Abstract
Perinatal depression is one of the leading causes of maternal morbidity and mortality. The biological etiology of this disorder remains in question, despite considerable research into the contributions of hormonal imbalance, the role of monoamines, and dysregulation of the HPA axis. Because inflammation is known to be associated with major depression in men and non-perinatal women as well as with other important morbidities of pregnancy (such as preeclampsia, preterm birth, and gestational diabetes), and because these morbidities may correlate with perinatal depression, inflammation may be a common physiological pathway that can also help explain perinatal depression. In this paper, we review the theoretical background of inflammation in perinatal depression and then review the literature concerning immune and inflammatory factors in the etiology and course of perinatal depression. We close with recommendations for future studies in this still relatively unexplored area. Identification and understanding of a common pathophysiology between other pregnancy morbidities and perinatal depression would link physical and mental well-being, likely leading to better treatment and prevention.
Keywords: Perinatal depression, postpartum depression, pregnancy, mood, stress, inflammation, immune, cytokine, biomarker
1. Introduction
Perinatal depression (depression during pregnancy or the immediate postpartum period) is an often disabling disorder that affects up to 15% of women in the developed world (Wisner et al. 2002, Bennet et al. 2004, Gaynes et al. 2005) and 20-40% of women in the developing world (World Health Organization, 2009). In fact, it is one of the leading causes of maternal perinatal morbidity and mortality (Mathers & Loncar 2006). Research on the biological etiology of perinatal depression has focused on hormonal imbalance, the role of monoamines, and dysregulation of the HPA axis (Hirschfeld 2000, Gold & Chrousos 2002, Gale & Harlow 2003, Kammerer et al. 2006), but relatively little has been written about the role of inflammation. Because inflammation is known to be associated with major depression in men and non-perinatal women as well as with other important morbidities of pregnancy (such as preeclampsia, preterm birth, and gestational diabetes), and because these pregnancy morbidities may correlate with perinatal depression (Langer & Langer 2000, Kim et al. 2005, Alder et al. 2007, Kozhimannil et al. 2009, Mautner et al. 2009, Bansil et al. 2010, Grote et al., 2010, Katon et al. 2011, Dalfra et al. 2012, Kharaghani et al. 2012, Palmsten et al. 2012), it is important to examine whether inflammation may be a common physiological pathway that also can contribute to the understanding of perinatal depression.
In this paper, we first summarize data demonstrating a role of inflammation and immune responses in (1) depression, (2) normal pregnancy, and (3) pregnancy morbidities. We then present a review of studies examining a role for immune and inflammatory factors in the etiology and course of perinatal depression, separating this disorder into its antenatal and postpartum variants. We close with recommendations for future studies in this still relatively unexplored area.
2. Background
2.1 Inflammation and Major Depression
Early research on the role of inflammation in major depression centered on the observation that some people with depression show elevated levels of pro-inflammatory cytokines, and in particular those released by activated macrophages (including IL-1, IL-6, and TNF-α) (Smith 1991). (See Table 1 for a review of the origin and function of cytokines studied in this paper.) [Table 1 about here] Further research, however, found that some depressed individuals showed elevated cytokines, but others did not, and still others who did not have depression had cytokine levels in the same range as those with depression. Recent reviews and pooled analyses have found general immune dysregulation (Miller et al. 2009) and/or positive associations between depression and various cytokines (IL-1, IL-6, and C-reactive protein (CRP) in Howren et al. 2009, TNF-α and IL-6 in Dowlati et al. 2010). Some researchers have speculated that the association may exist only for certain subsets of depressed people, those who have a particular sensitivity to pro-inflammatory signals (Raison et al. 2010).
Table 1. Cytokine Origin and Function.
| Cytokine | Source | General Activity | Specific Functions |
|---|---|---|---|
|
IL-1α IL-1β |
Monocytes, macrophages, endothelial cells, epithelial cells | Pro-inflammatory, innate immune response | Cell-mediated and humoral immunity, induces “sickness behaviors” (fever, fatigue, depression), stimulates T cells to produce IFN-γ, stimulates acute phase (1β) |
| IL-1Ra | Anti-inflammatory | IL-1 receptor antagonist, blocks IL-1 from binding to its receptors | |
| IL-2 | Th1 cells | Pro-inflammatory | Cell-mediated and humoral immunity, activates macrophages, T-cell proliferation |
| IL-4 | Th2 cells, mast cells | Pro- and anti-inflammatory | Cell-mediated, humoral and allergic immunity, Th2 cell differentiation, activates B cells |
| IL-6 | Macrophages, Th2 cells, CNS, endothelial cells | Pro- and anti-inflammatory, neuromodulator | Cell-mediated and humoral immunity, induction of IDO, stimulates acute phase |
| IL-6R | IL-6 receptor | forms a receptor-ligand complex with IL-6 and helps to regulate and coordinate inflammatory events mediated by IL-6 | |
| IL-8 | macrophages and other cells with toll-like receptors | Innate immune response | induction of chemotaxis in neutrophils and other target cells, stimulation of acute phase |
| IL-10 | Th2 CD4 cells | Pro- and anti-inflammatory | Cell-mediated and humoral immunity, inhibits macrophages |
| IL-12 | Macrophages, dendritic cells, antigen-presenting cells | Pro-inflammatory | Cell-mediated and humoral immunity, stimulates T cells to produce IFN-γ |
| IL-13 | Th2 cells, mast cells, epithelial cells, macrophages, fibroblasts | Anti-inflammatory | Cell-mediated, humoral and allergic immunity, similar to IL-4, mediates resistance to gastrointestinal nematodes, modulates tumor growth and asthma |
| IL-17 | T cells, neutrophils, esinophils, CD8 T cells | Pro-inflammatory | Cell-mediated immunity, recruitment of neutrophils, Th2 cytokine production, pulmonary inflammatory response |
| IL-18 | Macrophages, epithelial cells, adrenal cortex, pituitary gland | Pro-inflammatory | Cell-mediated immunity, induces production of IFN-γ |
| TNF-α | Th1 cells, macrophages | Pro-inflammatory | Cell-mediated immunity, activates macrophages, induces production of IFN-γ, activates the vascular endothelium and increases vascular permeability, stimulation of acute phase |
| TGF-β | Th-3 cells, macrophages | Pro- and anti-inflammatory | Humoral immunity, inhibits T-cell proliferation and effector functions, inhibits macrophages, stimulation of acute phase |
| IFN-γ | Th1 CD4 cells, NK cells | Pro-inflammatory | Cell-mediated and allergic immunity, activates macrophages, increases antigen presentation, crucial to the body's response to intravesicular bacteria, stimulation of acute phase |
| LIF | T lymphocytes, macrophages, fibroblasts, peripheral neurons, embryonal blastocyst cells | Pro-inflammatory | Stimulation of acute phase, pluripotency maintenance in embryonic stem cells |
| LIF-R | Embryonic stem cells, osteoblasts, megakaryocytes, cells that respond to LIF | LIF receptor | blocks the activities of LIF |
| CRP | Hepatocytes, stimulated by TNF-α, IL-1, and IL-6 | Acute-phase protein | enhances the fixation of complement to pathogens |
| CC-16 | Clara cells, epithelial cells of organs | Anti-inflammatory | down-regulates the activation of the Th1 cell immune system, suppresses IL-6, IL-1, and IFN-γ, inhibits the action of PLA2, |
| sCD8 | T lymphocytes | Coreceptor on the T-cell surface | cytotoxic T-cell antigen, marker of cell-mediated immunity |
| PEP | Hematopoietic cell cytoplasm | Anti-inflammatory | Inhibits TCR signaling |
| MIF | Macrophages | Pro-inflammatory | Proliferation of T cells, septic shock, can be induced by steroids |
References: Leonard and Maes (2012), Kuby et. al. (2007), Coico et. al (2003), Wynn (2003), Kawaguchi et. al (2004), Gabay et. al (1997), Bach et. al (2008), Dinarello (1999), Hasegawa et. al (2004), Metcalf (1991), Gabay and Kushner (1999), Johansson et. al (2009), Kazuhiko (2007)
Most recently, an extensive review by Leonard and Maes indicates that many different factors, both peripheral and central, that concern cell-mediated immunity, inflammation, and oxidative and nitrosative stress, are related to the onset of depressive symptoms (Leonard & Maes 2012). These factors include but are not limited to increased production of the Th1 cytokines IFN-γ and IL-2 and the pro-inflammatory cytokines produced by activated macrophages (IL-1β, IL-6, TNF-α), as well as an increased acute phase response. Leonard and Maes review the various mechanisms through which inflammation might contribute to depressive symptomatology, including cytokine-induced changes in 5-HT receptors and cytokine-induced activation of indoleamine 2,3-dioxygenase (IDO). IDO is the enzyme that activates the catabolism of tryptophan, and its activation is associated with lowered plasma tryptophan (a crucial precursor of monoamines) as well as with elevation of various tryptophan catabolites that may have depression-inducing effects in and of themselves (Leonard & Maes 2012). Moreover, although the connection is clearly bidirectional, there is compelling evidence of causality as well. Several trials have documented that the therapeutic administration of cytokines (for example, administration of interferon-α during treatment of cancer or infectious diseases) causes a high proportion of patients to experience symptoms that are responsive to standard treatment and are associated with changes in serotonin metabolism (Raison et al. 2006, Miller et al. 2009).
2.2 Inflammation and Immunity in Normal Pregnancy
It is increasingly clear that inflammation plays some role in depression, though perhaps only for certain subsets of people. Could pregnant women be one of those subsets? Before considering this question, we first must consider the state of inflammation in normal pregnancy. Researchers initially believed that pregnancy must be a state of immune suppression – a state generated to avoid maternal attack on the foreign body growing within her, but a state that left her vulnerable to pathogenic attack herself (Kraus et al. 2010, Chen et al. 2012, Kraus et al. 2012, Pazos et al. 2012). Early pregnancy offered evidence of a proinflammatory Th1 environment, essential to implantation, whereas later pregnancy seemed to be characterized by a decrease in Th1 activity and an increase in Th2 cells, which are both tolerant of paternal antigens and supported by progesterone (Larocca et al. 2008). The improvement of Th1 autoimmune diseases such as rheumatoid arthritis during pregnancy and the worsening of those characterized by Th2 dominance (such as lupus) was seen as evidence of this theory (Larocca et al. 2008, Groer et al. 2011). More recently, a more complex model has emerged, with macrophages as the central mediators of complex endocrine, neural, and immune mechanisms. Macrophages produce the major proinflammatory cytokines and contain receptors for both estrogen and serotonin. Their presence is essential at various key points of gestation (implantation, parturition), but an excess of macrophages at other points in pregnancy is a sign of excess inflammation that may lead to pregnancy morbidity (Larocca et al. 2008, Kraus et al. 2012).
2.3 Inflammation and Pregnancy Morbidities
While normal pregnancy is thus a time of altered immune and inflammatory responses, there are also a number of pregnancy complications that are associated with additionally modified inflammatory responses. The major pregnancy morbidities known to be associated with inflammation are preeclampsia, preterm birth, and gestational diabetes. Because these disorders may be related to perinatal depression via the common mechanism of inflammation, each will be briefly reviewed.
