also control plasma membrane resealing [12–18].
Figure 1.
A schematic diagram showing the early events of the cellular wound response. Immediately after plasma membrane damage, calcium rushes into the cell. This triggers vesicle–vesicle fusion in the center of the wound, creating a `patch'. The calcium inrush also triggers fusion of the vesicle with both the plasma membrane (PM) and the patch, thereby sealing the patch to the plasma membrane. Note that vesicle–vesicle fusion and vesicle–plasma membrane fusion could occur simultaneously. See [11•].
The above scheme explains plasma membrane resealing in a variety of cell types. However, many details of this process are still unclear. The most contentious issue is the identity of the membrane compartments (`sealing organelles') responsible for resealing. To date, the only unequivocal resealing compartment is the sea urchin egg yolk granule [15,18]. Yolk granules undergo heterotypic fusion with the plasma membrane and homotypic fusion with a calcium sensitivity that fits the profile of the healing process itself [15,19]. However, yolk granules have not been described in somatic cells, leading investigators to suggest two alternatives as the most likely candidates: lysosomes and enlargosomes.
Lysosomes were proposed to represent the resealing organelle because they undergo calcium-regulated exocytosis [20] and because manipulations that block lyso-some–plasma membrane fusion inhibit resealing [21]. For example, Synaptotagmin (syt) VII, a broadly expressed calcium-binding protein thought to regulate exocytosis in other contexts, localizes to lysosomes, and a dominant-negative syt VII suppresses lysosome exocytosis and resealing [21]. Further, lysosomal exocytosis and resealing are reduced in syt VII knockout mice [22]. On the other hand, syt VII is not exclusively localized to lysosomes [23] and dominant negative syt VII constructs are unlikely to be specific reagents [24•]. Moreover, lysosome exocytosis is actually enhanced in fibroblasts from syt VII knockout mice [25], and a small molecule inhibitor of lysosome exocytosis has no apparent effect on plasma membrane resealing ([26••], but see also [27••]). Finally, lysosome exocytosis following calcium elevation is much slower than the resealing response [28] and does not match the expected calcium sensitivity [29], although it should be noted that the assumed calcium sensitivity is based on what extracellular calcium concentration is required for resealing, rather than a direct measurement of the intracellular free calcium concentration in the wound region.
Equally controversial is the case for enlargosomes, organelles with a composition and morphology distinct from lysosomes, the Golgi, endosomes or the endoplasmic reticulum [30]. Like lysosomes, enlargosomes are broadly distributed, undergo plasma membrane fusion following calcium elevation, and were proposed to mediate plasma membrane resealing [30]. However, more recent work indicates that enlargosomes fuse with the plasma membrane at relatively low free calcium concentrations (1–3 uM) and do so in a manner that is insensitive to tetanus toxin [31], which was previously shown to block plasma membrane resealing in somatic cells [13].
Thus, the identity of animal cell sealing organelles remains unknown. Interestingly, a peroxisome-related organelle, the Woronin body, may play this role in fungi [32]. However, as these organelles have not been described in animal cells, it is not clear whether these findings will be broadly applicable.
Perhaps, however, it is a mistake to assume that plasma membrane resealing is the exclusive purview of a particular compartment. Instead, it might reflect rapid mobilization and fusion of a variety of organelles. Certainly, it is not difficult to imagine that raising the intracellular free calcium to 1 mM might provoke a local orgy of fusion.
The proteins involved in the resealing response have also been elusive. As noted above, the identification of syt VII as a key player has been challenged. Another candidate is dysferlin. Not only does disruption of dysferlin result in impaired plasma membrane repair in muscle (see above), it binds calcium [33], localizes to sites of membrane damage, and has been shown in other systems to participate in membrane fusion [34•]. Moreover, members of the ferlin family are broadly expressed [35], as expected given the conservation of resealing. Finally, in a very recent study, annexin A1, a calcium-binding plasma membrane protein, has been identified as another potential participant in the resealing response [36••]. Consistent with a role as a general mediator of the resealing response, annexin A1 localizes to damage sites. Moreover, inhibition of annexin A1 function via several independent approaches resulted in inhibition of plasma membrane resealing.