The best studied condition is preeclampsia (PE), defined as a hypertensive disorder of pregnancy that is accompanied by proteinuria and has its onset after 20 weeks of gestation. Preeclampsia occurs in up to 14% of pregnancies and is responsible for 15-20% of maternal and fetal morbidity and mortality in developed countries (Dolea & AbouZahr, 2000, Cudihy & Lee 2009, Ahn et al. 2011). The etiology of this disorder has been elusive. There is general agreement that the immediate spur for the disorder is a generalized activation of the vascular endothelium – but it is not entirely clear what factors lead to that activation. Considerable research has focused on abnormal cytotrophoblast invasion of the maternal spiral arteries, leading to improper remodeling and placental hypoxia, which in turn leads to the release of placental debris into the bloodstream. Others propose that placentation may occur normally, but the placenta may then deteriorate (for a variety of reasons), again leading to the release of placental debris. Recently, research has focused on the possible role of the immune and inflammatory systems in achieving this vascular endothelial activation. A recent review by Ahn et al. (2011) sums up the literature on this topic, covering the involvement of toll-like receptors, maternal cytokines, apoptotic bodies, macrophages, NK cells, T cells, thrombophilias, and autoimmunity. According to the Ahn et al. review, numerous studies show that in preeclamptic pregnancies, compared to normal pregnancies, there is an increased presence of pro-inflammatory cytokines. These include those produced by macrophages and NK cells, as well as those produced by Th1 cells. In fact, the ratio of Th1 to Th2 cells is altered (favoring Th1, the opposite of the case in normal pregnancy) (Ahn et al. 2011).
There is also considerable research devoted to the connection between preterm birth and inflammation (often but not always in the context of infection). Preterm birth (i.e., birth prior to 37 weeks of gestation) is even more widespread than preeclampsia and is the leading cause of perinatal morbidity and mortality in the developed world, with 9.6% of births worldwide occurring prior to term (10.6% in North America) (Beck et al. 2010). It leads to three-fourths of all neonatal deaths and about half of all long-term neurologic disability, and it is alarmingly on the rise in developed countries (Wei et al 2010). This rise is due both to an increase in indications for iatrogenic preterm birth (i.e., preeclampsia and intrauterine growth retardation) as well as an increase in factors that lead to spontaneous preterm birth, including infection or inflammation, vascular disease, very low or very high BMI, and uterine overdistention (Goldenberg et al. 2008, Torloni et al. 2009). The increase in multiple pregnancies associated with assisted reproductive technologies contributes to both types of preterm birth (Goldenberg et al. 2008). It is increasingly clear that inflammation may play an important role. This inflammation can sometimes be shown to be associated with infection (clinical or subclinical), but may also be associated with other trends that are increasing in developed countries (for example, sleep disturbance and obesity). Wei and colleagues recently reviewed the literature on inflammatory cytokines and spontaneous preterm birth. They performed a systematic review of 17 studies comprising 6,270 participants, and concluded that spontaneous preterm birth was strongly associated with increased markers of local but not systemic inflammation (specifically, IL-6 in midtrimester cervicovaginal fluid and amniotic fluid and CRP in midtrimester amniotic fluid). There was no association for these markers in plasma specimens, and there were insufficient data on other inflammatory cytokines.
There is also evidence of a link between inflammation and gestational diabetes, which occurs in 2-10% of all pregnancies and is associated with risks to both mother and fetus and with a 35-60% chance of developing Type II diabetes at some point after pregnancy (National Diabetes Statistics, 2011). Adipocytes produce both pro and anti-inflammatory cytokines. Recent research indicates that lean adipocytes activate macrophages that preferentially produce anti-inflammatory cytokines, while the adipocytes of obese people instead favor the production of pro-inflammatory cytokines (Olefksy & Glass 2010). This shift to a pro-inflammatory environment is one cause of increased insulin resistance, and provides a link between obesity and Type 2 diabetes. Even normal pregnancy is characterized by some insulin resistance, and for those who develop gestational diabetes the degree of insulin resistance is greater than that in Type 2 diabetes not associated with pregnancy. (Lowe et al. 2010). This insulin resistance in pregnancy has been shown to be related to inflammation, independent of BMI (Wolf et al. 2004, Bo et al. 2005, Kinalski et al. 2005). Moreover, as the recent Hyperglycemia and Adverse Pregnancy Outcome Study showed, levels of inflammatory mediators (CRP and plasminogen activator inhibitor type 1) were associated with glucose levels in pregnant women regardless of whether they developed frank diabetes (Lowe et al. 2010).
2.4 Pregnancy Morbidities and Perinatal Depression
The three pregnancy morbidities discussed above also may be associated with perinatal depression, although the evidence is mixed. For preeclampsia, some studies showed no relationship between preeclampsia and antenatal depressive symptoms (Sikkema et al. 2001, Andersson et al. 2004), but one study showed an odds ratio of 2.5 for developing preeclampsia among women with symptoms of anxiety and depression (Kurki et al. 2000). Another very recent study also found an increased risk of preeclampsia among women with depression (Kharaghani et al. 2012), while Palmsten et al. (2012) found an elevated risk for preeclampsia in women treated with antidepressants but not among women with untreated depression. Similarly, for preterm birth (as summarized in Alder 2007), some studies have found an association between preterm birth and antenatal depressive and anxiety symptoms (Steer et al. 1992, Hoffman and Hatch 2000, Orr et al. 2002) whereas several others have not (Andersson et al. 2004a, Berle et al. 2005). For both morbidities, most studies have looked at antenatal depressive symptoms rather than at the risk of developing postpartum depression.
The state of the research for gestational diabetes is similar. Several small studies have shown no relationship between diabetes during pregnancy and mood (Langer & Langeret 2000, Kim et al. 2005, Mautner et al. 2009). Katon et al. 2011, in a very large cross-sectional study, also found no relationship between antenatal depression and diabetes. However, several studies that included postpartum as well as antenatal depression did find an association (a two-fold increase in Kozhimannil et al. 2009, and an increase in severity of depressive symptoms in Dalfra et al. 2012). These results are not specific to gestational diabetes, but rather to all diabetes in pregnancy. Finally, Bansil et al. 2010, in a large nationwide sample of over 32,000,000 women hospitalized for childbirth from 1998-2005, found an increased incidence of all three morbidities (along with numerous others not discussed here) among women with a diagnosis of depression.
2.5 Background Summary
Inflammation is associated with the onset of major depression outside the context of pregnancy and childbirth. Pregnancy is an altered state of immune functioning and it is against this altered background that inflammation also plays a role in several disorders that contribute to maternal and fetal morbidity, and that themselves may be associated with perinatal depression. What role, then, might inflammation play in perinatal depression? What follows is a comprehensive review of the literature on this important question.
3. Methods
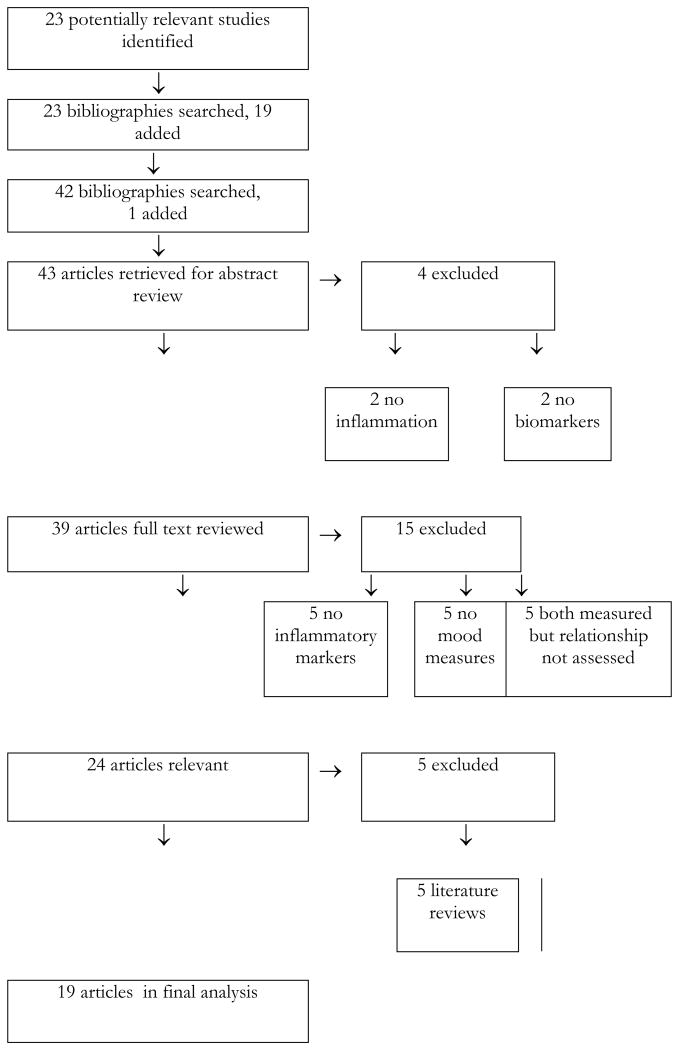
There are no available consensus guidelines on the conduct and reporting of qualitative literature reviews of observational studies. The authors therefore chose to adapt the methods outlined by the QUORUM statement and the MOOSE Group, both of which were designed for the reporting of meta-analyses (Moher et al. 1999, Stroup et al. 2000). The literature review began with a PubMed search conducted by one author (Osborne, an advanced resident physician in psychiatry) and completed in October 2012. Figure 1 contains a flow-chart outlining the search strategy. [Figure 1 about here.] The search was not limited by either date of publication or language of the study, nor by age of subjects, human vs. animal studies, journal of publication, or whether full text was available online. Three searches were combined, using the terms 1) inflammation, inflammatory, cytokine, immune 2) depression, mood and 3) pregnancy, pregnant, antenatal, postnatal, postpartum, perinatal. The search was conducted using these terms as keywords and through use of MESH categories that came closest to matching these terms. Reference librarians at Columbia University Medical Center assisted with the initial search design.
Figure 1. Flow-Chart of Study.
4. Results
The combined PubMed searches yielded 113 articles. Titles were reviewed, and of this number 23 were selected for abstract review. Excluded articles were those that did not address all three components of interest (the perinatal period, inflammatory factors, and mood). There were no abstracts without associated articles and no unpublished data were identified. The bibliographies of these 23 studies were hand-checked by Dr. Osborne and 19 additional studies were identified. The bibliographies of these 19 were also hand-checked, and one additional study was identified. Abstracts of the resulting 43 studies were then examined. Despite the lack of a limit on language of study, all of the identified studies were in English. To be included, articles needed to report experimental results, to use humans or animals as subjects, and to include both measurement of antenatal or postpartum mood states or related symptoms and measurement of markers of inflammation or immune system function. We did not exclude any articles on the basis of quality, study design, number or type of population studied, or specific tools used for measurement of outcomes. Four studies were excluded at this stage because they did not cover inflammatory markers (Gavin et al. 2005, McCoy et al. 2006, Oppo et al. 2009, Brummelte & Galea 2010).
Full text of the remaining 39 articles was then reviewed. An additional 15 studies were excluded at this point – five because they did not directly measure mood (Corwin et al. 2003, Coussons-Read et al. 2005, Groer et al. 2005b, Coussons-Read et al. 2007, Coussons-Read et al. 2012,), five because they did not directly measure inflammation (Abou-Saleh et al. 1999, Groer et al. 2005, Groer 2005a, Kohl et al. 2005, Albacar et al. 2011), and five because they measured both but did not attempt to correlate the two (Maes et al. 2001a, Maes et al. 2002, Maes et al. 2004, Groer et al. 2011, Enayati et al. 2012). The remaining 24 studies were directly concerned with the subject matter in question, but five additional studies were excluded at this point because they were literature reviews and did not contain original data (Kendall-Tackett 2007, Corwin & Pager 2008, Yonkers et al. 2011, Meltzer-Brody 2011, Christian 2012).
The remaining 19 articles included one animal study (O’Mahony et al. 2006); the rest were all cohort or case-control human studies. The rat study (O’Mahony et al. 2006) exposed 8 pregnant rats to conditions of stress in pregnancy, and compared them to 8 control rats. The primary outcome was behaviors consistent with postpartum depression, and the stressed group exhibited more such behaviors. In addition, IL-1β, TNF-α, and IL-10 were compared between the two groups. No significant difference between groups was found for TNF-α and IL-10 (though there was a trend toward higher levels in the stressed group). IL-1β levels were higher in the stressed group (p <.05). Results were not provided on the relation, if any, between this immune marker and “depressive” behaviors.
The remaining 18 articles examined the association between perinatal depression and various measures of inflammation (see Table 1). Most studies examined more than one marker. We have chosen to group these articles based on whether they measured mood and inflammation in the antenatal period, both antenatally and postnatally, or in the postpartum period only.
In addition to studying an extremely heterogeneous group of inflammatory markers, the articles used a wide variety of instruments to measure mood symptoms. No two sets of researchers used the same instruments, and only one instrument was used by more than one group (the Edinburgh Postnatal Depression Scale, EPDS). Other instruments included the Postpartum Blues Questionnaire (PPBQ), the Structured Interview Guide for the Hamilton Depression Rating Scale-Seasonal Affective Disorder (SIGH-SAD), the Centers for Epidemiologic Studies Depression Scale (CES-D), the Hamilton Depression Scale (HAM-D, the Beck Depression Inventory (BDI), the Denver Maternal Health Assessment (DMHA), the Spielberger State-Trait-Anxiety Inventory (STAI), the Zung Depression Rating Scale (ZDS), the Profile of Mood States (POMS), the Diagnostic Interview Schedule for Children 2.1 (DISC), the Stein Blues Scale (SBS), the Revised Pregnancy Distress Questionnaire (NUPDQ), the Positive and Negative Affect Schedule (PANAS), and the Hospital Anxiety and Depression Scale (HADS). Table 2 summarizes these results. [Table 2 about here.]
Table 2. Summary of Results.
| Period | Study | Sample Characteristics | Measures of Mood/Stress/Fatigue | Time Points | Measures of Inflammation (In serum unless indicated otherwise) | Results (Correlation of Mood with Inflammatory Measure) | Comments |
|---|---|---|---|---|---|---|---|
| Antenatal | Blackmore 2011 | 145 healthy pregnant women from a low-income, high-psychosocial risk population | EPDS, cut-off 9, SCID (mood episodes section, anxiety section, PTSD section), STAI | 18 weeks gestation and 32 weeks gestation | IL-6 TNF-α |
NS NS (depression, anxiety, PTSD), ↑ p<.05 (trauma exposure) |
|
| Cassidy-Bushrow 2012 | 187 African-American women | CES-D | 1× at 13-28 weeks gestation | hs-CRP IL-6 IL-10 IL-1β TNF-α |
NS ↑p=.04 NS ↑p=.03 NS |
BMI with inflammation? | |
| Christian 2010 | 22 pregnant women | CES-D | CES-D at baseline, MIF at 1 week after influenza virus vaccination | MIF | ↑p=.035 | CES-D does not diagnose clinical depression | |
| Christian 2009 | 60 pregnant women | CES-D | 1× at an average of 15 weeks gestation | IL-6 TNF-α |
↑p=.05 (CES-D), NS NS |
Marginally significant = NS? | |
|
| |||||||
| Postpartum | Boufidou 2009 | 56 Greek women parturients at 35-38 weeks gestation | PPBQ, cut-off 8.2 EPDS, cut-off 11 |
PPBQ at admission and 1-4d postpartum; EPDS at 1 and 6 wks postpartum | IL-6 (CSF) d1-4 IL-6 (serum) d1-4 TNF-α (CSF) d1-4 TNF-α (serum) d1-4 IL-6 (CSF) wk1 IL-6 (serum) wk1 TNF-α (CSF) wk1 TNF-α (serum) wk1 IL-6 (CSF) wk6 IL6 (serum) wk 6 TNF-α (CSF) wk6 TNF-α (serum) wk6 |
↑ (p <.04) NS ↑ (p=.03) ↑ (p =.02 ↑ (p = .04) NS NS NS ↑ (p = .01) NS NS NS |
CSF available from only 33 participants; EPDS cut-off lower than standard, PPBQ based on mean |
| Corwin et al. 2008 | 26 postpartum women | CES-D; score ≥ 11 = depression | 4× for inflammatory markers, at weekly intervals, beginning day 0 postpartum; 1× (day 28) for mood | IL-1β (urine) IL-6 (urine) |
↑ day 14 (p < .05) NS |
92% white; 2ndary analysis of study primarily looking at fatigue; urinary markers may not reflect blood levels; not gold standard instrument for postpartum women; depressive sx measured only once; not clinical diagnosed with depression | |
| Groer & Davis 2006 | 181 healthy postpartum women | ISLE, PSS, POMS Depressed = top decile, >21 on POMS |
1× at 4-6 weeks postpartum | IL-10 IFN-γ |
NS ↓ (p<.01) in formula feeders for PSS ↓ (p < .05) for formula feeders for POMS |
Sample chosen to compare breast to formula feeding; instrument not gold standard; # depressed moms small | |
| Groer & Morgan 2007 | 199 postpartum American women; 30 depressed, 169 not | ISLE, PSS, POMS Depressed = top decile, >21 on POMS |
1× at 4-6 weeks postpartum | IFN-γ | ↓ (p<.001) | 2ndary analysis; sample chosen to compare breast to formula feeding; instrument not gold standard; # depressed moms small | |
| Hucklebridge et al. 1994 | 75 postpartum English women | SBS, HADS, EPDS, combined with factor analysis for “dysphoria” | 1×, 4 days postpartum | Total lymphocytes T cells NK cells |
↓ (p<.03) ↓ (p<.03) NS |
T-tests one-tailed; measures lymphocytes, not cytokines; mood measure not validated | |
| Fransson 2011 | 27 Swedish women delivering preterm and 37 Swedish women delivering at term | PANAS, reported depressive symptoms | 1× at labor for cytokines, 1× within 5 days of delivery for affectivity variables | IL-1α IL-1β IL-2 IL-4 IL-6 IL-8 IL-10 IL-12 p70 IL-13 IL-17 IL-18 IFN-γ |
NS NS NS NS ↑p<.05 (PANAS, maternal), ↑p<.01 (PANAS, cord) ↑p<.01 (depressive, maternal), ↑p<.05 (PANAS, cord) ↑p<.05 (PANAS, cord) NS ↑p<.05 (PANAS, cord) NS ↑p<.01 (PANAS, cord), ↑p<.05 (depressive, cord) NS |
Depressive symptoms reported through interviews | |
| Okun et al. 2011 | 56 pregnant American women with past hx of MDD/PPD but not currently depressed | PSQI >5 = poor sleep; HAM-D scored for recurrence (2 consecutive ratings >15) | PSQI and HAM-D 8× during first 17 weeks; IL-6 only a single sample, chosen post-hoc | IL-6 | NS for PSQI NS for HAM-D |
Primarily sleep only, not mood; 2ndary analysis for trial in which all participants on medication vs. placebo; PSQI not previously validated in pregnant women; IL-6 measured posthoc | |
| Skalkidou et al. 2009 | 347 Swedish women at time of delivery (67 with PPD, 280 controls) | EPDS | Serum at delivery, EPDS 3×: 5 days, 6 weeks, 6 months after delivery | IL-6 | NS | Nested case control; main aim was to measure leptin levelsalso measured leptin; IL-6 levels trended higher in cases, but did not reach significance; adjustments made for smoking, BMI; IL-6 measured only at delivery and not at later time points | |
|
| |||||||
| Mixed | Maes et al. 2000 | 91 pregnant Belgians 22 nonpregnant controls |
STAI and ZDS scored as “reactors” (change in score >q75) at delivery | 3×: 3-5 days before EDD, 1 day postpartum, 3 days postpartum | IL-6 IL-1ra LIFR IL-6r |
↑ STAI 1d postpartum (p=.0001); ↑ZDS no time given (p = .03) ↑ STAI 1d postpartum (p=.006) ↓ STAI prepartum (p =.004) ↑ZDS all 3× (p = .001, .001, .002) |
Small # depressed mothers; main purpose was pregnant vs. postpartum diffs, not mood; applicable only to early puerperium (and not to PPD) |
| Maes et al. 2001 | 50 pregnant Belgians with no history MDD 16 pregnant Belgians with history MDD |
ZDS; SCID | 3×: 3-5 days before EDD, 1 day postpartum, 3 days postpartum for ZDS; SCID 6-10 months later | IL-6 IL-1ra IL-6r |
↑ 1d and 3d postpartum (p=.007, .02) ↑ 1d and 3d postpartum (p<10-3 for both) ↑ no time point given (p = .003) |
Small # with history; past hx based on telephone recollections; no difference in ZDS scores found for those w an | |
| Schmeelk et al. 1999 | 58 pregnant American adolescents | DISC 2.1 | 1× only, between 9 and 21 weeks of gestation | IL-1ra IL-1β |
CRH related to depression, and CRH significantly predicts IL-1ra (p = .05) | 98% white; depression not directly compared to IL-1ra; path model used that predicts effects on IL-1ra mediated by CRH; measured during pregnancy only; also looked at postpartum complications; results on IL-1β not reported | |
| Scrandis et al. 2008 | 27 American women at high risk of PPD | SIGH-SAD | 3×: 35-38 weeks gestation, 1-5d postpartum; 5-6 weeks postpartum | IL-6 CRP |
NS ↑ total (p = .03) and atypical (p =.002) scores prepartum ↓atypical (p=.03) early postpartum |
85% African American; not gold standard instrument for pregnant/ postpartum women; study also measured KYN/TRP ratio (indirect marker) to estimate IDO activity; scores as reported in abstract, text, and table do not match (table reported here) | |
Abbreviations for statistical procedures and result outcomes: p = probability; t = T-test; NA = not applicable; NS = not significant; ↑ indicates an increase/positive correlation; ↓ indicates a decrease/negative correlation
Abbreviations for psychological assessment measures: Edinburgh Postnatal Depression Scale (EPDS); Postpartum Blues Questionnaire (PPBQ); Structured Interview Guide for the Hamilton Depression Rating Scale-Seasonal Affective Disorder (SIGH-SAD); Denver Maternal Health Assessment (DMHA); Spielberger State-Trait-Anxiety Inventory (STAI); Zung Depression Rating Scale (ZDS), Beck Depression Inventory (BDI); Inventory of Small Life Events (ISLE); Perceived Stress Scale (PSS); Profile of Mood States (POMS); Modified Fatigue Symptom Checklist (MFSC); Diagnostic Interview Schedule for Children 2.1 (DISC); Stein Blues Scale (SBS); Hospital Anxiety and Depression Scale (HADS), Center for Epidemiologic Studies Depression Scale (CES-D), Revised Pregnancy Distress Questionnaire (NUPDQ), Positive and Negative Affect Schedule (PANAS)
Abbreviations for biological outcome measures (in alphabetical order): Clara Cell 16 protein (CC16), C-reactive protein (CRP), interferon gamma (IFN-γ), interleukin 1-beta (IL-1β), interleukin 1-receptor antagonis (IL-1ra), interleukin 6 (IL-6), interleukin 10 (IL-10), kynurenine (KYN), leukemia inhibitory factor receptor (LIF-R), natural killer cells (NK cells), tryptophan (TRP), tumor necrosis factor alpha (TNF-α).