Finally, in the first systematic effort to find proteins required for plasma membrane resealing, a complex required for tethering of yolk granules prior to homotypic fusion was purified from sea urchin eggs [37••]. This complex is comprised of seven major proteins, localizes to yolk granules in fixed samples and is required for formation of large patching compartments from yolk granules in vitro. Identification of the individual components of this complex will be of great interest.
The cytoskeletal response to plasma membrane damage
It is not enough for cells to simply reseal the plasma membrane after wounding. They must also restore the wound site to its original state, which entails repopulating the cortical cytoskeleton. It also entails replacement of the patching membrane, which is derived from membranes of organelles (like yolk granules) that presumably differ from the plasma membrane in terms of their lipid and protein composition. Indeed, measurement of ion transport in wounded cells indicates that it remains abnormal well after resealing has finished [38•]. Diffusion into and out of the patch area from the surrounding cytoplasm is too slow to explain the eventual recovery of fully functional plasma membrane, showing that other, more active processes are required.
The cytoskeleton response to wounding underlies these active processes. 30–60s post-wounding, F-actin and myosin-2 accumulate around the wound and then coalesce into a circular array that contracts inward until the original damaged area is covered (Figure 2a). Two features of this array are of particular interest. First, F-actin and myosin-2 accumulation reflects a sharp local increase in assembly, implying that it is templated by localized signals [39]. Second, the array undergoes progressive segregation, concentrating myosin-2 and stable F-actin on the interior and more dynamic actin around the outside [39,40] (Figure 2a). The formation and segregation of the actomyosin array is accompanied by formation of a radial array of microtubules around the wound and microtubules perpendicular to the plasma membrane on the interior of the wound. The microtubule array arises from both new microtubule assembly and physical transport of microtubules by the actomyosin array [40,41]. Conversely, microtubules modulate the actomyosin array. The relative importance of this modulation varies according to wound size, with actomyosin accumulation and closure around the largest wounds being severely compromised by microtubule disruption while smaller wounds are visibly affected, but still capable of closing. Similar scaling effects have been reported for the membrane fusion response [38•].
Figure 2.
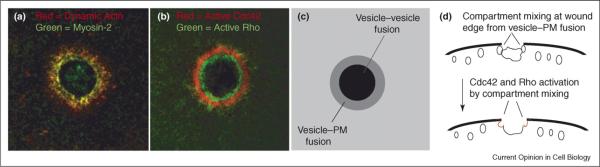
Signal transduction and the cytoskeletal response to plasma membrane (PM) damage. (a) F-actin (red) and myosin-2 (green) segregate into distinct regions around a plasma membrane wound with myosin-2 concentrated on the interior of the array and dynamic actin being more abundant in the outer portion of the array. See [38•,39,40]. (b) Active Cdc42 (red) and active Rho (green) sort into distinct activity zones around wounds. The distribution of active Cdc42 parallels that of dynamic actin while the distribution of active Rho parallels that of myosin-2; compare with (a). See [46•]. (c) A schematic showing the expected position of the different membrane fusion events when viewed from the same orientation as (a) and (b). The grey ring, corresponding to where plasma membrane–vesicle fusion occurs [11•], is in the same region where the zones of active Cdc42 and Rho are found (b). (d) Schematic showing the expected position of different membrane fusion events in the same orientation as Figure 1. Cdc42 and Rho activation are hypothesized to be activated as a result of compartment mixing caused by plasma membrane–vesicle fusion at the seam between the patching membrane and the plasma membrane.
The cytoskeletal response to plasma membrane damage has been studied most intensively in amphibian oocytes, but there is good reason to think that it is widely conserved. First, F-actin accumulates around fibroblast wounds within 60s [42]. Second, disruption of myosin-2 expression impairs the wound healing response in mammalian cells [43,44]. Third, transected axons constrict at the transection site in a manner consistent with actomyosin-based contraction [45]. Fourth, kinesins have been implicated in the healing response of fibroblasts, indicating the involvement of microtubules [13], while direct visualization of microtubule and EB1 (a microtubule plus-end binding protein) dynamics has shown that microtubules form a radial array around fibroblast wounds by growing toward it [46•].
The above cytoskeletal reorganizations explain how the cortical cytoskeletal network is repaired: F-actin and myosin-2 first assemble around the wound and subsequently move into the wound. They also suggest mechanisms by which plasma membrane function may be fully restored. First, the closing contractile array could physically expel the patched membrane into the extracellular space while pulling undamaged plasma membrane over the wound. Second, microtubules focused around the wound site could serve as transport tracks for vesicles moving to and from the patched area. Consistent with this notion, membranous compartments track toward the wound site on microtubules [46•].
Signal transduction during cellular wound healing
One of the most astonishing features of cellular wound repair is that what seems like the crudest of all possible stimuli — a plasma membrane hole — elicits a highly precise signal transduction response. Within ~15–20s of oocyte wounding, Rho and Cdc42 are activated. These small GTPases, which are well known to control various aspects of the actomyosin cytoskeleton, subsequently sort into concentric activity zones with active Cdc42 circumscribing active Rho [47••] (Figure 2b). Rho and Cdc42 are required for accumulation of F-actin and myosin-2 around wounds and, perhaps more interestingly, may be responsible for the observed organization of the contractile array. Specifically, experimental disruption of Rho activity inhibits the concentration of active myosin-2 on the interior of the array as well as contractility of the array, while failing to prevent local actin assembly [47••].
Neither Rho nor Cdc42 is activated in the absence of extracellular calcium; thus, either calcium itself or the events triggered by calcium initiate Rho and Cdc42 activation. We favor the latter possibility as we are unaware of any evidence that calcium binds Rho GTPases directly. Further, there is a 10–15s latency between calcium increase, which is thought to be over within a few seconds (e.g. [15,38•]), and Rho GTPase activation, implying the existence of several steps downstream of calcium elevation.
Strikingly, in Xenopus eggs, exocytosis of cortical secretory granules (CGs), an event which provides the slow block to polyspermy, triggers activation of Cdc42 on CGs with a latent period of 10–15s after fusion with the plasma membrane [48,49•]. Since membrane damage triggers exocytosis, we propose that at least some of the observed Rho and Cdc42 activation results from wound induced exocytosis via `compartment mixing'. During compartment mixing, local signals are generated in membranes as a result of fusion of two distinct membrane compartments [49•,50•]. In Xenopus eggs, following calcium elevation, levels of plasma membrane DAG transiently increase and DAG incorporates into CG membranes as a result of diffusion when the CGs fuse with the plasma membrane [50•]. Once in the CG membrane, DAG directs sustained recruitment of protein kinase Cβ, which, in turn, activates Cdc42, which is localized to the CGs prior to exocytosis. Thus, in a similar manner, fusion of other compartments with the plasma membrane following wounding could also lead to local Rho GTPase activation.
While this hypothesis remains to be tested, it has several attractive features. First, it explains why PKC agonists such as phorbol esters promote cellular wound repair [51,52]. Second, it explains the delay between wounding and the onset of Rho and Cdc42 activation — compartment mixing has a built in fuse [49•]. Third, it can also explain why the Rho GTPase activation, and thus actin and myosin-2 assembly, are restricted to the region around the edge of the original wound, rather than over the entire wound area: the middle of the wound is sealed over via membrane generated by vesicle–vesicle fusion while the edges are sealed via membrane fusion of these vesicles with the plasma membrane [11•]. Compartment mixing-dependent signal generation is implicitly dependent on such heterotypic fusion, in that it is the union of membrane components previously kept separate that acts as the trigger [50•].
Conclusions
In conclusion, several key questions are likely to occupy those who study cellular repair in the immediate future: identification of the sealing organelle(s); identification of the proteins directly involved in plasma membrane resealing; characterization of the roles played by the cytoskeleton during healing; and characterization of the signal transduction pathways that link calcium inrush to cytoskeletal reorganization.