Antenatal Depression
Four studies examined cytokines in antenatal depression. Three found an association between depressed mood and at least one marker of inflammation; one did not. In Christian et al. 2009, 60 pregnant women from a low-income, high psychosocial risk group were enrolled at an average gestational age of 15 weeks. At the single study visit, subjects completed the Center for Epidemiologic Studies Depression Scale (CES-D) and the Perceived Stress Scale (PSS) and gave blood to measure IL-6 and TNF-α. IL-6 is produced in the periphery by macrophages and T cells, and in the central nervous system (CNS) by astrocytes and microglia. It is capable of crossing the blood-brain barrier through both passive and active transport. Once there, it acts as a neuromodulator, with both pro and anti-inflammatory properties, and may also be involved in the induction of IDO, which is associated with a decrease in CNS serotonin (Leonard & Maes 2012). TNF-α is a pro-inflammatory cytokine produced by activated macrophages. It activates the vascular endothelium and increases vascular permeability. After controlling for pre-pregnancy BMI, IL-6 levels were found to be significant and positively associated with CES-D score (p = .05). There was no significant association with TNF-α. Neither stress nor any of a number of health behaviors was associated with either marker of inflammation. There was also a small effect size relationship between both inflammatory markers and severity of symptoms.
In Christian et al. 2010, the authors extended the work reported in their prior study to a comparison of the inflammatory response to an in vivo antigen challenge among depressed and non-depressed women. Mood was again measured using the CES-D, at the baseline visit only (conducted at an average of 17 weeks gestation). Macrophage migration inhibitory factor, a wide-ranging cytokine that inhibits the antinflammatory effects of glucocorticoids in a dose-response fashion and is known to be implicated in preeclampsia, was measured at the baseline visit and one week later, following vaccination against the influenza virus. Women in the highest quartile of CES-D scores were compared with those in the lowest quartile, and were found to have a significantly higher level of MIF one week after vaccination (p < .04). The groups did not differ in demographic characteristics, including BMI, nor in health behaviors.
Blackmore et al. (2011) observed 145 low-income women and assessed mood and inflammatory markers (IL-6 and TNF-α) in detail at approximately 18 and 32 weeks gestation. Mood was assessed using the Edinburgh Postnatal Depression Scale (EPDS), the State-Trait Anxiety Inventory (STAI), and the Structured Clinical Interview for DSM Disorders (SCID). No significant association was found between depressed mood or anxiety symptoms and either cytokine. There was, however, a significant association between elevated TNF-α and trauma exposure (p < .03).
Cassidy-Bushrow et al. (2012) recruited 187 African American women to examine the association between depressive symptoms and four pro-inflammatory biomarkers (hs-CRP, IL-6, IL-1β, TNF-α) and one anti-inflammatory biomarker (IL-10). CRP is an acute phase protein produced by hepatocytes and stimulated by TNF-α, IL-1, and IL-6. CRP in turn enhances the fixation of complement to pathogens. IL-1β is the most potent known mediator of inflammation in humans. It is one of the culprits in the constellation of symptoms known as “sickness behaviors” (Dantzer et al. 2008) that accompany many acute and chronic illnesses. These symptoms include most significantly fatigue and fever, as well as depression, anorexia, and myalgias. IL-1β is produced by activated macrophages and is known to be elevated in normal pregnancy. It is also associated with the synthesis of prostaglandins, so may affect uterine contractility and the onset of labor. IL-10 is an anti-inflammatory cytokine produced by Th2 CD4 cells. It is involved in the maintenance of pregnancy and progesterone production, and low levels of IL-10 have been found to increase the risk of preeclampsia (Redman 1991, Greer et al. 1994, Wadhwa et al. 2001).
Mood was measured with the CES-D. Women were recruited during the second trimester of pregnancy, and both mood assessment and blood draws were performed once. Both IL-6 and IL-1β were significantly and positively associated with depressive symptomatology (p < .04 and .03, respectively). The other markers were not significantly associated with CES-D score, nor was BMI. BMI did moderate the relationship between depressive symptoms and IL-6, however; IL-6 was significantly associated with depressive symptoms in lean women but that association disappeared in women of high BMI. In addition, IL-10 was higher in lean depressed women, but lower in heavy depressed women, likely reflecting a feedback loop (higher IL-10 in lean women in response to the increased proinflammatory burden, whereas those with depressive symptoms added to the already proinflammatory state of obesity are less likely to be able to mount an anti-inflammatory response).
In sum, two of these studies found significant associations between depression and IL-6 while one did not. Two found no association with TNF-α, one found an association with MIF, and one found an association with IL-1β. The three positive studies all measured mood with the CES-D, a screening tool that is not used to diagnose depression clinically, whereas the one negative study used a clinical tool, the EPDS.
Mixed Antenatal and Postpartum
There are 5 studies examining mood and inflammatory measures in both the antepartum and postpartum periods. All five found a significant association between mood and at least one inflammatory marker. Schmeelk et al. (1999) studied 58 pregnant adolescents. Blood samples for IL-1β and IL-1ra were drawn between 9 and 21 weeks of gestation. IL-1ra is an endogenous receptor antagonist for IL-1β. Within 24 hours of childbirth subjects were assessed for labor and delivery complications as well as for maternal depression. (No blood was drawn at the time of delivery, nor was mood measured during pregnancy.) Maternal depression was determined by scores on the Diagnostic Interview Schedule for Children (DISC) 2.1. The study authors do not comment on the reliability and validity of this scale for maternal depression, and it is notable that it was administered within 24 hours postpartum only. In addition, the authors do not mention whether they used a cutoff score or examined depressive symptoms as a continuous variable. The authors report that IL-1β levels were measured, but do not compare these levels to depression scores. It is possible that they declined to make this comparison because detectable IL-1β was found in only 4 of the 58 participants, but the authors do not directly address this. The authors also measured IL-1ra. They did not directly examine the link between depression and IL-1ra, but instead used a hierarchical multiple regression analysis to test paths in a model linking depression, CRH, IL-1ra, and postpartum complications. They state that “CRH and depression were found to predict significant levels of IL-1ra” (p=.05), and state elsewhere that lower CRH predicted lower IL-1ra (no significance level stated).
There are three relevant studies by Maes et al., all variations in the reporting of data collected at the same time on the same group of participants (up to 98 pregnant Belgian women with up to 22 nonpregnant controls). Of note, there are several other papers by the same group concerning the same set of data, but those papers were excluded because they did not directly compare mood and inflammatory measures.
In the first study, in 1999, Maes et al. examined Clara Cell Protein 16 (CC16), an antinflammatory protein produced by cells of the bronchiolar epithelium and urogenital system that suppresses IL-6, IL-1, and IFN-γ. Mood was assessed with the State-Trait Anxiety Inventory (STAI), the Zung Depression Rating Scale (ZDS), and the SCID (true for all the Maes studies). For the STAI and ZDS, women whose scores increased in the postpartum were deemed STAI or ZDS “reactors,” defined as those with a residualized score >q75. The SCID was administered 6-10 months after delivery by telephone. Serum samples were collected 3-5 days before expected delivery and at 1 and 3 days postpartum. The researchers decided to use 3 months as the cut-off for postpartum onset of depression (rather than the 4 weeks defined by DSM-IV). Women with postpartum depression (as recalled 6-10 months later) had significantly lower levels of CC16 than did those without (p=.002) at all three time points. There were no significant differences in CC16 concentration between STAI and ZDS reactors and nonreactors.
In 2000, the group examined the correlation between IL-6 (measured at the same time points as above), LIF-R (a versatile cytokine that works along with IL-6 to induce the acute phase response), IL-1ra, and IL-6r (a soluble receptor that forms a receptor-ligand complex with IL-6 and helps to regulate and coordinate inflammatory events mediated by IL-6) with STAI and ZDS reactor status. STAI reactor status was correlated with increased IL-6 level (p = .0001) at 1 day postpartum, as was ZDS reactor status (p = .03), though timing was not specified for ZDS. For LIF-R, prepartum serum LIF-R was significantly lower in STAI reactors than in non-reactors (p=.004). No significant relationship was found for ZDS reactors. IL-1ra was significantly higher in STAI reactors than in nonreactors one day after delivery (p =.006). ANOVA analysis showed a significant effect of time x STAI reactor status x parity interaction (p=.02). No significant differences were found for ZDS reactors. IL-6r was also found to be significantly increased in ZDS reactors when measured at 3-5 days before EDD (p = .001), at 1 day postpartum (p = .001), and at 3 days postpartum (p = .002); no relationship was found for STAI reactors. These correlations did not become more positive with higher scores – in other words, reactor status predicted whether inflammatory variables were elevated, but severity of symptoms did not.
In 2001, Maes et al. looked at a subset of the same sample to ask whether women with a lifetime history of major depression had greater increases in inflammatory markers in the early puerperium than women without such a history. Within 6-10 months after delivery, women had telephone interviews using the Structured Clinical Interview of the DSM to assess past history of depression. Sixteen women had a past history and 50 did not. Women with a history of depression had significantly higher levels of serum IL-6 at 1 and 3 days after delivery (p = .007 and .02, respectively) and of IL-1ra at the same time points (p<10-3 for both). IL-6r was found to be significantly increased in those with a history of major depression (no time point was given) when compared to those without such a history. The authors also looked at current ZDS scores and found no difference between women with and without a history of depression. They therefore conclude that current symptoms cannot be responsible for the difference in inflammatory response between the two groups. Of note, they did not look at differences in STAI scores; and as their prior study demonstrates, these were associated with IL-1ra levels but ZDS scores were not.
Scrandis et al. (2008) studied 27 women at high risk for developing postpartum depression. They measured mood using the Hamilton Depression Rating Scale-Seasonal Affective Disorder (SIGH-SAD), and collected scores at three points: 35-38 weeks gestation, 1-5 days postpartum, and 5-6 weeks postpartum. No information is provided about why this scale was chosen or whether it is reliable or validated for the purpose of screening for postpartum depression. Blood was drawn to measure IL-6 and CRP at the same time points. There were no significant relationships between IL-6 levels and total or atypical depression scores at any of the time points measured. CRP, however, was found to be positively correlated with total (p = .03) and atypical (p=.002) depression scores in the prepartum. In contrast, CRP was negatively correlated with atypical depression scores in the early postpartum (p=.03), and no relationship was found in the late postpartum or with total depression scores in any postpartum period.
In sum, two of these studies of mixed prepartum and postpartum measures found an association between mood and IL-1ra. One study found a positive association for CC16. One study found an association between mood and IL-6, while another study did not. One found a relationship between mood and IL-6r. One found a relationship between mood and CRP. While the balance thus favors an association between mood and inflammation, the markers used ranged widely and represented different elements of the inflammatory response. The same is true of mood measures, which include the DISC 2.1, a combination of the SCID with the STAI and the ZDS, and the SIGH-SAD. No studies used the EPDS, the most widely used depression screen in the perinatal period.
Postpartum
Nine studies have examined the link between mood and inflammatory state in the postpartum period. Seven studies have found an association between depressed mood and at least one measure of inflammation (though in some of these studies the association holds true only for a subset of women). Two studies did not find an association.
Hucklebridge et al. (1994) examined the relationship between dysphoria and circulating immune cell populations in 75 postpartum women. On day 4 postpartum, blood was drawn and participants completed three mood questionnaires – the Stein Blues Scale (SBS), the EPDS, and the Hospital Anxiety and Depression Scale (HADS), with subscales for depression and for anxiety. Blood was examined for the presence of T helper cells, cytotoxic T cells, and natural killer (NK) cells. HADS Anxiety, HADS Depression, and the EPDS were all negatively correlated with total lymphocyte count (p<.05, one-tailed, for all). Because the four mood measurements were highly intercorrelated, the authors combined the results into a single factor they termed “dysphoria.” Dysphoria was significantly negatively correlated with total lymphocyte count (p<.03 one tailed). When divided into T cells and NK cells, the T cell count remained significantly negatively correlated with dysphoria (p<.03 one-tailed), but NK cell count did not.
In 2006, Groer and Davis sought to analyze the relationships among stress, immunity, and mood in breast-feeding as compared to formula-feeding mothers. They examined 181 healthy mothers at 4-6 weeks postpartum. Mood was measured using the Profile of Mood States (POMS) and a Total Mood Disturbance Score (TMDS) was calculated. This instrument (along with stress measures) was mailed to the mothers for self-administration at home prior to blood draws for IL-10 and IFN-γ. IFN-γ is the characteristic pro-inflammatory cytokine produced by CD4 Th1 cells. It activates macrophages and is crucial to the body's response to intravesicular pathogens. The authors note that the validity of the POMS is excellent but its reliability and consistency less so; they make no comment on validity or reliability of the TMDS. Neither breastfeeding women nor formula feeders showed any correlation between IL-10 and any of the mood measures. No regression model provided a significant source of variance in IL-10. The POMS depression score had a Pearson correlation of -0.24 (p<.05) with IFN-γ but was nonsignificant with the ratio. TMDS score correlation coefficient was -0.24 with IFN-γ and -0.27 with the ratio (p<.05 for both).
Groer and Morgan (2007) sought to compare the demographic, health, stress, immune, and endocrine factors in depressed postpartum women compared to their non-depressed counterparts. The POMS was administered to 200 women, and those in the top-scoring decile (n=25, mean score 32.1, range 21-47) were deemed to be depressed. The remaining 175 women had a mean score of 5.7 (range 0-20). This study is a secondary analysis using the same data collected in Groer's 2006 study. Depressed mothers had lower IFN-γ concentrations in serum (p<.001). In addition, they had a lower IFN-γ/IL-10 ratio in both serum (p<.04) and whole blood culture supernatants (p<.009).
Corwin et al. (2008) examined 26 women recruited on day 0 postpartum for a larger study on fatigue. Women provided clean-catch urine samples on postpartum days 0, 7, 14, and 28, and completed the CES-D on day 28. There were no differences in urinary IL-6 levels based on the level of depressive symptoms measured on day 28. IL-6 was found to fall over time in women without, but not in women with, depression (p = .001), but the authors themselves question this finding because IL-6 was undetectable in most women in days 14 and 28. Women with depressive symptoms on day 28 as measured by the CES-D had elevated levels of urinary IL-1β on day 14, but there was no significant change over time in the depressed group or the control group.
Boufidou et al. (2009) studied cerebrospinal fluid (CSF) and plasma cytokines in 56 Greek parturients. Mood was measured using the Posptartum Blues Questionnaire (PPBQ) at 1-4 days postpartum and the EPDS at 1 and 6 weeks postpartum. Those with PPBQ scores >8.2 or EPDS scores >11 were deemed to have depressed mood. Of note, the EPDS scoring instructions indicate that >10 is possible depression and >13 is likely depression; for the PPBQ, 3-8 indicates mild-moderate blues and >9 severe blues. There was no significant relationship between mood and serum IL-6 levels, though the authors do report a trend result in the immediate postpartum (p = 0.09). The authors report this result as significant by using a cutoff of p <.1. There was a positive association between IL-6 measured in the CSF at postpartum days 1-4 (p < .04), postpartum week 1 (p < .04), and postpartum week 6 (p <.01). CSF TNF-α at delivery was significantly correlated with a high score on the PPBQ in days 1-4 (p=.03), as was serum TNF-α at delivery (p = .02). There was also a significant association between increased CSF TNF-α at delivery and EPDS score at 1 week postpartum (p = .009); no other significant associations with TNF-α were found.
The Skalkidou et al. (2009) study involved 347 women, with study visits both at delivery and in the postpartum. Mood was measured by a structured questionnaire including the EPDS at five days, six weeks, and six months after delivery. Women were deemed to have postpartum depression if they scored 14 or greater at 5 days or 12 or greater on the EPDS at either of the other two time points. Sixty-seven women were thus deemed to have postpartum depression. Serum was drawn at delivery only. There was no difference in mean IL-6 levels between cases and controls.
Okun et al. (2011) looked at 56 pregnant women with a past history of either MDD or PPD, but not depressed at time of enrollment. At eight points during the first 17 weeks postpartum, blood was drawn to measure various hormones and the women were evaluated for depressive symptoms (Hamilton Depression Rating Scale, or HAM-D). The primary aim of the study was to assess the relationship among sleep, depressive symptoms, and hormone levels, but a post-hoc analysis was also done of a single measure of IL-6 (unspecified when). There was no significant relationship between IL-6 level and recurrence of depression (though a trend was noted for the interaction among these two factors and sleep quality).
Kondo et al. (2011) enrolled 139 lactating Japanese mothers, who filled out a questionnaire and provided samples of breast milk at their children's 3-month checkups. Breast milk was assayed for TGF-β, a central cytokine in the regulation of the immune system. TGF-β has an important role in developing the mucosal immune system in infants, and seems to play a dual role: decreased TGF-β is thought to render the individual more susceptible to immune dysregulation and the rise of a pro-inflammatory state, and that dysregulation can then lead to increased levels of TGF-β and other anti-inflammatory cytokines (Saxena et al. 2008). Mood in the lactating mothers was assessed using the EPDS, using 9 as the cut-off for defining postpartum depression. Depressed mothers were found to have a 4.4-fold (CI 1.5-13) higher likelihood to have increased levels of TGF-β in their breast milk.
Finally, Fransson et al. (2012) measured the relationship between maternal emotions and cytokines at birth in both maternal serum and cord blood, and whether those parameters differed between women who delivered preterm and those who delivered at term. The sample included 27 women who delivered preterm and 37 who delivered at term. Emotions were measured within 5 days of birth using the Positive and Negative Affect Scale (PANAS) and a structured clinical interview with questions about depression. Blood was drawn for cytokines during labor, and cord blood was sampled immediately after delivery. IL-1a, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12 p70, IL-13, IL-17, IL-18, and IFN-γ were all measured. Significant associations were found for the preterm group only between depressive symptoms and maternal IL-8 (p = .013) and cord IL-18 (p = .038) and between negative affect and maternal IL-6 (p = .016), cord IL-6 (p = .001) and cord IL-18 (p = .023). IL-8 is produced by macrophages and other cells with toll-like receptors. It is an important mediator of the innate immune response, and its primary purpose is the induction of chemotaxis in neutrophils and other target cells. IL-18 is a pro-inflammatory cytokine produced by macrophages and epithelial cells, among others; it induces the production of IFN-γ.
In sum, one study found an association between total lymphocyte count and depressed mood. One found a correlation between IFN-γ and the IFN-γ/IL-10 ratio, but no correlation with IL-10 alone. One study found a correlation between IL-1β and mood. One study found an association between negative affect and maternal and cord IL-6, but four studies failed to find an association between IL- 6 (three in serum, one in urine) and mood. One study found an association between depressive symptoms and maternal IL-8 and between depressive symptoms and negative affect and maternal and cord IL-18. One study found an association between CSF IL-6 and mood, and between CSF TNF-α and mood. One study found an association between depression and increased TGF-β in breast milk. One study found an association between urinary IL-1β and mood. One study each used the SBS, the PANAS, the HADS, the HAM-D, and the PPBQ, and one used a self-created “dysphoria score.” Two each used the POMS and TMDS, and three used the EPDS.
5. Discussion
The studies reviewed above demonstrate that a number of researchers over the last twenty years have attempted to explore the relationships among mediators of inflammation and perinatal mood and anxiety symptoms. These initial attempts at building a literature in this young field suffer, however, from a lack of coordination in terms of how to measure both mood and inflammation and from a conflation of antenatal with postpartum depression.
The antenatal group of articles, all published within the last four years, show significant strengths in research design. The two studies by Christian et al. (2009 and 2010) include a high psychosocial risk population and consider racial differences, as well as possible confounders including BMI and health behaviors. Moreover, the authors take care to examine cytokines that are already implicated in other pregnancy morbidities. The second study utilizes a design unique in this literature, of measuring a provoked inflammatory response, which may provide results more applicable to clinical situations. Both studies are limited, however, by small sample sizes, a restricted range of socioeconomic status, and lack of a healthy non-pregnant control group. The first study is limited by collection of data at only a single time point early in gestation, and the second by measurement of a cytokine that is rarely used in other comparable studies and therefore limits comparison. The Cassidy-Bushrow et al. study (2012) has similar strengths, with its inclusion of an African American population (important because African Americans suffer disproportionately from the other inflammatory morbidities of pregnancy) and its large variety of cytokines studied; it also has a large sample size. All three studies rely on the CES-D for mood measures. This is a valid and reliable screening tool that is not used in clinical diagnosis. While the broader literature on inflammation and depression does make use of such screening tools, information about both clinical diagnosis and severity of symptoms would be ideal. Moreover, the reliance on a tool that measures somatic symptoms – important when considering inflammation, as “sickness behaviors” include the somatic – is nevertheless problematic, because somatic symptoms of depression and those of pregnancy can be similar. This reliance emphasizes the need for a new instrument, one that can take these differences into account.
The Blackmore (2011) study is a promising harbinger of the future, with many strengths including a large sample, a prospective design, measurements taken at multiple time points, use of the most widely used clinical tool to diagnose depression in pregnancy, inclusion of birth outcomes, and within-participant comparisons. Limitations include the use of a medically low-risk population (thus excluding those very women for whom the inflammatory link may be most profound) and the lack of socioeconomic diversity in the sample.
The studies that measure both antenatal and postpartum mood have many strengths – though all conflate what may be two separate states. While we know that the greatest risk factor for postpartum depression is antenatal depression, there is nevertheless some evidence that these may be two separate disorders, as a portion of women develop postpartum depression independent of any depressive symptoms during pregnancy (Wisner et al. 2013); they certainly represent different endocrine and inflammatory states. Both the Scrandis et al. (2008) and Schmeelk et al. (1999) studies used blood draws at multiple time points in an attempt to account for changes across time. In the Scrandis et al. study, however, the IL-1β sample was usable in only 4 subjects, blood was drawn many weeks before mood was assessed, inflammatory markers were measured during pregnancy and mood after, and the authors did not attempt a direct correlation between mood and inflammatory markers. The Schmeelk group did assess mood at the same time as inflammatory markers, and also attempted to account separately for typical and atypical symptoms. The small size of the study, however, meant that only two women met criteria for major depression during the study (indicating, perhaps, that we might best conceive of studies in this field by looking at type or severity of symptoms rather than particular diagnostic categories). Moreover, these two inflammatory measures are both mediators of the acute-phase response and thus do not help us to understand the complex inflammatory shifts that may be occurring among different types of cytokines.
The strengths of all the Maes et al. studies (1999, 2000, 2001) are the measurement of mood and cytokines at multiple points, and the administration of mood measures at the same time as blood draws. However, there are limitations: Mood was measured with instruments not specifically designed for the perinatal period, and depression history was retrospectively recalled by women 6-10 months after delivery. The cytokines measured, while all plausible to have a role in depressive symptomatology, do not follow any pattern, with some being produced by activated macrophages and others having different origins (such as epithelial cells). Moreover, these studies look specifically at mood and anxiety in the puerperium, rather than at the possibly longer-lasting symptoms characteristic of antenatal and postpartum depression. Finally, in analyses they combine symptoms and inflammatory measures that occur prenatally with those that occur postpartum, thus eliding any distinction in inflammatory status between the pregnant and postpartum periods.
The final group of studies was limited to mood changes in the postpartum period only. The Hucklebridge study, from 1994 by far the earliest of the studies reviewed, is unique in that it looks at lymphocytes rather than cytokines. Strengths include the relatively large sample size, the use of multiple mood measures, and employment of a large variety of inflammatory measures. Yet several limitations make it difficult to group the results of this study with the others reviewed here. The authors created a new “dysphoria” factor, impossible to compare to the more traditional mood measures used in other studies, did not report on cytokines, and did not attempt to distinguish between Th1 and Th2 cells, a crucial distinction in pregnancy); they also used one-tailed t-tests.