Acknowledgements
This work has been supported by the National Institutes of Health award GM 52932.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.McNeil PL, Steinhardt RA. Plasma membrane disruption: repair, prevention, adaptation. Annu Rev Cell Dev Biol. 2003;19:697–731. doi: 10.1146/annurev.cellbio.19.111301.140101. [DOI] [PubMed] [Google Scholar]
- 2.Darenfed H, Mandato CA. Wound-induced contractile ring: a model for cytokinesis. Biochem Cell Biol. 2005;83:711–720. doi: 10.1139/o05-164. [DOI] [PubMed] [Google Scholar]
- 3.Miyake K, McNeil PL. Mechanical injury and repair of cells. Crit Care Med. 2003;31(8 Suppl):S496–S501. doi: 10.1097/01.CCM.0000081432.72812.16. [DOI] [PubMed] [Google Scholar]
- 4.Mehendale HM, Limaye PB. Calpain: a death protein that mediates progression of liver injury. Trends Pharmacol Sci. 2005;26:232–236. doi: 10.1016/j.tips.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Beiner JM, Jokl P. Muscle contusion injury and myositis ossificans traumatica. Clin Orthop Relat Res. 2002;(403 Suppl):S110–S119. doi: 10.1097/00003086-200210001-00013. [DOI] [PubMed] [Google Scholar]
- 6.Bansal D, Miyake K, Vogel SS, Groh S, Chen CC, Williamson R, McNeil PL, Campbell KP. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- 7.Lennon NJ, Kho A, Bacskai BJ, Perlmutter SL, Hyman BT, Brown RH., Jr Dysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing. J Biol Chem. 2003;278:50466–50473. doi: 10.1074/jbc.M307247200. [DOI] [PubMed] [Google Scholar]
- 8.Laval SH, Bushby KM. Limb-girdle muscular dystrophies — from genetics to molecular pathology. Neuropathol Appl Neurobiol. 2004;30:91–105. doi: 10.1111/j.1365-2990.2004.00555.x. [DOI] [PubMed] [Google Scholar]
- 9.Yasuda S, Townsend D, Michele DE, Favre EG, Day SM, Metzger JM. Dystrophic heart failure blocked by membrane sealant poloxamer. Nature. 2005;436:1025–1029. doi: 10.1038/nature03844. [DOI] [PubMed] [Google Scholar]; •• This paper reveals that cardiac failure resulting from muscular dystrophy can be relieved by an exogenously applied agent that seals the plasma membrane, both confirming that unrepaired membrane damage is directly responsible for one of the most serious consequences of muscular dystrophy and providing a promising therapeutic approach.
- 10.Miyake K, Tanaka T, McNeil PL. Disruption-induced mucus secretion: repair and protection. PLoS Biol. 2006;4:e276. doi: 10.1371/journal.pbio.0040276. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper reveals that plasma membrane damage triggers local mucous exocytosis, such that the wound site is likely to be protected from further physical damage.
- 11.McNeil PL, Kirchhausen T. An emergency response team for membrane repair. Nat Rev Mol Cell Biol. 2005;6:499–505. doi: 10.1038/nrm1665. [DOI] [PubMed] [Google Scholar]; • This review clearly describes the relationship between membrane patching and resealing following plasma membrane wounds and proposes a model for the re-establishment of one continuous lipid bilayer at the wound site.