The two studies by Groer and collaborators (2006 (2007), by contrast, do attempt to assess the Th1/Th2 distinction. Both studies include a large sample size and measure the ratio between characteristic cytokines for the two types of T cells. Conclusions are limited by the fact that data were collected at only one time point while mood measures (which were self-administered) did not occur at the same time as the blood draw. In addition, there were demographic differences between the two groups; women in the breastfeeding group were older, more likely to be married, and of higher socioeconomic status.
Four of the postpartum studies look at levels of IL-6. While three of the four did not find an association between IL-6 and mood, it is difficult to pool the results of these studies due to the significant differences in design and measurement. The Corwin et al. (2008) study, which did measure inflammatory markers at multiple time points, is limited by a small sample size (yielding only nine depressed women) and by the use of urinary rather than serum measures (while perfectly valid and in fact probably a better measurement of another cytokine measured, IL-1β, the method limits comparability to other studies). The Boufidou et al. study (2009) introduced measurements of both serum and CSF, thus allowing for a more nuanced view of brain-level inflammation – but measured cytokines only at the time of delivery, so no comment could be made about changing levels of cytokines across the early postpartum period. Moreover, the authors used lower than usual cut-off scores, thus possibly inflating their sample of depressed women. The Skalkidou et al. (2009) study had a large number of participants, including a large number of depressed women, and used the scale most commonly used clinically in the perinatal period (the EPDS). All three studies measured mood at different time points than inflammation, thus making it hard to determine the relationship, if any. The Okun et al. study (2011), by contrast, did make a direct comparison – but this comparison was a post-hoc analysis of a single time point, despite the fact that inflammatory markers were measured at multiple time points and the study had the rare strength of extending until 17 weeks.
The Kondo et al. (2011) and Fransson et al. (2012) studies, most recent of this group, did avoid some of the limitations of the earlier work in this subset. The Kondo et al. study has a relatively large sample size, controls for possible confounders such as smoking, alcohol use, and history of allergic disease, and is highly representative (the sample includes all lactating mothers in the city). Mood and inflammation were measured at the same time, and the result is compelling – but the study is limited by the lack of control of other factors such as infection and maternal genetic traits, by self-report of depressive symptoms, and by use of a low cut-off on the EPDS (9 as opposed to the recommended 13) to define postpartum depression. Moreover, the study uses a both a marker (TGF-β) and a medium (breast milk) that are unique in this literature, so it is hard to pool results with those of other studies. The Fransson et al. study attempts a more comprehensive immune profile than the others reviewed here, and includes cytokines from a broad range of sources (Th1, Th2, and activated macrophages among others), and the measurement of both serum and cord blood. Limitations include the small sample size and the use of single question to determine depression retrospectively (thus introducing the possibility of recall bias).
The most striking conclusion to be drawn from the above concerns the difficulty of using the existing literature to make any definitive statement about the relationship between perinatal mood and inflammatory markers. The studies have used such a broad range of mood instruments, using different cut-off points, that it is hard to compare their results. Moreover, no study uses an instrument (indeed, one does not exist) that can account for the important distinction between somatic symptoms of depression and those of pregnancy. In addition, a number of them measured mood and inflammation at different time points – even on either side of parturition – making it even more difficult to draw an etiological line between the two. In addition to this heterogeneity in tools to measure mood, there is enormous diversity and little pattern to which cytokines have been measured.
While the data thus far do not allow for conclusions or even generalizations about the role of inflammatory processes in perinatal depression, these studies do suggest the importance of future research in this area. What the field needs now is a more nuanced approach to define exactly what that relationship is. First, future studies should carefully distinguish between antenatal and postpartum depressed states, as these represent two radically different endocrine and inflammatory states and may in fact be two distinct disorders. Second, studies would benefit from a close consideration of the link between hormone states and inflammatory markers. We know that some women are more sensitive to abrupt hormone shifts than others (Bloch et al. 2000) – could inflammation be a possible mediator for this sensitivity? Current studies that measure inflammation do not measure hormones nor attempt to account for differences among women in baseline hormonal states. Third, future work would benefit from a reconsideration of how to measure mood changes in this period, particular in light of the possible conflation of somatic symptoms of depression with pregnancy symptoms (Ji et al. 2011). It is impossible to compare the mood results of the above studies due to different screens and different cut-off points – and we may be able to shed more light on this area by examining clusters of symptoms along a continuum and incorporating NIMH's dimensional approach to characterizing psychopathology as outlined in the RDoC initiative (http://www.nimh.nih.gov/research-funding/rdoc/index.shtml), rather than continuing to study only those who meet particular diagnostic categories.
Finally, and perhaps most important, a crucial element of future studies will include defining exactly what type of inflammatory shifts may have significance in this period. During both pregnancy and the postpartum, what inflammatory changes are important for mood states? Possibilities include the acute phase response, the cytokines released by activated macrophages, and the Th1/Th2 shift, as well as the activities of natural killer cells. It will also be important to distinguish between local and systemic inflammation, as studies of preterm birth and preeclampsia are doing already. (Admittedly, this poses a greater logistical challenge for brain research, as it will require CSF samples and/or brain imaging, a much more daunting proposition than, say, cervical swabs or placental samples.) Given the possible link between perinatal depression and other inflammatory disorders of pregnancy, however, it will be crucial to design studies that can measure inflammation in the same ways that it is already being measured in the much more extensive literature on preeclampsia and preterm birth.
Designing studies that look specifically at these distinct areas may help us to refine our theories and also to link perinatal depression more broadly to other inflammatory pregnancy morbidities. It will also help us to place depression in the context of other inflammatory disorders, such as autoimmune and atopic diseases, that have different patterns of improving and worsening symptoms during pregnancy. Understanding just how inflammatory changes in pregnancy affect mood may thus help us to think of novel anti-inflammatory treatment strategies that will affect not only mood disorders but the broader range of inflammatory disorders of pregnancy. Novel treatment strategies may include the definition of new treatment targets, such as cytokines, intracellular inflammatory messengers, glial cells, and neurogenesis (Maes et al. 2009, Kern et al. 2012). In exploring such links, we will likely find new ways to improve the inextricably linked disorders of the body, the womb, and the mind.
Contributor Information
Lauren M. Osborne, Department of Psychiatry, Columbia University/New York State Psychiatric Institute, New York, NY, USA.
Catherine Monk, Departments of Psychiatry and Obstetrics & Gynecology, Columbia University, New York, NY, USA.
References
- Abou-Saleh MT, Ghubash R, Karim L, Krumski M, Anderson DN. The role of pterins and related factors in the biology of early postpartum depression. Eur Neuropsychopharmacol. 1999;9:295–300. doi: 10.1016/s0924-977x(98)00038-8. [DOI] [PubMed] [Google Scholar]
- Ahn H, Park J, Gilman-Sachs A, Kwak-Kim J. Immunologic characteristics of preeclampsia: a comprehensive review. Am J Reprod Immunol. 2011;65:377–394. doi: 10.1111/j.1600-0897.2010.00913.x. [DOI] [PubMed] [Google Scholar]
- Albacar G, Sans T, Martín-Santos R, García-Esteve L, Guillamat R, Sanjuan J, Cañellas F, Gratacòs M, Cavalle P, Arija V, Gaviria A, Gutiérrez-Zotes A, Vilella E. An association between plasma ferritin concentrations measured 48 h after delivery and postpartum depression. J Affect Disord. 2011;131:136–142. doi: 10.1016/j.jad.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Alder J, Fink N, Bitzer J, Hösli I, Holzgreve W. Depression and anxiety during pregnancy: a risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. J Matern Fetal Neonatal Med. 2007;20:189–209. doi: 10.1080/14767050701209560. [DOI] [PubMed] [Google Scholar]
- Andersson L, Sundstrom-Poromaa I, Wulff M, Astrom M, Bixo M. Implications of antenatal depression and anxiety for obstetric outcome. Obstet Gynecol. 2004;104:467–476. doi: 10.1097/01.AOG.0000135277.04565.e9. [DOI] [PubMed] [Google Scholar]
- Andersson L, Sundstrom-Poromaa I, Wulff M, Astrom M, Bixo M. Neonatal outcome following maternal antenatal depression and anxiety: A population-based study. Am J Epidemiol. 2004a;159:872–881. doi: 10.1093/aje/kwh122. [DOI] [PubMed] [Google Scholar]
- Bach J, Rinn B, Meyer B, Dodel R, Bacher M. Role of MIF in inflammation and tumorigenesis. Oncology. 2008;75:127–33. doi: 10.1159/000155223. [DOI] [PubMed] [Google Scholar]
- Bansil P, Kuklina EV, Meikle SF, Posner SF, Kourtis AP, Ellington SR, Jamieson DJ. Maternal and fetal outcomes among women with depression. J Womens Health. 2010;19(2):329–334. doi: 10.1089/jwh.2009.1387. [DOI] [PubMed] [Google Scholar]
- Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, Menon R, Van Look PFA. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull 31 World Health Organ. 2010;88:31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. 2004;103:698–709. doi: 10.1097/01.AOG.0000116689.75396.5f. [DOI] [PubMed] [Google Scholar]
- Berle JO, Mykletun A, Daltveit AK, Rasmussen S, Holsten F, Dahl AA. Neonatal outcomes in offspring of women with anxiety and depression during pregnancy. A linkage study from The Nord-Trondelag Health Study (HUNT) and Medical Birth Registry of Norway. Arch Womens Ment Health. 2005;8:181–189. doi: 10.1007/s00737-005-0090-z. [DOI] [PubMed] [Google Scholar]
- Blackmore ER, Moynihan JA, Rubinow DR, Pressman EK, Gilchrist M, O’Connor TG. Psychiatric Symptoms and Proinflammatory Cytokines in Pregnancy. Psychosom Med. 2011;73:656–663. doi: 10.1097/PSY.0b013e31822fc277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157:924–30. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- Bo S, Signorile A, Menato G, Gambino R, Bardelli C, Gallo ML, Cassader M, Massobrio M, Pagano GF. C-reactive protein and tumor necrosis factor-alpha in gestational hyperglycemia. J Endocrinol Invest. 2005;28:779–786. doi: 10.1007/BF03347566. [DOI] [PubMed] [Google Scholar]
- Boufidou F, Lambrinoudaki I, Argeitis J, Zervas IM, Pliatsika P, Leonardou AA, Petropoulos G, Hasiakos D, Papadias K, Nikolaou C. CSF and plasma cytokines at delivery and postpartum mood disturbances. J Affect Disord. 2009;115:287–292. doi: 10.1016/j.jad.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LA. Depression during pregnancy and postpartum: contribution of stress and ovarian hormones. Prog Neuropsychpharmacol Biol Psychiatry. 2010;34:766–776. doi: 10.1016/j.pnpbp.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Cassidy-Bushrow AE, Peters RM, Johnson DA, Templin TN. Association of depressive symptoms with inflammatory biomarkers among pregnant African-American women. J Reprod Immunol. 2012;94:202–209. doi: 10.1016/j.jri.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Charney DS. Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry. 1998;59(Suppl. 14):11–14. [PubMed] [Google Scholar]
- Chen SJ, Liu YL, Sytwu HK. Immunologic regulation in pregnancy: from mechanism to therapeutic strategy for immunomodulation. Clin Dev Immunol. 2012 doi: 10.1155/2012/258391. Article ID 258391, 10 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Franco A, Glaser R, Iams JD. Depressive symptoms are associated with elevated serum proinflammatory cytokines among pregnant women. Brain Behav Immun. 2009;23:750–754. doi: 10.1016/j.bbi.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Franco A, Iams JD, Sheridan J, Glaser R. Depressive symptoms predict exaggerated inflammatory responses to an in vivo immune challenge among pregnant women. Brain Behav Immun. 2010;24:49–53. doi: 10.1016/j.bbi.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM. Psychoneuroimmunology in pregnancy: Immune pathways linking stress with maternal health, adverse birth outcomes, and fetal development. Neurosci Biobehav Rev. 2012;36:350–361. doi: 10.1016/j.neubiorev.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coico R, Sunshine G, Benjamini E. Immunology: A Short Course. ed 5. Hoboken, NJ: Wiley-Liss; 2003. [Google Scholar]
- Corwin EJ, Bozoky I, Pugh LC, Johnston N. Interleukin-1β elevation during the postpartum period. Ann Behav Med. 2003;25(1):41–47. doi: 10.1207/S15324796ABM2501_06. [DOI] [PubMed] [Google Scholar]
- Corwin EJ, Johnston N, Pugh L. Symptoms of postpartum depression associated with elevated levels of Interleukin-1β during the first month postpartum. Biol Res Nurs. 2008;10:128–133. doi: 10.1177/1099800408323220. [DOI] [PubMed] [Google Scholar]
- Corwin EJ, Pager K. The psychoneuroimmunology of postpartum depression. J Womens Health. 2008;17(9):1529–1534. doi: 10.1089/jwh.2007.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussons-Read ME, Okun ML, Schmitt MP, Glesse S. Prenatal stress alters cytokine levels in a manner that may endanger human pregnancy. Psychosom Med. 2005;67:625–631. doi: 10.1097/01.psy.0000170331.74960.ad. [DOI] [PubMed] [Google Scholar]
- Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun. 2007;21:343–350. doi: 10.1016/j.bbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Coussons-Read ME, Lobel M, Carey JC, Kreither MO, D’Anna K, Argys L, Ross RG, Brandt C, Cole S. The occurrence of preterm delivery is linked to pregnancy-specific distress and elevated inflammatory markers across gestation. Brain Behav Immun. 2012;26(4):650–9. doi: 10.1016/j.bbi.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudihy D, Lee RV. The pathophysiology of pre-eclampsia: current clinical concepts. J Obstet Gynecol. 2009;29:576–582. doi: 10.1080/01443610903061751. [DOI] [PubMed] [Google Scholar]
- Dalfra MG, Nicolucci A, Bisson T, Bonsembiante B, Lapolla A QLISG, Quality of Life Italian Study Group. Quality of life in pregnancy and postpartum: a study in diabetic patients. Qual Life Res. 2012;21(2):291–298. doi: 10.1007/s11136-011-9940-5. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolea C, AbouZahr C. Global Burden of Disease 2000. Evidence and Information for Policy (EIP), World Health Organization; Geneva: 2000. Global burden of hypertensive disorders of pregnancy in the year 2000. July 2003. www.who.int/healthinfo/…/bod_hypertensivedisordersofpregnancy.p…. [Google Scholar]
- Dowlati Y, Hermann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A Meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Enayati M, Solati J, Hosseini MH, Shahi HR, Saki G, Salari AA. Maternal infection during late pregnancy increases anxiety- and depression-like behaviors with increasing age in male offspring. Brain Res Bull. 2012;87(2-3):295–302. doi: 10.1016/j.brainresbull.2011.08.015. 10. [DOI] [PubMed] [Google Scholar]
- Fransson E, Dubicke A, Byström B, Ekman-Ordeberg G, Hjelmstedt A, Lekander M. Negative emotions and cytokines in maternal and cord serum at preterm birth. Am J Reprod Immunol. 2012;67:506–514. doi: 10.1111/j.1600-0897.2011.01081.x. [DOI] [PubMed] [Google Scholar]
- Gabay C, Smith MF, Eidlen D, Arend WP. Interleukin 1 receptor antagonist (IL-1Ra) is an acute-phase protein. J Clin Invest. 1997;99:2930–2940. doi: 10.1172/JCI119488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Gale S, Harlow BL. Postpartum mood disorders: a review of clinical and epidemiological factors. J Psychosom Obstet Gynecol. 2003;24(4):257–266. doi: 10.3109/01674820309074690. [DOI] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehener G, Swinson T. Perinatal depression: A systematic review of prevalence and incidence. Obstet Gynecol. 2005;106:1071–83. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, Brody S, Miller WC. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ) 2005;119:1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholid and atypical depression: high vs. low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer IA, Lyall F, Perera T, Boswell F, Macara LM. Increased concentration of cytokines interleukin-6 and interleukin-1 receptor antagonist in plasma of women with preeclampsia: a mechanism of endothelial dysfunction? Obstet Gynecol. 1994;84:937–940. [PubMed] [Google Scholar]
- Groer MW, Davis M, Casey K, Short B, Smith K, Groer S. Neuroendocrine and immune relationships in postpartum fatigue. Am J Mat Child Nursing. 2005;30:133–138. doi: 10.1097/00005721-200503000-00012. [DOI] [PubMed] [Google Scholar]
- Groer MW. Differences between exclusive breastfeeders, formula-feeders, and controls: a study of stress, mood, and endocrine variables. Biol Res Nurs. 2005a;7:106–117. doi: 10.1177/1099800405280936. [DOI] [PubMed] [Google Scholar]
- Groer MW, Davis MW, Smith K, Casey K, Kramer V, Bukovsky E. Immunity, inflammation and infection in post-partum breast and formula feeders. Am J Reprod Immunol. 2005b;54:222–231. doi: 10.1111/j.1600-0897.2005.00301.x. [DOI] [PubMed] [Google Scholar]
- Groer MW, Davis MW. Cytokines, infections, stress, and dysphoric moods in breastfeeders and formula feeders. J Obstet Gynecol Neonatal Nurs. 2006;35(5):599–607. doi: 10.1111/j.1552-6909.2006.00083.x. [DOI] [PubMed] [Google Scholar]
- Groer MW, Morgan K. Immune, health, and endocrine characteristics of depressed postpartum mothers. Psychoneuroendocrinology. 2007;32:133–139. doi: 10.1016/j.psyneuen.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Groër MW, Yolken RH, Xiao JC, Beckstead JW, Fuchs D, Mohapatra SS, Seyfang A, Postolache TT. Prenatal depression and anxiety in Toxoplasma gondii-positive women. Am J Obstet Gynecol. 2011;204:433.e1–7. doi: 10.1016/j.ajog.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote NK, Bridge JA, Gavin AR, Melville JL, Iyengar S, Katon WJ. A Meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012–1024. doi: 10.1001/archgenpsychiatry.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschfeld RM. History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry 61 Suppl. 2000;6:4–6. [PubMed] [Google Scholar]
- Hoffman S, Hatch MC. Depressive symptomatology during pregnancy: Evidence for an association with decreased fetal growth in pregnancies of lower social class women. Health Psychol. 2000;19:535–543. [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-Reactive Protein, IL-1, and IL-6: A meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Hucklebridge FH, Smith MD, Clow A, Evans P, Glover V, Taylor A, Adams D, Lydyard PM. Dysphoria and immune status in postpartum women. Biol Psychol. 1994;37:199–206. doi: 10.1016/0301-0511(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Ji S, Long Q, Newport DJ, Na H, Knight B, Zach EB, Morris NJ, Kutner M, Stowe ZN. Validity of depression rating scales during pregnancy and the postpartum period: impact of trimester and parity. J Psychiatr Res. 2011;45(2):213–19. doi: 10.1016/j.jpsychires.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson S, Kristjánsson S, Bjarnarson SP, Wennergren G, Rudin A. Clara cell protein 16 (CC16) serum levels in infants during respiratory syncytial virus infection. Acta Paediatr. 2009;98:579–581. doi: 10.1111/j.1651-2227.2008.01083.x. [DOI] [PubMed] [Google Scholar]
- Kammerer M, Taylor A, Glover V. The HPA axis and perinatal depression: a hypothesis. Arch Womens Ment Health. 2006;9:187–196. doi: 10.1007/s00737-006-0131-2. [DOI] [PubMed] [Google Scholar]
- Katon JG, Russo J, Gavin AR, Melville JL, Katon WJ. Diabetes and depression in pregnancy: is there an association? J Womens Health. 2011;20(7):983–989. doi: 10.1089/jwh.2010.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi M, Adachi M, Oda N, Kokubu F, Huang S. IL-17 cytokine family. J Allergy Clin Immunol. 2004;114:1265–1273. doi: 10.1016/j.jaci.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Kaxuhiko K, et al. Urinary protein1/Clara cell 16 concentrations and lung functions in male subjects with pneumoconiosis. Ann Clin Biochem. 2007;44:560–562. doi: 10.1258/000456307782268110. [DOI] [PubMed] [Google Scholar]
- Kendall-Tackett K. A new paradigm for depression in new mothers: The central role of inflammation and how breastfeeding and anti-inflammatory treatments protect maternal mental health. Int Breastfeed J. 2007;2(6) doi: 10.1186/1746-4358-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern N, Sheldrick AJ, Schmidt FM, Minkwitz J. Neurobiology of depression and novel antidepressant drug targets. Curr Pharm Des. 2012;18(36):5791–801. doi: 10.2174/138161212803523581. [DOI] [PubMed] [Google Scholar]
- Kharaghani R, Geranmaye M, Janani L, Hantooshzade S, Arbabi M, Rahmani Bilandi R, Bagheri F. Preeclampsia and depression: a case-control study in Tehran. Arch Gynecol Obstet. 2012;286(1):249–253. doi: 10.1007/s00404-012-2260-3. [DOI] [PubMed] [Google Scholar]
- Kim C, Brawarsky P, Jackson RA, Fuentes-Afflick E, Haas JS. Changes in health status experienced by women with gestational diabetes and pregnancy-induced hypertensive disorders. J Womens Health. 2005;14:729–736. doi: 10.1089/jwh.2005.14.729. [DOI] [PubMed] [Google Scholar]
- Kinalski M, Telejko B, KuYmicki M, Kretowski A, Kinalska I. Tumor necrosis factor system and plasma adiponectin concentration in women with gestational diabetes. Horm Metab Res. 2005;37:450–454. doi: 10.1055/s-2005-870238. [DOI] [PubMed] [Google Scholar]
- Kohl C, Walch T, Huber R, Kemmler G, Neurater G, Fuchs D, Solder E, Schrocksnadel H, Sperner-Unterweger B. Measurement of tryptophan, kynurenine and neopterin in women with and without postpartum blues. J Affect Disord. 2005;86:135–142. doi: 10.1016/j.jad.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Kondo N, Suda Y, Nakao A, Oh-Oka K, Suzuki K, Ishamaru K, Sato M, Tanaka T, Nagai A, Yamagata Z. Maternal psychosocial factors determining the concentrations of transforming growth factor-beta in breast milk. Pediatr Allergy Immunol. 2011;22:853–861. doi: 10.1111/j.1399-3038.2011.01194.x. [DOI] [PubMed] [Google Scholar]