- 12.Steinhardt RA, Bi G, Alderton JM. Cell membrane resealing by a vesicular mechanism similar to neurotransmitter release. Science. 1994;263:390–393. doi: 10.1126/science.7904084. [DOI] [PubMed] [Google Scholar]
- 13.Bi GQ, Alderton JM, Steinhardt RA. Calcium-regulated exocytosis is required for cell membrane resealing. J Cell Biol. 1995;131:1747–1758. doi: 10.1083/jcb.131.6.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyake K, McNeil PL. Vesicle accumulation and exocytosis at sites of plasma membrane disruption. J Cell Biol. 1995;131:1737–1745. doi: 10.1083/jcb.131.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terasaki M, Miyake K, McNeil PL. Large plasma membrane disruptions are rapidly resealed by Ca2+-dependent vesicle-vesicle fusion events. J Cell Biol. 1997;139:63–74. doi: 10.1083/jcb.139.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNeil PL, Miyake K, Vogel SS. The endomembrane requirement for cell surface repair. Proc Natl Acad Sci USA. 2003;100:4592–4597. doi: 10.1073/pnas.0736739100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNeil PL, Vogel SS, Miyake K, Terasaki M. Patching plasma membrane disruptions with cytoplasmic membrane. J Cell Sci. 2000;113:1891–1902. doi: 10.1242/jcs.113.11.1891. [DOI] [PubMed] [Google Scholar]
- 18.McNeil PL, Baker MM. Cell surface events during resealing visualized by scanning-electron microscopy. Cell Tissue Res. 2001;304:141–146. doi: 10.1007/s004410000286. [DOI] [PubMed] [Google Scholar]
- 19.Chestkov VV, Radko SP, Cho MS, Chrambach A, Vogel SS. Reconstitution of calcium-triggered membrane fusion using `reserve' granules. J Biol Chem. 1998;273:2445–2451. doi: 10.1074/jbc.273.4.2445. [DOI] [PubMed] [Google Scholar]
- 20.Andrews NW. Lysosomes and the plasma membrane: trypanosomes reveal a secret relationship. J Cell Biol. 2002;158:389–394. doi: 10.1083/jcb.200205110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy A, Caler EV, Andrews NW. Plasma membrane repair is mediated by Ca(2+)-regulated exocytosis of lysosomes. Cell. 2001;106:157–169. doi: 10.1016/s0092-8674(01)00421-4. [DOI] [PubMed] [Google Scholar]
- 22.Chakrabarti S, Kobayashi KS, Flavell RA, Marks CB, Miyake K, Liston DR, Fowler KT, Gorelick FS, Andrews NW. Impaired membrane resealing and autoimmune myositis in synaptotagmin VII-deficient mice. J Cell Biol. 2003;162:543–549. doi: 10.1083/jcb.200305131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang P, Chicka MC, Bhalla A, Richards DA, Chapman ER. Synaptotagmin VII is targeted to secretory organelles in PC12 cells, where it functions as a high-affinity calcium sensor. Mol Cell Biol. 2005;25:8693–8702. doi: 10.1128/MCB.25.19.8693-8702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen SS, Tucker WC, Chapman ER, Steinhardt RA. Molecular regulation of membrane resealing in 3T3 fibroblasts. J Biol Chem. 2005;280:1652–1660. doi: 10.1074/jbc.M410136200. [DOI] [PubMed] [Google Scholar]; • In this paper, a detailed characterization of wound repair in the presence of different dominant-negative syt VII constructs reveals that these are unlikely to act specifically on a particular syt isoform or even on syts alone.
- 25.Jaiswal JK, Chakrabarti S, Andrews NW, Simon SM. Synaptotagmin VII restricts fusion pore expansion during lysosomal exocytosis. PLoS Biol. 2004;2:E233. doi: 10.1371/journal.pbio.0020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cerny J, Feng Y, Yu A, Miyake K, Borgonovo B, Klumperman J, Meldolesi J, McNeil PL, Kirchhausen T. The small chemical vacuolin-1 inhibits Ca(2+)-dependent lysosomal exocytosis but not cell resealing. EMBO Rep. 2005;6:898. doi: 10.1038/sj.embor.7400243. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• In a direct test of the role of lysosomes in plasma membrane repair, it was found that a small molecule — vacuolin-1 — inhibits fusion of lysosomes with the plasma membrane but has no effect on plasma membrane resealing.