- Kozhimannil KB, Pereira MA, Harlow BL. Association between diabetes and perinatal depression among low-income mothers. JAMA. 2009;301:842–847. doi: 10.1001/jama.2009.201. [DOI] [PubMed] [Google Scholar]
- Kraus TA, Sperling RS, Engel SM, Lo Y, Kellerman L, Singh T, Loubeau M, Ge Y, Garrido JL, Rodríguez-García M, Moran TM. Peripheral blood cytokine profiling during pregnancy and postpartum periods. Am J Reprod Immunol. 2010;64:411–426. doi: 10.1111/j.1600-0897.2010.00889.x. [DOI] [PubMed] [Google Scholar]
- Kraus TA, Engel SM, Sperling RS, Kellerman L, Lo Y, Wallenstein S, Escribese MM, Garrido JL, Singh T, Loubeau M, Moran TM. Characterizing the pregnancy immune phenotype: Results of the viral imunity and pregnancy (VIP) study. J Clin Immunol. 2012;32:300–311. doi: 10.1007/s10875-011-9627-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurki T, Hiilesmaa V, Raitasalo R, Mattila H, Ylikorkala O. Depression and anxiety in early pregnancy and risk for preeclampsia. Obstet Gynecol. 2000;95:487–490. doi: 10.1016/s0029-7844(99)00602-x. [DOI] [PubMed] [Google Scholar]
- Langer N, Langer O. Comparison of pregnancy mood profiles in gestational diabetes and preexisting diabetes. Diabetes Educ. 2000;26:667–672. doi: 10.1177/014572170002600414. [DOI] [PubMed] [Google Scholar]
- Larocca L, Ramhorst R, Roca V, Calafat M, Eisenberg J, Franchi A, Perez Leiros C. Neuroimmune-endocrine enteractions during early pregnancy in an autoimmune context: Focus on macrophage activation. Neuroimmunomodulation. 2008;15:84–90. doi: 10.1159/000135628. [DOI] [PubMed] [Google Scholar]
- Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev. 2012;36(2):764–85. doi: 10.1016/j.neubiorev.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Lowe LP, Metzger BE, Lowe WL, Jr, Dyer AR, McDade TW, McIntyre HD. HAPO Study Cooperative Research Group, 2010. Inflammatory mediators and glucose in pregnancy: results from a subset of the hyperglycemia and adverse pregnancy outcome (HAPO) study. J Clin Endocrinol Metab. 95:5427–5434. doi: 10.1210/jc.2010-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Ombelet W, Libreccht I, Steves K, Kenis G, De Jongh R, Lin A, Cox J, Bosmans E. Effects of pregnancy and delivery on serum concentrations of Clara Cell Protein (CC16), an endogenous anticytokine: lower serum CC16 is related to postpartum depression. Psychiatry Res. 1999;87:117–127. doi: 10.1016/s0165-1781(99)00073-6. [DOI] [PubMed] [Google Scholar]
- Maes M, Lin AH, Ombelet W, Stevens K, Kenis G, De Jongh R, Cox J, Bosmans E. Immune activation in the early puerperium is related to postpartum anxiety and depressive symptoms. Psychoneuroendocrinology. 2000;25:121–137. doi: 10.1016/s0306-4530(99)00043-8. [DOI] [PubMed] [Google Scholar]
- Maes M, Ombelet W, De Jongh R, Kenis G, Bosmans E. The inflammatory response following delivery is amplified in women who previously suffered from major depression, suggesting that major depression is accompanied by a sensitization of the inflammatory response system. J Affect Disord. 2001;63:85. doi: 10.1016/s0165-0327(00)00156-7. [DOI] [PubMed] [Google Scholar]
- Maes M, Ombelet W, Verkerk R, Bosmans E, Scharpe S. Effects of pregnancy and delivery on the availability of plasma tryptophan to the brain: relationships to delivery-induced immune activation and postpartum anxiety and depression. Psychol Med. 2001a;31:847–858. doi: 10.1017/s0033291701004007. [DOI] [PubMed] [Google Scholar]
- Maes M, Verkerk R, Bonaccorso S, Ombelet W, Bosmans E, Scharpe S. Depressive and anxiety symptoms in the early puerperium are related to increased degradation of tryptophan into kynurenine, a phenomenon which is related to immune activation. Life Sci. 2002;71:1837–1848. doi: 10.1016/s0024-3205(02)01853-2. [DOI] [PubMed] [Google Scholar]
- Maes M, Bosmans E, Ombelet W. In the puerperium, primiparae exhibit higher levels of anxiety and serum peptidase activity and greater immune responses that multiparae. J Clin Psychiatry. 2004;65(1):71–76. doi: 10.4088/jcp.v65n0112. [DOI] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mautner E, Greimel E, Trutnovsky G, Daghofer F, Egger JW, Lang U. Quality of life outcomes in pregnancy and postpartum complicated by hypertensive disorders, gestational diabetes, and preterm birth. J Psychosom Obstet Gynaecol. 2009;30:231–237. doi: 10.3109/01674820903254757. [DOI] [PubMed] [Google Scholar]
- McCoy SJ, Beal JM, Shipman SB, Payton ME, Watson GH. Risk factors for postpartum depression: A retrospective investigation at 4 weeks postnatal and a review of the literature. J Am Osteopath Assoc. 2006;106:193–8. [PubMed] [Google Scholar]
- Meltzer-Brody S. New insights into perinatal depression: pathogenesis and treatment during pregnancy and postpartum. Dialogues Clin Neurosci. 2011;13(1):89–100. doi: 10.31887/DCNS.2011.13.1/smbrody. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354(9193):1896–900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- National Diabetes Statistics. 2011 http://diabetes.niddk.nih.gov/dm/pubs/statistics/#Gestational.
- Okun ML, Luther J, Prather AA, Perel JM, Wisniewski S, Wisner K. Changes in sleep quality but not hormones predict time to postpartum depression recurrence. J Affect Disord. 2011;130:378–384. doi: 10.1016/j.jad.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- O’Mahony SM, Myint A, Van den Hove D, Desbonnet K, Steinbusch H, Leonard B. Gestational stress leads to depressive-like behavioural and immunological changes in the rat. Neuroimmunomodulation. 2006;13:82–88. doi: 10.1159/000096090. [DOI] [PubMed] [Google Scholar]
- Oppo A, Mauri M, Ramacciotti D, Camilleri V, Banti S, Borri C, Rambelli C, Montagnani MS, Cortopassi S, Bettini A, Ricciardulli S, Montaresi S, Rucci P, Beck CT, Cassano GB. Risk factors for postpartum depression: the role of the Postpartum Depression Predictors Inventory – Revised (PDPI-R). Results from the Perinatal Depression – Research & Screening Unit (PNDReScU) study. Arch Womens Ment Health. 2009;12:239–249. doi: 10.1007/s00737-009-0071-8. [DOI] [PubMed] [Google Scholar]
- Orr ST, James SA, Prince CB. Maternal prenatal depressive symptoms and spontaneous preterm birth among African-American women in Baltimore, Maryland. Am J Epidemiol. 2002;156:797–802. doi: 10.1093/aje/kwf131. [DOI] [PubMed] [Google Scholar]
- Palmsten K, Setoguchi S, Margulis AV, Patrick AR, Hernández-Díaz S. Elevated risk of preeclampsia in pregnant women with depression: depression or antidepressants? Am J Epidemiol. 2012;175(10):988–97. doi: 10.1093/aje/kwr394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazos M, Sperling RS, Moran THM, Kraus TA. The influence of pregnancy of systemic immunity. Immunol Res. 2012 doi: 10.1077/s12026-012-8303-9. published online 24 March 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Lowry CA, Rook GAW. Inflammation, sanitation, and consternation: Loss of contact with coevolved, tolerogenic microorganisms and the pathophysiology and treatment of major depression. Arch Gen Psychiatry. 2010;67(12):1211–1224. doi: 10.1001/archgenpsychiatry.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman CW. Immunology of preeclampsia. Semin Perinatol. 1991;15:257–262. [PubMed] [Google Scholar]
- Rosier A, Dupont P, Peuskens J, Bormans G, Vandenberghe R, Maes M, de Groot T, Schiepers C, Verbruggen A, Mortelmans L. Visualisation of loss of 5-HT2A receptors with age in healthy volunteers using [18F]altanserin and positron emission tomographic imaging. Psychiatry Res. 1996;68:11–22. doi: 10.1016/s0925-4927(96)02806-5. [DOI] [PubMed] [Google Scholar]
- Saxena V, Lienesch DW, Zhou M, Bommireddy R, Azhar M, Doetschman T, Singh RR. Dual roles of immunoregulatory cytokine TGF-beta in the pathogenesis of autoimmunity-mediated organ damage. J Immunol. 2008;180(3):1903–12. doi: 10.4049/jimmunol.180.3.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeelk KH, Granger DA, Susman EJ, Chrousos GP. Maternal depression and risk for postpartum complications: Role of prenantal corticotropin-releasing hormone and interleukin-1 receptor antagonist. Behav Med. 1999;25:88–93. doi: 10.1080/08964289909595741. [DOI] [PubMed] [Google Scholar]
- Scrandis DA, Langenberg P, Tonelli LH, Sheikh TM, Manogura AC, Alberico LA, Hermanstyne T, Fuchs D, Mighty H, Hasday JD, Boteva K, Postolache TT. Prepartum depressive symptoms correlate positively with C-Reactive Protein levels and negatively with tryptophan levels: a preliminary report. Int J Child Health Hum Dev. 2008;1(2):167–174. [PMC free article] [PubMed] [Google Scholar]
- Sikkema JM, Robles de Medina PG, Schaad RR, Mulder EJ, Bruinse HW, Buitelaar JK, Visser GH, Franx A. Salivary cortisol levels and anxiety are not increased in women destined to develop preeclampsia. J Psychosom Res. 2001;50:45–49. doi: 10.1016/s0022-3999(00)00208-7. [DOI] [PubMed] [Google Scholar]
- Skalkidou A, Sylvén SM, Papadopoulos FC, Olovsson M, Larsson A, Sundström-Poromaa I. Risk of postpartum depression in association with serum leptin and interleukin-6 levels at delivery: A nested case-control study within the UPPSAT cohort. Psychoneuroendocrinology. 2009;34:1329–1337. doi: 10.1016/j.psyneuen.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Smith RS. The macrophage theory of depression. Med Hypotheses. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- Steer RA, Scholl TO, Hediger ML, Fischer RL. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45:1093–1099. doi: 10.1016/0895-4356(92)90149-h. [DOI] [PubMed] [Google Scholar]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Torloni MR, Betran AP, Daheri S, Widmer M, Dolan S, Menon R, Bergel E, Allen T, Merialdi M. Maternal BMI and preterm birth: A systematic review of the literature with meta-analysis. J Matern-Fetal Neonatal Med. 2009;22(11):957–970. doi: 10.3109/14767050903042561. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Culhane JF, Rauh V, Barve SS, Hogan V, Sandman CA, Hobel CJ, Chicz-DeMet A, Dunkel-Schetter C, Garite TJ, Glynn L. Stress, infection and preterm birth: a biobehavioural perspective. Paediatr Perinat Epidemiol. 2001;15(suppl 2):17–29. doi: 10.1046/j.1365-3016.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- Wei SQ, Fraser W, Luo ZC. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet Gynecol. 2010;116(2):393–401. doi: 10.1097/AOG.0b013e3181e6dbc0. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Parry BL, Pointek CM. Clinical practice: Postpartum depression. N Engl J Med. 2002;347(3):194–199. doi: 10.1056/NEJMcp011542. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Sit DKY, McShea MC, Rizzo DM, Zoretich RA, Hughes CL, Eng HF, Luther JF, Wisniewski SR, Costantino ML, Confer AL, Moses-Kolko EL, Famy CS, Hanusa BH. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry. 2013:E1–E9. doi: 10.1001/jamapsychiatry.2013.87. published online March 13, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M, Sauk J, Shah A, Vossen Smirnakis K, Jimenez-Kimble R, Ecker JL, Thadhani R. Inflammation and glucose tolerance: a prospective study of gestational diabetes mellitus. Diabetes Care. 2004;27:21–27. doi: 10.2337/diacare.27.1.21. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Mental health aspects of women's reproductive health: a global review of the literature. Geneva: World Health Organization; 2009. [Google Scholar]
- Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–56. doi: 10.1146/annurev.immunol.21.120601.141142. [DOI] [PubMed] [Google Scholar]
- Yonkers KA, Vigod S, Ross LE. Diagnosis, pathophysiology, and management of mood disorders in pregnant and postpartum women. Obstet Gynecol. 2011;117:961–77. doi: 10.1097/AOG.0b013e31821187a7. [DOI] [PubMed] [Google Scholar]