- 27.Huynh C, Andrews NW. The small chemical vacuolin-1 alters the morphology of lysosomes without inhibiting Ca2+-regulated exocytosis. EMBO Rep. 2005;6:843–847. doi: 10.1038/sj.embor.7400495. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• In contrast to [26], this paper reports that while vacuolin-1 does not inhibit plasma membrane repair, it also fails to inhibit fusion of lysosomes with the plasma membrane.
- 28.Jaiswal JK, Andrews NW, Simon SM. Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J Cell Biol. 2002;159:625–635. doi: 10.1083/jcb.200208154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodriguez A, Webster P, Ortego J, Andrews NW. Lysosomes behave as Ca2+-regulated exocytic vesicles in fibroblasts and epithelial cells. J Cell Biol. 1997;137:93–104. doi: 10.1083/jcb.137.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borgonovo B, Cocucci E, Racchetti G, Podini P, Bachi A, Meldolesi J. Regulated exocytosis: a novel, widely expressed system. Nat Cell Biol. 2002;4:955–962. doi: 10.1038/ncb888. [DOI] [PubMed] [Google Scholar]
- 31.Cocucci E, Racchetti G, Podini P, Rupnik M, Meldolesi J. Enlargeosome, an exocytic vesicle resistant to nonionic detergents, undergoes endocytosis via a nonacidic route. Mol Biol Cell. 2004;15:5356–5368. doi: 10.1091/mbc.E04-07-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jedd G, Chua NH. A new self-assembled peroxisomal vesicle required for efficient resealing of the plasma membrane. Nat Cell Biol. 2000;2:226–231. doi: 10.1038/35008652. [DOI] [PubMed] [Google Scholar]
- 33.Davis DB, Doherty KR, Delmonte AJ, McNally EM. Calcium-sensitive phospholipid binding properties of normal and mutant ferlin C2 domains. J Biol Chem. 2002;277:22883–22888. doi: 10.1074/jbc.M201858200. [DOI] [PubMed] [Google Scholar]
- 34.Doherty KR, Cave A, Davis DB, Delmonte AJ, Posey A, Earley JU, Hadhazy M, McNally EM. Normal myoblast fusion requires myoferlin. Development. 2005;132:5565–5575. doi: 10.1242/dev.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study shows that myoferlin, a member of the ferlin family, binds calcium and is essential for efficient myoblast fusion, supporting the notion that the ferlins are general participants in calcium-dependent membrane fusion.
- 35.Davis DB, Delmonte AJ, Ly CT, McNally EM. Myoferlin, a candidate gene and potential modifier of muscular dystrophy. Hum Mol Genet. 2000;9:217–226. doi: 10.1093/hmg/9.2.217. [DOI] [PubMed] [Google Scholar]
- 36.McNeil A, Rescher U, Gerke V, McNeil PM. The requirement for annexin A1 in plasma membrane repair. J Biol Chem. doi: 10.1074/jbc.M606406200. in press. [DOI] [PubMed] [Google Scholar]; •• This study provides a very nicely balanced demonstration of the likely importance of annexin A1 in the plasma membrane resealing process. Several complementary approaches are used for the inhibition studies, and several different means of resealing assessment are employed.
- 37.McNeil A, McNeil PL. Yolk granule tethering: a role in cell resealing and identification of several protein components. J Cell Sci. 2005;118:4701–4708. doi: 10.1242/jcs.02593. [DOI] [PubMed] [Google Scholar]; •• This study reports the first systematic attempt to identify proteins involved in the plasma membrane resealing response; the authors identify a protein complex required for tethering of yolk granules to each other.
- 38.Fein A, Terasaki M. Rapid increase in plasma membrane chloride permeability during wound resealing in starfish oocytes. J Gen Physiol. 2005;126:151–159. doi: 10.1085/jgp.200509294. [DOI] [PMC free article] [PubMed] [Google Scholar]; • In this study, the authors show that normal plasma membrane function is not recovered for at least a minute after damage, despite the fact that the membrane is resealed within a few seconds.
- 39.Mandato CA, Bement WM. Contraction and polymerization cooperate to assemble and close actomyosin rings around Xenopus oocyte wounds. J Cell Biol. 2001;154:785–797. doi: 10.1083/jcb.200103105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bement WM, Mandato CA, Kirsch MN. Wound-induced assembly and closure of an actomyosin purse string in Xenopus oocytes. Curr Biol. 1999;9:579–587. doi: 10.1016/s0960-9822(99)80261-9. [DOI] [PubMed] [Google Scholar]
- 41.Mandato CA, Bement WM. Actomyosin transports microtubules and microtubules control actomyosin recruitment during Xenopus oocyte wound healing. Curr Biol. 2003;13:1096–1105. doi: 10.1016/s0960-9822(03)00420-2. [DOI] [PubMed] [Google Scholar]
- 42.Miyake K, McNeil PL, Suzuki K, Tsunoda R, Sugai N. An actin barrier to resealing. J Cell Sci. 2001;114:3487–3494. doi: 10.1242/jcs.114.19.3487. [DOI] [PubMed] [Google Scholar]
- 43.Togo T, Steinhardt RA. Nonmuscle myosin IIA and IIB have distinct functions in the exocytosis-dependent process of cell membrane repair. Mol Biol Cell. 2004;15:688–695. doi: 10.1091/mbc.E03-06-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen SS, Steinhardt RA. The mechanisms of cell membrane resealing in rabbit corneal epithelial cells. Curr Eye Res. 2005;30:543–554. doi: 10.1080/02713680590968574. [DOI] [PubMed] [Google Scholar]
- 45.Krause TL, Fishman HM, Ballinger ML, Bittner GD. Extent and mechanism of sealing in transected giant axons of squid and earthworms. J Neurosci. 1994;14:6638–6651. doi: 10.1523/JNEUROSCI.14-11-06638.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Togo T. Disruption of the plasma membrane stimulates rearrangement of microtubules and lipid traffic toward the wound site. J Cell Sci. 2006;119:2780–2786. doi: 10.1242/jcs.03006. [DOI] [PubMed] [Google Scholar]; • This report shows that microtubules reorganize around fibroblasts, extending earlier work in frog oocytes [40].
- 47.Benink HA, Bement WM. Concentric zones of active RhoA and Cdc42 around single cell wounds. J Cell Biol. 2005;168:429–439. doi: 10.1083/jcb.200411109. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This paper shows that plasma membrane wounding elicits rapid activation of Rho and Cdc42 around wounds and that following activation, these two GTPases sort into concentric activity zones.
- 48.Sokac AM, Co C, Taunton J, Bement W. Cdc42-dependent actin polymerization during compensatory endocytosis in Xenopus eggs. Nat Cell Biol. 2003;5:727–732. doi: 10.1038/ncb1025. [DOI] [PubMed] [Google Scholar]
- 49.Sokac AM, Bement WM. Kiss-and-coat and compartment mixing: coupling exocytosis to signal generation and local actin assembly. Mol Biol Cell. 2006;17:1495–1502. doi: 10.1091/mbc.E05-10-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]; • In this review, the compartment mixing hypothesis is proposed as a mechanism for generating localized signals in membrane compartments undergoing fusion.
- 50.Yu H-Y, Bement W. Compartment mixing dependent activation of Cdc42 and actin assembly by diacylglycerol and protein kinase C-β. Nat Cell Biol. doi: 10.1038/ncb1527. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; • In this paper, the compartment mixing hypothesis is directly tested and shown to be correct for activation of Cdc42 and actin assembly during Xenopus egg exocytosis.
- 51.Bement WM, Capco DG. Analysis of inducible contractile rings suggests a role for protein kinase C in embryonic cytokinesis and wound healing. Cell Motil Cytoskeleton. 1991;20:145–157. doi: 10.1002/cm.970200207. [DOI] [PubMed] [Google Scholar]
- 52.Togo T, Alderton JM, Bi GQ, Steinhardt RA. The mechanism of facilitated cell membrane resealing. J Cell Sci. 1999;112:719–731. doi: 10.1242/jcs.112.5.719. [DOI] [PubMed] [Google Scholar]



